Abstract
Peripheral sensory afferents in the hand activate both excitatory and inhibitory intracortical circuits to potentially facilitate and prune descending motor commands. In this study, we characterized how afferent inputs modulate the excitability of cortical circuits in the leg area of the primary motor cortex by examining how stimulation of the tibial nerve (TN) at the ankle alters motor evoked potentials (MEPs) activated by transcranial magnetic stimulation (TMS). Resting MEPs in the tibialis anterior (TA) muscle were facilitated in response to heteronymous activation of the TN 45–50 ms earlier, whereas MEPs were inhibited at interstimulus intervals of 32.5–37.5 ms. Similar time-dependent modulation occurred in the soleus (SOL) muscle with stimulation of the homonymous posterior tibial nerve (PTN) at the knee. To determine the site of this afferent-evoked facilitation and inhibition (spinal or cortical), we compared the effects of afferent stimulation to responses evoked at subcortical sites. At interstimulus intervals where MEP facilitation was observed (near 50 ms), spinal H-reflexes and responses evoked from corticospinal tract stimulation at the brainstem were predominantly depressed by the sensory stimulus suggesting that the observed MEP facilitation was cortical in origin. At interstimulus intervals where MEP depression was observed (near 35 ms), brainstem evoked responses were depressed to a similar degree and, in contrast to the hand, this suggests that spinal rather than cortical circuits mediate short-latency afferent inhibition (SAI) of leg MEPs. When the MEP was facilitated by afferent inputs, short-interval intracortical inhibition (SICI) was reduced and intracortical facilitation (ICF) was increased, but long-interval intracortical inhibition (LICI) at a 100 ms interval was unchanged. In addition, sensory excitation increased the recruitment of early, middle and late descending corticospinal volleys as evidenced from increases in MEP facilitation at the corresponding I-wave periodicity. We propose that sensory activation from the leg has a diffuse and predominantly facilitatory effect on the leg primary motor cortex.
Integration of sensory afferent feedback into the generation of motor commands from the primary motor cortex is important given the severe disruption of gait and fine motor skills in patients with large-fibre sensory neuropathies (Rothwell et al. 1982; Sanes et al. 1985; Lajoie et al. 1996). Although the process by which sensory inputs shape the activity of cortical networks is unknown, studies in monkeys have shown that both proprioceptive and cutaneous sensory stimulation can strongly excite or inhibit ongoing neuronal activity in the primary motor cortex during precise voluntary movement (Lemon, 1981; Boudreau & Smith, 2001). In humans, altering sensory input to the motor cortex via repetitive peripheral nerve stimulation (Ridding et al. 2000; Knash et al. 2003) or transient limb deafferentation (Brasil-Neto et al. 1992) can temporarily alter motor cortex excitability as assessed by transcranial magnetic stimulation (TMS). Likewise, permanent nerve injury or limb amputation is thought to promote cortical reorganization (Chen et al. 2002), potentially via changes in the excitability of intracortical inhibitory interneurons that link functional motor areas (Jacobs & Donoghue, 1991; Schneider et al. 2002).
Recently, several human studies using paired-pulse TMS techniques have examined how sensory afferent inputs from the hand influence both excitatory and inhibitory cortical networks of the primary motor cortex. For instance, when afferent inputs from electrical stimulation first arrive at the motor cortex, motor evoked potentials (MEPs) from TMS are depressed in what is called ‘short-latency afferent inhibition’ or SAI (Tokimura et al. 2000). Since late descending corticospinal volleys are also depressed, SAI is thought to occur through supraspinal mechanisms (Tokimura et al. 2000) that involve both cholinergic and GABAergic systems distinct from networks producing short-interval intracortical inhibition (SICI) (Di Lazzaro et al. 2000, 2007). Other forms of afferent-induced inhibition of the motor cortex include long-latency afferent inhibition (LAI) that occurs when afferent inputs are stimulated 200 ms before a TMS pulse (Chen et al. 1999; Sailer et al. 2002) and surround inhibition from vibration applied to adjacent hand muscles which reduces MEPs and increases SICI (Rosenkranz & Rothwell, 2003).
In addition to the inhibitory effects of afferent feedback at the cortex, afferent input can strengthen ongoing EMG activity through a transcortical loop (for review see Christensen et al. 2000). Muscle stretch and/or cutaneous stimulation in the upper and lower limb can access corticospinal circuits to facilitate the MEP (Nielsen et al. 1997; Petersen et al. 1998; Rosenkranz & Rothwell, 2003; Kessler et al. 2005). Such facilitation is most pronounced in muscles supplied by the nerve, though similar changes can be observed in neighbouring muscles (Deletis et al. 1992). In the leg, afferent feedback contributes to muscle activity (Sinkjaer et al. 2000) and the long-latency reflex is thought to be involved in lifting the foot over obstacles and stabilizing the supporting limb in the stance phase of gait (Christensen et al. 2000). In terms of cortical networks, MEP facilitation from hand muscle afferents is associated with a reduction in SICI, but paradoxically in an increase in long-interval intracortical inhibition (LICI) (Rosenkranz & Rothwell, 2003; Rothwell & Rosenkranz, 2005). Increases in intracortical facilitation (ICF), a complex motor circuit whose origin is currently a matter of debate (Di Lazzaro et al. 2006), occur in wrist motor areas following vibration of homonymous afferents (Rosenkranz et al. 2003), whereas increases in ICF measured from muscles of the wrist are only observed when antagonist muscle afferents are electrically stimulated (Aimonetti & Nielsen, 2001). Currently, the role of sensory stimulation on intracortical networks in the leg area of the primary motor cortex has not been systematically studied.
In this study, we explored the time course of MEP facilitation and inhibition following electrical stimulation of the tibial (at ankle) and posterior tibial (at knee) nerves with the former having strong heteronymous inputs to ankle dorsi- and plantar-flexors (Pierrot-Deseilligny & Burke, 2005). Conditioning TMS with a prior nerve stimulus produced bidirectional changes in the MEP with depression at short intervals (similar to SAI in the hand) and potentiation at longer intervals, the latter occurring at latencies a few milliseconds after the arrival of afferent inputs at the somatosensory cortex. Responses elicited with direct corticospinal tract stimulation were depressed by the peripheral nerve stimulus at both short and long interstimulus intervals, suggesting that the MEP inhibition from peripheral nerve stimulation was mainly spinal in origin whereas MEP facilitation was mediated by cortical circuits. Because the effects of leg afferent stimulation appeared to be mainly excitatory on the motor cortex, we examined if MEP facilitation was associated with decreases in the activation of cortical networks producing SICI and LICI and increases in the excitability of ICF networks. We also examined which excitatory cortical networks were activated by leg afferents, i.e. those involved in the production of earlier or later corticospinal volleys, by using MEP conditioning techniques at the periodicity of indirect (I) waves (Ziemann et al. 1998). Parts of this study have been presented in abstract form (Roy & Gorassini, 2008).
Methods
Subjects
Nineteen healthy subjects (9 of whom were female) aged 21–53 years (29 ± 8 years, mean ±s.d.) participated in this study. All subjects gave written informed consent and the protocol was approved by the Human Ethics Research Board at the University of Alberta. The experiments conformed to the Declaration of Helsinki. Subjects were comfortably seated with the examined leg (17 right and 2 left) slightly bent at the knee and with the foot secured to a footplate. Responses were recorded in the left leg of two subjects because resting MEPs were more easily elicited compared to the right leg.
EMG recordings
Surface electromyography (EMG) was recorded from the tibialis anterior (TA), soleus (SOL) and abductor hallucis (AH) muscles using pairs of surface Ag–AgCl electrodes (Kendall, Chicopee, MA, USA). EMG signals were amplified 1000 times and filtered with a band-pass of 10–1000 Hz (Octopus, Bortec Technologies, Calgary, Canada). EMG signals were digitized at 5 kHz using Axoscope hardware and software (Digidata 1200 Series, Axon Instruments, Union City, CA, USA) and stored on a personal computer for off-line analysis. During voluntary contraction, EMG from the target muscle was rectified, low-pass filtered using a 100 ms time constant (NL703, Digitimer, Hertfordshire, UK) and displayed on an oscilloscope.
