Abstract
Starting from the clinical observations that moderate haemophiliacs experienced only few bleeding episodes and rarely developed significant joint deterioration (haemophilic arthropathy), and the pioneer experience in Sweden, prophylaxis (i. e. the regular and long-term administration of clotting factor concentrate in order to prevent bleeding) has been practiced for more than forty years in severe haemophilia and is currently recommended as the first choice of treatment by the World Health Organisation and World Federation of Hemophilia and by many national medical/scientific organizations. Observational studies clearly established the superiority of prophylaxis over on-demand treatment in reducing the risk of arthropathy, also showing that starting prophylaxis earlier in life and after very few joint bleeds was associated with better joint outcomes, and led to the current definitions of primary (started before the age of 2 yrs and after no more than one joint bleed) and secondary prophylaxis. More recently, evidences from randomized trials, which were previously lacking in this setting, were also provided.
This review summarizes available data from which current clinical practice of primary (and early secondary) prophylaxis in children with severe haemophilia was drawn. Open issues concerning optimal regimens and barriers to the implementation of prophylaxis are also discussed.
Keywords: bleeding, children, haemophilia, prophylaxis, treatment
Prophylaxis in haemophilia: definitions and objectives
In patients with severe haemophilia, prophylaxis, i.e. the infusion of factor concentrate replacement treatment in order to prevent bleeding episodes, has been used for more than 40 years in Northern Europe and more recently in other European countries and in Northern America, becoming the first-choice treatment recommended by the World Health Organisation (WHO) and the World Federation of Haemophilia since 19941–2. Starting from the clinical observations that patients with moderate or mild haemophilia, with FVIII:C or IX:C levels ≥1%, have a low frequency of joint bleeds and rarely develop severe arthropathy3–4, the main objective of the prophylactic replacement treatment since the Swedish pioneering regimens was (and remains) to minimise the number of joint bleeds since an early age by converting the severe form of haemophilia to a milder form. This is in order to prevent or reduce the muscle-skeletal impairment from haemophilic arthropathy, the main cause of morbidity and factor affecting quality of life in patients with severe haemophilia. Preventing or reducing the clinical impact of haemophilic arthropathy by prophylaxis means enabling normal life and psychosocial development for haemophilic children, including the possibility of physical activities, regular school attendance and, consequently, social and work opportunities. On the whole, severe haemophiliacs on prophylaxis and their families have a better quality of life than patients who receive on-demand treatment1–2,5.
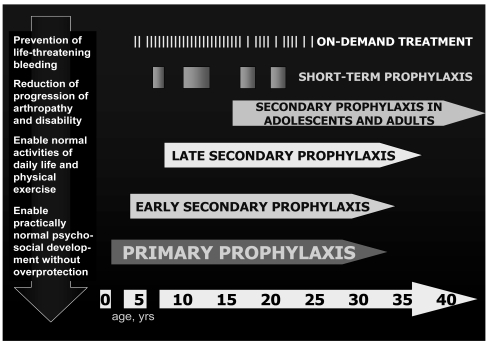
These clinical and social benefits seemed clearly demonstrated by the report of the 25-year Swedish experience in 19921 and numerous clinical studies confirmed these findings in the following years, showing that the earlier the start of prophylaxis, the better the results with regards to the patients’ joint status. However, prophylaxis was implemented in different ways and heterogeneous definitions were used, making it difficult to analyse and compare clinical outcomes. A revision of definitions was proposed by a Consensus Conference in 2002 (table I)6, encompassing the different strategies in Europe (prophylaxis started by the age of 2 years, regardless of bleeding tendency) and in North America (prophylaxis started after the first bleed). Despite the lack of evidence on the number of joint bleeds resulting in irreversible joint damage, the definition of primary prophylaxis is focused on the avoidance of any joint abnormality, in order to guarantee patients the best possible quality of life. The objective of secondary prophylaxis, whenever started, is to avoid (or delay) the progression of arthropathy; earlier treatment, however, results in better outcomes and quality of life. In some patients a secondary prophylaxis regimen is prescribed for short periods, usually to reduce the frequency of bleeds, in particular in target joints. The definitions of prophylaxis, therefore, reflect a wide spectrum of clinical conditions and objectives of the treatment, from the prevention of severe or life-threatening bleeds to the absence of arthropathy enabling patients to live a substantially normal life, without overprotection7 (Figure 1). In a congenital, chronic disease such as haemophilia, for which a definitive cure is still not available, the latter perspective is an important and reliable goal, achieved by primary prophylaxis. However, the choice of treatment regimens and their clinical implementation must take into account possible limitations related to the general resources of the healthcare system and to the specific conditions of each patient and his family.
