Abstract
The spatial resolution of hemodynamic-based neuroimaging techniques, including functional magnetic resonance imaging, is limited by the degree to which neurons regulate their blood supply on a fine scale. Here we investigated the spatial detail of neurovascular events with a combination of high spatiotemporal resolution two-dimensional spectroscopic optical imaging, multichannel electrode recordings and cytochrome oxidase histology in the rodent whisker barrel field. After mechanical stimulation of a single whisker, we found two spatially distinct cortical hemodynamic responses: a transient response in the “upstream” branches of surface arteries and a later highly localized increase in blood volume centered on the activated cortical column. Although the spatial representation of this localized response exceeded that of a single “barrel,” the spread of hemodynamic activity accurately reflected the neural response in neighboring columns rather than being due to a passive “overspill.” These data confirm hemodynamics are capable of providing accurate “single-condition” maps of neural activity.
INTRODUCTION
Neuroimaging procedures such as functional magnetic resonance imaging (fMRI) and optical recording of intrinsic signals can track regional changes in brain activity as tasks are being performed. Such techniques provide an indirect measure of neural activity based on the spatiotemporal coupling between local changes in neuronal firing and local changes in associated hemodynamic variables. Hence the interpretation of neuroimaging data is critically dependent on a good understanding of the mechanism(s) of neurovascular coupling (Logothetis 2002; Logothetis and Pfeuffer 2004). Recent reports have claimed both linear and/or nonlinear relationships between neuronal activity and blood supply depending on model system, stimulation paradigms, and measurement systems used (Devor et al. 2003; Hewson-Stoate et al. 2005; Jones et al. 2004; Nemoto et al. 2004; Sheth et al. 2004). However, an important unresolved issue concerns the fine spatial detail of neurally evoked hemodynamic responses. This is important because it will place an upper limit on the spatial resolution that can be attained by hemodynamically based neuroimaging techniques.
Various experimental procedures have been adopted to enhance the spatial resolution of neuroimaging studies. Typically in tasks with several functional components, stringent control conditions are deployed in which all components of the task are present except the one of interest. Subtractions of experimental and control imaging data then reveal maps of regional activity associated with the component of interest. On a finer spatial scale, investigations of cortical visual processing have performed subtractions on data derived from conditions where orthogonally oriented stimuli are presented (Malonek and Grinvald 1996; Malonek et al. 1997). However, in many circumstances, especially those involving higher cognitive processes, it is not always clear what an appropriate orthogonal control condition would be. Considerable advantage would therefore be derived from being able to perform imaging experiments without always having to subtract experimental and control data, i.e., single-stimulus condition experiments. However, because commonly used hemodynamic measurement procedures are unable to distinguish changes in different vascular compartments (arteries, veins, and capillaries in brain parenchyma), an important limit to the spatial resolution of hemodynamically based neuroimaging techniques will be set by how far stimulus-associated changes can be detected in the arterial feeding and venous drainage of an activated region. If two regions are functionally segregated but share a common vasculature, the hemodynamic responses will be difficult to assign to either region.
The purpose of the present investigation was therefore to analyze the fine spatiotemporal dynamics of blood-related responses evoked by selective activation of adjacent cortical columns. These architectural components are thought to represent the basic, spatially restricted functional processing units of the cerebral cortex (Hubel and Wiesel 1959; Rakic 2002). To address this issue, we used the somatotopically ordered columns of the rat barrel cortex as a model system (Woolsey and Van der Loos 1970). Two-dimensional (2D)-spectroscopic optical imaging and multi-channel electrophysiological recording were used to provide high spatial-resolution maps of hemodynamic and neural activity. We found that mechanical stimulation of a series of single adjacent whiskers produced hemodynamic responses that were centered on the successively activated cortical barrels. For longer-duration stimuli, these responses were characterized initially by widespread changes that involved vasculature shared by many barrels; however, as stimulation continued, the hemodynamic changes were reduced to a spatially restricted area centered on the single activated barrel. These results suggest there may be different but complementary neurovascular coupling mechanisms responsible for the “initial-diffuse” and “prolonged-restricted” hemodynamic responses. Some of the present results have appeared in abstract form (Berwick et al. 2005b).
METHODS
Animal preparation
All procedures were carried out in accordance with relevant Home Office legislation, the Animals (Scientific Procedures) Act 1986. Hooded Lister rats (n = 15) weighing between 200 and 300 g were kept in a 12-h dark/light cycle environment at a temperature of 22°C with ad libitum food and water. Prior to surgery, animals were anesthetized with urethane (1.25g/kg ip) with additional doses (0.1 ml) administered if required. Atropine was also administered (0.4 mg/kg sc) to reduce mucous secretions during surgery. A homoeothermic blanket (Harvard) maintained rectal temperature at 37°C throughout surgical and experimental procedures. Animals were tracheotomized to allow artificial ventilation with air and measurement of end-tidal CO2. The left femoral vein and artery were cannulated to allow drug infusion and measurement of mean arterial blood pressure, respectively. Phenylephrine (0.13–0.26 mg/h) was infused to maintain blood pressure between recognized physiological limits (MABP, ∼90–100 mmHg) (Golanov et al. 1994; Nakai and Maeda 1999). Animals were placed in a stereotaxic frame (Kopf Instruments), and the skull overlying the right somatosensory cortex was thinned to translucency with a dental drill under constant cooling with saline. A plastic “well” was attached to the thinned skull and filled with saline (37°C) to reduce specularities from the skull surface. Physiological parameters were continuously monitored and maintained within normal ranges [pO2 = 94.9 ± 2.9 (SE) mmHg; pCO2 = 32.1 ± 1.6 mmHg; arterial blood saturation = 97.8 ± 0.25% (mean ± SE)].
Stimulation paradigm
Individual whiskers were stimulated using a purpose-built computer-controlled mechanical device that comprised a hypodermic needle connected to an electrical relay. The whisker to be stimulated was placed inside the needle with each stimulation pulse moving the whisker 1–2 mm in the rostral-caudal plane. Five whiskers comprising the first whisker column (A1–E1) were stimulated individually in a randomized order for short (2 s) and long (16 s) duration at 5 Hz. The short stimulation paradigm entailed 60 trials of 23 s with stimulation after 8 s. From the end of one trial to the start of the next, there was a 2-s rest period resulting in a 25-s interval between stimulations. The long stimulation paradigm entailed 30 trials of 50 s with stimulation after 10 s. From the end of one trial to the start of the next, there was a 20-s rest period resulting in a 70-s interval between stimulations. The individual trials were averaged to create a mean trial that was subjected to the spectral analysis described in the following text.
2-Dimensional spectroscopy (2D-OIS) imaging
A Pentax 50-mm lens set at F-stop 4.0 together with 32 mm of spacers were used to form an image with a magnification of 0.64. A Dalsa 1M30P camera operating in 4 × 4 binning mode recorded the images; hence each image pixel represented 75 × 75 μm of the object. The camera has a quantum efficiency of 28% at 500 nm.
To generate spatial maps of cortical hemodynamic responses, the 2D-OIS technique used a Lambda DG-4 high-speed filter changer (Sutter Instrument, Novato, CA). The four wavelengths were specifically chosen as two pairs (495 ± 31 and 559 ± 16 nm FWHM; 575 ± 14 and 587 ± 9 nm FWHM). The wavelengths in each pair have a similar total absorption coefficient and thus sample the same tissue volume. However, they have specific absorption coefficients for total oxyhemoglobin (HbO2) and deoxyhemoglobin (Hbr) that are as different as possible to maximize signal to noise ratio. The frame rate of the camera was 32 Hz, which was synchronized to the filter switching, thereby giving an 8-Hz effective frame rate for each wavelength. The spectral analysis was based on the path-length scaling algorithm (PLSA) described previously (Mayhew et al. 1999) and used for 2D-OIS in Berwick et al. (2005a).
