Abstract
The slow delayed rectifier K+ current (IKs) is a major determinant of action potential repolarization in the heart. IKs channels are formed by coassembly of pore-forming KCNQ1 α-subunits and ancillary KCNE1 β-subunits. Two gain of function mutations in KCNQ1 subunits (S140G and V141M) have been associated with atrial fibrillation (AF). Previous heterologous expression studies found that both mutations caused IKs to be instantaneously activated, presumably by preventing channel closure. The purpose of this study was to refine our understanding of the channel gating defects caused by these two mutations located in the S1 domain of KCNQ1. Site-directed mutagenesis was used to replace S140 or V141 with several other natural amino acids. Wild-type and mutant channels were heterologously expressed in Xenopus oocytes and channel function was assessed with the two-microelectrode voltage clamp technique. Long intervals between voltage clamp pulses revealed that S140G and V141M KCNQ1-KCNE1 channels are not constitutively active as previously reported, but instead exhibit extremely slow deactivation. The slow component of IKs deactivation was decreased 62-fold by S140G and 140-fold by the V141M mutation. In addition, the half-point for activation of these mutant IKs channels was ∼50 mV more negative than wild-type channels. Other substitutions of S140 or V141 in KCNQ1 caused variable shifts in the voltage dependence of activation, but slowed IKs deactivation to a much lesser extent than the AF-associated mutations. Based on a published structural model of KCNQ1, S140 and V141 are located near E160 in S2 and R237 in S4, two charged residues that could form a salt bridge when the channel is in the open state. In support of this model, mutational exchange of E160 and R237 residues produced a constitutively open channel. Together our findings suggest that altered charge-pair interactions within the voltage sensor module of KCNQ1 subunits may account for slowed IKs deactivation induced by S140 or V141.
Atrial fibrillation (AF) affects over 2 million Americans and the number of affected individuals is estimated to increase to > 4 million by 2020 (Go et al. 2001). Recent studies have demonstrated that some forms of AF have a genetic basis (Fox et al. 2004; Ellinor et al. 2005; Arnar et al. 2006). Rare monogenic forms of AF are caused by mutations in several K+ channel subunit genes, including KCNQ1 (Chen et al. 2003b; Hong et al. 2005; Lundby et al. 2007), HERG (Sinner et al. 2008), KCNE2 (Yang et al. 2004), KCNJ2 (Xia et al. 2005) and KCNA5 (Olson et al. 2006).
KCNQ1 (Kv7.1) α-subunits can coassemble as a homotetramer to form a voltage-gated, delayed rectifier K+ channel. In the heart, KCNQ1 coassembles with KCNE1 β-subunits to form heteromultimeric channels that conduct the slow delayed rectifier K+ current, IKs (Barhanin et al. 1996; Sanguinetti et al. 1996). Compared to KCNQ1 channel current, IKs has a markedly slower rate of activation, has a larger unitary channel conductance and does not inactivate (Barhanin et al. 1996; Sanguinetti et al. 1996; Sesti & Goldstein, 1998; Yang & Sigworth, 1998).
Loss of function mutations in KCNQ1 are the most common cause of inherited long QT syndrome (Wang et al. 1996). In contrast, only three gain of function mutations in KCNQ1 have been described, and all cause AF (Chen et al. 2003b; Hong et al. 2005; Lundby et al. 2007). Two of these mutations, S140G (Chen et al. 2003b) and V141M (Hong et al. 2005), are located near the extracellular end of the S1 transmembrane domain, a region not usually considered to have a significant role in gating of voltage-gated potassium (Kv) channels. Indeed, these two mutations have no discernible effects on the gating of homomultimeric KCNQ1 channels. When S140G or V141M KCNQ1 α-subunits are coexpressed with KCNE1 β-subunits, the resulting heteromultimeric IKs channels appeared to be almost constitutively open (Chen et al. 2003b; Hong et al. 2005). Specifically, outward currents elicited by a step depolarization in voltage clamped cells were characterized by a large instantaneous component and only a small time-dependent component.
Here we used site-directed mutagenesis, functional expression of channels in Xenopus oocytes and two-microelectrode voltage clamp techniques to further characterize the functional consequences of the AF-associated mutations (S140G or V141M) in KCNQ1. When long interpulse intervals (100 s) were utilized to elicit IKs, mutant channels were not constitutively open. Instead, similar to WT IKs, mutant IKs channels were initially closed before activating very slowly in response to membrane depolarization. However, mutant S140G and V141M IKs channels activated at more negative potentials and with a markedly slowed rate of deactivation compared to WT IKs. Other substitutions of S140 or V141 also slowed IKs channel deactivation, but not nearly to the same extent as the AF-associated mutations. Thus, the disease-associated mutations S140G and V141M in KCNQ1 are particularly effective at disrupting deactivation of IKs.
Methods
Molecular biology
Human KCNQ1 was cloned into the pSP64 oocyte expression vector and 18 mutations of S140 or V141were introduced by site-directed mutagenesis with QuickChange (Stratagene, La Jolla, CA, USA). The presence of the desired mutation and absence of additional changes were verified by restriction mapping and DNA sequencing of the PCR-amplified segment. After transformation, the cDNA was purified using a QIAprep Spin Miniprep Kit (Qiagen, Valencia, CA, USA). Purification of the DNA construct after linearization with EcoRI was performed using a QIAquick PCR purification protocol (Qiagen). The cRNA used for oocyte injection was transcribed in vitro using SP6 Capscribe (Roche, Indianapolis, IN, USA) or the mMESSAGE mMACHINE SP6 kit (Ambion, Austin, TX, USA). The concentration of the cRNA was quantified with the RiboGreen assay (Invitrogen, Eugene, OR, USA).
Oocyte isolation and cRNA injection
The use of Xenopus laevis to harvest oocytes was approved by the University of Utah Institutional Animal Care and Use Committee. Frogs were anaesthetized with 0.2% tricaine methanesulphonate in deionized water before a small abdominal incision was made to remove ovarian lobes. The incision was sutured closed and the frog returned to its aquarium for a recovery period of at least 1 month before the procedure was repeated. After a maximum of three surgical procedures, tricaine-anaesthetized frogs were killed by pithing. Oocytes were manually dispersed from the lobes and the follicle cell layer was removed by treatment for 90–150 min with 1 mg ml−1 type II collagenase (Worthington, New York, NY, USA) in ND96 Ca2+-free solution that contained (in mm): 96 NaCl, 2 KCl, 1 MgCl2, 5 Hepes; pH was adjusted to 7.6 with NaOH.
