Abstract
Exposing rats to repeated restraint stress induces well-characterized adaptations in the expression of either corticotropin-releasing factor (CRF) or arginine-vasopressin (AVP) mRNA in the parvocellular neurons of the hypothalamic paraventricular nucleus (PVN). The effects of regulating corticosterone levels on this adaptation was studied in male rats. In intact rats, acute restraint stress increased the expression of CRF mRNA whilst AVP mRNA expression was no different to control. Repeated exposure resulted in habituation of CRF expression, whereas AVP mRNA increased above that seen in either non stressed or acutely stressed animals. In adrenalectomised rats with replacement pellets of corticosterone that replicated blood levels approximating to the daily trough (mean levels 37-65 ng/mL), basal CRF expression levels were raised, but the response to acute stress was still observed. However, the habituation seen in normal animals that had been repeatedly stressed was prevented, so that CRF mRNA levels continued to be raised after repeated stress. By contrast, the AVP response to both acute and repeated stress was unaltered in these low-dose corticosterone-treated rats compared with controls. Higher dose pellets, which resulted in blood levels around those of the daily maximum (mean 118-141 ng/mL) had the opposite effects. There was no change compared to intact rats in the expression of CRF mRNA following either acute or repeated stress, but the expected increase in AVP following repeated restraint was prevented. These experiments show that corticosterone has important modulating effects on the adaptive pattern of both CRF and AVP mRNA expression in the parvocellular PVN. The ‘set-point’ of corticosterone differs; for CRF, experiencing higher levels is necessary for subsequent adaptation to repeated restraint to occur, whereas for AVP a return to lower levels is necessary to allow this peptide to respond to repeated stress.
Keywords: corticosterone, corticotropin-releasing factor, arginine-vasopressin, paraventricular nucleus, stress, adaptation
Introduction
Repeated exposure to the same stress results in a process of adaptation; that is, a change in the response to the stressor which signals that the individual is making the necessary behavioural and physiological adjustments to cope with its demands (Harbuz & Lightman, 1992; Herbert, 1993). Altered gene expression in the brain in response to stress is now recognized as one indicator of this process. Acute stress, such as restraint, initiates the expression of immediate-early genes (IEG) such as c-fos in a number of structures in the basal forebrain and brainstem; this pattern identifies the location of the neural elements responsive to particular stressors (Bullitt, 1990; Chan et al., 1993; Cullinan et al., 1996). Repeated exposure modifies this pattern of gene expression: some areas (e.g. the septum) show slowly diminishing levels of response; in others, such as the hypothalamic paraventricular nucleus (PVN), adaptation in IEG expression is more rapid and complete; (Stamp & Herbert, 1999). The PVN is known to be highly responsive to stressors, and its outputs, including those to the brainstem, median eminence and pituitary, are important avenues for the response to stress (Swanson & Kuypers, 1980; Dallman et al., 1992).
Levels of corticotropin-releasing factor (CRF) and arginine vasopressin (AVP) have both been shown to be sensitive to stressful stimuli (Swanson et al., 1986; Watts, 1992). The expression of CRF mRNA in the parvocellular neurons of the PVN increases sharply following a variety of acute stressors (Ceccatelli et al., 1989; Jacobson et al., 1990; Ma et al., 1997a,b). The distribution of AVP in the PVN is complicated by expression in two populations of neurons. Magnocellular neurons contain high concentrations of AVP, and are responsive to specific demands, such as hypovolaemia or changes in blood osmolarity (Herbert et al, 1992). Parvocellular neurons also express AVP (Grino & Burgunder, 1992) and most stressors increase AVP expression in these neurons. (Herman & Sherman, 1993; Schmidt et al., 1996; Ma & Lightman, 1998). The importance of parvocellular AVP lies in its synergistic action, with CRF, on a number of dependent functions (Gillies et al., 1982). Adaptation to repeated stress seems to alter these two genes differentially (Ma & Aguilera, 1999). Most agree that the expression of CRF decreases with repeated stress, whereas that of AVP increases (in some cases, in neurons that also express CRF) (Makino et al., 1995; Ma et al., 1997a,b; Kovacs, 1998). This ‘gene-switch’ in the PVN is an example of adaptation of peptides that have a defined functional role in the response to stressful events.
Glucocorticoids represent one mechanism responsible for this sequence of events at the neuronal level. The PVN is a known target for glucocorticoids (Plotsky, 1991). Adrenalectomy (ADX) increases expression of both peptides, and this is reversed by replacing basal levels of glucocorticoids (Sawchenko, 1987; Akana & Dallman, 1997). Glucocorticoids are themselves increased following many varieties of acute stressor, though this response also tends to wane as the stress becomes chronic (Chen & Herbert, 1995). Increased CRF expression occurs after acute, single stress despite raised levels of glucocorticoids (Imaki et al., 1991; Lightman & Harbuz, 1993). A more uncertain topic is whether levels of glucocorticoid play an important role in adaptation to repeated stress, as represented by the CRF/AVP gene-switch in the PVN. As increased glucocorticoids follow, rather than proceed, initial CRF and AVP expression, it might be expected that these steroids would play a greater part in adaptive than in acute responses. In the experiments reported here, we have investigated whether clamping corticosterone levels at either a basal, unstressed level (resembling the diurnal trough), or at higher levels (similar to those seen during the diurnal maximum) could modulate the expression of either CRF or AVP in the PVN following either an acute or repeated stress. We show that both genes are markedly regulated by these treatments, in a manner that depends both on the nature of the stress (acute vs. chronic) and the gene (CRF vs. AVP). This suggests that glucocorticoids have an important, but differentiated, role to play in the adaptation to stress of effector (‘late’) peptide genes in the PVN.
Materials and methods
All experiments were carried out in accordance with the 1986 United Kingdom Animals (Scientific Procedures) Act.
Animals
Male Lister Hooded rats (Harlan, Oxon, UK) were used, weighing between 200 and 250 g at the start of the experiment. They were housed in groups of 5 per cage in a controlled environment (temperature 21 °C and humidity 55%) with ad lib access to food and tap water. After surgery all animals were given free access to both tap water and 0.9% saline. The volume of water and saline drunk was measured daily. Animals were kept on a 12-h reversed lighting cycle with lights on at 11 pm. All animals were handled daily for at least 1 week preceding the experiment, to acclimatize them to the new environment and handling.
Experimental groups
Animals were assigned to nine experimental groups (n = 5 per group): sham adrenalectomy - unstressed, acutely stressed, or chronically stressed; adrenalectomised - low dose corticosterone replacement - unstressed, acutely stressed or chronically stressed; adrenalectomised, high dose corticosterone - unstressed, acutely stressed or chronically stressed.