The spectral method is the same as described in the previous paper (Berwick et al. 2005a) with two modifications. The average blood volume concentration was increased from 75 to 104 μM. We have recently performed simultaneous fMRI and 2D-OIS experiments (Kennerley et al. 2005) in which it was possible to calculate the blood volume fraction of total blood volume (Hbt) in the rat somatosensory cortex. This measured 104 μM, and we have therefore used this new figure in the data analysis. The second modification of the spectral method was a better estimation of baseline saturation. In the previous paper, we used a range of set baseline saturations for the whole dataset. This is suboptimal due to the different saturations of the arteries, parenchyma, and veins. In this study, we estimated the baseline saturation on a pixel by pixel basis for the control first eight seconds. This produced a baseline saturation map. Although these values are only estimations, the fact that the map shows that the arteries have the highest saturation (∼0.7) and the veins the lowest (∼0.4) provides confidence that the algorithm is working well (for examples, see supplemental Fig. S1 )1. The spectral analysis produced 2D images over time, of HbO2, Hbr, and Hbt.
Selection of regions of interest and statistical analysis
The size and spatial spread of the hemodynamic responses (Hbt, Hbr, and HbO2) were analyzed for the long stimulation data. The data were split into two groups comprising the mean image from the first 4 s of the stimulation and the mean image from the last 4 s of stimulation. All pixels in each image that were >50% of the peak response were considered activated and were used to create masks of the response. Two statistics were calculated from the mask and recorded in Table 1. The first statistic was simply the area in mm2 of the mask. The second statistic, which we term the “spatial spread,” was based on the average distance (in mm), each pixel was from the center of the anatomical barrel from the warped histology sections (see following text) All the values ± SE are shown in Table 1.
TABLE 1.
Size and spatial spread of hemodynamic mask data from the first and last 4 s of stimulation
| Hbt First 4 s | Hbt Last 4 s | HbO2 First 4 s | HbO2 Last 4 s | Hbr First 4 s | Hbr Last 4 s | |
|---|---|---|---|---|---|---|
| Magnitude, mm2 | 0.73 ± 0.12 | 0.43 ± 0.03 | 1.25 ± 0.12 | 0.66 ± 0.04 | 1.21 ± 0.2 | 0.48 ± 0.07 |
| Spatial spread, mm | 0.60 ± 0.04 | 0.35 ± 0.04 | 0.59 ± 0.05 | 0.38 ± 0.02 | 1.06 ± 0.15 | 0.66 ± 0.07 |
Values are means±SE.
Electrophysiology
In 10 animals, electrophysiological measurements were obtained in an experimental paradigm that was similar to that described in the preceding text. All electrophysiology experiments were performed with the electrode inserted into barrel E1. To accurately place the electrode, whisker E1 was stimulated using the long stimulus duration (16 s) paradigm, and 2D-OIS was used to identify the barrel region as described in the preceding text. A small hole was drilled in the thinned skull, the dura overlying the active region removed, and electrode inserted. Electrophysiological recordings were made using a 16-channel electrode probe (100-μm spacing, area of each site: 177 μm2, impedance: 1.5–2.7 MΩ, probe width: 33 μm at tip; 123 μm at uppermost electrode, CNCT, University of Michigan) coupled to a data-acquisition device (Medusa Bioamp, TDT) with a custom-written Matlab (The Mathworks) interface. The electrode was inserted normal to the cortical surface under micromanipulator control to a depth of 1,600 μm.
Because neural responses are so much faster than hemodynamic responses, a different sampling duration and intertrial interval was employed for the electrophysiological data. Data were recorded for 2.7 and 16.7 s for the short (2 s) and long (16 s) stimulation paradigms, respectively, with whisker stimuli occurring 0.2 s into each recording period. The intertrial interval was set to 5 s for the short and 20 s for the long stimulation paradigm. Recordings were averaged over trials with stimulus onset “jittered” within a 20-ms window to reduce effects of 50-Hz mains noise. The resultant evoked field potential recordings were sampled at 6 kHz with 16-bit resolution. The CSD analysis has been described in detail previously (Martindale et al. 2003). Briefly, the field potentials were used to obtain spatiotemporal estimates of the major current sources and sinks within the cortical layers (Mitzdorf 1985; Nicholson and Freeman 1975). The current sink with the greatest amplitude was located ∼500 μm below the surface of the brain. This corresponds to layer IV, which receives direct somatosensory input from the ventral posterior thalamus.
Histology
After experimental testing, the animals were perfused transcardially with saline followed by 4% paraformaldehyde, and vessels were filled with photographic emulsion (Jessops). The method to stain tangential sections of the barrel cortex for cytochrome oxidase and then project the barrels in layer IV onto the surface has also been described previously (Zheng et al. 2001). The combined surface vessel/barrel cortex anatomical image was then projected onto an in vivo gray-scale image using the bifurcation of major vessels as fiducial points in the warping algorithm.
RESULTS
Spatiotemporal analysis of the hemodynamic response
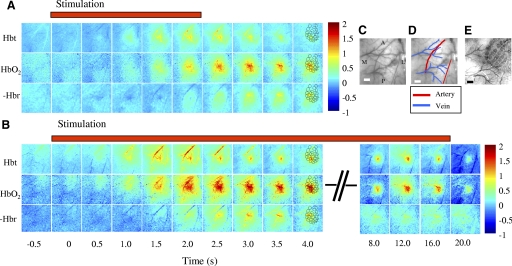
Hemodynamic responses were optically recorded from somatosensory (barrel) cortex of a urethane-anesthetized rat during periods of short (2 s) and long (16 s) mechanical stimulation of a single whisker of the first cortical column (A1–E1). Representations of the spatiotemporal characteristics of these responses to stimulation of whisker C1 from an individual animal are presented in Fig. 1. For short-duration stimulation (Fig. 1A), Hbt and HbO2 both increased above baseline, whereas the level of Hbr fell (the Hbr data were inverted so the red areas corresponded to the greatest negative change). The spatial structure of the Hbt response was dominated ∼1.0 s after stimulation onset by changes in a central area overlying whisker C1 and a branch of the middle cerebral artery (MCA) anterior to it. The HbO2 response was characterized by a large area, again centered on the whisker barrel C1, which peaked ∼2.5–3.0 s after stimulus onset. At ∼3.5 s, the HbO2 response became localized to a draining vein. The maximum decrease in Hbr occurred after ∼3 s and was localized to the draining cortical veins. The hemodynamic responses for prolonged stimulation of whisker C1 (Fig. 1B) had an early spatial structure that was similar to those recorded after short stimulation, albeit at a larger amplitude (reflecting between trial variability). The similarity of the magnitude of the hemodynamic response in the initial phase can be appreciated by comparing the averaged peak Micromolar changes in Hbt for the principal barrel for short stimulus durations (Fig. 4A) and long stimulus durations (Fig. 5A). However, qualitative differences between the two data sets only became evident >4.0 s after stimulation onset. Thus despite continuing mechanical activation in the long stimulation condition, the Hbt responses in the feeding arteries rapidly dissipated leaving a much smaller area of activity centered on the C1 cortical barrel. This restricted region remained relatively constant for the entire 16-s stimulation period. The HbO2 response followed the same pattern as Hbt but over a slightly larger area and was biased toward the draining veins. The Hbr reaction to stimulation remained consistently within the draining veins. Movies of the Hbt response for the long- and short-duration stimulation are supplied in the supplementary material.
