Abstract
Mammalian K+ homeostasis results from highly regulated renal and intestinal absorption and secretion, which balances the unregulated K+ intake. Aldosterone is known to enhance both renal and colonic K+ secretion. In mouse distal colon K+ secretion occurs exclusively via luminal KCa1.1 (BK) channels. Here we investigate if aldosterone stimulates colonic K+ secretion via BK channels. Luminal Ba2+ and iberiotoxin (IBTX)-sensitive electrogenic K+ secretion was measured in Ussing chambers. In vivo aldosterone was augmented via a high K+ diet. High K+ diet led to a 2-fold increase of luminal Ba2+ and IBTX-sensitive short-circuit current in distal mouse colonic mucosa. This effect was absent in BK α-subunit-deficient (BK−/−) mice. The resting and diet-induced K+ secretion was stimulated by luminal ionomycin. In BK−/− mice luminal ionomycin did not stimulate K+ secretion. In vitro addition of aldosterone likewise triggered a 2-fold increase in K+ secretion, which was inhibited by the mineralocorticoid receptor antagonist spironolactone and the BK channel blocker IBTX. Semi-quantification of mRNA from colonic crypts showed up-regulation of BK α- and β2-subunits in high K+ diet mice. The BK channel could be detected luminally in colonic crypt cells by immunohistochemistry. The expression level of the channel in the luminal membrane was strongly up-regulated in K+-loaded animals. Taken together, these data strongly suggest that aldosterone-induced K+ secretion occurs via increased expression of luminal BK channels.
The colon contributes to K+ homeostasis by either secretion or absorption of K+ under various dietary, hormonal or diseased states (Kunzelmann & Mall, 2002). Normally, the distal mammalian colon displays net K+ secretion (Binder & Sandle, 1994; Kunzelmann & Mall, 2002; Binder, 2003). In patients with end-stage renal disease faecal K+ excretion is directly proportional to dietary K+ intake (Hayes et al. 1967). This implies that colonic ‘K+ adaptation’ makes a substantial contribution to K+ homeostasis in this disease and underscores the importance of defining the elements of colonic K+ handling. Colonic K+ secretion occurs either via a paracellular or a transcellular route (Binder, 2003). The respective quantitative importance of these two K+ secretory pathways has yet to be established. Transcellular K+ secretion occurs according to the pump leak mechanism (Binder & Sandle, 1994). K+ enters the cell from the blood side via the basolateral Na+/K+-ATPase and the Na+/K+/2Cl− cotransporter (NKCC1). K+ exits via luminal K+ channels (Pacha et al. 1987; Sweiry & Binder, 1989; Binder & Sandle, 1994). Previous studies had suggested luminal BK channels as the K+ exit pathway in colonic and renal epithelia (Hunter et al. 1984; Butterfield et al. 1997). Recently, we have identified that BK channels indeed constitute the functionally relevant and only luminal K+ pathway for distal colonic K+ secretion (Sausbier et al. 2006). In the preceding study, Ussing chambers were used to measure ion transport in mice deficient in the BK channel α-subunit (BK−/−). BK−/− mice displayed a significant colonic epithelial phenotype with: (1) lack of Ba2+-sensitive resting K+ secretion; (2) absence of K+ secretion stimulated by luminal P2Y receptors; (3) absence of luminal Ca2+ ionophore-stimulated K+ secretion; and (4) reduced K+ and increased Na+ contents in the faeces. BK channels were shown to localize to the luminal membrane of colonic enterocytes. RT-PCR results confirmed the expression of the BK channel α-subunit in isolated distal colonic crypts (Sausbier et al. 2006).
Colonic K+ secretion is known to be enhanced by aldosterone (Pacha et al. 1987; Sweiry & Binder, 1989; Rechkemmer & Halm, 1989; Halm & Halm, 1994); its mechanism, however, remains incompletely defined. A likely cause of aldosterone-stimulated colonic K+ secretion could be the functional increase of the luminal K+ exit pathway. This could either be mediated by the luminal BK channel itself, or by introduction of an alternative luminal K+ conductance. We therefore used our BK−/− mouse to investigate if these animals treated on a high K+ diet up-regulate an alternative Ba2+-sensitive luminal K+ conductance. It is established that a high K+ diet dramatically increases plasma aldosterone in normal mice and especially BK−/− mice (Rieg et al. 2007). We find here that BK−/− mice on a high K+ diet develop no electrogenic Ba2+-sensitive colonic K+ secretion, despite a dramatically elevated plasma aldosterone (Rieg et al. 2007). Thus, it is very likely that a high K+ diet induces distal colonic K+ secretion via the BK channel. Here we also investigate if the BK channel mediates the increased colonic K+ secretion in mice fed a high K+ diet and in isolated tissue exposed to exogenous aldosterone. For this purpose we searched for a suitable mouse strain with a strong biological responsiveness to endogenous aldosterone. We used the established colonic epithelial aldosterone-responsive gene CHIF/FYXD4 (Wald et al. 1996) to quantify its expression in the NMRI outbred mouse strain and in the inbred B57/Bl6 mouse strain. We found that a high K+ diet strongly up-regulates aldosterone and CHIF in the NMRI mouse strain and to a much lesser degree in B57/Bl6 mice (see Fig. 1 in online Supplemental material). Therefore, we supposed the NMRI mice to be a suitable mouse model to investigate the role of aldosterone on BK channel-mediated distal colonic K+ secretion. We find that aldosterone activates K+ secretion via increased expression and function of luminal BK channels.
Methods
Mice
NMRI wild-type (WT) mice were obtained from Taconic. They were subsequently bred in-house. Experiments were performed on age-matched animals (2–4 months old) of either sex. BK−/− and BK+/+ on a SV129 × C57Bl6 hybrid background (F2 generation) (Sausbier et al. 2004) were bred in the Department of Pharmacology and Toxicology, Institute of Pharmacy, University of Tübingen, Germany. Mice were kept on two different diets both from Altromin (Lage, Germany): a standard diet (1310) with a K+ content of 10 g kg−1 and a high K+ diet (C1050) with a K+ content of 50 g kg−1 for a period of 3–5 days. Experiments were performed according to the Danish legislation on the protection of animals.
Serum aldosterone determinations
Blood sampling for serum aldosterone measurements was performed at 11.00 h in a group of control animals and a group of animals kept on the high K+ diet for 4 days. The animals were anaesthetized with a 3% isoflurane gas mixture, killed and completely drained of blood (approx. 1 ml). Blood was collected in Microtainers (Becton Dickinson) and the tubes were centrifuged at 12 000 g for 2 min to separate serum from the blood cells. The total serum aldosterone was assayed using a commercial radioimmunoassay (RIA, Coat-A-Count) kit (Siemens Medical Solutions Diagnostics, Ballerup, Denmark).
Ussing chamber experiments
Mice of either sex were killed by cervical dislocation. The distal 2 cm of the entire (non-stripped) colonic sheet was mounted in an Ussing chamber. The two halves of the chamber were continuously perfused by a bubble lift system. The solutions on the two sides were symmetrical and had the following composition (in mm): NaCl 120; NaHCO3 25; K2HPO4 1.6; KH2PO4 0.4; calcium gluconate 1.3; MgCl2 1; d-glucose 5; in addition 5 μm indomethacin. The reservoirs were bubbled with 5% CO2 and 95% O2 and kept at 37°C by water jackets. Initially, tetrodotoxin (TTX, 1 μm) was added to the serosal side to inhibit possible secretory activation by the enteric nervous system or other autonomous nerve cells. Subsequently, amiloride (100 μm) was added to the mucosal perfusate to abolish electrogenic Na+ absorption via the epithelial Na+ channel (ENaC).
