Abstract
Decreased oxygen availability (hypoxia) is a hallmark feature of the microenvironment in a number of chronic inflammatory conditions including arthritis and inflammatory bowel disease (IBD). Recent advances in our understanding of oxygen-dependent cell signalling have uncovered several mechanisms by which hypoxia impacts upon the development of inflammation through the coordinated expression of adaptive, inflammatory and apoptotic genes. Two central transcription factors involved in the regulation of this response are hypoxia inducible factor (HIF) and nuclear factor-κB (NF-κB) which display different degrees of sensitivity to activation during hypoxia. Furthermore, HIF and NF-κB demonstrate an intimate interdependence at several mechanistic levels. Recent studies indicate that these pathways may represent important new therapeutic targets in diseases characterized by hypoxic inflammation.
Hypoxic inflammation
Sites of chronic inflammation including arthritic joints and inflamed intestinal mucosae in patients suffering from inflammatory bowel disease (IBD) demonstrate distinct microenvironmental features including decreased oxygen availability (hypoxia; Cramer et al. 2003; Nathan, 2003; Taylor & Colgan, 2007). For example, in inflammatory bowel disease, oxygen levels in the chronically inflamed intestinal mucosa are significantly reduced when compared with healthy mucosal tissues (Hauser et al. 1988). Similarly, hypoxia is a feature of the inflamed arthritic joint (Murdoch et al. 2005). The likely causes of hypoxia during chronic inflammation are twofold. Firstly, a chronically inflamed tissue has increased metabolic activity due to infiltrating inflammatory cells as well as the highly metabolically active inflamed resident tissue resulting in elevated oxygen demand (Karhausen et al. 2005). Secondly, vasculopathy caused by extensive inflammation including blood vessel stenosis and microthrombosis leads to poor perfusion and subsequently decreased oxygen supply to the inflamed area (Hatoum et al. 2003; Wakefield et al. 1989). Thus, an imbalance between oxygen supply and demand renders chronically inflamed tissues hypoxic. Recently, it has become apparent that hypoxia is more than simply a bystander effect but can greatly impact upon the development of inflammation through the regulation of oxygen-dependent gene expression (Zinkernagel et al. 2007).
Hypoxia-responsive transcription factors
Hif
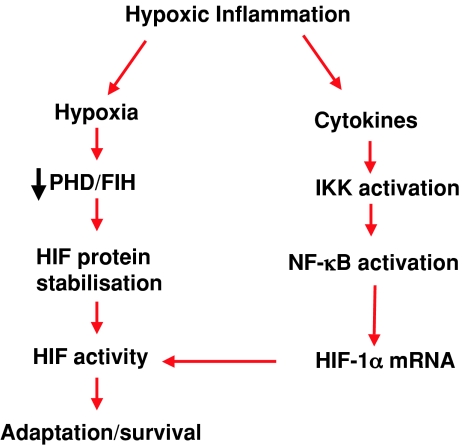
As molecular oxygen is the primary source of metabolic energy for all eukaryotic cells, it is not surprising that over the course of evolution, we have developed the ability to respond to hypoxia in a manner which is directed towards restoring tissue oxygenation as quickly as possible. The hypoxia-inducible factor (HIF) is a master regulator of this cellular response to hypoxia (Semenza, 2007). HIF is a ubiquitous, heterodimeric transcription factor, the oxygen sensitivity of which is conferred by a family of oxygen-dependent hydroxylase enzymes including three proline hydroxylases (PHDs) and one asparigine hydroxylase known as the factor inhibiting HIF (FIH). These hydroxylases promote oxygen-dependent hydroxylation of HIF leading to ubiquitylation by the Von Hipple Lindau (VHL) E3 ligase and subsequent degradation by the proteasome. This is reversed in hypoxia leading to HIF activation (Fig. 1). The specifics of hydroxylase-dependent oxygen-sensing pathways leading to activation of HIF in hypoxia have been recently reviewed elsewhere (Taylor, 2008; Kaelin & Ratcliffe, 2008).