Transcranial magnetic stimulation (TMS)
TMS was performed using two Magstim 200 stimulators connected to a Bistim module (Magstim, Dyfed, UK) and a custom-built figure-of-eight coil (P/N 15857: bat wing with 90 mm external wing diameter). The coil was placed over the leg area of the motor cortex. The optimal stimulus site was identified using a stimulation intensity that was slightly above threshold in the resting muscle. The coil was secured in place throughout the experiment and orientated to deliver anterior-posterior directed current in the brain. Single and paired pulses of TMS were delivered as described below.
Peripheral nerve stimulation
Peripheral nerves were stimulated using a constant-current stimulator (DS7A, Digitimer). The tibial nerve (TN), innervating the foot, was activated at the ankle with the cathode placed below the medial malleolus and a large anode on the lateral aspect of the ankle (0.2 ms pulse) as done by Yang & Stein (1990). The TN intensity was 1.5 × motor threshold (MT) in the AH muscle or 2 × MT if the M-wave was < 0.1 mV at the 1.5 MT intensity. Stimulation did not elicit cutaneous sensation on the lateral side of the foot via the sural nerve. The posterior tibial nerve (PTN) was stimulated using bipolar surface electrodes in the popliteal fossa (Poon et al. 2008) to minimize current spread to other nerves. The PTN stimulus intensity was just above MT (1 ms pulse). The H-reflex in the TA muscle was elicited by stimulating the common peroneal nerve near the head of the fibula with the anode placed on the medial side of the knee just below the patella (1 ms pulse). This arrangement provided more diffuse stimulation (compared to bipolar stimulation) and was used to test general spinal cord excitability to the TA muscle.
Corticospinal tract stimulation
The corticospinal tract was stimulated non-invasively at the cervicomedullary junction in five subjects. In three of the subjects, it was possible to elicit cervicomedullary evoked potentials (CMEPs) with TMS to the brainstem, which is less disturbing than direct electrical stimulation. In these subject, magnetic stimuli were delivered using a Magstim 200 stimulator and a double cone coil placed over the inion of the skull with the current flowing in a downward direction (Taylor & Gandevia, 2004). In the other two subjects, electrical stimulation of the corticospinal tract was done using a 100 μs electrical pulse (320–400 V, D185 stimulator, Digitimer) delivered through a pair of silver cup electrodes (10 mm diameter) fixed over the mastoids with the cathode on the left.
Somatosensory evoked potentials
Cortical potentials evoked by TN stimulation at the ankle were recorded with a pair of silver cup electrodes (10 mm diameter) placed on the scalp, with the anode over the vertex and the cathode 5 cm more anterior, a placement similar to that used in Nielsen et al. (1997). The signals were amplified 50 000 times and were filtered between 2 and 1000 Hz. Usually 200–300 sweeps were averaged. The arrival time of the evoked potential was measured as the time of the first negative peak, referred to as the P40 (Hauck et al. 2006).
Study design
Experiment 1: time course of afferent conditioning of TA MEPs
In eight subjects, heteronymous afferent inputs from the TN that innervates the medial and plantar surface of the foot were used to condition MEP responses evoked in the TA muscle. Electrical stimuli to the homonymous common peroneal nerve were not used because they strongly depressed the TA MEP, likely due to strong spinal inhibitory effects. The TMS intensity was set to approximately half the maximum MEP response in the resting TA muscle (0.2–0.6 mV). Fourteen conditioning–test intervals between the nerve stimulus and the TMS pulse were used from 25 to 100 ms (see Fig. 1). Six conditioning TN stimuli were delivered at each conditioning–test interval intermixed with 12 single test stimuli. For all trials, the time between consecutive stimuli was 5 s.
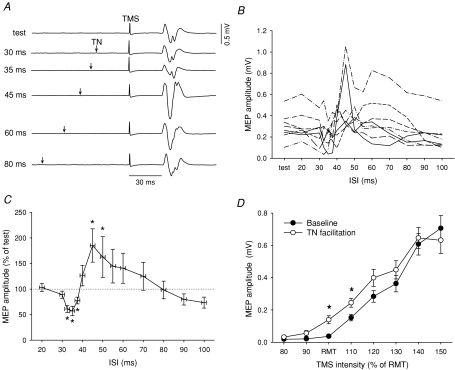
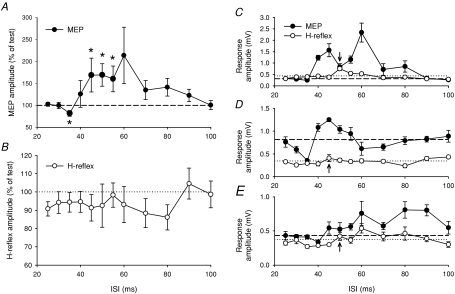
Figure 1. Effect of TN stimulation on MEP responses in TA.
A, raw sweeps showing average TA MEPs in one subject when preceded by a TN stimulus. The ISI influenced the size of the MEP by producing inhibition at 35 ms, facilitation at 45 and 60 ms, and no sizeable effect at 30 and 80 ms. B, individual subject data at ISIs from 20 to 100 ms. C, to account for each subject's conduction delay, the curves were shifted in time using the latency of the MEP in the AH muscle (see Methods). D, effect of TN stimulation on the TMS recruitment curve. The average RMT was 60 ± 3% MSO and the TN stimulus was delivered at a fixed interval that produced MEP facilitation in each subject. Data are from 8 subjects. MEPs at 150% RMT in D could only be elicited in 6 subjects. Asterisks indicate significant differences compared to baseline without TN stimulation (*P < 0.05).
TMS recruitment curves were collected for two conditions: without and with TN stimulation. The TN stimulus was delivered at a fixed conditioning–test interval that produced the largest MEP facilitation in each subject (at an ISI between 37.5 and 55 ms). Because absolute MEP amplitudes for a given intensity varied between subjects, the stimulation intensities used to produce the recruitment curves were normalized with respect to the resting TA motor threshold (RMT). RMT was determined before the recruitment curve was obtained and was defined as the lowest stimulus intensity that evoked a MEP with an amplitude > 50 μV in at least 3 of 5 consecutive stimuli (Perez et al. 2004). TMS intensities were then increased from 80% to 150% of the RMT in steps of 10%, with five MEPs collected at each intensity.
Experiment 2: site of afferent-induced MEP inhibition and facilitation
To investigate the site (i.e. cortex or spine) of afferent-induced MEP inhibition at short ISIs (near 35 ms) and MEP facilitation at longer ISIs (near 50 ms), TN stimulation was also used to condition H-reflexes and CMEPs. Since TA H-reflexes and CMEPs in the TA muscle are more readily evoked in the precontracted muscle, all responses were collected while subjects maintained a tonic dorsiflexion corresponding to 10% of their maximum voluntary contraction (MVC). During tonic contraction, the time course effect of TN stimulation on MEPs and H-reflexes was measured in 10 subjects. The H-reflex was adjusted to approximately half the maximum H-reflex response (0.2–0.6 mV) to show both inhibition and facilitation following the nerve stimulus. To compare similar-sized responses, the TMS stimulus intensity was adjusted to produce small, but reliable, MEPs of 0.5–0.75 mV in the contracted muscle. Twelve conditioning–test intervals from 25 to 100 ms were tested for both MEPs and H-reflexes (see Fig. 3). For each experiment, six conditioning TN stimuli were delivered at each conditioning–test interval intermixed with 12 single test stimuli. SEPs were also recorded in nine subjects following TN stimulation.
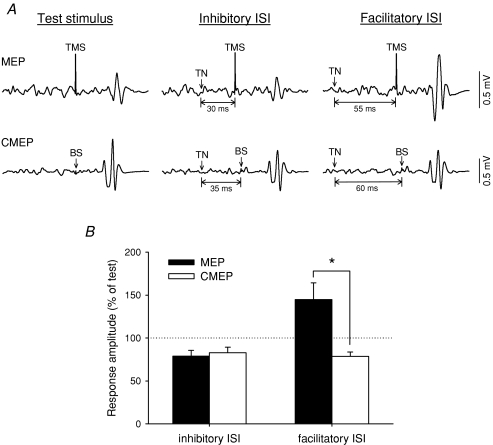
Figure 3. Effect of TN stimulation on MEPs and CMEPs.