The experiments with tissue from mice on control and high K+ diets were performed in open-circuit mode using chambers with an aperture of 0.126 cm2. The open circuit experiments in BK−/− mice fed a normal or a high K+ diet (Fig. 2) were performed with an aperture area of 0.283 cm2. The open-circuit recording is the standard method in our laboratory and has the advantage of leaving the tissue in its natural electrical status without applying external electrical forces. The transepithelial voltage (Vte) is given in reference to the serosal side. Transepithelial resistance (Rte) was calculated from the voltage deflections (ΔVte) induced by short current pulses (25 μA, 0.6 s). These deflections were corrected by values obtained in an empty chamber. The equivalent short-circuit current (Isc) was calculated by Ohm's law from Vte/Rte. The calculated Isc changes were derived from peak values. After an equilibration period of 30 min K+ channel activators or inhibitors were added luminally. The measurement of the effect of agonists or antagonists was read 4 min after their addition unless otherwise stated.
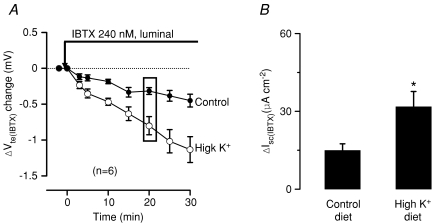
Figure 2. K+ loading increases luminal iberiotoxin-sensitive K+ secretion.
Luminal iberiotoxin (IBTX) slowly changed the transepithelial voltage (Vte) to more lumen-negative values. A is a summary of 6 experiments. B displays the corresponding changes of short-circuit current calculated 20 min after addition of IBTX. Note that the high K+ diet augmented the IBTX-sensitive short-circuit current.
Experiments with exogenous aldosterone were performed in a setup where the transepithelial voltage was clamped to zero (Model DVC-1000, dual voltage clamp, WPI, USA) and the short-circuit current was measured directly. The data were recorded with PowerLab 4/25 from ADInstruments and analysed with the Chart (version 5.1) program. The rationale for changing from open-circuit to direct short-circuit current measurements was to improve the signal-to-noise ratio because capacitance voltage deflections are avoided in short-circuit measurement mode. The Ussing chamber aperture area in the direct Isc measurements was 0.283 cm2. After mounting, the tissues were allowed to equilibrate for 30 min. Then, BaCl2 (5 mm) was added to the luminal solution and the Ba2+-sensitive Isc quantified. The tissues were then incubated for another 2 h with a specified concentration of aldosterone. After the aldosterone-containing solution was gently washed out and a short re-equilibration period of 10 min, BaCl2 (5 mm) was added once more to the luminal solution to quantify the Ba2+-sensitive Isc. In the experiments with spironolactone and iberiotoxin (IBTX) the antagonists were present during the entire aldosterone incubation period.
Note that the experiments in Figs 1 and 2 were performed in a small-aperture tissue holder (0.126 cm2). In contrast, in Figs 3, 6 and 7 a large-aperture tissue holder (0.283 cm2) was used. A smaller size aperture causes an underestimation of the true Rte values (significant edge leakiness of the tissue). This will lead to overestimation of the true equivalent Isc values. Thus, the absolute sizes of the blocker-sensitive K+ secretory conductances can only be compared within experimental series.
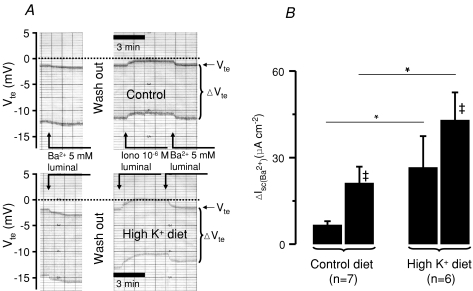
Figure 1. High K+ diet increases Ba2+-sensitive K+ secretion in mouse distal colon.
A, two original Ussing chamber traces are depicted, the upper one is from a control animal and the lower from a mouse fed a high K+ diet. The figure shows the resting and luminal Ba2+ and the luminal ionomycin-induced transepithelial voltage (Vte) changes in the continuous presence of luminal amiloride (100 μm). The upper line indicates Vte and the bandwidth of voltage deflections reflects the transepithelial resistance (Rte, see Methods). After testing the resting luminal Ba2+ effect the tissue was washed and the experiment continued to quantify the Ba2+ after addition of luminal ionomycin. B, summary of calculated Ba2+-sensitive short-circuit current. *Statistically significant difference between animals of a normal and high K+ diet. ‡Significant differences in the paired controls.
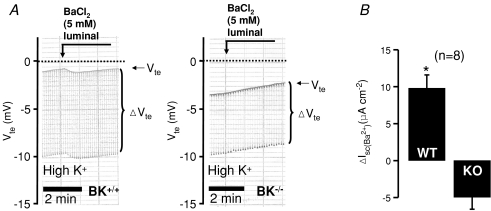
Figure 3. Luminal Ba2+-induced changes of transepithelial voltage in distal colon from BK+/+ and BK−/− mice treated on a high K+ diet for 4 days.
A shows resting and luminal Ba2+-induced Vte changes in the continuous presence of luminal amiloride (100 μm). The upper line indicates Vte and the bandwidth of voltage deflections reflects the transepithelial resistance (Rte, see Methods) B, summary of calculated Ba2+-sensitive short-circuit current. WT, wild-type; KO, knock-out mice. *Statistically significant differences between animals of normal and high K+ diet.
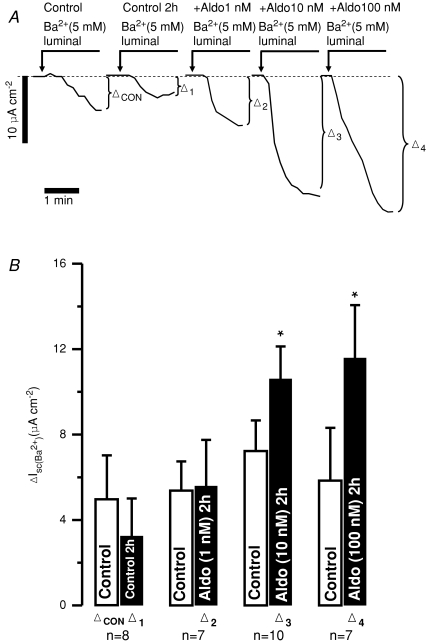
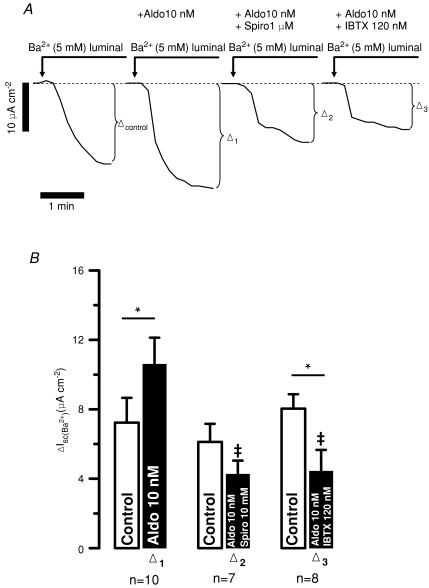
Figure 6. Exogenous aldosterone increases Ba2+-sensitive Isc in freshly isolated mouse distal colonic mucosa.
A, representative original traces from different experiments of luminal Ba2+-induced changes of Isc in time control after 2 h (Δ1) and in tissues treated for 2 h with increasing aldosterone (Aldo) concentrations (1 nm, Δ2; 10 nm, Δ3 and 100 nm, Δ4). B, summary. *Statistical significance (P <0.05) compared to the untreated time controls (2 h).
Figure 7. The Ba2+-sensitive Isc increased by exogenous aldosterone was inhibited by spironolactone (Spiro, 1 μm) and IBTX (120 nm) in freshly isolated mouse distal colonic mucosa.
