Abstract
Neural plasticity occurs throughout adult life in response to maturation, use and disuse. Recent studies have documented that H-reflex amplitudes increase following a period of immobilization. To elucidate the mechanisms contributing to the increase in H-reflex size following immobilization we immobilized the left foot and ankle joint for 2 weeks in 12 able-bodied subjects. Disynaptic reciprocal inhibition of soleus (SOL) motoneurons and presynaptic control of SOL group Ia afferents was measured before and after the immobilization as well as following 2 weeks of recovery. Following immobilization, maximal voluntary plantar- and dorsiflexion torque (MVC) was significantly reduced and the maximal SOL H-reflex amplitude increased with no changes in the maximal compound motor response (Mmax). Decreased presynaptic inhibition of the Ia afferents probably contributed to the increase of the H-reflex size, since we observed a significant decrease in the long-latency depression of the SOL H-reflex evoked by peroneal nerve stimulation (D2 inhibition) and an increase in the size of the monosynaptic Ia facilitation of the SOL H-reflex evoked by femoral nerve stimulation. These two measures provide independent evidence of changes in presynaptic inhibition of SOL Ia afferents and taken together suggest that GABAergic presynaptic inhibition of the SOL Ia afferents is decreased following 2 weeks of immobilization. The depression of the SOL H-reflex when evoked at intervals shorter than 10 s (homosynaptic post-activation depression) also decreased following immobilization, suggesting that the activity-dependent regulation of transmitter release from the afferents was also affected by immobilization. We observed no significant changes in disynaptic reciprocal Ia inhibition. Two weeks after cast removal measurements returned to pre-immobilization levels. Together, these observations suggest that disuse causes plastic changes in spinal interneuronal circuitries responsible for presynaptic control of sensory input to the spinal cord. This may be of significance for the motor disabilities seen following immobilization as well as the development of spasticity following central motor lesions.
It is well established that motor skill training may induce use-dependent functional and structural plasticity within the central nervous system. Experience-driven central nervous plasticity has been extensively documented at a cortical level in relation to motor learning (Buonomano & Merzenich, 1998; Sanes & Donoghue, 2000) and there is growing evidence that the cortical plasticity accompanying motor skill learning is paralleled by changes in the properties of the spinal neuronal circuitries as demonstrated in monkey (Wolpaw et al. 1983a,b), rats (Chen & Wolpaw, 1995), in the spinal central pattern generator properties in cats (de Leon et al. 1998; Pearson, 2000; Barriere et al. 2008) and human subjects following skill training (Perez et al. 2005; Meunier et al. 2007; Wolpaw, 2007).
It is less well investigated how and to what extent immobilization is also accompanied by changes in transmission in spinal neuronal circuitries. Spinal cord plasticity can occur at numerous neuronal and synaptic sites and through a variety of mechanisms. In humans, spinal cord plasticity has mostly been inferred from modifications in the size of H-reflexes (Perez et al. 2005, 2007; Gruber et al. 2007; Meunier et al. 2007). The H-reflex provides a gross measure of motoneuron pool excitability and transmission across the synapses of Ia afferents and thus reflects both presynaptic mechanisms, postsynaptic inhibition and excitation, intrinsic motoneuronal mechanisms and descending inputs.
Recent studies have provided evidence that H-reflex amplitudes increase following a period of immobilization. In the rat, Anderson et al. (1999) reported increases in the H-reflex gain following 3 weeks of hindlimb unloading and in human, Clark et al. (2006b) similarly observed increased H-reflex amplitudes at rest following 4 weeks of lower limb suspension. We have recently demonstrated an increase in the excitability of H-reflex following a period of 1 week of wrist and hand immobilization (Lundbye-Jensen & Nielsen, 2008). In this study, the size of the evoked H-reflexes increased with no significant changes in corticospinal excitability estimated from motor evoked potentials (MEPs) evoked by transcranial magnetic stimulation. Although this finding does not provide evidence of the specific mechanisms involved, it led to the hypothesis that immobilization may be accompanied by changes in the amount of presynaptic inhibition of the Ia afferents or post-activation depression (homosynaptic depression). It has previously been demonstrated that a considerable part of the H-reflex amplitude modulation is explained by changes in presynaptic inhibition of the synapses of Ia afferents on spinal motoneurons (Fig. 1; Hultborn et al. 1987a,b; Meunier & Pierrot-Deseilligny, 1989; Nielsen & Kagamihara, 1993b; Faist et al. 1996).
Figure 1. Spinal mechanisms.
During the experiments, EMG was obtained from SOL and TA muscles. Peripheral nerve electrical stimulation was delivered to the posterior tibial nerve (PTN), the common peroneal nerve (CPN) and the femoral nerve (FN) in order to investigate disynaptic reciprocal inhibition, ‘classical’ presynaptic inhibition and post-activation depression.
Presumably, gating of sensory inputs to spinal motoneurons is of functional importance in regulating the contribution of the stretch reflex circuitry to the ongoing motor activity (Nielsen & Sinkjaer, 2002; Rudomin, 2002). One advantage of regulating this contribution at the presynaptic level is that sensory inputs may be selectively gated, while allowing effective activation of the muscles by central commands (Rudomin, 2002). Furthermore, regulation of sensory information at the presynaptic level may permit conveyance of proprioceptive information to the cortex, which might contribute to adjusting supraspinal motor commands (Llewellyn et al. 1990; Morita et al. 1998). In line with this notion, both GABAergic presynaptic inhibition and post-activation depression have been demonstrated to increase following skill learning (Perez et al. 2005; Meunier et al. 2007), and it is possible that presynaptic control of sensory input changes during a period of immobilization. If this is the case it would be consistent with the findings of Manabe et al. (1989) who observed a disuse-induced enhancement of Ia synaptic transmission in spinal motoneurons of the rat.
The hypothesis that immobilization induces modifications of the transmission in the monosynaptic Ia pathway through changes in presynaptic control mechanisms was consequently tested in the present study.
We recorded the soleus H-reflexes before and after 2 weeks of ankle joint immobilization and following a corresponding period of recovery. Disynaptic reciprocal inhibition was evaluated through conditioning the SOL H-reflex by stimulation of the common peroneal nerve (CPN). To evaluate presynaptic inhibition of the soleus Ia afferents we measured the size of the femoral nerve monosynaptic Ia facilitation of the soleus H-reflex (Hultborn et al. 1987a,b) and the long-latency (D2) inhibition of the soleus H-reflex induced by CPN nerve stimulation (Mizuno et al. 1971). To assess post-activation (homosynaptic) depression we used the frequency-related depression of the Sol H-reflex (Crone & Nielsen, 1989b; Hultborn et al. 1996; Kohn et al. 1997).
Methods
Ethics approval
In 12 healthy volunteers (9 male and 3 female) with an average age of 25 ± 6 years (mean ±s.d.) the left foot and ankle joint was immobilized by application of a cast for 2 weeks. No volunteers had any history of neurological disease. Subjects gave informed written consent to the experiments which were approved by the local ethics committee (J. No. KF 100.1969/1991). The experimental procedures conformed to the standards set by the latest revision of the Declaration of Helsinki.
Experimental protocol
All subjects participated in four identical experimental sessions on four separate days. In order to assess day-to-day variability, measurements were obtained on two different days prior to immobilization (pre-tests). In addition, subjects were tested immediately after cast removal following 2 weeks of immobilization (post-test) and once again following 2 weeks of recovery after cast removal (re-test).
During all experimental sessions subjects were seated in a comfortable armchair with the head, torso and arms supported. The examined left leg was flexed in the hip (120 deg), knee (160 deg) and ankle (110 deg) and the foot was fixed to a pedal with a rotational axis located co-axially with the axis of rotation of the ankle joint. The pedal had a built-in potentiometer and was connected to a strain gauge transducer providing information on position and the torque applied. The pedal was fixed and the position of each individual was recorded and re-established in all tests.
Maximal voluntary contraction
In each test, maximal voluntary isometric contraction torque (MVC) was measured for the ankle plantar flexors and dorsi flexors, respectively, in order to evaluate alterations in the maximal strength of the subjects before and after immobilization. Subjects were instructed to perform a maximal contraction by increasing the torque to maximum within a few seconds and to exert maximal torque for 2 s, while maintaining the standardized position. Verbal encouragement and visual feedback of the torque exerted were provided. Typically, four or five successive trials were performed until the peak torque did not increase any further. The peak torque recorded in either of the trials was taken as the MVC. After completion of the strength tests electrophysiological testing procedures involving electrical stimulation of the peripheral nerves were performed.
Stimulation and recording
EMG recording
Electromyographic (EMG) activity was recorded from the soleus (SOL) and tibialis anterior (TA) muscles using a bipolar silver–silver chloride (Ag–AgCl) surface electrode configuration (0.5 cm diameter of electrodes; Blue Sensor, Ambu Inc., Ølstykke, Denmark) with an inter-electrode distance of 2 cm. The amplified EMG signals were filtered (band-pass, 25 Hz to 1 kHz), sampled at 2 kHz, and stored on a PC for off-line analysis (CED 1401+ with Signal & Spike 2.5 software, Cambridge Electronic Design Ltd, Cambridge, UK). In addition, all reflex responses were quantified, averaged and related to Mmax and/or unconditioned response amplitudes online using custom-built software (Winflex).