A, average TA MEPs (top traces) and CMEPs (bottom traces) in a single subject when conditioned by a preceding TN stimulus. MEPs are depressed at the inhibitory 30 ms ISI (middle top) and potentiated at the facilitatory 55 ms ISI (right top), whereas the CMEPs elicited by an appropriately timed brainstem stimulus (BS) were depressed (bottom traces). B, group data from 5 subjects showing the effect of TN stimulation on MEPs (black bars) and CMEPs (white bars) in the contracted TA muscle. The inhibitory and facilitatory ISIs were near 35 and 50 ms, respectively, and were different for each subject. MEPs and CMEPs were both significantly reduced at the inhibitory ISI. Asterisks indicate significant differences in the size of the MEP compared to the CMEP (*P < 0.05).
TN conditioning of evoked responses from direct activation of the corticospinal tract were also examined in 5 of the 10 subjects (the majority in separate experimental sessions) to determine if the MEP inhibition and/or facilitation were cortical in origin. CMEPs were elicited by stimulating the corticospinal tract at the brainstem (see Corticospinal tract stimulation, above). For brainstem stimulation, the duration of the conditioning–test interval was increased by 3–5 ms to account for the transmission delay from the motor cortex to the cervicomedullary junction and to match the arrival time of the MEP and CMEP volleys at the motoneuron pool (Taylor et al. 2002). Two ISIs were tested, one that produced MEP inhibition (near 35 ms) and one that produced MEP facilitation (near 50 ms). MEPs and CMEPs were investigated separately in a block of trials consisting of two randomly intermixed conditions (with test stimulus alone or conditioned by the TN stimulus), each with 10 responses. Responses were collected during voluntary contraction.
Experiment 3: intracortical mechanisms of afferent-induced MEP facilitation
Because it was determined that the major effect of leg afferent stimulation was to increase cortical excitability, we examined which cortical networks were associated with afferent-induced increases in MEP responses. The effect of afferent input on SICI, ICF, paired-pulse facilitation (PPF), LICI and I-wave facilitation was investigated using the appropriate paired-pulse TMS protocols (see below). These experiments were each performed at rest under two different conditions: with and without TN stimulation. TN stimulation was adjusted in each subject to a conditioning–test interval that produced MEP facilitation in the resting TA muscle (ISI near 50 ms), as described above. The afferent input was synchronized to arrive at the cortex at the same time as the test TMS stimulus as done by Aimonetti & Nielsen (2001). Because activity of intracortical circuits and corticospinal recruitment depend on the size of the test stimulus (Ziemann et al. 1998; Chen, 2004), the TN condition was also repeated (if necessary) with the test stimulus intensity adjusted (TS-ADJ) to match the MEP amplitude produced at baseline without TN stimulation.
The effect of TN stimulation on SICI and ICF was investigated in five subjects using a subthreshold conditioning stimulus preceding a suprathreshold test stimulus by 3 ms and 10 ms, respectively (Kujirai et al. 1993). The conditioning stimulus intensity was adjusted to 90–95% of the active motor threshold (AMT). AMT was defined as the lowest stimulus intensity that elicited a distinguishable MEP > 50–100 μV in at least 3 of 5 consecutive stimuli in the tonically contracted TA muscle. For both experimental conditions, 10 trials were recorded at each paired-pulse interval intermixed with 10 test stimuli.
The effect of afferent stimulation on paired-pulse TMS facilitation (PPF: ISIs from 10 to 50 ms) and LICI (ISIs from 60 to 150 ms) was investigated using pairs of suprathreshold TMS in eight subjects in a different experimental session (see Fig. 4C). The two pulses were given at the same intensity. For both experimental conditions, four paired-pulse stimuli were delivered at each interval intermixed with eight single-pulse test stimuli. LICI at 100 ms was further tested using a weaker conditioning stimulus such that the conditioned MEP was inhibited by approximately 40–60%. Ten paired-pulse stimuli and 10 test stimuli were delivered for each condition: without and with afferent input.
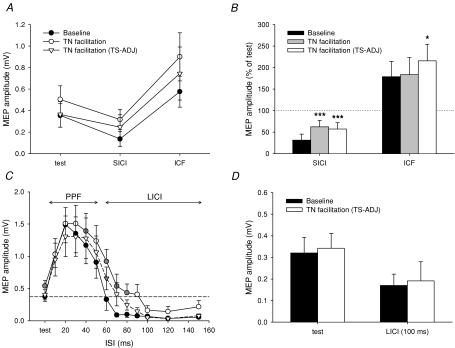
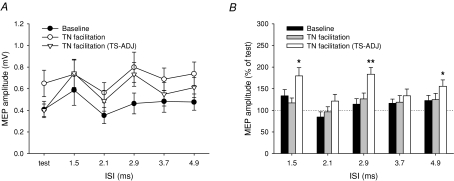
Figure 4. Effect of TN stimulation on intracortical circuits Influence of TN stimulation at facilitatory ISI on SICI and ICF.
(A and B) and the time course of PPF and LICI (C and D) in the resting TA muscle. SICI and ICF were evaluated at ISIs of 3 and 10 ms, respectively, and show the actual MEP amplitudes in A and expressed as a percentage of the test MEP in B. MEPs in TA were collected at baseline without TN stimulation (•; black bars), with TN facilitation (○; grey bars) and with TN facilitation when the test stimulus was adjusted (TS-ADJ) to match the baseline test MEP (▿; white bars). Only the intensity of the test stimulus was adjusted in the matched condition (compare the test MEP of • and ▿ in A and C). The TN input was given at a fixed interval of 40–55 ms before the test TMS pulse. The broken line in C represents the size of the average test MEP. D, LICI at an ISI of 100 ms was evaluated using a weaker conditioning stimulus which produced 40–60% inhibition. Data are from 5 subjects in (A and B) and 8 subjects in (C and D). Asterisks or grey symbols indicate significant differences compared to baseline without TN stimulation (*P < 0.05, ***P < 0.005).
I-wave facilitation (also known as short-interval intracortical facilitation: SICF) was investigated in six subjects (5 recorded in the same session as SICI and ICF) using a paired-pulse protocol described by Ziemann et al. (1998) with a suprathreshold test stimulus preceding a subthreshold conditioning stimulus. Five interstimulus intervals were tested: 1.5, 2.9 and 4.9 ms to capture the peaks of the I-wave periodicity and 2.1 and 3.7 ms to capture the intervening troughs (Chen & Garg, 2000). The first TMS pulse served as the test stimulus. The intensity of the second pulse was set to 95% of RMT. For both experimental conditions, a block of trials consisted of eight paired stimuli for each interval intermixed with 16 test stimuli.
Experiment 4: effect of posterior tibial nerve (PTN) stimulation on MEPs in SOL
The effect of afferent input on cortical excitability to SOL was investigated in eight subjects. The afferent input was provided by stimulating the homonymous PTN in the popliteal fossa. The conditioning–test protocol was similar to that described in Exp. 1 with ISIs from 20 to 100 ms (see above). Five subjects showing MEP decreases at an ISI of 30 ms were further tested at a variety of intervals between of 24 and 40 ms (see Fig. 6). The TMS intensity was adjusted to produce SOL MEPs of 0.1 mV, which was approximately half the size of the maximum resting MEP response. Since MEPs in SOL are much smaller than the antagonist TA, we ensured that the SOL MEPs had a different latency and waveform compared to the responses recorded in TA. Recruitment curves of increasing TMS intensities were collected for both conditions: without and with PTN stimulation. The PTN stimulus was delivered at a fixed conditioning–test interval that produced MEP facilitation in each subject (at an ISI between 40 and 55 ms). TMS intensities were then increased from 88% to 124% of the RMT (in steps of 4%) and at 130% RMT to measure the maximum MEP responses, with five MEPs collected at each intensity.
