Abstract
A crucial feature of the motor system is the ability to control some movements automatically. We have previously shown that all parts of the motor networks reduce their activity with automaticity, and, while this change may indicate increased efficiency in terms of neural processing, it is not clear how motor skill can be maintained after a reduction of neural activity. In the current study, we used functional MRI (fMRI) to investigate influences on the effective connectivity of the brain motor networks when movements become automatic. Subjects practiced a sequential movement until they could execute it automatically, and task-related brain fMRI activation was measured before and after they achieved automaticity. Using the psychophysiological interaction (PPI) method, we found that the cerebellum, cingulate motor area, supplementary motor area, and putamen had significantly greater connectivity, whereas the precuneus had less connectivity in the motor networks at the automatic stage. Our findings demonstrate that the importance of the attention networks decrease when movements become automatic. Moreover, the process of automaticity is accompanied by a strengthened interaction of central motor networks even though the magnitude of the activation is decreased. We speculate that this increase in connectivity reflects more efficient neural coding of movement at the automatic stage.
A general feature of the motor system is that people can perform some learned movements automatically. Automatic movements are performed without attention being clearly directed toward the details of the movement, particularly for movements that require low precision or that are commonly made (Bernstein, 1967). Numerous functional neuroimaging studies have revealed brain activity related to automaticity. These studies found that the pattern of neural activation is similar in the novel and automatic conditions, but with a reduction of brain activation in several regions, like the cerebellum, premotor area (PMA) and dorsolateral prefrontal cortex (DLPFC) concomitant with the acquisition of automaticity (Frith et al. 1991; Jueptner et al. 1997; Jansma et al. 2001; Wu et al. 2004; Lehericy et al. 2005; Poldrack et al. 2005). Some studies also found increased activation in specific regions, like the basal ganglia, during the execution of automatic tasks compared with the early learning phase (Jueptner et al. 1997; Hikosaka et al. 1999; Poldrack et al. 2005), but other research did not verify these findings (Jansma et al. 2001; Wu et al. 2004).
While these studies have provided important insights, the physiology of automaticity is still far from understood. All neuroimaging studies so far have focused on the magnitude of brain activity; however, investigations about interaction among human brain regions may play a more important role in understanding automaticity-related brain functional changes because multiple areas are likely to be involved in the control of a given task. In recent years, a great effort has been made in exploring interregional connectivity in a given task (Friston, 1995; Buchel & Friston, 1997; Buchel et al. 1999; Liu et al. 1999; Friston & Buchel, 2000; Buchel, 2004), which is usually characterized in terms of functional connectivity (Friston et al. 1993a) or effective connectivity (Friston et al. 1993b). This method has been extensively used in neuroscientific studies, and is commonly accepted as a powerful way to characterize neural interactions among brain regions during particular tasks. Some studies have investigated changes of brain networks during the motor learning process. For example, Toni et al. (2002) found that effective connectivity was enhanced in cortico-striatal circuits, but decreased among portions of the frontal cortex during visuomotor learning. Another study on motor sequence learning observed that sensorimotor cortex, PMA, and supplementary motor area (SMA) have significantly greater inter- and intrahemispheric coupling during the early condition compared with the late learning condition; and, in addition, there was greater connectivity between frontal regions and cortical motor regions in the early versus late learning stage (Sun et al. 2007). However, motor performances in these studies did not achieve the automatic stage. Automaticity-related effective connectivity of the brain networks has never been investigated.
It has been suggested that although similar brain regions are involved both in the novel and automatic performance, the functions associated with the regions within the networks during the process of achieving automaticity may differ (Little et al. 2004). We hypothesized that the acquisition of automaticity is not only related to the changes of the magnitude of neural activity, but also associated with a modification of the effective connectivity of brain networks. To investigate this we used functional MRI (fMRI) and the method of psychophysiological interaction (PPI) to analyse the changes of effective connectivity of neural motor networks when movements become automatic.
Methods
Experimental design and data collection have been reported previously (Wu et al. 2004), and will only be summarized here.
Subjects
Fifteen normal, right handed volunteers, partially overlapping with the subjects reported in our earlier report (Wu et al. 2004), participated in the study (10 males, 5 females; mean age 27.2; range 21–38). The experiment was performed according to the Declaration of Helsinki and was approved by the Institutional Review Board. All subjects gave their written informed consent.
Tasks
Subjects were asked to perform self-initiated, self-paced, sequential right finger movements at 0.5 Hz. The sequence was 1-4-3-2-2-4-1-3-4-1-2-3, in which 1, 2, 3 and 4 refer to the index, middle, ring and little fingers, respectively. Automaticity was evaluated by having subjects perform a visual letter-counting task simultaneously with the sequential movement (dual task). Before the first fMRI scan, all subjects practiced until they could move at the required rate, and briefly practiced each sequential movement for 10–20 min. After the first fMRI scan, subjects practiced the sequential movement until they could perform it from memory 10 times in a row without error, as well as the dual task accurately. Only at this level was the movement considered automatic. During the fMRI scanning, only sequential movement was performed.
Functional MRI acquisition
Data were obtained using a whole-body 1.5 tesla MRI scanner (Signa, General Electric, Milwaukee, WI, USA) and a standard head coil. Subjects lay supine in the MR scanner with a response device fixed to their right hand. The response device had four buttons, corresponding to the index, middle, ring and little fingers of the right hand and was used to record finger movements. The subjects viewed visual signals on a screen through a mirror built into the head coil. We used an EPI gradient echo sequence (21 slices, TE = 30 ms, TR = 2500 ms, flip angle = 90 deg, FOV = 22 × 22 cm, matrix = 64 × 64, slice thickness 5 mm, gap 2 mm) to obtain functional images.