Figure 1. Model of regulation of the HIF pathway during hypoxic inflammation.
During chronic inflammation, decreased tissue perfusion and increased energy demand causes hypoxia. This leads to inhibition of hydroxylases (PHD/FIH) and subsequent stabilization and transactivation of HIF. Simultaneous activation of the NF-κB pathway leads to transcriptional up-regulation of HIF mRNA expression which facilitates the activation of HIF signalling.
The development of tissue-specific gene knockout technology in mice has allowed the investigation of the role of HIF in hypoxic inflammation in vivo. HIF has a divergent effect on the progression of inflammation depending on in which cell type it is expressed. For example, HIF-1α expression in inflammatory cells of the myeloid lineage contributes to cell survival by allowing infiltrating macrophages and monocytes to switch to a glycolytic metabolic strategy and survive in the hypoxic milieu of the inflamed lesion. Thus, in this context, HIF-1α may be considered pro-inflammatory in that it promotes inflammatory cell survival (Cramer et al. 2003). In contrast to this pro-inflammatory role, HIF-1α expression in intestinal epithelial cells contributes to intestinal epithelial barrier function during inflammation. The intestinal epithelial barrier prevents the non-specific movement of luminal antigenic material into the subepithelial lamina propria and thus the HIF pathway can be considered to be anti-inflammatory in this context (Karhausen et al. 2004). As the primary function of the ubiquitous HIF pathway is adaptation to hypoxia, it is likely that the cell-specific roles of HIF in inflammation reflect the contribution of that particular cell type to the overall inflammatory response.
NF-kB
Although HIF is clearly central to the cellular response to hypoxia, it is not alone in displaying sensitivity to local oxygen concentrations. Indeed almost 20 different transcription factors have been reported to display direct or indirect hypoxic sensitivity (Cummins & Taylor, 2005). Principal among these is the nuclear factor-kappaB (NF-κB), which has been known for over 10 years to be activated in various cell types in response to hypoxia (Koong et al. 1994; Cummins & Taylor, 2005). NF-κB is a family of transcription factors which are typically activated following stimulation of cells with pro-inflammatory ligands including cytokines, antigens and bacterial products (Fig. 2; Rius et al. 2008). While this pathway is multi-pronged and complex, the majority of signalling to NF-κB involves the activation of the canonical pathway through activation of the IκB kinase (IKK) complex. Hypoxic activation of NF-κB is due at least in part to IKK activation in the canonical pathway but may also involve other mechanisms including tyrosine phosphorylation of IκBα (Koong et al. 1994; Cummins et al. 2006). NF-κB is primarily a regulator of inflammatory and anti-apoptotic gene expression and it is likely that the physiological reason for its activation in hypoxia is in the inhibition of apoptosis to enable a cell to survive a period of hypoxic insult. However, it may also play an important role in driving hypoxic inflammation through the expression of cytokines, adhesion molecules and pro-inflammatory enzymes. While the signalling events linking cellular hypoxia to transcriptional activation are less well understood for NF-κB than for HIF and are still under investigation, it has recently been appreciated that the same oxygen-sensing hydroxylases responsible for conferring hypoxic sensitivity to the HIF pathway may also regulate important components of the NF-κB pathway (Cockman et al. 2006; Cummins et al. 2006). However, it is important to note that while hypoxia is a strong activator of HIF-dependent pathways, it is a more mild and possibly modulatory stimulus for NF-κB signalling (Cummins et al. 2006; Rius et al. 2008). Similar to HIF-1α, mice which lack the canonical NF-κB signalling pathway in intestinal epithelial cells demonstrate increased susceptibility to inflammatory bowel disease (Chen et al. 2003; Greten et al. 2004) indicating a protective role for NF-κB in intestinal epithelial cells during hypoxic inflammation.
Figure 2. Model of regulation of the NF-κB pathway in hypoxic inflammation.
