Abstract
The purpose of this study was to delineate potential mechanisms initiating the onset of hepatic steatosis following the cessation of daily physical activity. Four-week-old, hyperphagic/obese Otsuka Long-Evans Tokushima Fatty rats were given access to voluntary running wheels for 16 weeks to prevent the development of hepatic steatosis. The animals were then suddenly transitioned to a sedentary condition as wheels were locked (wheel lock; WL) for 5 h (WL5), 53 h (WL53) or 173 h (WL173). Importantly after the cessation of daily exercise (5–173 h), no changes occurred in body weight, fat pad mass (omental and retroperitoneal), food intake, serum insulin, hepatic triglycerides or in the exercise-suppressed hepatic stearoyl-CoA desaturase-1 and peroxisome proliferator-activated receptor-γ protein content. However, complete hepatic fatty acid oxidation and mitochondrial enzyme activities were highest at WL5 and WL53 and dropped significantly to SED levels by WL173. In addition, cessation of daily exercise quickly increased the hepatic protein contents of fatty acid synthase and acetyl-coenzyme A carboxylase (ACC), reduced ACC phosphorylation status, and dramatically increased hepatic malonyl-CoA concentration. This study is the first to show that the sudden cessation of daily exercise in a hyperphagic/obese model activates a subgroup of precursors and processes known to initiate hepatic steatosis, including decreased hepatic mitochondrial oxidative capacity, increased hepatic expression of de novo lipogenesis proteins, and increased hepatic malonyl CoA levels; each probably increasing the susceptibility to non-alcoholic fatty liver disease.
Westernized societies are experiencing a weight gain epidemic, and recent epidemiological studies suggest an increased risk of coronary heart disease, type 2 diabetes and non-alcoholic fatty liver disease (NAFLD) in overweight and obese individuals. NAFLD is considered the hepatic manifestation of the metabolic syndrome (Farrell & Larter, 2006) and prevalence rates are as high as 75–100% in obese and morbidly obese individuals (Bellentani et al. 2000).
The Otsuka Long-Evans Tokushima Fatty (OLETF) rat is a commonly studied model of obesity and type 2 diabetes (Kawano et al. 1992). OLETF rats are selectively bred for null expression of the cholecystokinin-1 receptor and, thus, exhibit a within-meal feedback defect for satiety, resulting in hyperphagia and obesity (Moran & Bi, 2006), insulin resistance and type 2 diabetes (Moran & Bi, 2006), and multiple components of the metabolic syndrome (Miyasaka et al. 2003). In addition, our group and others have demonstrated that OLETF rats develop elevated liver mass (Miyasaka et al. 2003) and hepatic steatosis (Yeon et al. 2004; Rector et al. 2008).
Importantly, OLETF rats exhibit an inherent ability to maintain daily physical activity levels using voluntary running wheels, a quality which is absent in most obese animal models (Stern & Johnson, 1977; Bi et al. 2005). In the OLETF rat, voluntary wheel exercise suppresses increases in body weight (Miyasaka et al. 2003; Bi et al. 2005; Rector et al. 2008), enhances whole body insulin sensitivity (Shima et al. 1993), and prevents the development of type 2 diabetes (Shima et al. 1993). We have recently expanded these observations by reporting that daily physical activity on voluntary running wheels also significantly attenuates the development of hepatic steatosis in the OLETF rat by increasing hepatic fatty acid oxidation and reducing key de novo lipogenesis proteins acetyl-CoA carboxylase (ACC) and fatty acid synthase (FAS) (Rector et al. 2008).
Daily exercise prevents most chronic diseases and is commonly recommended for individuals diagnosed with NAFLD (Farrell & Larter, 2006). Conversely, recent cross-sectional studies in humans show that reduced habitual physical activity (Perseghin et al. 2007) and reduced cardiorespiratory fitness (Church et al. 2006) are associated with NAFLD. More importantly, while the lack of regular exercise or physical inactivity is an ‘actual’ cause of death (Mokdad et al. 2004), the effects of transitioning from high to low daily physical activity in a model known to develop hepatic steatosis is to our knowledge unknown.
The purpose of the current investigation was to determine if, and by which initiating molecules, the sudden removal of daily physical activity for 173 h in the OLETF rat would cause hepatic alterations in lipid metabolism implicated in the development of steatosis. We hypothesized that the sudden cessation of daily physical activity for 173 h in the OLETF rat would rapidly result in initiation of unique systemic and hepatic factors responsible for promoting hepatic steatosis.