Soleus H-reflexes
SOL H-reflexes were evoked by stimulating the tibial nerve through a monopolar electrode (1 ms rectangular pulse) in the popliteal fossa (constant-current stimulator model DS7A, Digitimer, UK). The indifferent electrode was placed proximal to the patella. The reflex response was measured as peak-to-peak amplitude of the non-rectified EMG reflex response. The interstimulus interval was 4 s. The stimulus intensity was randomly increased in steps of 0.05 mA, starting below H-reflex threshold (HT) and increasing up to supramaximal intensity to measure the maximal compound motor response (Mmax). SOL H-reflex recruitment curves were generated by averaging five responses at all obtained stimulus intensities. In a recent study we found Hmax to be increased following immobilization and special care was taken to identify Hmax (Lundbye-Jensen & Nielsen, 2008). Stimulus intensities ranged from 0 to 100 mA. In order to enable comparison between reflex responses obtained in different sessions, H-reflex amplitudes were normalized to the corresponding Mmax and stimulation intensities were normalized to Mthreshold in pre-test 1. The sensitivity of the H-reflex to facilitatory or inhibitory conditioning effects has been shown to depend crucially on its size (Crone et al. 1990). During measurements of the effect of a conditioning stimulus, the size of the SOL control reflex was therefore maintained at 20–25% of Mmax. Due to low Hmax amplitudes, a SOL control H-reflex of 15% of Mmax was used in two subjects in order to allow both inhibitory and facilitatory effects.
Disynaptic reciprocal inhibition
In order to assess the amount of disynaptic reciprocal inhibition, the SOL H-reflex was conditioned by previous stimulation of the common peroneal nerve (CPN) at rest. At conditioning test intervals of 2–3 ms (Crone et al. 1987) CPN stimulation elicits a depression of the SOL H-reflex, which is in all likelihood mediated by the disynaptic reciprocal Ia inhibitory pathway (Crone et al. 1987). The CPN was stimulated (1 ms rectangular pulse) through bipolar surface electrodes (Blue Sensor; diameter 0.5 cm) placed 1–3 cm distal to the neck of the fibula. Electrodes were placed in a position to evoke a threshold motor response in the TA muscle without activation of a motor response in the peroneal muscles. The specificity of this stimulation was checked several times during each experiment. The stimulus strength was expressed in multiples of the motor response threshold (1.1 × MT) in the TA muscle. This stimulation intensity was chosen because it elicited a small TA M-response, which could be monitored throughout the measurements and thereby ensure that the effect of the conditioning stimulus was comparable (Petersen et al. 1998). Higher stimulation intensities were avoided to minimize influence from other pathways than the disynaptic Ia reciprocal pathway to the measured inhibition (Petersen et al. 1998). Also, a stimulation intensity of 1.1 × MT is submaximal for activation of all inhibitory interneurones and thus permits both facilitatory and inhibitory effects on transmission in the pathway to be demonstrated (Petersen et al. 1998). In each experiment a time course of the effect of the CPN stimulation was recorded for every subject with conditioning–test intervals ranging from 1 to 6 ms. In a random sequence, 10 reflex responses were obtained at each conditioning–test interval with an inter-stimulus interval (ISI) of 4 s. Responses were averaged and expressed relative to the obtained unconditioned SOL H-reflex amplitude.
Presynaptic inhibition of the Ia afferent terminals
In order to assess the role of presynaptic inhibition of the Ia afferent terminals we applied two different protocols involving conditioning of the SOL H-reflex: femoral nerve (FN) facilitation and ‘D2’ inhibition. Changes in heteronymous Ia facilitation and D2 inhibition reflect changes in different Ia pathways to SOL motoneurones, heteronymous and homonymous, respectively. Previous studies have, however, documented that presynaptic inhibition of heteromymous and homonymous Ia pathways to SOL motoneurones is similarly controlled by descending and/or afferent input (Katz et al. 1988; Meunier & Pierrot-Deseilligny, 1989; Faist et al. 1996), suggesting that it is mediated by the same or similar primary afferent depolarization (PAD) interneurones (Hultborn et al. 1987a,b). It is, however, advantageous to obtain both measures in order to interpret the observed changes.
Heteronymous facilitation
The SOL H-reflex was conditioned by stimulating the femoral nerve (FN). This method was used to assess the ongoing presynaptic inhibition exerted on the Ia afferents mediating a monosynaptic conditioning excitatory volley (Hultborn et al. 1987a). In man, there are heteronymous, monosynaptic excitatory projections from Ia fibres in the FN nerve to SOL motoneurones and from Ia afferents in the PT nerve on quadriceps motoneurones (Hultborn et al. 1987a; Meunier et al. 1993). The FN was stimulated through a monopolar ball electrode placed over the femoral triangle. The indifferent electrode was placed just below the gluteus maximus muscle. The intensity for stimulating the FN was 1.5 × MT in the quadriceps muscle. In each experiment, a time course of the effect of the FN facilitation was recorded for every subject (Hultborn et al. 1987a,b) with conditioning–test intervals ranging from –8 to –3 ms (Fig. 5). The negative conditioning–test intervals designate that the conditioning stimulation was applied after the test stimulation. In a random sequence, 10 responses were obtained for each conditioning–test interval and the average response amplitudes were related to the unconditioned reflex amplitude. The onset of facilitation was considered to be the earliest interval at which the conditioned reflex was 10% larger than the test reflex. Following this procedure, a more detailed time course with steps of 0.2 ms for the 1 ms prior to the onset of facilitation was obtained. Using this procedure FN facilitation was quantified for the optimal conditioning–test interval for each subject.
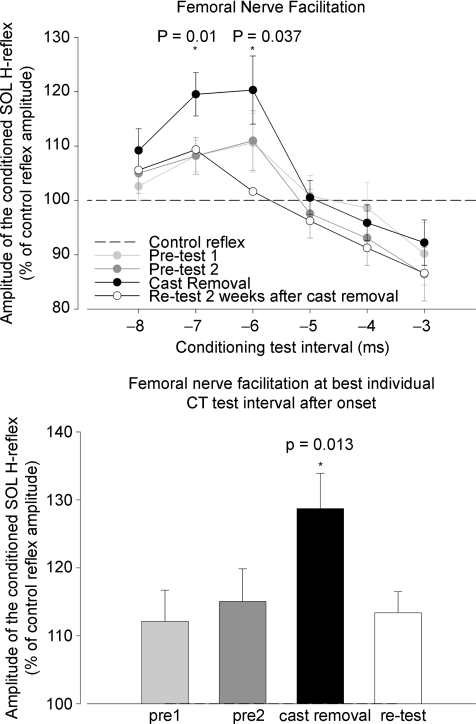
Figure 5. Presynaptic inhibition estimated from SOL H-reflex facilitation by conditioning stimulation of the femoral nerve (FN).
The soleus H-reflex was facilitated by a heteronymous Ia volley from quadriceps and the amount of reflex facilitation was used to estimate the size of the conditioning Ia excitatory postsynaptic potential (EPSP). The upper panel illustrates the time course (at rest) of the effect of conditioning femoral nerve (FN) stimulation (single 1 ms) stimuli applied to the FN at the femoral triangle at 1.5 × MT on the size of the soleus test H-reflex. Size of the unconditioned soleus H-reflex 20–25% of Mmax. Conditioning test intervals were –8 to –3 ms. The abscissa shows the interval in ms between the conditioning stimulus and the test stimulus. The ordinate shows the size of the conditioned test reflex expressed in percentage of the test reflex. The time course was obtained before immobilization (pre-test 1, light grey; and pre-test 2, dark grey) after immobilization (post-test, black) and 2 weeks after cast removal (re-test, white). Based on the onset of facilitation a detailed time course in steps of 0.2 ms was obtained for each subjects in each test. The bottom panel illustrates this facilitation at best individual conditioning test intervals before, after and 2 weeks after immobilization. All depicted values are group average ±s.d.* denotes P ≤ 0.05.
As demonstrated by Hultborn et al. (1987a,b), the short-latency facilitation of the SOL H-reflex produced by stimulation of femoral nerve Ia afferents can be ascribed to a monosynaptic heteronymous Ia excitatory postsynaptic potential (EPSP). Hultborn et al. (1987a) provided experimental evidence that during its first 0.5 ms this heteronymous Ia facilitation is only mediated through a monosynaptic pathway and not contaminated by input from oligo- or polysynaptic pathways (Pierrot-Deseilligny et al. 1981; Pierrot-Deseilligny, 1996). This is why in the present study the onset of the facilitation was examined in detail in the individual subjects with conditioning test intervals in steps of 0.2 ms to carefully measure the amount of facilitation within the initial 0.5 ms after this onset. Provided that stimulations were elicited at these appropriate conditioning–test intervals, this early heteronymous Ia facilitation depends only on the size of the conditioning monosynaptic Ia EPSP. Changes in the size of this early facilitation can therefore be used to assess ongoing presynaptic inhibition of Ia terminals to SOL motoneurones (Hultborn et al. 1987a,b). Accordingly, a constant conditioning stimulation of the femoral nerve, which in this study was adjusted to 1.5 × MT, should elicit an EPSP of a constant size in the motoneurones, and thus a constant reflex facilitation, unless presynaptic inhibition of Ia afferents mediating the conditioning Ia volley has changed. The smaller this ongoing presynaptic inhibition the larger the reflex facilitation.