FIG. 1.
The hemodynamic response for short (2 s) and long (16 s) whisker C1 stimulation. A: image montage for total blood volume (Hbt), oxyhaemoglobin (HbO2) and deoxyhemoglobin (Hbr) for short stimulation of whisker C1. The scale bar represents the micromolar change from baseline. Stimulation occurs at time 0. Both Hbt and HbO2 increase after stimulus onset, Hbr decreases (Hbr has been inverted so decreases appear as red regions). The Hbt increase is localized to the area surrounding barrel C1 and a branch of the middle cerebral artery lying anterior to it. The HbO2 response is less localized than Hbt and after 3.5 s is localized to the draining veins. The Hbr decrease is also localized to the draining veins. B: image montage for Hbt, HbO2 and Hbr for long stimulation of whisker C1. The onset responses for the long duration stimulation are similar to the short duration albeit at a slightly larger magnitude. For Hbt as the arterial response returns to baseline after 3 s a very localized region remains for the duration of the stimulation period, that overlies the whisker C1 barrel region. HbO2 is less localised than Hbt and is biased towards the draining veins and the Hbr decrease remains localized to the draining veins. C: and D: in vivo images of the surface vasculature show the branches of the middle cerebral artery (MCA) and draining veins. E: the relationship of the barrel cortex to the surface vasculature is shown in a combined post mortem image. This post mortem image was fitted to the in vivo image so that the barrels could then be superimposed over the activation maps as a reference (these are shown in the images 4 s after stimulation onset). Scale bar = 500 μm. M, medial; L, lateral; A, anterior; P, posterior.
FIG. 2.
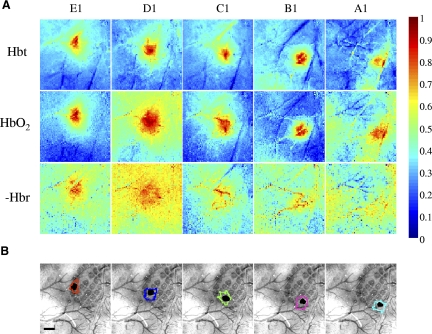
Hemodynamic responses for total blood volume (Hbt), oxyhemoglobin (HbO2) and deoxyhemoglobin (Hbr) for long duration stimulation of whiskers A1–E1. A: shows normalised mean response images for Hbt, HbO2 and the inverse of Hbr taken from 12–16 s after stimulation onset. The most localized responses are from the Hbt data. B: combined surface and barrel histological sections with the stimulated barrel highlighted in black. The contour round each stimulated barrel is the activated Hbt region calculated by including all pixels with 50% of the peak response from a mean image of the last 4 s of stimulation. Scale bar = 500 μm.
FIG. 3.
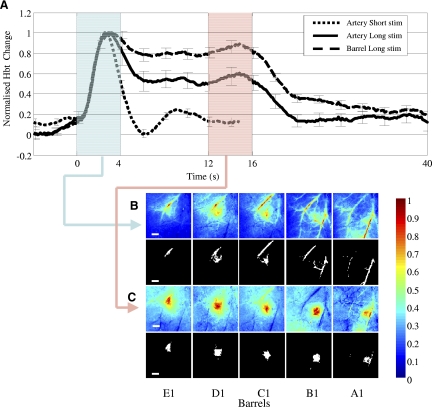
Transient arterial response. A: normalised average (across all barrel experiments) total blood volume (Hbt) times series responses for an artery region for short (2 s) and long (16 s) duration stimulation and Hbt time series for long stimulation taken from the barrel region, (mean of 40 barrels from 8 rats, error bars = SE). The transient nature of the arterial response is independent of stimulus duration, whereas the Hbt response from the barrel remains elevated for the period of stimulation. Highlighted regions are the time where the average was taken to produce the images in B and C. B: normalized average Hbt images and activated image region masks selected for subsequent analysis taken from the first 4 s after stimulus onset from the representative animal shown in Figs. 1 and 2. The masks were calculated by including all pixels with 50% of the peak response. For all whiskers stimulated the initial response is localized to branches of the MCA. C: normalised average Hbt images and activated image region masks taken from 12–16 s of the stimulation period again from the same representative animal shown in Figs. 1 and 2. These responses are localised to the individual barrel columns. Scale bar = 500 μm.
FIG. 4.
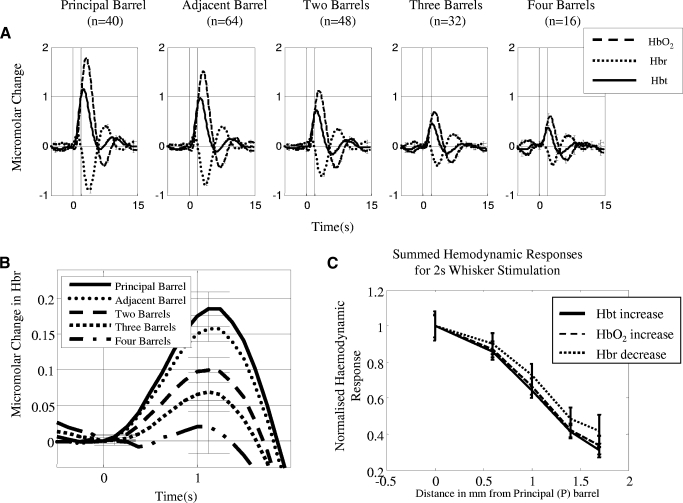
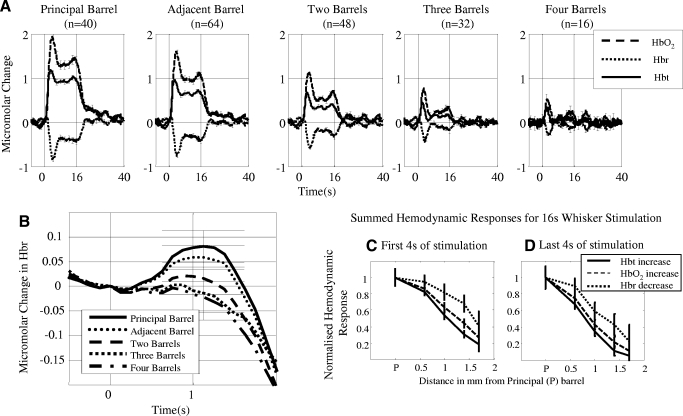
Average Micromolar time series changes from all experiments in total blood volume (Hbt), oxyhemoglobin (HbO2) and deoxyhemoglobin (Hbr) for short whisker stimulation as a function of distance relative to the principal barrel. A: responses for short (2 s) stimulus duration. The response can be characterized for all whiskers as a monophasic increase in Hbt and HbO2, with a transient increase in Hbr that the decreases below baseline. The size of the response is largest for the principal barrel and smallest for the barrel which is 4-distant. B: transient increase in deoxyhemoglobin for short (2 s) stimulation. The largest increase is from the principal barrel and the smallest in the barrel 4-distant. C: normalized hemodynamic response measurements for short duration stimulation. All aspects of the hemodynamic response decrease as a function of cortical distance.
FIG. 5.
