Abstract
Propulsion in both small and large intestine is largely mediated by the peristaltic reflex; despite this, transit through the shorter colon is at least 10 times slower. Recently we demonstrated that elongating a segment of colon releases nitric oxide (NO) to inhibit peristalsis. The aims of this study were to determine if colonic elongation was physiologically significant, and whether elongation activated polarized intrinsic neural reflexes. Video imaging monitored fecal pellet evacuation from isolated guinea-pig colons full of pellets. Recordings were made from the circular muscle (CM) and longitudinal muscle (LM) in flat sheet preparations using either intracellular microelectrode or Ca2+ imaging techniques. Full colons were 158.1 ± 6.1% longer than empty colons. As each pellet was expelled, the colon shortened and pellet velocity increased exponentially (full 0.34, empty 1.01 mm s−1). In flat sheet preparations, maintained circumferential stretch generated ongoing peristaltic activity (oral excitatory and anal inhibitory junction potentials) and Ca2+ waves in LM and CM. Colonic elongation (140% of its empty slack length) applied oral to the recording site abolished these activities, whereas anal elongation significantly increased the frequency and amplitude of ongoing peristaltic activity. Oral elongation inhibited the excitation produced by anal elongation; this inhibitory effect was reversed by blocking NO synthesis. Pelvic nerve stimulation elicited polarized responses that were also suppressed by NO released during colonic elongation. In conclusion, longitudinal stretch excites specific mechosensitive ascending and descending interneurons, leading to activation of polarized reflexes. The dominance of the descending inhibitory reflex leads to slowed emptying of pellets in a naturally elongated colon.
In guinea-pigs the proximal colon has a viscous liquid content, whereas the distal colon contains fecal pellets that are generated in the colonic flexure between the proximal and distal colon (see D'Antona et al. 2001). The isolated guinea-pig proximal colon exhibits both orally and aborally propagating waves necessary for mixing, whereas the distal colon generates intrinsic neural reflexes that underlie fecal pellet propulsion (Crema et al. 1970; Costa & Furness, 1976; Smith & McCarron, 1998; Smith & Robertson, 1998; Jin et al. 1999. Stevens et al. 2000; D'Antona et al. 2001). Interestingly, when the guinea-pig distal colon contains several fecal pellets emptying appears to be slowed, a phenomenon that has been attributed to descending inhibition generated by the radial stretch of more orally situated pellets (D'Antona et al. 2001), or as we have proposed to the activation of an intrinsic inhibitory reflex triggered specifically by elongation (Dickson et al. 2007).
We have shown that maintained circumferential stretch applied to a region of an isolated segment of guinea-pig distal colon generates ongoing excitatory (EJPs) and inhibitory (IJPs) junction potentials at both the oral and anal ends of the stretched region, respectively. The oral EJPs and anal IJPs are coordinated in both time and amplitude and occur simultaneously in both the longitudinal (LM) and circular muscle (CM) layers (Spencer et al. 2002, 2003, 2006; Dickson et al. 2007). For the oral EJP and anal IJP to be synchronized the excitatory and inhibitory muscle motor neurons at opposite ends of the stretched region must discharge at the same time, suggesting that activity in interneurons forming ascending excitatory and descending inhibitory nerve pathways are coordinated (Smith et al. 2007a,b; Spencer et al. 2006). We initially suspected that myenteric after-hyperpolarizing (AH) neurons would drive this activity, since it has been generally assumed that they are the only intrinsic sensory neuron or ‘intrinsic primary afferent neuron’ in the intestine of many different mammals (IPANs; see Hirst et al. 1974, 1975; Brookes et al. 1987, 1988; Furness et al. 1998; Lomax et al. 1999; Cornelissen et al. 2000, 2001; Mao et al. 2006). In fact, we found myenteric AH neurons in the guinea-pig colon to be electrically silent in these stretched preparations that exhibited ongoing peristaltic activity where electrical recordings were facilitated by paralysing the muscle with nifedipine (L-type Ca2+ channel antagonisti; Spencer & Smith, 2004). We found that the ongoing peristaltic activity appeared to be generated by mechanosenitive ascending and descending S (receive fast synaptic input) type interneurons that are stretch sensitive (Spencer & Smith, 2004; Spencer et al. 2006; Dickson et al. 2007; Smith et al. 2007b). It is perhaps not surprising that AH neurons in these paralysed colonic preparations exhibit a lack of spontaneous activity since they have been shown to respond largely to increases in muscle force (tension) generated by both stretch and contraction rather than changes in length per se (Kunze et al. 1998, 1999; see Smith et al. 2007b). Results from earlier studies did suggest that there was more than one class of intrinsic sensory neuron, at least in the guinea-pig intestine (Smith et al. 1991, 1992; see Smith et al. 2007b), and possibly sensory neurons with electrophysiological properties that differ from AH neurons in other gut regions or in similar regions in other species (Schemann & Wood, 1989; De Laet et al. 2002; Blackshaw et al. 2007).
In addition, we have also recently reported that another intrinsic neural reflex exists in the colon that is activated by longitudinal stretch (elongation) rather than circumferential stretch. Elongating circumferentially stretched preparations abolishes ongoing peristaltic reflex activity (Dickson et al. 2007; Smith et al. 2007a,b). We have called this reflex activated by colonic elongation an intrinsic ‘occult reflex’ (hidden from view) since it does not produce an output to the muscle but rather suppresses the ongoing peristaltic activity. Colonic elongation appears to cause the release of nitric oxide (NO) from interneurons that doesnD't directly relax the muscle but rather suppresses other mechanosensitive interneurons responsible for activating excitatory and inhibitory motor neurons underlying the peristaltic reflex (Dickson et al. 2007).
The mechanisms underlying colonic accommodation and slow transit are poorly understood (Phillips, 1984). In this study, we demonstrate that colonic elongation has true physiological significance since colons full of pellets are naturally elongated and as a consequence fecal pellet evacuation from the guinea-pig distal colon is slowed. Also, we report that the reflex pathways activated by colonic elongation are more complex than we previously thought since they are polarized. Furthermore, we also show that the polarized responses (oral EJP and anal IJP) to pelvic nerve stimulation are also suppressed by colonic elongation.
Methods
Animals
Male guinea pigs (200–350 g) were handled with care, then placed in a specially constructed chamber and killed by a rising concentration of CO2 followed by exsanguination. A midline incision was made and the distal colon removed between the flexure region and the rectum (i.e. 2 cm above the anus). These procedures were in accordance with National Institutes of Health guidelines and approved by the Animal Ethics Committee at the University of Nevada, Reno.
Video analysis of pellet movement
A segment of distal colon (∼100 mm long) filled with pellets was inserted into a glass tube (120 mm long, 6 mm diameter) to prevent the colon flexing during pellet expulsion. The colon was pinned at the oral end while the anal end was left free. The tube, which was drilled with holes to allow for oxygenation, was anchored to the base of an organ bath that was continuously perfused with oxygenated Krebs solution at 36.0 ± 0.5°C. The movements of fecal pellets and the length of the colon were viewed with a video camera (WV-BP330; Panasonic CCTV) and recorded directly to computer (PowerMac G4, Apple) and analysed using previously described methods (Hennig et al. 1999; D'Antona et al. 2001; Dickson et al. 2007).