Methods
Animal protocol
The animal protocol was approved by the Institutional Animal Care and Use Committee at the University of Missouri-Columbia. OLETF male rats at 4 week of age were kindly supplied by the Tokushima Research Institute, Otsuka Pharmaceutical (Tokushima, Japan). Upon arrival, each rat was randomly designated as either a runner or non-runner. The average initial body weights for runners and non-runners were 79.2 ± 2.5 g and 80.0 ± 3.9 g, respectively. The runners were immediately housed (at the age of 28 days) in cages equipped with voluntary running wheels outfitted with a Sigma Sport BC 800 bicycle computer (Cherry Creek Cyclery, Foster Falls, VA, USA) for measuring daily running activity. Voluntary running was selected to approximate the more natural activity state of the animal. Cages were in temperature-controlled animal quarters (21°C) with a 06.00–18.00 h light−18.00 − 06.00 h dark cycle that was maintained throughout the experimental period. All animals were provided standard rodent chow (Formulab 5008, Purina Mills, St Louis, MO, USA) in new cages at the beginning of each week when cages were changed and body weights obtained between 08.00 and 10.00 h. Body mass and food intake were measured weekly throughout the investigation. Running activity was obtained daily between 08.00 and 10.00 h, and rats in the running groups had daily access to wheels and food and water ad libitum until 20 week of age. At 20 week of age, runners were evenly divided by body weight into three additional experimental groups: wheel lock for 5 h (WL5), wheel lock for 53 h (WL53; which equals the cessation of running for ∼2 days), and wheel lock for 173 h (WL173; which equals the cessation of daily running for ∼7 days). The timing of 173 h of exercise cessation was based upon our previous work in this animal model (Morris et al. 2008). Also, additional age-matched, sedentary OLETF (SED) rats did not have access to running wheels throughout the duration of the study. Rat chow was removed on the day of kill at 06.00 h. At 20 weeks of age, rats were anaesthetized (ketamine (80 mg kg−1), xylazine (10 mg kg−1) and acepromazine (4 mg kg−1)) and killed by exsanguination by removal of the heart either 5 h, 53 h or 173 h after locking of wheels; the sedentary rats (SED) were killed at the same time. All animals were fasted for 5 h prior to kill.
Fat pad collection and serum assays
Retroperitoneal and omental adipose tissue fat pads were removed from exsanguinated animals and weighed. Serum glucose (Sigma, St Louis, MO, USA), TG (Sigma), free fatty acids (FFA; Wako Chemicals, Richmond, VA, USA), insulin (Linco Research, St Charles, MO, USA) and β-hydroxybutyrate (Stanbio, Boerne, TX, USA) were measured using commercially available kits according to the manufacturer's instructions. Serum alanine aminotransferase (ALT) concentrations were determined as reported previously (Rector et al. 2008).
Tissue homogenization procedure
Livers were quickly excised from anaesthetized rats and either flash frozen in liquid nitrogen, placed in 10% formalin, or placed in ice-cold isolation buffer (100 mm KCl, 40 mm Tris-HCl, 10 mm Tris-Base, 5 mm MgCl2.6H2O, 1 mm EDTA and 1 mm ATP; pH 7.4). Fresh tissue hepatic fatty acid oxidation assays were performed as previously described by our group (Rector et al. 2008).
Fatty acid oxidation
Fatty acid oxidation was measured with radiolabelled 1-14C palmitate (American Radiochemicals) in fresh liver homogenate preparations as reported previously (Rector et al. 2008). Briefly, the oxidation rate of 14C palmitate was measured by collecting and counting the 14CO2, representing complete fatty acid oxidation, and 14C-labelled acid-soluble metabolites, representing incomplete fatty acid oxidation.
Citrate synthase, β-hydroxyacyl-CoA dehydrogenase (β-HAD) and cytochrome c oxidase activity
Citrate synthase and β-HAD activities were determined using the methods of Srere (1969) and Bass et al. (1969), respectively, as previously described by our group (Rector et al. 2008). Cytochrome c oxidase activity was measured colourimetrically by a commercially available kit (Sigma). The assay measures the oxidation of ferrocytochrome c to ferricytochrome c by cytochrome c oxidase, and enzyme activity is expressed as units ml−1 (g protein)−1.
Intrahepatic lipid content, Oil-Red O staining and liver histology
Intrahepatic lipid content was extracted, quantified and expressed as nmol (g wet weight)−1 as previously described by our group (Rector et al. 2008). Oil-Red O staining was performed in frozen liver sections as previously described (Rector et al. 2008). The area of positive staining for Oil-Red O was calculated as a percentage of total section area, and an average lipid droplet size was calculated utilizing ImagePro Plus (Media Cybernetics, Bethesda, MD, USA) from five to seven views per animal. To examine liver morphology, formalin-fixed paraffin-embedded sections of liver were stained with haematoxylin and eosin (H&E).
Hepatic glycogen and malonyl-CoA concentrations
Liver glycogen was extracted as previously described (Aschenbach et al. 2002; Rector et al. 2008) and content was assessed in the samples using a glucose reagent kit (Thermo Electron, Louisville, CO, USA) and expressed as mg (g wet wt)−1. Hepatic malonyl-CoA was determined in extracts homogenized in deionized water as previously described (Chakravarthy et al. 2005). CoA esters were separated using reversed-phase HPLC on a 5 μm Supelco C18 column with a Waters HPLC system, monitoring 254 nm as the maximal absorbance for CoA. Buffers, gradients and flow rates were those previously described (Pizer et al. 2000).
Western blotting
AMP-activated protein kinase-α (AMPKα), AMPKα Thr172 phosphorylation-specific, cytochrome c, acetylcoenzyme A carboxylase (ACC), ACC Ser79 phosphorylation-specific, peroxisome proliferator-activated receptor γ (PPARγ), and fatty acid synthase (FAS) polyclonal antibodies were from Cell Signalling (Beverly, MA, USA). Stearoyl-CoA desaturase-1 (SCD-1) polyclonal antibody was from Alpha Diagnostics International (San Antonio, TX, USA). Content of phospho-proteins (using phospho-specific antibodies) was calculated from the density of the band of the phospho-protein divided by the density (content) of the protein (total) using the appropriate antibody.