Average Micromolar time series changes from all experiments in total blood volume (Hbt), oxyhemoglobin (HbO2) and deoxyhemoglobin (Hbr) for long whisker stimulation as a function of distance relative to the principal barrel. A: responses for long (16 s) stimulus duration. These responses can be characterized for Hbt and HbO2 as initial increases followed by a plateau phase that lasts for the duration of stimulation. For Hbr again there was a transient increase followed by a decrease below baseline and then a continued plateau below baseline for the duration of the stimulation period. (Error bars = SE). B: transient increase in deoxyhemoglobin for long (16 s) stimulation. The largest increase is from the principal barrel and the smallest in the barrel 4-distant. C: normalized hemodynamic response measurements for the first 4 s of long duration stimulation. D: normalized hemodynamic response measurements for the last 4 s of long duration stimulation. Although the results for the first and last 4 s are similar to the short stimulation there are differences. In both conditions the sharpest decline (smaller proportional response size) as a function of distance is from the Hbt measurement. The responses for all three variables decrease more sharply for the last 4 s of stimulation compared to the first 4 s.
Examples of late stage responses to the long period of stimulation for each of the five adjacent whiskers for the same single subject are shown in Fig. 2 A. Each image is a normalized (maximum response scaled to 1 and minimum to 0), average image from 12 to 16 s after stimulus onset to remove the arterial component of the response from the images (see following text). For each whisker, the Hbt data provide the most localized spatial response. The HbO2 response was again similar to the Hbt reaction but over a broader area and, as before, Hbr changes were localized to the draining veins. The localized region of Hbt responses (threshold at 50% of the peak response) for each whisker was superimposed on a combined surface vessel and anatomical image of the cortical surface (Fig. 2B). These regions corresponded closely to the surface projection of the relevant whisker barrels. These results were consistent across subjects. They show that with continuing stimulation the Hbt component of the initially diffuse hemodynamic response becomes spatially restricted almost to a region representing a single barrel column of somatosensory cortex. This effect was evident in the data of all subjects. Figures for the remaining seven animals are supplied in the supplementary material.
Initial arterial response
The hemodynamic responses involving the surface arteries for both short and long stimulation paradigms (Fig. 1, A and B) suggested that the initial arterial response may be largely independent of the duration of the initiating stimulus. To test this possibility, we took Hbt time series from one branch of the MCA for every barrel experiment across all animals for both the long and short stimulation paradigms. The normalized data (Fig. 3 A) show that the Hbt response in the feeding artery and barrel had a similar onset profile for both the long and short stimulation paradigms. It was characterized by a transient increase in Hbt that peaked within ∼2 s of stimulation onset. The short stimulus artery response then returned to baseline, whereas the long-duration arterial response fell to a plateau until the end of stimulation. This differed from the mean Hbt response recorded from the barrel regions, where the initial peak was followed by a plateau at a greater height than the artery response for the duration of stimulation. To emphasize this point, we compared the mean image taken from the first four seconds of stimulation (Fig. 3B) with the average image for the last 4 s (12–16 s) of stimulation period (Fig. 3C), separately for each of the five barrels. The initial 4.0-s response for each whisker was dominated by a Hbt response distributed in the branches of the middle cerebral artery. For the last 4 s, the active regions were largely restricted to the individual barrels (again the masks are taken are 50% of the peak response).
Spatial analysis of hemodynamic response
We wanted to compare the changes in magnitude and “spatial spread” of the hemodynamic response during the long-duration stimulation period. To do this, we first created two separate average images of the first and last 4 s of the stimulation period for all three (Hbt, HbO2, and Hbr) hemodynamic variables. We considered all pixels within 50% of the peak response to be activated in these images and used this criterion to create image masks. The magnitude of the response was the number of activated pixels converted to an area measurement (mm2), whereas the spatial spread was the average distance (in mm) of each activated pixel from the center of the stimulated cortical barrel. All results are presented in Table 1. Each barrel response was considered a separate data set and all the barrel responses (5 per animal as we stimulated 5 barrels in each) were analyzed together. One-way repeated-measures (poststimulus time, defined as first or last 4 s of stimulation) MANOVAs with three dependent variables (Hbt, HbO2, and Hbr) suggested that both mask size and spatial spread differed depending on “poststimulus time” (size: Wilks' Lambda = 0.56, hypothesis df = 3, error df = 36, F = 9.33 P < 0.001; spread: Wilks' Lambda = 0.48, hypothesis df = 3, error df = 38, F = 13.864 P < 0.001;). Subsequent univariate's ANOVA suggested that mask size and spatial spread differed depending on poststimulus time for all dependent variables even if Bonferroni corrected.(size: Hbt: F = 7.042, P = 0.012; HbO: F = 27.082, P < 0.001; Hbr: F = 12.253, P = 0.001; spatial spread: Hbt: F = 29.319, P = 0.0001; HbO2: F = 18.141, P = 0.0001; Hbr: F = 11.256, P = 0.002). These results confirm that reductions in magnitude and spatial spread of the hemodynamic response between the beginning and end of our long stimulation condition were statistically reliable.
Time series analysis
The absolute spatial resolution of neuroimaging techniques will be determined by the degree of spatial correspondence between hemodynamic and neural responses. To characterize hemodynamic responses as a function of cortical distance from the stimulated barrel, a region was defined for each of the barrels corresponding to the stimulated whiskers (A10–E1) for each animal. This region was all pixels within 50% of the peak response from a mean image of the last 4 s of the long-duration stimulation period (see previous section).
In the following analysis, the barrel corresponding to the stimulated whisker will be termed the principal barrel. Further barrels are designated, with respect to their distance from the principal barrel, as “adjacent” or two-, three-, or four-distant. Note that there are different numbers of responses averaged for each barrel due to the spatial constraints (there are more adjacent barrels than 4-distant barrels).
The short stimulation time series responses for Hbt, HbO2, and Hbr for each barrel region (Fig. 4A ) showed that the largest responses were from the principal barrel and smallest from barrels four-distant. The shape of the response from all regions was the same with an increase in Hbt and HbO2 that reached a peak ∼3 s after stimulus onset. The Hbr response contained a transient increase immediately after stimulation onset followed by a decrease below baseline ∼1–2 s later. The size of the early transient Hbr increase was dependent on barrel region with the principal barrel having the largest increase and the barrel four-distant the smallest (Fig. 4B).
To quantify hemodynamic responses as a function of cortical distance, we took three measures of the hemodynamic response: the peak response for Hbt and HbO2 and Hbr decrease. The results were then normalized by dividing every result by the principal whisker response. There was a linear relationship (Fig. 4C) with all the four measures of the hemodynamic response decreasing as a function of barrel distance.
The long stimulation results followed the same pattern as the short stimulation (Fig. 5A ); however, the shape of the responses varied. For the principal barrel, there was a peak response in HbO2 and Hbt, followed by a plateau, then a return to baseline after the end of stimulation. The Hbr response again contained a transient increase in Hbr (Fig. 5B) followed by a decrease below baseline which returned after the end of stimulation.
Because the mask data were split into the first and last 4 s of stimulation, we applied the same logic to the time series data. For the Hbt, HbO2, and Hbr, we took the area under the curve for the first and last 4 s of stimulation. Again the results were then normalized by dividing every result by the principal whisker response. The responses were similar between the first 4 s (Fig. 5C) and last 4 s (Fig. 5D) responses. Importantly in both data sets the Hbt has the smallest response size as a function of whisker distance, suggesting a more localized response compared with the Hbr and HbO2 (which agrees with the mask data analysis).