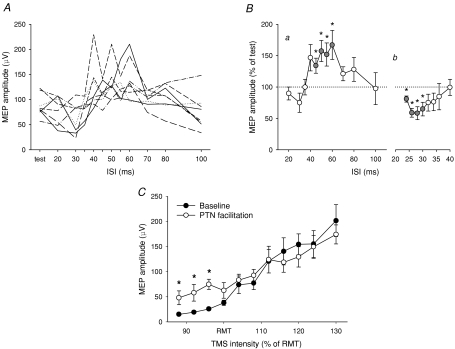
Figure 6. Effect of PTN stimulation on MEP responses in SOL.
A, individual subject data showing the effect of PTN stimulation on SOL MEPs. The non-adjusted group average is shown in Ba. Five subjects showing decreases at an ISI of 30 ms were further tested at a variety of ISIs between 24 and 40 ms (Bb). C, graph showing the TMS recruitment curve in SOL recorded at baseline without TN stimulation (•) and with TN stimulation (○). The TN stimulus was given at a fixed conditioning–test interval that produced MEP facilitation in each subject, based on A. The TMS intensity is expressed as a percentage of the RMT in SOL, which was 67 ± 3% MSO on average. Data in A and C are from 8 subjects. Asterisks indicate significant differences compared to the test MEP (B) and between the two conditions (C) (*P < 0.05).
Data analysis and statistics
The size of the MEP, H-reflex and CMEP was measured as the peak-to-peak amplitude of the non-rectified response. The level of background activity was measured from the rectified EMG in a 50 ms period before the stimulus and assessed to ensure there was a comparable level of EMG activity across conditions. Statistical analysis was performed on either the actual responses or after the data were expressed as a percentage of the test response. Both peak inhibition and facilitation were evaluated in each subject around the ISIs the produced significant changes in the MEP.
The time course of the effect of TN stimulation on resting MEP responses in Exp. 1 was adjusted in each subject to accommodate for differences in the time required for the afferent input to reach the motor cortex (see Fig. 1B and C). The afferent-conditioned MEP profile (over all the ISIs) was first interpolated in steps of 0.5 ms using a linear interpolation technique. A given MEP profile was then shifted to the right or left by an amount that was equal to the difference between a subject's MEP latency in the AH muscle and the latency of the group average, which was 40.4 ± 1.3 ms. For example, the conditioned MEP profile from a subject with an MEP latency of 42.4 ms was shifted to the left by 2 ms. After shifting the profiles (by 2.6 ± 0.7 ms on average), the values on the curves were averaged at the original ISI values of 20, 30, 32.5 ms, etc., all the way up to 100 ms. In the example subject, the interpolated values at 22, 32, 34.5 ms and so on were used when calculating the group average. MEP profiles during voluntary contraction (Exp. 2) were not adjusted because the facilitatory window was broader. Similarly, MEP profiles in the SOL muscle in response to PTN stimulation were not adjusted because the variability in SOL MEP latencies across subjects, and probably the variability in the time afferent inputs reached the motor cortex, was much less compared to TN stimulation.
For single pulse MEP and H-reflex data collected with TN stimulation over the range of ISIs used, Friedman analysis of variance (ANOVA) with ‘ISI’ as within-subject factor was used since the data were non-normally distributed across the intervals. For these data, post hoc comparisons were done using Wilcoxon's test. The recruitment curve data and the paired-pulse data (SICI, ICF, PPF, LICI and I-wave facilitation) were analyzed using two-way repeated measures ANOVAs on either the actual or normalized MEP amplitudes, with post hoc comparisons done using Student's paired t test (two-tailed). Differences in the effect of TN stimulation on brainstem and cortical responses were analyzed using a two-way repeated measures ANOVA with ‘site of stimulation’ (cortex and brainstem) and ‘type of ISI’ as within-subject factors. MEP data in SOL collected with PTN stimulation were analyzed using a one-way repeated measures ANOVA with ‘ISI’ as within-subject factor. The significance level was set at P < 0.05 and data are given as means ±s.e.m.
Results
Experiment 1: time course of afferent conditioning on TA MEPs
Electrical stimulation of the TN at the ankle modified the size of the peak-to-peak TA MEP. The intensity of the test stimulus (70 ± 3% of the maximum stimulator output: MSO) was adjusted to elicit MEP responses on the steep portion of the recruitment curve, which was 0.30 ± 0.04 mV on average in the resting muscle. Figure 1A shows the effect of TN stimulation on the test MEP in a single subject. Stimulating the TN 35 ms before applying a pulse of TMS to the motor cortex depressed the test MEP, whereas a conditioning stimulation at ISIs of 45 and 60 ms facilitated the test MEP. Surrounding these conditioning–test intervals, the MEP showed little change at ISIs of 30 and 80 ms. This trend was similar for all subjects in Fig. 1B, showing individual subject data across all ISIs.
In the averaged group data (Fig. 1C; see data analysis in Methods), a Friedman ANOVA showed a significant effect of the ISI (P < 0.001). TA MEPs were significantly depressed at intervals from 32.5 to 37.5 ms (by −55 ± 6% at peak inhibition) and facilitated at 45 and 50 ms (by +123 ± 42% at peak facilitation) (Wilcoxon's test; P < 0.05). All subjects showed a > 20% reduction and a > 20% increase in the MEP at one of the inhibitory and facilitatory ISIs, respectively. MEPs in AH and SOL muscles were also modulated in a parallel manner, showing strong significant inhibition and non-significant facilitation (data not shown). To characterize how TN afferents excite the corticospinal tract, we evaluated its effects on the TMS recruitment curve (Fig. 1D). Afferent stimulation at an ISI near 50 ms significantly increased MEP responses at TMS intensities of RMT and 110% RMT (P < 0.05). Over these intensities, the MEP was increased by 0.10 ± 0.02 mV on average.
Experiment 2: site of afferent-induced MEP inhibition and facilitation
To determine whether the MEP inhibition and facilitation shown in Fig. 1 occurred at the level of the cortex and/or the spinal cord, we examined the effect of TN stimuli on the excitability of subcortical networks using both H-reflexes and CMEPs. Because H-reflexes and CMEPs are more readily evoked in the precontracted TA muscle, the effect of afferent stimulation on TA MEPs was repeated during a small voluntary contraction of 10% MVC. The modulation in the MEP was similar in the contracted muscle (Fig. 2A; see below). When examining the excitability of spinal interneurons and motoneurons to the TA muscle using the H-reflex, TN conditioning resulted in non-significant changes to the size of the TA H-reflex (Fig. 2B; Friedman ANOVA; P= 0.64). At most ISIs, the TN conditioning input had a depressive effect on the H-reflex, though 5 out of 10 subjects revealed a small increase at conditioning–test intervals 5–15 ms longer than the ISIs that first produced MEP facilitation. The small reflex facilitation is shown as arrows for three example subjects in Fig. 2C–E. The size of the average test H-reflex was 0.44 ± 0.05 mV and was smaller than the average test MEP of 0.62 ± 0.05 mV (P < 0.05).
Figure 2. Effect of TN stimulation on MEPs and H-reflexes.
A and B, size of the average peak-to-peak MEP (A) and H-reflex (B) in TA when conditioned by a TN stimulus during background contraction. Data are from 10 subjects. C–E, individual subject data showing MEP (•) and H-reflex responses (○) and the size of the unconditioned test MEP (black dashed line) and test H-reflex (black dotted line). The arrows show the increase in the H-reflex that occurred after the initial increase in the MEP response. Asterisks indicate significant differences from the test MEP (*P < 0.05).
