Abstract
Patients with severe forms of von Willebrand’s disease (VWD) may have frequent haemarthroses, especially when factor VIII (FVIII) levels are below 10 U/dL, so that some of them develop target joints like patients with severe haemophilia A. Some patients have recurrent gastrointestinal bleeding, often without lesions in the gastrointestinal tract, and need treatment every day or every other day. Finally, there are children who have epistaxis frequently and severely enough to cause anaemia. In these frequent and severe bleeders, the optimal therapy may be secondary long-term prophylaxis with von Willebrand factor (VWF)/FVIII concentrates rather than on-demand treatment on the occasion of bleeding episodes. The largest experience on such prophylaxis in VWD has been in Sweden in 35 patients with severe forms of VWD. Long-term prophylaxis was also implemented in a cohort of Italian patients with VWD: prophylaxis was used in seven patients with types 3 (n=1), 2A (n=4), 2M (n=1) and type 1 (n=1) VWD because of recurrent gastrointestinal bleeds and in four patients with type 3 VWD because of joint bleeds. Prophylaxis prevented bleeding completely in eight patients and largely reduced hospitalisation for blood transfusions in the remaining three. The cost-effectiveness of these prophylaxis regimens versus on-demand therapy will now be investigated in one large international study.
Keywords: von Willebrand’s disease, von Willebrand factor/factor VIII concentrates, bleeds, secondary long-term prophylaxis, retrospective and prospective clinical trials
Introduction
Von Willebrand’s disease (VWD) is the most common inherited bleeding disorder, with a prevalence of 66 to 100 cases per million in the general population, taking patients referred for clinical manifestations of bleeding as a basis of the estimate1. A much higher prevalence (1 per 100) is reported in population-based studies, but the clinical relevance of many of these cases is uncertain1. The year 2006 marked the 80th anniversary of the first description of the disease by the Finnish paediatrician Erik von Willebrand, who had the opportunity to examine a 5-year old girl from Föglo on the island of Åland in the Gulf of Bothnia. The patient was admitted to hospital in Helsinki for investigation of severe bleeding from the nose and gums. Her parents were cousins and there was a bleeding history within the family: 11 siblings had a history of bleeding and three had died from massive menorrhagia and gastrointestinal bleeds. In contrast to haemophilia, the epitome of inherited bleeding disorders, both sexes were affected, and mucosal bleeding was the predominant symptom. A prolonged bleeding time with a normal platelet count was the most important laboratory abnormality and a functional disorder of platelets associated with a systemic lesion of the vessel wall was suggested as a possible cause of the disease. Erik von Willebrand called this novel clinical disorder “hereditary pseudo-haemophilia” and this disease was named after him since then2. Throughout nearly a century, much progress has been made in the understanding of von Willebrand factor (VWF), the protein deficient or defective in VWD, and the molecular basis, natural history and treatment of the disease3.
Definition and classification of VWD
The current classification of VWD was recently updated4. Six different types of VWD have been proposed: VWD 1, 3, 2A, 2B, 2M, and 2N. Type 1 is caused by a partial quantitative defect in VWF, whereas type 3 is characterised by the almost complete absence of VWF in plasma and platelets. Type 1 is easily distinguished from type 3 because the former is associated with a milder deficiency of VWF (usually in the range of 10–30 U/dL), an autosomal dominant pattern of inheritance and a milder bleeding tendency1. In the past type 1 was reported to be the most frequent form of VWD, accounting for approximately 70% of cases. However, a study in which diagnoses of type 1 VWD were reappraised after 10 years (1994–2004) in 1,234 patients followed by 16 Italian Centres established that only 671/1234 (54%) of the cases were truly type 1 VWD, because many cases previously diagnosed as type 1 were re-diagnosed as being type 2 due to discrepant VWF measurements (ratio of ristocetin cofactor activity (VWF:RCo) to VWF: Ag <0.7)5,6. VWF defects were recently found in 154 families with previously diagnosed VWD type 1 evaluated prospectively in a European study7.