During chronic inflammation, elevated cytokine levels promote inflammation through activation of the canonical (IKK complex-dependent) NF-κB pathway. Simultaneous hypoxia facilitates this response by decreasing hydroxylase activity which serves to de-repress NF-κB signalling.
Cross talk between NF-κB and HIF
It has recently become apparent that HIF and NF-κB, as well as acting independently in the regulation of gene expression in hypoxic inflammation, have a significant and profound level of cross-talk (Fig. 3). It is now clear that HIF, as well as being responsive to hypoxia, can also be activated in response to a number of non-hypoxic stimuli including bacterial lippopolysaccharide, microtubule disruption, tumour necrosis factor α, hepatocyte growth factor, reactive oxygen species and interleukin-18 (Figueroa et al. 2002; Jung et al. 2003a,b; Zhou et al. 2003; Tacchini et al. 2004; Frede et al. 2006; Bonello et al. 2007; Sun et al. 2007; Belaiba et al. 2007; Kim et al. 2008; van Uden et al. 2008). A common factor for all of these non-hypoxic stimuli of HIF is that the mechanism involves NF-κB-dependent up-regulation of HIF-1α at the mRNA level rather than at the protein level (as usually occurs during hypoxia). NF-κB has also been reported to play a role in hypoxia-induced HIF-1α mRNA expression (Belaiba et al. 2007). Furthermore, NF-κB has recently been shown to be important in basal levels of HIF-1α gene expression (Rius et al. 2008; van Uden et al. 2008). Importantly, this NF-κB dependence of HIF-1α has been demonstrated to occur in vivo (Rius et al. 2008). The HIF-1α promoter contains an active NF-κB binding site in a position −197/188 upstream of the transcription start site (van Uden et al. 2008). A further mechanism by which NF-κB can regulate HIF signalling is through an interaction between the IKKγ subunit of the IKK complex and HIF-2α which increases HIF-2α transcriptional activity through promoting interaction with CREB binding protein (CBP)/p300 (Bracken et al. 2005). In summary, convincing evidence now exists that the HIF pathway is heavily reliant upon NF-κB signalling in response to non-hypoxic stimuli and in the regulation of basal HIF-1α mRNA expression.
Figure 3. Model of crosstalk between the HIF and NF-κB pathways in hypoxic inflammation.
In chronic inflammatory disease, tissue hypoxia leads to decreased hydroxylase activity with the subsequent activation of HIF-dependent adaptive gene expression (1). Simultaneously, signalling in response to inflammatory ligands leads to NF-κB activity and subsequent inflammatory and anti-apoptotic gene expression (2). An extensive level of cross-talk between the two pathways also exists including NF-κB-dependent up-regulation of HIF-1α mRNA expression (3), HIF-dependent regulation of NF-κB activity (4) and regulation of NF-κB signalling by HIF-hydroxylases (5).
While the effects of inflammatory mediators on HIF-1 transcriptional activity as outlined above are predominantly positive, recent evidence has indicated that inflammatory stimuli may also elicit the transcription of small inhibitory micro-RNAs which are involved in the resolution of HIF-dependent signalling. Briefly, O'Connell et al. have demonstrated that the micro-RNA miR-155, which is up-regulated in haematopoeietic stem cells in response to LPS (a potent activator of NF-κB), targets HIF-1α for silencing (O’Connell et al. 2008). This represents the first evidence that there may be a negative feedback loop between the NF-κB and HIF pathways mediated at the levels of micro-RNAs.
While the transcriptional regulation of HIF-1α is under the control of NF-κB it has also been reported that HIF-1α can also regulate NF-κB signalling. For example, mice overexpressing HIF-1α in keratinocytes demonstrate increased NF-κB activity and increased expression of pro-inflammatory NF-κB gene targets leading to hyper-responsiveness to inflammatory stimuli (Scortegagna et al. 2008). Furthermore mice lacking HIF-1α in neutrophils demonstrated a role for HIF-dependent NF-κB signalling in the regulation of neutrophil survival (Walmsley et al. 2005). In summary, NF-kB and HIF appear to share an intimate co-dependency which is complex and occurs at a number of mechanistic levels.