Neurovascular coupling as a function of cortical distance
To provide corresponding measures of neural activity, we performed electrophysiological recording in the barrel cortex of 10 animals. Electrode placement was guided by 2D-OIS data (Hbt, long stimulation) and later confirmed via post mortem histology. We performed current source density analysis on the evoked potentials to identify the main current sources and sinks in the data. The main current sink in layer IV, which is believed to be a result of excitatory post synaptic activity (EPSP) in the barrel, was taken as our neural activity measure and will be referred to as the CSD-sink. We inserted the electrode into a single barrel only (barrel E1 for every animal), to allow measurement of neural activity at a distance of four barrels (to be the same as the data from the 2D-OIS study). Thus the principal barrel response for the neural activity measurements was obtained by stimulating whisker E1, the adjacent barrel response obtained from stimulating whisker D1, and so on.
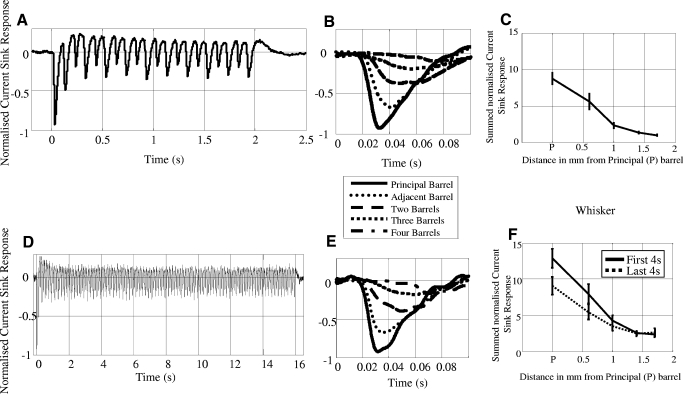
The average CSD-sink time series across the 10 animals for both the short (Fig. 6 A) and long (D) durations for the principal barrel are shown. The stimulation frequency was set at 5 Hz; however, there was a separate neural response to both the backward and forward movements of the whisker stimulator (resulting in an effective response frequency of 10 Hz). For both stimulation periods, the response to the first backward movement of the stimulator produced the largest CSD-sink (Fig. 6, B and E) with subsequent whisker deflections being at a reduced but constant level. The latency of the first CSD-sink was calculated by taking the minima of the first CSD-sink for both stimulation periods; the results showed that the principal barrel had the smallest latency and the barrel four distant had the largest (principal barrel = 34 ± 1.7 ms, adjacent barrel = 38 ± 1.4 ms, 2-distant = 44 ± 1.4 ms, 3-distant = 59 ± 4.7 ms, 4-distant = 62 ± 2.4 ms). To calculate a measure of neural activity in each barrel, we summed the magnitude of each CSD-sink from each deflection. To reduce interanimal variability, all responses were normalized by the response to the first deflection. This summed neural activity from all deflections of the 2-s data and from the first and last 4 s of stimulation of the long stimulation period was used in our analyses. The summed neural activity showed similar spatial characteristics for both the short (Fig. 6C) and long duration (F) stimulation paradigms, with a consistent reduction in response amplitude as a function of distance from the principal barrel.
FIG. 6.
Averaged evoked neural response using current source density sinks (CSD-Sink) time series for short (2 s) and long (16 s) whisker stimulation normalized with respect to the first impulse response on the principal whisker (n = 10 animals). A: CSD-Sink time series for short stimulation from the principal barrel. B: enlarged image of the first impulse response for the short stimulation for all five whiskers. The largest response was from the principal barrel and smallest from the barrel 4-distant C: summed CSD-Sink activity for the short stimulus duration, this measure was taken from the sum of all deflections (±SE). The response magnitude decreases as a function of cortical distance. D: CSD-Sink time series for long stimulation from the principal barrel. E: enlarged image of the first impulse response for the long stimulation for all five whiskers. The largest response was from the principal barrel and smallest from the barrel four distant. F: summed CSD-Sink activity for the long stimulus duration. This measure was taken from the sum of all deflections split into the first 4 s of stimulation and the last 4 s of stimulation (±SE). Note that magnitudes of these responses differ from the 2 s stimulation data in (6c) because the times series have been summed over 4 s rather than 2 s of data.
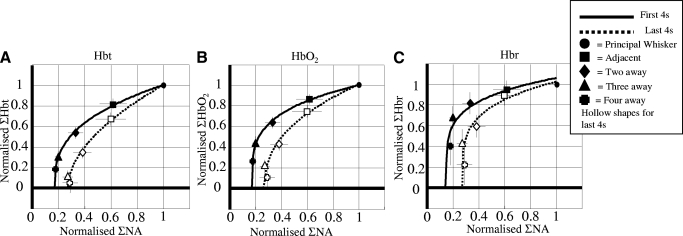
To assess neurovascular coupling for the long duration stimulus, the normalized neural and hemodynamic results from the first and last 4 s of stimulation were plotted against each other (Fig. 7, A–C). Lines were superimposed on the data where the hemodynamic response (Hx) was fitted to the neural response (E) using the equation
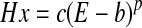 |
because small values of E failed to produce any hemodynamic response, which meant the relationship did not go through the origin, hence the need for the parameter b. Parameter p is used because the relationship is nonlinear while parameter c is a scaling constant. Note that the function is monotonic and so it enforces compliance with the expectation that the hemodynamic response increases with the neural response. All of the values used in the equation for all the hemodynamic responses are detailed in the supplementary materials. Responses in the last 4 s for all three conditions displayed a larger fall as a proportion of neural activity compared with the first 4 s.
FIG. 7.
Neural and hemodynamic responses for long stimulation. Normalized neural and (a) Hbt, (b) Hbo2 and (c) Hbr responses plotted against one another for the first and last 4 s of stimulation. All the responses were non-linear in shape and fitted with a monotonic relationship defined by the equation Hx = c (E − b)p. For all cases there was a greater hemodynamic response in the first 4 s compared to the last 4 s for a proportional measure of neural activity (NA). Strongly suggesting the hemodynamic measurements taken for the last 4 s are more localized.
DISCUSSION
The principal findings of the present study for long stimulation are first that in response to single whisker sensory stimulation, the hemodynamic response of rat somatosensory cortex was characterized by two distinct phases: an initial transient response represented by changes in the large up-stream branches of the middle cerebral artery and a later and more restricted region of increased blood volume centered on the stimulated barrel. This second phase of more localized activity was maintained throughout the 16-s period of stimulation.
The two distinct phases of the hemodynamic response of somatosensory cortex is a novel finding and may have not been reported previously for several reasons: prior investigations typically used only brief stimulation periods (Chen-Bee et al. 2007; Devor et al. 2005; Erinjeri and Woolsey 2002; Polley et al. 1999; Sheth et al. 2005); they lacked the spatial resolution required to discriminate responses in different vascular compartments; or they failed to use single whisker stimulation (Berwick et al. 2005a; Dunn et al. 2005; Sheth et al. 2005). Indeed our own previous investigation (Berwick et al. 2005a) applied electrical stimulation to the entire whisker pad, which, while producing a large response in the feeding artery, also elevated hemodynamic levels throughout the whole barrel field for the duration of stimulation. This made it difficult to disambiguate distinct arterial and parenchymal responses. It is only the use of discrete single whisker stimulation in the present study that revealed the transient arterial and prolonged response confined to single barrels.