All subjects were scanned both before and after they achieved automaticity. A time course series of 100 images per slice was acquired for each scanning session, in an OFF–ON cycle protocol of ‘rest’ and ‘active’ condition. Each condition lasted 25 s and was repeated 5 times in a session. In the rest condition, subjects relaxed and focused on the screen in front of them without moving in the scanner. In the active condition, subjects were asked to perform the sequential movement.
Data analysis
Each subject's performance of the sequential movement task was recorded during fMRI scans, and wrong button presses were considered as errors. fMRI data analysis was performed with SPM2 software (Wellcome Institute of Cognitive Neurology, London, UK). Functional images were aligned to the first image of each session for motion correction. After spatial normalization, all images were resampled into voxels that were 2 × 2 × 2 mm in size, and smoothed with a Gaussian filter of 6 mm full width at half-maximum (FWHM). Data were analysed for each single subject separately on a voxel-by-voxel basis using the principles of the general linear model extended to allow the analysis of fMRI data as a time series (Friston et al. 1995). The data were modelled using a fixed-effect boxcar design, convolved with a haemodynamic response function (HRF) chosen to represent the relationship between neuronal activation and blood flow changes. The model had the same ON-OFF frequency as the alternation frequency of the ‘active’ and ‘rest’ conditions, and was constructed for analysis of task-dependent activation, identical for all subjects. A contrast representing the effect of the active condition compared with the rest condition was defined and contrast images were calculated individually. These contrast images were used in the second level for random-effects analyses. The group result was calculated by a one-sample t test model to identify the brain activity before and after achieving automaticity (P < 0.05, with correction for multiple comparisons). A paired t test model was used to compare the results before and after achieving automaticity (P < 0.05, corrected). The numbers of voxels activated above threshold were determined, and the centre of the activation cluster was defined as the maximum activated voxel within the cluster and expressed in Talairach coordinates.
Automaticity-dependent changes in effective connectivity were assessed using a PPI model (Friston et al. 1997). PPI is defined as the change in contribution of one brain area to another due to a change in experimental condition or psychological context (Friston et al. 1997), and aims to explain regionally specific responses in terms of the interaction between the psychological variable and the activity in a specific index area. PPI computes whole-brain connectivity between the time series of the index area and the time series of all other voxels. The analysis is constructed to test for the differences in the regression slope of activity in all areas, on the activity in the index area, under the two conditions (automatic versus novel condition in the present study). The bilinear term in PPI represents the interaction between physiological activity and a psychological context input, which modulates the connectivity between the index area and the other brain regions, and has a directional character (Stephan, 2004). In the current study, the PPI identifies areas in which the degree of coupling with the index region is modulated significantly by the process of automaticity.
We chose the left primary motor cortex (M1), bilateral dorsal PMA, bilateral DLPFC, bilateral cerebellum, left putamen, SMA, and cingulate motor area (CMA), and precuneus as index areas because these regions may be involved in the process of automaticity or are important in motor learning (Jenkins et al. 1994; Pascual-Leone et al. 1994; Karni et al. 1995; Nudo et al. 1996; Jueptner et al. 1997; Sakai et al. 1998; Toni et al. 1998; Hikosaka et al. 1999; Nakamura et al. 1999; Doyon et al. 2002; Muellbacher et al. 2002; Wu et al. 2004; Lehericy et al. 2005; Poldrack et al. 2005). The SMA contains two separate areas, the SMA proper in the caudal portion and the pre-SMA in the rostral portion (Picard & Strick, 1996). Similarly, the CMA also includes the rostral (RCZ) and the caudal (CCZ) parts (Picard & Strick, 1996). Our previous study (Wu et al. 2004) suggested that the main portions related to automaticity in these areas are the anterior SMA and rostral CMA; therefore, we used pre-SMA and RCZ as the index areas in the current study. Separate PPI analyses were conducted for each index area. Following the described procedure (Stephan et al. 2003; Garraux et al. 2005), the mean corrected and high-passed-filtered time series in each index area were obtained on a subject-by-subject basis by extracting the first principal component from all voxel time series in a 5 mm radius sphere centred at the coordinates of the subject specific activations. The psychophysiological interaction term (referred to as ‘PPI regressor’) was computed as the element-by-element product of the deconvolved extracted time series of selected index area and a vector coding for the main effect of task (1 for automatic condition, –1 for before automatic condition, 0 elsewhere) (Gitelman et al. 2003; Stephan et al. 2003; Garraux et al. 2005). The PPI regressor was mean corrected to remove subject-specific effects and convolved by the canonical HRF to account for possible haemodynamic lag. For each subject, the PPI regressor, the task regressor (representing the automatic minus novel contrast for the main effect of automaticity), and the extracted time series were entered in a first-level model of effective connectivity in which the PPI regressor was orthogonalized with regard to the main effect of task and the regional time series. Brain areas receiving context-dependent influences from the index areas that were greater during the automatic stage than the novel stage were determined by testing for positive slopes of the PPI regressor, i.e. by applying a t-contrast that was 1 for the PPI regressor and 0 elsewhere. Conversely, brain areas receiving context-dependent influences from the index areas that were greater during the novel stage than the automatic stage were determined by testing for negative slopes of the PPI regressor, i.e. by applying a t-contrast that was –1 for the PPI regressor and 0 elsewhere. Contrast images from the first-level PPI analysis in each subject were entered into a second-level random-effect model. At the second level, to detect the regions that receive greater influences from each index area during the automatic stage, the contrast images from each subject shown greater influences during the automatic stage than the novel stage were calculated by a one-sample t test (P < 0.05, corrected). Then, contrast images from each subject showing greater influence during the novel stage than the automatic stage were calculated by another one-sample t test to detect the regions that receive greater influences from each index area during the novel stage (P < 0.05, corrected).