Adrenalectomy and corticosterone replacement
Surgery was performed under Halothane/N2O anaesthesia. Bilateral adrenalectomy was carried out using a ventral approach. Control animals underwent sham surgery where the adrenal glands were exposed but not excised. Adrenalectomised animals were implanted subcutaneously with either one 30% corticosterone : cholesterol pellet (low dose) or two 80% (high dose) pellets. Pellets (≈ 200 mg) were made by melting weighed amounts of the steroids in silicone electron-microscope specimen molds (Sigma, Dorset, UK). Control animals received subcutaneous cholesterol pellets. Low corticosterone pellets were implanted in all adrenalectomised animals at the time of surgery. One week after recovery from surgery, animals designated to receive high-dose corticosterone had pellets replaced with two 80% corticosterone pellets, while low corticosterone and sham animals underwent sham pellet replacement. High-dose corticosterone pellets were inserted after 1 week, as implantation at time of adrenalectomy noticeably impaired wound healing. The modified procedure proved to be more successful than administering the high dose pellets at the time of surgery.
Restraint stress
One week after the second surgery, animals were subjected to daily 60-min restraint sessions (chronic experiment) every day for 10 days, whereas the single-stress animals were subjected to one period of 1-h restraint at the end of the chronically stressed groups sessions (i.e. 17 days after final surgery). All animals were placed in a Plexiglas tube (internal diameter 5.5 cm) in the supine position in the testing cage as described previously (Chen & Herbert, 1995). Control animals were handled on that day. At the end of each stress session, animals were returned to their home cages. All animals were weighed both at the start of the chronic stress sessions and again 10 days later.
Blood samples and radioimmunoassay
Animals (10-week-old) were killed with an overdose of pentobarbitone sodium (75 mg/kg body weight), 5 h into the light cycle. Blood samples were collected at the time of sacrifice by cardiac puncture to measure corticosterone levels. The samples were collected in heparinized tubes and were centrifuged at 2500 r.p.m. for 10 min. Plasma was stored at -20 °C until assayed. Corticosterone was measured by radioimmunoassay using a validated procedure. Plasma was thawed and extracted with 75 volumes of ethanol. The supernatant was dried and re-suspended in 500 μL of 0.025 m phosphate-buffered saline (Sigma, Dorset, UK) at pH 7.4 with 0.2% gelatin (Sigma, Dorset, UK), and 100 μL of the re-suspended solution was used in the subsequent radioimmunoassay. Standards of corticosterone at 4.000-15.625 pg per 100 μL were set up by double dilution. (intra-assay variation 4.5%). (Chen & Herbert, 1995)
In situ hybridization
Brains were sectioned at 10 μm at -20 °C on to poly lysine coated microscope slides (Sigma, Dorset, UK). Sections were allowed to air dry at room temperature and were then fixed with 4% paraformaldehyde (Sigma, Dorset, UK) for 5 min, washed in PBS and then dehydrated in 70% ethanol for 5 min, 95% ethanol for 5 min, before finally storing in fresh 95% ethanol at 4 °C. Forty eight and thirty base oligonucleotides complimentary to exonic mRNA coding for CRF and AVP were designed using a special suite of programmes. (Oligo-NBI, Plymouth, USA) and the sequences checked for specificity by basic BLAST search on NCBI. Both probes were labelled with 35S as follows: 2 μL of purified oligonucleotide (5 ng/μL) was added to 1.25 μL of 10 × One for All Buffer (Pharmacia, Buckinghamshire, UK). DEPC-treated water (7.25 μL) was added, followed by 1 μL of terminal 35S deoxyadenosine 5′ (α-thio) triphosphate (10 mCi/mL) (Amersham, Buckinghamshire, UK) and 1 μL (15-20 U) of terminal deoxynucleotide transferase enzyme (Pharmacia, Buckinghamshire, UK). The reaction was incubated at 32 °C in a water bath for 1 h: 40 μL of DEPC water was added to terminate the reaction. Purification of the labelled probe from unincorporated nucleotides was accomplished by centrifugation through a G-50 sephadex spin column (3000 r.p.m., 3 min, 4 °C). G-50 sephadex (Sigma, Dorset, UK) was preswollen and autoclaved in 0.2 μm filtered TENS buffer (140 mm NaCl, 20 mm Tris-HCl at pH 7.5) 5 mm ethylene diamine tetraacetic acid disodium salt 0.1% sodium dodecylsulphate; all Sigma, Dorset (UK). Probes were then evaluated for incorporation of radio-label by scintillation counting. All hybridizations were carried out at 2500-5000 c.p.m./μL in hybridization buffer (50% deionized formamide 4 × SSC, 5 × Denhardt’s, 100 μg/mL polyadenylic (potassium salt) acid, 200 μg/mL salmon sperm DNA, 120 μg/mL heparin (BDH, Leicestershire. UK), 25 mm sodium phosphate pH 7.0, 1 mm sodium pyrophosphate, 10% (w/v) dextran sulphate in DEPC-treated water (all Sigma, Dorset, UK). After incubation at 42 °C overnight with probe, slides were washed as follows. Slides were rinsed with 1 × SSC at room temperature, washed twice for 30 min at 55 °C with 1 × SSC, and then rinsed at room temperature for 5 s each in 1 × SSC, 0.1 × SSC, 18 Ω water, 50% ethanol, 70% ethanol, and 95% ethanol (BDH, Leicestershire, UK). The sections are then thoroughly air-dried at room temperature before exposure to dry X-ray film (Amersham, Buckinghamshire UK). Some slides were designated for emulsion autoradiography and were treated as follows. Slides were twice dipped into LM-1 emulsion (Amersham, Buckinghamshire, UK) heated to 43 °C in complete darkness, stored at 4 °C in a light proof box for a pre designated length of time and were developed in 50% D19 Kodak developer for 5 min, stopped in 1% acetic acid for 2 min, fixed in 25% AL4 Kodak fixer for 5 min, and finally rinsed in running water for 15 min. Developed slides were then counter stained using a standard cresyl violet Nissl stain (Sigma, Dorset, UK), dehydrated and coverslipped with DPX mountant (BDH, Leicestershire, UK).
Analysis and quantification
For quantification of CRF mRNA in the PVN, the section and C14-labelled standards of known radioactivity (Amersham, Buckinghamshire, UK) were placed in X-ray cassettes and then exposed to autoradiographic film for 7 days. For cellular localization, of AVP mRNA the slides were subsequently dipped in nuclear emulsion (Ilford LM1) (Amersham, Buckinghamshire, UK) and exposed for an appropriate time (5 days). The slides were counter-stained with cresyl violet acetate (Sigma, Dorset, UK). The optical density of the autoradiographic images was measured by using a computerized Macintosh-based image analysis system (NIH Image). The optical densities were obtained in three/four consecutive sections per rat, the average value for each rat was used to calculate group means. CRF mRNA, as expected, was found principally in the dorsomedial part of the PVN and all signal was quantified. Analysis of the AVP mRNA in the parvocellular subdivision of the PVN was carried out in the cresyl violet counter-stained sections using a ×40 objective with a bright-field condenser. The parvocellular cells were differentiated histologically from the magnocellular neurons on the basis of their overall size and their small dense staining nuclei.