Four forms of type 2 VWD have been identified, reflecting different pathophysiological mechanisms4. Types 2A and 2B are marked by the absence of high molecular weight VWF multimers in plasma but in type 2B there is also an increased affinity of VWF for GpIbα. Type 2M is characterised by qualitatively abnormal variants with decreased platelet-dependent function and a normal multimeric structure. In type 2N there is a full array of multimers, with the defect being in the N-terminal region of the VWF where the binding domain for FVIII is located4. This type is phenotypically distinguishable from mild haemophilia A only by the abnormal binding of FVIII to VWF(VWF:FVIIIB).
Clinical manifestations
The clinical manifestations of VWD are excessive mucocutaneous bleeding and prolonged oozing after surgical procedures. In women menorrhagia may be the only clinical manifestation. Soft tissue and joint bleeds are rare, except in patients with type 3 VWD and severe deficiencies of VWF and FVIII (prevalence approximately 1 per million in the general population). The clinical expression of the disease is usually mild in most patients with type 1, whereas severity increases in type 2 and particularly in type 3. Generally, the severity of bleeding correlates with the degree of reduction of VWF:RCo and FVIII. Todate, only a few detailed descriptions of symptoms are available5,6,8,9. Several attempts have recently been made to evaluate sensitivity and specificity of bleeding symptoms, especially in the mild cases with type 1 VWD and VWF:RCo levels >20 U/dL. In a multicentre study carried out in obligatory carriers of type 1 VWD, menorrhagia and epistaxis were poor predictors of the disease while cutaneous bleeding and bleeding after dental extractions were more sensitive symptoms for the diagnosis10. A bleeding severity score was developed and validated in affected and non-affected members of 154 families enrolled prospectively in a large European study, as well as in 200 normal individuals11. This bleeding severity score is derived from the answers in a questionnaire collecting detailed information about 12 different types of bleeds, which should be administered at the time of diagnosis in every new patient. Despite the fact that this bleeding severity score was investigated prospectively in patients with type 1 VWD, it can be applied in all VWD patients. Preliminary data collected in a prospective study among Italian Haemophilia Centres suggest that the bleeding severity score can be a useful parameter for predicting bleeding in all VWD types12.
General principle for the treatment of VWD
The goal of treatment is to correct the dual defects of haemostasis, i.e., abnormal platelet adhesion due to low or defective VWF and abnormal intrinsic coagulation due to lowFVIII13. Two main therapeutic approaches are available: desmopressin, which releases endogenous VWF from endothelial cells, and exogenous VWF contained in VWF/FVIII plasma-derived concentrates.
Desmopressin (l-deamino-8-D-arginine vasopressin, DDAVP) is a synthetic analogue of vasopressin originally designed for the treatment of diabetes insipidus. DDAVP raises FVIII and VWF plasma concentrations with no major side effects in healthy volunteers or patients with mild haemophilia and VWD14. Its mechanism of action has been investigated15. The first successful clinical trial with DDAVP was in 1977, when it was used with the aim of avoiding administration of blood products to patients with mild haemophilia or VWD who needed dental extractions or other surgical procedures16. Following this early experience, DDAVP has been widely used for the treatment of these diseases17. The obvious advantages are that DDAVP is relatively inexpensive and carries no risk of transmitting blood-borne viruses. DDAVP is usually injected intravenously at a dose of 0.3 μg/Kg diluted in 50 mL saline, infused over 30 minutes. This increases plasma FVIII/VWF three to five times above the basal levels within 30–60 minutes and, in general, the FVIII/VWF concentrations remain high for 6 to 8 hours. Since the responses in a given patient are consistent on different occasions17, a test dose of DDAVP at the time of diagnosis helps to establish the individual response patterns. Infusions can be repeated every 12 to 24 hours depending on the type and severity of the bleeding episode. However, most patients treated repeatedly with DDAVP become less responsive to therapy17. The drug is also available in concentrated forms for subcutaneous and intranasal administration, which can be convenient for home treatment17. The protocol of the infusion test, with the clinical and laboratory parameters to be measured, was reported in detail in the European Study onDDAVP18.
