Abstract
Recent evidence suggests that adenosine triphosphate (ATP) can inhibit vasoconstrictor responses to endogenous noradrenaline release via tyramine in the skeletal muscle circulation, similar to what is observed in contracting muscle. Whether this involves direct modulation of postjunctional α-adrenoceptor responsiveness, or is selective for α1- or α2-receptors remains unclear. Therefore, in Protocol 1, we tested the hypothesis that exogenous ATP can blunt direct postjunctional α-adrenergic vasoconstriction in humans. We measured forearm blood flow (FBF; Doppler ultrasound) and calculated the vascular conductance (FVC) responses to local intra-arterial infusions of phenylephrine (α1-agonist) and dexmedetomidine (α2-agonist) during moderate rhythmic handgrip exercise (15% maximum voluntary contraction), during a control non-exercise vasodilator condition (adenosine), and during ATP infusion in eight young adults. Forearm hyperaemia was matched across all conditions. Forearm vasoconstrictor responses to direct α1-receptor stimulation were blunted during exercise versus adenosine (ΔFVC =−11 ± 3%versus−39 ± 5%; P< 0.05), and were abolished during ATP infusion (−3 ± 2%). Similarly, vasoconstrictor responses to α2-receptor stimulation were blunted during exercise versus adenosine (−13 ± 4%versus−40 ± 8%; P< 0.05), and were abolished during ATP infusion (−4 ± 4%). In Prototol 2 (n= 10), we tested the hypothesis that graded increases in ATP would reduce α1-mediated vasoconstriction in a dose-dependent manner compared with vasodilatation evoked via adenosine. Forearm vasoconstrictor responses during low dose adenosine (−38 ± 3%) and ATP (−33 ± 2%) were not significantly different from rest (−40 ± 3%; P < 0.05). In contrast, vasoconstrictor responses during moderate (−22 ± 6%) and high dose ATP (−8 ± 5%) were significantly blunted compared with rest, whereas the responses during adenosine became progressively greater (moderate =−48 ± 4%, P= 0.10; high =−53 ± 6%, P< 0.05). We conclude that exogenous ATP is capable of blunting direct postjunctional α-adrenergic vasoconstriction, that this involves both α1- and α2-receptor subtypes, and that this is graded with ATP concentrations. Collectively, these data are consistent with the conceptual framework regarding how muscle blood flow and vascular tone are regulated in contracting muscles of humans.
Blood flow and oxygen delivery increase in proportion to the oxygen demand of contracting skeletal muscle, a complex response involving competing influences of local vasodilator signals and sympathetic neural vasoconstriction particularly as the intensity of exercise and amount of muscle mass engaged increase (Saltin et al. 1998). Although it is clear that sympathetic restraint of active muscle blood flow is imperative for appropriate blood pressure regulation (Marshall et al. 1961; Rowell, 1997), it has also been repeatedly demonstrated that the vasoconstrictor responses in contracting muscle are significantly blunted compared with the responses under resting (quiescent) conditions (Remensnyder et al. 1962; Anderson & Faber, 1991; Thomas & Victor, 1998; Buckwalter et al. 2001; Tschakovsky et al. 2002; Dinenno & Joyner, 2003). A variety of substances/mechanisms have been proposed in this local modulation of sympathetic vasoconstriction including activation of ATP-sensitive potassium channels (KATP) (Thomas et al. 1997; Keller et al. 2004), adenosine (Nishigaki et al. 1991), nitric oxide (NO) (Thomas & Victor, 1998; Chavoshan et al. 2002), and vasodilating prostaglandins (PGs) (Faber et al. 1982). However, recent data indicate that independent inhibition of NO and PGs does not influence the ability of contractions to blunt sympathetic vasoconstriction (Dinenno & Joyner, 2003, 2004), and that combined inhibition of these substances only slightly augments the constrictor response to α-adrenoceptor stimulation (Dinenno & Joyner, 2004). Further, exogenous infusion of adenosine to mimic exercise hyperaemia does not blunt sympathetic vasoconstriction (Tschakovsky et al. 2002). Thus, identifying the sympatholytic factor associated with muscle contractions has proven difficult.
It has recently been proposed that circulating adenosine triphosphate (ATP) could be involved in matching muscle perfusion to oxygen demand during exercise (Ellsworth, 2000; Gonzalez-Alonso et al. 2002; Ellsworth, 2004). Although ATP can be produced in many cells, one source of ATP release during mismatches in oxygen delivery and demand appears to be the red blood cell, where ATP release is coupled with the level of deoxygenated haemoglobin (Ellsworth, 2000, 2004; Jagger et al. 2001; Gonzalez-Alonso et al. 2002), and can also be augmented by hypercapnia, acidosis and mechanical deformation (Bergfeld & Forrester, 1992; Ellsworth et al. 1995; Sprague et al. 1998). In addition to directly evoking vasodilatation via binding to P2y-receptors on the endothelium (Burnstock & Kennedy, 1986; Rongen et al. 1994), work by Rosenmeier et al. (2004) indicate that ATP could assist in matching oxygen delivery with tissue demand by modulating local sympathetic vasoconstrictor tone. Indeed, data derived from this study suggest that circulating ATP can override sympathetic vasoconstriction in the leg circulation evoked via intra-arterial administration of tyramine (evokes endogenous noradrenaline (NA) release), similar to that observed in contracting muscle (Rosenmeier et al. 2004). This occurred despite similar increases in femoral venous NA concentrations, suggesting that this modulatory effect of ATP is at the level of postjunctional α-adrenoceptors. However, it must be emphasized that changes in venous NA concentrations do not always accurately reflect NA release from sympathetic nerve endings especially when there are marked changes in regional blood flow (Esler et al. 1990). Therefore, whether circulating ATP modulates direct postjunctional α-adrenoceptor responsiveness and whether this is selective for α1- or α2-adrenoceptors is unclear.
An additional question related to the role of circulating ATP in modulating sympathetic vasoconstriction is whether this is graded with the levels of circulating ATP. In this context, several studies utilizing isolated limb models have clearly demonstrated that the magnitude of sympatholysis during exercise is graded with exercise intensity, such that mild levels of muscle contraction (< 10% maximal effort) do not interfere with sympathetic vasoconstriction whereas progressive increases in exercise intensity above this level lead eventually to robust blunting of the vasoconstrictor response (Thomas et al. 1994; Buckwalter et al. 2001; Tschakovsky et al. 2002; Kirby et al. 2005). Thus, if circulating ATP does indeed play a role in muscle blood flow regulation during contractions, one would predict that low levels of ATP are not sympatholytic, whereas increasing circulating levels of ATP would lead to a progressive reduction in sympathetic vasoconstrictor responsiveness. This is an important hypothesis to test, as the only data regarding how ATP interacts directly with sympathetic vasoconstriction indicate that ATP completely overrides the vasoconstrictor response (Rosenmeier et al. 2004). As such, if ATP release from red blood cells occurred in all active muscle during high intensity large muscle mass exercise, and this completely abolishes sympathetic vasoconstriction as recently demonstrated (Rosenmeier et al. 2004), the vasodilatory capacity of exercising muscle would outstrip cardiac pumping capacity and arterial pressure would fall (Joyner & Thomas, 2003; Calbet et al. 2004). In healthy humans, this does not occur.
Therefore, in the present study we tested the hypothesis that exogenous ATP infusion blunts direct postjunctional α-adrenergic vasoconstriction in humans and determined whether this is selective for α1- or α2-adrenoceptors. Further, given that the ability of muscle contractions to blunt a known sympathetic vasoconstrictor stimulus is graded with exercise intensity, we also tested the hypothesis that graded increases in arterial ATP concentrations would cause graded inhibition of sympathetic α-adrenergic vasoconstriction.