As stated above, the effect of TN stimulation on MEPs was similar in the contracted muscle (Fig. 2A), but with the afferent input producing facilitation over a broader window of ISIs during contraction compared to rest. TA MEPs were significantly affected by the conditioning stimulus (Friedman ANOVA; P < 0.005) and were significantly facilitated at ISIs of 45–55 ms (by +145 ± 60% at peak facilitation) (Wilcoxon's test; P < 0.05). This corresponded to intervals that were a few to several milliseconds longer than the average SEP latency (41.5 ± 0.9 ms, n= 9), indicating that MEP facilitation occurred after the arrival of the afferent input at the somatosensory cortex. Prior to the onset of the facilitation, MEPs were depressed at the 35 ms ISI (by –31 ± 5% at peak inhibition) (P < 0.05). Likewise, the size of the MEP in the homonymous AH was modified over a large range of intervals (Friedman ANOVA; P < 0.005), reflecting significant inhibition at 35 ms and a broad facilitatory window from 45 to 90 ms (data not shown). During dorsiflexion, MEPs in the antagonist SOL muscle were also significantly modulated (Friedman ANOVA; P < 0.01) and showed significant facilitation at ISIs of 45 and 55 ms (data not shown). The congruent results described in the three muscles were not likely to be due to cross-talk since the MEP waveforms and latencies differed in each muscle.
TN conditioning of responses evoked from stimulating the corticospinal tract at the cervicomedullary junction (i.e. CMEPs) were also measured and compared to TN conditioning of MEPs elicited over the motor cortex (Fig. 3). As shown for the single subject in Fig. 3A, MEP (top traces) and CMEP (bottom traces) responses were equally suppressed at a short ISI (near 30 ms, see legend for details), suggesting that suppression of MEP responses by TN stimulation at this ISI occurred at a subcortical site. In contrast, TN stimulation near 55 ms prior to stimulating the brainstem failed to facilitate the CMEP, even though the cortical MEP was facilitated by +115%, suggesting that MEP facilitation at this longer interval occurred at a cortical site. In the group data (Fig. 3B), a two-way repeated measures ANOVA showed a significant interaction effect between ‘stimulation site’ (cortex and brainstem) בtype of ISI’ (inhibitory and facilitatory ISIs) (F1,4= 17.65, P < 0.05). At the facilitatory ISI, the conditioned MEP was significantly larger than the conditioned CMEP (P < 0.05), the latter in fact being suppressed compared to the unconditioned CMEP. At the inhibitory ISI, there was no significant difference in the size of the conditioned MEP compared to the conditioned CMEP (P= 0.7), and both responses were significantly reduced compared to 100% (P < 0.05). Average test MEP and CMEP amplitudes were 0.66 ± 0.07 mV and 0.58 ± 0.15 mV, respectively, and were not significantly different.
Experiment 3: intracortical mechanisms of afferent-induced MEP facilitation
Because MEP facilitation at the longer ISIs is likely to be cortical in origin (re Exp. 2), we evaluated the influence of the afferent inputs on the excitability of intracortical circuits. First, the amount of SICI and ICF was examined when the TN stimulus was timed to arrive at the motor cortex at the same time as the test pulse. When the size of the test stimulus was matched to the unconditioned test MEP amplitude (TS-ADJ, see legend for details), TN stimulation reduced SICI and increased ICF (Fig. 4A). Normalizing the data by the test MEP size (Fig. 4B), there was a significant interaction effect between ‘condition’ (without and with TN stimulation) and ‘ISI’ (two-way ANOVA: F1,4= 26.81, P < 0.01). The afferent input reduced SICI from 69 to 43% (P < 0.05) and increased ICF from 179 to 215% (P < 0.05).
The effect of TN stimulation on PPF and LICI was also examined in the resting TA muscle. In unconditioned trials (Fig. 4C, filled circles), paired pulses of suprathreshold TMS produced a sequence of strong MEP facilitation (>200%) at PPF intervals of 10–40 ms followed by a suppression of test MEP responses at LICI intervals of 60–150 ms, as shown previously (Wassermann et al. 1996; Nakamura et al. 1997). When the second cortical stimulus (test stimulus) was conditioned by an appropriately timed TN stimulation, there was enhancement of the MEP response at select intervals from 40 to 80 ms (Fig. 4C, grey circles). When the size of the test MEP was matched in the conditioned trials (TS-ADJ, see legend), a two-way repeated measures ANOVA showed a significant interaction effect ‘condition’בISI’ (F12,84= 2.96, P < 0.005) showing that neuronal populations mediating PPF or LICI were affected by the afferent input (Fig. 4C, triangles). Post hoc paired t tests showed significant MEP facilitation at the 60 ms ISI (grey triangles) and half of the subjects still showed a > 20% increase at 70 and 80 ms. To test LICI at conditioning intensities that did not fully depress the MEP, the conditioning stimulus at 100 ms was decreased so that the MEP was depressed by only 40–60%. Using equal-sized test MEPs (Fig. 4D), there was no effect on LICI following the afferent stimulus (P= 0.6).
To estimate the effect of afferent excitation on the recruitment of cortical inputs producing the descending corticospinal volleys, or I-waves from TMS, we examined the effect of TN stimulation on MEP facilitation at the I-wave periodicity. TN stimulation facilitated MEP responses at ISIs of 1.5, 2.7 and 4.9 ms, probably corresponding to the peak I-wave activity (Fig. 5A). Facilitation persisted when the size of the test MEP was matched to levels without afferent stimulation (TS-ADJ, see legend). When the data were normalized to the test MEP (Fig. 5B, white bars), a two-way repeated measures ANOVA showed a significant ‘condition’ effect (F1,5= 15.06, P < 0.05), revealing that the TN stimulus modified the amount of MEP facilitation at the I-wave periodicity. Compared to the pre-TN condition (baseline), facilitation using the adjusted test MEP size was significantly enhanced at ISIs of 1.5, 2.7, 4.9 ms (all P < 0.05) with 5 out of 6 subjects showing an average > 20% increase. Intervals between the three presumed I-wave peaks (i.e. 2.1 and 3.7 ms) were non-significantly enhanced (all P > 0.09).
Figure 5. Effect of TN stimulation on I-wave facilitation.
Effect of TN inputs on MEP facilitation at the I-wave periodicity in the TA muscle. Experimental details are described in the caption of Fig. 4. Data are from 6 subjects. Asterisks indicate significant differences compared to baseline without TN stimulation (*P < 0.05, **P < 0.01).
Experiment 4: Effect of PTN stimulation on SOL MEPs
The profile of inhibition and facilitation of SOL MEPs following stimulation of the PTN at the knee was similar to that measured in the TA muscle in response to TN stimulation at the ankle. Figure 6A shows the effect of the PTN stimulus on SOL MEPs in all the subjects. In the averaged group data (Fig. 6Ba), there was significant effect of the peripheral input on the SOL MEPs (two-way ANOVA: F11,77= 5.61, P < 0.001) and MEPs were significantly increased at ISIs of 45–60 ms (P < 0.05). In Fig. 6Bb, a larger number of conditioning–test intervals between 24 and 40 ms were examined and there was a significant effect of the ‘ISI’ over these inhibitory intervals (one-way ANOVA: F9,36= 3.65, P < 0.005) with post hoc t tests showing significant MEP depression at intervals of 24–30 ms (P < 0.05). The facilitatory PTN input also altered the recruitment curve in the SOL muscle (Fig. 6C; two-way ANOVA: F10,70= 2.66, P < 0.01). Conditioning the SOL MEP at low TMS intensities, ranging from 88% to 96% RMT, significantly increased MEP amplitudes by 41 ± 13 μV on average (P < 0.05). In contrast, at high TMS intensities (≥ 116% RMT), MEPs in the SOL muscle tended to be slightly, but not significantly, depressed by the peripheral stimulus.
Discussion
In agreement with previous studies, MEP responses in lower limb muscles were both suppressed and increased by a preceding sensory nerve stimulus depending on the conditioning–test interval that was used (Deletis et al. 1992; Kasai et al. 1992; Nielsen et al. 1997). Similar time-dependent changes were observed in muscles with heteronymous (TN→TA and SOL) and homonymous (TN→AH; PTN→SOL) nerve activation. A novel finding was that the facilitation of corticospinal tract connections was only enhanced when activated at the cortex and not at the pyramidal decussation, supporting the evidence that MEP facilitation was cortical in origin. We further show that the MEP facilitation was associated with afferent-induced depression of intracortical inhibitory circuits (SICI) and facilitation of putative intracortical excitatory circuits (ICF). The present data further suggest that sensory afferents from the leg have access to excitatory cortical networks that synapse close to pyramidal tract neurons as evidenced from increases in paired pulse facilitation at early, as well as middle and late cycles of I-wave periodicity. Finally, responses elicited from stimulation to the motor cortex and brainstem by conditioning afferent inputs at short ISIs (near 35 ms) were depressed to a similar degree suggesting that afferent-induced depression of MEPs (i.e. SAI in leg) mainly occurs via a spinal mechanism. We discuss below that afferent inputs from the leg have a mainly excitatory and diffuse action on the leg area of the primary motor cortex.