Transfusion therapies
Until the mid 1980s cryoprecipitate was the mainstay of treatment of patients with VWD who were unresponsive to DDAVP. The advent of virally-inactivated VWF/FVIII concentrates, originally devoted to haemophiliacs, provided a better therapeutic approach to VWD and these concentrates were, therefore, introduced into the clinical management of this disease in most Centres in Europe. VWF/FVIII concentrates are the first choice for the treatment of patients with type 3 VWD, for patients with type 2B (because DDAVP can induce transient thrombocytopenia), and for those patients with types 1 and 2 who are unresponsive to DDAVP or have contraindications to its use17. The minimal requirements for plasma-derived VWF/FVIII concentrates for use in VWD are the following: (i) they must contain active VWF and FVIII; (ii) they should be treated by virucidal methods; and (iii) before clinical use, their pharmacokinetics and efficacy should have been tested in retrospective and prospective clinical trials in relatively large numbers of VWD patients5. Among several concentrates containing VWF, few have been extensively evaluated in pharmacokinetic trials as well as in retrospective or prospective studies19.
HaemateP®/Humate-P®, a pasteurised plasma-derived VWF/FVIII concentrate widely used in VWD, is characterised by a very high content of VWF (VWF:RCo IU, 2.4 for each IU of FVIII:C) with a relatively high percentage of large molecular weight VWF muMmers20,21. Haemate® P has also demonstrated an excellent safely record with regards to blood-borne infections over the past 25 years of clinical use22–25. Clinical efficacy data were collected for Haemate-P® through a large retrospective study organised by the Canadian Haemophilia Centres26. Other published studies include a retrospective analysis of the efficacy and safely of Haemate® P used to prevent bleeding during surgery or invasive procedures in 26 Italian VWD patients27, as well as two prospective, multicentre, open-label, non-randomised studies conducted in the USA on Humate-P® used in urgent bleeding and urgent surgical events28,29. Another large retrospective study on the clinical use of Haemate-P in 100 Italian patients was published recently30: the distribution and severity of the disease in the patients enrolled is shown in Figure 1.
Figure 1.
Summary of the retrospective study on 100 Italian patients with VWD treated with Haemate-P. Note the distribution on VWD types and the relatively high percentage (71%) of cases with a severe phenotype.
The data derived from pharmacokinetic and clinical studies have contributed to help in the clinical choice of VWF/FVIII concentrates. The accumulation of FVIII, between that which is exogenously infused together with that endogenously synthesised and stabilised by infused VWF, may cause very high FVIII levels when multiple infusions are given to cover major surgery13,19. Sustained high levels of VWF/FVIII may increase the risk of deep vein thrombosis: however, this is a rare event reported only in patients with concomitant riskfactors13,19,31. When repeated injections of VWF/FVIII concentrates are used to control recurrent bleeding episodes and to prevent excessive bleeding after major surgery, daily monitoring of FVIII levels and adjustment of the dosage of the concentrate are necessary to keep FVHI levels between 50 and 150 U/dL. The minimal VWF:RCo levels needed to ensure haemostasis are not well established. Prospective data from a large cohort of well-characterised Italian patients suggest that levels > 30 U/dL are associated with a low rate of spontaneous mucosal bleeding12.
Retrospective studies on secondary long-term prophylaxis
Patients with severe forms of VWD may have frequent haemarthroses, especially when FVIII levels are below 10 U/dL, so that some of them develop target joints like patients with severe haemophilia A. Some patients have recurrent gastrointestinal bleeding, often without lesions in the gastrointestinal tract, and need treatment every day or every other day. Finally, there are children who have epistaxis frequently and severely enough to cause anaemia. In these frequent and severe bleeders, the optimal therapy may be regular prophylaxis with VWF/FVIII concentrates rather than on-demand treatment on the occasion of bleeding episodes. The largest experience on secondary long-term prophylaxis in VWF was gained in Sweden in 35 patients with severe forms of VWD32. Secondary prophylaxis was also implemented in a cohort of Italian patients with VWD33. Among 89 patients who needed treatment with VWF/FVIII concentrates in the preceding 2 years because of one or more bleeding episodes, 11 (12%) were included in a programme of prophylaxis because of frequent recurrence of bleeding at the same sites33.