From PPI analysis, we found that five index areas, including the bilateral cerebellum, CMA, pre-SMA, and left putamen, have greater influence on other brain regions during the automatic stage than the novel stage. Then, we applied a conjunction analysis to find out the brain areas commonly receiving stronger automaticity-dependent influences from various index areas. While performing conjunction analysis, we used the one-way ANOVA optionin SPM2. We specified five groups in one-way ANOVA, each group included the contrasts from one of the above five index areas in each subject. Once having estimated the model, we specified contrasts for each of the five groups. Then, we selected all contrasts together to perform the conjunction analysis, and followed suggestions proposed by Nichols et al. (2005). A contrast of the PPI results from CMA was used as the inclusive mask (P < 0.05, uncorrected).
Results
Task performance
At the novel stage, all subjects initially committed several errors during performing sequential finger movement (11.4 ± 12.1) and dual task (19.3 ± 10.4/7.7 ± 8.2 for sequential movement/letter counting). After training (2.1 ± 0.6 h), all subjects reported that they could execute the motor sequence without paying attention to the task, even during performing dual task. Their performance was significantly improved (paired t test, P < 0.05), and they made no errors in executing sequential movement (0.0 ± 0.0), and performed the dual task with high accuracy (1.0 ± 2.1/0.0 ± 0.0). In addition, there was no difference of the rate of sequential movement before and after achieving automaticity stage (0.54 ± 0.03 and 0.53 ± 0.04 Hz, respectively). Thus, movement rate had no effect on our observed automaticity-related changes.
fMRI activity
As previously described, the pattern of brain activity while performing sequential movement was similar at the novel and the automatic stage, and was associated with activations at the left primary sensorimotor cortex, bilateral PMAs, bilateral parietal cortex, bilateral inferior frontal gyrus, DLPFC, pre-SMA, SMA proper, CMA, precuneus, basal ganglia, bilateral insular cortex and bilateral cerebellum (one-sample t test, P < 0.05, FWE corrected). The bilateral cerebellum, bilateral PMAs, bilateral parietal cortex, left DLPFC, pre-SMA, CMA, precuneus and left putamen were less activated as the sequential movement became automatic (paired t test, P < 0.05, FWE corrected). No additional activation was observed at the automatic stage.
Effective connectivity analyses
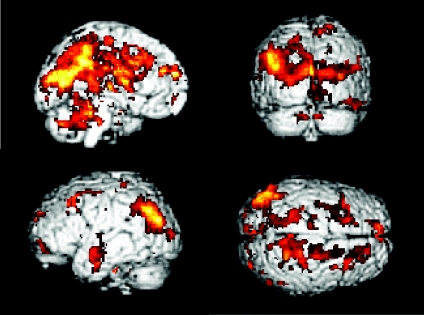
PPI analysis showed that the bilateral cerebellum, CMA, pre-SMA and left putamen had significantly stronger psychophysiological interactions (P < 0.05, FWE corrected) with a number of brain regions at the automatic stage compared to the novel condition (Table 1). Figure 1 shows the areas that receive a significant automaticity process-dependent influence from the CMA.
Table 1.
Brain areas receiving significantly greater influences from the index areas of cerebellum bilaterally, CMA, SMA, and left putamen at the automatic stage compared to the novel learning stage
| Index | Coordinates | ||||
|---|---|---|---|---|---|
| area Areas receiving stronger influence | Cluster size | x | y | Z | Z-value |
| RCB | |||||
| L Cerebellum, anterior lobe | 471 | −10 | −34 | −13 | 5.59 |
| R Cerebellum, posterior lobe | 655 | 44 | −60 | −31 | 4.73 |
| Right anterior cingulate area | 3688 | 12 | 15 | 36 | 5.55 |
| L Middle temporal gyrus | 161 | −50 | −26 | −14 | 5.08 |
| L Precuneus | 296 | −16 | −50 | 50 | 4.84 |
| L Parietal cortex | 238 | −36 | −68 | 36 | 4.35 |
| L Cerebellum, posterior lobe | 531 | −38 | −75 | −30 | 4.01 |
| L Dorsal premotor area | 144 | −31 | −6 | 60 | 3.94 |
| R Inferior parietal lobule | 96 | 30 | −45 | 53 | 3.90 |
| R Dorsal premotor area | 76 | 21 | −5 | 62 | 3.72 |
| LCB | |||||
| R Cerebellum, posterior lobe | 796 | 18 | −43 | − 35 | 5.68 |
| L Precuneus | 2960 | −16 | −50 | 43 | 5.05 |
| L Parietal cortex | 321 | −38 | −70 | 32 | 4.22 |
| L Anterior cingulate area | 1136 | −16 | 15 | 29 | 4.76 |
| L Cerebellum, posterior lobe | 415 | −14 | −56 | −33 | 4.28 |
| L Middle temporal gyrus | 119 | −51 | −18 | −14 | 4.11 |
| R Middle frontal gyrus | 123 | 36 | 23 | 41 | 3.98 |