To characterize IKs, stage IV and V oocytes were coinjected with nearly equimolar amounts of KCNQ1 and KCNE1 cRNAs: 6 ng of wild-type (WT) or mutant KCNQ1 plus 0.6 ng of WT human KCNE1 cRNA. Oocytes were subsequently stored for 2 days at 18°C in Barth's saline solution that contained (in mm): 88 NaCl, 1 KCl, 0.41 CaCl2, 0.33 Ca(NO3)2, 1 MgSO4, 2.4 NaHCO3, 10 Hepes, 1 pyruvate plus gentamycin (50 mg l−1); pH adjusted to 7.4 with NaOH.
Two electrode voltage clamp
Oocytes were placed in a 0.2 ml recording chamber and perfused at 1 ml min−1 with an external bath solution at room temperature (22–24°C) that contained (in mm): 96 NaCl, 4 KCl, 1 MgCl2, 1 CaCl2 and 5 mm Hepes; pH adjusted to 7.6 with NaOH. Standard two-microelectrode voltage-clamp techniques (Stühmer, 1992) and a GeneClamp 500 amplifier (Molecular Devices, Union City, CA, USA) were used to measure ionic currents from single oocytes. Details of the voltage clamp protocols are described in the figure legends. In general, to record the current–voltage (I–V) relationship for IKs, oocytes were voltage clamped at a negative holding potential (−90 to −120 mV). Test potential were applied to a variable potential with a fixed incremental increase between successive pulses (e.g. −80 to +60 mV in 20 mV steps). A brief prepulse, applied to the same potential used to measure tail currents, preceded each test pulse. Tail currents were measured upon repolarization of the membrane to a specific return potential as described for each experiment. The interval between test pulses was 20, 100 or 300 s as indicated.
Data analysis
Data acquisition was performed with Clampex 8.2 (Molecular Devices, Union City, CA, USA) and off-line data analysis was performed with Clampfit 8.2 (Molecular Devices), Origin 7.5 (OriginLab Corp., Northampton, MA, USA), Microsoft Excel and SPSS 12 (SPSS, Inc., Chicago, IL, USA) software.
The voltage dependence of isochronal current activation was estimated by tail current analysis. The tail current amplitude (Itail) was plotted as a function of the test potential (Vt) and the data for individual oocytes fitted with a Boltzmann function. When Itail did not saturate at the most positive Vt, then the maximum Itail was estimated by extrapolation of the Boltzmann function to more positive potentials. Data were normalized to the estimated maximum Itail for each oocyte to obtain a conductance–voltage (G–V) relationship that was fitted with a Boltzmann function:
| (1) |
where z is the effective valence, F is Faraday's constant, R is the gas constant, T is room temperature in kelvins and V0.5 is the potential at which the current is half-activated.
The fitted parameters from the Boltzmann relationships were used to calculate the Gibbs free energy, ΔG0 (expressed in kcal mol−1) according to:
| (2) |
and the mutation-induced changes in ΔG0 (ΔΔG0), according to:
| (3) |
A two-exponential function (eqn (4)) was used to fit the time course of tail currents to determine the fast and slow time constants of current deactivation (τf and τs):
| (4) |
Af and As represent the amplitudes of the fast and slow components of tail current.
All data are expressed as means ±s.e.m. (n= number of oocytes). The comparison between groups was performed using Student's t test and the differences were considered statistical significant for a P-value < 0.05.
Results
S140G and V141M mutations in KCNQ1 slow IKs deactivation
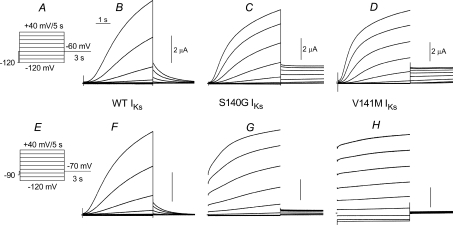
IKs channels expressed in Xenopus oocytes were activated from a holding potential of −120 mV with 5 s depolarizing pulses applied in 20 mV incremental steps from −120 to +40 mV (Fig. 1A). For our initial experiments, the interpulse interval was set at 100 s to ensure that channels were completely closed between successive test pulses. After each test pulse, the membrane was repolarized to a potential of −60 mV to record deactivating tail currents. Using this pulse protocol, WT IKs was elicited at test potentials of −20 mV or more positive and activated very slowly. Currents did not reach a steady-state level during the 5 s depolarizing pulses, whereas deactivation at −60 mV was nearly completely after just 3 s (Fig. 1B). IKs conducted by channels formed by S140G KCNQ1 + KCNE1 subunits (S140G IKs) were activated at more negative potentials, with a voltage threshold near −60 mV (Fig. 1C). Accounting for the shift in activation, the rate of S140G IKs was relatively normal; however, tail currents did not deactivate during the 3 s pulse to −60 mV. The kinetics and voltage dependence of IKs conducted by channels formed by V141M KCNQ1 + KCNE1 subunits (V141M IKs) was similar to S140G IKs (Fig. 1D). The same oocytes used for these recordings were subjected to another pulse protocol where the holding potential and interpulse interval was altered. For the currents shown in Fig. 1F–H, the oocytes were clamped to a less negative holding potential of −90 mV and a shorter interpulse interval of 20 s was used. Test pulses were applied to the same potentials (from −120 to +40 mV), but the tail currents were measured at −70 mV (Fig. 1E). The kinetics and voltage dependence of WT IKs activation was unaltered using this other voltage clamp protocol (Fig. 1F). Tail current amplitudes were smaller due to the reduced driving force for K+ efflux and currents deactivated a little faster because of the more negative return potential. In contrast, S140G IKs exhibited a prominent instantaneous current component and a reduced time-dependent component during the test pulses (Fig. 1G). The relative amplitude of the instantaneous component was even larger for V141M IKs (Fig. 1H). In addition, inward currents were elicited at the test potentials of −100 and −120 mV. Together these experiments demonstrate that the ‘instantaneous’ currents conducted by mutant IKs channels, as illustrated in Fig. 1G and H and reported previously (Chen et al. 2003b; Hong et al. 2005), results from accumulation of channels in the open state when the interpulse interval is too short relative to their markedly slowed rate of deactivation.
Figure 1. S140G and V141M KCNQ1–KCNE1 (IKs) channels deactivate very slowly.