A, representative original traces of different luminal Ba2+-induced changes of Isc in control, in aldosterone (10 nm, Δ1), in aldosterone (10 nm) plus spironolactone (1 μm, Δ2) and in aldosterone (10 nm) plus IBTX (120 nm, Δ3)-treated tissues. B, summary. ‡Statistical significance compared to tissue treated with aldosterone only (P <0.05). *Statistical significance (P <0.05) compared to the untreated pre-controls.
Crypt preparations
For preparation of colonic crypts a 2 cm piece of mouse distal colon was everted and rinsed with ice-cold Ca2+-free Ringer-type solution with the following composition (in mm): NaCl 127; KCl 5; sodium pyruvate 5; d-glucose 5; 4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid (Hepes) 10; ethylenediaminetetraacetic acid (EDTA) 5; MgCl2 1. Both ends were tied to obtain a sac preparation. This sac was filled with the same Ca2+-free solution. The sacs were then incubated in the-above mentioned solution for 10 min at 37°C. Isolated crypts were obtained by shaking the sacs and this preparation thus comprised almost pure epithelial enterocytes. Approximately 100 crypts were collected from this preparation. Crypts were centrifuged for 1 min at 1520g and the pellet was immediately re-suspended in lysis buffer (RNeasy Mini Kit, Qiagen), vortexed and frozen in liquid nitrogen.
Semi-quantitative PCR analysis of BK channel α- and β-subunits
Semi-quantitative PCR analysis was used to investigate mRNA levels of the murine BK α- and -β1–4-subunits. Total RNA was extracted from isolated colonic crypts (7 control and 7 K+-loaded animals) with the RNeasy Mini Kit (Qiagen). To remove possible genomic DNA contamination, total RNA was treated with RNase-free DNase (Qiagen). RNA was quantified with a Quant-iT RiboGreen RNA reagent (Stratagene). cDNAs were then synthesized from 300 ng of total RNA using SuperScriptIII reverse transcriptase (Invitrogen) and random decamer primers according to the manufacturer's instructions. The primers were validated for non-specific products with SYBR Green. qPCR with TaqMan probes (5′-labelled FAM and 3′-labelled BHQ1) was performed with the ExTaq enzyme (Takara) on an Mx3000P Quantitative PCR System (Stratagene). All reactions were carried out in duplicates. For the β1, β3 and β4 subunits cDNA from a mixture of brain, testis and kidney were used as positive controls. Sequences and final concentrations of primers and probes are shown in Table 1.
Table 1.
Primer and probe sequences and concentrations for qPCR
| Gene name | Sequence | Used concentration (nm) |
|---|---|---|
| KCNMA1 (α) | ||
| Forward primer 5′–3′ | CTCCAATGAAATGTACACAGAATATCT | 300 |
| Reverse primer 3′–5′ | CTATCATCAGGAGCTTAAGCTTCACA | 300 |
| Probe | CCTTCGTGGGTCTGTCCTTCCCTACTGTT | 200 |
| KCNMB1 (β1) | ||
| Forward primer 5′–3′ | TAGTGTGTGCTGCCATCACC | 100 |
| Reverse primer 3′–5′ | GCTCTTCCTGGTCCTTGATATTAG | 300 |
| Probe | CGTCCTGGGTACAACTGTGCTGCC | 300 |
| KCNMB2 (β2) | ||
| Forward primer 5′–3′ | TGCAGGACCAACATCCTCTAAG | 300 |
| Reverse primer 3′–5′ | CTTCAGAGCTGTCACAGTTTTCC | 300 |
| Probe | TGGACCAGTGGCCGGACCTCTTCA | 100 |
| KCNMB3 (β3) | ||
| Forward primer 5′–3′ | ACTCGGGACAGAAAGTGCTTC | 500 |
| Reverse prime 3′–5′r | GATGTCCAGGACGCTGTTGAG | 300 |
| Probe | ACGACGACGAGGCCATCAGGACCA | 300 |
| KCNMB4 (β4) | ||
| Forward primer 5′–3′ | CAACTCCGAGTCCAACTCCAG | 300 |
| Reverse primer 3′–5′ | TCTCTTACAGGGCGGGATATAGG | 100 |
| Probe | ACACAGCGACCAGCACCAGCTCCT | 200 |
| CHIF (corticosteroid hormone-induced factor) | ||
| Forward primer 5′–3′ | TGACCCAGTTGATAAAGACAGTCC | 300 |
| Reverse primer 3′–5′ | CATTTGCACTTGCCACTCAGG | 300 |
| Probe | TTCCAGCGATGCACAGGAGCCCTC | 200 |
| β-Actin | ||
| Forward primer 5′–3′ | TTTGCAGCTCCTTCGTTGCC | 100 |
| Reverse primer 3′–5′ | CACGATGGAGGGGAATACAGC | 500 |
| Probe | TCCACACCCGCCACCAGTTCGCC | 300 |
| HPRT | ||
| Forward primer 5′–3′ | CTTCCTCCTCAGACCGCTTTT | 300 |
| Reverse primer 3′–5′ | TCTAGGTCATAACCTGGTTCATCA | 300 |
| Probe | CGCTAATCACGACGCTGGGACTGC | 300 |
The cycling profile for each run was: 95°C 10 min; 45 cycles 95°C 15 s followed by 60°C for 1 min. The expression of genes of interest was analysed in reference to the endogenous reference genes hypoxanthine-guanine phosphoribosyl transferase (HPRT) and β-actin. As a positive control we chose the corticosteroid hormone-induced factor (CHIF or FXYD4), a γ-subunit of the Na+/K+-ATPase, whose expression level is regulated by aldosterone in colonic epithelium (Wald et al. 1996). Initial relative quantities of the genes of interest were normalized to the reference genes. HPRT and β-actin expression levels were similar in all tested groups.
Immunohistochemistry of BK channels
On-slide 10 μm cryostat-slices from non-fixed BK+/+ and BK−/− mouse distal colon segments were used. After pre-incubation with 10% normal donkey serum in buffer (1% bovine serum albumin, 0.5% Triton X-100, 0.05 m Tris-buffered saline (TBS)) and rinsing with TBS, the slices were incubated with anti-BKα(674–1115) (1 : 1000 in buffer) and tagged with Alexa 555-conjugated donkey anti-rabbit IgG (1 : 1000 in buffer). This antibody was produced in the laboratory of Peter Ruth (Tübingen, Germany). BK channel immunofluorescence was analysed using a confocal laser-scanning microscope (Biorad MRC1000 attached to Nikon Diaphot 300 and equipped with a krypton–argon laser). For histological analysis of distal colon segments from BK+/+ and BK−/− mice the common Masson-Goldner staining method was performed and evaluated using a Leica Aristoplan microscope equipped with a digital camera. No apparent differences were observed (data not shown).
Solutions and chemicals
IBTX was purchased from Latoxan (Valence, France). All other chemicals were supplied from Sigma Aldrich or Merck.
Statistics
The data shown are means ±s.e.m.n refers to the number of tissue preparations and only one preparation was used from each animal. Data were tested for normal distribution (Kolmogorov–Smirnov Test) and Student's t test was used to compare mean values between the experimental series. Two effects in the same tissue were tested paired and effects obtained from different tissues were tested unpaired. ANOVA was used to allow multiple comparisons (data in Figs 1, 3 and 4). P values of < 0.05 were accepted as statistically significant.
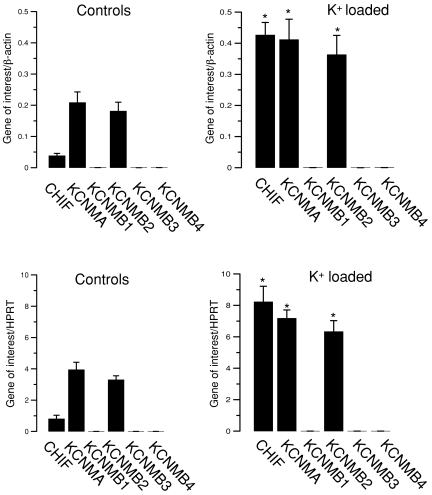
Figure 4. K+ loading increases the mRNA expression levels of BK α- and β2-subunits in isolated colonic crypts (from 7 male animals in each group).