Site of sensory afferent induced facilitation of the MEP
The evidence from direct corticospinal tract stimulation suggests that afferent-induced MEP facilitation at ISIs between 45 and 55 ms occurs through cortical mechanisms since responses were only facilitated when elicited over the motor cortex and not at the pyramidal decussation in the brainstem, which is thought to recruit similar descending corticospinal axons (Taylor et al. 2002). In fact, TN stimulation at an ISI near 50 ms markedly depressed the CMEP (Fig. 3B), and thus the magnitude of TN facilitation of descending volleys evoked from TMS over the motor cortex was probably underestimated because of the simultaneously occurring spinal inhibition. Similar decreases in evoked responses from transcranial electric stimulation have been reported when the sural nerve is stimulated 50 ms earlier (Nielsen et al. 1997). Further evidence supporting a lack of MEP facilitation by spinal networks at ISIs near 50 ms was provided by the lack of TA H-reflex facilitation by TN stimulation (Fig. 2B), assuming that there was some degree of overlap between H-reflex and corticospinal tract inputs onto TA motoneurons (Morita et al. 1999; Petersen et al. 2002). It is interesting that in several subjects there was an abrupt, but small, recovery in the size of the H-reflex at conditioning intervals that were 5–15 ms longer than the intervals that first produced the MEP facilitation. This increase probably involves contributions from the transcortical reflex loop (Nielsen et al. 1997) and requires several extra milliseconds for the afferent input to depolarize and activate pyramidal tract neurons. Finally, the latency of the MEP facilitation is also consistent with a cortical mechanism because the shortest conditioning–test interval that facilitated each subject's MEP was on average 4.1 ± 1.4 ms longer than the latency of the respective somatosensory evoked potential from TN stimulation (41.5 ms). This suggests the presence of an afferent pathway that passes through the somatosensory cortex to the motor cortex and corroborates a previously reported central processing delay of 4–10 ms (Nielsen et al. 1997; Petersen et al. 1998; Mrachacz-Kersting et al. 2007).
Site of sensory afferent induced inhibition of the MEP
With both TN and PTN stimulation, there was a period of MEP inhibition that preceded the facilitatory window analogous to SAI in the hand (Tokimura et al. 2000). However, unlike SAI in the hand, the inhibition of MEPs in leg muscles was probably not mediated by afferent inhibition of cortical networks given that TN stimulation equally depressed brainstem and cortically evoked responses in the TA muscle. SAI in the hand at an ISI of 20 ms is in line with a fast thalamo-cortical pathway (Tokimura et al. 2000; Kessler et al. 2005). If cortical in origin, inhibition of leg MEPs with TN stimulation occurring at ISIs near 35 ms would also have to go through a fast thalamo-cortical route because the latency for afferent inputs to the sensory cortex was 40 ms or more (see above). A spinal postsynaptic mechanism is more likely given recent findings where TMS and PTN inputs inhibit the SOL H-reflex to the same degree when delivered at an ISI of 30–40 ms (Poon et al. 2008). Because the amount of inhibition was the same for the two different conditioning presynaptic inputs, the common depression is likely to be occurring at a postsynaptic site. For instance, in conditioning of MEP responses, the motoneurons that are first activated by the peripheral stimulus are likely to be in a state of refractoriness when the descending corticospinal inputs arrive 30–40 ms later. Although the brainstem stimulation and H-reflex data provide strong arguments for a spinal mechanism, it is nonetheless possible that SAI might not be as prominent in the contracted muscle given that suppression of the TA MEP was less pronounced in the active muscle (–31 ± 5%) compared to rest (–55 ± 6%). Thus, it would be worthwhile to record from epidural electrodes over the spinal cord to examine whether any of the descending corticospinal volleys are in fact depressed by the leg afferents at these short ISIs (Tokimura et al. 2000).
Effect of afferent input on cortical circuits
The activation of cortical networks by afferent inputs from the leg was also demonstrated by the modification of SICI and ICF by TN stimulation. At an ISI that produced MEP facilitation in the TA muscle, SICI was decreased and ICF was increased, suggesting that increases in MEP responses by peripheral nerve stimulation were mediated by a decrease in the activation of inhibitory cortical networks and an increase in the activation of excitatory cortical networks. However, there has been some question as to whether ICF at 10 ms (used in this study) is in fact mediated by cortical networks given that descending volleys evoked from ICF at 10–15 ms are not altered despite increases in the size of the MEP (Di Lazzaro et al. 2006). Because the brainstem and H-reflex data show that the site of afferent-induced MEP facilitation in the leg is mainly cortical in origin, the fact that ICF was increased by afferent inputs suggest that the neurons mediating ICF at 10 ms are indeed cortical in origin.
When probing the excitability of neuronal populations mediating PPF and LICI, MEPs of large amplitude (∼1.5 mV) were produced at ISIs of 10–40 ms followed by inhibition starting at 60 ms. When comparing MEPs at intervals exhibiting maximal PPF, the afferent input produces little enhancement in the MEP response (except at 40 ms). This is potentially due to a ceiling effect as the recruitment curve data indicate that TA MEPs > 0.5 mV on average were not enhanced by the sensory input. At longer ISIs, the afferent excitation increased the size of the MEPs normally susceptible to long-interval inhibition. The observed MEP facilitation at the 60 ms ISI, evaluated using a matched test MEP, could have arisen either by a direct disinhibition of LICI networks or by a prolongation of the PPF window by the afferent input. To further examine this question, we tested LICI at a longer 100 ms ISI when the MEP was only partially depressed by the conditioning TMS pulse. In such a case, LICI was unchanged by the peripheral stimulus. Hence, it seems likely that continued activation of PPF neurons triggered from the first TMS pulse could have been potentiated by afferent inputs arriving more than 80 ms later to facilitate the second MEP. This result may also elucidate the underlying mechanism behind our previous findings where repetitively pairing cortical and peripheral nerve inputs in an intervention of paired-associative stimulation (PAS) facilitated MEPs in lower leg muscles when afferent inputs arrived at the motor cortex up to 90 ms after the TMS pulse (Roy et al. 2007).
Effect of afferent input on I-wave facilitation
In the upper and lower limb, the effect of afferent excitation on I-wave facilitation has not been reported previously. In the present study, afferent inputs potentiated the amount of MEP facilitation at early (1.5 ms), middle (2.9 ms) and late (4.9 ms) intervals of I-wave periodicity. On the basis that MEP facilitation reflects the periodicity of indirect waves (i.e. I1, I2, I3 waves) initiated by the first TMS pulse (Di Lazzaro et al. 1999; Hanajima et al. 2002), the facilitation of the MEP at an ISI of 1.5 ms suggests that afferent inputs synapse onto interneurons that can potentiate the earlier descending volleys. Excitatory inputs from hand afferents are likely to also activate interneurons that enhance the early I-wave cycle but this remains to be tested. So far only a reduction of I-wave facilitation at 1.5 ms has been reported following stimulation of the digit, which further depresses the MEP (Zittel et al. 2007). Similar to hand afferents, leg afferents also access more distant cortical interneurons as evidenced by MEP facilitation at 2.9 and 4.9 ms intervals and by the modification of SICI, which preferentially reduces the size of later I-wave inputs onto corticospinal neurons (Nakamura et al. 1997; Di Lazzaro et al. 1998; Hanajima et al. 1998).