Prophylaxis was started because of gastrointestinal bleeds in seven patients with types 3 (n= 1), 2A (n=4), 2M (n= 1) and type 1 (n= 1) VWD and for joint bleeds in four patients with type 3 VWD (n= 4). Prophylaxis prevented bleeding completely in eight patients and largely reduced hospitalisation for blood transfusions in the remaining three.
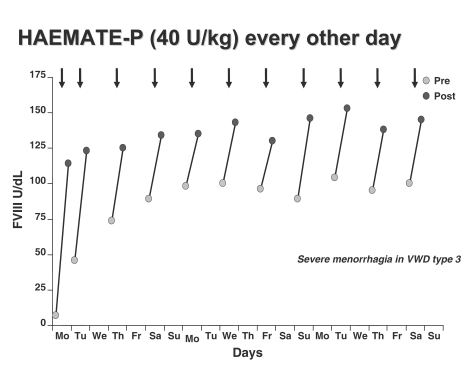
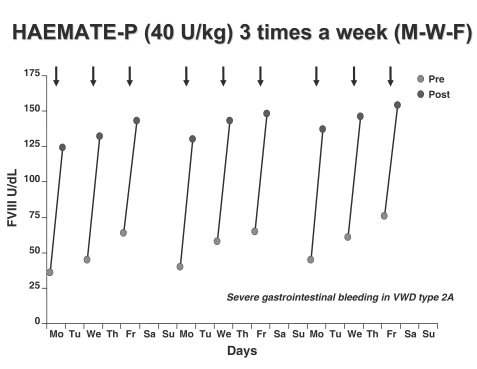
When prophylaxis was compared with previous on-demand regimens in all the 11 cases the annual total consumption of concentrate, the number of transfused blood units and days spent in hospital were significantly reduced33. FVIII levels were always higher than 180 U/dL but no side effects, including thrombosis, were observed (Figures 2 and 3).
Figure 2.
Changes of FVIII:C levels before and after repeated injections of the VWF/FVIII concentrate. A dose of 40 FVIII U/Kg of Haemate-P® was given every other day to prevent menorrhagia in a patient with type 3 VWD.
Figure 3.
Changes of FVIII:C levels before and after repeated injections of the VWF/FVIII concentrate. A dose of 40 FVIII U/Kg of Haemate-P® was given three times a week to prevent gastrointestinal bleeding in patient with type 2A VWD.
In the retrospective study on 100 Italian VWD patients33, 12 (types 1=1, 2B=1, 2M VIC1, 3=9), with a median age of 34.5 years, range 11–71 years) also underwent 17 long-term secondary prophylaxis regimens in order to prevent recurrent bleedings at the same site (47% in the gastrointestinal tract, 35% in joints). Patients received 5.60 × 106 IUVWF:RCo in 1,424 infusions of Haemate® P, given three times (53.0%) or twice (47.0%) a week, with clinical response rated as excellent/good response in 100%. Among cases on prophylaxis, 70% had VWD type 3 and received 54% of the overall product infused. During the 4,358 days of prophylaxis (median, 201 days; range, 30–730 days) only four bleeding episodes were observed. These three retrospective studies suggested that the cost-effectiveness of prophylactic regimens should be further evaluated in larger prospective studies and compared with that of on-demand treatment.