| R Dorsal premotor area | 156 | 21 | −10 | 58 | 3.84 |
| L Dorsal premotor area | 93 | −28 | −4 | 63 | 3.66 |
| CMA | |||||
| L Precuneus | 4928 | −14 | −42 | 48 | 6.13 |
| L Parietal cortex | 178 | −42 | −63 | 32 | 4.94 |
| R Anterior cingulate area | 899 | 14 | 44 | 27 | 5.74 |
| L Middle temporal gyrus | 141 | −50 | −12 | −12 | 5.06 |
| L Angular gyrus | 620 | −48 | −62 | 36 | 4.82 |
| L Cerebellum, posterior lobe | 634 | −4 | −76 | −13 | 4.46 |
| R Middle frontal gyrus | 123 | 36 | 23 | 41 | 4.32 |
| R Cerebellum, posterior lobe | 168 | 18 | −43 | −35 | 4.24 |
| L Basal ganglia, caudate nucleus | 58 | −12 | −3 | 15 | 4.12 |
| R Dorsal premotor area | 132 | 18 | −16 | 58 | 3.98 |
| L Dorsal premotor area | 76 | −22 | −4 | 61 | 3.65 |
| SMA | |||||
| R Precuneus | 6341 | 10 | −51 | 62 | 6.02 |
| L Inferior parietal lobule | 465 | −46 | −60 | 38 | 5.26 |
| L Anterior cingulate area | 695 | −8 | 11 | 34 | 5.12 |
| R Parietal cortex, postcentral gyrus | 122 | 38 | −19 | 47 | 4.76 |
| L Cerebellum, posterior lobe | 311 | −6 | −67 | −25 | 4.64 |
| L Dorsal premotor area | 131 | −24 | −7 | 64 | 3.97 |
| R Cerebellum, anterior lobe | 54 | 10 | −42 | −15 | 3.72 |
| L Middle temporal gyrus | 52 | −38 | −71 | 18 | 3.70 |
| R Dorsal premotor area | 106 | 28 | −10 | 58 | 3.40 |
| L Putamen | |||||
| L Precuneus | 2398 | −38 | −72 | 37 | 6.11 |
| R Anterior cingulate area | 1855 | 4 | 19 | 34 | 5.45 |
| R Medial frontal gyrus | 131 | 4 | 42 | 22 | 5.02 |
| L Cerebellum, anterior lobe | 251 | −12 | −61 | −24 | 4.94 |
| R Cerebellum, anterior lobe | 233 | 6 | −48 | −28 | 4.36 |
| L Middle temporal gyrus | 124 | −54 | −28 | −12 | 4.30 |
| L Cerebellum, posterior lobe | 155 | −26 | −43 | −40 | 4.21 |
| R Dorsal premotor area | 84 | 33 | −8 | 59 | 4.06 |
| L Dorsal premotor area | 112 | −24 | −12 | 64 | 3.86 |
The coordinates are given as stereotaxic coordinates referring to the atlas of Talairach & Tournoux (1988). Cluster size is the number of voxels. All areas were significant at P < 0.05, corrected. Abbreviations: L: left; R: right; CB: cerebellum; CMA: cingulate motor area; SMA: supplementary motor area.
Figure 1. Psychophysiological interaction (PPI) results from the cingulate motor area (CMA).
Brain regions are shown that receive a significantly more influence from the CMA at the automatic stage compared to the novel stage (P < 0.05, corrected for multiple comparisons).
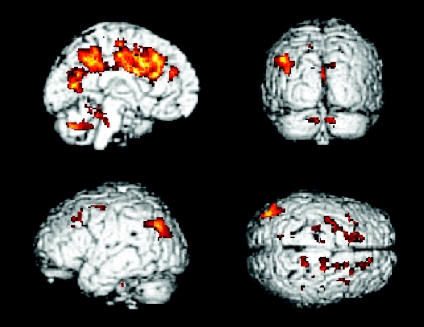
The conjunction analysis showed that the cerebellum bilaterally, CMA, pre-SMA and left putamen all have a significantly stronger influence on the cerebellar cortex bilaterally, the dorsal PMA bilaterally, the left anterior cingulate cortex, left precuneus and left parietal cortex at the automatic stage compared to the novel condition (conjunction analysis, P < 0.05, FWE corrected; Table 2 and Fig. 2).
Table 2.
Results from conjunction analysis (P < 0.05, corrected). Showing brain areas that receive significantly more influence from all of the five index areas (the cerebellum bilaterally, CMA, pre-SMA, and left putamen) at the automatic stage compared to the novel stage
| Coordinates | |||||
|---|---|---|---|---|---|
| Brain area | Cluster size | x | y | Z | Z-value |
| L Anterior cingulate area | 3533 | −10 | 11 | 34 | 5.22 |
| L Precuneus | 435 | −16 | −46 | 48 | 4.92 |
| L Parietal cortex | 312 | −40 | −74 | 36 | 4.72 |
| L Cerebellum, posterior lobe | 143 | −8 | −65 | −27 | 3.94 |
| R Cerebellum, anterior lobe | 89 | 14 | −65 | −25 | 3.88 |
| R Dorsal premotor area | 61 | 14 | −14 | 60 | 3.42 |
| L Dorsal premotor area | 52 | −20 | −5 | 62 | 3.22 |
The coordinates are given as stereotaxic coordinates referring to the atlas of Talairach & Tournoux (1988). Cluster size is the number of voxels. All areas were significant at P < 0.05, corrected. Abbreviations: L: left; R: right; CB: cerebellum; CMA: cingulate motor area; SMA: supplementary motor area.
Figure 2. Brain regions commonly receive automatic-dependent contribution from the index areas.
Brain areas that commonly receive significantly more influence from the bilateral cerebellum, CMA, supplementary motor area (SMA) and left posterior putamen at the automatic stage compared to the novel stage (conjunction analysis, P < 0.05, corrected).
