Introduction
Recombinant activated factor VII (rFVIIa, NovoSeven® , Novo Nordisk, Bagsvaerd, Denmark) is a haemostatic agent that was originally developed for the treatment of haemophiliacs with inhibitors and then used successfully for treating haemorrhages in patients with acquired haemophilia1–4.
In the last few years, rFVIIa has also been used with benefit as a “universal haemostatic agent” in many other non-haemophilic bleeding situations including congenital factor VII deficiencies, hepatic failure, liver transplantation, surgery and trauma5–7.
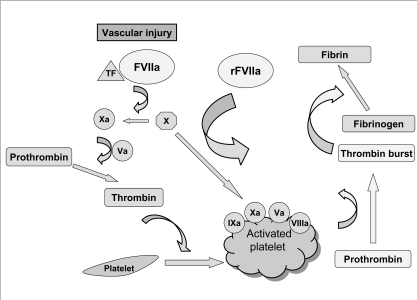
The haemostatic effect of pharmacological doses of rFVIIa seems to be that of enhancing the rate of thrombin generation on thrombin-activated platelet surfaces, thus providing the thrombin necessary for the formation of a stable fibrin haemostatic plug8. Based on this information, rFVIIa has also been employed in disorders characterised by impaired thrombin generation, such as quantitative and qualitative platelet defects9. Figure 1 illustrates the mechanisms of action of rFVIIa.
Figure 1.
Mechanisms of action of rFVIIa
Following injury to a vessel, exposed tissue factor (TF) forms a complex with factor VII activated (FVIIa). The TF-FVIIa complex activates FX leading to the conversion of prothrombin to thrombin. The initial limited amount of thrombin formed subsequently activates FVIII, FV and platelets so that the tenase complex (FVIIIa/ FIXa) and the prothrombinase complex (FXa/FVa) assembled on the activated platelet surface lead to full thrombin generation. Besides this TF-dependent mechanism there is a second pathway which involves a TF-independent mechanism. In fact, recent data suggest that rFVIIa at high pharmacological doses can activate FX directly, thus leading to an additional burst of thrombin generation.
The current clinical experience regarding the use of rFVIIa for the treatment of bleeding in patients with congenital or acquired platelet disorders is analysed briefly in this review.
rFVIIa in congenital platelet disorders
rFVIIa is being increasingly used in certain disorders of platelet dysfunction, particularly Glanzmann’s thrombasthenia, which is a rare autosomal recessive inherited platelet disorder characterised by impaired platelet aggregation and due to defects in the platelet membrane glycoprotein (GP) IIb-IIIa. Bleeding manifestations, which usually appear in early childhood, range from easy bruising, purpura, epistaxis, gingival bleeds, haematuria, haemarthrosis, muscle haematomas to central nervous system bleed. When bleeding does not respond to local measures and/or antifibrinolytic drugs, platelet transfusion is currently the standard treatment. However, repeated platelet transfusions may result in GP IIb-IIIa and/or HLA immunisation and the development of refractoriness to platelets10. Tengborn and Petruson11 first reported the successful use of rFVIIa (110 μg/kg) in the management of intractable epistaxis in a 2-year old child with Glanzmann’s thrombasthenia. Following this report, several other authors substantiated the efficacy of rFVIIa in the management of bleeding or surgical procedures, using doses ranging from 120 to 300 μg/kg12–22. Poon and colleagues13 successfully used rFVIIa to treat 24 bleeding episodes and to prevent bleeding during surgery in four children with Glanzmann’s thrombasthenia, administering 89 to 116 μg/kg every 2 hours in association with antifibrinolytic drugs. On behalf of the International Data Collection on rFVIIa and Congenital Platelet Disorders Study Group, the same authors analysed the use of rFVIIa during 34 surgical/invasive procedures and 108 bleeding episodes in 59 patients with Glanzmann’s thrombasthenia, including 29 with platelet antibodies. rFVIIa was effective in stopping bleeding in 29 of 31 (94%) evaluable procedures and in 77 of 103 (75%) evaluable bleeding episodes. There was one failure in a minor surgical procedure and another in a major surgical procedure, and 8 recurrences and 26 failures in bleeding episodes. Among