Exit velocities in an elongated colon were normalized to the length of an empty colon following expulsion of all pellets. This normalization procedure takes into account the relative distance travelled by a fecal pellet under different levels of colonic elongation. For example, a pellet travelling the whole length of an elongated piece of colon (150% of slack length, e.g. 100 mm) at a measured velocity of 1 mm s −1 is considered to have the same relative velocity as a pellet travelling the whole length of an unstretched piece of colon (100% of slack length, e.g. 6.6 cm) at a measured velocity of 0.66 mm s −1. Measured velocities in stretched preparations were converted to relative velocities using the formula:
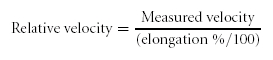 |
Preparation used for intracellular recording and Ca2+ imaging
A 6 cm-long segment of distal colon was cut open along the mesenteric border in the middle of the segment (20 mm long) and this region pinned either circumferentially (10–12 mm wide) or unstretched. In some experiments the tissue was threaded through two greased partitions (7 mm apart), which divided the organ bath into three chambers that were separately perfused with warm oxygenated Krebs solution. This allowed drugs to be selectively added to the middle third of the preparation (Dickson et al. 2007). Multiple small pins (diameter = 100 μm) were inserted into the preparation along the outer edges of the stretched region so as to avoid mechanical interactions between the recording sites and the portion of the colon that was longitudinally stretched. In other experiments a small balloon (Fogarty Embolectomy Catheter 0.2 cc; Irvine Life Sciences, Irvine, CA, USA), was inserted approximately 10 mm from the nearest recording site at both the oral and anal ends of the intact segment, and was inflated with a syringe (300 μl; Hamilton Industries, Reno, NV, USA). Longitudinal stretch (colonic elongation) was applied to either the oral or anal intact tubes or to either end of an opened flat sheet preparation using a pulley system to which weights were added. We use the term colonic elongation throughout the manuscript as a way of describing mechanical stretch in the longitudinal axis. The mucosa and submucosa were then removed from the opened region by sharp dissection. The dissected region was pinned serosal side down in a Sylgard (Dow Corning) lined organ bath, which was perfused with warmed oxygenated Krebs solution (36.0 ± 0.5°C) containing nifedipine (1–2 μm) to paralyse the smooth muscle. When pinned out in the recording bath, the preparations had a circumferential axis measurement of 10–12 mm and an overall length of 50 mm.
Intracellular recordings
Simultaneous microelectrode recordings were made from CM cells at both the oral and the anal ends of the stretched middle segment (20 mm long, 10–12 mm wide) as previously described (Dickson et al. 2007).
Calcium imaging of muscle layers
Thin strips of longitudinal muscle (LM) were removed from a segment of colon to expose the underlying CM. The preparation was opened and the two edges pinned in the middle to mimic the diameter of a fecal pellet. The preparation was stretched at either the oral or the anal end using a rake system that was attached via a thread to a pulley system to which weights could be added (Dickson et al. 2007). Both the LM and CM in the preparation were loaded with the Ca2+ indicator Fluo-4 (50 nm) and viewed under 20× water immersion objectives. Regions of interest (ROI) were selected over each muscle layer so that its characteristic Ca2+ wave activity muscle could be recorded as previously described (Stevens et al. 2000; Dickson et al. 2007; Lee et al. 2007). At this magnification, Ca2+ wave activity in the muscle, has a characteristic propagation pattern specific to the orientation of the longitudinal and circular smooth muscle cells, and has a far greater intensity than that observed in myenteric ganglia or ICC (Stevens et al. 2000; Dickson et al. 2007; Lee et al. 2007).
Pelvic nerve stimulation
The method described by Gillespie (1962) was used to dissect out a 20 mm-long segment of colon that was stretched in the circumferential axis (10–12 mm) with the attached pelvic and perivascular nerves. Single shot focal electrical field stimulation (0.5 ms, 40–50 V) was applied to the pelvic nerves with silver chloride wires (250 μm apart) placed across and near the cut ends of the nerves, using a Grass SD9 stimulator (Grass Instruments, Quincy, MA, USA). Following each experiment the pelvic nerves were cut directly adjacent to the partition and restimulated to ensure that the responses in the preparation were not due to stimulus spread.
Drugs and solutions
Hexamethonium was used to block nicotinic transmission, nifedipine was used to paralyse the muscle, Nω-nitro-l-arginine (l-NA; 100 μm) was used to block NO synthesis and tetrodotoxin (TTX) was used to block conduction along nerves (Sigma). The Krebs solution contained (mm): NaCl, 120.35; KCl, 5.9; NaHCO3, 15.5; NaH2PO4, 1.2; MgSO4, 1.2; CaCl2, 2.5; and glucose, 11.5 (gassed with 3% CO2–97% O2, pH 7.3–7.4).
Statistical analysis
Analysis of spontaneous junction potentials (sJPs) was performed using custom-written routines as previously described (Spencer & Smith, 2001, 2003; Spencer & Smith, 2004,2006; Dickson et al. 2007). Regression analysis was used to summarize the variability in the amplitude of JPs between the two recordings and was expressed as the correlation coefficient, r2. All results are expressed as means ±s.e.m. The use of n in Results refers to the number of animals on which observations were made. Measurements of amplitude and half-width, and time to peak response were made using Axoscope 8.0 (Axon Instruments, Union City, CA, USA). One-way and two-way factorial analysis of variance statistical tests were used to compare differences between groups. Scheffé's post hoc test was used. Student's paired t test was also used where appropriate. A minimum significance level of P < 0.05 was used throughout.
Results
Changes in colonic length during evacuation of pellets
Propulsion of a fecal pellet along the isolated guinea-pig distal colon is dependent upon intrinsic neural reflexes, since it was blocked by tetrodotoxin (1 μm; n= 3) and hexamethonium (100 μm; n= 3) (Jin et al. 1999; D'Antona et al. 2001).