Physiological considerations
We have shown that cortical excitability is enhanced using afferent stimulation from the lower leg in a manner consistent with the transcortical loop of the long-latency reflex. Interestingly, the afferent facilitation of the leg motor cortex was predominantly non-specific and diffuse, occurring from stimulation of mixed nerves supplying both homotopic (TN→AH; PTN→SOL) and distant heterotopic (TN→TA and SOL) muscles. This is in contrast to the upper limb where the facilitation of the motor cortex by peripheral nerve stimulation, even of mixed nerves, is more muscle specific (Classen et al. 2000; Aimonetti & Nielsen, 2001; Tamburin et al. 2001). Further studies are required to determine if more specific activation of leg muscle afferents by vibration produces more focal, or even inhibitory, activation of cortical networks as has been shown in the wrist and hand (Rosenkranz et al. 2003; Rosenkranz & Rothwell, 2003). Moreover, afferent activation of the leg motor cortex is predominantly excitatory, potentially mediated by both disinhibition and potentiation of excitatory intracortical circuits. In trauma such as spinal cord injury, much of this facilitation is likely to be abolished due to damage of ascending connections (Hayes et al. 1992). As afferent sensory input from the leg is important for lower limb motor function such as standing and walking (Thoumie & Do, 1996), motor dysfunction after spinal cord injury may result not only from disruption of descending efferents but also from a lack of afferent-induced facilitation of cortical motor networks. Thus, regulating the amount of sensorimotor integration may be important in enhancing motor rehabilitation following injury to the central nervous system. Based on the present results, SICI and ICF may be involved in shaping these changes when muscle afferent input is provided from the lower leg.
Acknowledgments
We thank the subjects who participated in the study. We appreciate the technical assistance of Dean Jeffery and thank Dr Richard Stein for helpful comments and discussions. This research was funded by the Canadian Institute for Health Research and the Alberta Heritage Foundation for Medical Research. F.D.R. is supported by a postgraduate scholarship from the Natural Sciences and Engineering Research Council of Canada.
References
- Aimonetti JM, Nielsen JB. Changes in intracortical excitability induced by stimulation of wrist afferents in man. J Physiol. 2001;534:891–902. doi: 10.1111/j.1469-7793.2001.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau MJ, Smith AM. Activity in rostral motor cortex in response to predictable force-pulse perturbations in a precision grip task. J Neurophysiol. 2001;86:1079–1085. doi: 10.1152/jn.2001.86.3.1079. [DOI] [PubMed] [Google Scholar]
- Brasil-Neto JP, Cohen LG, Pascual-Leone A, Jabir FK, Wall RT, Hallett M. Rapid reversible modulation of human motor outputs after transient deafferentation of the forearm: a study with transcranial magnetic stimulation. Neurology. 1992;42:1302–1306. doi: 10.1212/wnl.42.7.1302. [DOI] [PubMed] [Google Scholar]
- Chen R. Interactions between inhibitory and excitatory circuits in the human motor cortex. Exp Brain Res. 2004;154:1–10. doi: 10.1007/s00221-003-1684-1. [DOI] [PubMed] [Google Scholar]
- Chen R, Cohen LG, Hallett M. Nervous system reorganization following injury. Neuroscience. 2002;111:761–773. doi: 10.1016/s0306-4522(02)00025-8. [DOI] [PubMed] [Google Scholar]
- Chen R, Corwell B, Hallett M. Modulation of motor cortex excitability by median nerve and digit stimulation. Exp Brain Res. 1999;129:77–86. doi: 10.1007/s002210050938. [DOI] [PubMed] [Google Scholar]
- Chen R, Garg R. Facilitatory I wave interaction in proximal arm and lower limb muscle representations of the human motor cortex. J Neurophysiol. 2000;83:1426–1434. doi: 10.1152/jn.2000.83.3.1426. [DOI] [PubMed] [Google Scholar]
- Christensen LO, Petersen N, Andersen JB, Sinkjaer T, Nielsen JB. Evidence for transcortical reflex pathways in the lower limb of man. Prog Neurobiol. 2000;62:251–272. doi: 10.1016/s0301-0082(00)00007-1. [DOI] [PubMed] [Google Scholar]
- Classen J, Steinfelder B, Liepert J, Stefan K, Celnik P, Cohen LG, Hess A, Kunesch E, Chen R, Benecke R, Hallett M. Cutaneomotor integration in humans is somatotopically organized at various levels of the nervous system and is task dependent. Exp Brain Res. 2000;130:48–59. doi: 10.1007/s002210050005. [DOI] [PubMed] [Google Scholar]
- Deletis V, Schild JH, Beric A, Dimitrijevic MR. Facilitation of motor evoked potentials by somatosensory afferent stimulation. Electroencephalogr Clin Neurophysiol. 1992;85:302–310. doi: 10.1016/0168-5597(92)90106-l. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Pennisi MA, Di Giovanni S, Zito G, Tonali P, Rothwell JC. Muscarinic receptor blockade has differential effects on the excitability of intracortical circuits in the human motor cortex. Exp Brain Res. 2000;135:455–461. doi: 10.1007/s002210000543. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Dileone M, Profice P, Ranieri F, Ricci V, Bria P, Tonali PA, Ziemann U. Segregating two inhibitory circuits in human motor cortex at the level of GABAA receptor subtypes: a TMS study. Clin Neurophysiol. 2007;118:2207–2214. doi: 10.1016/j.clinph.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Oliviero A, Dileone M, Saturno E, Mazzone P, Insola A, Profice P, Ranieri F, Capone F, Tonali PA, Rothwell JC. Origin of facilitation of motor evoked potentials after paired magnetic stimulation: direct recording of epidural activity in conscious humans. J Neurophysiol. 2006;96:1765–1771. doi: 10.1152/jn.00360.2006. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC. Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Exp Brain Res. 1998;119:265–268. doi: 10.1007/s002210050341. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Rothwell JC, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P. Intracortical origin of the short latency facilitation produced by pairs of threshold magnetic stimuli applied to human motor cortex. Exp Brain Res. 1999;129:494–499. doi: 10.1007/s002210050919. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Enomoto H, Shiio Y, Mochizuki H, Furubayashi T, Uesugi H, Iwata NK, Kanazawa I. Mechanisms of intracortical I-wave facilitation elicited with paired-pulse magnetic stimulation in humans. J Physiol. 2002;538:253–261. doi: 10.1113/jphysiol.2001.013094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Sakai K, Furubayashi T, Machii K, Kanazawa I. Paired-pulse magnetic stimulation of the human motor cortex: differences among I waves. J Physiol. 1998;509:607–618. doi: 10.1111/j.1469-7793.1998.607bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauck M, Baumgartner U, Hille E, Hille S, Lorenz J, Quante M. Evidence for early activation of primary motor cortex and SMA after electrical lower limb stimulation using EEG source reconstruction. Brain Res. 2006;1125:17–25. doi: 10.1016/j.brainres.2006.09.041. [DOI] [PubMed] [Google Scholar]
- Hayes KC, Allatt RD, Wolfe DL, Kasai T, Hsieh J. Reinforcement of subliminal flexion reflexes by transcranial magnetic stimulation of motor cortex in subjects with spinal cord injury. Electroencephalogr Clin Neurophysiol. 1992;85:102–109. doi: 10.1016/0168-5597(92)90075-m. [DOI] [PubMed] [Google Scholar]
- Jacobs KM, Donoghue JP. Reshaping the cortical motor map by unmasking latent intracortical connections. Science. 1991;251:944–947. doi: 10.1126/science.2000496. [DOI] [PubMed] [Google Scholar]
- Kasai T, Hayes KC, Wolfe DL, Allatt RD. Afferent conditioning of motor evoked potentials following transcranial magnetic stimulation of motor cortex in normal subjects. Electroencephalogr Clin Neurophysiol. 1992;85:95–101. doi: 10.1016/0168-5597(92)90074-l. [DOI] [PubMed] [Google Scholar]