Relative expression levels of mRNA of the genes of interest are shown in comparison to β-actin (upper panels) and to HPRT (lower panels). Control data are shown in the left panels and those obtained from K+-loaded mice on the right. Corticosteroid hormone-induced factor (CHIF) served as a positive control for the action of aldosterone. *Statistically significant difference (P <0.05) between groups.
Results
High K+ diet increases K+ secretion in mouse distal colon
Our preceding publication identified luminal BK channels as mediators of resting and Ca2+-activated K+ secretion in mouse distal colon (Sausbier et al. 2006). Electrogenic K+ secretion ex vivo measured as the Ba2+-sensitive short-circuit current was completely absent in BK−/− mice (Sausbier et al. 2006). Here we confirm that Ba2+ added to the luminal side of freshly isolated distal colonic mucosa from NMRI mice changed the transepithelial voltage instantly to more lumen-negative values (Fig. 1A). In the following experiments the size of the Ba2+-sensitive K+ secretion was quantified in NMRI mice on a normal diet compared to mice fed a high K+ diet for 4 days. This manoeuvre is a well-established way to increase ENaC-mediated electrogenic Na+ absorption in an aldosterone-dependent fashion (Will et al. 1985). As expected, this approach resulted in the induction of a significant amiloride-sensitive Isc (18.3 ± 6.7 μA cm−2 in mice on control diet, n= 7, versus 188.8 ± 48.7 μA cm−2 in mice on high K+ diet, n= 6, see Supplemental Fig. 2). Plasma aldosterone levels after 4 days on a high K+ diet increased from a resting value of 336 ± 80 to 4625 ± 474 pg ml−1 (n= 7).
Figure 1A shows two original Ussing chamber traces after the addition of luminal amiloride (100 μm), one from a control animal and the other from an animal on a high K+ diet. The basal electrical parameters in normal tissue were: Vte−1.9 ± 0.6 mV; Rte 33.1 ± 5.7 Ω cm2 and Isc−62.4 ± 21.6 μA cm−2 (n= 8). Luminal Ba2+ changed Vte by −0.2 ± 0.03 mV to more lumen-negative values. The Ba2+-sensitive Isc amounted to 6.7 ± 1.3 μA cm−2 (Fig. 1B). In animals on a high K+ diet the basal parameters were: Vte−0.3 ± 0.5 mV; Rte 29.2 ± 2.7 Ω cm2 and Isc−7.5 ± 21.0 μA cm−2 (n= 6). Luminal Ba2+ changed Vte to more lumen-negative values by −0.7 ± 0.2 mV. Under these conditions the Ba2+-sensitive Isc had increased to 26.6 ± 10.9 μA cm−2 (Fig. 1B). In summary, these results show that electrogenic distal colonic K+ secretion is significantly increased in NMRI mice fed a high K+ diet. This is consistent with known increased faecal K+ excretion in mice treated on a high K+ diet (Arrighi et al. 2001).
Ba2+-sensitive K+ secretion is activated by luminal ionomycin
In the following experiment we used luminal ionomycin to investigate Ca2+-activated K+ secretion in NMRI mice fed a high K+ diet. Luminal ionomycin is assumed to preferentially increase subapical [Ca2+]i in colonic enterocytes (see Discussion). Figure 1A shows the effect of luminal stimulation with the Ca2+ ionophore ionomycin (1 μm) in the control group as well as in the group on high K+ diet. In control animals addition of luminal ionomycin did not significantly change the transepithelial electrical parameters (Vte: −1.0 ± 0.2 versus−0.8 ± 0.2 mV; Rte 25.4 ± 2.5 versus 24.4 ± 2.2 Ω cm2 and Isc−39.1 ± 8.0 versus−30.9 ± 8.5 μA cm−2, n= 7). Subsequent inhibition of K+ secretion with luminal Ba2+ (5 mm) showed a Ba2+-sensitive Isc of 21.2 ± 5.7 μA cm−2 (Fig. 1B). In the high K+ diet group, addition of luminal ionomycin induced a significant change of transepithelial voltage to more lumen- positive values from 0.5 ± 0.3 to 1.1 ± 0.3 mV (n= 6). Note the positive resting Vte values in the K+-loaded animals. Rte remained unchanged (26.0 ± 4.0 versus 25.3 ± 3.8 Ω cm2) and Isc changed from 21.3 ± 14.3 to 46.4 ± 14.3 μA cm−2. These animals showed a greatly increased Ba2+-sensitive Isc of 44.5 ± 8.9 μA cm−2 (Fig. 1B). These results show that luminal ionomycin in mice on a control diet and in those fed a high K+ diet triggered an increase of luminal Ba2+-sensitive Isc. The magnitude of this luminal ionomycin effect is similar in both experimental series. This indicates that the same amount of additional BK channel-dependent current was activated when [Ca2+]i was increased.
Importantly, BK−/− mice did not show any luminal ionomycin (1 μm)-induced K+ secretion. Basal parameters in BK+/+ mucosa activated with ionomycin were: Vte−1.6 ± 0.3 mV; Rte 27.3 ± 0.9 Ω cm2 and Isc−65.1 ± 12.8 μA cm−2 (n= 9). Luminal Ba2+ changed Vte by −0.7 ± 0.1 mV and Isc by 24.8 ± 2.3 μA cm−2. Rte remained unchanged (0.00 ± 0.52 Ω cm2). In BK−/− preparations electrical values before ionomycin were: Vte−6.0 ± 1.4 mV; Rte 52.7 ± 9.2 Ω cm2; and Isc−127.6 ± 27.5 μA cm−2 (n= 5) and luminal ionomycin had no effect (Vte−6.0 ± 1.4 mV; Rte 52.0 ± 9.0 Ω cm2; and Isc−128.4 ± 28.7 μA cm−2). These data indicate that the K+ diet-induced up-regulated luminal K+ conductance can be activated by an elevation of intracellular Ca2+.
K+ loading increases luminal iberiotoxin-sensitive K+ secretion
In the context of our previous study these results strongly imply that the increased K+ secretion in animals on a high K+ diet occurs via increased activity of luminal BK channels. To investigate this pharmacologically the following experiments were performed to quantify the iberiotoxin-sensitive K+ secretion in control and K+-loaded animals. IBTX is a specific BK channel blocker (Galvez et al. 1990). IBTX-sensitive short-circuit current is taken as a measure of BK channel-mediated K+ secretion. Figure 2A shows the effect of luminal IBTX (240 nm) on the transepithelial voltage. The effect of luminal IBTX compared to luminal Ba2+ is very slow, probably because the diffusion of IBTX to its site of action is impeded by the mucous layer or the complex geometry of the infolded epithelium. Luminal IBTX changed Vte by −0.3 ± 0.1 mV measured after 20 min in controls (n= 6) and −0.8 ± 0.1 mV in K+-fed animals (n= 6). These voltage changes correspond to changes in current by 14.9 ± 2.6 μA cm−2 and 31.8 ± 5.9 μA cm−2, respectively (Fig. 2B). The summary of the IBTX experiments (Fig. 2B) clearly shows that the IBTX-sensitive short circuit current is greatly increased in colonic mucosa from K+-loaded mice. Note that IBTX had no effect on Vte when applied to the luminal surface of BK−/− mouse preparations (Sausbier et al. 2006).