Statistical analysis
Data were analysed by one- or two-way anovas, log-transformed if the variances were not homogeneous (Levene test). Post hoc comparisons (following a significant one-way anova) were based on the Bonferroni test.
Results
Corticosterone
Blood samples were taken 6 h after the end of the stress (in restrained animals) so plasma corticosterone levels do not represent responses to restraint (Table 1). A two-way anova (log-transformed data) showed that both stress (F2,36 = 3.7, P < 0.03) and corticosterone treatment (F2,36 = 66.7, P < 0.001) had significant main effects, but there was no interaction between these two factors (F = 2.3, not significant). As expected, there were no differences between stressed and unstressed intact rats (Bonferroni test, P > 0.05). There were also no differences between stressed and unstressed low-dose corticosterone-treated rats, or between those receiving the higher dose. The values in intact control (unstressed) rats were greater than those in the low-does (unstressed) group (P < 0.05), but not the higher-dose group. The two adrenalectomised (unstressed: low-dose vs. high-dose) groups were significantly different from each other (P < 0.05).
Table 1.
Corticosterone levels in the nine groups of rats
| Corticosterone levels (ng/mL) |
|||
|---|---|---|---|
| Intact controls | ADX, low dose corticosterone | ADX, high dose corticosterone | |
| Unstressed | 84.4 ± 7.95 | 37.2 ± 3.40 | 117.6 ± 4.18 |
| Acute stress (1 day) | 78.0 ± 10.27 | 50.8 ± 4.72 | 140.7 ± 11.42 |
| Chronic stress (10 days) | 86.2 ± 9.61 | 65.4 ± 7.59 | 132.4 ± 3.87 |
Data are means ± SEM. Samples taken 6 h poststress in the ‘stressed’ groups.
Saline intake
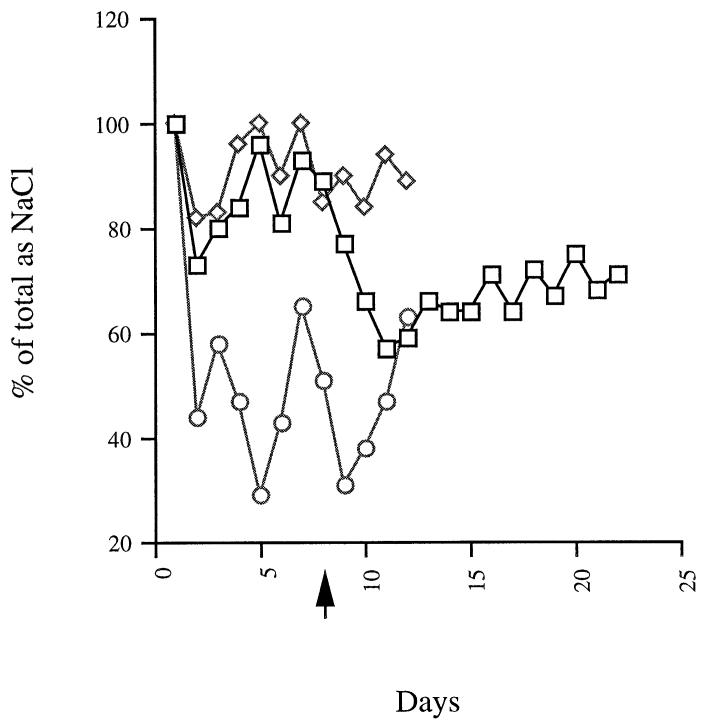
Figure 1 shows data from unstressed rats. Low-dose adrenalectomised (unstressed) rats drank significantly more 0.9% saline than either intact rats, or those that received the higher dose (the latter two groups did not differ from each other). The figure also shows that adding extra corticosterone pellets to low dose adrenalectomised rats (and, hence, converting them into high-dose animals) reduced their saline intake to control levels. Data from stressed rats (not shown) was similar. Stress itself had no effect on saline intake.
Fig. 1.
Daily intake of 0.9% sodium chloride in control (intact) rats (○), in adrenalectomised rats receiving a single low-does corticosterone pellet (◇).The arrow indicates the day on which the low dose was replaced by a high dose pellet in one group. (□). n = 5 rats per group.
Body weight
A repeated-measures anova (start and end weight as repeat factors) showed that there were significant effects of both stress (F2,36 = 12.9, P < 0.001) and corticosterone treatment (F2,36 = 10.1, P < 0.001), and an interaction between them (F = 3.3, P = 0.02), on weight gain during the experiment (Table 2). Split anovas showed this to apply to body weights at both time points. By the end of the experiment, there were no differences in body weight between the unstressed groups (Bonferroni, not significant), but for both acutely and chronically stressed groups, only the high-dose animals weighed less than controls (P < 0.05).
Table 2.
Body weights of controls or ADX rats after 14 days of corticosterone treatment (start), and after acute or chronic stress (end) 10 days later
| Mean body weights (g) |
||||||
|---|---|---|---|---|---|---|
| Intact controls |
ADX, low dose corticosterone |
ADX, high dose corticosterone |
||||
| Start | End | Start | End | Start | End | |
| Unstressed | 251 ± 4.0 | 288 ± 6.04 | 235 ± 5.1 | 267 ± 5.0 | 250 ± 4.5 | 289 ± 1.9 |
| Acute stress | 241 ± 5.1 | 279 ± 5.8 | 218 ± 4.9 | 257 ± 7.5 | 236 ± 3.7 | 232 ± 3.7 |
| Chronic stress | 247 ± 6.4 | 272 ± 6.0 | 237 ± 4.6 | 267 ± 5.8 | 237 ± 4.9 | 244 ± 5.1 |
Data are means ± SEM.