Possible mechanisms underlying a dual phase hemodynamic response
It is likely that distinct neurovascular coupling mechanisms are responsible for the transient and prolonged responses observed in the present study. First, theoretical support for dual coupling mechanisms has emerged from the mathematical models of measured hemodynamic responses (Martindale et al. 2005; Rosengarten et al. 2003; Ureshi et al. 2004). Second, an upstream arterial response has been reported in the peripheral microcirculation in which a dilation signal in the capillary bed was shown to spread upstream via gap junctions in the vessel wall (Cohen et al. 2000; Sarelius et al. 2000). The present results are consistent with this mechanism having been conserved in the cerebral circulation. Supporting evidence for this view comes from reports of arterial dilation ≤2 mm upstream of evoked neural activity in the rat cerebellum (Iadecola et al. 1997). Therefore although retrograde dilation of arteries feeding activated areas has been reported in other model systems, its transitory nature in rodent barrel cortex is novel. The discovery of this initial transitory response will therefore constrain what mechanisms or mediators are responsible for maintaining increased blood volume within an activated barrel column throughout a period of stimulation. There are several possibilities.
First, the restricted increase in blood volume could reflect local increases in capillary perfusion and/or local increases of red blood cell stacking. Both mechanisms have been reported after electrical stimulation of rat cerebral cortex (Schulte et al. 2003). Prearteriole sphincters (pericytes) have also been hypothesized to play a contributory role in local changes in blood volume (Peppiatt et al. 2006; Vanzetta et al. 2005).
Second, initial data (unpublished observations) suggest the localized response could be mediated by a network of smaller arterioles that directly overlie individual barrel columns. We have increased the magnification of our images by a factor of four by developing new procedures to perform 2D-OIS through a stereo microscope. With this system, we have seen a small network of arterioles on the surface of the cortex that lie between the main branches of the middle cerebral artery [as recently shown by Kleinfeld's group (Nishimura et al. 2007)]. These arterioles appear to remain dilated after the main branch of the MCA has returned to baseline.
Third, an interesting observation from the present data is that the restricted response to prolonged stimulation was usually spherical. Perhaps this is indicative of a diffusible mediator (e.g., nitric oxide) being released from the activated barrel. We are currently conducting experiments involving the inhibition of several potential mediators to identify the cause of the “transient” and “prolonged” hemodynamic responses.
Fourth, spatial contraction of the hemodynamic response after long-duration stimulation could be a consequence of the phenomenon of surround inhibition. Using optical procedures to observe hemodynamic activity in squirrel monkey cortex, Simons et al. (2006) reported that longer periods (from 1 to 5 s) of vibrotactile stimulation restricted the size of the evoked cortical response surrounded by a region of negative hemodynamic activity. It was suggested that for stimulation exceeding 1 s, a process of lateral inhibition could spread from the principal site of activation. There are several differences between these results and our findings, and in the present data, there is no evidence for this suggestion. For example, the initially diffuse arterial response lasted considerably longer than 1 s, peaking 2–3 s post stimulus onset. Moreover, we found no evidence of a surround negative response in either of our stimulus conditions. Interpretation of the negative responses reported by Simons et al. (2007) are, however, not straightforward because their use of 830-nm illumination provides an absorption measure that would preclude light scattering and blood volume changes being distinguished. Clearly to permit direct comparison between rat and monkey data, it would be important to use 2D-OIS procedures in the monkey. However, 2D-OIS has been used to detect decreases in blood volume signals surrounding areas of activation in whisker barrel cortex (Devor et al. 2005). Interestingly in this study, the hemodynamic responses to the movement of a single whisker were significantly smaller than the ones reported in the present study and were surrounded by a negative response in cortical regions adjacent to the entire barrel field. We have only seen negative responses in regions surrounding barrel cortex when a strong electrical stimulus is applied to the entire whisker pad (unpublished findings).
When considering the generality of our results, it is important to bear in mind that the vasculature structure of rat somatosensory cortex highlights the organization of capillaries seen in most brain regions in mammals, including humans (Woolsey and Rovainen 1991). That is, the density of capillaries is greater in regions showing greater metabolic activity (Harrison et al. 2002; Zheng et al. 1991). Consequently, the modular concentrations of cytochrome oxidase, the metabolic indicator used to define the barrel structure, correspond to the high-density organization of blood capillaries. These metabolic modules are thought to represent a fundamental unit of cortical functional processing (Hubel and Wiesel 1959; Rakic 2002). Interestingly, analogous metabolically related “cytochrome oxidase blobs” have been described in other areas of cortex in various species (Murphy et al. 1995). However, it is possible that the present dual phase mechanisms of blood recruitment could be independent of any particular arrangement of arteries and veins. Thus the extent to which neurovascular coupling principles described in the present report generalize to other brain regions and other species remains to be determined.
Spatial resolution of hemodynamic response parameters
The results of the present study may have general implications for functional neuroimaging studies. There has been controversy about which aspect of the hemodynamic response (Hbt, HbO2, or Hbr) can provide the most spatially localized information. The transient increase in Hbr, commonly termed the deoxy-dip, has long been considered to be the most spatially localized hemodynamic response (Vanzetta and Grinvald 2001). However, compared with later responses, the deoxy-dip is typically small and brief, which often makes it difficult to detect. For example, in the current study, we never observed a spatially localized deoxy-dip on individual stimulation trials, although averaging across experiments did reveal an early localized Hbr effect. A possible reason for this is that our 2D-OIS technique can only measure hemodynamic changes to a cortical depth of ∼400 μm (see Berwick et al. 2005a).
Regardless of difficulties in measuring the deoxy-dip, both the present and recent studies (Culver et al. 2005; Sheth et al. 2004) found that changes in blood volume provide the most spatially localized, (and easily measurable) hemodynamic response. Thus in the current study, the spatial resolution of the Hbt response was significantly greater than the decrease in Hbr in the surface draining veins. This decrease in Hbr is believed to be one of the main signal sources in BOLD fMRI. Better spatial resolution imaging results may therefore be attained by measuring the blood volume response in the later stages of long stimulation periods when early transient arterial responses have dissipated. Also experiments investigating interactions between adjacent cortical areas will need to consider how the initial widespread arterial response (which may overspill from one region to the next) may complicate interpretation of data (Blood and Toga 1998; Blood et al. 2002).
Relationship between the neural and hemodynamic response
There was a nonlinear monotonic relationship between hemodynamic and electrophysiological measures of neural activity as a function of distance from the single cortical barrel as the sensory response progressively weakened (Fig. 7). Most previous studies determined the neurovascular coupling relationship from a single cortical locus (Hewson-Stoate et al. 2005; Jones et al. 2004; Sheth et al. 2004). However, when measuring hemodynamic and neural responses at progressive distances from an activated barrel, we observed that during the initial phase (1st 4 s), there was a slower decrease in hemodynamic response relative to the reduction in associated neural activity compared with that seen in the later stages of stimulation (last 4 s) when the hemodynamic response was more spatially restricted.
An alternative interpretation of this result is that our measure of neural activity (CSD-Sink) reflects the passive detection of a constant, discrete neural response moving progressively further away from the recording electrode and therefore weakening; as opposed to a weakening neural response of the recorded barrel to suboptimal sensory stimulation (i.e., activation of distant whiskers). If this were the case, the potentially misleading correlation would reflect a comparatively nonspatially specific hemodynamic response, decreasing in magnitude at its periphery, associated with a highly localized but shifting neural response. A key observation inconsistent with the passive detection suggestion was that the latency of the sensory-evoked neural response increased progressively as whiskers further along the row were activated (34 ms in principal barrel to 62 ms at barrel 4-distant away). The average distance between the principal barrel E1 and barrel C1 (2-distant, see Fig. 6, C and F) was 1 ± 0.03 mm. The neural response latency for barrel E1 when whisker C1 was stimulated was 10 ms longer than its response to stimulation of whisker E1. This indicates transmission speeds of ∼0.1 mm/ms far slower than the 1 mm/ms recorded for transmission through cortical tissue after direct electrical stimulation of cerebral cortex (Barth and Sutherling 1988).