Table I.
Definitions of replacement treatment regimens in haemophilia (Consensus Perspectives on Prophylactic Therapy for Haemophilia, London 20–21 September 2002, updated by the European paediatric Network for Haemophilia Management in 2006)
| Regimen | Definition |
|---|---|
| Primary prophylaxis | Long-term continuous (at least 46 weeks/year)* treatment started |
| by age | before the age of 2 years and prior to any clinically evident joint bleeding |
| by first bleed | after no more than one joint bleed and before the age of 2 years |
| Secondary prophylaxis | Long-term continuous (at least 46 weeks/year)* treatment not fulfilling the criteria for primary prophylaxis |
| Short-term prophylaxis | Short-term regular treatment to prevent bleeding |
| On demand therapy | Treatment given when bleeding occurs |
with the aim of treating 52 weeks/year up to adulthood
Figure 1.
Choices of treatment regimens in haemophiliacs and the different ages at which they are implemented affect the patients clinical and psychosocial outcomes. From Ljung7, modified.
Primary prophylaxis: from clinical observations to evidence-based medicine
Despite long clinical experience and the extensive use of prophylaxis, in particular in some European countries, data supporting the benefits of prophylaxis in haemophiliacs come mostly from retrospective or non-controlled studies. These studies evaluated the effects of different regimens of treatment on frequency of bleeding, in particular joint bleeds, and on long-term clinical outcome in terms of arthropathy, assessed by clinical and radiological scores8–9, and, in some cases, of patients’ quality of life, by reporting the number of visits, hospital admissions and work/school days lost. Table II summarises some relevant studies from which the current definitions and clinical choices of primary prophylaxis in children have been drawn. The different cohorts of patients described in the Malmö experience reflect the evolution in clinical implementation of prophylaxis1: the oldest patients (24–32 years at study evaluation) started to receive prophylaxis at an age between 3 and 13 years old (median, 7 years) with a regimen with lower factor doses and a frequency of infusion (10–20 IU/Kg every 3–5 days); they experienced 1.6–16 joint bleeds per year and had radiological scores ranging from 0 to41. Patients aged 13–17 years, who started prophylaxis between 1 and 4.5 years old (median, 2.6 years), despite more intensive regimens, showed a similar frequency of joint bleeds (0.1–16 per year) and progression of orthopaedic and radiological scores. Only younger children (3–12 years) receiving higher doses (25–40 IU/Kg thrice weekly) earlier in life, before the age of 2 (median, 1.2 years), did not have any bleeding episodes at all and showed no signs of arthropathy at orthopaedic and radiological evaluations1. These findings were confirmed by subsequent reports, indicating that there was no detectable arthropathy in children starting prophylaxis before 2 years old or after no more than one joint bleed10 and that, irrespective of the regimens, starting prophylaxis at an age of about 4 years was associated with significant benefits in the reduction of frequency of joint bleeds (a major predictor of clinical outcome in the prospective multinational 6-year Orthopaedic Outcome Study5), but did not prevent the development of arthropathy in most patients11–13.
Table II.