A, slow voltage pulse protocol used to elicit currents shown in panels B–D. From a holding potential of −120 mV, test pulses were applied once every 100 s to potentials ranging from −120 mV to +40 mV. Tail currents were measured at −60 mV. B–D, IKs recorded from Xenopus oocytes expressing wild-type (WT), S140G or V141M KCNQ1 α-subunits plus KCNE1 β-subunits. E, voltage pulse protocol used to elicit currents shown in panels F–H. Test currents were elicited once every 20 s from a holding potential of −90 mV; tail currents were measured at −70 mV. F–H, IKs recorded from the same three oocytes used in panels B–D, but using pulse protocol shown in panel E. The more rapid pulsing rate prevented complete current deactivation between pulses in oocytes expressing S140G or V141M KCNQ1–KCNE1 channels. The reduced tail current amplitudes for the mutant channels in panels G and H likely reflects a diminished driving force for K+ that results from extracellular K+ accumulation. Capacity transient currents were nulled in all traces. The vertical scale bars represent 2 μA.
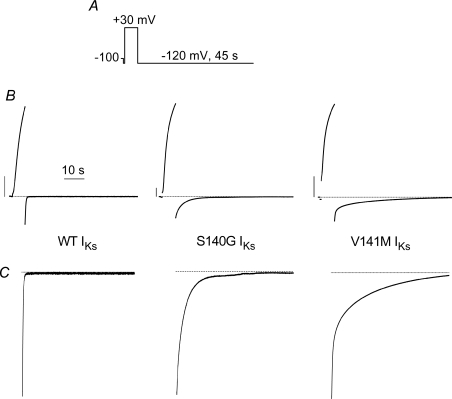
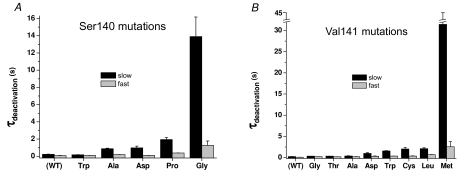
The rate of IKs deactivation for the mutant channels was too slow to accurately quantify at −70 mV. Therefore, we determined the effect of the S140G and V141M mutations on IKs deactivation at −120 mV (Fig. 2). Currents were activated with a 7 s pulse to +30 mV and deactivating tail currents were measured for 45 s at a return potential of −120 mV. The time required for tail currents to decay at this potential was quantified by fitting the traces to a two-exponential function. The fast and slow time constants (τf, τs) for deactivation of WT IKs were 83 ± 18 ms and 224 ± 31 ms (n= 7). The S140G mutation increased τf and τs by 15-fold and 62-fold, respectively (Fig. 3A). The V141M mutation slowed deactivation even more, causing a 30-fold and 140-fold increase in τf and τs, respectively (Fig. 3B).
Figure 2. Mutant IKs channels deactivate slowly at −120 mV.
A, voltage pulse protocol. B, currents were activated during a 7 s pulse to +30 mV. Deactivating (tail) currents were recorded for 45 s at a return potential of −120 mV. C, tail current traces from panel B are shown on an expanded Y-axis scale.
Figure 3. Time constants for deactivation of WT and mutant IKs channel currents determined at −120 mV.
Time constants for fast and slow components of deactivation are plotted for IKs channels containing mutations of S140 (A) or V141 (B) (n= 4–7).
S140G and V141M mutations in KCNQ1 cause a negative shift in the voltage dependence of IKs activation
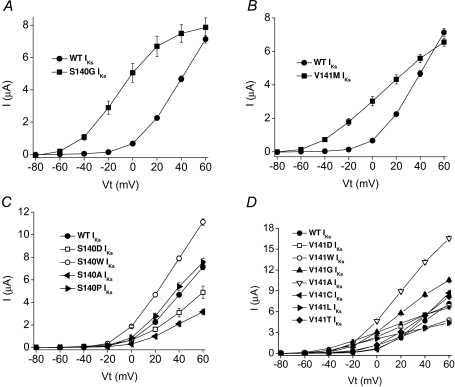
The isochronal I–V relationship for IKs was determined using 5 s pulses to potentials ranging from −80 mV to +60 mV and an interpulse interval of 100 s. As noted above, the threshold for activation of S140G IKs and V141M IKs was about 40 mV more negative than for WT IKs. However, when injected with equivalent amounts of KCNQ1 and KCNE1 cRNA, the amplitude of IKs at +60 mV was nearly the same for WT and the mutant channels (Fig. 4A and B).
Figure 4. Current–voltage (I–V) relationships for WT and mutant IKs channels. A and B, I–V relationships for WT and S140G or V141M KCNQ1–KCNE1 (IKs) channel currents as indicated (n= 6–10). C and D, I–V relationships for WT and mutant channels carrying the indicated substitutions of S140 and V141 (n= 6–10).
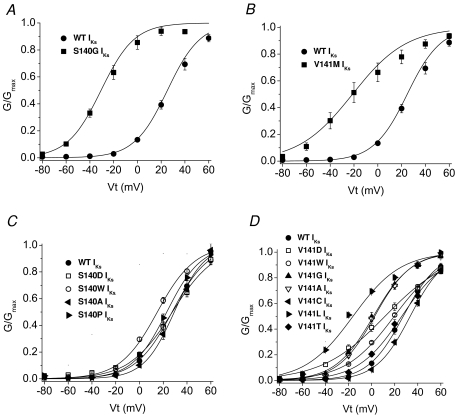
The voltage dependence of current activation was determined from analysis of peak tail current amplitudes measured at −60 mV. When compared to WT IKs, S140G and V141M mutations shifted the V0.5 by −57 and −53 mV, respectively (Fig. 5A and B; Table 1). In addition, the slope of the G–V relationship for V141M IKs was shallow compared to WT IKs.
Figure 5. Voltage dependence of activation for WT and mutant IKs channels.
A and B, G–V relationships for WT, S140G and V141M IKs channel currents as indicated (n= 6–10). C and D, G–V relationships for WT and mutant IKs channels carrying the indicated substitutions of S140 and V141 (n= 6–10). In all panels, data were fitted to a Boltzmann function (smooth curves). Values for V0.5 and z are listed in Table 1.
Table 1.