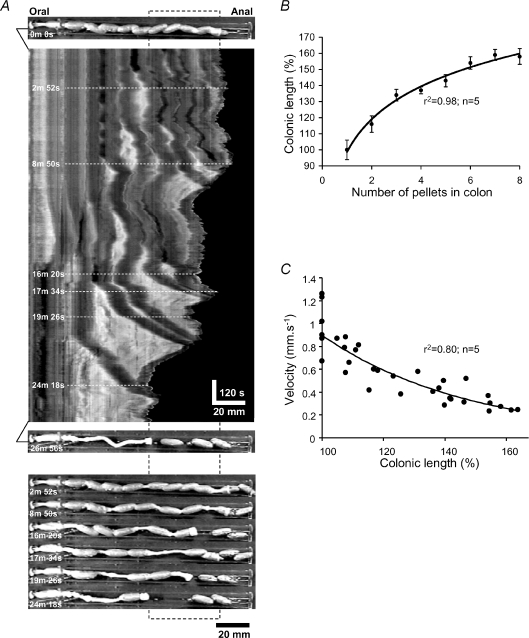
When an isolated segment of distal colon full of fecal pellets was allowed to spontaneously evacuate its contents, it took 16.8 ± 1.4 min (n= 5) to expel all its fecal pellets (8.8 ± 0.4 pellets; Fig. 1A). In contrast, the time it takes for a single pellet with a velocity of 1.1 mm s−1 (see below) to traverse the empty colon is only ∼55 s. A colon that was filled with fecal pellets was 158.1 ± 6.1% (n= 8) longer than a slack colon that had expelled all its pellets. As each pellet was evacuated from the anal end, the colon recoiled back to a shorter resting length (Fig. 1A). In fact, the colon shortened in a logarithmic manner with the evacuation of each pellet (Fig. 1B). Pellets were expelled periodically (every 143.3 ± 22.0 s, n= 5) from the distal colon, sometimes in groups of two or three (Fig. 1A). Also, as each pellet was expelled from the colon the velocity of expulsion of the next pellet increased in an exponential manner (Fig. 1C). The first pellet to evacuate from the filled colon had a velocity of 0.3 ± 0.1 mm s−1, whereas the last pellet to evacuate had a significantly higher velocity of 1.0 ± 0.1 mm s−1 (P < 0.01; n= 5). During expulsion the contraction (*) behind the pellet caused the colon to transiently lengthen (compare top image 0 min 0 s with last image at 26 min 56 s). This was likely to be due to mechanical interactions between the two muscle layers (see Hennig et al. 1999) since the intrinsic neural circuit is ‘wired’ to ensure that both muscle layers either contract together or relax together (Smith & Robertson, 1998; Spencer & Smith, 2001). Also, in a full colon there appeared to be little tone in the LM and CM, evident by overlapping pellets (compare top and bottom frames in Fig. 1A). The average maximal diameter of a full colon was 5.9 ± 0.1 mm, which was significantly greater than that of an empty colon 3.6 ± 0.1 mm (P≤ 0.05; n= 5) and greater than the mid diameter of a fecal pellet (4.6 ± 0.1 mm) that had an average length 14.4 ± 0.7 mm.
Figure 1. Expulsion of fecal pellets from a full, isolated segment of distal colon.
A, spatio-temporal map (ST Map) of spontaneous emptying of fecal pellets from a full colon; dark areas show the dilated regions of colon produced by fecal pellets. Lower panels show images of pellet expulsion at different times, which correspond to those on the ST Map (dashed lines). B, there is a logarithmic relationship between the number of pellets in a full colon and its overall length (y= 29.5ln(x) + 98.5). The correlation between the raw data and the logarithmic curve fit was r2= 0.98 (P < 0.001; n= 5). C, as pellets were expelled sequentially from the full colon, colonic shortening was associated with an exponential (y= 0.9e−0.02x) increase in pellet velocity; the correlation between the raw data and the exponential curve fit was r2= 0.80 (P < 0.001; n= 5).
Ongoing peristaltic reflex activity generated by circumferential stretch
We have shown previously that if a region (20 mm long) of isolated guinea-pig distal colon without the mucosa and submucosal plexus is maintained under circumferential stretch it generates ongoing peristaltic reflex activity consisting of ongoing, coordinated EJPs and IJPs at the oral and anal ends of the preparation, respectively (Fig. 2A: Spencer et al. 2002, 2003, 2004, 2006).
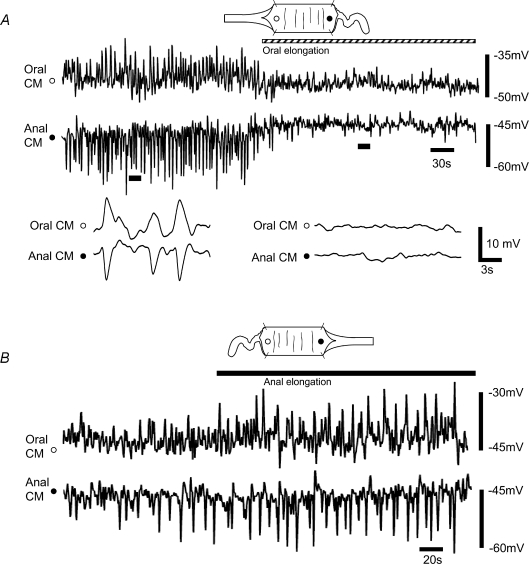
Figure 2. Polarized nervous reflexes generated by colonic elongation.
A, the polarized peristaltic reflex is activated by circumferential stretch, giving a synchronized oral EJP and anal IJP in the CM (see expanded traces). When elongation was applied to the oral end of the colon under circumferential stretch, the oral EJP and anal IJP were depressed. Open and filled circles indicate oral and anal recording sites in the CM, respectively. B, when the preparation was elongated in the anal direction there was a significant increase in amplitude and frequency of firing of the coordinated junction potentials.
Polarized nervous reflexes generated by colonic elongation
We have previously reported that the addition of maintained longitudinal stretch to these preparations suppresses this ongoing peristaltic activity activated by circumferential stretch (Dickson et al. 2007; Smith et al. 2007a,b). However, whether colonic elongation also exhibits a polarized response has not been determined.
Following expulsion of all pellets from a full colon it was observed to have shortened by 40% of its original full length, and therefore in the experiments described below we applied a 40% increase in the longitudinal length to either the oral or the anal end of the isolated segment of distal colon in order to determine whether elongation evokes polarized reflex responses.
Control activity
When the middle (20 mm long) of the preparation was stretched circumferentially it generated ongoing peristaltic reflex activity, i.e. oral EJPs coordinated with anal IJPs in the CM, even though muscle tone was abolished with nifedipine (1 μm; Fig. 2B: Spencer et al. 2002, 2003, 2006).
Oral elongation with applied circumferential stretch
Colonic elongation (to 140% of its slack length) of the intact tube applied oral of the recording sites caused an immediate suppression of oral EJPs and anal IJPs in CM (Fig. 2A). Junction potentials (oral EJPs and anal IJPs) were uncorrelated (Fig. 3A and D) and depressed to within the noise (Fig. 3D and G). This descending ‘occult’ inhibitory reflex also did not appear to fatigue since the suppression of activity also lasted for the duration of the applied stimulus (up to 20 min tested). The power of this inhibitory reflex is supported by previous studies where we showed that pulling on an oral segment of colon slows fecal pellet propulsion (Dickson et al. 2007).
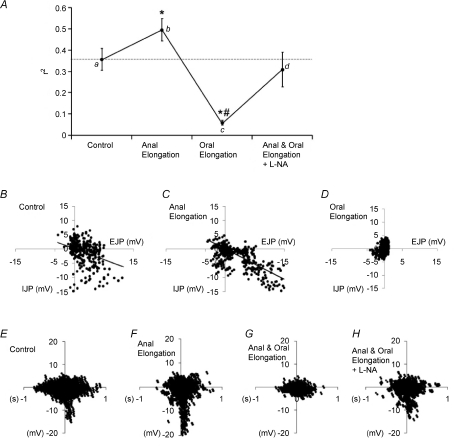
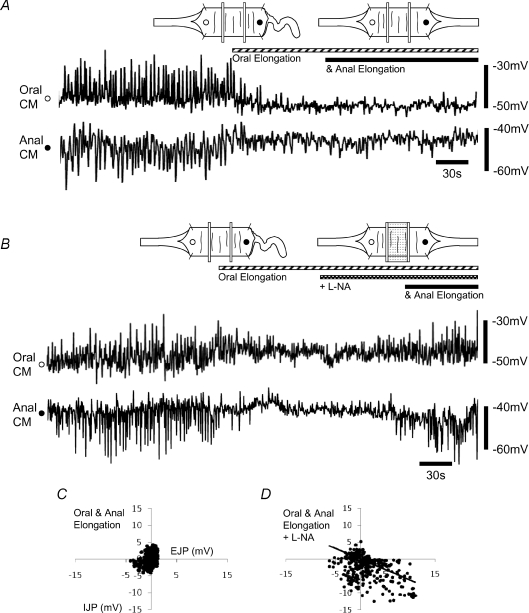
Figure 3. Correlation between EJPs and IJPs following anal and oral stretch.