Some clinical studies of primary/early secondary prophylaxis in children with haemophilia
| Author, year Country [ref.] | Patients | Median age at start of prophylaxis, years; (range) | Main results |
|---|---|---|---|
| Nillsson, 1992 Sweden [1] | 35 (30 HA, 5 HB)* 3 cohorts, age at study evaluation 3–6, 7–12 and 13–17 years | 1.1 (1–1.5), n=6 1.2 (0.5–2), n=9 2.6 (1–4.5), n=20 | The two younger cohorts, starting prophylaxis before the age of 2, minimised joint bleeds (median 0.1/year) and maintained OJS and PS 0, with higher F VIII/IX consumption compared to patients who started prophylaxis later, and experienced a higher frequency of joint bleeds (median 3/year) and worse joint status (median OJS and PS 1.2 and 4.8, respectively). |
| Aledort, 1994 Multi-national [5] | 48 HA** | na. | At the 6-year follow-up absence of progression of OJS and PS was correlated to significantly lower frequency of joint bleeds (mean, 1.8 vs. 11.2, p=0.0001) and higher use of prophylaxis longer than 45 weeks/year (9 vs 0, p=0.002). |
| Kreuz, 1998 | 14 (11 HA, 3 HB)* | 1.75 (1–2.5), n=8 | At study entry the median OJS and PS was 0 in both groups; at the 4-year follow-up the frequency of joint bleeds was similar (0. 14 vs 0.22/year) but the joint outcome was better (median PS 0 vs 4) in the first group, starting prophylaxis earlier and with history of = 1 joint bleed, than in the other group with a median of 6 joint bleeds before prophylaxis. |
| Van den Berg, 2001 the Netherlands[11] | 22 HA | 4.0±0.5 | At a mean age of 14.7 years, patients, who started prophylaxis after 2–5 joint bleeds and at a mean of 2.3 years after the first bleed, had a mean of 3.2 joint bleeds/year and a mean PS of 2.3. |
| Panicker, 2002 US [12] | 20 (17 HA, 3 HB) | 4.5 | A reduction of the mean frequency of major bleeds from 15.4 to 1.9/year at a median age of 11.4 years, with significant reductions in target joints and number of visits and hospital admissions, was observed. |
| Yee, 2002 UK [13] | 29 (24 HA, 5 HB) | 4.0(2–12.7) | At a median follow-up of 4.1 years (0.3–11.5), median joint bleeds decreased from 3.5 to 0.5/year, 20 children (70%) retained an OJS of 0, while in the other 9 the median OJS was 1.5. |
Abbreviations: n.a.: not available; HA: haemophilia A; HB: haemophilia B; OJS: orthopaedic joint score; PS: Pettersson score
only patients considered on primary or early secondary prophylaxis from the whole study population (60 patients, 52 HA, 8 HB, in the Swedish study, 21 patients, 18 HA, 3 HB in the German study) are mentioned.
the subgroup of children with orthopaedic and Pettersson scores 0 at study entry is considered.
The superiority of primary prophylaxis over early secondary prophylaxis was confirmed in a further Swedish study, in which age at the start of prophylaxis was as an independent predictor for the development of arthropathy14; however no significant difference in the joint outcome was detectable between children starting high-dose prophylaxis (i.e. 25–10 IU/Kg 2–3 times weekly) before the age of 3 and those starting between 3 and 5 years old, indicating that an early start is important but individualisation is possible on the basis of the patient’s bleeding pattern. The key point is, therefore, likely to be the number of joint bleeds before the start of prophylaxis, as also shown in the Dutch experience with lower dose regimens (15–25 IU/Kg 1–2 times weekly), tailored to the patients’ bleeding tendency11, 15. Long-term clinical outcome was more favourable in patients starting prophylaxis before the age of 4 (no arthropathy in 50% of patients vs. 21%in those starting between 3 and 7 years old), with an estimated 8% higher Pettersson score per year of postponed prophylaxis from birth, and the best results (70% of patients with score 0) found in patients with fewer than three bleeds before the start of prophylaxis15.
The relative rarity of the disease and the difficulty in performing randomised controlled studies (especially in countries in which consolidated experience on prophylaxis raises ethical considerations on possible randomisation of children to on-demand treatment) until recently, led the Cochrane reviewers in 200516 to identify only four small randomised cross-over trials (three of them carried out in the 1970s, three in haemophilia A and one in haemophilia B), comparing a prophylactic regimen with placebo17 or two different prophylactic regimens18–20. The heterogeneity of the study designs, interventions and patients’ characteristics hampered a meta-analysis of these studies, involving only a total of 44 patients. No randomised studies were found providing a direct comparison of prophylaxis to on-demand treatment. As a conclusion, the authors stated that there was insufficient evidence supporting clinical benefits of prophylaxis over on-demand treatment and remarked on the need for well-designed randomised controlled trials addressing this issue16.