Baseline (control) CRF and AVP mRNA expression in the PVN
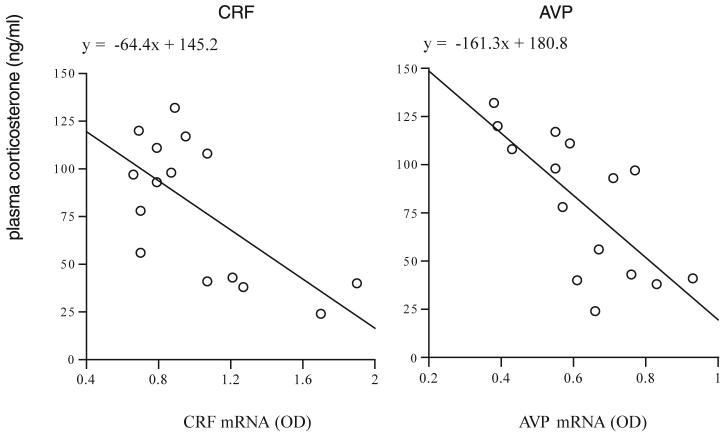
Some indication of the relative feedback effects of the implanted corticosterone pellets on basal CRF and AVP mRNA expression in the PVN was obtained by analysing separately the data from the control (unstressed) rats. This showed that, for CRF, there was a significant difference between the three groups (F2,12 = 16.3, P < 0.003), which was due to levels of CRF mRNA in the low-dose group being higher than the other two (Bonferroni, P < 0.05). The controls and the high-dose group did not differ. For AVP, the results were not the same: the difference between the groups (F2,12 = 10.4, P < 0.003) was due to levels of AVP mRNA in the high-dose group being significantly less than in the other two (P < 0.05), which did not differ from each other. Taking all the unstressed rats together (n = 15), there was a significant negative correlation (Spearman) between plasma corticosterone and CRF mRNA (-0.54, P < 0.04) but a more marked one for AVP (-0.75, P < 0.001). The scatterplots for each are shown in Fig. 2; the relation between AVP mRNA and plasma corticosterone is clearly more predictable than for CRF.
Fig. 2.
Scatter plot showing the association between integrated OD for CRF mRNA (left) and AVP (right) with plasma corticosterone in the unstressed rats from the three groups of treatments. n = 15 rats.
To check whether the treatments altered magnocellular AVP, images of the magnocellular subdivision of the PVN were analysed from each of the three groups of unstressed animals (intact, ADX + Low corticosterone, ADX + High corticosterone). Optical density was measured in the circular of 160 μ. As expected, there was no difference between the 3 groups (anova, F = 2.03, not significant).
Response to stress
CRF
The expression of CRF mRNA in the PVN of the nine groups is shown in Fig. 3. The effects of both stress and corticosterone replacement on levels of CRF mRNA are shown in Fig. 4. There were significant main effects of both stress (F2,36 = 271.9 P < 0.001) and corticosterone treatment (F2,36 = 103.6, P < 0.001) on CRF mRNA levels in the PVN; there was also a significant interaction between these two factors (F4,36 = 62.4, P < 0.001).
Fig. 3.
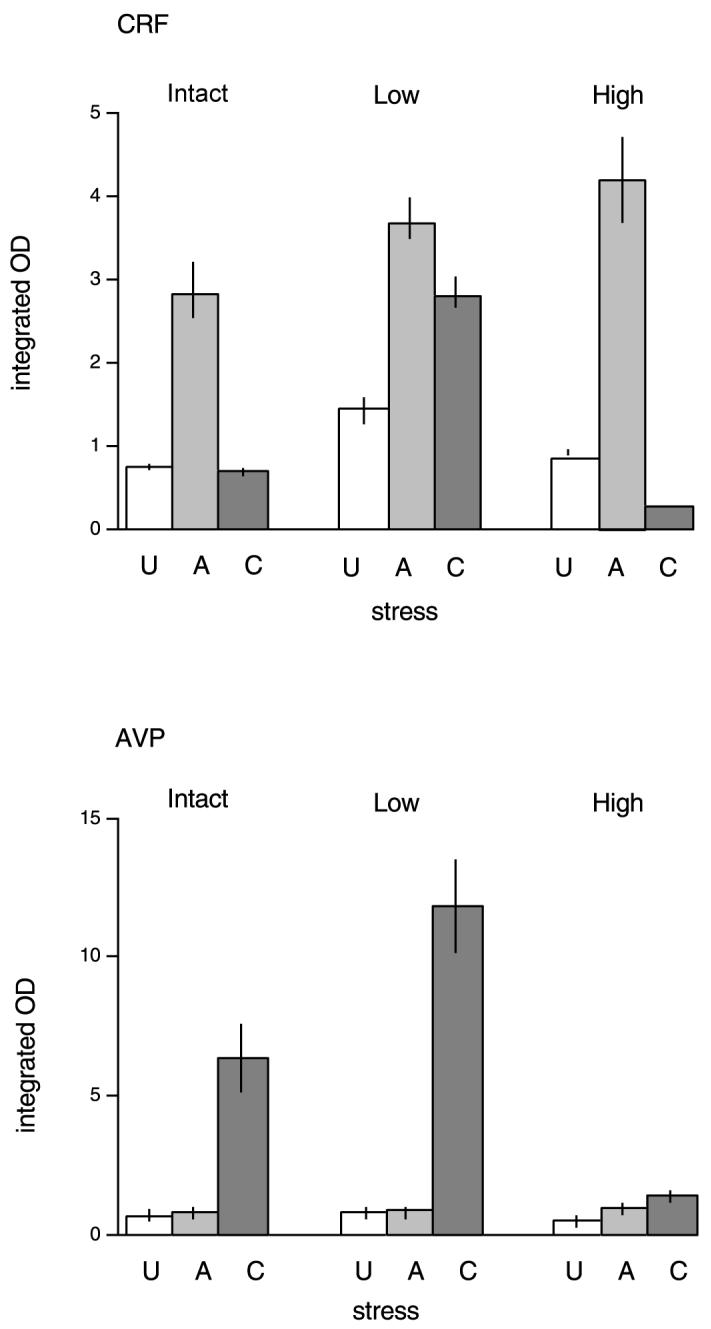
Mean integrated OD for CRF (above) or AVP (below) mRNA in the paraventricular nucleus in either no stress (U), acutely stressed (A) or chronically stressed (C) rats. The results following three treatments are shown: intact (control), low-dose and high-dose corticosterone replacement in adrenalectomised rats. n = 5 rats per group.
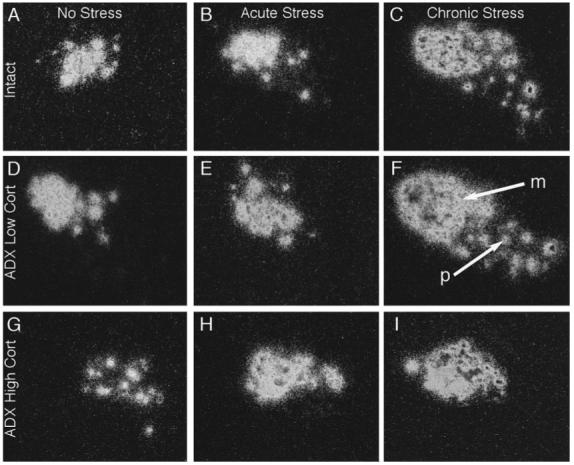
Fig. 4.

Dark-field photomicrographs of emulsion dipped autoradiographs from similar rostrocaudal levels of the PVN showing CRF mRNA signals in control (Intact) rats (A, B and C), and adrenalectomised (ADX) rats replaced with either low (D, E and F) or high (G, H and I) levels of corticosterone, exposed to no stress (A, D and G), acute stress (B, E and H), or chronic stress (C, F and I).