Liver samples were homogenized using lysis buffer. Protein (20–40 μg) was loaded in SDS-PAGE gel and probed with primary antibodies. After washing, the membrane was incubated with HRP-conjugated secondary antibodies. Protein bands were quantified using a laser densitometer (Molecular Dynamics, Sunnyvale, CA, USA). In order to control for equal protein loading and transfer, the membranes were then stained with 1% amido-black (Sigma) as previously described (Rector et al. 2008).
Statistics
Each outcome measure was examined in six to eight animals. For each outcome measure, a one-way analysis of variance was performed (SPSS/15.0, SPSS, Chicago, IL, USA). A significant main effect (P < 0.05) was followed-up with Student–Newman–Kuel post hoc comparisons. Values are reported as means ± standard error of the mean (s.e.m.), and a P value less than 0.05 denotes a statistically significant difference.
Abbreviations
ACC, acetyl-coenzyme A carboxylase; ALT, alanine aminotransferase; AMPK, AMP-activated protein kinase; β-HAD, β-hydroxyacyl-CoA dehydrogenase; FAS, fatty acid synthase; FFA, free fatty acids; NAFLD, non-alcoholic fatty liver disease; OLETF, Otsuka Long-Evans Tokushima Fatty rats; TG, triglycerides; SCD-1, stearoyl-coA desaturase-1; PPARγ, peroxisome proliferator-activated receptor γ.
Results
Animal characteristics
Average daily running distance during the entire study did not differ among WL5, WL53 and WL173 groups (7.13 ± 0.27 km day−1, 7.11 ± 0.33 km day−1 and 8.04 ± 0.32 km day−1, respectively). Consistent with our previous report (Rector et al. 2008), voluntary running suppressed (P < 0.001) body mass and fat pad mass (omental and retroperitoneal) gain compared with SED (Table 1); however, body mass and fat pad mass did not differ among the WL groups and was unchanged during the 173 h of wheel lock. Absolute food intake did not differ among groups, but food consumption relative to body weight was significantly higher in the running (WL groups) animals compared with SED (Table 1); however, during the wheel lock period, daily food intake also did not differ among the WL animals. In the WL53 and WL173 animals, daily food intake during the respective wheel lock periods did not differ from daily food intake during the preceding week (WL53: 30.4 ± 2.0 g day−1versus 32.7 ± 0.89 g day−1, respectively, P = 0.319; WL173: 33.7 ± 1.4 g day−1versus 34.1 ± 1.2 g day−1, respectively, P = 0.550).
Table 1.
Animal characteristics
| Variable | WL5 | WL53 | WL173 | SED |
|---|---|---|---|---|
| Body weight | 449.2 ± 9.7b | 450.0 ± 12.5b | 451.2 ± 10.6b | 631.1 ± 14.7a |
| (g) | ||||
| Absolute food consumption | 210.0 ± 2.9 | 209.5 ± 2.8 | 214.5 ± 2.5 | 219.5 ± 5.5 |
| (g week−1) | ||||
| Relative food consumption | 0.47 ± 0.006b | 0.47 ± 0.009b | 0.44 ± 0.008b | 0.35 ± 0.003a |
| (g per week (g body weight)−1) | ||||
| Fat pad mass | 14.7 ± 1.7b | 15.3 ± 2.4b | 19.9 ± 1.4b | 44.3 ± 1.7a |
| (g) | ||||
| Serum glucose | 633.7 ± 36.2b | 594.8 ± 40.2b | 678.5 ± 50.3a,b | 745.8 ± 35.1a |
| (mg dl−1) | ||||
| Serum insulin | 0.49 ± 0.03b | 0.52 ± 0.04b | 0.46 ± 0.01b | 1.77 ± 0.59a |
| (ng ml−1) | ||||
| Serum TG | 48.1 ± 5.5b | 60.4 ± 4.0b,c | 64.3 ± 4.9c | 208.7 ± 42.7a |
| (mg dl−1) | ||||
| Serum FFA | 164.0 ± 21.9a,b | 129.5 ± 16.6b | 172.4 ± 27.5a,b | 187.0 ± 16.3a |
| (μmol l−1) | ||||
| Serum β-hydroxybutyrate | 342.9 ± 27.8a,b | 293.0 ± 32.6b | 388.6 ± 17.7a | 399.9 ± 30.4a |
| (μmol l−1) | ||||
| Serum ALT | 55.2 ± 3.1 | 53.3 ± 3.4 | 62.4 ± 4.3 | 63.6 ± 6.6 |
| (U l−1) | ||||
| Heart weight/body weight | 3.69 ± 0.23b | 3.67 ± 0.15b | 3.53 ± 0.08b | 2.67 ± 0.04a |
| (mg g−1) |
Values are means ±s.e.m. (n= 6–8). Values with different superscripts are significantly different (P < 0.05). TG, triglycerides; FFA, free fatty acids; ALT, alanine aminotransferase. Fat pad mass was the combination of omental and retroperitoneal fat pads.