Methods
Subjects
With Institutional Review Board approval and after written informed consent, a total of 18 young healthy adults (12 men, 6 women; age = 22 ± 1 years; weight = 72.6 ± 3.0 kg; height = 176 ± 2 cm; body mass index = 23.1 ± 0.7 kg m−2; means ±s.e.m.) participated in the present study. All were non-smokers, non-obese, normotensive, and not taking any medications. Studies were performed after a 4 h fast with the subjects in the supine position. All studies were performed according to the Declaration of Helsinki.
Arterial catheterization
A 20 gauge, 7.6 cm catheter was placed in the brachial artery of the non-dominant arm under aseptic conditions after local anaesthesia (2% lidocaine) for local administration of study drugs. The catheter was connected to a 3-port connector as well as a pressure transducer for mean arterial pressure (MAP) measurement and continuously flushed at 3 ml h−1 with heparinized saline (Dinenno & Joyner, 2003, 2004; Dinenno et al. 2005). The two side ports were used for infusions of vasoactive drugs.
Forearm blood flow and vascular conductance
A 4 MHz pulsed Doppler probe (Model 500V, Multigon Industries, Mt Vernon, NY, USA) was used to measure brachial artery mean blood velocity (MBV) with the probe securely fixed to the skin over the brachial artery proximal to the catheter insertion site as previously described by our laboratory (Dinenno & Joyner, 2003, 2004; Dinenno et al. 2005). The probe insonation angle relative to the skin was 45 deg. A linear 12 MHz echo Doppler ultrasound probe (GE Vingmed Ultrasound Vivid7, Horten, Norway) was placed in a holder securely fixed to the skin immediately proximal to the velocity probe to measure brachial artery diameter. Forearm blood flow was calculated as:
where the FBF is in ml min−1, the MBV is in cm s−1, the brachial diameter is in cm, and 60 is used to convert from ml s−1 to ml min−1. Forearm vascular conductance (FVC) was calculated as (FBF/MAP) × 100, and expressed as ml min−1 (100 mmHg)−1.
Rhythmic handgrip exercise
Maximum voluntary contraction (MVC) was determined for each subject as the average of at least three maximal squeezes of a handgrip dynamometer (Stoelting, Chicago, IL, USA) that were within 3% of each other. For the exercise trials, weights corresponding to 15% MVC were attached to a pulley system and lifted 4–5 cm over the pulley at a duty cycle of 1 s contraction–2 s relaxation (20 contractions per minute) using audio and visual signals to ensure the correct timing (Dinenno & Joyner, 2003, 2004). We chose this moderate workload because it significantly blunts, but does not abolish, sympathetic vasoconstriction in contracting muscle (Tschakovsky et al. 2002; Dinenno & Joyner, 2003; Dinenno et al. 2005).
Sympathetic α-adrenergic vasoconstrictor drugs
In male subjects, phenylephrine (a selective α1-agonist; Baxter, Irvine, CA, USA) was infused at 0.0625 μg (dl forearm volume)−1 min−1 and dexmedetomidine (a selective α2-agonist; Hospira, Lake Forest, IL, USA) was infused at 6.25 ng (dl forearm volume)−1 min−1. The doses of phenylephrine and dexmedetomidine were chosen based on our experience at rest (Dinenno et al. 2002; Smith et al. 2007) and during handgrip exercise (Dinenno & Joyner, 2003, 2004; Rosenmeier et al. 2003a). Because young women typically have reduced vasoconstrictor responses to α-receptor stimulation (Kneale et al. 2000), the doses of phenylephrine and dexmedetomidine were doubled for the female participants. All vasoconstrictor drug infusions were adjusted for the hyperaemic conditions as previously described (see below) (Dinenno & Joyner, 2003, 2004).
Given that exercise increases forearm blood flow, adenosine was infused to elevate resting forearm blood flow to similar levels observed during exercise. We have previously demonstrated that exercise blunts the vasoconstrictor responses to direct α1- and α2-adrenoceptor stimulation, whereas these vasoconstrictor responses are maintained when blood flow is elevated with adenosine and hence it was used to create a ‘high flow’ control state (Dinenno & Joyner, 2003, 2004; Rosenmeier et al. 2003a). In an effort to normalize the concentration of each vasoconstricting drug in the blood perfusing the forearm, the infusions were adjusted on the basis of forearm blood flow and forearm volume (measured via regional analysis of whole-body DEXA scans). Various concentrations of each compound were available to keep the absolute infusion rates less than 3 ml min−1 in every trial.
Experimental protocols
General experimental protocol
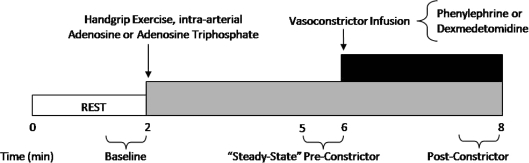
Figure 1 is an example of a time-line for the specific trials. In the supine position, subjects either performed a bout of handgrip exercise, or received intra-arterial adenosine (Sicor, Irvine, CA, USA) or ATP (Sigma, USA); the total time for each trial was 8 min. After 2 min of baseline measurements, exercise or vasodilator infusion was initiated and steady-state FBF was reached within 3 min. Between 3 and 4 min of hyperaemia (minutes 5 and 6 of Fig. 1) the dose of the α1- or α2-agonist (vasoconstrictor) was calculated on the basis of forearm volume and blood flow. The vasoconstrictor infusion began at the 6-minute mark and lasted for 2 min.
Figure 1. General experimental trial.
Each trial consisted of a 2 min baseline period. After this time period, subjects either began rhythmic handgrip exercise or received intra-arterial adenosine or adenosine triphosphate (ATP) to elevate resting forearm blood flow to levels observed during exercise. During minutes 5–6 (pre-constrictor), the doses of the α1- or α2-adrenoceptor agonists (phenylephrine or dexmedetomidine, respectively) were calculated on the basis of steady-state hyperaemic forearm blood flow and forearm volume. Subsequently, the α-agonist was infused for 2 min until minute 8. An average of forearm blood flow and mean arterial blood pressure during the final 30 s of α-agonist infusion was used to calculate the vasoconstrictor effect during all hyperaemic conditions
Protocol 1. Effects of exogenous ATP on postjunctional α-adrenergic vasoconstrictor responsiveness
The purpose of this protocol was to determine whether exogenous ATP blunts direct postjunctional α-adrenergic responsiveness, and whether this is selective for α1- or α2-adrenoceptors. Therefore, in eight subjects (6 men, 2 women), the vasoconstrictor responses to direct α1- and α2-adrenoceptor stimulation (via phenylephrine and dexmedetomidine, respectively) were assessed during control vasodilator infusion of adenosine, during moderate rhythmic handgrip exercise (15% MVC), and during infusion of ATP. In total, there were six experimental trials for each subject. In this protocol, the goal was to match steady-state FBF during infusion of adenosine or ATP with that observed during exercise. To do so, adenosine (45 nmol (dl forearm volume)−1 min−1) and ATP (5 nmol (dl forearm volume)−1 min1) were initially infused and doses were increased to elevate FBF accordingly. The final average doses of adenosine and ATP were 73 ± 8 and 11 ± 2 nmol (dl forearm volume)−1 min−1, respectively. The order of the adenosine, exercise and ATP trials were counterbalanced across subjects. Thus, for subjects that did not perform the exercise trial first, we had them perform 3–4 min of rhythmic handgrip exercise prior to any experimental trials with α-agonists to determine their individual steady-state FBF for this exercise intensity. Additionally, in one-half of the subjects, vasoconstrictor responses to α1-adrenoceptor stimulation were determined under each hyperaemic condition, followed by the trials for α2-receptor stimulation. This order was reversed in the other four subjects, and all subjects rested for 15 min between each trial.