Absence of Ba2+-sensitive K+ secretion in K+-loaded BK−/− mice
The increased K+ secretion on a high K+ diet could be caused by induction of the existing BK channel or alternatively the introduction of a new luminal K+ conductance. Therefore, we investigated the Ba2+-sensitive Isc in BK+/+ and BK−/− mice treated on a high K+ diet for 4 days. Tissue resistances between the two genotypes were not different (BK+/+: 51.9 ± 3.7 Ω cm2, BK−/−: 45.6 ± 2.1 Ω cm2). As reported previously (Sausbier et al. 2006), the resting transepithelial voltages were more lumen-negative in BK−/− mice (BK+/+: −1.6 ± 0.3 mV, BK−/−: −3.8 ± 0.7 mV). The effectiveness of high K+ treatment can be seen as induction of amiloride-sensitive Isc. BK−/− mice under a normal diet have an increased plasma aldosterone level (Sausbier et al. 2005) and this can be indirectly seen as an increased colonic amiloride-sensitive Isc (Sausbier et al. 2006). We confirm these data and find that ΔIsc(amil) was 16.4 ± 3.1 μA cm−2 in BK+/+ (n= 8) versus 46.5 ± 14.8 μA cm−2 in BK−/− (n= 5). After 4 days of a high K+ diet, BK+/+ mice showed a large increase of ΔIsc(amil) to 179.0 ± 40.7 μA cm−2 (n= 8) and BK−/− mice present a dramatic increase of ΔIsc(amil) to 394.3 ± 97.5 μA cm−2 (n= 8, see Supplemental Fig. 2). This has to be viewed in the light of a dramatically increased plasma aldosterone in BK−/− mice on a high K+ diet (Rieg et al. 2007). Importantly, BK−/− mice on a high K+ diet do not show a luminal Ba2+-sensitive Isc (Fig. 3). These results strongly indicate that in the presence of elevated plasma aldosterone no alternative Ba2+-sensitive luminal K+ conductance has appeared. They support the idea that the BK channel is the relevant luminal K+ secretory channel in mouse distal colon.
Semi-quantitative PCR analysis of BK channel gene transcripts
Since aldosterone is a strong modulator of gene expression it is likely that the observed functional effects are caused by transcriptional up-regulation of the relevant K+ channel mRNAs. Therefore, we investigated if a high K+ diet affects the mRNA levels of the α-subunit and the related four β-subunits of the BK channel (KCNMA1, KCa1.1). As a positive control we tested the known aldosterone-induced gene corticosteroid hormone-induced factor (CHIF, FXYD4) (Wald et al. 1996). The genes of interest were compared to the reference genes β-actin and HPRT. The strong up-regulation (8-fold) of CHIF in NMRI mice on a high K+ diet confirmed the strength of the method. K+-loaded animals show a relative 2-fold increase in mRNA expression of the pore-forming BK α-subunit (KCNMA) and the auxiliary BK β2-subunit (KCNMB2) (n= 7). These 2-fold increases of α- and β2-subunit mRNA were identical when compared to both reference genes (Fig. 4). Both, the β1- and β4-subunits showed very low expression in the control colonic preparations and the β3-subunit could not be detected at all. None of these genes changed their expression level after K+ loading.
Immunolocalization of BK channels
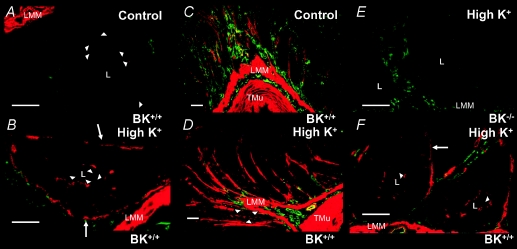
Figure 5 shows the localization of BK channel α-subunits in mouse distal colonic mucosa on a normal diet and after a high K+ diet. The green colour represents intrinsic autofluorescence and provides a crude morphological image of the colonic tissue. The red stain reflects localization of BK channels and the specificity is ensured by the complete lack of staining in BK−/− tissue (Fig. 5E). Under the normal diet weak staining is visible at the luminal membrane domain of crypt cells (Fig. 5A, arrowheads). Animals on a high K+ diet show a marked increase of the staining associated with the luminal membrane of crypt enterocytes (Fig. 5B, D and F; see arrowheads). Unexpectedly, animals on a high K+ diet show strong staining in the tissue surrounding the colonic crypts (Fig. 5). The anatomical structure of this staining remains to be defined. It should be noted that distinct staining of basolateral membrane domains can be seen in Fig. 5B and F, which has newly appeared in mice treated with a high K+ diet.
Figure 5. Immunohistochemical localization of BK channel α-subunits in mouse distal colon under normal and high K+ diet.
The green colour represents intrinsic autofluorescence providing crude morphological orientation. The red stain reflects localization of BK channel α-subunits and is specific as indicated by the complete absence of staining in BK−/− tissue (E). Under normal diet, red staining is faintly visible in the luminal membrane domain of crypt cells (A, arrowheads). Animals on a high K+ diet show a marked increase of red staining associated with the luminal membrane of crypt enterocytes (B, D and F; arrowheads). Mucosa from animals on a high K+ diet showed additional enhanced staining in the tissue surrounding the colonic crypts (D). The anatomical structure of this remains to be established. Note that novel BK staining associated with the basolateral membrane domains can be seen in high K+-treated tissues (arrows, B and F). Scale bars in A, B, E and F: 25 μm; C and D: 50 μm. Abbreviations: L, crypt lumen; LMM, laminal muscularis mucosae; TMu, tunica muscularis mucosae.
Exogenous aldosterone increases colonic K+ secretion in a spironolactone- and iberiotoxin-sensitive manner
In the following experiments we investigated if aldosterone added ex vivo to freshly isolated colonic mucosa is able to mimic the effect of a high K+ diet. Electrogenic K+ transport was measured directly (short circuit) as the Ba2+-sensitive Isc. The size of Ba2+-sensitive K+ secretion was quantified in distal colon before and after 2 h of aldosterone incubation. The resting Ba2+-sensitive Isc in freshly isolated tissue was 4.9 ± 2.1 μA cm−2 and amounted to 3.2 ± 1.8 μA cm−2 (n= 8) after 2 h in the time control experiments (Fig. 6). A concentration of 1 nm aldosterone was not enough to increase K+ secretion after 2 h; however, it appeared to counteract the decay in K+ secretion seen in the time controls. The values were 5.4 ± 1.4 μA cm−2 before and 5.6 ± 2.2 μA cm−2 (n= 7) after the incubation period (Fig. 6). Incubation with 10 nm aldosterone resulted in an increase of Ba2+-sensitive Isc to 10.6 ± 1.5 μA cm−2 (n= 10). The same results were seen with an aldosterone concentration of 100 nm (11.6 ± 2.5 μA cm−2; n= 7; Fig. 6).
Subsequently, we investigated if the effect of aldosterone was mediated via mineralocorticoid receptors (MRs). In the presence of spironolactone (1 μm), 10 nm aldosterone no longer induced K+ secretion. The Ba2+-sensitive Isc was not changed over the 2 h incubation (6.1 ± 1.0 μA cm−2versus 4.3 ± 0.8 μA cm−2, n= 7, Fig. 7). This increase in Ba2+-sensitive K+ secretion occurred via BK channels because IBTX (120 nm) prevented the 10 nm aldosterone-induced Isc increase. The initial Ba2+-sensitive Isc was 8.0 ± 0.8 μA cm−2 and after the incubation it had changed to 4.4 ± 1.2 μA cm−2 (n= 8, Fig. 7). These results show that high K+ diet and exogenously added aldosterone resulted in a similar K+ handling phenotype in mouse distal colon. This K+ secretion is inhibited by MR receptor blockage.