Serum TGs increased by 15% from WL5 to WL173 (Table 1), but still were 69% less than SED, while serum measures of glucose, insulin, FFA, β-hydroxybutyrate, ALT (Table 1) and insulin sensitivity as assessed by homeostasis model assessment (HOMA) (Matthews et al. 1985) (data not shown) were unchanged from WL5 to WL173 with several of these measures significantly below the SED values (Table 1). A marker of exercise training, higher heart weight to body weight ratio, was found in all WL animals compared with SED (Table 1). In addition, liver glycogen content was significantly elevated at WL53 (Table 2).
Table 2.
Hepatic responses to daily exercise cessation
| Variable | WL5 | WL53 | WL173 | SED |
|---|---|---|---|---|
| Hepatic TG | 1.79 ± 0.15b | 1.62 ± 0.42b | 1.68 ± 0.10b | 2.99 ± 0.69a |
| (nmol (g wet wt)−1) | ||||
| Hepatic Oil-Red O | 1.25 ± 0.86b | 0.41 ± 0.33b | 1.38 ± 0.79b | 7.44 ± 0.96a |
| (% staining) | ||||
| Hepatic Oil-Red O | 26.8 ± 3.6b | 20.3 ± 2.8b | 22.7 ± 1.9b | 45.8 ± 3.4a |
| (droplet size, pixels) | ||||
| Hepatic glycogen | 31.4 ± 2.8a | 45.6 ± 4.7b | 37.7 ± 3.3a,b | 30.8 ± 2.9a |
| (mg g−1) |
Values are means ±s.e.m. (N= 5–8). Values with different superscripts are significantly different (P < 0.05). TG, triglycerides.
Hepatic steatosis
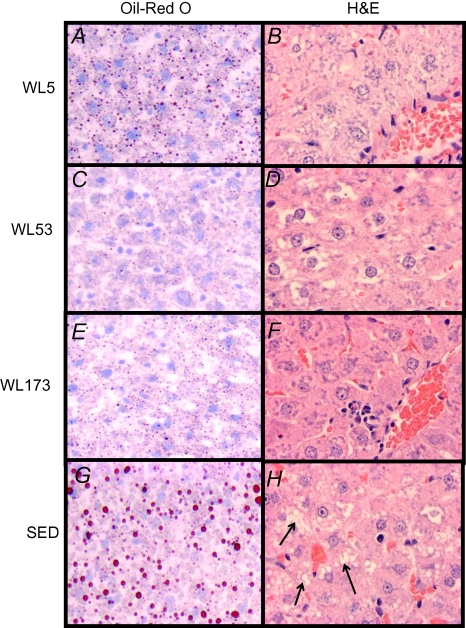
Representative images of randomly selected sections of the liver stained for H&E and Oil-Red O are shown in Fig. 1 at a 40× magnification. There were notable histological differences between the WL and the SED animals (Fig. 1), including significantly elevated numbers of vacuoles in the SED images (P < 0.001; Fig. 1H; see arrows), most probably indicating lipid moieties. Although dramatically reduced compared with the SED animals, cessation of daily physical activity did not significantly alter percent positive staining for neutral lipid, lipid droplet size or hepatic TG accumulation among the WL groups (Fig. 1 and Table 2).
Figure 1. Representative images of haematoxylin and eosin (H&E) and Oil-Red O staining.
Oil-Red O staining in WL5 (A), WL53 (C), WL173 (E) and SED (G). Red droplets indicate neutral lipid staining. Quantification of Oil-Red O lipid staining is shown in Table 2. H&E staining from WL5 (B), WL53 (D), WL173 (F) and SED (H).
Fatty acid oxidation
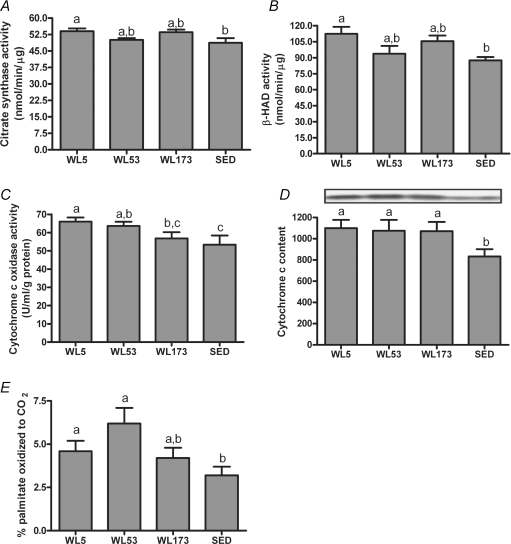
Hepatic citrate synthase and β-HAD activities were significantly higher at WL5 compared with SED, whereas the values for WL53 and W173 were intermediate and not significantly different from SED (Fig. 2A and B). The activity of the electron transport chain enzyme cytochrome c oxidase at WL5 and WL53 was significantly higher compared with SED, whereas activity in the WL173 group was not significantly different from the SED (Fig. 2C). However, liver cytochrome c protein content remained significantly elevated in all WL groups compared to the SED (Fig. 2D). Reductions in these enzymatic activities were associated with reductions in percent palmitate oxidized completely to CO2 such that at WL173 there was no significant difference compared with SED (Fig. 2E). Total (incomplete (label incorporation into chain-shortened acid soluble metabolites, which provide an index of incomplete β-oxidation) + complete to CO2) palmitate oxidation did not differ significantly among SED and WL groups (data not shown).