A, correlation coefficient (r2; n= 6) between an oral EJP and its corresponding anal IJP evoked by circumferential stretch. a, under circumferential stretch only. b, following the addition of anal elongation (to 140% of slack length). c, following the further addition of oral elongation (140% of slack length). d, plus the addition l-NA (100 μm) to the middle chamber. Significance level: *P < 0.05 compared to control; #P < 0.001 compared to anal elongation. B, amplitude of the oral EJP plotted against the corresponding anal IJP. C, following the addition of anal elongation (140%; n= 6). D, following the further addition of oral stretch (140%). E–H, the temporal coordination between an oral EJP and its corresponding IJP (sum of n= 6 preparations). E, control, during only circumferential stretch. F, following the addition of anal elongation; note the narrowing of the ‘funnel’ indicating higher temporal correlation. G, following the further addition of oral elongation. H, following the further addition of l-NA (100 μm).
Anal elongation with applied circumferential stretch
When the intact segment of colon was elongated to 140% of its slack length anal to the recording site, there was an immediate and significant increase in the amplitude between coordinated oral EJPs and anal IJPs (control EJP 8.55 ± 0.34, IJP 8.27 ± 0.52 mV; following anal elongation EJP 10.98 ± 0.43, IJP 11.76 ± 0.63 mV; P < 0.05, n= 6) for as long as the elongation was maintained (tested up to 20 min; Fig. 2B). Furthermore, elongation also decreased the interval (increased frequency) between successive EJP–IJP complexes (time interval: control 3.5 ± 0.18 s; following anal elongation 2.65 ± 0.11 s; P < 0.05; n= 6). Further analysis of the stretch evoked ongoing junction potentials in response to anal elongation revealed that not only did the amplitudes of the oral EJP and anal IJP significantly increase (compare Fig. 3A and C) but also that the events became more tightly correlated in time, i.e. the time between an individual oral EJP and an anal IJP was decreased (compare Fig. 3E and F). This tight coupling is illustrated by the narrowing of the funnel in the scatter plots (Fig. 3F).
There was found to be no significant change in resting membrane potential of circular muscle cells after either oral or anal elongation (control: oral electrode −44.7 ± 2.4 mV, anal electrode −45.3 ± 3.1 mV; stretch: −45.7 ± 1.9 mV, anal electrode −43.9 ± 2.5 mV, P < 0.01, n = 6).
Effect of nicotinic antagonist on intrinsic polarized reflexes
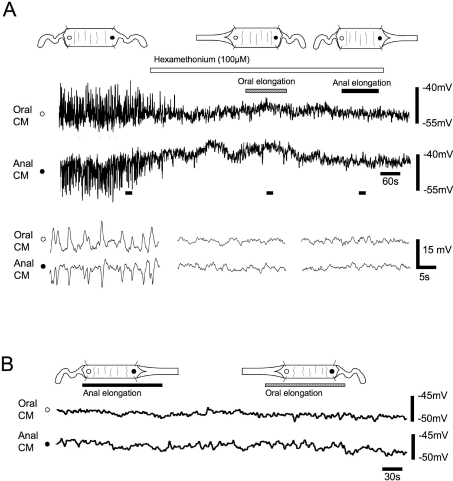
Hexamethonium (100 μm; n= 4) applied to the whole preparation or to just the middle chamber in a partitioned bath abolished the ongoing oral EJPs and anal IJPs in the CM activated by circumferential stretch (see Fig. 4A and Spencer et al. 2002). In the presence of hexamethonium (100 μm) neither oral nor anal longitudinal stretch had any detectable output to the muscle. This suggests that the polarized neural pathways activated by longitudinal stretch project onto interneurons involved in peristaltic reflex pathways rather than directly synapsing with muscle motor neurons. Hexamethonium (100 μm; p > 0.0, n= 3) applied only to the oral chamber had no effect on the suppression of ongoing oral EJPs and anal IJPs produced by oral elongation. In contrast, when hexamethonium (100 μm; p > 0.001, n= 3) was added to the anal chamber the excitatory effects of anal stretch were blocked.
Figure 4. Effect of nicotinic antagonism and no circumferential stretch on elongation reflexes.
A, circumferential stretch activates intrinsic polarized reflexes, consisting of an EJP at the oral electrode and an IJP at the anal electrode. Application of hexamethonium (100 μm) applied to the whole preparation abolished ongoing peristaltic reflex activity. Neither oral nor elongation had any detectable effect in the presence of hexamethonium. B, in a colon with no circumferential stretch, there was no polarized reflex present and colonic elongation (to 140% of slack length) of the anal or oral ends gave no detectable response in the CM. When elongation is applied to the oral end of the colon under circumferential stretch, the oral EJP and anal IJP are depressed. Open and filled circles indicate oral and anal recording sites in the CM, respectively
Anal and oral elongation without circumferential stretch
In a colonic preparation that was pinned loosely in the middle without circumferential stretch we found no ongoing peristaltic reflex activity (Dickson et al. 2007, Spencer et al. 2002). Also, colonic elongation (to 140% of its slack length) applied at either the anal or the oral end failed to generate any response in the CM (Fig. 4B; n= 5).
Peristaltic reflex evoked by distension of an intraluminal balloon
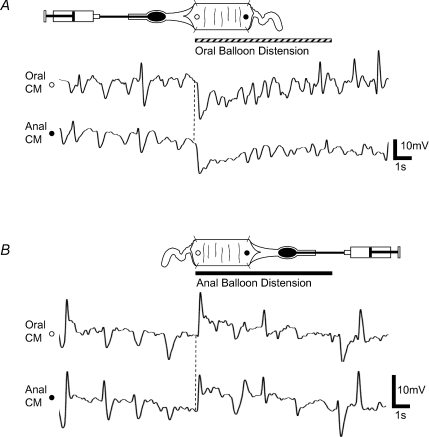
We were interested to see whether distension of an intraluminal balloon, which would be expected to cause both circumferential stretch and compression of the mucosa, applied oral or anal to the intact segment elicited similar responses to those evoked by colonic elongation. Orally applied distension, in contrast to oral elongation, evoked a transient inhibitory junction potential (IJP) (oral IJP 15.4 ± 2.6 mV; anal IJP 12.4 ± 2.4 mV; n= 6) in the CM at both the oral and anal recording sites rather than withdrawing activity from the muscle (Fig. 5A). Anal distension evoked a brief robust EJP (oral EJP 16.5 ± 2.7 mV; anal EJP 11.6 ± 23.5 mV; n= 6) in the CM at both the oral and anal recording sites of the stretched segment, rather than potentiating ongoing oral EJP–anal IJP complexes caused by circumferential stretch (Fig. 5B).
Figure 5. Responses to balloon distension are different from the responses to colonic elongation.
A, oral balloon distension applied to the intact segment evoked a transient IJP (descending inhibition) in the CM at both the oral and the anal electrodes. B, anal balloon distension evoked a transient EJP (ascending excitation) in the CM at both the oral and the anal electrodes.