Discussion
Aldosterone increases colonic K+ secretion via up-regulation of luminal BK channel
Colonic K+ handling is one determinant of whole-body K+ homeostasis. Net colonic K+ handling is defined by the balance of K+ absorption via the luminal colonic H+/K+-ATPase and the amount of secreted K+. The ion channel responsible for K+ secretion in the distal colon was recently defined as the Ca2+-activated BK channel in the luminal membrane of colonic crypt epithelium. BK−/− mice showed neither resting nor [Ca2+]i-stimulated luminal K+ secretion, which was reflected in reduced faecal K+ excretion (Sausbier et al. 2006). Colonic K+ secretion is known to be activated by aldosterone (Pacha et al. 1987; Sweiry & Binder, 1989; Rechkemmer & Halm, 1989; Halm & Halm, 1994; Binder & Sandle, 1994). Recently, it was found that knock-out mice for the regulatory KCNE1 subunit of the voltage-dependent IKs K+ channel show significantly elevated plasma aldosterone levels (Arrighi et al. 2001). Interestingly, these mice display profound hypokalaemia and significant faecal K+ wasting (Arrighi et al. 2001). These results support the idea that aldosterone is also an important activator of intestinal K+ excretion in the mouse.
In this study we asked if aldosterone-induced K+ secretion occurs via BK channels. Several lines of evidence support this conclusion: (1) a high K+ diet led to a 2-fold increase of the luminal Ba2+- and IBTX-sensitive Isc in distal mouse colonic mucosa; (2) a high K+ diet in BK−/− mice failed to induce a Ba2+-sensitive K+ secretion; (3) the resting and diet-induced enhanced K+ secretion is stimulated by luminal ionomycin; (4) in BK−/− mice luminal ionomycin was completely ineffective in stimulating K+ secretion; (5) in vitro addition of aldosterone likewise triggered a 2-fold increase in K+ secretion which was sensitive to the MR blocker spironolactone and IBTX; (6) semi-quantitative RT-PCR from isolated colonic crypts showed significant up-regulation of BK α- and β2-subunit mRNA; (7) BK immunohistochemistry confirmed the previous results of the luminal localization in colonic crypt cells and showed that this luminal staining of BK channels is strongly up-regulated in K+-loaded animals. These combined results define that the luminal BK channel is the target of the aldosterone-induced colonic K+ secretion and it is suggested that this occurs via the classic action of aldosterone through its mineralocorticoid receptor.
The nature and localization of the K+ secretory channel in distal colon
The nature of the K+ secretory channel in colonic mucosa was until recently a matter of debate. Several candidates had been suggested including the SK, IK and BK potassium channels (Butterfield et al. 1997; Joiner et al. 2003) and, in addition, many other K+ channels known to be expressed luminally in other segments of the gastrointestinal tract could be putative players (Warth & Barhanin, 2003). The notion that the BK channel is the relevant candidate originated from the following observations: (1) BK channels are present in the luminal membrane of mammalian K+ secretory epithelia (Hunter et al. 1984; Woda et al. 2001; Frindt & Palmer, 2004), and (2) BK channels are found in isolated surface enterocytes from rat distal colon (Butterfield et al. 1997). Our study using the BK−/− mouse provided strong evidence that this channel is the crucial player in mouse distal colonic K+ secretion (Sausbier et al. 2006). This paper also presented evidence that leaves little room for other luminal K+ channels relevant for colonic K+ secretion. However, controversial data exist with regard to the localization of the BK channel along the crypt-to-surface epithelial axis, with several immunolabelling studies supporting BK expression in surface epithelial cells (Grunnet et al. 1999; Hay-Schmidt et al. 2003; Sandle et al. 2007; Puntheeranurak et al. 2007; Flores et al. 2007). In sharp contrast, our preceding results present evidence that luminal BK channels preferentially localize to the crypt epithelium and to a much lesser degree to surface enterocytes (Sausbier et al. 2006). The current immunohistochemical data support the idea that K+ loading does not promote a strong expression in surface cell luminal membranes. Noteworthy, our immunolocalization studies of BK channels in colonic epithelium are currently the only ones available with the important negative control performed in BK−/− tissue. Active K+ secretion can be inhibited by interfering with the basolateral K+ uptake step with either ouabain to inhibit the Na+/K+-ATPase or with loop diuretics to block NKCC1 (Sweiry & Binder, 1989; Rechkemmer et al. 1996). NKCC1 is localized preferentially to the basolateral membranes of the crypt and not to surface colonic epithelium (Pena-Munzenmayer et al. 2005). In summary, this supports the idea that BK channel-dependent K+ secretion is a prominent function of colonic crypts and not surface cells. An unexpected finding demonstrated in Fig. 5B and F indicates that a high K+ diet might provoke BK channel expression in the basolateral membrane of colonic crypt cells. Interestingly, we have recently found that BK channels are not functionally relevant for cholinergic (via intracellular Ca2+)-stimulated anion secretion in colon from animals on a normal diet (Matos et al. 2007). The above-mentioned data indicate that basolateral BK channels may become functionally relevant for [Ca2+]i-activated anion secretion under high K+ diet treatment. This needs to be further investigated.
BK channel-mediated K+ secretion in other secretory epithelia
A comparative view towards other epithelia provides the intriguing observation that K+-secretory tissues in general use this ion channel as one relevant luminal K+ exit pathway. There is circumstantial evidence for this in salivary glands (Romanenko et al. 2007) and several studies highlight the role of BK channels in renal flow-stimulated K+ secretion (Woda et al. 2001; Bailey et al. 2006). In addition to the fundamental role of ROMK channels for renal K+ secretion under basal conditions (Hebert et al. 2005) BK channel-mediated renal K+ secretion works in parallel and becomes active under high tubular flow conditions (Woda et al. 2001; Bailey et al. 2006; Pluznick & Sansom, 2006). BK channels are composed of the pore-forming α-subunit and one associated β-subunit (β1–4). The β-subunit renders the channel functional under physiological membrane voltages and [Ca2+]i concentrations when coexpressed with the α-subunit. It is therefore believed that functional BK channels form hetero-octamers composed of four α-subunits and four associated β-subunits (Salkoff et al. 2006; Torres et al. 2007). We find clear evidence that the associated regulatory β-subunit in colonic enterocytes is β2. Also mRNA of this protein is up-regulated in vivo under conditions of high aldosterone. Intriguingly, the β2-subunit causes rapid inactivation of the human BK channel (Wallner et al. 1999). In mouse distal colon luminal nucleotides via luminal P2Y2 or P2Y4 receptors cause rapid and transient K+ secretory bursts (Matos et al. 2005; Sausbier et al. 2006). It is therefore tempting to speculate that this transient K+ secretion could relate to β2-subunit-mediated fast deactivation of BK channels. Alternatively, the transient K+ secretory burst could merely mirror a fast and transient subapical [Ca2+]i transient.
Our data are in agreement with semi-quantitative RT-PCR data from microdissected rabbit cortical collecting ducts which indicate up-regulation of α- and β2-subunits in animals on a high K+ diet (Najjar et al. 2005). In that study the β3- and β4-subunits were also found to be up-regulated (Najjar et al. 2005), an observation we had not seen in distal colonic mucosa. In renal epithelia the issue of the associated β-subunits in the K+-secretory distal tubule continues to be complex as has been reviewed recently (Pluznick & Sansom, 2006). The β1-subunit has been suggested to be functionally relevant for connecting tubule high flow-induced K+ secretion (Pluznick et al. 2005) and that the β4-subunit may be critical for collecting duct-mediated K+ secretion (Grimm et al. 2007). Further studies are necessary to outline which BK β-subunits are present and functionally relevant in renal and other K+-secretory epithelia.