These conclusions confirmed the perplexities concerning the implementation of prophylaxis in children in countries requiring evidence-based data for the acceptance of healthcare approaches and reimbursements. As expected, many criticisms were triggered concerning the rigid emphasis on randomised controlled trials, which could deprive patients of improvement in care recognised as safe and effective by other research approaches21–24. Such trials were considered unjustified and substantially unfeasible in a chronic disease such as haemophilia, with a life-time perspective, in order to assess the long-term impact of a treatment, in terms of outcomes and cost-effectiveness ratio (in the case of prophylaxis, joint outcome, disability, labour force participation, health-care costs for hospital admissions, physiotherapy, orthopaedic procedures and the overall quality of life of patients)24. Moreover, the conclusions of the Cochrane Reviewers seemed rather untimely: it was recognised that two randomised controlled trials were on-going, and preliminary results of one of them were available16. The first RCT on prophylaxis vs. on-demand treatment, the Joint Outcome Study (JOS), was published in August 200725.
This study, whose primary end-point focused on the prevention of joint deterioration by prophylaxis started prior to or at the time of the second joint bleed (between 6 and 30 months old) provided evidence of an about 6-fold reduction (85%) in the risk of joint damage in children on prophylaxis (25 IU/Kg every other day) compared to those on intensive on-demand treatment, as assessed at the age of 6 years by radiography and/or by the most advanced approach for evaluating joint structure, magnetic resonance imaging (MRI)26. The main results of the JOS are reported in Table III
Table III.
Main findings from the Joint Outcome Study25
| Prophylaxis | On-demand | p | |
|---|---|---|---|
| Enrolled patients | 32 | 33 | |
| Evaluable patients (primary end-point)* | 28 | 29 | |
| OUTCOME DATA | |||
| Joint bleeds (n. per year, median) | 0.2 | 4.35 | <0.001 |
| Total bleeds (n. per year, median) | 1.15 | 17.1 | <0.001 |
| rFVIII use (IU per year, mean) | 352,793 | 113,237 | <0.001 |
| No joint damage at RX (n, %) | 27/28 (96%) | 22/27 (81%) | 0.002 |
| No joint damage at MRI (n, %) | 25/27 (93%) | 16/29 (55%) | 0.1 |
| SEVERE ADVERSE EVENTS | |||
| High-titre inhibitors (n. of patients) | 2 | 0 | 0.24 |
| Life-threatening bleeds (n. of patients) | 0 | 3 | 0.24 |
| Hospital admissions# (n. per patient per year, median) | 0.25 | 0.24 | 0.9 |
| CVAD (n. of patients, %) | 29 (91%) | 25 (76%) | 0.2 |
| CVAD-related infections (n. of patients) | 6 | 6 | 0.95 |
Abbreviations: RX: plain-film radiography; MRI: magnetic resonance imaging; CVAD: central venous access devices.
Patients with RX and/or MRI documenting joint status at the completion of the study, i.e. at the age of 6 years.
for causes related to haemophilia.
Interestingly, only MRI assessment showed a significant difference in the joint outcome, with more than half of the joint abnormalities not being detectable by the traditional radiological evaluation. Despite the debate on standardising the interpretation and scoring of findings27, MRI was confirmed as the preferable imaging technique in haemophilic children, revealing joint deterioration also in the absence of a history of haemarthroses and overt abnormalities at physical examination25 (the role of microhaemorrhages without clinical evidence of joint bleeds has been suggested, as also previously hypothesized5), In Table IV the patients’ characteristics and interventions of the JOS are compared with those of the other randomised trial carried out in Italy, the ESPRIT (Evaluation Study on Prophylaxis: a Randomized Italian Trial)28. The final results from this study should soon be available and will provide data on the long-term outcome (10 years) of children receiving early secondary prophylaxis, according to the tendency in some countries, in particular in the 1990s, to evaluate patients’ bleeding phenotype prior to starting prophylaxis.