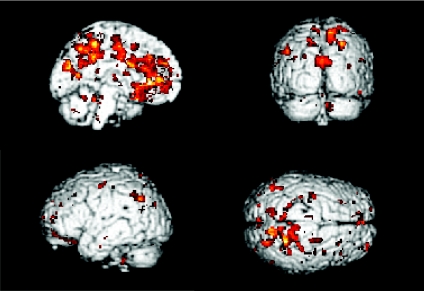
We did not detect a stronger influence from other index areas, including the M1, PMA, and DLPFC on the brain networks at the automatic stage compared to the novel condition. In addition, we found that the precuneus had a stronger influence on the motor networks at the novel stage than at the automatic stage (P < 0.05, FWE corrected, Fig. 3).
Figure 3. PPI results from the precuneus.
Brain regions are shown that receive significantly more influence from the precuneus at the novel stage compared to the automatic stage (P < 0.05, corrected).
Discussion
In our previous study (Wu et al. 2004), we found extensively decreased brain activity as movements become automatic. One factor that should be considered in explaining our finding is the repetition effect of fMRI. Previous studies have found reduced activation of cortical motor areas with repeated simple motor tasks, even with only 10–30 min between the scans (Ramsey et al. 1996). This repetition effect may influence our findings. However, as the changes of brain activity between the novel and automatic stage are extensive, and consistent with other investigations, we suppose those brain activation changes observed in our studies are mainly due to automaticity.
The novel finding of the current study is that the development of automaticity is accompanied by a modification of the effective connectivity of the brain networks. The CMA, pre-SMA, putamen and cerebellum have strengthened psychophysiological interaction with numerous of brain areas at the automatic stage (Table 1), and these regions all have stronger automaticity process-dependent influences on the cingulate cortex, precuneus, bilateral cerebellum, PMA and parietal cortex. These findings suggest that the process of automaticity is accompanied by a strengthened interaction within the central motor networks even though the magnitude of the activation is decreased. In contrast, we observed less influence from the precuneus on the motor networks at automatic stage.
The mesial frontal cortical structures (the CMA and SMA) have strong anatomical connections, and a similar pattern of changes of brain activity during the motor learning process has been reported (Jueptner et al. 1997; Debaere et al. 2004; Poldrack et al. 2005). The SMA has a role in storing learned motor sequences in monkeys as well as in human subjects (Grafton et al. 1994; Jenkins et al. 1994; Tanji & Shima, 1994; van Mier et al. 1998). It may be involved in preparing and executing highly practiced, remembered movement sequences, especially in the programming and execution of movement sequences (Grafton et al. 1994; Jenkins et al. 1994; Tanji & Shima, 1994; Fink et al. 1997; Nakamura et al. 1999). The role of the CMA is not fully defined, but may include both motor and cognitive functions (Picard & Strick, 2001). Monkey studies have demonstrated that the CMA is involved more than any other medial area in preparing and executing highly practiced, remembered movement sequences (Picard & Strick, 1996, 2001). It was suggested that the motor functions of the medial wall that were traditionally attributed to the SMA in human subjects, such as programming and execution of movement sequences, actually may involve the CMA alone or in combination with the SMA (Picard & Strick, 1997).
Our observation of the stronger effective connectivity between the basal ganglia and several cortical areas in the automatic stage is consistent with previous finding that motor learning is associated with increased effective connectivity in cortico-striatal circuits (Toni et al. 2002). The basal ganglia are involved in movement programming and executing (Alexander & Crutcher, 1990). They project to motor cortical areas including the M1, PMA, SMA and CMA. These connections are thought to be involved in acquiring and coordinating motor sequences (Nakano, 2000). In monkeys, it was shown that caudate nucleus neurons are preferentially activated for learning a new motor sequence (Hikosaka et al. 1999; Inase et al. 2001; Miyachi et al. 2002). The basal ganglia may be involved in building a repertoire of motor actions that can be triggered in response to appropriate environmental stimuli (Laforce & Doyon, 2001). The role of the basal ganglia in shifting a learned performance to the automatic stage is supported by observations in patients with Parkinson's disease (Wu & Hallett, 2005).
The cerebellum is another area that develops stronger connectivity with other brain areas as movement becomes more automatic. The cerebellum is also important for learning of skilled movements (Jenkins et al. 1994; Doyon et al. 1998; Thach, 1998; Lang & Bastian, 2002), and critical in both switching learned motor tasks into a more automatic stage and execution of automatic movements (Thach et al. 1992; Jenkins et al. 1994; Doyon et al. 1996; Jueptner et al. 1997; Shadmehr & Holcomb, 1997; Thach, 1998; Toni et al. 1998; Lang & Bastian, 2002; Wu et al. 2004). This region may play an important role in combining learned movements together to produce a well-executed motor skilled behaviour (Laforce & Doyon, 2001).
In contrast to these areas, the precuneus has decreased effective connectivity at the automatic stage. The function of the precuneus has been suggested to be related to attention (Culham et al. 2001), orientation, monitoring (Gusnard & Raichle, 2001), working memory (Callicott et al. 1999), preparation (Astafiev et al. 2003) and motor learning process (Sakai et al. 1998). This area is involved in directing attention in space, as well as in attentive tracking (Culham et al. 2001). It is more activated in performing dual tasks than in performing single tasks, possibly due to increased demands of attention (Wenderoth et al. 2005). The precuneus is important in gathering information continuously, and this broad information-gathering activity decreases only when the successful performance of a task demands focused attention (Gusnard & Raichle, 2001). Thus, less connectivity from the precuneus to other motor regions may indicate that cortical attention networks is no longer critical as movement becomes automatic.