A prospective study on prophylaxis: the VIP study
The von Willebrand Disease Prophylaxis Network is an international study group made up of leading bleeding disorder experts from more than 70 centres around the world. In research conducted previously, the VWD Prophylaxis Network found that, over the course of 1 year, 77% of patients with type 3 VWD had bleeding events that required treatment with plasma-derived products. The Network also found that 22% of patients with type 3 VWD had bleeding patterns severe enough to warrant preventive therapy. These findings were derived from the first international census to examine the use of prophylaxis in treating VWD. The VIP Study will enrol up to 200 people in this multicentre, prospective study. Each of four study arms will include up to 50 participants. Each of the arms focuses on an important bleeding indication: joint bleeds, gastrointestinal bleeds, menorrhagia, and epistaxis. Participants will undergo an escalation of treatment as needed, from receiving replacement factor treatment once a week to three times a week. All subjects will begin at the level 1 dosing frequency and remain on this dose for 1 year of follow-up, or until they meet the criteria for escalation to level 2 or 3. In the case of joint bleeding, gastrointestinal bleeding and epistaxis, patients will begin with 50 U VWF:RCo/kg of a VWF/FVIII product once a week, escalating to twice a week at level 2, and three times a week at level 3. Females with menorrhagia will commence with 50 U VWF:RCo/kg on day one of menses for two cycles, 50 U VWF:RCo/kg on days one and two of menses for two cycles in level 2, and 50 U VWF:RCo/kg on days one, two and three of menses in level 3. The choice of which VWF/FVIII product will be used is at the discretion of the investigator. Four of the 14 centres have completed all necessary ethical committee, administrative, and contractual reviews and have begun enrolment. Eight participants have been recruited: four in the prospective study and four in the retrospective study. Enrolment logs from ten of the 14 centres show 45 potentially available subjects for the retrospective study of the effect of prophylaxis. We will be contacting individual centres in the coming weeks to identify a timeline for completion of these enrolments. An additional ten centres have confirmed interest in joining the network (Table 2). As indicated, several of these have begun ethical committee preparation, as well as the contractual process.
Acknowledgements
Some data on the diagnosis and management of VWD are from the Italian Registry of VWD sponsored by a grant from the Italian Ministry of Health. We wish to thank all the Members of the Italian Association of Haemophilia Centres who participated in this Registry and contributed to the preparation of the Guidelines for the diagnosis and therapy of VWD in Italy. We also wish to thank all the Members who participated in the retrospective clinical study. The VIP study is posted on the Haemophilia and Thrombosis Research Society (HTRS) website. Finally, we thank Dr. Erik Berntorp for providing data on the VIP study.
Footnotes
Conflicts of interest disclosure
The Author declares that he receives fees as speaker in educational activities and for occasional expert opinion by Baxter, Bayer, CSLBehring, Grifols and Kedrion.
References
- 1.Federici AB, Mannucci PM. Management of Inherited von Willebrand disease in 2007. Ann Med. 2007;39:133–9. doi: 10.1080/07853890701513738. [DOI] [PubMed] [Google Scholar]
- 2.Lee CA, Kessler CM. Proceedings of a Nordic von Willebrand symposium. Haemophilia. 1999;5(suppl 2):1–12. [Google Scholar]