Table IV.
| JOS | ESPRIT | |
|---|---|---|
| Enrolment | 08/1996–04/2005 | 12/1996–06/1998 |
| Enrolled patients, n. (prophylaxis/on-demand) | 65 (32/33) | 45 (23/22) |
| Evaluable patients*, n. (prophylaxis/on-demand) | 57 (28/29) | 40 (20/20) |
| Median age, years | 1.6 | 4.0 |
| Follow-up, months^ | 49 | 120 |
| ELEGIBILITY CRITERIA | ||
| Age, months | < 30 | < 84 |
| FVIII activity | < 2 | < 1 |
| Joint bleeds | ≤2 | ≥1° |
| Baseline orthopaedic and Pettersson scores | 0 | 0 |
| INTERVENTIONS | ||
| Prophylactic regimen, FVIII | 25 IU/Kg e.o.d. | 25 IU t.i.w. (target FVIII>1%) |
| On-demand treatment, FVIII | 40 IU/Kg, 20 IU/Kg at 24 and 72 hrs and e.o.d. until recovery | ≥ 25 IU/Kg, repeated until complete recovery |
in the JOS, number of patients with radiological data for the assessment of the primary end-point; in the ESPRIT, number of patients following the treatment regimen assigned by randomisation.
median follow-up in the JOS, in which patients were evaluated at the age of 6 years old; planned follow-up in the ESPRIT.
at least one joint or muscle bleed in the 6 months before randomisation.
Primary prophylaxis in haemophilia: open issues and perspectives
Despite the general lack of high-level evidence supporting clinical choices for the optimal prophylactic regimen, convincing findings from observational studies show that the early start of prophylaxis may be individualised on the basis of the bleeding pattern5,10,11,14,15. This led to tailored doses and/or frequencies of prophylactic infusions, in order to improve the compliance of families to such a highly demanding treatment (whose perceived need and knowledge of benefits are often poor29), and, in particular, to reduce the need for central venous access devices, reaching full-dose regimens when children may have good peripheral vein accesses.
According to the Swedish approach, prophylaxis is started in children between 1 and 2 years old or after the first joint bleed, with a single weekly infusion at the Haemophilia Centre. The frequency of infusions is escalated to two and then three times weekly, based on the availability of adequate peripheral veins (in parallel parents’ training for home treatment is carried out) or, more rarely, on the bleeding frequency. With this approach, home treatment with 500IU every other day was achieved in 6–18 months and no clinical influence of maintaining a trough level of >1% was shown30. Another tailored dose approach was proposed in Canada, where children generally receive 50 IU/Kg once weekly, started when they are between 1 and 2 years old, and the bleeding frequency is evaluated by clinical follow-up every 3 months. In patients experiencing three bleeds in the same joint or four total bleeds over a 3 -month period, the prophylactic infusion dose is increased to 30 IU/Kg twice weekly and then to 25 IU/Kg every other day. According to the recently published experience, with a median follow-up of 4.1 years, 40% of children remained on the once weekly infusion and 16% reached the full-dose prophylactic regimen31. The development of target joints in 22.5% of these children raised some perplexities31; however, both the Swedish and the Canadian approaches enabled a remarkable reduction in the need for central venous accesses (24% and 40%, respectively)30–31, compared to the highest frequency reported in the JOS (91%)25. The perspective of new factor concentrates with longer half-lives32 is encouraging for regimens with less frequent infusions, better acceptance and compliance of patients and, probably, lower consumption of replacement factors.
The issue of availability and, in particular, of costs of clotting factor concentrates remains the main barrier to the diffusion of prophylaxis, since these costs are prohibitive particularly for developing countries30. The comparison of different dose regimens for prophylaxis showed that the median annual amount of factor concentrates required for the intermediate-dose regimen used in the Netherlands is approximately half that required with the high-dose regimen used in Sweden (table V). When long-term outcomes are considered, the frequency of joint bleeds and orthopaedic and radiological scores were significantly lower in Swedish patients than in Dutch ones33. The latter did, however, have a more favourable orthopaedic outcome than French patients treated on-demand, with almost comparable clotting factor consumption34.
Table V.