Voltage dependence of activation for WT and mutant IKs channel currents
| IKs | n | V0.5 (mV) | ΔV0.5* (mV) | z | ΔG0 (kcal mol−1) |
|---|---|---|---|---|---|
| WT | 10 | 26.0 ± 0.3 | — | 1.76 ± 0.09 | 1.06 ± 0.06 |
| S140G | 6 | −31.2 ± 0.7† | −57.2 | 1.87 ± 0.22 | −1.34 ± 0.18† |
| S140W | 10 | 14.4 ± 0.5 | −11.6 | 1.58 ± 0.15 | 0.52 ± 0.08 |
| S140P | 10 | 22.8 ± 0.4 | −3.2 | 1.77 ± 0.11 | 0.93 ± 0.07 |
| S140A | 8 | 33.9 ± 0.4 | 7.8 | 1.68 ± 0.09 | 1.31 ± 0.08 |
| S140D | 6 | 35.0 ± 0.5 | 9 | 1.26 ± 0.04 | 1.02 ± 0.05 |
| V141M | 7 | −27.2 ± 1.5† | −53.2 | 0.88 ± 0.13‡ | −0.55 ± 0.12† |
| V141L | 7 | −22.6 ± 1.8† | −48.6 | 1.05 ± 0.23‡ | −0.54 ± 0.17† |
| V141G | 8 | 0.2 ± 0.3† | −25.8 | 1.52 ± 0.08 | 0.01 ± 0.03† |
| V141A | 7 | 1.8 ± 0.5† | −24.2 | 1.54 ± 0.12 | 0.06 ± 0.05† |
| V141D | 7 | 8.9 ± 0.6† | −17.1 | 0.83 ± 0.05† | 0.17 ± 0.03† |
| V141W | 8 | 18.9 ± 0.5‡ | −7.1 | 1.17 ± 0.06‡ | 0.51 ± 0.05‡ |
| V141T | 8 | 24.9 ± 0.8 | −1.2 | 1.33 ± 0.12‡ | 0.76 ± 0.10 |
| V141C | 7 | 33.4 ± 0.5 | 7.4 | 1.71 ± 0.10 | 1.32 ± 0.10 |
Half-point (V0.5) and effective valence (z) for activation were determined from fitting normalized tail current amplitudes to a Boltzmann function (Fig. 4C and D).
Shift in V0.5 (compared to WT IKs channels).
P < 0.002,
P< 0.05.
Other substitutions of S140 and V141 in KCNQ1
The slowed IKs deactivation induced by S140G or V141M could be a gain of function specific to these mutations. Alternatively, a Ser or Val at these positions could be specifically required for normal IKs channel function and any mutation of these residues could lead to altered IKs gating. To discriminate between these two possible mechanisms, we next determined the biophysical properties of channels formed by KCNE1 + KCNQ1 subunits harbouring mutations in S140 or V141 other than Gly or Met.
We mutated S140 to Pro or other residues that were polar (Thr, Cys), hydrophobic (Ala, Met, Trp) or charged (Asp, Arg). Four of the S140 mutations in KCNQ1 (Cys, Met, Arg, Thr) resulted in IKs amplitudes (∼2 μA for 5 s pulses to +60 mV) that were indistinguishable from current induced by injection of oocytes with KCNE1 cRNA alone, suggesting that these mutations caused a complete loss of KCNQ1 function without a dominant-negative suppression of endogenous KCNQ1 subunits. S140P IKs was indistinguishable from WT IKs, whereas S140A and S140D IKs were smaller and S140W IKs was larger compared to WT IKs (Fig. 4C). These S140 mutant channel currents had a voltage dependence of activation that was relatively similar to WT IKs with a V0.5 shift < 12 mV (Fig. 5C). All mutations except S140W also slowed the rate of current deactivation, albeit not anywhere near the extent observed for S140G (Fig. 3A).
We mutated V141 to other residues that were polar (Thr, Cys), hydrophobic (Gly, Ala, Leu, Trp) or charged (Asp). V141L and V141W IKs were smaller and V141A and V141G IKs were larger compared to WT IKs (Fig. 4D). Mutation of Val141 to Leu, Gly, Ala and Asp shifted the V0.5 for activation to more negative potentials (Fig. 5D). The shift induced by V141L was the largest (−49 mV) and was similar to the shift measured for the naturally occurring mutation V141M (−53 mV). All mutations except V141T also slowed the rate of current deactivation, albeit not anywhere near the extent observed for V141M (Fig. 3B). Moreover, the slowing of deactivation was not correlated with the shift in V0.5 for activation.
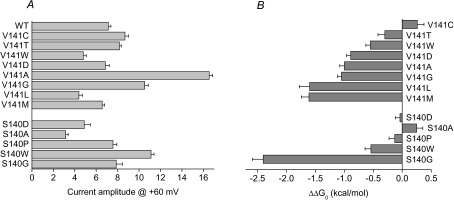
The effects of all the S140 and V141 mutations on the voltage dependence of activation, expressed as ΔV0.5 and ΔG0, are summarized in Table 1. Figure 6 summarizes the effects of all the mutations on IKs amplitude (measured at +60 mV, Fig. 6A) and the mutation-induced change in Gibbs free energy for activation, ΔΔG0 (Fig. 6B). All mutations except V141C, V141T, S140D, S140A and S140P decreased ΔG0 > 0.5 kcal mol−1.
Figure 6. Effects of V141 and S140 mutations on IKs amplitude and ΔG0.
A, bar graph of IKs magnitude measured at the end of a 5 s test pulse to +60 mV for WT and mutant channels. B, bar graph of mutation-induced changes in ΔG0 for IKs channel activation. Number of oocytes (n) are reported in Table 1.