Protocol 2. Effects of graded infusions of ATP on postjunctional α-adrenergic vasoconstrictor responsiveness
The ability of muscle contractions to blunt a known sympathetic vasoconstrictor stimulus is graded with exercise intensity, such that greater inhibition of α-mediated vasoconstriction (greater sympatholysis) is observed with increasing workloads (Thomas et al. 1994; Buckwalter et al. 2001; Tschakovsky et al. 2002; Kirby et al. 2005). Therefore, the purpose of this protocol was to determine whether graded increases in exogenous ATP caused graded sympatholysis as has been observed during exercise. In 10 subjects (6 men, 4 women), we determined the vasoconstrictor responses to direct α1-adrenoceptor stimulation via phenylephrine at rest (saline control), as well as during graded increases in ATP and adenosine. In total, there were seven experimental trials for each subject and each trial was performed in a similar fashion as outlined in Protocol 1. For this protocol, the doses of ATP were calculated (based on resting forearm blood flow) to increase arterial concentrations by 500, 1000 and 2000 nmol l−1 (‘low’, ‘moderate’, and ‘high’) provided no ATP degradation were to occur, and this was based on data obtained from the femoral vein during graded knee extensor exercise (Gonzalez-Alonso et al. 2002). Similar to Protocol 1, we infused adenosine as a control vasodilator at concentrations required to match the increase in FBF evoked via these doses of ATP. Thus, to do so, ATP trials were always performed prior to adenosine trials, but the order of ATP and adenosine doses (low, moderate, and high) were counterbalanced across subjects. All trials were separated by 15 min of rest.
Data acquisition and analysis
Data were collected and stored on computer at 250 Hz and analysed off-line with signal-processing software (WinDaq, DATAQ Instruments, Akron, OH, USA). Mean arterial pressure (MAP) was determined from the arterial pressure waveform. Baseline FBF, HR and MAP represent an average of the last minute of the resting time period, the steady-state hyperaemic values represent an average of minutes 3–4 (minutes 5–6 of Fig. 1; pre-vasoconstrictor) during exercise, adenosine, or ATP, and the effects of the α-agonists represent an average of the final 30 s of drug infusion (post-vasoconstrictor). The percentage reduction in FBF during vasoconstrictor administration was calculated as:
We also calculated percentage reduction in FVC as our standard index to compare vasoconstrictor responses to the α-agonists across conditions, as this appears to be the most appropriate way to compare vasoconstrictor responsiveness under conditions where there might be differences in vascular tone (Lautt, 1989; O’Leary, 1991; Thomas et al. 1994; Tschakovsky et al. 2002). In an effort to be comprehensive, we have also presented absolute values of forearm haemodynamics for all conditions in tabular form.
Statistics
All values are reported as means ±s.e.m. Specific hypothesis testing within each of the exercise, adenosine, or ATP trials with the two different α-agonist infusions was performed using repeated measures ANOVA. Comparison of the haemodynamic values at specific time points between the exercise, adenosine and ATP conditions was made with Student's t test for unpaired data, and the values within each hyperaemic condition (exercise, adenosine, or ATP) with Student's t test for paired data. Significance was set at P< 0.05.
Results
Protocol 1. Effects of exogenous ATP on postjunctional α-adrenergic vasoconstrictor responsiveness
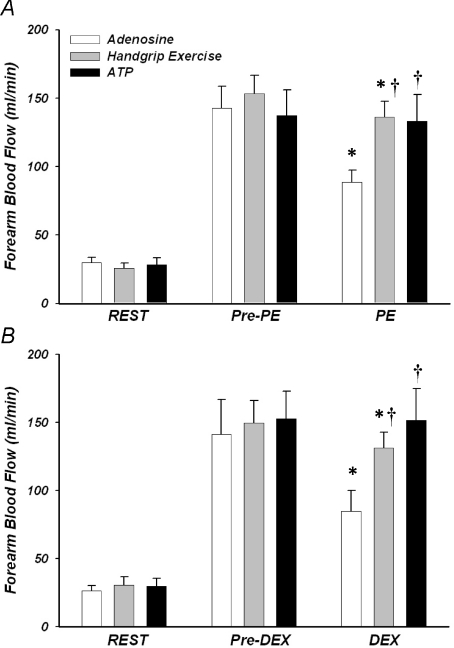
Forearm haemodynamics, HR, and MAP for Protocol 1 are presented in Table 1. Intra-arterial infusion of both adenosine and ATP, as well as handgrip exercise, significantly increased FBF and FVC from baseline (P< 0.05). As desired by experimental design, steady-state (pre-vasoconstrictor) FBF responses to adenosine and ATP infusion were effectively matched to that observed during 15% MVC handgrip exercise within both phenylephrine (Fig. 2A) and dexmedetomidine conditions (Fig. 2B; P= 0.5–0.9). Infusion of phenylephrine (α1-agonist) significantly reduced FBF from steady-state hyperaemia during adenosine and exercise (P< 0.05), whereas FBF was unchanged during ATP (NS; Fig. 2A). Similarly, infusion of dexmedetomidine (α2-agonist) significantly reduced FBF from steady-state hyperaemia during adenosine and exercise (P< 0.05), whereas FBF was unchanged during ATP (NS; Fig. 2B).
Table 1.
Protocol 1. Forearm and systemic haemodynamics
| Condition | Forearm blood flow (ml min−1) | Mean arterial pressure (mmHg) | Forearm vascular conductance (ml min−1 (100 mmHg)−1) | Heart rate (beats min−1) |
|---|---|---|---|---|
| A. Phenylephrine trials | ||||
| Baseline | ||||
| Adenosine | 30 ± 4 | 92 ± 3 | 32 ± 4 | 56 ± 3 |
| Exercise | 26 ± 4 | 94 ± 3 | 28 ± 4 | 55 ± 4 |
| ATP | 29 ± 5 | 92 ± 2 | 31 ± 5 | 54 ± 4 |
| Pre-phenylephrine | ||||
| Adenosine | 143 ± 16* | 93 ± 3 | 156 ± 19* | 57 ± 3 |
| Exercise | 154 ± 13* | 96 ± 3 | 160 ± 12* | 60 ± 4* |
| ATP | 138 ± 19* | 94 ± 2 | 149 ± 21* | 56 ± 3 |
| Phenylephrine | ||||
| Adenosine | 89 ± 9*† | 98 ± 3* | 92 ± 10*† | 56 ± 3 |
| Exercise | 136 ± 12*†‡ | 96 ± 3 | 141 ± 10*†‡ | 60 ± 3* |
| ATP | 133 ± 19*‡ | 93 ± 1 | 143 ± 21*‡ | 54 ± 3 |
| B. Dexmedetomidine trials | ||||
| Baseline | ||||
| Adenosine | 27 ± 4 | 95 ± 2 | 28 ± 4 | 53 ± 3 |
| Exercise | 30 ± 6 | 92 ± 3 | 33 ± 7 | 54 ± 4 |
| ATP | 30 ± 6 | 97 ± 2 | 31 ± 6 | 54 ± 3 |
| Pre-dexmedetomidine | ||||
| Adenosine | 141 ± 25* | 98 ± 3 | 144 ± 25* | 54 ± 4 |
| Exercise | 150 ± 16* | 94 ± 2 | 158 ± 15* | 57 ± 4* |
| ATP | 153 ± 20* | 95 ± 2 | 162 ± 21* | 54 ± 3 |
| Dexmedetomidine | ||||
| Adenosine | 85 ± 15*† | 101 ± 3 | 84 ± 15*† | 55 ± 3 |
| Exercise | 132 ± 12*†‡ | 95 ± 2 | 136 ± 12*†‡ | 57 ± 4* |
| ATP | 152 ± 23*‡ | 96 ± 2 | 159 ± 24*‡ | 53 ± 3 |
P< 0.05 vs baseline within condition;
P< 0.05 vs steady-state (Pre-vasoconstrictor; Phenylephrine/Dexmedetomidine) within condition;
P< 0.05 vs adenosine during α-agonist infusion. Forearm vascular conductance was calculated as (forearm blood flow/mean arterial pressure) × 100.