Prophylactic regimens in children with haemophilia
| High-dose regimens (Sweden, North America)1,14,25,30–31,33–34 | ||
| Haemophilia A: | 25–40 IU/Kg | 3 times weekly or every other day |
| Haemophilia B: | 25–40 IU/Kg | 2 times weekly |
| Intermediate-dose regimens (the Netherlands)11,15,33–34 | ||
| Haemophilia A | 15–25 IU/Kg | 2–3 times weekly |
| Haemophilia B | 30–50 IU/Kg | 1–2 times weekly |
| Guidelines of the Italian Association of Haemophilia Centres (AICE)28,39 | ||
| Haemophilia A | 25–30 IU/Kg | 3 times weekly |
| Haemophilia B | 30–40 IU/Kg | 2 times weekly |
In the light of the lack of prospective and controlled data, individualisation of regimens may help to tailor more cost-effective treatments19,30. In this respect, longer follow-ups are required when considering outcomes in haemophilia and economic analyses should not be confined to costs of concentrates, but should take into account all the additional health resources related to the treatment of haemophilia and its orthopaedic complications35. Moreover newer outcome measures, useful for determining the overall benefits to patients, should be added to the clinical outcomes, including Health-Related Quality of Life, for which specific validated instruments have now been developed35–36.
Recent data suggest new potential benefits of early initiation of prophylaxis in haemophilic children. A protective effect against the development of inhibitors has been shown in a case-control Italian study37, with a 70% reduction of inhibitor risk in children starting prophylaxis at a median age of 35 months. Similar findings were shown in the larger multinational CANAL study, in which early regular prophylaxis (started at a median age of 20 months) was an independent negative predictor associated with a 60% lower risk of inhibitor development than on-demand treatment38. This additional benefit could further help to overcome barriers to the acceptance and the diffusion of this strategy of treatment which, irrespective of evidence-based medicine, has undeniably transformed the lives of children with severe haemophilia and their families over the last four decades.
Footnotes
Conflicts of interest disclosure
The Author declares that he received lecture fees from CSL Behring and Bayer Schering Pharma.
References
- 1.Nilsson IM, Berntorp E, Löfqvist T, Pettersson H. Twenty-five years experience of prophylactic treatment in severe haemophilia A and B. J Intern Med. 1992;232:25–32. doi: 10.1111/j.1365-2796.1992.tb00546.x. [DOI] [PubMed] [Google Scholar]
- 2.Berntorp E, Boulyjenkov V, Brettler D, et al. Modern treatment of haemophilia. Bull World Health Organ. 1995;73:691–701. [PMC free article] [PubMed] [Google Scholar]
- 3.Ramgren O. Haemophilia in Sweden. III. Symptomatology, with special reference to differences between haemophilia A and B. Acta Med Scand. 1962;171:237–42. [PubMed] [Google Scholar]
- 4.Ahlberg A. Haemophilia in Sweden. VII. Incidence, treatment and prophylaxis of arthropathy and other musculo-skeletal manifestations of haemophilia A and B. Acta Orthop Scand Suppl. 1965;(Suppl 77):3–132. doi: 10.3109/ort.1965.36.suppl-77.01. [DOI] [PubMed] [Google Scholar]
- 5.Aledort L, Haschmeyer RH, Pettersson H. A longitudinal study of orthopaedic outcomes for severe factor-VIII-deficient haemophiliacs. The Orthopaedic Outcome Study Group. J Intern Med. 1994;236:391–9. doi: 10.1111/j.1365-2796.1994.tb00815.x. [DOI] [PubMed] [Google Scholar]
- 6.Berntorp E, Astermark J, Bjorkman S, et al. Consensus perspectives on prophylaxis therapy for haemophilia: summary statement. Haemophilia. 2003;9 (Suppl 1):1–4. doi: 10.1046/j.1365-2516.9.s1.17.x. [DOI] [PubMed] [Google Scholar]