The precise nature of the spread of neural information through cortical tissue remains a matter of much debate, for example, to what extent might the observed neural responses arise from cortico-cortical as opposed to thalamo-cortical processing (Fox et al. 2003; Petersen and Diamond 2000; Wirth and Luscher 2004). However, our data do not distinguish whether intracortical or thalamocortical processes are responsible for the observed latencies, although our results do suggest that local hemodynamic responses serve to supply local neuronal responses.
Conclusions
The value of hemodynamic measures of brain function will be limited, in part, by their ability to report local changes in neural activation with high spatial fidelity. In this regard, the results of the present study make two important and novel contributions. First, for prolonged stimuli, the discovery of two temporally distinct phases of the cortical hemodynamic response, with the latter phase more spatially restricted, has important implications for hemodynamically based imaging studies. Our data suggest that spatial resolution in functional imaging studies could be improved by adopting the simple expedient of using longer periods of neuronal activation with dependent measures taken during the later stages of stimulation. Second, the quantitative measures of hemodynamic and neural changes from the same tissue reported in the present study provide a more precise description of the neurovascular coupling relationship after sensory stimulation, without which the mechanisms underlying the observed changes will be difficult to discern.
GRANTS
The authors acknowledge the support of the MRC New Investigator Grant G0601581, co-operative group Grant G9825307. J. Martindale and D. Johnston were supported by National Institute of Neurological Disorders and Stroke Grant R01-NS-445671-01.
Supplementary Material
Acknowledgments
The authors thank the technical staff of the Psychology Department: M. Simkins, M. Benn, N. Kennerley, M. Port, and A. Ham.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Barth and Sutherling 1988.Barth DS, Sutherling W. Current source-density and neuromagnetic analysis of the direct cortical response in rat cortex. Brain Res 450: 280–294, 1988. [DOI] [PubMed] [Google Scholar]
- Berwick et al. 2005a.Berwick J, Johnston D, Jones M, Martindale J, Redgrave P, McLoughlin N, Schiessl I, Mayhew JE. Neurovascular coupling investigated with two-dimensional optical imaging spectroscopy in rat whisker barrel cortex. Eur J Neurosci 22: 1655–1666, 2005a. [DOI] [PubMed] [Google Scholar]
- Berwick et al. 2005b.Berwick J, Jones M, Martindale J, Mayhew JEW. High resolution 2 dimensional optical imaging spectroscopy of single whisker representations in rat somatosensory cortex. Soc Neurosci Abstr 2005b.
- Blood et al. 2002.Blood AJ, Pouratian N, Toga AW. Temporally staggered forelimb stimulation modulates barrel cortex optical intrinsic signal responses to whisker stimulation. J Neurophysiol 88: 422–437, 2002. [DOI] [PubMed] [Google Scholar]
- Blood and Toga 1998.Blood AJ, Toga AW. Optical intrinsic signal imaging responses are modulated in rodent somatosensory cortex during simultaneous whisker and forelimb stimulation. J Cereb Blood Flow Metab 18: 968–977, 1998. [DOI] [PubMed] [Google Scholar]
- Chen-Bee et al. 2007.Chen-Bee CH, Agoncillo T, Xiong Y, Frostig RD. The triphasic intrinsic signal: implications for functional imaging. J Neurosci 27: 4572–4586, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen et al. 2000.Cohen KD, Berg BR, Sarelius IH. Remote arteriolar dilations in response to muscle contraction under capillaries. Am J Physiol Heart Circ Physiol 278: H1916–1923, 2000. [DOI] [PubMed] [Google Scholar]
- Culver et al. 2005.Culver JP, Siegel AM, Franceschini MA, Mandeville JB, Boas DA. Evidence that cerebral blood volume can provide brain activation maps with better spatial resolution than deoxygenated hemoglobin. NeuroImage 27: 947–959, 2005. [DOI] [PubMed] [Google Scholar]
- Devor et al. 2003.Devor A, Dunn AK, Andermann ML, Ulbert I, Boas DA, Dale AM. Coupling of total hemoglobin concentration, oxygenation, and neural activity in rat somatosensory cortex. Neuron 39: 353–359, 2003. [DOI] [PubMed] [Google Scholar]
- Devor et al. 2005.Devor A, Ulbert I, Dunn AK, Narayanan SN, Jones SR, Andermann ML, Boas DA, Dale AM. Coupling of the cortical hemodynamic response to cortical and thalamic neuronal activity. Proc Natl Acad Sci USA 102: 3822–3827, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn et al. 2005.Dunn AK, Devor A, Dale AM, Boas DA. Spatial extent of oxygen metabolism and hemodynamic changes during functional activation of the rat somatosensory cortex. NeuroImage 27: 279–290, 2005. [DOI] [PubMed] [Google Scholar]
- Erinjeri and Woolsey 2002.Erinjeri JP, Woolsey TA. Spatial integration of vascular changes with neural activity in mouse cortex. J Cereb Blood Flow Metab 22: 353–360, 2002. [DOI] [PubMed] [Google Scholar]
- Fox et al. 2003.Fox K, Wright N, Wallace H, Glazewski S. The origin of cortical surround receptive fields studied in the barrel cortex. J Neurosci 23: 8380–8391, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golanov et al. 1994.Golanov EV, Yamamoto S, Reis D. Spontaneous waves of cerebral blood flow associated with a pattern of electrocortical activity. J Am Physiol Regulatory Integrative Comp Physiol 266: R204–R214, 1994. [DOI] [PubMed] [Google Scholar]
- Harrison et al. 2002.Harrison RV, Harel N, Panesar J, Mount RJ. Blood capillary distribution correlates with hemodynamic-based functional imaging in cerebral cortex. Cereb Cortex 12: 225–233, 2002. [DOI] [PubMed] [Google Scholar]
- Hewson-Stoate et al. 2005.Hewson-Stoate N, Jones M, Martindale J, Berwick J, Mayhew J. Further nonlinearities in neurovascular coupling in rodent barrel cortex. NeuroImage 24: 565–574, 2005. [DOI] [PubMed] [Google Scholar]
- Hubel and Wiesel 1959.Hubel DH, Wiesel TN. Receptive fields of single neurones in the cat's striate cortex. J Physiol 148: 574–591, 1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola et al. 1997.Iadecola C, Yang G, Ebner TJ, Chen G. Local and propagated vascular responses evoked by focal synaptic activity in cerebellar cortex. J Neurophysiol 78: 651–659, 1997. [DOI] [PubMed] [Google Scholar]
- Jones et al. 2004.Jones M, Hewson-Stoate N, Martindale J, Redgrave P, Mayhew J. Nonlinear coupling of neural activity and CBF in rodent barrel cortex. NeuroImage 22: 956–965, 2004. [DOI] [PubMed] [Google Scholar]
- Kennerley et al. 2005.Kennerley AJ, Berwick J, Martindale J, Johnston D, Papadakis N, Mayhew JE. Concurrent fMRI and optical measures for the investigation of the hemodynamic response function. Magn Reson Med 54: 354–365, 2005. [DOI] [PubMed] [Google Scholar]