- 3.Federici AB, Berntorp E, Lee CA. The 80th anniversary of von Willebrand disease: history, management, research. Haemophilia. 2006;12:563–72. doi: 10.1111/j.1365-2516.2006.01393.x. [DOI] [PubMed] [Google Scholar]
- 4.Sadler JE, Budde U, Eikenboom JCJ, et al. Update on the pathophysiology and classification of von Willebrand disease. A report of the Subcommittee on von Willebrand factor. J Thromb Haemost. 2006;4:2103–14. doi: 10.1111/j.1538-7836.2006.02146.x. [DOI] [PubMed] [Google Scholar]
- 5.Federici AB, Castaman G, Mannucci PM. Guidelines for the diagnosis and management of VWD in Italy. Haemophilia. 2002;8:607–21. doi: 10.1046/j.1365-2516.2002.00672.x. [DOI] [PubMed] [Google Scholar]
- 6.Federici AB. Clinical diagnosis of von Willebrand disease. Haemophilia. 2004;10:169–76. doi: 10.1111/j.1365-2516.2004.00991.x. [DOI] [PubMed] [Google Scholar]
- 7.Goodeve A, Eikenboom J, Castaman G, et al. Phenotype and genotype of a cohort of families historically diagnosed with type 1 von Willebrand disease in the European study, Molecular and Clinical Markers for the Diagnosis and Management of Type 1 von Willebrand Disease (MCMDM-1VWD) Blood. 2007;109:112–21. doi: 10.1182/blood-2006-05-020784. [DOI] [PubMed] [Google Scholar]
- 8.Silwer J. von Willebrand’s disease in Sweden. Acta Paediat Scand. 1973;238:1–159. [PubMed] [Google Scholar]
- 9.Lak M, Peyvandi F, Mannucci PM. Clinical manifestations and complications of childbirth and replacement therapy in 348 Iranian patients with type 3 von Willebrand disease. Br J Haematol. 2000;111:1223–9. doi: 10.1046/j.1365-2141.2000.02507.x. [DOI] [PubMed] [Google Scholar]
- 10.Rodeghiero F, Castaman G, Tosetto A, et al. The discriminant power of bleeding history for the diagnosis of von Willebrand disease type 1: an international multicenter study. J Thromb Haemost. 2005;3:2619–26. doi: 10.1111/j.1538-7836.2005.01663.x. [DOI] [PubMed] [Google Scholar]
- 11.Tosetto A, Rodeghiero F, Castaman G, et al. A quantitative analysis of bleeding symptoms in type 1 of von Willebrand disease: results from a multicenter European Study (MCMDM-1 VWD) J Thromb Haemost. 2006;4:766–73. doi: 10.1111/j.1538-7836.2006.01847.x. [DOI] [PubMed] [Google Scholar]
- 12.Federici AB, Bucciarelli P, Castaman G, et al. Prevalence and determinants of bleeding in different types of von Willebrand disease: results of the first prospective multicenter study on 814 Italian patients. Blood. 2007;111:219a. Abstract 714. [Google Scholar]
- 13.Mannucci PM. Treatment of von Willebrand disease. N Eng J Med. 2004;351:683–94. doi: 10.1056/NEJMra040403. [DOI] [PubMed] [Google Scholar]
- 14.Cash JD, Garder AMA, Da Costa J. The release of plasminogen activator and fector VIII by LVP, AVP, DDAVP, ATIII and OT in man. Br J Haematol. 1974;27:363–4. [PubMed] [Google Scholar]
- 15.Hashemi S, Palmer DS, Aye MT, Ganz PR. Platelet-activating factor secreted by DDAVP-treated monocytes mediates von Willebrand factor releases from endothelial cells. J Cell Physiol. 1993;154:496–505. doi: 10.1002/jcp.1041540307. [DOI] [PubMed] [Google Scholar]
- 16.Mannucci PM, Ruggeri ZM, Pareti FI, Capitanio A. Anew pharmacological approach to the management of hemophilia and von Willebrand disease. Lancet. 1977;1:869–72. doi: 10.1016/s0140-6736(77)91197-7. [DOI] [PubMed] [Google Scholar]
- 17.Federici AB. The use of desmopressin in von Willebrand disease: the first 30 years (1977–2007) Haemophilia. 2008;14 (Suppl 1):5–14. doi: 10.1111/j.1365-2516.2007.01610.x. [DOI] [PubMed] [Google Scholar]
- 18.Federici AB, Mazurier C, Berntorp E, et al. Biological response to desmopressin in patients with severe type 1 and type 2 von Willebrand disease: results of a multicenter European study. Blood. 2004;103:2032–8. doi: 10.1182/blood-2003-06-2072. [DOI] [PubMed] [Google Scholar]