Figure 2. Forearm blood flow at rest, during each hyperaemic condition and during infusion of α-agonists.
Steady-state hyperaemia was similar during rhythmic handgrip exercise, adenosine and ATP infusions for trials involving the α1-agonist phenylephrine (A; Pre-PE) and the α2-agonist dexmedetomidine (B; Pre-Dex). Forearm blood flow was reduced significantly with both α-agonists during adenosine and exercise, but the response was attenuated during exercise. In contrast, α-agonist infusion did not significantly reduce forearm blood flow during ATP. *P< 0.05 vs. steady state (Pre-vasoconstrictor; PE/Dex) within condition; †P< 0.05 vs. adenosine during α-agonist infusion.
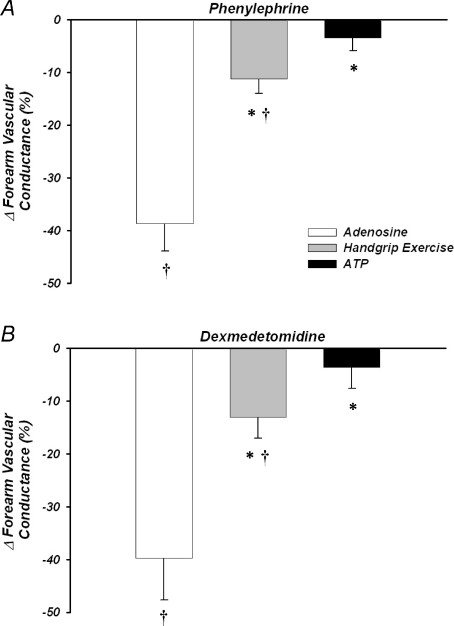
The forearm vasoconstrictor responses to direct α1-adrenoceptor stimulation were blunted during steady-state exercise versus adenosine (ΔFVC =–11 ± 3%versus−39 ± 5%; P< 0.05), and were abolished during ATP infusion (−3 ± 2%; P= 0.2 versus zero; Fig. 3A). Similarly, vasoconstrictor responses to α2-receptor stimulation were blunted during exercise versus adenosine (−13 ± 4%versus−40 ± 8%; P< 0.05), and were abolished during ATP infusion (−4 ± 4%; P= 0.5 versus zero; Fig. 3B). MAP changed minimally within and between conditions (Table 1A and B), thus FBF responses were similar to FVC. Heart rate increased in response to exercise (P< 0.05), but otherwise was not significantly between or within trials and conditions (Table 1).
Figure 3. Forearm vasoconstrictor responses to α1- and α2-adrenoceptor stimulation.
Percentage reductions in forearm vascular conductance to phenylephrine (α1-agonist) (A) were significantly blunted during exercise and abolished during ATP compared with adenosine infusions. Similar data were obtained in response to α2-adrenoceptor stimulation via dexmedetomidine (B). *P< 0.05 vs. adenosine; †P< 0.05 vs. zero.
Protocol 2. Effects of graded infusions of ATP on postjunctional α-adrenergic vasoconstrictor responsiveness
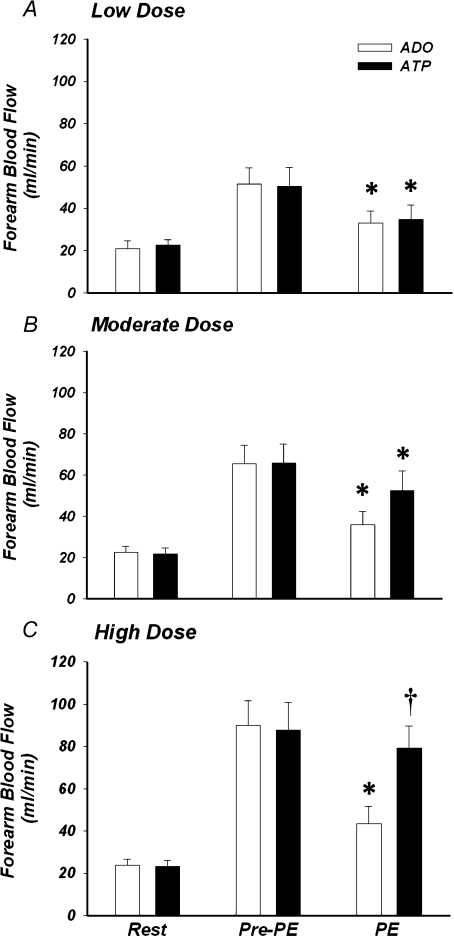
Forearm haemodynamics, HR, and MAP for Protocol 2 are presented in Table 2. Graded increases in exogenous ATP evoked a dose-dependent increase in FBF (Fig. 4). Specifically, increasing the arterial concentration by 500, 1000 and 2000 nmol l−1 increased FBF by ∼2, 3, and 4-fold, respectively (all P< 0.05 versus baseline). As desired by experimental design, steady-state (prevasoconstrictor) FBF responses to adenosine infusion were effectively matched to that observed within each dose condition of ATP (Fig. 4; P= 0.9–1.0). Infusion of phenylephrine (α1-agonist) significantly reduced FBF from steady-state hyperaemia during low and moderate dose adenosine and ATP (both P< 0.05; Fig. 4A and B). However, although phenylephrine also reduced FBF during high dose adenosine (P< 0.05), it did not significantly affect FBF during high dose ATP (P= 0.16; Fig. 4C).
Table 2.
Protocol 2. Forearm and systemic haemodynamics during graded infusions of ATP and adenosine
| Condition/dose | Forearm blood flow (ml min−1) | Mean arterial pressure (mmHg) | Forearm vascular conductance (ml min−1 (100 mmHg)−1) | Heart rate (beats min−1) |
|---|---|---|---|---|
| Baseline | ||||
| Adenosine 1 | 21 ± 4 | 86 ± 3 | 25 ± 5 | 58 ± 5 |
| ATP 1 | 23 ± 3 | 86 ± 3 | 27 ± 4 | 59 ± 5 |
| Adenosine 2 | 23 ± 3 | 86 ± 3 | 27 ± 4 | 58 ± 5 |
| ATP 2 | 22 ± 3 | 86 ± 3 | 26 ± 4 | 59 ± 4 |
| Adenosine 3 | 23 ± 3 | 86 ± 3 | 28 ± 4 | 60 ± 5 |
| ATP 3 | 24 ± 3 | 86 ± 3 | 28 ± 4 | 59 ± 5 |
| Pre-phenylephrine | ||||
| Adenosine 1 | 51 ± 8* | 86 ± 3 | 61 ± 9* | 60 ± 5 |
| ATP 1 | 50 ± 9* | 84 ± 3 | 61 ± 12* | 58 ± 5 |
| Adenosine 2 | 66 ± 9* | 87 ± 2 | 77 ± 11* | 59 ± 5 |
| ATP 2 | 66 ± 9* | 86 ± 3 | 78 ± 12* | 59 ± 5 |
| Adenosine 3 | 90 ± 12* | 87 ± 3 | 105 ± 14* | 60 ± 5 |
| ATP 3 | 88 ± 13* | 84 ± 3 | 106 ± 17* | 59 ± 5 |
| Phenylephrine | ||||
| Adenosine 1 | 33 ± 6*† | 88 ± 4 | 38 ± 7*† | 60 ± 5 |
| ATP 1 | 35 ± 7*† | 87 ± 3 | 41 ± 9*† | 59 ± 5 |
| Adenosine 2 | 36 ± 6*† | 90 ± 3* | 41 ± 8*† | 59 ± 5 |
| ATP 2 | 53 ± 10*† | 88 ± 4 | 62 ± 12*† | 58 ± 5 |
| Adenosine 3 | 43 ± 8*† | 90 ± 4* | 50 ± 10*† | 60 ± 5 |
| ATP 3 | 79 ± 11*‡ | 86 ± 3 | 94 ± 14*‡ | 59 ± 5 |
P< 0.05 vs baseline within condition;
P< 0.05 vs steady-state (Pre-vasoconstrictor; Phenylephrine) within condition;
P< 0.05 vs adenosine during phenylephrine (α1-agonist) infusion. 1 = Low dose condition; 2 = Moderate dose condition; 3 = High dose condition (see text for details). Forearm vascular conductance was calculated as (forearm blood flow/mean arterial pressure) × 100.