D2 inhibition
Using CPN conditioning of the SOL H-reflex at longer conditioning–test intervals than those applied when investigating disynaptic reciprocal inhibition, CPN stimulation elicits a pronounced depression of the SOL H-reflex reaching a maximum for conditioning–test intervals around 15–20 ms. At conditioning–test intervals around 60 ms a second, more pronounced depression occurs. Although it is thought of as one long depression separated by a period of facilitation, the early and late depressions are referred to as D1 and D2, respectively (Mizuno et al. 1971). Previous findings have suggested that both D1 and D2 are in all likelihood influenced/caused by presynaptic inhibition of the terminal of Ia afferents on SOL motoneurones. Considering D2, there is no inhibition in the EMG corresponding to the D2 time course as it would have been in the case of postsynaptic inhibition (Iles & Roberts, 1986). In this study, we looked at D2 inhibition primarily because pilot experiments demonstrated D2 to have a better reproducibility than D1 between experimental sessions. Secondly, postsynaptic inhibition may last 20–30 ms (Baldissera et al. 1981) and may therefore interfere with D1.
In the present experiment, D2 inhibition was assessed by conditioning the SOL H-reflex by CPN stimulation at conditioning–test intervals of 40, 50, 60, 70, 90 and 100 ms. In a random sequence, 10 responses were obtained for each conditioning–test interval and the average response amplitudes were related to the unconditioned reflex amplitude.
FN facilitation and D2 inhibition provide independent information about presynaptic inhibition and help us to exclude changes in recruitment gain in the soleus motoneurones as a cause of changes in the H-reflex size (Nielsen & Kagamihara, 1993a,b). D2 (and D1) inhibition reflects the level of presynaptic inhibition evoked by peripheral nerve stimulation and FN facilitation reflects the level of ongoing presynaptic inhibition of FN Ia afferents (which is paralleled by SOL Ia afferents).
Post-activation depression
Another presynaptic mechanism that could be affected by immobilization, is post-activation (or homosynaptic) depression originally described by Curtis & Eccles (1960). Post-activation depression reflects a reduced Ia afferent transmitter release probability due to previous activation (Crone & Nielsen, 1989a; Nielsen et al. 1993, 1995; Aymard et al. 2000) and it is therefore only observed when the Ia afferents have been previously activated by a conditioning stimulus of the same afferents (e.g. vibration, tendon tap, electrical nerve stimulation; Crone & Nielsen, 1989a). The depression of the monosynaptic Ia EPSP results primarily from mechanisms operating within the presynaptic terminals, which depends on the history of activation of the synapse (Lev-Tov & Pinco, 1992; Pinco & Lev-Tov, 1993). Usually, post-activation depression is observed for more than 10 s following a preceding activation (conditioning stimulus), compared with only 300–400 ms for ‘classical’ presynaptic inhibition (Hultborn et al. 1996). Unlike GABAergic presynaptic inhibition, post-activation depression is also not widely distributed among the afferent fibres (Hultborn et al. 1996).
In the present study, post-activation depression was induced by electrical activation of the soleus Ia afferents and evaluated by frequency-related changes in the H-reflex. Ten SOL H-reflexes (15–25% of Mmax) were evoked with an inter-stimulus interval of 10 s. After this, 10 SOL H-reflexes were evoked with an identical stimulus intensity and an inter-stimulus interval of 1 s. Since the first reflex in this series was not influenced by post-activation depression it was excluded and the remaining nine responses were averaged. Post-activation depression was quantified as the difference between the average reflex amplitudes obtained at 10 s and 1 s inter-stimulus interval. That is, the depression of the SOL H-reflex elicited by shortening the inter-stimulus interval from 10 s to 1 s.
Contractile properties
In order to test changes in muscular contractile properties, supra-maximal electrical stimulation was delivered to the PTN and CPN to elicit maximal twitch contractions. Stimulation consisted of a 1 ms rectangular pulse delivered by a constant-current stimulator (model DS7A, Digitimer). The elicited twitch torque was registered and peak torque, time-to-peak torque and half-relaxation time were calculated during offline analysis.
Immobilization
At the end of the second pre-test, a custom-moulded circular ankle cast (X-lite, Camp Scandinavia) was configured for each subject. The subject's left ankle and foot was covered by a stockinette and foam padding and the cast was positioned around the ankle, foot and toes, maintaining the ankle joint in a neutral position restricting ankle joint movements. Subjects were not able to remove the cast themselves, ensuring that joint immobilization was effective for the full period. Subjects were instructed to walk with crutches and to avoid ground contact with the immobilized foot throughout the immobilization period. After 2 weeks of immobilization the cast was removed by the experimenter immediately before the beginning of the post-test.
Data analysis and statistics
Reflex measurements
The mean and standard error of the mean (s.e.m.) were calculated for all parameters and conditions.
Considering SOL H-reflexes, all stimulation intensities were normalized to Mthreshold in pre-test 1 and all response amplitudes were normalized to the corresponding Mmax. Hmax was quantified from the H-reflex recruitment curve.
One-way repeated measures ANOVA test was used to determine the effect of immobilization on MVC, twitch torque, time to peak, half-relaxation time, Mmax, Hmax/Mmax ratios, disynaptic reciprocal inhibition, femoral nerve facilitation, D2 inhibition and post-activation depression with time of measurements as a factor. Tukey post hoc test was performed on significant comparisons. All values are reported as mean ±s.d. unless stated otherwise. In all tests, statistical significance was assumed if the P < 0.05.
Results
Following immobilization, maximal voluntary plantar and dorsiflexion torque decreased significantly (Fig. 2). Plantar flexion torque decreased ∼15% from 69.1 ± 21.2 Nm in pre-test 1 and 69.3 ± 19.8 Nm in pre-test 2 to 59 ± 20 Nm immediately after cast removal (P < 0.001 and P < 0.001). After 2 weeks of recovery plantar flexion, MVC returned to baseline level (69.9 ± 20.5 Nm; P < 0.001 compared to post-immobilization, P = 0.42 and P = 0.46 compared to pre-test 1 and 2, respectively). For dorsiflexion MVC torque also decreased significantly ∼23% from 44.1 ± 9.2 Nm in pre-test 1 and 43.7 ± 11 Nm in pre-test 2 to 33.6 ± 8.1 Nm immediately after cast removal (P < 0.001). After 2 weeks of recovery, MVC torque returned to 40.4 ± 11.5 Nm (P = 0.004 compared to post-immobilization, P = 0.753 and 0.65 compared to pre-test 1 and 2, respectively).
Figure 2. Maximal voluntary contraction torque (MVC) was measured during ankle joint plantar flexion and dorsiflexion.
Measures of maximal isometric muscle strength (MVC) before immobilization (grey bars), after immobilization (black bars) and after 1 week of recovery (white bars). The figure illustrates group mean peak torque (Nm) ±s.d. for plantar flexion and dorsiflexion. * denotes P ≤ 0.05, *** denotes P < 0.001
Following supra-maximal PTN stimulation, three parameters were quantified from the elicited twitch contraction in order to assess changes in contractile properties. These three parameters were twitch contraction torque (TT), contraction time (CT) (time-to-peak torque) and half-relaxation time (HRT). TT was 5.34 ± 2.83 and 5.24 ± 2.83 Nm in pre-test 1 and 2, respectively. Following immobilization, TT increased significantly to 8.29 ± 4.6 Nm (P = 0.03 and P = 0.048, respectively). After 2 weeks of recovery, TT decreased to 6.72 ± 2.83 Nm (P = 0.084 compared to post-immobilization, P = 0.994 and 0.994 compared to pre-test 1 and 2, respectively). There were no significant changes in the CT from before immobilization (116.5 ± 21 ms and 111.4 ± 17 ms) to cast removal (114.7 ± 19 ms) or following 2 weeks of recovery (106.1 ± 9 ms) (P = 0.206). There was, however, a tendency towards a change in half-relaxation time between tests. Before immobilization, HRT was 83 ± 21 ms and 86 ± 23 ms. Following immobilization HRT was prolonged to 101.6 ± 16 ms (P = 0.067) After 2 weeks of recovery HRT decreased to 93.7 ± 20 ms.