Figure 2. Effects of 5 h, 53 h and 173 h of physical inactivity on liver citrate synthase (A), β-HAD (B) and cytochrome c oxidase (C) activities, cytochrome c protein content (D), and the complete oxidation of palmitate (E).
Values (means ±s.e.m.; n= 6–8) with different letters are significantly different (P < 0.05).
Key protein intermediates in lipid metabolism
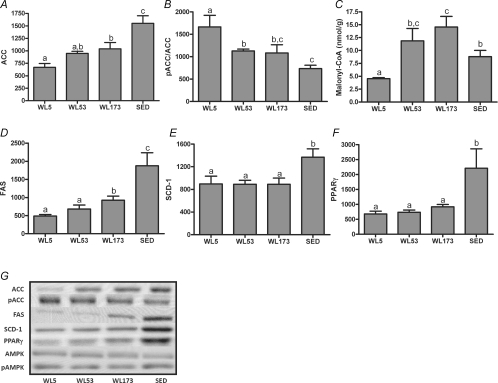
WL173 animals exhibited a ∼35% increase in hepatic ACC and FAS protein content when compared with WL5 (Fig. 3A and D), but their WL173 levels still were significantly less than SED. In addition, there was a robust decline in ACC Ser79 phosphorylation (pACC/ACC) in WL53 and WL173 animals compared with WL5 such that WL173 values were not statistically different from SED (Fig. 3B). Hepatic malonyl-CoA content showed the appropriate reciprocal pattern: content was dramatically increased in WL53 and WL173 animals compared with WL5 and overshot SED at WL173 (Fig. 3C). The protein contents of liver SCD-1 and PPARγ were not significantly altered between WL5 and WL173 (Fig. 3E and F), but all WL animals were markedly lower than SED. Hepatic AMPKα total protein (α1 and α2) or AMPKα Thr172 phosphorylation did not differ among groups (representative Western blots are shown in Fig. 3G).
Figure 3. Effects of 5 h, 53 h and 173 h of physical inactivity on liver ACC (A), pACC (B), malonyl-CoA (C), FAS (D), SCD-1 (E) and PPARγ (F) protein content.
Representative Western blots for each and AMPKα and pAMPK are shown in G. Values (means ±s.e.m.; n= 6–8) with different letters are significantly different (P < 0.05).
Discussion
While the global benefits of exercise on NAFLD are becoming more apparent in animal models and humans (Ueno et al. 1997; Gauthier et al. 2003; Tamura et al. 2005; Rector et al. 2008), little is known about initial detrimental hepatic alterations that probably ensue after ceasing daily physical activity and undertaking a sedentary lifestyle. Here we provide novel mechanistic insight into initial detrimental effects of the cessation of daily exercise on hepatic lipid metabolism in a hyperphagic/obese animal model. This is the first study to report that cessation of daily exercise dramatically activates specific precursors linked in other reports to hepatic steatosis. Intriguingly, a number of peripheral factors previously associated with hepatic steatosis were not changed in the early days after exercise cessation including body weight, fat pad mass, food intake or serum insulin levels. Unexpectedly, numerous hepatic factors also potentially playing a role in initiating hepatic steatosis also were not changed: they are PPARγ, SCD-1 and cytochrome c protein as well as AMPK protein content and phosphorylation status; on the other hand, hepatic fatty acid oxidation decreased and key mediators in hepatic fatty acid synthesis increased independently of those factors in the initial 173 h after exercise cessation, providing key insights into the early events that acutely follow exercise cessation. These data have important clinical significance as the OLETF rat model might be assimilated to hyperphagic/obese humans who continually stop and start exercise programs.
In the current investigation, there was a rapid decline in the complete oxidation of palmitate in liver and hepatic mitochondrial enzymes (citrate synthase, β-HAD and cytochrome c oxidase activities) in the days after the cessation of daily exercise. However, cytochrome c protein content remained elevated above SED, suggesting there was not a generalized decrease in hepatic mitochondrial content, but rather a decrease in mitochondrial quality and function within the first 173 h after exercise cessation. With the apparent uncoupling and reductions in complete fatty acid oxidation, incomplete oxidation products (acetyl CoA and acetyl-carnitine) are probably being directed to fatty acid biosynthesis pathways (Aureli et al. 1999).