Interactions between polarized nervous reflexes generated by colonic elongation
We next attempted to determine whether the inhibitory (occult) reflex activated by oral longitudinal stretch was more dominant than the ascending reflex activated by anal longitudinal stretch.
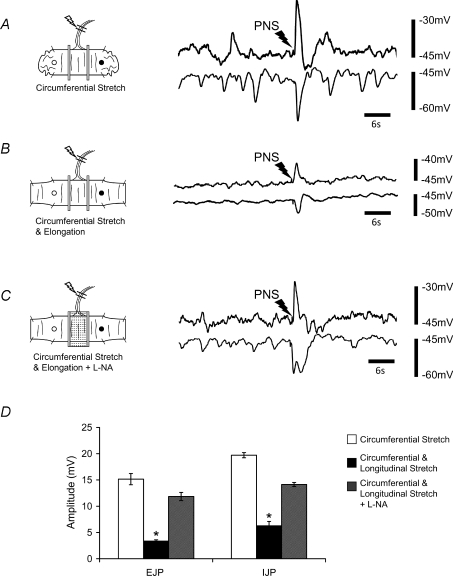
When anal elongation was maintained and then oral elongation applied, there was a subsequent withdrawal of all activity from the CM (Fig. 6), which was similar to the result we obtained when we elongated the entire length of colonic segment (Dickson et al. 2007). In fact, when the colon was elongated at both the anal and oral tubular ends, no additional increase in the amplitude of JPs was observed, and junction potentials were depressed to within the noise (< 5 mV; see Fig. 3A and G). While maintaining both the oral and anal colonic elongation, application of l-NA (100 μm; n= 5) to the middle chamber between the recording sites reversed the inhibitory effect of oral elongation and increased both the amplitude and the temporal synchronization between the oral EJP and the anal IJP complexes (Figs 3H and 7).
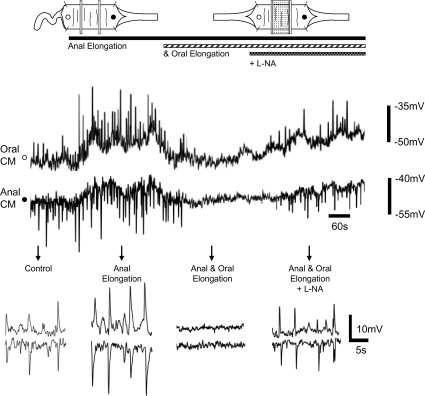
Figure 6. Interactions between anal and oral elongation reflexes.
Maintained circumferential stretch generated oral EJPs and anal IJPs in CM. Anal elongation (140%) increased the amplitude, frequency and degree of synchronization between the oral EJP and anal IJP. Oral elongation (140%) applied in conjunction with anal elongation depressed the junction potentials. The addition of l-NA (100 μm) reversed the inhibitory effects of oral elongation.
Figure 7. Dominant inhibitory action of oral versus anal elongation.
A and C, maintained circumferential stretch generated oral EJPs and anal IJPs in CM. Maintained oral elongation (140% increase in slack length) immediately suppressed this activity. Anal elongation (140%) in the presence of oral elongation had no effect. B and D, maintained oral elongation (140%) immediately suppressed this activity. Following the addition of l-NA (100 μm) between the partitions, which restored coordinated EJPs and anal IJPs, the further addition of anal elongation increased the amplitude, frequency and degree of synchronization between oral EJPs and anal IJPs.
Conversely, when oral elongation was applied first, it immediately suppressed the ongoing oral EJPs and anal IJPs in the CM (Fig. 7A), and subsequent anal elongation had no detectable excitatory effect (see Fig. 7A and C). Similarly, inhibition of NO synthesis with l-NA (100 μm; n= 5) between the recording sites revealed coordinated oral EJPs and anal IJPs that had been suppressed by oral elongation (Fig. 7B). Furthermore, in the presence of l-NA (100 μm) the addition of anal elongation increased the amplitude of oral EJPs and anal IJPs (amplitude: before oral elongation: control EJP 8.6 ± 0.3, IJP 8.3 ± 0.5 mV; following anal elongation in l-NA EJP 10.0 ± 0.6, IJP 10.3 ± 0.2 mV; n= 5, P < 0.03) and decreased the interval between ongoing oral EJP–anal IJP complexes (time interval: control 3.5 ± 0.2 s; l-NA 3.2 ± 0.2 s, n= 5), despite maintained oral elongation (Fig. 7B and D).
Calcium imaging of LM and CM in circumferentially stretched preparations: response to colonic elongation
The above experiments that investigated elongation reflexes were carried out in preparations that were paralysed with nifedipine to block muscle contraction. In calcium imaging experiments, we investigated whether similar elongation reflexes could be evoked when muscle tone was present. In these circumferentially stretched preparations we removed a thin strip of LM to reveal the underlying CM. Ca2+ waves, which are generated by action potentials propagating through the muscle, were observed in both the LM (11.1 waves min−1) and underlying CM (8.3 waves min−1) of a circumferentially stretched preparation. Following oral elongation to 140% of the slack length, Ca2+ waves in both the LM and CM were abolished at the same time (Fig. 8A, n= 5; P < 0.001). In contrast, anal elongation to 140% of the slack length significantly increased Ca2+ waves in both the LM (from 13.6 waves. min−1 to 17.4 waves. min−1) and CM (in the example shown in Fig. 7B from 10.9 waves. min−1 to 17.1 waves. min−1; P < 0.05, n= 5), which became synchronized in the two muscles throughout the duration of the stimulus (Fig. 8B).
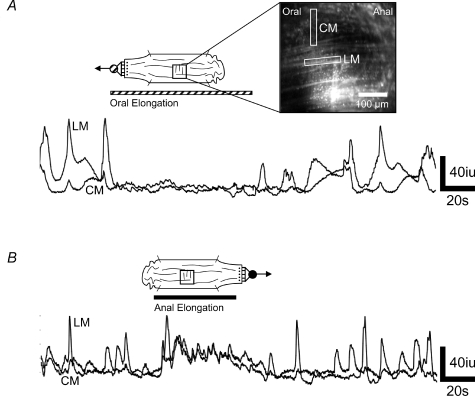
Figure 8. Effect of oral and anal elongation on calcium waves in the muscle.
Strips of LM were stripped away to reveal underlying CM. Ca2+ transients were measured in adjacent regions of interest aligned with either the LM or the CM. In the absence of nifedipine, oral elongation suppressed Ca2+ transients in both the LM and CM at the same time. B, in contrast, anal elongation increased the Ca2+ transients in the LM and CM at the same time.
Does pelvic nerve stimulation overcome the inhibitory effect of longitudinal stretch?
Pelvic nerve stimulation produces an increase in the velocity of propulsion of an intraluminal balloon in the guinea-pig distal colon (Crema et al. 1970). It has been reported that pelvic nerve stimulation evokes EJPs in LM and CM of the guinea-pig distal colon (Furness, 1969). We postulated that parasympathetic nerve stimulation could directly excite ascending interneurons or excitatory motor neurons that would remove the inhibitory ‘occult’ reflex activated by colonic elongation.