Conclusions and perspectives
Hypoxia is a microenvironmental feature of chronically inflamed tissues which can impact upon the progression of inflammation in a number of ways. HIF and NF-κB are two hypoxia-responsive transcription factors which, as well as controlling independent cohorts of adaptive and inflammatory genes, demonstrate a high degree of interdependence. Central to the activation of both the HIF and NF-κB pathways in hypoxia appear to be the oxygen-sensing hydroxylases. A greater understanding of the crosstalk between these two ancient pathways will uncover new therapeutic targets in a range of inflammatory disorders which currently have limited therapeutic repertoires including rheumatoid arthritis and inflammatory bowel disease. Indeed recent data indicate a potential therapeutic role for inhibitors of hydroxylases in a number of models of inflammation including diseases of the kidney and gastrointestinal tract (Bernhardt et al. 2006; Cummins et al. 2008; Robinson et al. 2008; Hill et al. 2008).
References
- Belaiba RS, Bonello S, Zähringer C, Schmidt S, Hess J, Kietzmann T, Görlach A. Hypoxia up-regulates hypoxia-inducible factor-1α transcription by involving phosphatidylinositol 3-kinase and nuclear factor κB in pulmonary artery smooth muscle cells. Mol Biol Cell. 2007;18:4691–4697. doi: 10.1091/mbc.E07-04-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt WM, Câmpean V, Kany S, Jürgensen JS, Weidemann A, Warnecke C, Arend M, Klaus S, Günzler V, Amann K, Willam C, Wiesener MS, Eckardt KU. Preconditional activation of hypoxia-inducible factors ameliorates ischemic acute renal failure. J Am Soc Nephrol. 2006;17:1970–1978. doi: 10.1681/ASN.2005121302. [DOI] [PubMed] [Google Scholar]
- Bonello S, Zähringer C, BelAiba RS, Djordjevic T, Hess J, Michiels C, Kietzmann T, Görlach A. Reactive oxygen species activate the HIF-1α promoter via a functional NFκB site. Arterioscler Thromb Vasc Biol. 2007;27:755–761. doi: 10.1161/01.ATV.0000258979.92828.bc. [DOI] [PubMed] [Google Scholar]
- Bracken CP, Whitelaw ML, Peet DJ. Activity of hypoxia-inducible factor 2a is regulated by association with the NF-κB essential modulator. J Biol Chem. 2005;280:14240–14251. doi: 10.1074/jbc.M409987200. [DOI] [PubMed] [Google Scholar]
- Chen LW, Egan L, Li ZW, Greten FR, Kagnoff MF, Karin M. The two faces of IKK and NF-κB inhibition: prevention of systemic inflammation but increased local injury following intestinal ischemia-reperfusion. Nat Med. 2003;9:575–581. doi: 10.1038/nm849. [DOI] [PubMed] [Google Scholar]
- Cockman ME, Lancaster DE, Stolze IP, Hewitson KS, McDonough MA, Coleman ML, Coles CH, Yu X, Hay RT, Ley SC, Pugh CW, Oldham NJ, Masson N, Schofield CJ, Ratcliffe PJ. Posttranslational hydroxylation of ankyrin repeats in IκB proteins by the hypoxia-inducible factor (HIF) asparaginyl hydroxylase, factor inhibiting HIF (FIH) Proc Natl Acad Sci U S A. 2006;103:14767–14772. doi: 10.1073/pnas.0606877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer T, Yamanishi Y, Clausen BE, Förster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber HP, Ferrara N, Johnson RS. HIF-1α is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins EP, Berra E, Comerford KM, Ginouves A, Fitzgerald KT, Seeballuck F, Godson C, Nielsen JE, Moynagh P, Pouyssegur J, Taylor CT. Prolyl hydroxylase-1 negatively regulates IκB kinase-β, giving insight into hypoxia-induced NFκB activity. Proc Natl Acad Sci U S A. 2006;103:18154–18159. doi: 10.1073/pnas.0602235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins EP, Seeballuck F, Keely SJ, Mangan NE, Callanan JJ, Fallon PG, Taylor CT. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology. 2008;134:156–165. doi: 10.1053/j.gastro.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Cummins EP, Taylor CT. Hypoxia-responsive transcription factors. Pflugers Arch. 2005;450:363–371. doi: 10.1007/s00424-005-1413-7. [DOI] [PubMed] [Google Scholar]
- Figueroa YG, Chan AK, Ibrahim R, Tang Y, Burow ME, Alam J, Scandurro AB, Beckman BS. NF-κB plays a key role in hypoxia-inducible factor-1-regulated erythropoietin gene expression. Exp Hematol. 2002;30:1419–1427. doi: 10.1016/s0301-472x(02)00934-7. [DOI] [PubMed] [Google Scholar]
- Frede S, Stockmann C, Freitag P, Fandrey J. Bacterial lipopolysaccharide induces HIF-1 activation in human monocytes via p44/42 MAPK and NF-κB. Biochem J. 2006;396:517–527. doi: 10.1042/BJ20051839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKβ links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Hatoum OA, Miura H, Binion DG. The vascular contribution in the pathogenesis of inflammatory bowel disease. Am J Physiol Heart Circ Physiol. 2003;285:H1791–H1796. doi: 10.1152/ajpheart.00552.2003. [DOI] [PubMed] [Google Scholar]
- Hauser CJ, Locke RR, Kao HW, Patterson J, Zipser RD. Visceral surface oxygen tension in experimental colitis in the rabbit. J Lab Clin Med. 1988;112:68–71. [PubMed] [Google Scholar]
- Hill P, Shukla D, Tran MG, Aragones J, Cook HT, Carmeliet P, Maxwell PH. Inhibition of hypoxia inducible factor hydroxylases protects against renal ischemia-reperfusion injury. J Am Soc Nephrol. 2008;19:39–46. doi: 10.1681/ASN.2006090998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y, Isaacs JS, Lee S, Trepel J, Liu ZG, Neckers L. Hypoxia-inducible factor induction by tumour necrosis factor in normoxic cells requires receptor-interacting protein-dependent nuclear factor κB activation. Biochem J. 2003a;370:1011–1017. doi: 10.1042/BJ20021279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung YJ, Isaacs JS, Lee S, Trepel J, Neckers L. Microtubule disruption utilizes an NFκB-dependent pathway to stabilize HIF-1α protein. J Biol Chem. 2003b;278:7445–7452. doi: 10.1074/jbc.M209804200. [DOI] [PubMed] [Google Scholar]
- Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest. 2004;114:1098–1106. doi: 10.1172/JCI21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karhausen J, Haase VH, Colgan SP. Inflammatory hypoxia. Role for hypoxia-inducible factor. Cell Cycle. 2005;4:256–258. [PubMed] [Google Scholar]
- Kim J, Shao Y, Kim SY, Kim S, Song HK, Jeon JH, Suh HW, Chung JW, Yoon SR, Kim YS, Choi I. Hypoxia-induced IL-18 increases hypoxia-inducible factor-1α expression through a Rac1-dependent NF-κB pathway. Mol Biol Cell. 2008;19:433–444. doi: 10.1091/mbc.E07-02-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koong AC, Chen EY, Giaccia AJ. Hypoxia causes the activation of nuclear factor κB through the phosphorylation of IκBα on tyrosine residues. Cancer Res. 1994;54:1425–1430. [PubMed] [Google Scholar]
- Murdoch C, Muthana M, Lewis CE. Hypoxia regulates macrophage functions in inflammation. J Immunol. 2005;175:6257–6263. doi: 10.4049/jimmunol.175.10.6257. [DOI] [PubMed] [Google Scholar]