the successful treatments, the median doses of rFVIIa as a bolus were 74–150 μg/kg (1–33 injections) in 23 surgical procedures and 28–238 μg/kg (1–48 injections) in 68 bleeding episodes; continuous infusions of rFVIIa were used in the remaining successful treatments. Two serious adverse events were reported – one was a deep vein thrombosis with pulmonary embolism, and the other was development of a thrombus in a ureter16. Based on the positive results, the authors concluded that rFVIIa seems to be a valid alternative to platelet transfusion in patients with Glanzmann’s thrombasthenia, especially in those refractory to platelet transfusions. Chuansumrit and colleagues19 reported on two children with Glanzmann’s thromboasthenia undergoing invasive dental procedures. rFVIIa at a dose of 180–200 μg/kg, along with local measures including fibrin glue and mouth rinses with tranexamic acid, was successful in both cases. d’Oiron and colleagues21 reported on the use of rFVIIa (an initial bolus dose of 70–110 μg/kg followed by a continuous infusion at a rate of 9–20 μg/kg/hour for 3–15 days) in three patients with Glanzmann’s thrombasthenia undergoing invasive procedures. The treatment resulted in excellent clinical efficacy and tolerance in two cases, while in the third patient bleeding persisted for 10 days and was complicated by a thromboembolic event occurring 5 days after discontinuation of the drug. Valentino described four children with Glanzmann’s thrombasthenia in whom surgical or traumatic bleeding was successfully prevented or controlled by rFVIIa22. In contrast to the above reports of success, Almeida and colleagues20 found that rFVIIa was less satisfactory in the management of 33 episodes (28 acute bleeds and 5 surgical interventions) in seven children with inherited platelet function disorders (5 Glanzmann’s thrombasthenia, 1 Bernand-Soulier syndrome and 1 storage pool disease). Most of the children received three doses of 100 μg/kg of rFVIIa at 90-minute intervals and tranexamic acid. While the patients with Bernand-Soulier syndrome and storage pool disease responded well to the rFVIIa therapy, children with Glanzmann’s thrombasthenia had variable results with an excellent or good response during surgery or when the severity of bleeding was mild and a poor or ineffective response in severe bleeding episodes.
Similarly Peters and colleagues23 reported on a 5-year-old boy with Bernand-Soulier syndrome and severe epistaxis who was not responsive to standard therapy but was successfully treated with rFVIIa. Finally, a patient with platelet-type von Willebrand’s disease was reported to have been treated effectively with rFVIIa24.
Based on the evidence from the literature, rFVIIa is currently approved in Europe for prophylaxis and treatment of bleeding in patients with Glanzmann’s thrombasthenia with antibodies to GP IIb-IIIa and/or HLA, and past or present refractoriness to platelet transfusion. The recommended dose is a bolus injection of 90 μg/kg at 2 hour intervals for a minimum of three doses.
rFVIIa in acquired platelet bleeding disorders
The are a few reports describing the haemostatic effects of rFVIIa in thrombocytopenic patients25,26. One report27 presented the case of an 8-year old girl with idiopathic thrombocytopenic purpura whose refractory epistaxis was successfully on two occasions with rFVIIa (at a dose of 85 μg/kg). Wrobel and colleagues28 reported the cases of two patients with refractory chronic idiopathic thrombocytopenic purpura who underwent splenectomy and were prepared for surgery with a regimen including rFVIIa. The largest case series was published by Kristensen and colleagues29 who studied 74 patients with moderate to severe thrombocytopenia due to impaired platelet production or immune destruction to evaluate the effect of rFVIIa administration. Given at a dose of 50 or 100 μg/kg, rFVIIa shortened the Ivy bleeding time in approximately 50 percent of the patients and all the eight patients with overt bleeding had a clinical benefit from rFVIIa administration.