- 7.Ljung R. Prevention of bleeding in haemophilia: trends, overcoming barriers and future treatment options. Haemophilia. 2007;13 (Suppl 2):1–3. [Google Scholar]
- 8.Gilbert MS. Prophylaxis: musculoskeletal evaluation. Semin Hematol. 1993;3 (Suppl2):3–6. [PubMed] [Google Scholar]
- 9.Pettersson H, Ahlberg A, Nilsson IM. A radiologic classification of hemophilic arthropathy. Clin Orthop Relat Res. 1980;149:153–9. [PubMed] [Google Scholar]
- 10.Kreuz W, Escuriola-Ettingshausen C, Funk M, et al. When should prophylactic treatment in patients with haemophilia A and B start? The German experience. Haemophilia. 1998;4:413–7. doi: 10.1046/j.1365-2516.1998.440413.x. [DOI] [PubMed] [Google Scholar]
- 11.van den Berg HM, Fischer K, Mauser-Bunschoten EP, et al. Long-term outcome of individualized prophylactic treatment of children with severe haemophilia. Br J Haematol. 2001;112:561–5. doi: 10.1046/j.1365-2141.2001.02580.x. [DOI] [PubMed] [Google Scholar]
- 12.Panicker J, Warrier I, Lusher J. The impact of prophylaxis on children with severe haemophilia. Journal. issue; pages. [Google Scholar]
- 13.Yee TT, Beeton K, Griffioen A, et al. Experience of prophylaxis treatment in children with severe haemophilia. Haemophilia. 2002;8:76–82. doi: 10.1046/j.1365-2516.2002.00630.x. [DOI] [PubMed] [Google Scholar]
- 14.Astermark J, Petrini P, Tengborn L, et al. Primary prophylaxis in severe haemophilia should be started at an early age but can be individualized. Br J Haematol. 1999;105:1109–13. doi: 10.1046/j.1365-2141.1999.01463.x. [DOI] [PubMed] [Google Scholar]
- 15.Fischer K, van der Bom JG, Mauser-Bunschoten EP, et al. The effects of postponing prophylactic treatment on long-term outcome in patients with severe haemophilia. Blood. 2002;99:2337–41. doi: 10.1182/blood.v99.7.2337. [DOI] [PubMed] [Google Scholar]
- 16.Stobart K, Iorio A, Wu JK. Clotting factor concentrates given to prevent bleeding and bleeding-related complications in people with hemophilia A or B. Cochrane Database Syst Rev. 2006;2:CD003429. doi: 10.1002/14651858.CD003429.pub3. [DOI] [PubMed] [Google Scholar]
- 17.Aronstam A, Arblaster PG, Rainsford SG, et al. Prophylaxis in haemophilia: a double-blind controlled trial. Br J Haematol. 1976;33:81–90. doi: 10.1111/j.1365-2141.1976.tb00973.x. [DOI] [PubMed] [Google Scholar]
- 18.Aronstam A, Kirk PJ, McHardy, et al. Twice weekly prophylactic therapy in haemophilia A. J Clin Pathol. 1977;30:65–7. doi: 10.1136/jcp.30.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carlsson M, Berntorp E, Bjorkman S, et al. Improved cost-effectiveness by pharmacokinetic dosing of factor VIII in prophylactic treatment of haemophilia A. Haemophilia. 1997;3:96–101. doi: 10.1046/j.1365-2516.1997.00091.x. [DOI] [PubMed] [Google Scholar]
- 20.Morfini M, Mannucci PM, Mariani G, et al. Evaluation of prophylactic replacement in haemophilia B. Scand J Haematol. 1976;16:41–7. doi: 10.1111/j.1600-0609.1976.tb01115.x. [DOI] [PubMed] [Google Scholar]
- 21.Mannucci PM. Need for randomized trials in haemophilia. J Thromb Haemost. 2006;4:501–2. doi: 10.1111/j.1538-7836.2006.01852.x. [DOI] [PubMed] [Google Scholar]
- 22.Aledort L, Ljung R, Blanchette V. Are randomized clinical trials the only truth? Not always. J Thromb Haemost. 2006;4:503–4. doi: 10.1111/j.1538-7836.2006.01841.x. [DOI] [PubMed] [Google Scholar]
- 23.The Medical and Scientific Advisory Council of the National Haemophilia Foundation. More on: are randomized clinical trials the only truth? Not always. J Thromb Haemost. 2006;4:1167–8. doi: 10.1111/j.1538-7836.2006.01938.x. [DOI] [PubMed] [Google Scholar]
- 24.Fischer K, Grobbee DE, van den Berg HM. RCTs and observational studies to determine the effect of prophylaxis in severe haemophilia. Haemophilia. 2007;13:345–50. doi: 10.1111/j.1365-2516.2007.01521.x. [DOI] [PubMed] [Google Scholar]