In a segment of colon that was maintained under circumferential stretch, a single maximal electric shock (0.5 ms, 40–50 V) applied to the pelvic nerves elicited a polarized response consisting of a large amplitude oral EJP (15.2 ± 1.1 mV; n= 5) and anal IJP (16.7 ± 0.5 mV; n= 5) in the CM at either end of the preparation (see Fig. 9A). Although of larger amplitude, these were similar in duration to the ongoing oral EJPs and anal IJPs in CM evoked by maintained circumferential stretch. To our surprise, when the pelvic nerves were stimulated after stretching the preparation in the longitudinal axis (to 140% of slack length), there was a significant decrease in the amplitude of the evoked oral EJP (3.4 ± 0.2 mV) and anal IJP (4.3 ± 0.8 mV), along with the ongoing stretch activated peristaltic reflex activity (Fig. 9B). When l-NA (100 μm; n= 5) was added between the two partitions (7 mm wide) the oral EJP (11.9 ± 0.8 mV) and anal IJP (14.1 ± 0.4 mV) responses in the CM to pelvic nerve stimulation were significantly restored (Fig. 9C). These results are summarized in Fig. 9D.
Figure 9. Effect of elongation on the responses to pelvic nerve stimulation.
A, circumferential stretch generated coordinated oral EJPs and anal IJPs in CM. Pelvic nerve stimulation (PNS; single pulse at 0.5 ms, 50 V) evoked an oral EJP and anal IJP of large amplitude. B, following the addition of maintained longitudinal stretch to the whole preparation the ongoing oral EJPs–anal IJPs were depressed, as were the responses to PNS. C, l-NA (100 μm) added to the middle chamber (between the partitions) restored both the ongoing activity and the response to PNS, despite the maintained elongation. D, summary of the effects of elongation on the responses to PNS.
Discussion
The current study shows that when the guinea-pig distal colon is full of fecal pellets it is on average 160% longer than an empty colon. We demonstrate that this physiological degree of elongation can activate polarized reflexes: a descending inhibitory reflex and an ascending excitatory reflex. As discussed below, these elongation reflexes are different from traditional peristaltic reflexes since they donD't elicit a direct output to the muscle but rather appear to inhibit or potentiate intrinsic neurons in persistaltic nerve pathways.
Physiological relevance of colonic elongation
The natural elongation of the guinea-pig distal colon when full of pellets had not apparently been appreciated in past studies because the colonic segment was arbitrarily pinned at both ends (D'Antona et al. 2001). This ability of the colon to increase in length allows the colon to accommodate more pellets. The expulsion of each pellet led to a gradual shortening in colonic length that was associated with an increase in emptying velocity of subsequent pellets. Initially, pellet evacuation velocity from a full colon was slow (0.3 mm s−1) compared to the velocity of the last fecal pellet along the empty colon (1.1 mm s−1). The final fecal pellet had a velocity that was similar to those reported for pellet propulsion along an empty guinea-pig colon (Jin et al. 1999). Our data suggests that there is a direct correlation between the degree of elongation of the guinea-pig distal colon and the exit velocity of a fecal pellet from the anal end of the colon. Presumably, this is because as the colon shortens with the expulsion of each pellet, the dominant descending inhibitory ‘occult reflex’, which is graded according to the amount of longitudinal stretch (Dickson et al. 2007), is gradually reduced in strength.
Oral and anal intrinsic reflexes activated by colonic elongation
We have recently suggested that AH neurons are not the only mechanosensory intrinsic sensory neuron in the colon since different classes of the multiple interneurons in the large bowel (see Lomax & Furness, 2000) may also be mechanosensitive in response to stretch (Spencer et al. 2006; Dickson et al. 2007; Smith et al. 2007a,b). Some interneurons appear to respond to graded amounts of circumferential stretch while others appear to respond only to longitudinal stretch (Spencer et al. 2006; Dickson et al. 2007; Smith et al. 2007a,b; Fig. 10).
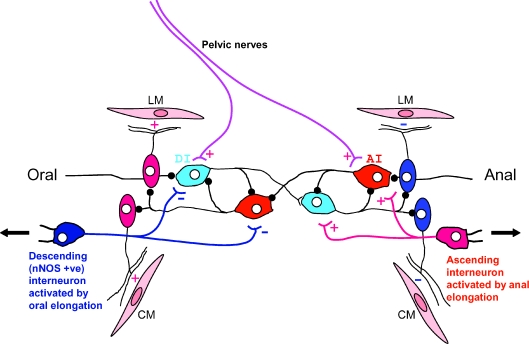
Figure 10. Proposed neural circuit underlying polarized reflexes activated by colonic elongation.
Circumferential stretch activates mechanosensitive ascending interneurons (AI) and descending interneurons (DI) underlying ongoing peristaltic reflex activity. AIs and DIs synapse with one another, activating excitatory motor neurons orally and inhibitory motor neurons anally to both the LM and CM layers (see Smith et al. 2007b). Following colonic elongation descending (NOS positive) and ascending (ACh) mechanosensitive interneurons are activated by longitudinal stretch. Descending NOS-positive interneurons release NO that generate IPSPs in AI and DI, thus depressing their activity. In contrast, the ascending interneurons activated by elongation release ACh, evoking FEPSPs in AI and DI, thus enhancing their activity. Pelvic nerve stimulation also activates both AI and DI, probably by also generating FEPSPs in these neurons.
Ongoing peristaltic activity – oral EJPs coordinated with anal IJPs – is generated by stretch sensitive ascending and descending interneurons; whereas AH sensory neurons that respond to muscle tension or tone and mucosal stimuli are electrically silent in these preparations where the mucosa has been removed and the smooth muscle tension eliminated by nifedipine (an L-Type Ca2+ channel blocker; Spencer & Smith, 2004; Smith et al. 2007a,b). These mechanosensory interneurons that respond to circumferential stretch may have mechanosensitive dendrites running in parallel to CM fibres (Spencer & Smith, 2004; Spencer et al. 2006). The coordination between the oral EJPs and anal IJPs implies that these mechanosensory ascending and descending interneurons are likely to synapse with one another (Spencer et al. 2006; Smith et al. 2007a,b; Fig. 10).
Our current studies suggest that reflexes activated by colonic elongation are more complex than we previously thought (Dickson et al. 2007; Smith et al. 2007a,b), since both descending inhibitory (occult) and ascending excitatory intrinsic nerve pathways are activated by longitudinal stretch (elongation). The descending inhibitory elongation reflex is likely to be mediated by descending nNOS-positive interneurons, since these are the only class of the four descending interneurons that donD't contain acetylcholine (see Lomax & Furness, 2000), whereas the ascending excitatory (potentiating) reflex activated by colonic elongation is likely to be one of the three classes of ascending cholinergic interneurons, since the response to anal elongation was blocked by hexamethonium. Intrinsic elongation reflexes, which continue for as long as the stimulus is maintained, donD't appear to generate a direct output to muscle motor neurons, but rather decrease (oral elongation – occult reflex) or potentiate (anal elongation – ascending potentiating reflex) activity in mechanosensory interneurons underling ongoing peristaltic activity (Fig. 10). Therefore, reflexes elicited largely by colonic elongation are different in nature from the ‘classic’ peristaltic ascending excitatory and descending inhibitory reflex that evokes a single oral EJP or a single anal IJP of the muscle layers at both oral and anal recording sites. These ‘classic’ peristaltic reflex responses are probably elicited largely by mucosal compression that probably activates AH neurons (compare Figs 2 and 5) (Smith & Robertson, 1998; Smith & McCarron, 1998; Smith et al. 2007b; Spencer & Smith, 2001).