We did not find automatic-dependent changes of brain activation in M1 (Wu et al. 2004). In contrast, several studies in humans and monkeys have shown changes of activation in M1 related to motor practice (Pascual-Leone et al. 1994; Karni et al. 1995; Nudo et al. 1996; Muellbacher et al. 2002). A possible reason for the inconsistency is that those studies compared early learning to ‘mid-stage’ learning, not ‘mid-stage’ to automatic. Moreover, there was no automaticity-related effective connectivity change in M1. Our observations indicate that while the primary motor cortex may contribute to early motor learning and consolidation, it is subsequently involved mostly in execution and not in automaticity.
In agreement with previous findings (Frith et al. 1991; Jueptner et al. 1997; Jansma et al. 2001; Wu et al. 2004; Lehericy et al. 2005), we found that the automatic process is accompanied by less activity in extensive brain regions. This finding suggests that the motor networks becomes more efficient as movements become more automatic. Among the regions that show less activity, several areas (i.e. the DLPFC, CMA, PMA and cerebellum) have critical roles, like attention, rehearsal, or monitoring (Deiber et al. 1991, 1997; Halsband et al. 1993; Petrides et al. 1993; Jueptner et al. 1997; Jansma et al. 2001) at the motor learning stage.
With automaticity, brain regions become less active, but some of them increase their effective connectivity. More efficient connectivity may indicate an increased efficacy of connections, which presumably allows the brain to function more efficiently in a given task, even with a reduced level of activation. The network that does become more connected includes the basal ganglia and cerebellum. In contrast, some cortical regions, like the DLPFC, PMA and M1, did not show stronger automaticity-related effective connectivity. These findings provide evidence for the previously poorly supported, but widely held view that the execution of automatic movements is shifted more subcortically. The importance of the cortical attention network decreases when movements become automatic. The primary motor cortex itself is not a part of the automatic networks, perhaps indicating that at this stage it is acting largely in execution mode, carrying out the directions sent to it.
Acknowledgments
T. Wu was supported by a National Institute of Neurological Disorders and Stroke Intramural Competitive Fellowship. This work was partially supported by the National Science Foundation of China, Grant no. 30570530. In addition, this work was supported in part by the Intramural Research Program of the NINDS, NIH.
References
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Astafiev SV, Shulman GL, Stanley CM, Snyder AZ, van Essen DC, Corbetta M. Functional organization of human intraparietal and frontal cortex for attending, looking, and pointing. J Neurosci. 2003;23:4689–4699. doi: 10.1523/JNEUROSCI.23-11-04689.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein N. The Co-ordination and Regulation of Movements. London: Pergamon Press; 1967. [Google Scholar]
- Buchel C. Perspectives on the estimation of effective connectivity from neuroimaging data. Neuroinformatics. 2004;2:169–174. doi: 10.1385/NI:2:2:169. [DOI] [PubMed] [Google Scholar]
- Buchel C, Coull JT, Friston KJ. The predictive value of changes in effective connectivity for human learning. Science. 1999;283:1538–1541. doi: 10.1126/science.283.5407.1538. [DOI] [PubMed] [Google Scholar]
- Buchel C, Friston KJ. Modulation of connectivity in visual pathways by attention: cortical interactions evaluated with structural equation modelling and fMRI. Cereb Cortex. 1997;7:768–778. doi: 10.1093/cercor/7.8.768. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Bertolino A, Finn K, Coppola R, Frank JA, Goldberg TE, Weinberger DR. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex. 1999;9:20–26. doi: 10.1093/cercor/9.1.20. [DOI] [PubMed] [Google Scholar]
- Culham JC, Cavanagh P, Kanwisher NG. Attention response functions: characterizing brain areas using fMRI activation during parametric variations of attentional load. Neuron. 2001;32:737–745. doi: 10.1016/s0896-6273(01)00499-8. [DOI] [PubMed] [Google Scholar]
- Debaere F, Wenderoth N, Sunaert S, Van Hecke P, Swinnen SP. Changes in brain activation during the acquisition of a new bimanual coordination task. Neuropsychologia. 2004;42:855–867. doi: 10.1016/j.neuropsychologia.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Passingham RE, Colebatch JG, Friston KJ, Nixon PD, Frackowiak RS. Cortical areas and the selection of movement: a study with positron emission tomography. Exp Brain Res. 1991;84:393–402. doi: 10.1007/BF00231461. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Wise SP, Honda M, Catalan MJ, Grafman J, Hallett M. Frontal and parietal networks for conditional motor learning: a positron emission tomography study. J Neurophysiol. 1997;78:977–991. doi: 10.1152/jn.1997.78.2.977. [DOI] [PubMed] [Google Scholar]
- Doyon J, Laforce R, Bouchard G, Gaudreau D, Roy J, Poirer M, Bedard PJ, Bedard F, Bouchard JP. Role of the striatum, cerebellum, and frontal lobes in the automatization of a repeated visuomotor sequence of movements. Neuropsychologica. 1998;36:625–641. doi: 10.1016/s0028-3932(97)00168-1. [DOI] [PubMed] [Google Scholar]