The peak-to-peak amplitude of the SOL maximal compound action potential (Mmax) did not change significantly following immobilization (P = 0.28). The group average Mmax±s.d. was 33.5 ± 12.9 mV and 31.9 ± 12.5 mV in pre-tests, 30.9 ± 14 mV following immobilization and 31.6 ± 9.1 mV after 2 weeks of recovery.
H-reflexes
SOL H-reflex amplitudes measured at rest increased significantly following immobilization (Fig. 3). We recently demonstrated how Hmax/Mmax ratios and H-reflex recruitment curve properties changed significantly following immobilization. Because of this, and in order to avoid an excessive number of stimulations, we focused on determining the Hmax/Mmax ratio in the present study (Lundbye-Jensen & Nielsen, 2008). The SOL Hmax/Mmax ratio increased significantly from 0.566 ± 0.21 and 0.553 ± 0.2 in pre-tests to 0.644 ± 0.23 after immobilization (P = 0.03 and P = 0.021). Following recovery the Hmax/Mmax ratio returned to pre-immobilization levels of 0.565 ± 0.24 (P = 0.032 compared to post-immobilization, P = 0.999 and P = 0.988 compared to pre-test 1 and 2, respectively). There were no significant changes in the H-reflex latency from 31.7 ± 2.7 and 31.8 ± 3.1 ms in pre-tests to 32.3 ± 2.9 ms following immobilization. In the re-test the H-reflex latency was 32.2 ± 3.3 ms (P = 0.2).
Figure 3. Hoffmann reflex and M-wave recruitment curves, and Hmax/Mmax ratios.
Hoffmann reflexes were elicited in SOL before (grey), after (black) and 2 weeks after immobilization (white). The upper panel illustrates Hoffmann reflex (circles) and M-wave (squares) recruitment curves for a representative subject. The abscissa represents the stimulation intensity normalized to the M-wave threshold in pre-test 1, the ordinate illustrates response amplitude normalized to the corresponding Mmax. The lower panel illustrates group average Hmax/Mmax ratios (±s.d.) before (pre1 and pre2), after (cast removal) and 2 weeks after (re-test) immobilization. * denotes P ≤ 0.05.
Disynaptic reciprocal inhibition
Figure 4 illustrates the time course of the inhibition of the SOL H-reflex at rest following single stimuli at 1.1 × MT to the common peroneal nerve expressed relative to the unconditioned H-reflex size. The inhibition was apparent when the conditioning CPN stimulation preceded the PTN test stimulus by 2 ms (88.7 ± 11%; 90 ± 7.2%; 90.9 ± 8.7%; 90.9 ± 13%), 3 ms (88.2 ± 13%; 86.9 ± 9.1%; 92.4 ± 10.3%; 84.8 ± 14%) and 4 ms (89.1 ± 10.9%; 89.2 ± 14.6%; 91 ± 14.4%; 85.6 ± 9.2%) in the pre-, post- and re-tests, respectively (Fig. 4). No significant differences in disynaptic reciprocal inhibition were observed between the pre-, post- and re-test data at any of the conditioning–test intervals (P = 0.39–0.94).
Figure 4. Measurement of disynaptic reciprocal inhibition from ankle dorsiflexors to ankle plantar flexors by the H-reflex technique at rest.
The time course (at rest) of the effect of conditioning peroneal nerve stimulation (single 1 ms) stimuli applied to the peroneal nerve 2–3 cm distal to the caput fibulae at 1.1 × MT on the size of the soleus test H-reflex. (This was evoked by single 1 ms stimuli applied to the tibial nerve in the popliteal fossa. Size of the unconditioned soleus H-reflex 20–25% of Mmax.) The abscissa shows the interval in ms between the conditioning stimulus and the test stimulus. The ordinate shows the size of the conditioned test reflex expressed in percentage of the test reflex. The time course was obtained before immobilization (pre-test 1, light grey; and pre-test 2, dark grey) after immobilization (post-test, black) and 2 weeks after cast removal (re-test, white). The short-latency, presumed disynaptic, reciprocal inhibition was seen at conditioning-test (CT) intervals between 2 and 4 ms. It was expressed as the size of the conditioned H-reflex as a percentage of the control H-reflex size. The figure illustrates group average ±s.d.
Femoral nerve facilitation
Figure 5 illustrates the early facilitation of the SOL H-reflex evoked by stimulation of the femoral nerve stimulation at 1.5 × MT for quadriceps expressed relative to the unconditioned H-reflex size. The facilitation of the SOL H-reflex induced by femoral nerve stimulation had an onset at a conditioning–test interval around –5.5 ms (negative conditioning–test intervals designate that the control stimulus preceded the conditioning stimulus). At a conditioning–test interval of –6 ms the average facilitation was 110.6 ± 18.7%, 111 ± 19%, 120.3 ± 21%, 101.6 ± 18% in pre-, post- and re-tests, respectively, with a significantly increased facilitation following immobilization (P = 0.037). At a conditioning–test interval of –7 ms, the facilitation was 108.2 ± 9.9% and 108.2 ± 11.7% in pre-test 1 and 2. It increased significantly to 119.5 ± 13.8% following immobilization (P = 0.01) and decreased to 109.3 ± 9% after 2 weeks of recovery (P = 0.089 compared to post- and P = 0.916 compared to pre-tests)
In order to ensure that the facilitation was caused solely by transmission in the Ia monosynaptic pathway from the quadriceps to SOL motoneurones, an individually optimal conditioning–test interval was applied with 0.2 ms accuracy to evaluate the size of the early facilitation before and after immobilization and recovery (Hultborn et al. 1987a) (bottom panel in Fig. 5). The average size of the FN facilitation from all subjects at this individual interval was 112.2 ± 4.6% and 115 ± 5.2% in the pre-tests (size of the conditioned reflex expressed as a percentage of the control reflex). After immobilization the facilitation increased to 128.2 ± 5.2% (P = 0.013). After 2 weeks of recovery the facilitation returned to baseline values of 113.4 ± 3.1% (P = 0.033 compared to post-, P = 0.995 and P = 0.989 compared to pre-test 1 and 2, respectively.
D2 inhibition
Figure 6 illustrates the D2 inhibition which could be observed at conditioning test intervals of 70, 80, 90 and 100 ms. At a 80 ms conditioning–test interval, the conditioned SOL H-reflex was 69.8 ± 22.2% and 72.7 ± 28% of the unconditioned reflex amplitude before immobilization and 84.5 ± 22.9% (P = 0.156) following immobilization. After recovery the conditioned response returned to pre-immobilization level of 70.6 ± 36%. At a 90 ms conditioning–test interval, the conditioned reflex was depressed to 58.9 ± 21.5% and 59.7 ± 25.7% before immobilization. This inhibition decreased following immobilization to 72.6 ± 17.2% (P = 0.08) but returned to pre-immobilization level (59.6 ± 35%) following 2 weeks of recovery.
Figure 6. D2 inhibition.
Time course of the long-latency depression of the SOL H-reflex (D2 inhibition) evoked at 40–100 ms conditioning-test interval by conditioning peroneal nerve stimulation (single 1 ms) stimuli applied to the peroneal nerve 2–3 cm distal to the caput fibulae at 1.1 × MT. The abscissa shows the interval in ms between the conditioning stimulus and the test stimulus. The ordinate shows the size of the conditioned test reflex expressed in percentage of the test reflex. The time course was obtained before immobilization (pre-test 1, light grey; and pre-test 2, dark grey) after immobilization (post-test, black) and 2 weeks after cast removal (re-test, white). All depicted values are group average ±s.d.* denotes P ≤ 0.05.
At a 100 ms conditioning–test interval, the conditioned H-reflex was 52.9 ± 19.8% and 51.7 ± 25.7% of the unconditioned H-reflex before immobilization. Following immobilization the response was significantly larger (71.1 ± 26.9%; P = 0.024 and P = 0.015). After 2 weeks of recovery the conditioned response returned to the pre-immobilization level of 54.4 ± 34% (P = 0.063 compared to post-immobilization, P = 0.996 and P = 0.998 compared to pre-tests).
Post-activation depression
Figure 7 illustrates the depression of the SOL H-reflex observed when decreasing inter-stimulus intervals from 10 s to 1 s. At 10 s inter-stimulus interval, the SOL H-reflex amplitude was 27.7 ± 7% and 26.9 ± 7.6% of Mmax in pre-test 1 and 2, 26.8 ± 7% of Mmax after immobilization and 27.7 ± 8.2% of Mmax in the re-test. There was no significant difference in this parameter between tests (P = 0.935).
Figure 7. Post-activation depression.
The figure illustrates the frequency-related depression of the SOL H-reflex at interstimulus intervals of 1 s relative to 10 s. In A, the ordinate shows the average amplitude of the SOL H-reflex obtained at 1 s ISI in percentage of the average SOL H-reflex amplitude obtained at 10 s ISI. In B, the ordinate shows the amount of the frequency-related (post-activation) depression from the control reflex obtained at 10 s ISI. The data were obtained before immobilization (pre-test 1, light grey; and pre-test 2, dark grey) after immobilization (post-test, black) and 2 weeks after cast removal (re-test, white). All depicted values are group average ± s.d.* denotes P ≤ 0.05.