Elongation reflexes can only be detected in preparations that are under maintained circumferential stretch, suggesting that the mechanosensory neurons responding to elongation are likely to synapse with mechanosensory neurons driving ongoing peristaltic activity, rather than directly affecting excitatory and inhibitory motor neurons to the LM and CM (Fig. 10). The descending inhibitory (occult) reflex activated by oral elongation is more powerful since it suppresses the ascending excitatory reflex activated by anal elongation, a phenomena that is reversed by inhibiting the release of NO by blocking NO synthesis. Thus it appears that the dominant response is the nitrergic suppression of interneurons in peristaltic pathways by generating NO mediated inhibitory postsynaptic potentials (IPSPs) in the soma of these neurons (Fig. 10: Dickson et al. 2007; Smith et al. 2007a,b). This would render them less excitable to fast excitatory postsynaptic potentials (FEPSPs) generated by cholinergic transmission from orally projecting mechanosensitive interneurons activated by anal longitudinal stretch (Fig. 10). This likely explains why overall maintained colonic elongation suppresses oral EJP and anal IJP complexes (Dickson et al. 2007).
Using Ca2+ imaging of the two muscle layers in intact tissues, these elongation reflexes were also observed in unparalysed muscle preparations with the mucosa intact, suggesting the possibility that AH sensory neurons may also have been inhibited by oral elongation (Kunze et al. 1998; Smith et al. 2007a). Some AH neurons, like mechanosensitive interneurons activated by circumferential stretch, also receive IPSPs (Lomax et al. 1999) that may also be mediated by NO (see Dickson et al. 2007).
Extrinsic pelvic nerves synapse with interneurons
In response to pelvic (parasympathetic) nerve stimulation both excitatory and inhibitory responses of colonic smooth muscle have been observed (Tonini et al. 2003). Crema et al. (1970) showed that pelvic nerve stimulation increased the velocity of propulsion of an intraluminal balloon and decreased the amount of distension (lowered threshold) necessary to elicit propulsion along empty segments of guinea-pig and cat distal colon. In the cat they found an increase in contraction above the bolus, while the degree of descending inhibition below the bolus was only ‘slightly affected’. We found that pelvic nerve stimulation produced not just an EJP in the muscle (Furness, 1969), but a large coordinated oral EJP and anal IJP in the CM at either end of the preparation. This could explain why Crema et al. (1970) saw an apparently polarized response over the balloon. Much to our surprise, longitudinal stretch reduced not only the coordinated oral EJPs and anal IJPs generated by circumferential stretch, but also the similar enhanced responses evoked by pelvic nerve stimulation. These inhibitory effects of elongation were also reversed by blocking NO synthesis between the recording sites. The fact that pelvic nerve stimulation evokes an oral EJP and anal IJP and its effects are inhibited by longitudinal stretch suggests that the pelvic nerves also directly excite ascending and descending mechanosensitive interneurons driving peristaltic nerve pathways (Fig. 10). It is likely then that these interneurons are involved in not only generating intrinsic peristaltic reflexes but also integrating extrinsic nervous inputs. Previously we have shown that stimulation of sympathetic nerves also reduced ACh release from ascending and descending interneurons in peristaltic reflex circuits (Spencer et al. 1999).
In summary, when the guinea-pig distal colon is full of fecal pellets it is normally elongated and transit is slowed. Under these conditions, colonic elongation would be likely to activate both descending depressing (occult) and ascending potentiating reflexes. Therefore colonic transit of fecal pellets would be subject to a balance between these two reflexes activated by elongation, as well as by peristaltic reflexes activated by circumferential stretch. The dominance of the descending occult reflex means that there is usually a net reduction in the excitability of myenteric neurons in peristaltic circuits, favouring accommodation and slow transit of fecal pellets.
Acknowledgments
Supported by a grant from the National Institute of Health, USA: Grant Number ROI NIDDK 45713.
References
- Blackshaw LA, Brookes SJ, Grundy D, Schemann M. Sensory transmission in the gastrointestinal tract. Neurogastroenterol Motil. 2007;19:1–19. doi: 10.1111/j.1365-2982.2006.00871.x. [DOI] [PubMed] [Google Scholar]
- Brookes SJ, Ewart WR, Wingate DL. Intracellular recordings from myenteric neurones in the human colon. J Physiol. 1987;390:305–318. doi: 10.1113/jphysiol.1987.sp016702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes SJ, Ewart WR, Wingate DL. Intracellular recordings from cells in the myenteric plexus of the rat duodenum. Neuroscience. 1988;24:297–307. doi: 10.1016/0306-4522(88)90332-6. [DOI] [PubMed] [Google Scholar]
- Cornelissen W, De Laet A, Kroese AB, Van Bogaert PP, Scheuermann DW, Timmermans JP. Electrophysiological features of morphological Dogiel type II neurons in the myenteric plexus of pig small intestine. J Neurophysiol. 2000;84:102–111. doi: 10.1152/jn.2000.84.1.102. [DOI] [PubMed] [Google Scholar]
- Cornelissen W, de Laet A, Kroese AB, van Bogaert PP, Scheuermann DW, Timmermans JP. Excitatory synaptic inputs on myenteric Dogiel type II neurones of the pig ileum. J Comp Neurol. 2001;432:137–154. doi: 10.1002/cne.1093. [DOI] [PubMed] [Google Scholar]
- Costa M, Furness JB. The peristaltic reflex: an analysis of the nerve pathways and their pharmacology. Naunyn Schmiedebergs Arch Pharmacol. 1976;16:47–60. doi: 10.1007/BF00692784. [DOI] [PubMed] [Google Scholar]
- Crema A, Frigo GM, Lecchini S. A pharmacological analysis of the peristaltic reflex in the isolated colon of the guinea-pig or cat. Br J Pharmacol. 1970;39:334–345. doi: 10.1111/j.1476-5381.1970.tb12897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Antona G, Hennig GW, Costa M, Humphreys CM, Brookes SJ. Analysis of motor patterns in the isolated guinea-pig large intestine by spatio-temporal maps. Neurogastroenterol Motil. 2001;13:483–492. doi: 10.1046/j.1365-2982.2001.00282.x. [DOI] [PubMed] [Google Scholar]
- De Laet A, Cornelissen W, Adriaensen D, Van Bogaert PP, Scheuermann DW, Timmermans JP. Ca2+ involvement in the action potential generation of myenteric neurones in the rat oesophagus. Neurogastroenterol Motil. 2002;14:161–172. doi: 10.1046/j.1365-2982.2002.00315.x. [DOI] [PubMed] [Google Scholar]
- Dickson EJ, Spencer NJ, Hennig GW, Bayguinov PO, Ren J, Heredia DJ, Smith TK. An enteric occult reflex underlies accommodation and slow transit in the distal large bowel. Gastroenterology. 2007;132:1912–1924. doi: 10.1053/j.gastro.2007.02.047. [DOI] [PubMed] [Google Scholar]