A double mutation (V141M/R237A) in KCNQ1 locks IKs channels in the open state
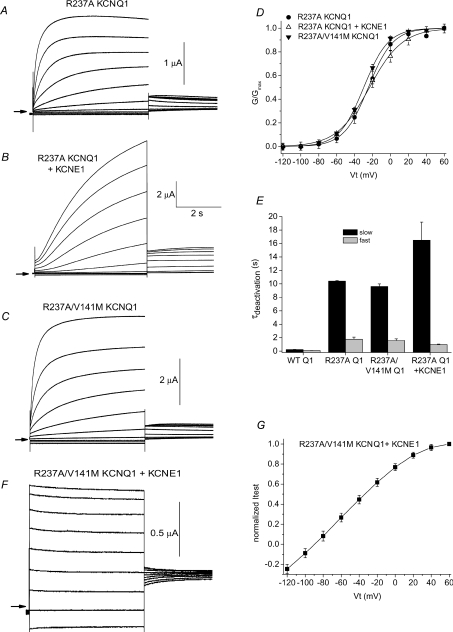
R237 is one of three basic residues in the S4 domain of the KCNQ1 subunit that play an important role in voltage sensing. Recently, Panaghie & Abbott (2007) reported that mutation of R237 to Ala greatly slowed KCNQ1 channel deactivation. Moreover, when coexpressed with KCNE1 subunits, R237A KCNQ1 channels appeared to be constitutively open. We confirmed that R237A KCNQ1 channels deactivated very slowly (Fig. 7A). At −120 mV, τf= 1.8 ± 0.31 s and τs= 10.4 ± 0.07 s (n= 4). The voltage dependence of activation of this channel was right-shifted by ∼10 mV compared to WT KCNQ1, with a V0.5 of −23.0 ± 2.6 mV and a z of 1.53 ± 0.13 (n= 4). However, we found that when coexpressed with KCNE1, the resulting R237A IKs channels were capable of closing if the interpulse interval was sufficiently long; e.g. 90 s (Fig. 7B). R237A IKs channels activated slowly as normal, but deactivation was very slow (at −120 mV, τf= 0.98 ± 0.06 s, τs= 16.5 ± 2.7 s; n= 7). In addition, the V0.5 for activation of R237A IKs channels was sifted to more negative potentials (V0.5=−22.0 ± 1.5 mV; z= 1.24 ± 0.11, n= 7) compared to WT IKs, similar to the shift induced by the single mutations S140G and V141M. Thus, in the presence of KCNE1, the R237A mutation in the S4 domain of KCNQ1 mimics the effects of the AF-associated point mutations in the S1 domain. This finding suggested the possibility that the S140G and V141M mutations might alter IKs gating by interfering with channel gating functions normally subserved by R237. To test this hypothesis, we determined the biophysical properties of channels containing two mutations in KCNQ1: R237A and V141M.
Figure 7. R237A mutation slows channel deactivation and R237A/V141M IKs channels are constitutively open.
A–C, current traces recorded from Xenopus oocytes expressing R237A KCNQ1 alone, R237A KCNQ1 plus KCNE1, or R237A/V141M KCNQ1 subunits. From a holding potential of −100 mV, 5 s test pulses were applied once every 90 s, in 20 mV increments to potentials ranging from −120 mV to +60 mV. Tail currents were measured at −60 mV. Arrows indicate the 0 current level. D, voltage dependence of current activation for oocytes expressing the indicated subunits. V0.5 and z for G–V relationships were as follows: R237A KCNQ1: −23.0 ± 2.6 mV, 1.53 ± 0.13 (n= 4); R237A KCNQ1 + KCNE1: −22.0 ± 1.5 mV, 1.24 ± 0.11 (n= 7); R237A/V141M KCNQ1: −29.7 ± 1.6 mV, 1.85 ± 0.05 (n= 5). E, time constants for deactivation determined at −120 mV for WT KCNQ1 (WT Q1, n= 7), R237A KCNQ1 (n= 4), R237A/V141M KCNQ1 (n= 5) and R237A KCNQ1-KCNE1 (n= 7) channel currents. F, currents recorded from an oocyte expressing R237A/V141M KCNQ1 plus KCNE1 subunits. From a holding potential of −100 mV, 5 s test pulses were applied once every 90 s, in 20 mV increments to potentials ranging from −120 mV to +40 mV. G, I–V relationship for R237A/V141M IKs channels (n= 4). Currents were normalized to currents at +60 mV.
R237A/V141M KCNQ1 channel currents (Fig. 7C) had altered biophysical properties similar to that induced by R237A alone (Fig. 7D and E). This is consistent with our previous findings that V141M alone does not alter gating of KCNQ1 channels (Hong et al. 2005). However, R237A/V141M IKs channels did not deactivate (Fig. 7F) and the I–V relationship for 5 s test pulses was ohmic between 0 and −120 mV (Fig. 7G), even when activated with an interpulse interval of 300 s. Thus, R237A and V141M mutations were synergistic with respect to disruption of IKs channel deactivation.
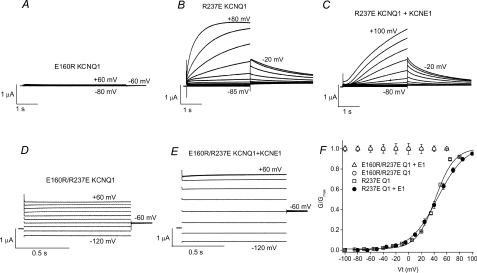
Mutational exchange of two charged residues (E160R/R237E) locks KCNQ1 and IKs channels in the open state
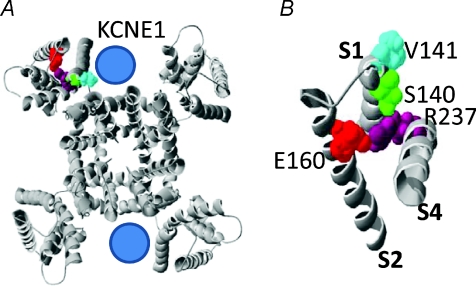
Smith et al. (2007) have proposed a structural model for the closed and open states of the KCNQ1 channel. In this model, mutations in KCNQ1 that cause a gain-of-function of IKs gating (including S140G and V141M) are located near the outer portion of the crevice proposed to be occupied by a KCNE1 subunit in an IKs channel. In the closed state, R231 of the S4 segment is adjacent to E160 in the S2 segment, whereas in the open state, rearrangement of the voltage sensor places R237 in close proximity to E160 (Fig. 8). To determine if E160 and R237 interaction is important for KCNQ1 or IKs channel gating, we swapped the residues at these two positions and examined the effects of single and the double mutations. Channels with the single mutation E160R were non-functional (Fig. 9A). E160A KCNQ1 channels were also non-functional (not shown). Channels with the single mutation R237E activated at much more positive potentials (V0.5=+42.0 ± 0.7 mV, n= 5) compared to WT KCNQ1 (V0.5=−31.5 ± 1.2 mV, n= 6), but deactivated relatively normally (Fig. 9B). Coexpression of R237E KCNQ1 with KCNE1 produced channels with slow activation kinetics typical for IKs (Fig. 9C). In contrast, channels containing both charge-reversing mutations (E160R/R237E) were constitutively open when expressed with or without KCNE1 subunits. Currents were time independent, even when activated with test pulses applied once every 300 s (Fig. 9D and E). The G–V relationships for KCNQ1 or IKs channels containing the R237E mutation are summarized in Fig. 9F. To summarize, the R237E mutation rescued the loss of function induced by E160R and together these mutations prevented channel closure, similar to the phenotype observed for R237A/V141M IKs channels.