Before immobilization, the depression of the SOL H-reflex amplitude was 61.7 ± 5% in pre-test 1 and 59.6 ± 6.6% in pre-test 2. Following immobilization, the amount of post-activation depression decreased significantly to 43.7 ± 9.4% (P = 0.001 and P = 0.005 compared to pre-tests). After 2 weeks of recovery, post-activation depression increased to pre-immobilization level of 58.6 ± 11.8% (P = 0.008 compared to post-test, P = 0.874 and P = 0.995 compared to pre-tests).
Discussion
The primary purpose of the present study was to elucidate adaptations in spinal neuronal circuitries in relation to immobilization. The main behavioural finding was that 2 weeks of ankle joint immobilization leads to significant decreases of maximal voluntary strength. This was accompanied by an increase in the SOL H-reflex excitability in the immobilized leg as has previously been reported (Duchateau, 1995; Anderson et al. 1999; Clark et al. 2006b; Lundbye-Jensen & Nielsen, 2008). Based on the applied conditioning–test protocols of the SOL H-reflex, it appears that the observed increase of H-reflex excitability at least in part relates to changes in presynaptic control of group Ia afferents following immobilization whereas disynaptic reciprocal inhibition was unaffected by the immobilization.
MVC and twitch contractions
In the present study, immobilization led to a 14% reduction of plantar flexion MVC and a 23% reduction of dorsiflexion MVC. These changes are in agreement with the findings in previous studies reporting 17% and 19% decreases in triceps surae MVC following 2 weeks (Gondin et al. 2004) and 3 weeks (Davies et al. 1987) of immobilization, respectively. Depending on the duration of the immobilization period, the observed decrease in voluntary strength has been attributed partly to changes in neural activation and partly to changes in muscle contractile properties (White et al. 1984; Davies et al. 1987; Duchateau & Hainaut, 1987; Duchateau, 1995; Seki et al. 2001a,b; Gondin et al. 2004; Clark et al. 2006a,b). Several studies have found decreases in voluntary activation following immobilization (Vandenborne et al. 1998; Kawakami et al. 2001; Gondin et al. 2004). Seki et al. (2001a) reported a restriction of motoneurone firing to lower rates following immobilization and reduced maximal motoneuronal firing rates following only 1 week of immobilization (Seki et al. 2001a, 2007). Judged from the plantar flexor twitch measures used in the present study, it is not unlikely that changes in muscle contractile properties may have contributed to the reduced voluntary strength observed following immobilization. The finding of increased twitch contraction torque (TT) and the tendency toward prolonged half-relaxation time (HRT) following immobilization is indeed in agreement with previous studies on short-term immobilization (White et al. 1984; Davies et al. 1987; Seki et al. 2001b; Gondin et al. 2004). The trend towards increased HRT has previously been suggested to relate to reduction in the reuptake of Ca2+ by the sarcoplasmatic reticulum following immobilization (Thom et al. 2001). Although immobilization was accompanied by changes in twitch contraction parameters in the present study, the exact origin of these alterations remains to be determined.
Interestingly, immobilization was accompanied by a larger reduction of the MVC for dorsiflexion compared to plantar flexion. One possible reason for this could be differences in muscle characteristics, fibre-type distribution and myosin heavy chain (MHC) expression demonstrating different sensitivities to inactivity. Secondly, ankle joint immobilization, while restricting TA and SOL contractions, does not completely immobilize the biarticular gastrocnemius muscle. Although twitch contraction measures changed for triceps surae as a whole, the fact that contraction of gastrocnemius was possible during the immobilization may have counteracted loss of plantar flexion MVC following immobilization. In line with this, Gondin et al. (2004) found SOL, but not gastrocnemius, EMG activity to be significantly reduced following ankle joint immobilization.
Increased Hmax/Mmax ratio
In the present study we observed an increase of the Hmax/Mmax ratio at rest following immobilization. It has previously been reported that the Hmax/Mmax ratio is increased following a period of immobilization in man (Duchateau, 1995; Clark et al. 2006b; Lundbye-Jensen & Nielsen, 2008) and hindlimb suspension in the rat (Anderson et al. 1999) and that the gain (Hslope/Mslope ratio) of the H-reflex is increased (Lundbye-Jensen & Nielsen, 2008).
Modulation of the H-reflex amplitude can result from a number of factors, including changes in GABAergic presynaptic inhibition of the Ia afferents, variation in the amount of Ia neurotransmitter release, and changes in the excitability of the motoneurones due to changes in either the membrane potential arising from excitatory or inhibitory input (postsynaptic) or the intrinsic properties of the neurones. Cormery et al. (2005) recently observed changes in the intrinsic properties of rat motoneurones following 2 weeks of hindlimb unloading. This was signified by increased rheobase, decreased spike amplitudes and deceased membrane time constants. Some properties of ‘slow’ motoneurones resembled those of ‘fast’ motoneurones following hindlimb unloading (Cormery et al. 2005). This finding indicates that the motoneurones are not more excitable or can be more easily activated. However, a selective change in the properties of the ‘slow’ motoneurones means that the changes observed in H-reflex following immobilization in the present study could be due to changes in the input–output relation or ‘recruitment gain’ of the motoneurone pool following immobilization (Kernell & Hultborn, 1990; Hultborn et al. 2004).
In a recent study, we observed increased H-reflex amplitudes following 1 week of wrist immobilization with no significant changes in corticospinal excitability estimated from transcranial magnetic stimulation measures (Lundbye-Jensen & Nielsen, 2008). Although many mechanisms may potentially be involved in this phenomenon, we hypothesized, based on this finding, that presynaptic control of Ia afferent input to the spinal cord could change (decrease) following a period of immobilization. If so, this would be in agreement with Manabe et al. (1989) who observed a disuse-induced enhancement of monosynaptic Ia afferent transmission (EPSPs) to rat spinal motoneurones following conduction block with tetrodotoxin and nerve section (Manabe et al. 1989).
Presynaptic inhibition of Ia afferents
It has previously been demonstrated that part of the H-reflex modulation during voluntary movement may be explained by changes in presynaptic inhibition of the synapses of Ia afferents on spinal motoneurones (Hultborn et al. 1987a,b; Meunier & Pierrot-Deseilligny, 1989; Nielsen & Kagamihara, 1993b; Faist et al. 1996; Perez et al. 2005). Presumably, gating of sensory input to spinal motoneurones is of functional importance in regulating the contribution of the stretch reflex circuitry to the ongoing motor activity (Rudomin & Schmidt, 1999; Nielsen & Sinkjaer, 2002). One advantage of regulating this contribution at the presynaptic level is that sensory inputs may be selectively gated while allowing effective activation of the muscles by central commands (Rudomin, 2002). Furthermore, regulation of sensory information at the presynaptic level may permit conveyance of proprioceptive information to the cortex which might contribute to adjusting of supraspinal motor commands (Llewellyn et al. 1990; Morita et al. 1998). There is good evidence from previous studies that changes in presynaptic inhibition of the synapses between sensory afferents and motoneurones is fundamental in the adaptation of the reflex circuitry during motor learning (Kandel et al. 2000; Wolpaw, 2007). Recently, Perez et al. (2005) demonstrated that presynaptic inhibition of Ia afferents from SOL was increased following a period of visuomotor learning. However, it has not to our knowledge been investigated whether presynaptic inhibition changes following a period of immobilization.
Presynaptic inhibition consists of a GABA-mediated depolarization of the terminals of primary afferents, which results in a decreased transmitter release (Schmidt, 1971; Rudomin, 1990). In animal experiments, the most direct demonstration of presynaptic inhibition of Ia afferents (see Eccles, 1964) is the depression of the monosynaptic EPSP evoked in motoneurones by a constant stimulation of Ia afferents without a change in the motoneurone membrane potential and conductance (Frank & Fuortes, 1957). In human studies, information about presynaptic inhibition can only be obtained indirectly.
In the present study, we observed significant increases in the heteronymous facilitation of the SOL H-reflex evoked by femoral nerve stimulation (Hultborn et al. 1987a) and a reduction in the amount of D2 inhibition. These two measures provide independent evidence of changes in presynaptic inhibition of SOL Ia afferents and taken together with the observation of unchanged disynaptic reciprocal inhibition these findings strongly suggest that presynaptic GABAergic inhibition of the SOL Ia afferents is reduced following 2 weeks of ankle joint immobilization.