Figure 4. Forearm blood flow at rest, during the adenosine and ATP hyperaemic conditions, and during infusion of the α1-agonist.
Forearm blood flow was significantly elevated in a dose-dependent manner with exogenous ATP (P < 0.05), and forearm hyperaemia was effectively matched via infusion of adenosine. α1-adrenoceptor stimulation with phenylephrine (PE) significantly reduced forearm blood flow during all doses (low, moderate, high) of adenosine. In contrast, phenylephrine significantly reduced forearm blood flow during low and moderate dose ATP, whereas this was not significant during high dose ATP. *P< 0.05 vs. steady state (Pre-PE) within condition; †P< 0.05 vs. adenosine during phenylephrine.
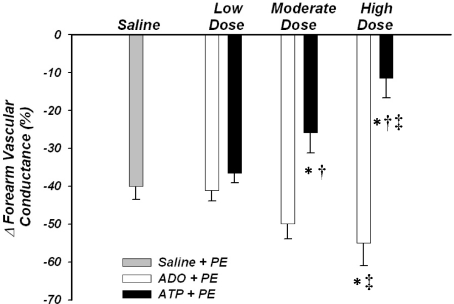
The forearm vasoconstrictor responses to direct α1-adrenoceptor stimulation (ΔFVC) under control resting conditions were −40 ± 3%. The vasoconstrictor responses during low dose adenosine (−38 ± 3%) and ATP (−33 ± 2%) were not significantly different from one another, and were similar to the responses observed at rest (P= 0.3–0.9). The vasoconstrictor responses during the moderate dose of adenosine tended to be greater versus resting conditions (−48 ± 4%; P= 0.07), and the responses during high dose adenosine were significantly greater than rest conditions (−53 ± 6%; P< 0.05), most likely due to the absolute amount of phenylephrine infused as part of the flow adjustment process. In contrast, the vasoconstrictor responses during moderate dose ATP (−22 ± 6%) were significantly blunted compared with rest, and the responses during high dose ATP (−8 ± 5%) were significantly blunted compared with rest and those observed during low dose ATP (both P< 0.05; Fig. 5). Importantly, the vasoconstrictor responses during moderate and high dose ATP were significantly blunted compared with the responses during the conditions of matched forearm hyperaemia via adenosine (both P <0.05). MAP changed minimally within and between conditions, and thus FBF responses were similar to FVC. Heart rate and MAP were not significantly different between trials or conditions (Table 2).
Figure 5. Forearm vasoconstrictor responses to α1-adrenoceptor stimulation.
Percentage reduction in forearm vascular conductance in response to α1-adrenoceptor stimulation (via phenylephrine; PE) during low dose infusion of adenosine and ATP were not significantly different from during saline (control) conditions. α1-mediated vasoconstriction was greater during high dose infusion of adenosine compared with control, whereas the vasoconstrictor responses were blunted during moderate and high dose ATP. *P< 0.05 vs. saline (control); †P< 0.05 vs. adenosine within dose condition; ‡P< 0.05 vs. low dose within drug condition.
Discussion
The primary findings from the present investigation are as follows. First, exogenous infusions of ATP required to match steady-state hyperaemia observed during moderate intensity dynamic handgrip exercise (15% MVC) abolishes postjunctional α-adrenoceptor mediated vasoconstriction in humans. Second, the ability of ATP to modulate α-mediated vasoconstriction under these conditions is not selective for α1- or α2-adrenoceptors, as both were similarly abolished by exogenous ATP. Third, increasing arterial ATP concentrations to mimic levels observed within the physiological range during dynamic exercise elevates resting forearm blood flow in a dose-dependent manner. Finally, low dose ATP infusion is not sympatholytic in the human forearm, but graded increases in ATP infusions progressively blunt postjunctional α-adrenergic vasoconstriction. Importantly, these data cannot be explained simply by the vasodilator properties per se of ATP, as forearm hyperaemia was matched via infusions of adenosine, which did not blunt sympathetic α-adrenergic vasoconstriction. The physiological implications of these findings will now be discussed.
Exogenous ATP and postjunctional α-adrenergic vasoconstriction
Data derived from experimental studies using a variety of approaches in both animals and humans have clearly demonstrated a unique ability of contracting muscle to blunt sympathetic vasoconstriction, a phenomenon believed to optimize blood flow and oxygen delivery to active muscle when the sympathetic nervous system is engaged (Joyner & Thomas, 2003; VanTeeffelen & Segal, 2003). Several potential modulators of sympathetic vasoconstriction have been suggested to contribute to this phenomenon including adenosine, NO and PGs, although elucidating a clear mechanism in humans has proved difficult (Dinenno & Joyner, 2003, 2004). Recently, however, Rosenmeier et al. (2004) demonstrated that exogenous ATP infusions abolished regional vasoconstriction in the skeletal muscle circulation evoked via intra-arterial tyramine, similar to what was observed during isolated knee extensor exercise at 25% of peak power output. These data are of significant interest in that infusions of other vasodilators such as adenosine and sodium nitroprusside (NO donor) to mimic exercise hyperaemia do not interfere with sympathetic vasoconstriction in humans (Tschakovsky et al. 2002; Dinenno & Joyner, 2003; Rosenmeier et al. 2003b). Further, emerging evidence that ATP released from red blood cells in proportion to deoxygenated haemoglobin might act to couple blood flow and oxygen delivery to demand during exercise make it an attractive candidate capable of directly causing vasodilatation and also limiting sympathetic vasoconstriction in active muscle. In this context, ATP appears to be a good candidate for explaining sympatholyis because it has a short half-life, and is released when erythrocytes become deoxygenated, which would occur in close proximity of active muscle fibres, and this would allow sympathetic vasoconstriction to occur in resistance vessels of less active fibres to maintain an appropriate match between oxygen demand and oxygen delivery at the microcirculatory level (Calbet et al. 2006; Lundby et al. 2008).
In the present study, we directly tested the hypothesis that exogenous ATP infusion blunts postjunctional α-adrenoceptor responsiveness similar to that observed during exercise. In the study by Rosenmeier et al. (2004), intra-arterial administration of tyramine was used to evoke endogenous NA release and cause subsequent vasoconstriction. Although we (Dinenno & Joyner, 2003, 2004) and others (Ruble et al. 2002; Wilkins et al. 2006) have also used this approach, it must be emphasized that measured changes in venous NA concentrations do not always accurately reflect NA release from sympathetic nerve endings especially when there are marked changes in regional blood flow (Esler et al. 1990). Therefore, we determined the forearm vasoconstrictor responses to direct α1- and α2-adrenoceptor simulation (via phenylephrine and dexmedetomidine, respectively) during moderate handgrip exercise (15% MVC), during control vasodilator infusion of adenosine, and during infusion of ATP required to match forearm hyperaemia during exercise (Protocol 1). As expected, there was marked vasoconstriction to both α-agonists during infusion of adenosine, whereas the responses were significantly blunted (but not abolished) during handgrip exercise. These data are consistent with our previous work in this area of investigation (Dinenno & Joyner, 2003, 2004). Somewhat similar to our observations during exercise, the vasoconstrictor responses to direct α-adrenoceptor stimulation were abolished during infusion of exogenous ATP. To the best of our knowledge, these data provide the first experimental evidence that exogenous ATP modulates direct postjunctional α-adrenoceptor vasoconstriction in humans.