- Doyon J, Owen AM, Petrides M, Sziklas V, Evans AC. Functional anatomy of visuomotor skill learning in human subjects examined with positron emission tomography. Eur J Neurosci. 1996;8:637–648. doi: 10.1111/j.1460-9568.1996.tb01249.x. [DOI] [PubMed] [Google Scholar]
- Doyon J, Song AW, Karni A, Lalonde F, Adams MM, Ungerleider LG. Experience-dependent changes in cerebellar contributions to motor sequence learning. Proc Natl Acad Sci USA. 2002;99:1017–1022. doi: 10.1073/pnas.022615199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink GR, Frackowiak RSJ, Pietrzyk U, Passingham RE. Multiple nonprimary motor areas in the human cortex. J Neurophysiol. 1997;77:2164–2174. doi: 10.1152/jn.1997.77.4.2164. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Functional and effective connectivity in neuroimaging: a synthesis. Hum Brain Mapp. 1995a;2:56–78. [Google Scholar]
- Friston KJ, Buchel C. Attentional modulation of effective connectivity from V2 to V5/MT in humans. Proc Natl Acad Sci U S A. 2000;97:7591–7596. doi: 10.1073/pnas.97.13.7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Frackowiak RS. Time-dependent changes in effective connectivity measured with PET. Hum Brain Mapp. 1993b;1:69–80. [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, Frackowiak RS. Functional connectivity: the principal component analysis of large (PET) data sets. J Cereb Blood Flow Metab. 1993a;13:5–14. doi: 10.1038/jcbfm.1993.4. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS. Analysis of fMRI time-series revisited. Neuroimage. 1995b;2:45–53. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- Frith CD, Friston KJ, Liddle PF, Frackowiak RSJ. Willed action and the prefrontal cortex in man: a study with PET. Proc R Soc Lond B Biol Sci. 1991;244:241–246. doi: 10.1098/rspb.1991.0077. [DOI] [PubMed] [Google Scholar]
- Garraux G, McKinney C, Wu T, Kansanku K, Nolte G, Hallett M. Shared brain areas but not functional connections controlling movement timing and order. J Neurosci. 2005;25:5290–5297. doi: 10.1523/JNEUROSCI.0340-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman DR, Penny WD, Ashburner J, Friston KJ. Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. Neuroimage. 2003;19:200–207. doi: 10.1016/s1053-8119(03)00058-2. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Woods RP, Tyszka M. Functional imaging of procedural motor learning: relating cerebral blood flow with individual subject performance. Hum Brain Mapp. 1994;1:221–234. doi: 10.1002/hbm.460010307. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Halsband U, Ito N, Tanji J, Freund HJ. The role of premotor cortex and the supplementary motor area in the temporal control of movement in man. Brain. 1993;116:243–266. doi: 10.1093/brain/116.1.243. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Nakahara H, Rand MK, Sakai K, Lu X, Nakamura K, Miyachi S, Doya K. Parallel neural networks for learning sequential procedures. Trends Neurosci. 1999;22:464–471. doi: 10.1016/s0166-2236(99)01439-3. [DOI] [PubMed] [Google Scholar]
- Inase M, Li BM, Takashima I, Iijima T. Pallidal activity is involved in visuomotor association learning in monkeys. Eur J Neurosci. 2001;14:897–901. doi: 10.1046/j.0953-816x.2001.01701.x. [DOI] [PubMed] [Google Scholar]
- Jansma JM, Ramsey NF, Slagter HA, Kahn RS. Functional anatomical correlates of controlled and automatic processing. J Cognit Neurosci. 2001;13:730–743. doi: 10.1162/08989290152541403. [DOI] [PubMed] [Google Scholar]
- Jenkins IH, Brooks DJ, Nixon PD, Frackowiak RSJ, Passingham RE. Motor sequence learning: a study with positron emission tomography. J Neurosci. 1994;14:3775–3790. doi: 10.1523/JNEUROSCI.14-06-03775.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jueptner M, Frith CD, Brooks DJ, Frackowiak RSJ, Passingham RE. Anatomy of motor learning. II. Subcortical structures and learning by trail and error. J Neurophysiol. 1997;77:1325–1337. doi: 10.1152/jn.1997.77.3.1325. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature. 1995;377:155–158. doi: 10.1038/377155a0. [DOI] [PubMed] [Google Scholar]
- Laforce RJ, Doyon J. Distinct contribution of the striatum and cerebellum to motor learning. Brain Cogn. 2001;45:189–211. doi: 10.1006/brcg.2000.1237. [DOI] [PubMed] [Google Scholar]
- Lang CE, Bastian AJ. Cerebellar damage impairs automaticity of a recently practiced movement. J Neurophysiol. 2002;87:1336–1347. doi: 10.1152/jn.00368.2001. [DOI] [PubMed] [Google Scholar]
- Lehericy S, Benali H, Van de Moortele PF, Pelegrini-Issac M, Waechter T, Ugurbil K, Doyon J. Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. Proc Natl Acad Sci U S A. 2005;102:12566–12571. doi: 10.1073/pnas.0502762102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little DM, Klein R, Shobat DM, McClure ED, Thulborn KR. Changing patterns of brain activation during category learning revealed by functional MRI. Cognitive Brain Res. 2004;22:84–93. doi: 10.1016/j.cogbrainres.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Liu YJ, Gao JH, Liotti M, Pu Y, Fox PT. Temporal dissociation of parallel processing in the human subcortical outputs. Nature. 1999;400:364–367. doi: 10.1038/22547. [DOI] [PubMed] [Google Scholar]