Figure 8. Location of S140 and V141 residues in the KCNQ1 channel.
A, entire KCNQ1 channel viewed from extracellular side. In one of the subunits, R237, E160, S140 and V141 are coloured. Location of KCNE1 subunits is speculative. B, extracellular view of S1, S2 and S4 segments from a single KCNQ1 subunit. R237 in S4 segment, E160 in S2 segment, and S140 and V141 in S1 segment are labelled. Open state KCNQ1 models modified from Smith et al. (2007).
Figure 9. E160R/R237E KCNQ1 and IKs channels are constitutively open.
A, lack of currents recorded in an oocyte injected with E160R KCNQ1 cRNA. Currents were recorded from a holding potential of −90 mV and 5 s pulses were applied to test potentials ranging from −80 to +60 mV, applied in 20 mV increments. B, currents recorded from an oocyte expressing R237E KCNQ1 channel subunits. Holding potential was −90 mV and 3 s pulses were applied to test potentials ranging from −85 to +80 mV, applied in 15 mV increments. Tail currents were measured at −20 mV. C, currents recorded from an oocyte expressing R237E KCNQ1 + KCNE1 subunits. Holding potential was −90 mV and 3 s pulses were applied to test potentials ranging from −80 to +100 mV, applied in 15 mV increments. Tail currents were measured at −20 mV. D and E, currents recorded from oocyte expressing either E160R/R237E KCNQ1 subunits alone (D) or with KCNE1 subunits (E). currents were recorded from a holding potential of −90 mV and 1 s pulses were applied to test potentials ranging from −120 to +60 mV, applied in 20 mV increments. Tail currents were measured at −60 mV. For panels A–E, a few traces are labelled with the corresponding potential (in mV) used to elicit the currents. F, G–V relationships for oocytes expressing mutant KCNQ1 (Q1) with or without KCNE1 (E1) subunits as indicated. E160R/R237E KCNQ1 + KCNE1 (n= 6) and E160R/R237E KCNQ1 (n= 15) channels were constitutively open. For R237E KCNQ1 channels, V0.5= 42.0 ± 0.7 mV, z= 1.91 ± 0.03 (n= 5). For R237E KCNQ1-KCNE1 channels, V0.5= 45.1 ± 2.7 mV, z= 1.37 ± 0.03 (n= 7).
Discussion
Previous studies from Chen et al. (2003b) and our laboratory (Hong et al. 2005) concluded that when coexpressed with KCNE1 β-subunits, S140G or V141M mutations in the S1 domain of KCNQ1 α-subunits caused IKs channels to be constitutively open. These conclusions were based on experiments where depolarizing pulses were applied at a rate of 6 min−1 or faster. Thus, even at very slow physiological heart rates, these mutant channels would be expected to be functionally trapped in their open configuration. In the present study we used extremely slow pulsing (1 pulse every 100 or 300 s) to reveal that these mutant IKs channels actually can deactivate to a fully closed state, albeit ultra-slowly.
Our experiments used cloned channels expressed in oocytes and currents were recorded at room temperature. Thus, extrapolation of our findings to the intact human heart has obvious limitations. Nonethelessess, it is clear that the naturally occurring point mutations in S140 and V141 that cause AF (and short QT syndrome for V141M) slowed IKs channel deactivation more effectively than other substitutions of the native residues. Both mutations shifted the voltage dependence of IKs gating by about −50 mV. Based solely on this shift in voltage dependence of gating, IKs deactivation would be expected to be slowed by ∼3-fold (Splawski et al. 1997). This is far less than the 62-fold observed for S140G IKs or 140-fold slowing measured for V141M IKs, indicating that deactivation was slowed by a mechanism unrelated to the shift in the voltage dependence of activation gating.
Based on multiple lines of evidence, KCNQ1 tetramers can only accommodate two KCNE1 subunits (Chen et al. 2003a; Morin & Kobertz, 2008). The full range of interactions between KCNQ1 and KCNE1 are uncertain, but previous studies concluded that the α-helical transmembrane domain of KCNE1 associates with the pore region of KCNQ1 (Tai & Goldstein, 1998; Tapper & George, 2000). More recent studies have reported that the α-helical transmembrane domain of a KCNE1 subunit may be positioned in a crevice formed by the intersection of the S1 segment of the voltage sensor domain of one KCNQ1 subunit and the pore domain of an adjacent KCNQ1 subunit (Smith et al. 2007; Shamgar et al. 2008; Xu et al. 2008). Specifically, Xu et al. (2008) showed that residue 145 in the S1 domain of KCNQ1 interacts with residues in position 40 and 41 of KCNE1 in a state-specific manner. Situated in this position, it is not difficult to imagine that a KCNE1 protein could influence the coupling between voltage sensing and channel opening to cause slowed channel activation. However, precisely how KCNE1 subunits alter KCNQ1 channel gating is unknown. An equally perplexing question is how S140G or V141M mutations in the S1 domain of KCNQ1 slow deactivation of IKs in a KCNE1-obligate manner.
Combining the Smith et al. (2007) structural model with the functional consequences that result from mutation of charged residues in the voltage sensor module of KCNQ1 and other Kv channels may provide clues into the molecular mechanism for slowed KCNQ1 activation induced by KCNE1 and slowed IKs deactivation caused by S140G or V141M. One or more salt bridges formed between specific basic residues in S4 and acidic residues in S2 or S3 are required for proper subunit folding and channel assembly of several Kv channels, including Shaker (Papazian et al. 1995; Tiwari-Woodruff et al. 1997; Tiwari-Woodruff et al. 2000; Silverman et al. 2003), EAG (Silverman et al. 2003; Silverman et al. 2004), and ERG (Liu et al. 2003; Fernandez et al. 2005). By analogy, E160 may form a salt bridge with R231 in single KCNQ1 subunits when the channel is in the closed state or with R237 in the open state. The likely importance of E160–R231 interaction for KCNQ1 and IKs channel assembly and deactivation is supported by our finding that mutation of E160 to Arg or Ala renders the channels non-functional, and that R231A KCNQ1 (Panaghie & Abbott, 2007) and R231W KCNQ1 (Shamgar et al. 2008) channels are constitutively open. The loss of function caused by E160R was rescued by the additional charge-reversal mutation R237E. E160R/R237E KCNQ1 channels were constitutively open in the presence or absence of KCNE1, presumably because the ability to form a salt bridge between residues 160 and 237 would be retained in the double mutant channel (favouring the open state), whereas charge repulsion between R231 and R160 would preclude channel closure. Single mutations of R237 were also informative. The R237A mutation markedly slowed KCNQ1 channel deactivation without much affect on the rate or voltage dependence of activation. In contrast, R237E caused a +74 mV shift in the V0.5 for KCNQ1 activation, but did not significantly alter the rate of deactivation. Together these findings confirm the importance of R237 in KCNQ1 channel gating (Panaghie & Abbott, 2007) and support the proposal that E160–R237 intrasubunit interactions facilitates activation and stabilizes the open state of KCNQ1 channels. Based on previously published findings and our new mutagenesis results, we suggest that binding of KCNE1 to KCNQ1 subunits slows IKs channel activation by impeding dissociation of the proposed R231–E160 salt bridge (formed in the closed state) and/or delaying the formation of the R237–E160 salt bridge (formed in the open state).