- 19.Federici AB. Management of von Willebrand disease with factor VIII/von Willebrand factor concentrates: results from current studies and surveys. Blood Coagulation and Fibrinolysis. 2005;16:S17–S21. doi: 10.1097/01.mbc.0000167658.85143.49. [DOI] [PubMed] [Google Scholar]
- 20.Dobrovska A, Krzensk U, Chediak JR. Pharmacokinetics, efficacy and safety of Humate-P in von Willebrand disease. Haemophilia. 1998;4:33–9. doi: 10.1046/j.1365-2516.1998.0040s3033.x. [DOI] [PubMed] [Google Scholar]
- 21.Lethagen S, Carlson M, Hillarp A. A comparative in vitro evaluation of six von Willebrand factor concentrates. Haemophilia. 2004;10:243–9. doi: 10.1111/j.1365-2516.2004.00893.x. [DOI] [PubMed] [Google Scholar]
- 22.Schimpf K, Mannucci PM, Kreutz W. Absence of hepatitis after treatment with a pasteurised factor VIII concentrate and no previous transfusions. N Engl J Med. 1987;316:918–22. doi: 10.1056/NEJM198704093161505. [DOI] [PubMed] [Google Scholar]
- 23.Schimpf K, Brackmann H H, Kreutz W. Absence of anti human immunodeficiency virus types 1 and 2 seroconversion after the treatment of haemophilia A or VWD with pasteurized factor VIII concentrate. N Engl J Med. 1989;321:1148–52. doi: 10.1056/NEJM198910263211702. [DOI] [PubMed] [Google Scholar]
- 24.Kreuz W, Auerswald G, Bruckmann C, et al. Prevention of hepatitis C virus infection in children with haemophilia A and B and von Willebrand’s disease. Thromb Haemost. 1992;67:184. [PubMed] [Google Scholar]
- 25.Federici AB, Santagostino E, Rumi MG, et al. The natural history of hepatitis C virus infection in Italian patients with von Willebrand’s disease: a cohort study. Haematologica. 2006;91:503–8. [PubMed] [Google Scholar]
- 26.Lillicrap D, Poon MC, Walker I, et al. Efficacy and safety of the factor VIII/von Willebrand factor concentrate, Haemate-P/Humate P: ristocetin cofactor unit dosing in patients with von Willebrand disease. Thromb Haemost. 2002;87:224–30. [PubMed] [Google Scholar]
- 27.Franchini M, Rossetti G, Tagliaferri A, et al. Efficacy and safety of factor VIII/von Willebrand factor concentrate, Haemate-P, in preventing bleeding during surgery or invasive procedures in patients with von Willebrand’s disease. Haematologica. 2003;88:1279–83. [PubMed] [Google Scholar]
- 28.Cox Gill J, Ewenstein BM, Thompson AR, et al. Successful treatment of urgent bleeding in von Willebrand disease with factor VIII/von Willebrand factor concentrate (Humate-P): use of the ristocetin cofactor assay (VWF:RCo) to measure potency and to guide therapy. Haemophilia. 2003;9:688–95. doi: 10.1046/j.1351-8216.2003.00816.x. [DOI] [PubMed] [Google Scholar]
- 29.Thompson AR, Gill JC, Ewenstein BM, et al. Successful treatment for patients with von Willebrand disease undergoing urgent surgery using factor VIII/von Willebrand factor concentrate (Humate-P) Haemophilia. 2004;10:42–51. doi: 10.1046/j.1351-8216.2003.00809.x. [DOI] [PubMed] [Google Scholar]
- 30.Federici AB, Castaman G, Franchini M, et al. Clinical use of Haemate-P in inherited von Willebrand’s disease: a cohort study on 100 Italian patients. Haematologica. 2007;92:944–51. doi: 10.3324/haematol.11124. [DOI] [PubMed] [Google Scholar]
- 31.Mannucci PM. Venous thromboembolism in von Willebrand disease. Thromb Haemost. 2002;88:378–9. [PubMed] [Google Scholar]
- 32.Berntorp E, Petrini P. Long-term prophylaxis in von Willebrand disease. Blood Coag Fibrin. 2005;16:S23–S26. doi: 10.1097/01.mbc.0000167659.23262.18. [DOI] [PubMed] [Google Scholar]
- 33.Federici AB, Gianniello F, Canciani MT, Mannucci PM. Secondary long-term prophylaxis in severe patients with von Willebrand disease: an Italian cohort study. Blood. 2005;106:507a. abstract 1782. [Google Scholar]