- Miyachi S, Hikosaka O, Lu X. Differential activation of monkey striatal neurons in the early and late stages of procedural learning. Exp Brain Res. 2002;146:122–126. doi: 10.1007/s00221-002-1213-7. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Wissel J, Dang N, Kofler M, Facchini S, Boroojerdi B, Poewe W, Hallett M. Early consolidation in human primary motor cortex. Nature. 2002;415:640–644. doi: 10.1038/nature712. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Sakai K, Hikosaka O. Effects of local inactivation of monkey medial frontal cortex in learning of sequential procedures. J Neurophysiol. 1999;82:1063–1068. doi: 10.1152/jn.1999.82.2.1063. [DOI] [PubMed] [Google Scholar]
- Nakano K. Neural circuits and topographic organization of the basal ganglia and related regions. Brain Dev Suppl. 2000;1:S5–S16. doi: 10.1016/s0387-7604(00)00139-x. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Nudo R, Milliken G, Jenkins W, Merzenich M. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci. 1996;16:785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Grafman J, Hallett M. Modulation of motor output maps during development of implicit and explicit knowledge. Science. 1994;263:1287–1289. doi: 10.1126/science.8122113. [DOI] [PubMed] [Google Scholar]
- Petrides M, Alivisatos B, Evans AC, Meyer E. Dissociation of human mid-dorsolateral from posterior dorsolateral frontal cortex in memory processing. Proc Natl Acad Sci U S A. 1993;90:873–877. doi: 10.1073/pnas.90.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard N, Strick PL. Medial wall motor areas: a review of their location and functional activation. Cereb Cortex. 1996;6:342–353. doi: 10.1093/cercor/6.3.342. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL. Activation on the medial wall during remembered sequences of reaching movements in monkeys. J Neurophysiol. 1997;77:2197–2201. doi: 10.1152/jn.1997.77.4.2197. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL. Imaging the premotor areas. Curr Opin Neurobiol. 2001;11:663–672. doi: 10.1016/s0959-4388(01)00266-5. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Sabb FW, Foerde K, Tom SM, Asarnow RF, Bookheimer SY, Knowlton BJ. The neural correlates of motor skill automaticity. J Neurosci. 2005;25:5356–5364. doi: 10.1523/JNEUROSCI.3880-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey NF, Tallent K, Van Gelderen P, Frank JA, Moonen CT, Weinberger DR. Reproducibility of human 3D fMRI brain maps acquired during a motor task. Hum Brain Mapp. 1996;4:113–121. doi: 10.1002/(SICI)1097-0193(1996)4:2<113::AID-HBM3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Sakai K, Hikosaka O, Miyauchi S, Takino R, Sasaki Y, Pütz B. Transition of brain activation from frontal to parietal areas in visuomotor sequence learning. J Neurosci. 1998;18:1827–1840. doi: 10.1523/JNEUROSCI.18-05-01827.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Holcomb HH. Neural correlates of motor memory consolidation. Science. 1997;277:821–825. doi: 10.1126/science.277.5327.821. [DOI] [PubMed] [Google Scholar]
- Stephan KE. On the role of general system theory for functional neuroimaging. J Anat. 2004;205:443–470. doi: 10.1111/j.0021-8782.2004.00359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Marshall JC, Friston KJ, Rowe JB, Ritzl A, Zilles K, Fink GR. Lateralized cognitive processes and lateralized task control in the human brain. Science. 2003;301:384–386. doi: 10.1126/science.1086025. [DOI] [PubMed] [Google Scholar]
- Sun FT, Miller LM, Rao AA, D'Esposito M. Functional connectivity of cortical networks involved in bimanual motor sequence learning. Cereb Cortex. 2007;17:1227–1234. doi: 10.1093/cercor/bhl033. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. Stuttgart, Germany: Thieme; 1988. [Google Scholar]
- Tanji J, Shima K. Role for supplementary motor area cells in planning several movements ahead. Nature. 1994;371:413–416. doi: 10.1038/371413a0. [DOI] [PubMed] [Google Scholar]
- Thach WT. A role for the cerebellum in learning and movement coordination. Neurobiol Learn Mem. 1998;70:177–188. doi: 10.1006/nlme.1998.3846. [DOI] [PubMed] [Google Scholar]
- Thach WT, Goodkin HP, Keating JG. Cerebellum and the adaptative coordination of movement. Annu Rev Neurosci. 1992;15:403–442. doi: 10.1146/annurev.ne.15.030192.002155. [DOI] [PubMed] [Google Scholar]
- Toni I, Krams M, Turner R, Passingham RE. The time course of changes during motor sequence learning: a whole-brain fMRI study. Neuroimage. 1998;8:50–61. doi: 10.1006/nimg.1998.0349. [DOI] [PubMed] [Google Scholar]
- Toni I, Rowe J, Stephan KE, Passingham RE. Changes of cortico-striatal effective connectivity during visuomotor learning. Cereb Cortex. 2002;12:1040–1047. doi: 10.1093/cercor/12.10.1040. [DOI] [PubMed] [Google Scholar]
- van Mier H, Tempel LW, Perlmutter JS, Raichle ME, Petersen SE. Changes in brain activity during motor learning measured with PET: effects of hand of performance and practice. J Neurophysiol. 1998;80:2177–2199. doi: 10.1152/jn.1998.80.4.2177. [DOI] [PubMed] [Google Scholar]
- Wenderoth N, Debaere F, Sunaert S, Swinnen SP. The role of anterior cingulate cortex and precuneus in the coordination of motor behaviour. Eur J Neurosci. 2005;22:235–246. doi: 10.1111/j.1460-9568.2005.04176.x. [DOI] [PubMed] [Google Scholar]
- Wu T, Hallett M. A functional MRI study of automatic movements in patients with Parkinson's disease. Brain. 2005;128:2250–2259. doi: 10.1093/brain/awh569. [DOI] [PubMed] [Google Scholar]
- Wu T, Kansaku K, Hallett M. How self-initiated memorized movements become automatic: a fMRI study. J Neurophysiol. 2004;91:1690–1698. doi: 10.1152/jn.01052.2003. [DOI] [PubMed] [Google Scholar]