- 25.Manco-Johnson MJ, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357:535–44. doi: 10.1056/NEJMoa067659. [DOI] [PubMed] [Google Scholar]
- 26.Kilcoyne RF, Nuss R. Radiological assessment of haemophilic arthropathy with emphasis on MRI findings. Haemophilia. 2003;9 (Suppl 1):57–64. doi: 10.1046/j.1365-2516.9.s1.11.x. [DOI] [PubMed] [Google Scholar]
- 27.Doria AS, Lundin B, Kilcoyne RF, et al. Reliability of progressive and additive MRI scoring systems for evaluation of haemophilic arthropathy in children: expert MRI Working Group of the International Prophylaxis Study Group. Haemophilia. 2005;11:245–53. doi: 10.1111/j.1365-2516.2005.01097.x. [DOI] [PubMed] [Google Scholar]
- 28.Gringeri A. Prospective controlled study on prophylaxis: an Italian approach. Haemophilia. 2003;9 (Suppl 1):38–43. doi: 10.1046/j.1365-2516.9.s1.6.x. [DOI] [PubMed] [Google Scholar]
- 29.Petrini P. Identifying and overcoming barriers to prophylaxis in the management of haemophilia. Haemophilia. 2007;13 (Suppl 2):16–22. doi: 10.1111/j.1365-2516.2007.01501.x. [DOI] [PubMed] [Google Scholar]
- 30.Petrini P. What factors should influence the dosage and interval of prophylactic treatment in patients with severe haemophilia A and B? Haemophilia. 2001;7:99–102. doi: 10.1046/j.1365-2516.2001.00471.x. [DOI] [PubMed] [Google Scholar]
- 31.Feldman BN, Pai M, Rivard GE, et al. Tailored prophylaxis in severe hemophilia A: interim results from the first 5 years of the Canadian Hemophilia Primary Prophylaxis Study. J Thromb Haemost. 2006;4:1228–36. doi: 10.1111/j.1538-7836.2006.01953.x. [DOI] [PubMed] [Google Scholar]
- 32.Spira J, Plyushch OP, Andreeva TA, Andreev Y. Prolonged bleeding-free period following prophylactic infusion of recombinant F VIII reconstituted with pegylated liposomes. Blood. 2006;108:3668–73. doi: 10.1182/blood-2006-03-008276. [DOI] [PubMed] [Google Scholar]
- 33.Fischer K, Astermark J, van der Bom JG, et al. Prophylactic treatment for severe haemophilia: comparison of an intermediate-dose to a high-dose regimen. Haemophilia. 2002;8:753–60. doi: 10.1046/j.1365-2516.2002.00694.x. [DOI] [PubMed] [Google Scholar]
- 34.van den Berg HM, Fischer K, van der Bom JG. Comparing outcomes of different treatment regimens for severe haemophilia. Haemophilia. 2003;9 (Suppl 1):27–31. doi: 10.1046/j.1365-2516.9.s1.10.x. [DOI] [PubMed] [Google Scholar]
- 35.Bohn RL, Schramm W, Bullinger M, et al. Outcome measures in haemophilia: more than just factor levels. Haemophilia. 2004;10 (Suppl 1):2–8. doi: 10.1111/j.1355-0691.2004.00873.x. [DOI] [PubMed] [Google Scholar]
- 36.Remor E, Young NL, von Mackensen S, Lopatina EG. Disease-specific quality of life measurements tools for haemophilia patients. Haemophilia. 2004;10:34–41. doi: 10.1111/j.1365-2516.2004.01004.x. [DOI] [PubMed] [Google Scholar]
- 37.Santagostino E, Mancuso ME, Rocino A, et al. Environmental risk factors for inhibitor development in children with haemophilia A: a case-control study. Br J Haematol. 2005;130:422–7. doi: 10.1111/j.1365-2141.2005.05605.x. [DOI] [PubMed] [Google Scholar]
- 38.Gouw SC, van der Bom JG, van den Berg HM. Treatment-related risk factors of inhibitor development in previously untreated patients with hemophilia A: the CANAL cohort study. Blood. 2007;109:4648–54. doi: 10.1182/blood-2006-11-056291. [DOI] [PubMed] [Google Scholar]
- 39.Santagostino E, Mannucci PM for the Italian Association of Hemophilia Centers (AICE) Guidelines for replacement treatment of haemophilia and inherited coagulation disorders in Italy. Haemophilia. 2000;6:1–10. doi: 10.1046/j.1365-2516.2000.00361.x. [DOI] [PubMed] [Google Scholar]