The early facilitation of the SOL H-reflex evoked by femoral nerve stimulation provides indirect information about the heteronymous Ia EPSPs evoked in the motoneurone pool (Hultborn et al. 1987a). It is essential for the method that the investigated facilitation of the SOL H-reflex evoked by FN stimulation is monosynaptic in origin, as a change in facilitation may otherwise be caused by postsynaptic changes at an interneuronal level, meaning that changes in facilitation of the test reflex cannot be ascribed unequivocally to changes in the size of the conditioning Ia EPSP. This is why in the present study the onset of facilitation was examined in detail with conditioning–test intervals in steps of 0.2 ms. Considering the homonymous D2 inhibition, PAD interneurones mediating presynaptic inhibition of the Ia afferent volley of the test reflex are activated by a conditioning group I volley. Although the D1 and D2 inhibition (Mizuno et al. 1971) is probably contaminated by other effects (Meunier & Pierrot-Deseilligny, 1998), the size of these depressions can be assessed to estimate the excitability of PAD interneurones mediating presynaptic inhibition. Following immobilization, D2 inhibition decreased significantly, which also indicates that presynaptic inhibition is decreased. Decreased inhibition could result from decreased excitability of PAD interneurones or occlusion following activation by another excitatory input (Faist et al. 1996). However, in the lower limb, Iles (1996), using very weak stimuli, has shown that a cortically evoked decrease in CPN-induced inhibition is not due to occlusion in PAD pathways. Furthermore, in the present experiments, the decreased D2 inhibition was associated with increased heteronymous facilitation, which cannot be due to an occlusion in PAD pathways (see Hultborn et al. 1987b).
As previously mentioned, it may be argued that an increased heteronymous Ia facilitation could be observed as a consequence of skewed input to or effects within the motoneurone pool. This could potentially affect the recruitment gain by increasing the slope of the input–output relation of the H-reflex (Kernell & Hultborn, 1990; Nielsen & Kagamihara, 1993b). This would be in agreement with the findings of Cormery et al. (2005). However, if recruitment gain had been changed by a skewed distribution of effects within the motoneurone pool, this would be expected to enhance the effect of all synaptic input to the motoneurones thereby increasing D1 and D2 inhibition. In effect, the larger the recruitment gain the larger should be the D1 and D2 inhibition. The present finding of increased heteronymous Ia facilitation and decreased D2 inhibition following immobilization is in other words not consistent with a change in the recruitment gain. In conclusion, the observed changes are most probably caused by changes in presynaptic inhibition.
Post-activation depression
Post-activation depression also decreased following immobilization. Post-activation depression of the elicited reflex is caused by a frequency-dependent decrease in the probability of transmitter release from previously activated synapses. This depression of synaptic efficiency following previous synaptic activation of Ia afferents has been well characterized in both animal (Curtis & Eccles, 1960; Hultborn et al. 1996) and human studies (Crone & Nielsen, 1989a; Hultborn et al. 1996). We suggest that the long-term changes we observe in the frequency-related depression of the SOL H-reflex following immobilization reflect plastic changes at the level of the synapse between Ia afferents and SOL motoneurones causing an increase in the efficacy of the synapse. The molecular mechanisms underlying post-activation depression are not yet fully understood and subsequently we can only speculate about the possible mechanisms contributing to the modifications in the amount of transmitter released following immobilization.
It has, however, recently been reported by Meunier et al. (2007) that post-activation depression is subject to use-dependent plasticity. They reported that post-activation depression increased immediately following and 1 day after a single bout of skilful bicycle training. Contrary to the GABAergic presynaptic inhibition, there is so far no evidence for a descending control of post-activation depression, but some effects of, for instance, monoaminergic systems cannot be excluded. It is also not unlikely that structural modifications of the synapses between Ia afferents and SOL motoneurons have occurred during the 2 weeks of immobilization.
Post-activation depression is decreased in spastic patients (Faist et al. 1994; Nielsen et al. 1995) and Hultborn & Nielsen (1998) previously pointed out that the decreased post-activation depression in patients with cerebral or spinal lesions may (at least in part) be secondary to the disuse of motoneurons and Ia afferents accompanying the primary disorder. This notion is emphasized by the results of the present study and further supported by the findings of Gallego et al. (1979) that in the cat, prolonged disuse of the sensory fibres causes an increase in synaptic efficacy (EPSPs) of primary afferents in triceps surae motoneurons. Conversely, Meunier et al. (2007) demonstrated that motor training increased post-activation depression.
As already mentioned post-activation depression differs from the classical presynaptic inhibition transmitted through GABAergic axo-axonal synapses (see review by Hultborn & Nielsen, 1998). Even though both mechanisms contribute to the gating of sensory input to the central nervous system through modulation of synaptic transmitter release, the two mechanisms have distinct characteristics. Nevertheless, it is possible that an interaction between GABAergic presynaptic inhibition and post-activation depression contributes to the observed changes following immobilization. It has previously been suggested from animal studies (Enriquez-Denton et al. 2002) that reduced presynaptic inhibition also results in reduced post-activation depression and vice versa.
It is not possible from our data to make any conclusions regarding the exact mechanism(s) of the reduced presynaptic inhibition following immobilization, but some speculations may be made. There is good evidence from cat experiments that primary afferent synapses on ascending neurons and motoneurons are inhibited by different populations of interneurons (Jankowska & Padel, 1984). Indeed, the network responsible for controlling presynaptic inhibition of primary afferents seems in general to be organized to ensure selective control of afferent input to specific populations of both motoneurons and ascending neurons (Rudomin & Schmidt, 1999; Rudomin, 2002) and it is not impossible that changes in sensory input to the spinal interneurons that convey the presynaptic inhibition lead to plastic changes during a period of immobilization. According to afferent input and the functional requirements to the system, the CNS may increase or decrease the level of presynaptic inhibition of Ia afferents and thereby the central gain of the monosynaptic stretch reflex (Capaday & Stein, 1986; Hultborn et al. 1987a; Nielsen & Kagamihara, 1993b).
Based on the findings of the present study, it seems plausible that the decreased presynaptic inhibition occurred in response to the decreased motor activity accompanying limb immobilization. The changes in presynaptic inhibition may be a direct consequence of a reduced voluntary motor activity. It is, however, also possible that immobilization is accompanied by decreased proprioceptive input to the central nervous system. Reducing the amount of presynaptic inhibition of Ia afferents may in this way be a means of the central nervous system to increase the gain of the actual incoming afferent input. Since stimulation of low-threshold cutaneous mechanoreceptors by light brushing of both distal dorsal and plantar surfaces of the foot has also been demonstrated to decrease presynaptic inhibition (Iles, 1996), it may also be possible that the changes observed following immobilization occur in response to wearing the ankle cast. Measurements were, however, obtained after removal of the cast.
Functional and clinical perspectives
Although the present study involved limb immobilization in able-bodied subjects, the findings may also be of clinical relevance. This is especially the case in relation to neurological disorders leading to physical inactivity. It is noteworthy that the findings of increased H-reflexes, decreased GABAergic presynaptic inhibition and decreased post-activation depression following immobilization to some extent matches the findings of previous studies in spastic patients (Nielsen et al. 1993, 1995; Faist et al. 1994; Grey et al. 2008) and it is worth considering the effects of reduced physical activity in itself. As mentioned previously, it is possible that the decreased presynaptic inhibition and post-activation depression observed in patients with cerebral or spinal lesions may at least in part be a consequence of the disuse of motoneurons and Ia afferents.
Sensory feedback mechanisms through spinal reflex circuits help to ensure that the muscle activity is optimally adjusted to the immediate environment during movement. From a functional perspective it is interesting how changes in the functional properties of the central nervous system following a period of immobilization relate to functional motor control and further how subsequent training or rehabilitation affects these changes.