Univariate analysis confirmed that, in the intact groups, acute stress increased CRF levels (Bonferroni, P < 0.05), but this was not observed following repeated (chronic) stress (not significant). Low-dose corticosterone replacement resulted in a different pattern. Acute stress still increased CRF mRNA (P < 0.05 vs. unstressed low-dose group), but levels remained elevated even after chronic stress (P < 0.05, low-dose controls vs. chronic stress). The two stressed groups did not differ from each other. Adrenalectomised rats receiving the higher dose of corticosterone, however, showed a pattern very similar to intact rats. Acute stress increased CRF mRNA (P < 0.05 vs. unstressed high-dose group), but this was no longer seen after 10 days repeated restraint. In fact, CRF levels at this time were lower than in either control or acutely stressed high-dose groups (P < 0.05, both cases).
AVP
The effects of these treatments on AVP mRNA are shown in Figs. 3 and 5. As for CRF, there were main effects of stress (F2,36 = 236.6, P < 0.001) and corticosterone treatment (F2,36 = 36.7, P < 0.001), and a significant interaction between them (F4,36 = 25.4, P < 0.001). Inspection of Fig. 3, and the univariant analysis, shows a very different pattern of response by AVP mRNA compared to CRF both to stress and to corticosterone treatment. In all groups, acute stress had no effect on AVP mRNA (Bonferroni, not significant). However, there was a large increase after repeated restraint (P < 0.05). in the intact and adrenalectomised low-dose corticosterone-treated groups (P < 0.05). The higher-dose group showed a different pattern. There was a very small (but significant) increase after both acute and chronic stress (P < 0.05), but these groups did not differ from each other.
Fig. 5.
Dark-field photomicrographs of emulsion dipped autoradiographs from similar rostrocaudal levels of the PVN showing AVP mRNA signals in control (Intact) rats (A, B and C), and adrenalectomised (ADX) rats replaced with either low (D, E and F) or high (G, H and I) levels of corticosterone, exposed to no stress (A, D and G), acute stress (B, E and H), or chronic stress (C, F and I). p, parvocellular subdivision of the PVN, m, magnocellular subdivision of the PVN.
Discussion
These experiments show that: (i) acute (single) restraint stress has differing effects 6 h later on the expression of the mRNAs for CRF and AVP in the PVN; (ii) this pattern changes in brains examined following 10 days repeated (chronic) stress; and (iii) clamping corticosterone levels at either around 50 ng/mL (≈ 140 nm/L) or around 140 ng/mL (c 400 nm/L) had very distinct effects on this pattern of adaptation to repeated stress. As we analysed only one time point, we do not know whether corticosterone altered the half life of either CRF or AVP mRNA.
Restraint represents a form of ëpsychologicalí stress; that is, one involving emotional and cognitive processing of an adverse experience, without associated physical discomfort or pain, or direct challenge to physiological well being. It has been widely used for this reason, and also because it is easy to apply and to control. However, it does not represent a ëreal-lifeí stressor, in the sense that restraint is not a model for any credible form of stress that a rat (or any other animal) is likely to encounter. More elaborate procedures, for example exposing rats to conspecific, aggressive strangers in a novel environment (e.g. Martinez et al., 1998; Chung et al., 1999) may represent processes more closely allied to that occurring under natural conditions and give additional information, but have their own problems in terms of standardization and complexity. The neural responses to different forms of stress, though closely allied, may be appreciably different: for example, the patterns of c-fos expression following either repeated restraint or repeated exposure to social stress, though similar, are not identical (Chen & Herbert, 1995; Martinez et al., 1998).
There is general agreement that CRF mRNA is elevated in the PVN during the hours following an acute episode of restraint. CRF hnRNA increases within 30-60 min, whereas mRNA takes longer, reaching a maximum 3-6 h later (Ma et al., 1997a,b; Ma & Lightman, 1998) and is associated with increased CRF levels (Chappell et al., 1986). Similar results follow exposure to other forms of acute stress, such as cold or hypertonic saline (Harbuz et al., 1994; Ma & Aguilera, 1999). This was confirmed in the present study: CRF mRNA optical density increased about three-fold in intact rats 6 h after a single 60 min episode of restraint stress. The demonstration that stress activates a subset of CRF axons containing AVP in the median eminence pointed to a close association between these two peptides (Whitnall, 1989). Whether parvocellular AVP is itself activated by an acute stress is more debatable. A study on three strains of rats showed no change in AVP mRNA 4 h after a single restraint in two of them (Sprague-Dawley, Wistar) but a small increase in the third (CFY); hypertonic saline (a rather different category of stress) had a somewhat greater effect (Harbuz et al., 1994) and this was confirmed by a later studies (Ma & Lightman, 1998) However, others report that a single period of restraint increases both AVP hnRNA and mRNA in the parvocellular PVN (Herman & Sherman, 1993; Herman, 1995). Different categories of stressors may have stress-specific effects on AVP (Canny et al., 1989). We found no increase in AVP mRNA 6 h after restraint, though other (earlier) time points might have given somewhat different results. Nevertheless, there was a striking distinction between the expression of CRF and AVP mRNAs after a single stress in our experiments.
There was an equally clear distinction in intact rats between CRF and AVP 6 h following the last stress after 10 days of repeated restraint. CRF mRNA was no longer elevated, and this confirms some previous results on restraint stress (Ma & Lightman, 1998) but not hypertonic saline (Ma & Aguilera, 1999). The exact nature of the stressor may be important. 13 days of repeated, varied and unpredictable stress resulted in persistently elevated CRF immunoreactivity in the PVN (Chappell, 1986), and chronic hypoglycaemia (Brown & Sawchenko, 1997), social stress (Albeck et al., 1997) or exposure to cold (Akana & Dallman, 1997) had similar effects on CRF mRNA. The boundary between ëacuteí and ëchronicí stress will always be indistinct; nevertheless, it seems that there is general agreement that persistent stress is associated with upregulation of parvocellular AVP mRNA in the PVN. This includes both footshock (Sawchenko et al., 1996) and restraint (Makino et al., 1995); repeated hypertonic saline resulted in less obvious activation though acute responses were more prolonged than for CRF mRNA (Ma & Aguilera, 1999). In the experiments reported here, there was marked stimulation of AVP mRNA in intact rats after 10 days of repeated restraint, which agrees well with previous work. The reciprocal change in CRF and AVP mRNAs during adaptation to repeated restraint means that the neurochemical phenotype in the parvocellular PVN is very different at the beginning and end of this process. Of course, there may also be changes in the other peptides that are characteristic of these neurons, so that the real picture may be more complex than is suggested by measuring only two constituents.