Time course of up-regulated K+ secretion in colonic epithelium
Mineralocorticoid action on Na+- and K+-transporting epithelia can be divided into early (1.5–3 h) and late (6 h to days) responses (Verrey, 1995, 1999). Non-genomic, very fast aldosterone actions are not discussed here. Transcriptional regulation is the underlying cause of both the ‘regulatory’ fast effects and the ‘anabolic-type’ late effects (Verrey, 1999). Numerous aldosterone-induced and repressed genes are identified today, some of which show expression changes in different aldosterone-sensitive model epithelia within the first 30 min (e.g. SGK1) (Naray-Fejes-Toth & Fejes-Toth, 2000; Robert-Nicoud et al. 2001). Colonic up-regulation of ENaC-mediated Na+ absorption is an example of a late aldosterone response (Epple et al. 2000). In contrast, the effect of aldosterone on the distal colonic K+ secretion reaches maximal values within the first 2 h, which clearly preceded its effect on Na+ transport (Halm & Halm, 1994). In our study aldosterone stimulated colonic K+ secretion in vitro 2-fold after 2 h, which was similar in magnitude to the increase observed after feeding a high K+ diet for 4 days. The fast aldosterone response was completely inhibited with spironolactone demonstrating that transcriptional regulation is involved. The protein and mRNA level of the BK channel proteins were not investigated after 2 h of aldosterone treatment. The cause of the rapid up-regulation of BK channel function remains to be elucidated.
In summary, this study extends our previous finding that BK channels are the sole luminal K+ ion channel relevant for colonic K+ secretion. The aldosterone-induced increase of electrogenic K+ secretion occurs via increased abundance of BK channels in the luminal membrane of colonic crypts and not in colonic surface cells.
No conflict of interest exists.
Supplementary material
Online supplemental material for this paper can be accessed at:
http://jp.physoc.org/cgi/content/full/jphysiol.2008.156968/DC1
References
- Arrighi I, Bloch-Faure M, Grahammer F, Bleich M, Warth R, Mengual R, Drici MD, Barhanin J, Meneton P. Altered potassium balance and aldosterone secretion in a mouse model of human congenital long QT syndrome. Proc Natl Acad Sci U S A. 2001;98:8792–8797. doi: 10.1073/pnas.141233398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey MA, Cantone A, Yan Q, MacGregor GG, Leng Q, Amorim JB, Wang T, Hebert SC, Giebisch G, Malnic G. Maxi-K channels contribute to urinary potassium excretion in the ROMK-deficient mouse model of Type II Bartter's syndrome and in adaptation to a high-K diet. Kidney Int. 2006;70:51–59. doi: 10.1038/sj.ki.5000388. [DOI] [PubMed] [Google Scholar]
- Binder HJ. Intestinal fluid and electrolyte movement. In: Boron W, Boulpaep EL, editors. Medical Physiology. Philadelphia: Saunders; 2003. pp. 931–946. [Google Scholar]
- Binder HJ, Sandle GI. Electrolyte transport in the mammalian colon. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. New York: Raven Press; 1994. pp. 2133–2171. [Google Scholar]
- Butterfield I, Warhurst G, Jones MN, Sandle GI. Characterization of apical potassium channels induced in rat distal colon during potassium adaptation. J Physiol. 1997;501:537–547. doi: 10.1111/j.1469-7793.1997.537bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epple HJ, Amasheh S, Mankertz J, Goltz M, Schulzke JD, Fromm M. Early aldosterone effect in distal colon by transcriptional regulation of ENaC subunits. Am J Physiol Gastrointest Liver Physiol. 2000;278:G718–G724. doi: 10.1152/ajpgi.2000.278.5.G718. [DOI] [PubMed] [Google Scholar]
- Flores CA, Melvin JE, Figueroa CD, Sepulveda FV. Abolition of Ca2+-mediated intestinal anion secretion and increased stool dehydration in mice lacking the intermediate conductance Ca2+-dependent K+ channel Kcnn4. J Physiol. 2007;583:705–717. doi: 10.1113/jphysiol.2007.134387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frindt G, Palmer LG. Apical potassium channels in the rat connecting tubule. Am J Physiol Renal Physiol. 2004;287:F1030–F1037. doi: 10.1152/ajprenal.00169.2004. [DOI] [PubMed] [Google Scholar]
- Galvez A, Gimenez-Gallego G, Reuben JP, Roy-Contancin L, Feigenbaum P, Kaczorowski GJ, Garcia ML. Purification and characterization of a unique, potent, peptidyl probe for the high conductance calcium-activated potassium channel from venom of the scorpion Buthus tamulus. J Biol Chem. 1990;265:11083–11090. [PubMed] [Google Scholar]
- Grimm PR, Foutz RM, Brenner R, Sansom SC. Identification and localization of BK-β subunits in the distal nephron of the mouse kidney. Am J Physiol Renal Physiol. 2007;293:F350–F359. doi: 10.1152/ajprenal.00018.2007. [DOI] [PubMed] [Google Scholar]
- Grunnet M, Knaus HG, Solander C, Klaerke DA. Quantification and distribution of Ca2+-activated maxi K+ channels in rabbit distal colon. Am J Physiol Gastrointest Liver Physiol. 1999;277:G22–G30. doi: 10.1152/ajpgi.1999.277.1.G22. [DOI] [PubMed] [Google Scholar]
- Halm DR, Halm ST. Aldosterone stimulates K secretion prior to onset of Na absorption in guinea pig distal colon. Am J Physiol Cell Physiol. 1994;266:C552–C558. doi: 10.1152/ajpcell.1994.266.2.C552. [DOI] [PubMed] [Google Scholar]
- Hayes CP, Jr, McLeod ME, Robinson RR. An extravenal mechanism for the maintenance of potassium balance in severe chronic renal failure. Trans Assoc Am Physicians. 1967;80:207–216. [PubMed] [Google Scholar]
- Hay-Schmidt A, Grunnet M, Abrahamse SL, Knaus HG, Klaerke DA. Localization of Ca2+-activated big-conductance K+ channels in rabbit distal colon. Pflugers Arch. 2003;446:61–68. doi: 10.1007/s00424-002-0983-x. [DOI] [PubMed] [Google Scholar]
- Hebert SC, Desir G, Giebisch G, Wang W. Molecular diversity and regulation of renal potassium channels. Physiol Rev. 2005;85:319–371. doi: 10.1152/physrev.00051.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter M, Lopes AG, Boulpaep EL, Giebisch GH. Single channel recordings of calcium-activated potassium channels in the apical membrane of rabbit cortical collecting tubules. Proc Natl Acad Sci U S A. 1984;81:4237–4239. doi: 10.1073/pnas.81.13.4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner WJ, Basavappa S, Vidyasagar S, Nehrke K, Krishnan S, Binder HJ, Boulpaep EL, Rajendran VM. Active K+ secretion through multiple KCa-type channels and regulation by IKCa channels in rat proximal colon. Am J Physiol Gastrointest Liver Physiol. 2003;285:G185–G196. doi: 10.1152/ajpgi.00337.2002. [DOI] [PubMed] [Google Scholar]
- Kunzelmann K, Mall M. Electrolyte transport in the mammalian colon: mechanisms and implications for disease. Physiol Rev. 2002;82:245–289. doi: 10.1152/physrev.00026.2001. [DOI] [PubMed] [Google Scholar]
- Matos JE, Robaye B, Boeynaems JM, Beauwens R, Leipziger J. K+ secretion activated by luminal P2Y2 and P2Y4 receptors in mouse colon. J Physiol. 2005;564:269–279. doi: 10.1113/jphysiol.2004.080002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos JE, Sausbier M, Beranek G, Sausbier U, Ruth P, Leipziger J. Role of cholinergic-activated KCa1.1 (BK), KCa3.1 (SK4) and KV7.1 (KCNQ1) channels in mouse colonic Cl− secretion. Acta Physiol (Oxf) 2007;189:251–258. doi: 10.1111/j.1748-1716.2006.01646.x. [DOI] [PubMed] [Google Scholar]