Acknowledgments
This work was supported by grants from The Danish Health Science Research Council, The Danish Ministry of Culture, The Novo Nordisk Foundation, The Carlsberg Foundation, The Elsass Foundation and the Danish Society of Multiple Sclerosis
References
- Anderson J, Almeida-Silveira MI, Perot C. Reflex and muscular adaptations in rat soleus muscle after hindlimb suspension. J Exp Biol. 1999;202:2701–2707. doi: 10.1242/jeb.202.19.2701. [DOI] [PubMed] [Google Scholar]
- Aymard C, Katz R, Lafitte C, Lo E, Penicaud A, Pradat-Diehl P, Raoul S. Presynaptic inhibition and homosynaptic depression: a comparison between lower and upper limbs in normal human subjects and patients with hemiplegia. Brain. 2000;123:1688–1702. doi: 10.1093/brain/123.8.1688. [DOI] [PubMed] [Google Scholar]
- Baldissera F, Hultborn H, Illert M. Integration in spinal neuronal systems. In: Brooks VB, editor. Handbook of Physiology, section 1, The Nervous System. Vol. 2. Bethesda, MD, USA: American Physiological Society; 1981. pp. 509–595. Motor Control. [Google Scholar]
- Barriere G, Leblond H, Provencher J, Rossignol S. Prominent role of the spinal central pattern generator in the recovery of locomotion after partial spinal cord injuries. J Neurosci. 2008;28:3976–3987. doi: 10.1523/JNEUROSCI.5692-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- Capaday C, Stein RB. Amplitude modulation of the soleus H-reflex in the human during walking and standing. J Neurosci. 1986;6:1308–1313. doi: 10.1523/JNEUROSCI.06-05-01308.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XY, Wolpaw JR. Operant conditioning of H-reflex in freely moving rats. J Neurophysiol. 1995;73:411–415. doi: 10.1152/jn.1995.73.1.411. [DOI] [PubMed] [Google Scholar]
- Clark BC, Fernhall B, Ploutz-Snyder LL. Adaptations in human neuromuscular function following prolonged unweighting. J Appl Physiol. 2006a;101:256–263. doi: 10.1152/japplphysiol.01402.2005. I. Skeletal muscle contractile properties and applied ischemia efficacy. [DOI] [PubMed] [Google Scholar]
- Clark BC, Manini TM, Bolanowski SJ, Ploutz-Snyder LL. Adaptations in human neuromuscular function following prolonged unweighting. J Appl Physiol. 2006b;101:264–272. doi: 10.1152/japplphysiol.01404.2005. II. Neurological properties and motor imagery efficacy. [DOI] [PubMed] [Google Scholar]
- Cormery B, Beaumont E, Csukly K, Gardiner P. Hindlimb unweighting for 2 weeks alters physiological properties of rat hindlimb motoneurones. J Physiol. 2005;568:841–850. doi: 10.1113/jphysiol.2005.091835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C, Hultborn H, Jespersen B, Nielsen J. Reciprocal Ia inhibition between ankle flexors and extensors in man. J Physiol. 1987;389:163–185. doi: 10.1113/jphysiol.1987.sp016652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C, Hultborn H, Mazieres L, Morin C, Nielsen J, Pierrot-Deseilligny E. Sensitivity of monosynaptic test reflexes to facilitation and inhibition as a function of the test reflex size: a study in man and the cat. Exp Brain Res. 1990;81:35–45. doi: 10.1007/BF00230098. [DOI] [PubMed] [Google Scholar]
- Crone C, Nielsen J. Methodological implications of the post activation depression of the soleus H-reflex in man. Exp Brain Res. 1989a;78:28–32. doi: 10.1007/BF00230683. [DOI] [PubMed] [Google Scholar]
- Crone C, Nielsen J. Spinal mechanisms in man contributing to reciprocal inhibition during voluntary dorsiflexion of the foot. J Physiol. 1989b;416:255–272. doi: 10.1113/jphysiol.1989.sp017759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis DR, Eccles JC. Synaptic action during and after repetitive stimulation. J Physiol. 1960;150:374–398. doi: 10.1113/jphysiol.1960.sp006393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies CT, Rutherford IC, Thomas DO. Electrically evoked contractions of the triceps surae during and following 21 days of voluntary leg immobilization. Eur J Appl Physiol Occup Physiol. 1987;56:306–312. doi: 10.1007/BF00690897. [DOI] [PubMed] [Google Scholar]
- de Leon RD, Hodgson JA, Roy RR, Edgerton VR. Locomotor capacity attributable to step training versus spontaneous recovery after spinalization in adult cats. J Neurophysiol. 1998;79:1329–1340. doi: 10.1152/jn.1998.79.3.1329. [DOI] [PubMed] [Google Scholar]
- Duchateau J. Bed rest induces neural and contractile adaptations in triceps surae. Med Sci Sports Exerc. 1995;27:1581–1589. [PubMed] [Google Scholar]
- Duchateau J, Hainaut K. Electrical and mechanical changes in immobilized human muscle. J Appl Physiol. 1987;62:2168–2173. doi: 10.1152/jappl.1987.62.6.2168. [DOI] [PubMed] [Google Scholar]
- Eccles JC. Presynaptic inhibition in the spinal cord. Prog Brain Res. 1964;12:65–91. doi: 10.1016/s0079-6123(08)60618-4. [DOI] [PubMed] [Google Scholar]
- Enriquez-Denton M, Morita H, Christensen LO, Petersen N, Sinkjaer T, Nielsen JB. Interaction between peripheral afferent activity and presynaptic inhibition of ia afferents in the cat. J Neurophysiol. 2002;88:1664–1674. doi: 10.1152/jn.2002.88.4.1664. [DOI] [PubMed] [Google Scholar]
- Faist M, Dietz V, Pierrot-Deseilligny E. Modulation, probably presynaptic in origin, of monosynaptic Ia excitation during human gait. Exp Brain Res. 1996;109:441–449. doi: 10.1007/BF00229628. [DOI] [PubMed] [Google Scholar]
- Faist M, Mazevet D, Dietz V, Pierrot-Deseilligny E. A quantitative assessment of presynaptic inhibition of Ia afferents in spastics. Brain. 1994;117:1449–1455. doi: 10.1093/brain/117.6.1449. Differences in hemiplegics and paraplegics. [DOI] [PubMed] [Google Scholar]
- Frank K, Fuortes M. Presynaptic and postsynaptic inhibition of monosynaptic reflexes. Federation Proceeding. 1957. pp. 39–40.
- Gallego R, Kuno M, Nunez R, Snider WD. Disuse enhances synaptic efficacy in spinal mononeurones. J Physiol. 1979;291:191–205. doi: 10.1113/jphysiol.1979.sp012807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondin J, Guette M, Maffiuletti NA, Martin A. Neural activation of the triceps surae is impaired following 2 weeks of immobilization. EurJ Appl Physiol. 2004;93:359–365. doi: 10.1007/s00421-004-1225-z. [DOI] [PubMed] [Google Scholar]
- Grey MJ, Klinge K, Crone C, Lorentzen J, Biering-Sorensen F, Ravnborg M, Nielsen JB. Post-activation depression of soleus stretch reflexes in healthy and spastic humans. Exp Brain Res. 2008;185:189–197. doi: 10.1007/s00221-007-1142-6. [DOI] [PubMed] [Google Scholar]
- Gruber M, Taube W, Gollhofer A, Beck S, Amtage F, Schubert M. Training-specific adaptations of H- and stretch reflexes in human soleus muscle. J Mot Behav. 2007;39:68–78. doi: 10.3200/JMBR.39.1.68-78. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Brownstone RB, Toth TI, Gossard JP. Key mechanisms for setting the input-output gain across the motoneuron pool. Prog Brain Res. 2004;143:77–95. doi: 10.1016/s0079-6123(03)43008-2. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Illert M, Nielsen J, Paul A, Ballegaard M, Wiese H. On the mechanism of the post-activation depression of the H-reflex in human subjects. Exp Brain Res. 1996;108:450–462. doi: 10.1007/BF00227268. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Meunier S, Morin C, Pierrot-Deseilligny E. Assessing changes in presynaptic inhibition of I a fibres: a study in man and the cat. J Physiol. 1987a;389:729–756. doi: 10.1113/jphysiol.1987.sp016680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Meunier S, Pierrot-Deseilligny E, Shindo M. Changes in presynaptic inhibition of Ia fibres at the onset of voluntary contraction in man. J Physiol. 1987b;389:757–772. doi: 10.1113/jphysiol.1987.sp016681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Nielsen JB. Modulation of transmitter release from Ia afferents by their preceding activity – a ‘postactivation depression’. In: Rudomin PM, Romo R, Mendell L, editors. Presynaptic Inhibition and Neural Control. New York: Oxford University Press; 1998. pp. 178–191. [Google Scholar]
- Iles JF. Evidence for cutaneous and corticospinal modulation of presynaptic inhibition of Ia afferents from the human lower limb. J Physiol. 1996;491:197–207. doi: 10.1113/jphysiol.1996.sp021207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iles JF, Roberts RC. Presynaptic inhibition of monosynaptic reflexes in the lower limbs of subjects with upper motoneuron disease. J Neurol Neurosurg Psychiatry. 1986;49:937–944. doi: 10.1136/jnnp.49.8.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Padel Y. On the origin of presynaptic depolarization of group I muscle afferents in Clarke's column in the cat. Brain Res. 1984;295:195–201. doi: 10.1016/0006-8993(84)90967-3. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science. 4th edn. New York: McGraw-Hill; 2000. [Google Scholar]