CRF and AVP expression in parvocellular PVN neurons are regulated by glucocorticoids. Both increase after adrenalectomy (Sawchenko, 1987) and are counteracted by subsequent treatment with glucocorticoids (Plotsky, 1991; Kovacs, 1998). The corticoster-one pellets used in the experiments reported here had two functions: they clamped plasma levels to those resembling either the daily nadir or the daily peak in intact rats (Dallman et al., 1992), but they also abolished (it must be presumed) diurnal rhythmicity in these plasma levels. The significance of the amplitude of the diurnal rhythm in adrenal glucocorticoids, which is very marked, has not been well-studied, and was not the focus of these experiments, but the existence of this daily rhythm may well be functionally important for the stress response (Jacobson et al, 1988). CRF mRNA was higher in the low-dose adrenalectomised rats than the other two groups, which suggests that this dose did not entirely suppress the increased levels expected after adrenalectomy. Because of the daily rhythm of corticosterone in intact rats, it is difficult to compare such animals with those in which levels have been ëclampedí by implants (see above); but as the rats in these experiments were sampled about 5-7 h into the dark phase of a 12 : 12 h light-dark cycle, it is likely that levels in intact animals were intermediate between those at the diurnal peak and trough (Dallman et al., 1992). Results for AVP were different: the lower dose of corticosterone suppressed levels to those similar to intact controls, and the higher dose reduced AVP mRNA to below control levels. This suggests that the parvocellular AVP might be more sensitive to feedback than CRF. There may be other differences, as the relation between AVP mRNA and plasma corticosterone seemed more progressive and predictable than for CRF mRNA; however, these results should not be overemphasized, as they are based on a mixture of intact and ëclampedí animals, and the different diurnal dynamics of the two have already been discussed.
The major finding of this study, however, lies in the distinct results of either low or high-dose corticosterone on subsequent adaptation in CRF and AVP mRNA after repeated restraint stress. Neither dose of corticosterone in adrenalectomised rats had any effect on the response of CRF mRNA in the PVN to an acute stress. Stress-induced Fos expression in the PVN is not prevented by corticosterone implants replicating mean diurnal levels in adrenalectomised rats (Viau et al., 1999). The response of CRF mRNA to hypovolaemic stress in adrenalectomised rats was increased by low (sub physiological) corticosterone, but normal in those receiving physiological amounts (Tanimura & Watts, 1998). However, the effect that corticosterone has on stress responses may differ with time of day (Dallman et al., 1992). Within the range of corticosterone we used, there was no evidence that they regulated stress-induced mRNA after the first restraint. In repeatedly stressed rats, the results were very different. Clamping corticosterone levels at the lower limit effectively prevented CRF mRNA from showing the adaptive response observed in controls. This strongly suggests either that stress-induced hypercortisolaemia is necessary for adaptation in CRF mRNA to occur, or that it is inhibited by maintaining levels at the lower limit of the diurnal cycle throughout each 24 h. The evolution of stress-induced cortisolaemia is complex. Overall, the response wanes with repeated exposure (Chen & Herbert, 1995), so this decrement (which cannot occur in ëclampedí rats) might be a factor. However, adaptation was observed in clamped, higher-dose rats, which rules this out. The latter also suggests that experiencing higher levels of corticosterone (though those below maximal stress levels) is sufficient for this process to occur. Stress levels of corticosterone can reach 300-400 ng/mL immediately postrestraint, well above those in the higher-dose rats in these experiments. However, levels decline within an hour or so: it may be that the persistently elevated levels in the higher-dose group reproduced, overall, this period of elevated glucocorticoid.
The effects of clamping corticosterone on stress-induced AVP mRNA were quite different. As for CRF mRNA, there was no effect on acute responses (which did not occur in either of the adrenalectomised groups). But the characteristic increase in AVP mRNA observed after repeated stress in controls was also seen in low-dose corticosterone-treated rats, but prevented by the higher dose. This strongly suggests that the decline in corticosterone that occurs with repeated stress is necessary for the later adaptation in AVP mRNA to occur. Absence of a diurnal rhythm (which would have been expected in both adrenalectomised groups) did not, in itself, prevent AVP mRNA from responding to repeated stress. It seems clear that there is the role for corticosterone in regulating the response to repeated (not acute) stress, but that this differs for each peptide. Preventing elevated corticosterone may inhibit adaptation in CRF mRNA, but the absence of the subsequent decline may interfere with AVP mRNA. One complication of replacement treatment following adrenalectomy which does not include a mineralocorticoid (e.g. aldosterone) may be excess excretion of salt in the urine. This effect was clearly seen in our adrenalectomised animals with lower levels of replacement corticosterone, which drank excess amounts of 0.9% saline. However, this was normalized in the higher dose-treated animals, so is unlikely to play a part on the effects we describe on AVP mRNA. Whether it contributed to those observed on CRF mRNA in the lower dose also seems unlikely, as CRF was not activated to stress levels under such conditions in the nonstressed controls.
It is not possible to determine from these experiments whether or not the actions of corticosterone are directly on the expression of AVP or CRF mRNA. Unlike CRF, AVP has a glucocorticoid response element (GRE) about five hundred bases from the 5′ of the open reading frame (Kovacs, 1998); this might have been responsible for the suppressive effects of the higher dose of corticosterone on the activation otherwise seen following repeated stress. The promoter of the CRF has a cAMP response element (CRE) and glucocorticoids have been postulated to interact here to regulate transcription (Scatena & Adler, 1996). Whether this could also be responsible for the effects of clamping corticosterone at the lower levels on the adaptation of CRF mRNA is not known. However, it seems equally likely that glucocorticoids might have their prime action in this context on more distal parts of the neuraxis.
These findings are comparable to those on the effects of similar corticosterone treatment on the expression of immediate-early genes in response to stress. Rats receiving low-dose corticosterone showed increased Fos-b expression following 9 days stress in the lateral septum and in the dorsal and medial parts of the paraventricular nucleus compared to either control, stressed rats or those receiving the higher corticosterone dose and repeated stress (Stamp & Herbert, unpublished data). They are also directly relevant to Munck’s hypothesis, which suggests that that at low doses, glucocorticoids permit responses to stress while at high doses they protect the organism by toning down or shutting off these responses (Munck & Náray-Fejes-Tóth, 1994; Sapolsky et al. 2000). It seems that the trajectory of glucocorticoid responses following repeated stress has an important determinant role in subsequent gene expression in the brain.
Acknowledgements
Supported by a project grant from the BBSRC. We thank Rod Carter for his valuable assistance in surgery, Sarah Cleary and Helen Shiers for assays, Pam Stacey for technical assistance and Adrian Newman for photography.