To our knowledge this is the first report to demonstrate that cessation of daily exercise results in the up-regulation of the rate-limiting steps in hepatic fatty acid synthesis and malonyl-CoA formation prior to hepatic TG accumulation. Malonyl-CoA is formed by the carboxylation of acetyl-CoA by ACC and its intracellular accumulation is associated with increased lipid deposition and reduced fatty acid oxidation by two mechanisms: (1) malonyl-CoA is the substrate for the synthesis of long chain saturated fatty acids by FAS; and (2) increased formation of malonyl-CoA allosterically inhibits carnitine palmitoyltransferase 1 (CPT-1) and reduces fatty acid entry into the mitochondria for oxidation. With exercise cessation, FAS was reduced to the greatest degree at WL5, but hepatic malonyl-CoA had not accumulated, suggesting that daily exercise reduces the synthesis or increases the oxidation of lipogenic substrates. There upon, malonyl-CoA and de novo fatty acid synthesis increased with duration of exercise stoppage, suggesting that reduced hepatic fatty acid oxidation with exercise cessation could probably be due, in part, to an increased malonyl-CoA-induced inhibition of CPT-1 leading to reduced entry of fatty acids into the mitochondria.
These suggested explanations for alterations in fatty acid oxidation from WL5 to WL173 occurred in the absence of changes in AMPK protein content or phosphorylation status, which previously has been shown to channel acyl-CoAs towards β-oxidation and away from biosynthetic pathways in liver (Muoio et al. 1999). In addition, SCD-1 and PPARγ protein contents were unchanged with 173 h of inactivity. SCD-1, through the conversion of saturated fatty acids to monounsaturated fatty acids, contributes to abnormal partitioning of fatty acids by increasing ACC activity and decreasing fatty acid oxidation, shunting substrate towards fatty acid synthesis (Dobrzyn et al. 2004; Hulver et al. 2005). Furthermore, increased liver PPARγ activity is known to promote hepatic steatosis development (Gavrilova et al. 2003). Surprisingly, the lack of increase in SCD-1 and PPARγ protein or decrease in AMPK phosphorylation from WL5 to WL173 suggests that such changes are not an initial event on the pathway to hepatic steatosis in our model of exercise cessation.
In the current investigation, despite the significant increase in malonyl-CoA levels at WL53 and WL173, hepatic TG accumulation was not apparent with 173 h of exercise cessation. These findings are consistent with previous reports where 14 days of exercise cessation following 8 weeks of treadmill running did not result in significant hepatic lipid accumulation in female Sprague–Dawley rats being fed a normal chow diet (Yasari et al. 2006). The authors did, however, observe a significant increase in hepatic TG accumulation after 6 weeks of exercise cessation (Yasari et al. 2006). We attribute our novel findings to the sustained reduction in protein concentration below SED of factors known to increase TG synthesis and promote hepatic steatosis (FAS, SCD-1 and PPARγ) over the 173 h period (Evans et al. 2004; Reddy & Rao, 2006; Semple et al. 2006). However, it is unlikely that the beneficial effects of daily physical activity on hepatic TG accumulation will persist indefinitely, particularly in a hyperphagic/obese model. Exercise cessation may provide an opportunity to understand the time course of molecular/biochemical events that lead to excessive hepatic fat deposition and steatosis, and thus future time course studies in both healthy and obese models are warranted.
We postulate that the dramatic reduction in daily physical activity was responsible for the observed changes in hepatic lipid metabolism based on the following rationale. Daily food intake was not different in the wheel lock period compared with the preceding week; however, because energy expenditure was reduced during the wheel lock period, we cannot entirely eliminate the possibility that the positive energy balance caused by the reduced physical activity may have partially contributed to our findings. An exclusive contribution by positive energy balance seems less likely though as many traditional responses to caloric excess, including serum insulin, glucose and FFAs, and body and fat pad mass, were unaltered with the imposition of less physical activity. This is supported by previous findings from our group demonstrating the negative consequences of exercise cessation on gaining intra-abdominal fat even after the removal of excess food intake through pair feeding in Fischer–Brown Norway hybrid rats (Laye et al. 2007). Furthermore, it has been demonstrated that short-term high-fat feeding increases hepatic peroxisomal fatty acid oxidation and mitochondrial enzyme activities (Ishii et al. 1980), opposite to the responses observed in the current report. Collectively, these findings suggest that reduced physical activity, probably independent of a positive energy balance, is associated with rapid alterations in hepatic factors involved in NAFLD development.
In summary, exercise cessation in hyperphagic/obese rats allowed for the first time the separation of candidate molecules for the development of hepatic steatosis into those that change early in the non-steady-state period and those with delayed responses. In spite of no changes in body weight, fat pad mass or food intake, exercise cessation for 173 h appears to uncouple β-oxidation, TCA cycle and electron transport chain activities, resulting in reduced complete oxidation of fatty acids in the liver. In addition, several hepatic lipogenesis intermediates that also inhibit fatty acid oxidation were up-regulated following only 173 h of exercise cessation, whereas others were not altered. These data strongly suggest that a sudden transition to a sedentary lifestyle increases susceptibility to NAFLD. These findings also have important health and clinical applications as many populations have adopted increasingly intermittent weekly sedentary intervals at the same time that NAFLD is reaching epidemic proportions in the United States and westernized societies.
Acknowledgments
The OLETF rats were a generous gift of the Tokushima Research Institute, Otsuka Pharmaceutical (Tokushima, Japan). The authors would like to thank Dr James Turk, Dr Javad Habibi, Jennifer Casati and Suzie Clark for excellent technical assistance to this work. The authors also would like to thank the Veterinary Medicine Diagnostics Laboratory at the University of Missouri for help with the histological sections and serum ALT measurements. This work was supported by a Research Board Grant (F.W.B.) and College of Veterinary Medicine at University of Missouri-Columbia.