With respect to the α-adrenoceptor subtypes, we observed no difference in the ability of muscle contractions to blunt α1- and α2-mediated vasoconstriction in the human forearm. Similarly, exogenous ATP abolished both α1- and α2-mediated vasoconstriction with no apparent selectivity for the α-receptor subtypes. These data are consistent with previous studies indicating that both postjunctional α1- and α2-adrenoceptor responsiveness are significantly blunted during moderate forearm exercise in humans (Dinenno & Joyner, 2003; Rosenmeier et al. 2003a; Dinenno & Joyner, 2004). It should be noted that there are data in humans performing knee extensor exercise suggestive that α2-adrenoceptors are more sensitive to metabolic inhibition than α1-receptors (Wray et al. 2004), and this is conceptually similar with data derived from various experimental animal models (Anderson & Faber, 1991; Buckwalter et al. 2001). However, in this particular study in humans, there were marked pressor effects (and thus baroreflex activation) during infusion of the α-agonists that preclude clear interpretation of these data (Wray et al. 2004). Nevertheless, if α2-adrenoceptors are indeed more sensitive to metabolic inhibition in the leg circulation, it would be of interest to determine whether ATP has a greater modulatory effect on α2- versusα1-adrenoceptor mediated vasoconstriction in the leg vasculature.
Exogenous ATP and graded modulation of postjunctional α-adrenergic vasoconstriction
In Protocol 1, we demonstrated that exogenous ATP required to match steady-state hyperaemia observed during moderate dynamic handgrip exercise (15% MVC) completely abolished postjunctional α1- and α2-mediated vasoconstriction. From a physiological standpoint, however, if circulating ATP were to completely override sympathetic vasoconstriction in active muscle during large muscle mass exercise, excess vasodilatation would occur and arterial pressure would be compromised (Marshall et al. 1961; Rowell, 1997; Joyner & Thomas, 2003). Thus, in Protocol 2, we determined whether graded ATP infusions evoked a dose-dependent modulation of α-adrenergic vasoconstriction, as has been demonstrated during graded levels of exercise (Thomas et al. 1994; Buckwalter et al. 2001; Tschakovsky et al. 2002; Kirby et al. 2005). Interestingly, we found that low dose ATP infusion (500 nmol l−1) sufficient to increase resting forearm blood flow 2-fold did not blunt α-adrenergic vasoconstriction. This is strikingly similar to what is observed during very mild muscle contractions (5% MVC) that elevate forearm blood flow to a similar extent (Kirby et al. 2005). However, at moderate (1000 nmol l−1) and higher (2000 nmol l−1) doses of ATP selected to increase arterial concentrations similar to that observed in the femoral vein draining skeletal muscle during graded knee extensor exercise (Gonzalez-Alonso et al. 2002), α-adrenoceptor responsiveness was progressively blunted. In this context, the ability of graded ATP infusions to limit postjunctional α-adrenoceptor mediated vasoconstriction is quite similar to what is observed with graded exercise intensity, and provides further support for the hypothesis that circulating ATP could play a significant role in regulating muscle blood flow and vascular tone during exercise.
Potential mechanisms
The mechanism(s) by which circulating ATP can modulate sympathetic α-adrenergic vasoconstriction in the skeletal muscle circulation are at present unknown. What is clear, however, is that this does not simply reflect the vasodilator properties of ATP per se, as infusions of other vasodilators to mimic exercise hyperaemia do not blunt sympathetic vasoconstriction (Tschakovsky et al. 2002; Rosenmeier et al. 2003b). Indeed, in the present study (Protocol 2), adenosine was not capable of inhibiting α-adrenergic vasoconstriction. Thus, we speculate that cellular mechanisms by which ATP-induced P2y receptor activation evokes smooth muscle cell relaxation are involved. In this context, recent studies indicate that ATP-mediated vasodilatation in humans is independent of NO and PGs (van Ginneken et al. 2004), which is consistent with recent findings that independent inhibition of NO (Dinenno & Joyner, 2003) and PGs (Hansen et al. 2000; Dinenno & Joyner, 2004) does not have a significant impact on functional sympatholysis in humans. Further, when we performed combined NO and PG inhibition, sympathetic vasoconstriction in contracting muscle was only slightly augmented compared with control conditions, suggesting other modulatory factors were involved (Dinenno & Joyner, 2004). Taken together, it seems plausible to speculate that ATP evokes smooth muscle cell hyperpolarization, and this in turn blunts sympathetic α-adrenergic vasoconstriction. Future studies will be needed to determine the exact mechanism underlying the sympatholytic effect of circulating ATP.
Experimental considerations
In Protocol 2 of the present investigation, we chose to only use phenylephrine (α1-agonist) to test whether the ability of circulating ATP to blunt postjunctional α-adrenoceptor vasoconstriction was graded with ATP concentrations, as opposed to using both α1- and α2-adrenoceptor agonists. However, in Protocol 1, our data indicated that exogenous infusion of ATP at concentrations to match forearm hyperaemia observed during moderate intensity handgrip exercise abolished both α1- and α2-mediated vasoconstriction, with no apparent difference between the receptor subtypes. Additionally, we were concerned about giving dexmedetomidine (α2-agonist) over seven experimental trials, as this would significantly increase the risk of α2-adrenoceptor effects on the central nervous system (e.g. hypotension, sedation). This is important not only from a subject safety standpoint, but also if there were central α2 effects, this would inhibit basal sympathetic outflow (Lang et al. 1997) and cloud interpretation of the data for the remaining experimental trials.
An additional consideration for Protocol 2 relates to the dose adjustment of phenylephrine we employed based on changes in forearm blood flow, which was performed to reduce any potential ‘dilution effect’ of the α1-agonist during the various doses of adenosine and ATP. Our findings indicate that the vasoconstrictor responses during the moderate dose of adenosine tended to be greater, and that the vasoconstrictor responses during the high dose of adenosine were greater compared with control (saline) conditions. Although seemingly counterintuitive, this is consistent with what has been demonstrated when adjustments in the dose of tyramine were performed for similar reasons during hyperaemic conditions associated with adenosine infusions (Tschakovsky et al. 2002). Thus, although we were aware this might occur, we needed to be sure that any apparent sympatholytic effect of exogenous ATP was not due to a dilution effect, as this has never been determined before during ATP infusions. Regardless, our data clearly indicate a graded sympatholytic effect of exogenous ATP whether compared with the vasoconstriction observed during control conditions, or conditions of matched hyperaemia via adenosine.
Experimental limitations
One limitation of the present study relates to the use of moderate intensity exercise with a small muscle mass, and thus moderate hyperaemic conditions, to test our hypotheses. However, our experimental model allows for moderate intensity dynamic muscle contractions to be performed without increasing sympathetic outflow, and allows for well-controlled vasoactive drug infusions that do not alter arterial blood pressure and thus do not evoke baroreflex-mediated alterations in sympathetic outflow. This is important in that changes in sympathetic nervous system activity would cloud interpretation of our vasoconstrictor responses during the α-agonist infusions. Nevertheless, it is important to recognize that the potential interaction between ATP and sympathetic vasoconstriction during larger muscle mass, higher intensity exercise could be more complex than observed in our studies as indicated by recent data demonstrating that some degree of local vasoconstriction is necessary for the precise matching of oxygen delivery to oxygen demand under such conditions (Calbet et al. 2006; Lundby et al. 2008).