Abbreviations
- CRF
Corticotropin-releasing factor
- AVP
Argenine-vasopressin
- PVN
Paraventricular nucleus
- ADX
Adenalectomy
- U
Unstressed
- A
Acutely stressed
- C
Chronically stressed
- CRE
c-AMP response element
- hnRNA
heteronuclear ribonucleic acid
- mRNA
messenger ribonucleic acid
- DEPC
diethyl pyrocarbonate
References
- Akana SF, Dallman MF. Chronic cold in adrenaliextomized, corticosterone (B) -treated rats: facilitated corticotropin responses to acute restraint emerge as B increases. Endocrinology. 1997;138:3249–3258. doi: 10.1210/endo.138.8.5291. [DOI] [PubMed] [Google Scholar]
- Albeck DS, McKittrick CR, Blanchard DC, Blanchard RJ, Nikulina J, McEwen BS, Sakai RR. Chronic social stress alters levels of corticotropin-releasing factor and arginine vasopressin mRNA in rat brain. J. Neurosci. 1997;17:4895–4903. doi: 10.1523/JNEUROSCI.17-12-04895.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ER, Sawchenko PE. Hypophysiotropic CRF neurons display a sustained immediate-early gene response to chronic stress but not to adrenalectomy. J. Neuroendocrinol. 1997;9:307–316. doi: 10.1046/j.1365-2826.1997.00586.x. [DOI] [PubMed] [Google Scholar]
- Bullitt E. Expression of c-fos-like protein as a marker for neuronal activity following noxious stimulation on the rat. J. Comparative Neurology. 1990;296:517–530. doi: 10.1002/cne.902960402. [DOI] [PubMed] [Google Scholar]
- Canny BJ, Funder JW, Clarke IJ. Glucocorticoids regulate ovine hypophysial portal levels of corticotropin-releasing factor and arginine vasopressin in a stress-specific manner. Endocrinology. 1989;125:2532–2539. doi: 10.1210/endo-125-5-2532. [DOI] [PubMed] [Google Scholar]
- Ceccatelli S, Villar MJ, Goldstein M, Hokfelt T. Expression of c-Fos immunoreactivity in transmitter-characterized neurons after stress. Proc. Natl. Acad. Sci. 1989;86:9569–9573. doi: 10.1073/pnas.86.23.9569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan RKW, Brown ER, Ericsson A, Kovács KJ, Sawchenko PE. A comparison of two immediate-early genes, c-fos and NGFI-B, as markers for functional activation in stress-related neuroendocrine circuitry. J. Neurosci. 1993;13:5126–5138. doi: 10.1523/JNEUROSCI.13-12-05126.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell PB, Smith MA, Kilts CD, Bissette G, Ritchie J, Anderson C, Nemeroff CB. Alterations in corticotropin-releasing factor-like immunoreactivity in discrete rat brain regions after acute and chronic stress. J. Neurosci. 1986;6:2908–2914. doi: 10.1523/JNEUROSCI.06-10-02908.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Herbert J. Regional changes in c-fos expression in the basal forebrain and brainstem during adaptation to repeated stress: correlation’s with cardiovascular, hypothermic and endocrine responses. Neuroscience. 1995;64:675–685. doi: 10.1016/0306-4522(94)00532-a. [DOI] [PubMed] [Google Scholar]
- Chung KKK, Martinez M, Herbert J. Central serotonin depletion modulates the behavioural, endocrine and physiological responses to repeated social stress and subsequent c-fos expression in the brains of male rats. Neuroscience. 1999;92:613–625. doi: 10.1016/s0306-4522(99)00028-7. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Helmreich DL, Watson SJ. Fos expression in forebrain afferents to the hypothalamic paraventricular nucleus following swim stress. J. Comp. Neurol. 1996;368:88–99. doi: 10.1002/(SICI)1096-9861(19960422)368:1<88::AID-CNE6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Scribner KA, Bradbury MJ, Walker C-D, Strack AM, Cascio CS. Stress, feedback and facilitation in the hypothalamo-pituitary-adrenal axis. J. Neuroendocrinol. 1992;4:517–526. doi: 10.1111/j.1365-2826.1992.tb00200.x. [DOI] [PubMed] [Google Scholar]
- Gillies GE, Linton EA, Lowry PJ. Corticotropin releasing activity of the new CRF is potentiated several times by vasopressin. Nature. 1982;299:355–357. doi: 10.1038/299355a0. [DOI] [PubMed] [Google Scholar]
- Grino M, Burgunder J-M. Ontogeny of expression and glucocorticoid regulation of the arginine vasopressin gene in the rat hypothalamic paraventricular nucleus. J. Neuroendocrinol. 1992;4:71–77. doi: 10.1111/j.1365-2826.1992.tb00348.x. [DOI] [PubMed] [Google Scholar]
- Harbuz MS, Jessop DS, Lightman SL, Chowdrey HS. The effects of restraint or hypertonic saline stress on corticotropin-releasing factor, arginine vasopressin, and proenkephalin A mRNAs in the CFY, Sprague-Dawley and Wistar strains of rat. Brain Res. 1994;667:6–12. doi: 10.1016/0006-8993(94)91707-8. [DOI] [PubMed] [Google Scholar]
- Harbuz MS, Lightman SL. Stress and the hypothalamo-pituitary-adrenal axis: acute, chronic and immunological activation. J. Endocrinol. 1992;134:327–339. doi: 10.1677/joe.0.1340327. [DOI] [PubMed] [Google Scholar]
- Herbert J. Peptides in the limbic system: neurochemical codes for coordinated adaptive responses to behavioral and physiological demand. Prog. Neurobiol. 1993;41:723–791. doi: 10.1016/0301-0082(93)90033-o. [DOI] [PubMed] [Google Scholar]
- Herbert J, Forsling MK, Howes SR, Stacey PM, Shiers HM. Regional expression of c-fos antigen in the basal forebrain following intraventricular infusions of angiotensin and its modulation by drinking either water or saline. Neuroscience. 1992;51:867–882. doi: 10.1016/0306-4522(92)90526-8. [DOI] [PubMed] [Google Scholar]
- Herman JP. In situ hybidisation analysis of vasopressin gene transcription in the paraventricular and supraoptic nuclei of the rat: Regulation by stress and glucocorticoids. J. Comp. Neurol. 1995;363:15–27. doi: 10.1002/cne.903630103. [DOI] [PubMed] [Google Scholar]
- Herman JP, Sherman TG. Acute stress upregulates vasopressin gene expression in parvocellular neurons of the hypothalamic paraventricular nucleus. Ann. N.Y. Acad. Sci. 1993;689:546–549. doi: 10.1111/j.1749-6632.1993.tb55590.x. [DOI] [PubMed] [Google Scholar]
- Imaki T, Nahan J-L, Rivier C, Sawchenko PE, Vale W. Differential regulation of corticotropin-releasing factor mRNA in rat brain regions by glucocorticoids and stress. Endocrinology. 1991;11:585–599. doi: 10.1523/JNEUROSCI.11-03-00585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson LJ, Akana SF, Cascio CS, Shinsako J, Dallman MF. Circadian variations in plasma corticosterone permit normal termination of adrenocorticotropin responses to stress. Endocrinology. 1988;122:1343–1348. doi: 10.1210/endo-122-4-1343. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Sharp FR, Dallman MF. Induction of fos-like immunoreactivity in hypothalamic corticotropin-releasing factor neurons after adrenalectomy in the rat. Endocrinology. 1990;126:1709–1719. doi: 10.1210/endo-126-3-1709. [DOI] [PubMed] [Google Scholar]