- Nathan C. Immunology: Oxygen and the inflammatory cell. Nature. 2003;422:675–676. doi: 10.1038/422675a. [DOI] [PubMed] [Google Scholar]
- O’Connell RM, Rao DS, Chaudhuri AA, Boldin MP, Taganov KD, Nicoll J, Paquette RL, Baltimore D. Sustained expression of microRNA-155 in hematopoietic stem cells causes a myeloproliferative disorder. J Exp Med. 2008;205:585–594. doi: 10.1084/jem.20072108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, Johnson RS, Haddad GG, Karin M. NF-κB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1α. Nature. 2008;453:807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A, Keely S, Karhausen J, Gerich ME, Furuta GT, Colgan SP. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology. 2008;134:145–155. doi: 10.1053/j.gastro.2007.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scortegagna M, Cataisson C, Martin RJ, Hicklin DJ, Schreiber RD, Yuspa SH, Arbeit JM. HIF-1α regulates epithelial inflammation by cell autonomous NFκB activation and paracrine stromal remodeling. Blood. 2008;111:3343–3354. doi: 10.1182/blood-2007-10-115758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. Life with oxygen. Science. 2007;318:62–64. doi: 10.1126/science.1147949. [DOI] [PubMed] [Google Scholar]
- Sun HL, Liu YN, Huang YT, Pan SL, Huang DY, Guh JH, Lee FY, Kuo SC, Teng CM. YC-1 inhibits HIF-1 expression in prostate cancer cells: contribution of Akt/NF-κB signaling to HIF-1α accumulation during hypoxia. Oncogene. 2007;26:3941–3951. doi: 10.1038/sj.onc.1210169. [DOI] [PubMed] [Google Scholar]
- Tacchini L, De Ponti C, Matteucci E, Follis R, Desiderio MA. Hepatocyte growth factor-activated NF-κB regulates HIF-1 activity and ODC expression, implicated in survival, differently in different carcinoma cell lines. Carcinogenesis. 2004;25:2089–2100. doi: 10.1093/carcin/bgh227. [DOI] [PubMed] [Google Scholar]
- Taylor CT. Mitochondria and cellular oxygen sensing in the HIF pathway. Biochem J. 2008;409:19–26. doi: 10.1042/BJ20071249. [DOI] [PubMed] [Google Scholar]
- Taylor CT, Colgan SP. Hypoxia and gastrointestinal disease. J Mol Med. 2007;85:1295–1300. doi: 10.1007/s00109-007-0277-z. [DOI] [PubMed] [Google Scholar]
- van Uden P, Kenneth NS, Rocha S. Regulation of hypoxia-inducible factor-1α by NF-κB. Biochem J. 2008;412:477–484. doi: 10.1042/BJ20080476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield AJ, Sawyerr AM, Dhillon AP, Pittilo RM, Rowles PM, Lewis AA, Pounder RE. Pathogenesis of Crohn's disease: multifocal gastrointestinal infarction. Lancet. 1989;2:1057–1062. doi: 10.1016/s0140-6736(89)91078-7. [DOI] [PubMed] [Google Scholar]
- Walmsley SR, Print C, Farahi N, Peyssonnaux C, Johnson RS, Cramer T, Sobolewski A, Condliffe AM, Cowburn AS, Johnson N, Chilvers ER. Hypoxia-induced neutrophil survival is mediated by HIF-1α-dependent NF-κB activity. J Exp Med. 2005;201:105–115. doi: 10.1084/jem.20040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Schmid T, Brüne B. Tumor necrosis factor-a causes accumulation of a ubiquitinated form of hypoxia inducible factor-1α through a nuclear factor-κB-dependent pathway. Mol Biol Cell. 2003;14:2216–2225. doi: 10.1091/mbc.E02-09-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel AS, Johnson RL, Nizit V. Hypoxia inducible factor (HIF) function in innate immunity and infection. J Mol Med. 2007;85:1339–1346. doi: 10.1007/s00109-007-0282-2. [DOI] [PubMed] [Google Scholar]