In the open state model of KCNQ1 (Fig. 8), V141 and S140 in the S1 segment are in close proximity to E160 in S2 and R237 in S4 of the same subunit. The R237A mutation markedly slowed deactivation and thus, mimicked the effect of V141M on IKs. However, R237A, and not V141M, slowed deactivation of KCNQ1 channels in the absence of KCNE1, indicating that different molecular mechanisms could mediate disruption of channel deactivation by the two mutations. Moreover, combining the two mutations (R237A/V141M) in KCNQ1 produced a synergistic effect, completely preventing the closure of IKs channels. The S140G and V141M mutations might slow deactivation by interfering with the R231–E160 interaction, or stabilizing the R237–E160 interaction in a KCNE1-dependent manner. Alternatively, S140G and V141M could alter how KCNE1 binds to S1 and thereby disrupt deactivation via an altered interaction between a KCNE1 subunit and the pore domain (S5, S6) of KCNQ1. Obviously, a crystal structure of the IKs channel would greatly facilitate our understanding of the molecular mechanisms underlying the KCNE1-induced slowing of KCNQ1 activation and the S140G or V141M-induced slowing of IKs deactivation.
In summary, we report that S140G and V141M IKs channels retain a capacity to close in a voltage-dependent manner. Functionally, these mutant channels are constitutively open when activated repetitively at rates that approximate physiological conditions. As described before (Hong et al. 2005), these S1 point mutations disrupt IKs channel gating sufficiently to enhance net outward current during the plateau phase and shorten cardiac action potentials. Finally, we suggest that altered intrasubunit charge-pair interactions between E160 in S2 and R231 and/or R237 of KCNQ1, may account for the slowed IKs deactivation induced by S140 or V141.
Acknowledgments
We thank Kam Hoe Ng for isolation and cRNA injection of Xenopus oocytes. This study was supported by the National Institutes of Health, National Heart Lung and Blood Institute grant number R01 HL065299.
References
- Arnar DO, Thorvaldsson S, Manolio TA, Thorgeirsson G, Kristjansson K, Hakonarson H, Stefansson K. Familial aggregation of atrial fibrillation in Iceland. Eur Heart J. 2006;27:708–712. doi: 10.1093/eurheartj/ehi727. [DOI] [PubMed] [Google Scholar]
- Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. KvLQT1 and IsK (minK) proteins associate to form the IKs cardiac potassium channel. Nature. 1996;384:78–80. doi: 10.1038/384078a0. [DOI] [PubMed] [Google Scholar]
- Chen H, Kim LA, Rajan S, Xu S, Goldstein SA. Charybdotoxin binding in the IKs pore demonstrates two MinK subunits in each channel complex. Neuron. 2003a;40:15–23. doi: 10.1016/s0896-6273(03)00570-1. [DOI] [PubMed] [Google Scholar]
- Chen YH, Xu SJ, Bendahhou S, Wang XL, Wang Y, Xu WY, Jin HW, Sun H, Su XY, Zhuang QN, Yang YQ, Li YB, Liu Y, Xu HJ, Li XF, Ma N, Mou CP, Chen Z, Barhanin J, Huang W. KCNQ1 gain-of-function mutation in familial atrial fibrillation. Science. 2003b;299:251–254. doi: 10.1126/science.1077771. [DOI] [PubMed] [Google Scholar]
- Ellinor PT, Yoerger DM, Ruskin JN, MacRae CA. Familial aggregation in lone atrial fibrillation. Hum Genet. 2005;118:179–184. doi: 10.1007/s00439-005-0034-8. [DOI] [PubMed] [Google Scholar]
- Fernandez D, Ghanta A, Kinard KI, Sanguinetti MC. Molecular mapping of a site for Cd2+-induced modification of human ether-a-go-go-related gene (hERG) channel activation. J Physiol. 2005;567:737–755. doi: 10.1113/jphysiol.2005.089094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox CS, Parise H, D’Agostino RB, Sr, Lloyd-Jones DM, Vasan RS, Wang TJ, Levy D, Wolf PA, Benjamin EJ. Parental atrial fibrillation as a risk factor for atrial fibrillation in offspring. JAMA. 2004;291:2851–2855. doi: 10.1001/jama.291.23.2851. [DOI] [PubMed] [Google Scholar]
- Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- Hong K, Piper DR, Diaz-Valdecantos A, Brugada J, Oliva A, Burashnikov E, Santos-de-Soto J, Grueso-Montero J, Diaz-Enfante E, Brugada P, Sachse F, Sanguinetti MC, Brugada R. De novo KCNQ1 mutation responsible for atrial fibrillation and short QT syndrome in utero. Cardiovasc Res. 2005;68:433–440. doi: 10.1016/j.cardiores.2005.06.023. [DOI] [PubMed] [Google Scholar]
- Liu J, Zhang M, Jiang M, Tseng GN. Negative charges in the transmembrane domains of the HERG K channel are involved in the activation- and deactivation-gating processes. J Gen Physiol. 2003;121:599–614. doi: 10.1085/jgp.200308788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundby A, Ravn LS, Svendsen JH, Olesen SP, Schmitt N. KCNQ1 mutation Q147R is associated with atrial fibrillation and prolonged QT interval. Heart Rhythm. 2007;4:1532–1541. doi: 10.1016/j.hrthm.2007.07.022. [DOI] [PubMed] [Google Scholar]
- Morin TJ, Kobertz WR. Counting membrane-embedded KCNE β-subunits in functioning K+ channel complexes. Proc Natl Acad Sci U S A. 2008;105:1478–1482. doi: 10.1073/pnas.0710366105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson TM, Alekseev AE, Liu XK, Park S, Zingman LV, Bienengraeber M, Sattiraju S, Ballew JD, Jahangir A, Terzic A. Kv1.5 channelopathy due to KCNA5 loss-of-function mutation causes human atrial fibrillation. Hum Mol Genet. 2006;15:2185–2191. doi: 10.1093/hmg/ddl143. [DOI] [PubMed] [Google Scholar]