- Kovacs KJ. Functional neuroanatomy of the parvocellular vasopressinergic system: transcriptional responses to stress and glucocorticoid feedback. Prog. Brain Res. 1998;119:31–43. doi: 10.1016/s0079-6123(08)61560-5. [DOI] [PubMed] [Google Scholar]
- Lightman SL, Harbuz MS. Expression of corticotropin-releasing factor mRNA in response to stress. Ciba Foundation Symposium. 1993;172:173–198. doi: 10.1002/9780470514368.ch9. [DOI] [PubMed] [Google Scholar]
- Ma X-M, Aguilera G. Transcriptional responses of the vasopressin and corticotropin-releasing hormone genes to acute and repeated intraperitoneal hypertonic saline injection in rats. Mol. Brain Res. 1999;68:129–140. doi: 10.1016/s0169-328x(99)00080-7. [DOI] [PubMed] [Google Scholar]
- Ma X-M, Levy A, Lightman SL. Rapid changes in heteronuclear RNA for corticotrophin-releasing hormone and arginine vasopressin in response to acute stress. J. Endocrinol. 1997a;152:81–89. doi: 10.1677/joe.0.1520081. [DOI] [PubMed] [Google Scholar]
- Ma X, Levy A, Lightman SL. Emergence of an isolated arginine vasopressin (AVP) response to stress after repeated restraint: a study of both AVP and corticotropin-releasing hormone messenger ribonucleic acid (RNA) and heteronuclear RNA. Endocrinology. 1997b;138:4351–4357. doi: 10.1210/endo.138.10.5446. [DOI] [PubMed] [Google Scholar]
- Ma XM, Lightman SL. The arginine vasopressin and corticotrophin-releasing hormone gene transcription responses to varied frequencies of repeated stress in rats. J. Physiol. (Lond.) 1998;510:605–614. doi: 10.1111/j.1469-7793.1998.605bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S, Smith MA, Gold PW. Increased expression of corticotropin-releasing hormone and vasopressin messenger ribonucleic acid (mRNA) in the hypothalamic paraventricular nucleus during repeated stress: association with reduction in glucocorticoid receptor mRNA levels. Endocrinology. 1995;136:3299–3309. doi: 10.1210/endo.136.8.7628364. [DOI] [PubMed] [Google Scholar]
- Martinez M, Phillips PJ, Herbert J. Adaptation in patterns of c-fos expression in the brain associated with exposure to either single or repeated social stress in male rats. Eur. J. Neurosci. 1998;10:20–33. doi: 10.1046/j.1460-9568.1998.00011.x. [DOI] [PubMed] [Google Scholar]
- Munck M, Náray-Fejes-Tóth A. Glucocorticoids and stress: Permissive and suppressive actions. Ann. N.Y. Acad. Science. 1994;746:115–130. doi: 10.1111/j.1749-6632.1994.tb39221.x. [DOI] [PubMed] [Google Scholar]
- Plotsky PM. Pathways to the secretion of adrenocorticotrophin: a view from the portal. J. Neuroendocrinol. 1991;3:1–9. doi: 10.1111/j.1365-2826.1991.tb00231.x. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimularory, and preparative actions. Endocrine Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE. Adrenalectomy-induced enhancement of CRF and basopressin immunoreactivity in parvocellular neurosecretory neurons: anatomic peptide, and steroid specificity. J. Neurosci. 1987;7:1093–1106. doi: 10.1523/JNEUROSCI.07-04-01093.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawchenko PE, Brown ER, Chan RKW, Ericsson A, Li HY, Roland BL, Kovacs KJ. The paraventricular nucleus of the hypothalamus and the functional neuroanatomy of visceromotor responses to stress. Prog. Brain Res. 1996;107:201–222. doi: 10.1016/s0079-6123(08)61866-x. [DOI] [PubMed] [Google Scholar]
- Scatena CD, Adler S. Trans-acting factors dictate the species-specific placental expression of corticotropin-releasing factor genes in choriocarcinoma cell lines. Endocrinology. 1996;37:3000–3008. doi: 10.1210/endo.137.7.8770924. [DOI] [PubMed] [Google Scholar]
- Schmidt ED, Binnekade R, Janszen JW, Tilders FJH. Short stressor induced long-lasting increases of vasopressin stores in hypothalamic corticotropin-releasing hormone (CRH) neurons in adult rats. J. Neuroendocrinol. 1996;8:703–712. [PubMed] [Google Scholar]
- Stamp JA, Herbert J. Multiple immediate-early gene expression during physiological and endocrine adaptation to repeated stress. Neuroscience. 1999;94:1313–1322. doi: 10.1016/s0306-4522(99)00368-1. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Kuypers HGJM. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organisation of projections to the pituitary, dorsal vagal complex and spinal cord as demonstrated by retrograde fluorescence double-labelling methods. J. Comp. Neurol. 1980;194:555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Lind RW. Regulation of multiple peptides in CRF parvocellular neurosecretory neurons: implications for the stress response. Prog. Brain Res. 1986;68:169–190. doi: 10.1016/s0079-6123(08)60238-1. [DOI] [PubMed] [Google Scholar]
- Tanimura SM, Watts AG. Corticosterone can facilitate as well as inhibit corticotropin-releasing hormone gene expression in the rat hypothalamic paraventricular nucleus. Endocrinology. 1998;139:3830–3836. doi: 10.1210/endo.139.9.6192. [DOI] [PubMed] [Google Scholar]
- Viau V, Chu A, Soriano L, Dallman MF. Independent and overlapping effects of corticosterone and testosterone on corticotropin-releasing hormone and arginine vasopressin mRNA expression in the paraventricular nucleus of the hypothalamus and stress-induced adrenocorticotropic hormone release. J. Neurosci. 1999;19:6684–6693. doi: 10.1523/JNEUROSCI.19-15-06684.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts AG. Disturbance of fluid homeostasis leads to temporally and anatomically distinct responses in neuropeptide and tyrosine hydrozylase mRNA levels in the paraventricular and supraoptic nuclei of the rat. Neuroscience. 1992;46:859–879. doi: 10.1016/0306-4522(92)90190-d. [DOI] [PubMed] [Google Scholar]
- Whitnall MH. Stress selectivity activates the vasopressin-containing subset of corticotropin-releasing hormone neurons. Neuroendocrinology. 1989;50:702–707. doi: 10.1159/000125302. [DOI] [PubMed] [Google Scholar]