References
- Aschenbach WG, Hirshman MF, Fujii N, Sakamoto K, Howlett KF, Goodyear LJ. Effect of AICAR treatment on glycogen metabolism in skeletal muscle. Diabetes. 2002;51:567–573. doi: 10.2337/diabetes.51.3.567. [DOI] [PubMed] [Google Scholar]
- Aureli T, Puccetti C, Di Cocco ME, Arduini A, Ricciolini R, Scalibastri M, Manetti C, Conti F. Entry of [(1,2–13C2)acetyl]-L-carnitine in liver tricarboxylic acid cycle and lipogenesis: a study by 13C NMR spectroscopy in conscious, freely moving rats. Eur J Biochem. 1999;263:287–293. doi: 10.1046/j.1432-1327.1999.00524.x. [DOI] [PubMed] [Google Scholar]
- Bass A, Brdiczka D, Eyer P, Hofer S, Pette D. Metabolic differentiation of distinct muscle types at the level of enzymatic organization. Eur J Biochem. 1969;10:198–206. doi: 10.1111/j.1432-1033.1969.tb00674.x. [DOI] [PubMed] [Google Scholar]
- Bellentani S, Saccoccio G, Masutti F, Croce LS, Brandi G, Sasso F, Cristanini G, Tiribelli C. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000;132:112–117. doi: 10.7326/0003-4819-132-2-200001180-00004. [DOI] [PubMed] [Google Scholar]
- Bi S, Scott KA, Hyun J, Ladenheim EE, Moran TH. Running wheel activity prevents hyperphagia and obesity in Otsuka Long-Evans Tokushima Fatty rats: role of hypothalamic signaling. Endocrinology. 2005;146:1676–1685. doi: 10.1210/en.2004-1441. [DOI] [PubMed] [Google Scholar]
- Chakravarthy MV, Pan Z, Zhu Y, Tordjman K, Schneider JG, Coleman T, Turk J, Semenkovich CF. ‘New’ hepatic fat activates PPARα to maintain glucose, lipid, and cholesterol homeostasis. Cell Metab. 2005;1:309–322. doi: 10.1016/j.cmet.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Church TS, Kuk JL, Ross R, Priest EL, Biltoft E, Blair SN. Association of cardiorespiratory fitness, body mass index, and waist circumference to nonalcoholic fatty liver disease. Gastroenterology. 2006;130:2023–2030. doi: 10.1053/j.gastro.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Dobrzyn P, Dobrzyn A, Miyazaki M, Cohen P, Asilmaz E, Hardie DG, Friedman JM, Ntambi JM. Stearoyl-CoA desaturase 1 deficiency increases fatty acid oxidation by activating AMP-activated protein kinase in liver. Proc Nat Acad Sci U S A. 2004;101:6409–6414. doi: 10.1073/pnas.0401627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RM, Barish GD, Wang YX. PPARs and the complex journey to obesity. Nat Med. 2004;10:355–361. doi: 10.1038/nm1025. [DOI] [PubMed] [Google Scholar]
- Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99–S112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- Gauthier MS, Couturier K, Latour JG, Lavoie JM. Concurrent exercise prevents high-fat-diet-induced macrovesicular hepatic steatosis. J Appl Physiol. 2003;94:2127–2134. doi: 10.1152/japplphysiol.01164.2002. [DOI] [PubMed] [Google Scholar]
- Gavrilova O, Haluzik M, Matsusue K, Cutson JJ, Johnson L, Dietz KR, Nicol CJ, Vinson C, Gonzalez FJ, Reitman ML. Liver peroxisome proliferator-activated receptor γ contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J Biol Chem. 2003;278:34268–34276. doi: 10.1074/jbc.M300043200. [DOI] [PubMed] [Google Scholar]
- Hulver MW, Berggren JR, Carper MJ, Miyazaki M, Ntambi JM, Hoffman EP, Thyfault JP, Stevens R, Dohm GL, Houmard JA, Muoio DM. Elevated stearoyl-CoA desaturase-1 expression in skeletal muscle contributes to abnormal fatty acid partitioning in obese humans. Cell Metab. 2005;2:251–261. doi: 10.1016/j.cmet.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii H, Fukumori N, Horie S, Suga T. Effects of fat content in the diet on hepatic peroxisomes of the rat. Biochim Biophys Acta. 1980;617:1–11. doi: 10.1016/0005-2760(80)90218-0. [DOI] [PubMed] [Google Scholar]
- Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications. Diabetes. 1992;41:1422–1428. doi: 10.2337/diab.41.11.1422. Otsuka Long-Evans Tokushima Fatty (OLETF) strain. [DOI] [PubMed] [Google Scholar]