- Kessler KR, Ruge D, Ilic TV, Ziemann U. Short latency afferent inhibition and facilitation in patients with writer's cramp. Mov Disord. 2005;20:238–242. doi: 10.1002/mds.20295. [DOI] [PubMed] [Google Scholar]
- Knash ME, Kido A, Gorassini M, Chan KM, Stein RB. Electrical stimulation of the human common peroneal nerve elicits lasting facilitation of cortical motor-evoked potentials. Exp Brain Res. 2003;153:366–377. doi: 10.1007/s00221-003-1628-9. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajoie Y, Teasdale N, Cole JD, Burnett M, Bard C, Fleury M, Forget R, Paillard J, Lamarre Y. Gait of a deafferented subject without large myelinated sensory fibers below the neck. Neurology. 1996;47:109–115. doi: 10.1212/wnl.47.1.109. [DOI] [PubMed] [Google Scholar]
- Lemon RN. Functional properties of monkey motor cortex neurones receiving afferent input from the hand and fingers. J Physiol. 1981;311:497–519. doi: 10.1113/jphysiol.1981.sp013601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita H, Baumgarten J, Petersen N, Christensen LO, Nielsen J. Recruitment of extensor-carpi-radialis motor units by transcranial magnetic stimulation and radial-nerve stimulation in human subjects. Exp Brain Res. 1999;128:557–562. doi: 10.1007/s002210050881. [DOI] [PubMed] [Google Scholar]
- Mrachacz-Kersting N, Fong M, Murphy BA, Sinkjaer T. Changes in excitability of the cortical projections to the human tibialis anterior following paired associative stimulation. J Neurophysiol. 2007;97:1951–1958. doi: 10.1152/jn.01176.2006. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H. Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. J Physiol. 1997;498:817–823. doi: 10.1113/jphysiol.1997.sp021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J, Petersen N, Fedirchuk B. Evidence suggesting a transcortical pathway from cutaneous foot afferents to tibialis anterior motoneurones in man. J Physiol. 1997;501:473–484. doi: 10.1111/j.1469-7793.1997.473bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez MA, Lungholt BK, Nyborg K, Nielsen JB. Motor skill training induces changes in the excitability of the leg cortical area in healthy humans. Exp Brain Res. 2004;159:197–205. doi: 10.1007/s00221-004-1947-5. [DOI] [PubMed] [Google Scholar]
- Petersen N, Christensen LO, Morita H, Sinkjaer T, Nielsen J. Evidence that a transcortical pathway contributes to stretch reflexes in the tibialis anterior muscle in man. J Physiol. 1998;512:267–276. doi: 10.1111/j.1469-7793.1998.267bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen NT, Taylor JL, Gandevia SC. The effect of electrical stimulation of the corticospinal tract on motor units of the human biceps brachii. J Physiol. 2002;544:277–284. doi: 10.1113/jphysiol.2002.024539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Burke D. The Circuitry of the Human Spinal Cord: Its Role in Motor Control and Movement Disorders. Cambridge: Cambridge University Press; 2005. pp. 81–83. [Google Scholar]
- Poon DE, Roy FD, Gorassini MA, Stein RB. Interaction of paired cortical and peripheral nerve stimulation on human motor neurons. Exp Brain Res. 2008;188:13–21. doi: 10.1007/s00221-008-1334-8. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Brouwer B, Miles TS, Pitcher JB, Thompson PD. Changes in muscle responses to stimulation of the motor cortex induced by peripheral nerve stimulation in human subjects. Exp Brain Res. 2000;131:135–143. doi: 10.1007/s002219900269. [DOI] [PubMed] [Google Scholar]
- Rosenkranz K, Pesenti A, Paulus W, Tergau F. Focal reduction of intracortical inhibition in the motor cortex by selective proprioceptive stimulation. Exp Brain Res. 2003;149:9–16. doi: 10.1007/s00221-002-1330-3. [DOI] [PubMed] [Google Scholar]
- Rosenkranz K, Rothwell JC. Differential effect of muscle vibration on intracortical inhibitory circuits in humans. J Physiol. 2003;551:649–660. doi: 10.1113/jphysiol.2003.043752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell JC, Rosenkranz K. Role of afferent input in motor organization in health and disease. IEEE Eng Med Biol Mag. 2005;24:40–44. doi: 10.1109/memb.2005.1384099. [DOI] [PubMed] [Google Scholar]
- Rothwell JC, Traub MM, Day BL, Obeso JA, Thomas PK, Marsden CD. Manual motor performance in a deafferented man. Brain. 1982;105:515–542. doi: 10.1093/brain/105.3.515. [DOI] [PubMed] [Google Scholar]
- Roy FD, Gorassini MA. Effect of peripheral sensory input on excitability in the human leg motor cortex. Lake Louise, AB, Canada: Proc Canadian Physiol Soc; 2008. p. 23. [Google Scholar]
- Roy FD, Norton JA, Gorassini MA. Role of sustained excitability of the leg motor cortex after transcranial magnetic stimulation in associative plasticity. J Neurophysiol. 2007;98:657–667. doi: 10.1152/jn.00197.2007. [DOI] [PubMed] [Google Scholar]
- Sailer A, Molnar GF, Cunic DI, Chen R. Effects of peripheral sensory input on cortical inhibition in humans. J Physiol. 2002;544:617–629. doi: 10.1113/jphysiol.2002.028670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C, Devanne H, Lavoie BA, Capaday C. Neural mechanisms involved in the functional linking of motor cortical points. Exp Brain Res. 2002;146:86–94. doi: 10.1007/s00221-002-1137-2. [DOI] [PubMed] [Google Scholar]
- Sanes JN, Mauritz KH, Dalakas MC, Evarts EV. Motor control in humans with large-fiber sensory neuropathy. Hum Neurobiol. 1985;4:101–114. [PubMed] [Google Scholar]
- Sinkjaer T, Andersen JB, Ladouceur M, Christensen LO, Nielsen JB. Major role for sensory feedback in soleus EMG activity in the stance phase of walking in man. J Physiol. 2000;523:817–827. doi: 10.1111/j.1469-7793.2000.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamburin S, Manganotti P, Zanette G, Fiaschi A. Cutaneomotor integration in human hand motor areas: somatotopic effect and interaction of afferents. Exp Brain Res. 2001;141:232–241. doi: 10.1007/s002210100859. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Gandevia SC. Noninvasive stimulation of the human corticospinal tract. J Appl Physiol. 2004;96:1496–1503. doi: 10.1152/japplphysiol.01116.2003. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Petersen NT, Butler JE, Gandevia SC. Interaction of transcranial magnetic stimulation and electrical transmastoid stimulation in human subjects. J Physiol. 2002;541:949–958. doi: 10.1113/jphysiol.2002.016782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoumie P, Do MC. Changes in motor activity and biomechanics during balance recovery following cutaneous and muscular deafferentation. Exp Brain Res. 1996;110:289–297. doi: 10.1007/BF00228559. [DOI] [PubMed] [Google Scholar]
- Tokimura H, Di Lazzaro V, Tokimura Y, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P, Rothwell JC. Short latency inhibition of human hand motor cortex by somatosensory input from the hand. J Physiol. 2000;523:503–513. doi: 10.1111/j.1469-7793.2000.t01-1-00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann EM, Samii A, Mercuri B, Ikoma K, Oddo D, Grill SE, Hallett M. Responses to paired transcranial magnetic stimuli in resting. active. and recently activated muscles. Exp Brain Res. 1996;109:158–163. doi: 10.1007/BF00228638. [DOI] [PubMed] [Google Scholar]
- Yang JF, Stein RB. Phase-dependent reflex reversal in human leg muscles during walking. J Neurophysiol. 1990;63:1109–1117. doi: 10.1152/jn.1990.63.5.1109. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Tergau F, Wassermann EM, Wischer S, Hildebrandt J, Paulus W. Demonstration of facilitatory I wave interaction in the human motor cortex by paired transcranial magnetic stimulation. J Physiol. 1998;511:181–190. doi: 10.1111/j.1469-7793.1998.181bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zittel S, Baumer T, Liepert J. Modulation of intracortical facilitatory circuits of the human primary motor cortex by digital nerve stimulation. Exp Brain Res. 2007;176:425–431. doi: 10.1007/s00221-006-0624-2. [DOI] [PubMed] [Google Scholar]