- Furness JB. An electrophysiological study of the innervation of the smooth muscle of the colon. J Physiol. 1969;205:549–562. doi: 10.1113/jphysiol.1969.sp008982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness JB, Kunze WAA, Bertrand PP, Clerc N, Bornstein JC. Intrinsic primary afferent neurons of the intestine. Neurobiology. 1998;54:1–18. doi: 10.1016/s0301-0082(97)00051-8. [DOI] [PubMed] [Google Scholar]
- Gillespie JS. The electrical and mechanical responses of intestinal smooth muscle cells to stimulation of their extrinsic parasympathetic nerves. J Physiol. 1962;162:76–92. doi: 10.1113/jphysiol.1962.sp006915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig GW, Costa M, Chen BN, Brookes SJ. Quantitative analysis of peristalsis in the guinea-pig small intestine using spatio-temporal maps. J Physiol. 1999;517:575–590. doi: 10.1111/j.1469-7793.1999.0575t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GD, Holman ME, McKirdy HC. Two descending nerve pathways activated by distension of guinea-pig small intestine. J Physiol. 1975;244:113–127. doi: 10.1113/jphysiol.1975.sp010786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GD, Holman ME, Spence I. Two types of neurones in the myenteric plexus of duodenum in the guinea-pig. J Physiol. 1974;236:303–326. doi: 10.1113/jphysiol.1974.sp010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin JG, Foxx-Orenstein AE, Grider JR. Propulsion in guinea pig colon induced by 5-hydroxytryptamine (HT) via 5-HT4 and 5-HT3 receptors. J Pharmacol Exp Ther. 1999;288:93–97. [PubMed] [Google Scholar]
- Kunze WA, Clerc N, Bertrand PP, Furness JB. Contractile activity in intestinal muscle evokes action potential discharge in guinea-pig myenteric neurons. J Physiol. 1999;517:547–561. doi: 10.1111/j.1469-7793.1999.0547t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze WA, Furness JB, Bertrand PP, Bornstein JC. Intracellular recording from myenteric neurons of the guinea-pig ileum that respond to stretch. J Physiol. 1998;506:827–842. doi: 10.1111/j.1469-7793.1998.827bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HT, Hennig GW, Fleming NW, Keef KD, Spencer NJ, Ward SM, Sanders KM, Smith TK. Mechanism and spread of pacemaker activity through myenteric interstitial cells of Cajal in human small intestine. Gastroenterology. 2007;132:1852–1865. doi: 10.1053/j.gastro.2007.02.049. [DOI] [PubMed] [Google Scholar]
- Lomax AE, Furness JB. Neurochemical classification of enteric neurons in the guinea-pig distal colon. Cell Tissue Res. 2000;302:59–72. doi: 10.1007/s004410000260. [DOI] [PubMed] [Google Scholar]
- Lomax AE, Sharkey KA, Bertrand PP, Low AM, Bornstein JC, Furness JB. Correlation of morphology, electrophysiology and chemistry of neurons in the myenteric plexus of the guinea-pig distal colon. J Auton Nerv Syst. 1999;16:45–61. doi: 10.1016/s0165-1838(99)00008-9. [DOI] [PubMed] [Google Scholar]
- Mao Y, Wang B, Kunze W. Characterization of myenteric sensory neurons in the mouse small intestine. J Neurophysiol. 2006;96:998–1010. doi: 10.1152/jn.00204.2006. [DOI] [PubMed] [Google Scholar]
- Phillips SF. Functions of the large bowel: an overview. Scand J Gastroenterol Suppl. 1984;93:1–12. [PubMed] [Google Scholar]
- Schemann M, Wood JD. Electrical behaviour of myenteric neurones in the gastric corpus of the guinea-pig. J Physiol. 1989;417:501–518. doi: 10.1113/jphysiol.1989.sp017815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TK, Bornstein JC, Furness JB. Interactions between reflexes evoked by distension and mucosal stimulation: electrophysiological studies of guinea-pig ileum. J Auton Nerv Syst. 1991;34:69–75. doi: 10.1016/0165-1838(91)90009-r. [DOI] [PubMed] [Google Scholar]
- Smith TK, Bornstein JC, Furness JB. Convergence of reflex pathways excited by distension and mechanical stimulation of the mucosa onto the same myenteric neurons of the guinea pig small intestine. J Neurosci. 1992;12:1502–1510. doi: 10.1523/JNEUROSCI.12-04-01502.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TK, Dickson EJ, Hennig GW, Bayguinov PO, Spencer NJ. Colonic elongation activates an intrinsic reflex that underlies slow transit and accommodation. Physiol News. 2007a;69:33–35. [Google Scholar]
- Smith TK, McCarron S. Nitric oxide modulates cholinergic reflex pathways to the longitudinal and circular muscle in the isolated guinea-pig distal colon. J Physiol. 1998;512:893–906. doi: 10.1111/j.1469-7793.1998.893bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TK, Robertson WJ. Synchronous movements of the longitudinal and circular muscle during peristalsis in the isolated guinea-pig distal colon. J Physiol. 1998;506:563–577. doi: 10.1111/j.1469-7793.1998.563bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TK, Spencer NJ, Hennig GW, Dickson EJ. Recent advances in enteric neurobiology: mechanosensitive interneurons. Neurogastroenterol Motil. 2007b;19:869–878. doi: 10.1111/j.1365-2982.2007.01019.x. [DOI] [PubMed] [Google Scholar]
- Spencer NJ, Dickson EJ, Hennig GW, Smith TK. Sensory elements within the circular muscle are essential for mechanotransduction of ongoing peristaltic reflex activity in guinea-pig distal colon. J Physiol. 2006;576:519–531. doi: 10.1113/jphysiol.2006.109561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer NJ, Hennig GW, Smith TK. A rhythmic motor pattern activated by circumferential stretch in guinea-pig distal colon. J Physiol. 2002;545:629–648. doi: 10.1113/jphysiol.2002.028647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer NJ, Hennig GW, Smith TK. Stretch activated neuronal pathways in longitudinal and circular muscle of guinea-pig distal colon. Am J Physiol Gastrointest Liver Physiol. 2003;284:G231–G241. doi: 10.1152/ajpgi.00291.2002. [DOI] [PubMed] [Google Scholar]
- Spencer N, McCarron SL, Smith TK. Sympathetic inhibition of ascending and descending interneurones during the peristaltic reflex in the isolated guinea-pig distal colon. J Physiol. 1999;519:539–550. doi: 10.1111/j.1469-7793.1999.0539m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer NJ, Smith TK. Simultaneous intracellular recordings from longitudinal and circular muscle during the peristaltic reflex in guinea-pig distal colon. J Physiol. 2001;533:787–799. doi: 10.1111/j.1469-7793.2001.00787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer NJ, Smith TK. Mechanosensory S-neurons rather than AH neurons appear to generate a rhythmic motor pattern in guinea-pig distal colon. J Physiol. 2004;558:577–596. doi: 10.1113/jphysiol.2004.063586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens RJ, Publicover NG, Smith TK. Propagation and neural regulation of calcium waves in longitudinal and circular muscle layers of guinea pig small intestine. Gastroenterology. 2000;118:892–904. doi: 10.1016/s0016-5085(00)70175-2. [DOI] [PubMed] [Google Scholar]
- Tonini M, De Ponti F, Frigo G, Crema F. Phamacology of the enteric nervous system. In: Brookes S, Costa M, editors. Innervation of the GI Tract. London and New York: Taylor & Francis; 2003. pp. 213–294. [Google Scholar]