- Laye MJ, Thyfault JP, Stump CS, Booth FW. Inactivity induces increases in abdominal fat. J Appl Physiol. 2007;102:1341–1347. doi: 10.1152/japplphysiol.01018.2006. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Miyasaka K, Ichikawa M, Kawanami T, Kanai S, Ohta M, Sato N, Ebisawa H, Funakoshi A. Physical activity prevented age-related decline in energy metabolism in genetically obese and diabetic rats, but not in control rats. Mech Ageing Dev. 2003;124:183–190. doi: 10.1016/s0047-6374(02)00118-5. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Moran TH, Bi S. Hyperphagia and obesity of OLETF rats lacking CCK1 receptors: developmental aspects. Dev Psychobiol. 2006;48:360–367. doi: 10.1002/dev.20149. [DOI] [PubMed] [Google Scholar]
- Morris RT, Laye MJ, Lees SJ, Rector RS, Thyfault JP, Booth FW. Exercise-induced attenuation of obesity, hyperinsulinemia, and skeletal muscle lipid peroxidation in the OLETF rat. J Appl Physiol. 2008;104:708–715. doi: 10.1152/japplphysiol.01034.2007. [DOI] [PubMed] [Google Scholar]
- Muoio DM, Seefeld K, Witters LA, Coleman RA. AMP-activated kinase reciprocally regulates triacylglycerol synthesis and fatty acid oxidation in liver and muscle: evidence that sn-glycerol-3-phosphate acyltransferase is a novel target. Biochem J. 1999;338:783–791. [PMC free article] [PubMed] [Google Scholar]
- Perseghin G, Lattuada G, De Cobelli F, Ragogna F, Ntali G, Esposito A, Belloni E, Canu T, Terruzzi I, Scifo P, Del Maschio A, Luzi L. Habitual physical activity is associated with intrahepatic fat content in humans. Diabetes Care. 2007;30:683–688. doi: 10.2337/dc06-2032. [DOI] [PubMed] [Google Scholar]
- Pizer ES, Thupari J, Han WF, Pinn ML, Chrest FJ, Frehywot GL, Townsend CA, Kuhajda FP. Malonyl-coenzyme-A is a potential mediator of cytotoxicity induced by fatty-acid synthase inhibition in human breast cancer cells and xenografts. Cancer Res. 2000;60:213–218. [PubMed] [Google Scholar]
- Rector RS, Thyfault JP, Morris RT, Laye MJ, Borengasser SJ, Booth FW, Ibdah JA. Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Am J Physiol Gastrointest Liver Physiol. 2008;294:G619–G626. doi: 10.1152/ajpgi.00428.2007. [DOI] [PubMed] [Google Scholar]
- Reddy JK, Rao MS. Lipid metabolism and liver inflammation. Am J Physiol Gastrointest Liver Physiol. 2006;290:G852–G858. doi: 10.1152/ajpgi.00521.2005. II. Fatty liver disease and fatty acid oxidation. [DOI] [PubMed] [Google Scholar]
- Semple RK, Chatterjee VK, O'Rahilly S. PPARγ and human metabolic disease. J Clin Invest. 2006;116:581–589. doi: 10.1172/JCI28003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima K, Shi K, Sano T, Iwami T, Mizuno A, Noma Y. Is exercise training effective in preventing diabetes mellitus in the Otsuka-Long-Evans-Tokushima fatty rat, a model of spontaneous non-insulin-dependent diabetes mellitus? Metabolism. 1993;42:971–977. doi: 10.1016/0026-0495(93)90009-d. [DOI] [PubMed] [Google Scholar]
- Srere PA. Citrate synthase. Meth Enzymol. 1969;13:3–5. [Google Scholar]
- Stern JS, Johnson PR. Spontaneous activity and adipose cellularity in the genetically obese Zucker rat (fafa) Metabolism. 1977;26:371–380. doi: 10.1016/0026-0495(77)90104-4. [DOI] [PubMed] [Google Scholar]
- Tamura Y, Tanaka Y, Sato F, Choi JB, Watada H, Niwa M, Kinoshita J, Ooka A, Kumashiro N, Igarashi Y, Kyogoku S, Maehara T, Kawasumi M, Hirose T, Kawamori R. Effects of diet and exercise on muscle and liver intracellular lipid contents and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab. 2005;90:3191–3196. doi: 10.1210/jc.2004-1959. [DOI] [PubMed] [Google Scholar]
- Ueno T, Sugawara H, Sujaku K, Hashimoto O, Tsuji R, Tamaki S, Torimura T, Inuzuka S, Sata M, Tanikawa K. Therapeutic effects of restricted diet and exercise in obese patients with fatty liver. J Hepatol. 1997;27:103–107. doi: 10.1016/s0168-8278(97)80287-5. [DOI] [PubMed] [Google Scholar]
- Yasari S, Paquette A, Charbonneau A, Gauthier MS, Savard R, Lavoie JM. Effects of ingesting a high-fat diet upon exercise-training cessation on fat accretion in the liver and adipose tissue of rats. Appl Physiol Nutr Metab. 2006;31:367–375. doi: 10.1139/h06-032. [DOI] [PubMed] [Google Scholar]
- Yeon JE, Choi KM, Baik SH, Kim KO, Lim HJ, Park KH, Kim JY, Park JJ, Kim JS, Bak YT, Byun KS, Lee CH. Reduced expression of peroxisome proliferator-activated receptor-α may have an important role in the development of non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2004;19:799–804. doi: 10.1111/j.1440-1746.2004.03349.x. [DOI] [PubMed] [Google Scholar]