Another limitation of the present study relates to the lack of measurement of circulating arterial or venous plasma ATP concentrations during the experimental trials. In Protocol 1, our goal was to match forearm blood flow to that observed during steady-state exercise hyperaemia, and thus we were not concerned with how much exogenous ATP was required to achieve this. However, in Protocol 2, our doses were chosen to mimic average increases in ATP concentrations observed in the venous circulation during graded knee extensor exercise in humans (Gonzalez-Alonso et al. 2002). We used data from the venous circulation (versus arterial) as ATP release from red blood cells would occur at the level of the microcirculation and thus venous concentrations would provide a better estimate of this. Using this approach, we were able to demonstrate a 2- to 4-fold increase in forearm blood flow across this range of exogenous ATP and further, that increasing ATP concentrations within this predicted range caused graded sympatholysis. However, it still remains unclear what exact concentrations of ATP at the level of the resistance vessel network are ultimately observed during exercise, and thus are sympatholytic under these conditions. A final limitation of the present investigation relates to the inability to inhibit P2y-receptors in humans due to the lack of an available pharmacological agent. In this context, to definitively determine if ATP is mechanistically linked with the ability of muscle contractions to blunt sympathetic vasoconstriction, similar studies will need to be performed before and after P2y-receptor blockade and demonstrate that muscle contractions are incapable of modulating sympathetic vasoconstriction under conditions of P2y-receptor inhibition.
Conclusions
The results from the present investigation demonstrate that exogenous ATP infusions required to match the hyperaemic responses observed during moderate handgrip exercise abolish postjunctional α1- and α2-adrenoceptor responsiveness in the human forearm. Importantly, graded increases in arterial concentrations of ATP within the physiological range that evoke moderate limb hyperaemia causes graded inhibition of α-mediated vasoconstriction, such that low levels are not sympatholytic whereas progressive reductions in α-adrenoceptor mediated vasoconstriction are observed with increasing ATP concentrations. Collectively, these data are consistent with the conceptual framework regarding how muscle blood flow and vascular tone are regulated in contracting muscles of humans.
Acknowledgments
We would like to thank Carrie Simpson, Whitney Lewis, and Anne Crecelius for assistance in these studies, as well as the subjects who volunteered to participate. This research was supported by National Institutes of Health awards AG022337, AG027150, and HL087952 (F.A.D.).
References
- Anderson KM, Faber JE. Differential sensitivity of arteriolar α1- and α2-adrenoceptor constriction to metabolic inhibition during rat skeletal muscle contraction. Circ Res. 1991;69:178–184. doi: 10.1161/01.res.69.1.174. [DOI] [PubMed] [Google Scholar]
- Bergfeld GR, Forrester T. Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovasc Res. 1992;26:40–47. doi: 10.1093/cvr/26.1.40. [DOI] [PubMed] [Google Scholar]
- Buckwalter JB, Naik JS, Valic Z, Clifford PS. Exercise attenuates α-adrenergic-receptor responsiveness in skeletal muscle vasculature. J Appl Physiol. 2001;90:172–178. doi: 10.1152/jappl.2001.90.1.172. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Kennedy C. A dual function for adenosine 5-triphosphate in the regulation of vascular tone: excitatory cotransmitter with noradrenaline from perivascular nerves and locally released inhibitory intravascular agent. Circ Res. 1986;58:319–330. doi: 10.1161/01.res.58.3.319. [DOI] [PubMed] [Google Scholar]
- Calbet JA, Jensen-Urstad M, van Hall G, Holmberg HC, Rosdahl H, Saltin B. Maximal muscular vascular conductances during whole body upright exercise in humans. J Physiol. 2004;558:319–331. doi: 10.1113/jphysiol.2003.059287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calbet JA, Lundby C, Sander M, Robach P, Saltin B, Boushel R. Effects of ATP-induced leg vasodilation on VO2 peak and leg O2 extraction during maximal exercise in humans. Am J Physiol Regul Integr Comp Physiol. 2006;291:R447–RR453. doi: 10.1152/ajpregu.00746.2005. [DOI] [PubMed] [Google Scholar]
- Chavoshan B, Sander M, Sybert TE, Hansen J, Victor RG, Thomas GD. Nitric oxide-dependent modulation of sympathetic neural control of oxygenation in exercising human skeletal muscle. J Physiol. 2002;540:377–386. doi: 10.1113/jphysiol.2001.013153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinenno FA, Dietz NM, Joyner MJ. Aging and forearm postjunctional α-adrenergic vasoconstriction in healthy men. Circulation. 2002;106:1349–1354. doi: 10.1161/01.cir.0000028819.64790.be. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Joyner MJ. Blunted sympathetic vasoconstriction in contracting skeletal muscle of healthy humans: is nitric oxide obligatory? J Physiol. 2003;553:281–292. doi: 10.1113/jphysiol.2003.049940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinenno FA, Joyner MJ. Combined NO and PG inhibition augments α-adrenergic vasoconstriction in contracting human skeletal muscle. Am J Physiol Heart Circ Physiol. 2004;287:H2576–H2584. doi: 10.1152/ajpheart.00621.2004. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Masuki S, Joyner MJ. Impaired modulation of sympathetic α-adrenergic vasoconstriction in contracting forearm muscle of ageing men. J Physiol. 2005;567:311–321. doi: 10.1113/jphysiol.2005.087668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellsworth ML. The red blood cell as an oxygen sensor: what is the evidence? Acta Physiol Scand. 2000;168:551–559. doi: 10.1046/j.1365-201x.2000.00708.x. [DOI] [PubMed] [Google Scholar]
- Ellsworth ML. Red blood cell-derived ATP as a regulator of skeletal muscle perfusion. Med Sci Sports Exerc. 2004;36:35–41. doi: 10.1249/01.MSS.0000106284.80300.B2. [DOI] [PubMed] [Google Scholar]
- Ellsworth ML, Forrester T, Ellis CG, Dietrich HH. The erythrocyte as a regulator of vascular tone. Am J Physiol Heart Circ Physiol. 1995;269:H2155–H2161. doi: 10.1152/ajpheart.1995.269.6.H2155. [DOI] [PubMed] [Google Scholar]
- Esler MD, Jennings G, Lambert G, Meredith I, Horne M, Eisenhofer G. Overflow of catecholamine neurotransmitters to the circulation: source, fate, and functions. Physiol Rev. 1990;70:963–985. doi: 10.1152/physrev.1990.70.4.963. [DOI] [PubMed] [Google Scholar]
- Faber JE, Harris PD, Joshua IG. Microvascular response to blockade of prostaglandin synthesis in rat skeletal muscle. Am J Physiol Heart Circ Physiol. 1982;243:H51–H60. doi: 10.1152/ajpheart.1982.243.1.H51. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Alonso J, Olsen DB, Saltin B. Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: role of circulating ATP. Circ Res. 2002;91:1046–1055. doi: 10.1161/01.res.0000044939.73286.e2. [DOI] [PubMed] [Google Scholar]