- Logothetis 2002.Logothetis NK The neural basis of the blood-oxygen-level-dependent functional magnetic resonance imaging signal. Philos Trans R Soc Lond B Biol Sci 357: 1003–1037, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis and Pfeuffer 2004.Logothetis NK, Pfeuffer J. On the nature of the BOLD fMRI contrast mechanism. Magn Reson Imaging 22: 1517–1531, 2004. [DOI] [PubMed] [Google Scholar]
- Malonek et al. 1997.Malonek D, Dirnagl U, Lindauer U, Yamada K, Kanno I, Grinvald A. Vascular imprints of neuronal activity: relationships between the dynamics of cortical blood flow, oxygenation, and volume changes following sensory stimulation. Proc Natl Acad Sci USA 94: 14826–14831, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malonek and Grinvald 1996.Malonek D, Grinvald A. Interactions between electrical activity and cortical microcirculation revealed by imaging spectroscopy: implications for functional brain mapping. Science 272: 551–554, 1996. [DOI] [PubMed] [Google Scholar]
- Martindale et al. 2005.Martindale J, Berwick J, Martin C, Kong Y, Zheng Y, Mayhew J. Long duration stimuli and nonlinearities in the neural-haemodynamic coupling. J Cereb Blood Flow Metab 25: 651–661, 2005. [DOI] [PubMed] [Google Scholar]
- Martindale et al. 2003.Martindale J, Mayhew J, Berwick J, Jones M, Martin C, Johnston D, Redgrave P, Zheng Y. The hemodynamic impulse response to a single neural event. J Cereb Blood Flow Metab 23: 546–555, 2003. [DOI] [PubMed] [Google Scholar]
- Mayhew et al. 1999.Mayhew J, Zheng Y, Hou Y, Vuksanovic B, Berwick J, Askew S, Coffey P. Spectroscopic analysis of changes in remitted illumination: the response to increased neural activity in brain. NeuroImage 10: 304–326, 1999. [DOI] [PubMed] [Google Scholar]
- Mitzdorf 1985.Mitzdorf U Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiol Rev 65: 37–100, 1985. [DOI] [PubMed] [Google Scholar]
- Murphy et al. 1995.Murphy KM, Jones DG, Van Sluyters RC. Cytochrome-oxidase blobs in cat primary visual cortex. J Neurosci 15: 4196–4208, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai and Maeda 1999.Nakai M, Maeda M. Scopolamine-sensitive and resistant components of increase in cerebral cortical blood flow elicited by periaqueductal gray matter of rats. Neurosci Lett 270: 173–176, 1999. [DOI] [PubMed] [Google Scholar]
- Nemoto et al. 2004.Nemoto M, Sheth S, Guiou M, Pouratian N, Chen JW, Toga AW. Functional signal- and paradigm-dependent linear relationships between synaptic activity and hemodynamic responses in rat somatosensory cortex. J Neurosci 24: 3850–3861, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson and Freeman 1975.Nicholson C, Freeman JA. Theory of current source-density analysis and determination of conductivity tensor for anuran cerebellum. J Neurophysiol 38: 356–368, 1975. [DOI] [PubMed] [Google Scholar]
- Nishimura et al. 2007.Nishimura N, Schaffer CB, Friedman B, Lyden PD, Kleinfeld D. Penetrating arterioles are a bottleneck in the perfusion of neocortex. Proc Natl Acad Sci 104: 365–370, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppiatt et al. 2006.Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature 443: 700–704, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen and Diamond 2000.Petersen RS, Diamond ME. Spatial-temporal distribution of whisker-evoked activity in rat somatosensory cortex and the coding of stimulus location. J Neurosci 20: 6135–6143, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley et al. 1999.Polley DB, Chen-Bee CH, Frostig RD. Varying the degree of single-whisker stimulation differentially affects phases of intrinsic signals in rat barrel cortex. J Neurophysiol 81: 692–701, 1999. [DOI] [PubMed] [Google Scholar]
- Rakic 2002.Rakic P Evolving concepts of cortical radial and areal specification. Prog Brain Res 136: 265–280, 2002. [DOI] [PubMed] [Google Scholar]
- Rosengarten et al. 2003.Rosengarten B, Lutz H, Hossmann KA. A control system approach for evaluating somatosensory activation by laser-Doppler flowmetry in the rat cortex. J Neurosci Methods 130: 75–81, 2003. [DOI] [PubMed] [Google Scholar]
- Sarelius et al. 2000.Sarelius IH, Cohen KD, Murrant CL. Role for capillaries in coupling blood flow with metabolism. Clin Exp Pharmacol Physiol 27: 826–829, 2000. [DOI] [PubMed] [Google Scholar]
- Schulte et al. 2003.Schulte ML, Wood JD, Hudetz AG. Cortical electrical stimulation alters erythrocyte perfusion pattern in the cerebral capillary network of the rat. Brain Res 963: 81–92, 2003. [DOI] [PubMed] [Google Scholar]
- Sheth et al. 2004.Sheth SA, Nemoto M, Guiou M, Walker M, Pouratian N, Toga AW. Linear and nonlinear relationships between neuronal activity, oxygen metabolism, and hemodynamic responses. Neuron 42: 347–355, 2004. [DOI] [PubMed] [Google Scholar]
- Sheth et al. 2005.Sheth SA, Nemoto M, Guiou MW, Walker MA, Toga AW. Spatiotemporal evolution of functional hemodynamic changes and their relationship to neuronal activity. J Cereb Blood Flow Metab 25: 830–841, 2005. [DOI] [PubMed] [Google Scholar]
- Simons et al. 2007.Simons SB, Chiu J, Favorov OV, Whitsel BL, Tommerdahl M. Duration-dependent response of SI to vibrotactile stimulation in squirrel monkey. J Neurophysiol 97: 2121–2129, 2007. [DOI] [PubMed] [Google Scholar]
- Ureshi et al. 2004.Ureshi M, Matsuura T, Kanno I. Stimulus frequency dependence of the linear relationship between local cerebral blood flow and field potential evoked by activation of rat somatosensory cortex. Neurosci Res 48: 147–153, 2004. [DOI] [PubMed] [Google Scholar]
- Vanzetta and Grinvald 2001.Vanzetta I, Grinvald A. Evidence and lack of evidence for the initial dip in the anesthetized rat: implications for human functional brain imaging. NeuroImage 13: 959–967, 2001. [DOI] [PubMed] [Google Scholar]
- Vanzetta et al. 2005.Vanzetta I, Hildesheim R, Grinvald A. Compartment-resolved imaging of activity-dependent dynamics of cortical blood volume and oximetry. J Neurosci 25: 2233–2244, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth and Luscher 2004.Wirth C, Luscher HR. Spatiotemporal evolution of excitation and inhibition in the rat barrel cortex investigated with multielectrode arrays. J Neurophysiol 91: 1635–1647, 2004. [DOI] [PubMed] [Google Scholar]
- Woolsey and Rovainen 1991.Woolsey TA, Rovainen CM. Whisker barrels: A model for direct observation of changes in the cerebral microcirculation with neuronal activity. Alfred Benzon Symp 31: 189–198, 1991. [Google Scholar]
- Woolsey and Van der Loos 1970.Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res 17: 205–242, 1970. [DOI] [PubMed] [Google Scholar]
- Zheng et al. 1991.Zheng D, LaMantia AS, Purves D. Specialized vascularization of the primate visual cortex. J Neurosci 11: 2622–2629, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng et al. 2001.Zheng Y, Johnston D, Berwick J, Mayhew J. Signal source separation in the analysis of neural activity in brain. NeuroImage 13: 447–458, 2001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.