- Najjar F, Zhou H, Morimoto T, Bruns JB, Li HS, Liu W, Kleyman TR, Satlin LM. Dietary K+ regulates apical membrane expression of maxi-K channels in rabbit cortical collecting duct. Am J Physiol Renal Physiol. 2005;289:F922–F932. doi: 10.1152/ajprenal.00057.2005. [DOI] [PubMed] [Google Scholar]
- Naray-Fejes-Toth A, Fejes-Toth G. The sgk, an aldosterone-induced gene in mineralocorticoid target cells, regulates the epithelial sodium channel. Kidney Int. 2000;57:1290–1294. doi: 10.1046/j.1523-1755.2000.00964.x. [DOI] [PubMed] [Google Scholar]
- Pacha J, Popp M, Capek K. Potassium secretion by neonatal rat distal colon. Pflugers Arch. 1987;410:362–368. doi: 10.1007/BF00586512. [DOI] [PubMed] [Google Scholar]
- Pena-Munzenmayer G, Catalan M, Cornejo I, Figueroa CD, Melvin JE, Niemeyer MI, Cid LP, Sepulveda FV. Basolateral localization of native ClC-2 chloride channels in absorptive intestinal epithelial cells and basolateral sorting encoded by a CBS-2 domain di-leucine motif. J Cell Sci. 2005;118:4243–4252. doi: 10.1242/jcs.02525. [DOI] [PubMed] [Google Scholar]
- Pluznick JL, Sansom SC. BK channels in the kidney: Role in K+ secretion and localization of molecular components. Am J Physiol Renal Physiol. 2006;291:F517–F529. doi: 10.1152/ajprenal.00118.2006. [DOI] [PubMed] [Google Scholar]
- Pluznick JL, Wei P, Grimm PR, Sansom SC. BK-β1 subunit: immunolocalization in the mammalian connecting tubule and its role in the kaliuretic response to volume expansion. Am J Physiol Renal Physiol. 2005;288:F846–F854. doi: 10.1152/ajprenal.00340.2004. [DOI] [PubMed] [Google Scholar]
- Puntheeranurak S, Schreiber R, Spitzner M, Ousingsawat J, Krishnamra N, Kunzelmann K. Control of ion transport in mouse proximal and distal colon by prolactin. Cell Physiol Biochem. 2007;19:77–88. doi: 10.1159/000099194. [DOI] [PubMed] [Google Scholar]
- Rechkemmer G, Frizzell RA, Halm DR. Active potassium transport across guinea-pig distal colon: action of secretagogues. J Physiol. 1996;493:485–502. doi: 10.1113/jphysiol.1996.sp021398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechkemmer G, Halm DR. Aldosterone stimulates K secretion across mammalian colon independent of Na absorption. Proc Natl Acad Sci U S A. 1989;86:397–401. doi: 10.1073/pnas.86.1.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieg T, Vallon V, Sausbier M, Sausbier U, Kaissling B, Ruth P, Osswald H. The role of the BK channel in potassium homeostasis and flow-induced renal potassium excretion. Kidney Int. 2007;72:566–573. doi: 10.1038/sj.ki.5002369. [DOI] [PubMed] [Google Scholar]
- Robert-Nicoud M, Flahaut M, Elalouf JM, Nicod M, Salinas M, Bens M, Doucet A, Wincker P, Artiguenave F, Horisberger JD, Vandewalle A, Rossier BC, Firsov D. Transcriptome of a mouse kidney cortical collecting duct cell line: effects of aldosterone and vasopressin. Proc Natl Acad Sci U S A. 2001;98:2712–2716. doi: 10.1073/pnas.051603198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanenko VG, Nakamoto T, Srivastava A, Begenisich T, Melvin JE. Regulation of membrane potential and fluid secretion by Ca2+-activated K+ channels in mouse submandibular glands. J Physiol. 2007;581:801–817. doi: 10.1113/jphysiol.2006.127498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkoff L, Butler A, Ferreira G, Santi C, Wei A. High-conductance potassium channels of the SLO family. Nat Rev Neurosci. 2006;7:921–931. doi: 10.1038/nrn1992. [DOI] [PubMed] [Google Scholar]
- Sandle GI, Perry MD, Mathialahan T, Linley JE, Robinson P, Hunter M, MacLennan KA. Altered cryptal expression of luminal potassium (BK) channels in ulcerative colitis. J Pathol. 2007;212:66–73. doi: 10.1002/path.2159. [DOI] [PubMed] [Google Scholar]
- Sausbier M, Arntz C, Bucurenciu I, Feil S, Zhao H, Sausbier U, Kamm S, Zhou X-B, Essin K, Sailer CA, Krippeit-Drews P, Feil R, Hofmann F, Knaus H-G, Kenyon C, Shipston MJ, Storm JF, Neuhuber W, Korth M, Schubert R, Gollasch M, Ruth P. Elevated blood pressure linked to primary hyperaldosteronism and impaired vasodilation in BK channel deficient mice. Circulation. 2005;112:60–88. doi: 10.1161/01.CIR.0000156448.74296.FE. [DOI] [PubMed] [Google Scholar]
- Sausbier M, Hu H, Arntz C, Feil S, Kamm S, Adelsberger H, Sausbier U, Sailer CA, Feil R, Hofmann F, Korth M, Shipston MJ, Knaus HG, Wolfer DP, Pedroarena CM, Storm JF, Ruth P. Cerebellar ataxia and Purkinje cell dysfunction caused by Ca2+-activated K+ channel deficiency. Proc Natl Acad Sci U S A. 2004;101:9474–9478. doi: 10.1073/pnas.0401702101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sausbier M, Matos JE, Sausbier U, Beranek G, Arntz C, Neuhuber W, Ruth P, Leipziger J. Distal colonic K+ secretion occurs via BK channels. J Am Soc Nephrol. 2006;17:1275–1282. doi: 10.1681/ASN.2005101111. [DOI] [PubMed] [Google Scholar]
- Sweiry JH, Binder HJ. Characterization of aldosterone-induced potassium secretion in rat distal colon. J Clin Invest. 1989;83:844–851. doi: 10.1172/JCI113967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres YP, Morera FJ, Carvacho I, Latorre R. A marriage of convenience: β-subunits and voltage-dependent K+ channels. J Biol Chem. 2007;282:24485–24489. doi: 10.1074/jbc.R700022200. [DOI] [PubMed] [Google Scholar]
- Verrey F. Transcriptional control of sodium transport in tight epithelial by adrenal steroids. J Membr Biol. 1995;144:93–110. doi: 10.1007/BF00232796. [DOI] [PubMed] [Google Scholar]
- Verrey F. Early aldosterone action: toward filling the gap between transcription and transport. Am J Physiol Renal Physiol. 1999;277:F319–F327. doi: 10.1152/ajprenal.1999.277.3.F319. [DOI] [PubMed] [Google Scholar]
- Wald H, Goldstein O, Asher C, Yagil Y, Garty H. Aldosterone induction and epithelial distribution of CHIF. Am J Physiol Renal Physiol. 1996;271:F322–F329. doi: 10.1152/ajprenal.1996.271.2.F322. [DOI] [PubMed] [Google Scholar]
- Wallner M, Meera P, Toro L. Molecular basis of fast inactivation in voltage and Ca2+-activated K+ channels: a transmembrane β-subunit homolog. Proc Natl Acad Sci U S A. 1999;96:4137–4142. doi: 10.1073/pnas.96.7.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warth R, Barhanin J. Function of K+ channels in the intestinal epithelium. J Membr Biol. 2003;193:67–78. doi: 10.1007/s00232-002-2001-9. [DOI] [PubMed] [Google Scholar]
- Will PC, Cortright RN, DeLisle RC, Douglas JG, Hopfer U. Regulation of amiloride-sensitive electrogenic sodium transport in the rat colon by steroid hormones. Am J Physiol Gastrointest Liver Physiol. 1985;248:G124–G132. doi: 10.1152/ajpgi.1985.248.1.G124. [DOI] [PubMed] [Google Scholar]
- Woda CB, Bragin A, Kleyman TR, Satlin LM. Flow-dependent K+ secretion in the cortical collecting duct is mediated by a maxi-K channel. Am J Physiol Renal Physiol. 2001;280:F786–F793. doi: 10.1152/ajprenal.2001.280.5.F786. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.