- Hansen J, Sander M, Hald CF, Victor RG, Thomas GD. Metabolic modulation of sympathetic vasoconstriction in human skeletal muscle: role of tissue hypoxia. J Physiol. 2000;527:387–396. doi: 10.1111/j.1469-7793.2000.00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagger JE, Bateman RM, Ellsworth ML, Ellis CG. Role of erythrocyte in regulating local O2 delivery mediated by hemoglobin oxygenation. Am J Physiol Heart Circ Physiol. 2001;280:H2833–H2839. doi: 10.1152/ajpheart.2001.280.6.H2833. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Thomas GD. Having it both ways? Vasoconstriction in contracting muscles. J Physiol. 2003;550:333. doi: 10.1113/jphysiol.2003.044628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller DM, Ogoh S, Greene S, Olivencia-Yurvati A, Raven PB. Inhibition of KATP channel activity augments baroreflex-mediated vasoconstriction in exercising human skeletal muscle. J Physiol. 2004;561:273–282. doi: 10.1113/jphysiol.2004.071993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BS, Markwald RR, Smith EG, Dinenno FA. Mechanical effects of muscle contraction do not blunt sympathetic vasoconstriction in humans. Am J Physiol Heart Circ Physiol. 2005;289:H1610–H1617. doi: 10.1152/ajpheart.00391.2005. [DOI] [PubMed] [Google Scholar]
- Kneale BJ, Chowienczyk PJ, Brett SE, Coltart DJ, Ritter JM. Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. J Am Coll Cardiol. 2000;36:1233–1238. doi: 10.1016/s0735-1097(00)00849-4. [DOI] [PubMed] [Google Scholar]
- Lang CC, Rayos GH, Chomsky DB, Wood AJJ, Wilson JR. Effect of sympathoinhibition on exercise performance in patients with heart failure. Circulation. 1997;96:238–245. doi: 10.1161/01.cir.96.1.238. [DOI] [PubMed] [Google Scholar]
- Lautt WW. Resistance or conductance for expression of arterial vascular tone. Microvasc Res. 1989;37:230–236. doi: 10.1016/0026-2862(89)90040-x. [DOI] [PubMed] [Google Scholar]
- Lundby C, Boushel R, Robach P, Moller K, Saltin B, Calbet JA. During hypoxic exercise some vasoconstriction is needed to match O2 delivery with O2 demand at the microcirculatory level. J Physiol. 2008;586:123–130. doi: 10.1113/jphysiol.2007.146035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall RJ, Schirger A, Shepherd JT. Blood pressure during supine exercise in idiopathic orthostatic hypotension. Circulation. 1961;24:76–81. doi: 10.1161/01.cir.24.1.76. [DOI] [PubMed] [Google Scholar]
- Nishigaki K, Faber JE, Ohyanagi M. Interactions between α-adrenoceptors and adenosine receptors on microvascular smooth muscle. Am J Physiol Heart Circ Physiol. 1991;260:H1655–H1666. doi: 10.1152/ajpheart.1991.260.5.H1655. [DOI] [PubMed] [Google Scholar]
- O’Leary DS. Regional vascular resistance vs conductance: which index for baroreflex responses? Am J Physiol Heart Circ Physiol. 1991;260:H632–H637. doi: 10.1152/ajpheart.1991.260.2.H632. [DOI] [PubMed] [Google Scholar]
- Remensnyder JP, Mitchell JH, Sarnoff SJ. Functional sympatholysis during muscular activity. Circ Res. 1962;11:370–380. doi: 10.1161/01.res.11.3.370. [DOI] [PubMed] [Google Scholar]
- Rongen GA, Smits P, Thien T. Characterization of ATP-induced vasodilation in the human forearm vascular bed. Circulation. 1994;90:1891–1898. doi: 10.1161/01.cir.90.4.1891. [DOI] [PubMed] [Google Scholar]
- Rosenmeier JB, Dinenno FA, Fritzlar SJ, Joyner MJ. α1- and α2-adrenergic vasoconstriction is blunted in contracting human muscle. J Physiol. 2003a;547:971–976. doi: 10.1113/jphysiol.2002.037937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmeier JB, Fritzlar SJ, Dinenno FA, Joyner MJ. Exogenous NO administration and α-adrenergic vasoconstriction in human limbs. J Appl Physiol. 2003b;95:2370–2374. doi: 10.1152/japplphysiol.00634.2003. [DOI] [PubMed] [Google Scholar]
- Rosenmeier JB, Hansen J, Gonzalez-Alonso J. Circulating ATP-induced vasodilatation overrides sympathetic vasoconstrictor activity in human skeletal muscle. J Physiol. 2004;558:351–365. doi: 10.1113/jphysiol.2004.063107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell LB. Neural control of muscle blood flow: importance during dynamic exercise. Clin Exp Pharmacol Physiol. 1997;24:117–125. doi: 10.1111/j.1440-1681.1997.tb01793.x. [DOI] [PubMed] [Google Scholar]
- Ruble SB, Valic Z, Buckwalter JB, Tschakovsky ME, Clifford PS. Attenuated vascular responsiveness to noradrenaline release during dynamic exercise in dogs. J Physiol. 2002;541:637–644. doi: 10.1113/jphysiol.2001.014738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltin B, Radegran G, Koskolou MD, Roach RC. Skeletal muscle blood flow in humans and its regulation during exercise. Acta Physiol Scand. 1998;162:421–436. doi: 10.1046/j.1365-201X.1998.0293e.x. [DOI] [PubMed] [Google Scholar]
- Smith EG, Voyles WF, Kirby BS, Markwald RR, Dinenno FA. Ageing and leg postjunctional α-adrenergic vasoconstrictor responsiveness in healthy men. J Physiol. 2007;582:63–71. doi: 10.1113/jphysiol.2007.130591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague RS, Ellsworth ML, Stephenson AH, Kleinhenz ME, Lonigro AJ. Deformation-induced ATP release from red blood cells requires CFTR activity. Am J Physiol Heart Circ Physiol. 1998;275:H1726–H1732. doi: 10.1152/ajpheart.1998.275.5.H1726. [DOI] [PubMed] [Google Scholar]
- Thomas GD, Hansen J, Victor RG. Inhibition of α2-adrenergic vasoconstriction during contraction of glycolytic, not oxidative, rat hindlimb muscle. Am J Physiol Heart Circ Physiol. 1994;266:H920–H929. doi: 10.1152/ajpheart.1994.266.3.H920. [DOI] [PubMed] [Google Scholar]
- Thomas GD, Hansen J, Victor RG. ATP-sensitive potassium channels mediate contraction-induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. J Clin Invest. 1997;99:2602–2609. doi: 10.1172/JCI119448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GD, Victor RG. Nitric oxide mediates contraction-induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. J Physiol. 1998;506:817–826. doi: 10.1111/j.1469-7793.1998.817bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschakovsky ME, Sujirattanawimol K, Ruble SB, Valic Z, Joyner MJ. Is sympathetic neural vasoconstriction blunted in the vascular bed of exercising human muscle? J Physiol. 2002;541:623–635. doi: 10.1113/jphysiol.2001.014431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ginneken EE, Meijer P, Verkaik N, Smits P, Rongen GA. ATP-induced vasodilation in human skeletal muscle. Br J Pharmacol. 2004;141:842–850. doi: 10.1038/sj.bjp.0705589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanTeeffelen JW, Segal SS. Interaction between sympathetic nerve activation and muscle fibre contraction in resistance vessels of hamster retractor muscle. J Physiol. 2003;550:563–574. doi: 10.1113/jphysiol.2003.038984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins BW, Schrage WG, Liu Z, Hancock KC, Joyner MJ. Systemic hypoxia and vasoconstrictor responsiveness in exercising human muscle. J Appl Physiol. 2006;101:1343–1350. doi: 10.1152/japplphysiol.00487.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray DW, Fadel PJ, Smith ML, Raven P, Sander M. Inhibition of α-adrenergic vasoconstriction in exercising human thigh muscles. J Physiol. 2004;555:545–563. doi: 10.1113/jphysiol.2003.054650. [DOI] [PMC free article] [PubMed] [Google Scholar]