- Katz R, Meunier S, Pierrot-Deseilligny E. Changes in presynaptic inhibition of Ia fibres in man while standing. Brain. 1988;111:417–437. doi: 10.1093/brain/111.2.417. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Akima H, Kubo K, Muraoka Y, Hasegawa H, Kouzaki M, Imai M, Suzuki Y, Gunji A, Kanehisa H, Fukunaga T. Changes in muscle size, architecture, and neural activation after 20 days of bed rest with and without resistance exercise. EurJ Appl Physiol. 2001;84:7–12. doi: 10.1007/s004210000330. [DOI] [PubMed] [Google Scholar]
- Kernell D, Hultborn H. Synaptic effects on recruitment gain: a mechanism of importance for the input-output relations of motoneurone pools? Brain Res. 1990;507:176–179. doi: 10.1016/0006-8993(90)90542-j. [DOI] [PubMed] [Google Scholar]
- Kohn AF, Floeter MK, Hallett M. Presynaptic inhibition compared with homosynaptic depression as an explanation for soleus H-reflex depression in humans. Exp Brain Res. 1997;116:375–380. doi: 10.1007/pl00005765. [DOI] [PubMed] [Google Scholar]
- Lev-Tov A, Pinco M. In vitro studies of prolonged synaptic depression in the neonatal rat spinal cord. J Physiol. 1992;447:149–169. doi: 10.1113/jphysiol.1992.sp018996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn M, Yang JF, Prochazka A. Human H-reflexes are smaller in difficult beam walking than in normal treadmill walking. Exp Brain Res. 1990;83:22–28. doi: 10.1007/BF00232189. [DOI] [PubMed] [Google Scholar]
- Lundbye-Jensen J, Nielsen JB. Central nervous adaptations following one week of wrist and hand immobilization. J Appl Physiol. 2008;105:139–151. doi: 10.1152/japplphysiol.00687.2007. [DOI] [PubMed] [Google Scholar]
- Manabe T, Kaneko S, Kuno M. Disuse-induced enhancement of Ia synaptic transmission in spinal motoneurons of the rat. J Neurosci. 1989;9:2455–2461. doi: 10.1523/JNEUROSCI.09-07-02455.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier S, Kwon J, Russmann H, Ravindran S, Mazzocchio R, Cohen L. Spinal use-dependent plasticity of synaptic transmission in humans after a single cycling session. J Physiol. 2007;579:375–388. doi: 10.1113/jphysiol.2006.122911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier S, Pierrot-Deseilligny E. Gating of the afferent volley of the monosynaptic stretch reflex during movement in man. J Physiol. 1989;419:753–763. doi: 10.1113/jphysiol.1989.sp017896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier S, Pierrot-Deseilligny E. Cortical control of presynaptic inhibition of Ia afferents in humans. Exp Brain Res. 1998;119:415–426. doi: 10.1007/s002210050357. [DOI] [PubMed] [Google Scholar]
- Meunier S, Pierrot-Deseilligny E, Simonetta M. Pattern of monosynaptic heteronymous Ia connections in the human lower limb. Exp Brain Res. 1993;96:534–544. doi: 10.1007/BF00234121. [DOI] [PubMed] [Google Scholar]
- Mizuno Y, Tanaka R, Yanagisawa N. Reciprocal group I inhibition on triceps surae motoneurons in man. J Neurophysiol. 1971;34:1010–1017. doi: 10.1152/jn.1971.34.6.1010. [DOI] [PubMed] [Google Scholar]
- Morita H, Petersen N, Nielsen J. Gating of somatosensory evoked potentials during voluntary movement of the lower limb in man. Exp Brain Res. 1998;120:143–152. doi: 10.1007/s002210050388. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Kagamihara Y. Differential projection of the sural nerve to early and late recruited human tibialis anterior motor units: change of recruitment gain. Acta Physiol Scand. 1993a;147:385–401. doi: 10.1111/j.1748-1716.1993.tb09515.x. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Kagamihara Y. The regulation of presynaptic inhibition during co-contraction of antagonistic muscles in man. J Physiol. 1993b;464:575–593. doi: 10.1113/jphysiol.1993.sp019652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J, Petersen N, Ballegaard M, Biering-Sorensen F, Kiehn O. H-reflexes are less depressed following muscle stretch in spastic spinal cord injured patients than in healthy subjects. Exp Brain Res. 1993;97:173–176. doi: 10.1007/BF00228827. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Petersen N, Crone C. Changes in transmission across synapses of Ia afferents in spastic patients. Brain. 1995;118:995–1004. doi: 10.1093/brain/118.4.995. [DOI] [PubMed] [Google Scholar]
- Nielsen JB, Sinkjaer T. Afferent feedback in the control of human gait. J Electromyogr Kinesiol. 2002;12:213–217. doi: 10.1016/s1050-6411(02)00023-8. [DOI] [PubMed] [Google Scholar]
- Pearson KG. Plasticity of neuronal networks in the spinal cord: modifications in response to altered sensory input. Prog Brain Res. 2000;128:61–70. doi: 10.1016/S0079-6123(00)28007-2. [DOI] [PubMed] [Google Scholar]
- Perez MA, Lundbye-Jensen J, Nielsen JB. Task-specific depression of the soleus H-reflex after cocontraction training of antagonistic ankle muscles. J Neurophysiol. 2007;98:3677–3687. doi: 10.1152/jn.00988.2007. [DOI] [PubMed] [Google Scholar]
- Perez MA, Lungholt BK, Nielsen JB. Presynaptic control of group Ia afferents in relation to acquisition of a visuo-motor skill in healthy humans. J Physiol. 2005;568:343–354. doi: 10.1113/jphysiol.2005.089904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen N, Morita H, Nielsen J. Evaluation of reciprocal inhibition of the soleus H-reflex during tonic plantar flexion in man. J Neurosci Methods. 1998;84:1–8. doi: 10.1016/s0165-0270(98)00044-2. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E. Transmission of the cortical command for human voluntary movement through cervical propriospinal premotoneurons. Prog Neurobiol. 1996;48:489–517. doi: 10.1016/0301-0082(96)00002-0. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Morin C, Bergego C, Tankov N. Pattern of group I fibre projections from ankle flexor and extensor muscles in man. Exp Brain Res. 1981;42:337–350. doi: 10.1007/BF00237499. [DOI] [PubMed] [Google Scholar]
- Pinco M, Lev-Tov A. Modulation of monosynaptic excitation in the neonatal rat spinal cord. J Neurophysiol. 1993;70:1151–1158. doi: 10.1152/jn.1993.70.3.1151. [DOI] [PubMed] [Google Scholar]
- Rudomin P. Presynaptic inhibition of muscle spindle and tendon organ afferents in the mammalian spinal cord. Trends Neurosci. 1990;13:499–505. doi: 10.1016/0166-2236(90)90084-n. [DOI] [PubMed] [Google Scholar]
- Rudomin P. Central control of information transmission through the intraspinal arborizations of sensory fibers examined 100 years after Ramon y Cajal. Prog Brain Res. 2002;136:409–421. doi: 10.1016/s0079-6123(02)36033-3. [DOI] [PubMed] [Google Scholar]
- Rudomin P, Schmidt RF. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp Brain Res. 1999;129:1–37. doi: 10.1007/s002210050933. [DOI] [PubMed] [Google Scholar]
- Sanes JN, Donoghue JP. Plasticity and primary motor cortex. Annu Rev Neurosci. 2000;23:393–415. doi: 10.1146/annurev.neuro.23.1.393. [DOI] [PubMed] [Google Scholar]
- Schmidt RF. Presynaptic inhibition in the vertebrate central nervous system. Ergeb Physiol. 1971;63:20–101. doi: 10.1007/BFb0047741. [DOI] [PubMed] [Google Scholar]
- Seki K, Kizuka T, Yamada H. Reduction in maximal firing rate of motoneurons after 1-week immobilization of finger muscle in human subjects. J Electromyogr Kinesiol. 2007;17:113–120. doi: 10.1016/j.jelekin.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Seki K, Taniguchi Y, Narusawa M. Effects of joint immobilization on firing rate modulation of human motor units. J Physiol. 2001a;530:507–519. doi: 10.1111/j.1469-7793.2001.0507k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki K, Taniguchi Y, Narusawa M. Alterations in contractile properties of human skeletal muscle induced by joint immobilization. J Physiol. 2001b;530:521–532. doi: 10.1111/j.1469-7793.2001.0521k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom JM, Thompson MW, Ruell PA, Bryant GJ, Fonda JS, Harmer AR, De Jonge XA, Hunter SK. Effect of 10-day cast immobilization on sarcoplasmic reticulum calcium regulation in humans. Acta Physiol Scand. 2001;172:141–147. doi: 10.1046/j.1365-201X.2001.00853.x. [DOI] [PubMed] [Google Scholar]
- Vandenborne K, Elliott MA, Walter GA, Abdus S, Okereke E, Shaffer M, Tahernia D, Esterhai JL. Longitudinal study of skeletal muscle adaptations during immobilization and rehabilitation. Muscle Nerve. 1998;21:1006–1012. doi: 10.1002/(sici)1097-4598(199808)21:8<1006::aid-mus4>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- White MJ, Davies CT, Brooksby P. The effects of short-term voluntary immobilization on the contractile properties of the human triceps surae. Q J Exp Physiol. 1984;69:685–691. doi: 10.1113/expphysiol.1984.sp002860. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR. Spinal cord plasticity in acquisition and maintenance of motor skills. Acta Physiol (Oxf) 2007;189:155–169. doi: 10.1111/j.1748-1716.2006.01656.x. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, Braitman DJ, Seegal RF. Adaptive plasticity in primate spinal stretch reflex: initial development. J Neurophysiol. 1983a;50:1296–1311. doi: 10.1152/jn.1983.50.6.1296. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, Kieffer VA, Seegal RF, Braitman DJ, Sanders MG. Adaptive plasticity in the spinal stretch reflex. Brain Res. 1983b;267:196–200. doi: 10.1016/0006-8993(83)91059-4. [DOI] [PubMed] [Google Scholar]