- Panaghie G, Abbott GW. The role of S4 charges in voltage-dependent and voltage-independent KCNQ1 potassium channel complexes. J Gen Physiol. 2007;129:121–133. doi: 10.1085/jgp.200609612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papazian DM, Shao XM, Sech S-A, Mock AF, Huang Y, Wainstock DH. Electrostatic interactions of S4 voltage sensor in Shaker K+ channel. Neuron. 1995;14:1293–1301. doi: 10.1016/0896-6273(95)90276-7. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL, Keating MT. Coassembly of KvLQT1 and minK (IsK) proteins to form cardiac IKs potassium channel. Nature. 1996;384:80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- Sesti F, Goldstein SA. Single-channel characteristics of wild-type IKs channels and channels formed with two minK mutants that cause long QT syndrome. J Gen Physiol. 1998;112:651–663. doi: 10.1085/jgp.112.6.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamgar L, Haitin Y, Yisharel I, Malka E, Schottelndreier H, Peretz A, Paas Y, Attali B. KCNE1 constrains the voltage sensor of Kv7.1 K+ channels. PLoS ONE. 2008;3:e1943. doi: 10.1371/journal.pone.0001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman WR, Bannister JP, Papazian DM. Binding site in Eag voltage sensor accommodates a variety of ions and is accessible in closed channel. Biophys J. 2004;87:3110–3121. doi: 10.1529/biophysj.104.044602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman WR, Roux B, Papazian DM. Structural basis of two-stage voltage-dependent activation in K+ channels. Proc Natl Acad Sci U S A. 2003;100:2935–2940. doi: 10.1073/pnas.0636603100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinner MF, Pfeufer A, Akyol M, Beckmann BM, Hinterseer M, Wacker A, Perz S, Sauter W, Illig T, Nabauer M, Schmitt C, Wichmann HE, Schomig A, Steinbeck G, Meitinger T, Kaab S. The non-synonymous coding IKr-channel variant KCNH2-K897T is associated with atrial fibrillation: results from a systematic candidate gene-based analysis of KCNH2 (HERG) Eur Heart J. 2008;29:907–914. doi: 10.1093/eurheartj/ehm619. [DOI] [PubMed] [Google Scholar]
- Smith JA, Vanoye CG, George AL, Jr, Meiler J, Sanders CR. Structural models for the KCNQ1 voltage-gated potassium channel. Biochemistry. 2007;46:14141–14152. doi: 10.1021/bi701597s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splawski I, Tristani-Firouzi M, Lehmann MH, Sanguinetti MC, Keating MT. Mutations in the hminK gene cause long QT syndrome and suppress IKs function. Nature Genet. 1997;17:338–340. doi: 10.1038/ng1197-338. [DOI] [PubMed] [Google Scholar]
- Stühmer W. Electrophysiological recording from Xenopus oocytes. Methods Enzymol. 1992;207:319–339. doi: 10.1016/0076-6879(92)07021-f. [DOI] [PubMed] [Google Scholar]
- Tai KK, Goldstein SAN. The conduction pore of a cardiac potassium channel. Nature. 1998;391:605–608. doi: 10.1038/35416. [DOI] [PubMed] [Google Scholar]
- Tapper AR, George AL., Jr MinK subdomains that mediate modulation of and association with KvLQT1. J Gen Physiol. 2000;116:379–390. doi: 10.1085/jgp.116.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari-Woodruff SK, Lin MA, Schulteis CT, Papazian DM. Voltage-dependent structural interactions in the Shaker K+ channel. J Gen Physiol. 2000;115:123–138. doi: 10.1085/jgp.115.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari-Woodruff SK, Schulteis CT, Mock AF, Papazian DM. Electrostatic interactions between transmembrane segments mediate folding of Shaker K+ channel subunits. Biophys J. 1997;72:1489–1500. doi: 10.1016/S0006-3495(97)78797-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Curran ME, Splawski I, Burn TC, Millholland JM, VanRaay TJ, Shen J, Timothy KW, Vincent GM, de Jager T, Schwartz PJ, Towbin JA, Moss AJ, Atkinson DL, Landes GM, Connors TD, Keating MT. Positional cloning of a novel potassium channel gene: KVLQT1 mutations cause cardiac arrhythmias. Nat Genet. 1996;12:17–23. doi: 10.1038/ng0196-17. [DOI] [PubMed] [Google Scholar]
- Xia M, Jin Q, Bendahhou S, He Y, Larroque MM, Chen Y, Zhou Q, Yang Y, Liu Y, Liu B, Zhu Q, Zhou Y, Lin J, Liang B, Li L, Dong X, Pan Z, Wang R, Wan H, Qiu W, Xu W, Eurlings P, Barhanin J. A Kir2.1 gain-of-function mutation underlies familial atrial fibrillation. Biochem Biophys Res Commun. 2005;332:1012–1019. doi: 10.1016/j.bbrc.2005.05.054. [DOI] [PubMed] [Google Scholar]
- Xu X, Jiang M, Hsu KL, Zhang M, Tseng GN. KCNQ1 and KCNE1 in the IKs channel complex make state-dependent contacts in their extracellular domains. J Gen Physiol. 2008;131:589–603. doi: 10.1085/jgp.200809976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Sigworth FJ. Single-channel properties of IKs potassium channels. J Gen Physiol. 1998;112:665–678. doi: 10.1085/jgp.112.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Xia M, Jin Q, Bendahhou S, Shi J, Chen Y, Liang B, Lin J, Liu Y, Liu B, Zhou Q, Zhang D, Wang R, Ma N, Su X, Niu K, Pei Y, Xu W, Chen Z, Wan H, Cui J, Barhanin J. Identification of a KCNE2 gain-of-function mutation in patients with familial atrial fibrillation. Am J Hum Genet. 2004;75:899–905. doi: 10.1086/425342. [DOI] [PMC free article] [PubMed] [Google Scholar]