rFVIIa has also been successfully used in oncohaematolgical patients with thrombocytopenia following chemotherapy associated or not with stem cell transplantation and severe bleeding refractory to standard haematological or haemostatic support30–37. Blatt and colleagues30 used rFVIIa (boluses of 90–270 μg/kg with subsequent doses of 90 μg/kg every 4–24 hours for 3–14 days) for the treatment of severe haemorrhage in three transplanted patients; two of them had transient clinical responses. De Fabritiis and colleagues31 reported on the use of rFVIIa for the treatment of severe bleeding episodes in seven patients with haematological malignancies and thrombocytopenia following chemotherapy: two had complete responses, three had partial responses and treatment failed in the other two patients. Hicks and colleagues32 documented the efficacy of rFVIIa for the treatment of diffuse alveolar haemorrhage following bone marrow transplantation. Vidarsson and Onudarson33 described a 27-year-old woman with acute myelogenous leukaemia and refractory thrombocytopenia, who developed a subdural haematoma, haemoptysis, and periorbital haematoma. rFVIIa (90 μg/kg) was administered every 2 hours for five doses, then every 4 hours for six doses. A rapid clinical improvement was noted, despite persistent thrombocytopenia.
Another report34 described two patients with severe thrombocytopenia and life-threatening haemorrhage. A 75-year-old man with Waldenstrom’s macroglobulinaemia and refractory thrombocytopenia presented with persistent epistaxis, which was unresponsive to platelet transfusion therapy. A single dose of rFVIIa (90 μg/kg) resulted in immediate cessation of haemorrhage. The second case, a 52-year old woman with acute lymphoblastic leukaemia, presented with severe thrombocytopenia and gastrointestinal bleeding unresponsive to platelet therapy. A single bolus dose of rFVIIa (90 μg/kg) substantially reduced the haemorrhage. The largest case series so far is that recently reported by Brenner and colleagues35. The authors, using an Internet-based registry, collected 24 cases in which rFVIIa was used in the management of haemorrhage in patients with thrombocytopenia associated with haematological malignancies. Patients received a median dose of rFVIIa of 85 μg/kg (range, 18–1040 μg/kg) and a median number of 1.6 doses (range, 1–8). Bleeding stopped in 11 of 24 patients (46%), markedly decreased in 8 of 24 patients (33%) and decreased in 4 of 24 patients (17%). In most patients, the response was achieved within 2.5 hours of administration of rFVIIa. On the basis of these results, the authors suggested that rFVIIa has a beneficial effect in this clinical setting. However, a case of ischaemic stroke, possibly related to the use of rFVIIa, was documented. Another severe rFVIIa-related adverse reaction was described by Mantzios and colleagues36, who reported massive pulmonary embolism after treatment with rFVIIa in a thrombocytopenic patient with acute myeloid leukaemia and refractory bleeding.
rFVIIa has also been used successfully in uraemic patients, who may develop a bleeding disorder as a consequence of an acquired defect in platelet function38,39.
Finally, rFVIIa use has been described in iatrogenic platelet disorders due to aspirin, clopidogrel and GP IIb/IIIa inhibitors in patients with cardiovascular disorders40.
Conclusions
The mechanisms of action of rFVIIa support its potential utility in bleeding conditions other than haemophilia characterised by impaired thrombin generation, such as quantitative and qualitative platelet deficiencies. However, while the data in the literature suggest that rFVIIa may be an alternative option to platelet transfusion in patients with Glanzmann’s thrombasthenia, the published experience with rFVIIa in controlling bleeding in thrombocytopenic patients is too limited to draw any conclusions. Clinical trials are, therefore, needed to assess the safety and cost-effectiveness of this expensive approach in inherited and acquired platelet disorders.
References
- 1.Jurlander B, Thim L, Klausen NK, et al. Recombinant activated factor VII (rFVIIa): characterization, manufacturing and clinical development. Semin Thromb Hemost. 2001;27:373–83. doi: 10.1055/s-2001-16971. [DOI] [PubMed] [Google Scholar]
- 2.Hedner U, Erhardtsen E. Potential role for rFVIIa in transfusion medicine. Transfusion. 2002;42:114–24. doi: 10.1046/j.1537-2995.2002.00017.x. [DOI] [PubMed] [Google Scholar]
- 3.Uhlmann EJ, Eby CS. Recombinant activated factor VIII for non-hemophiliac bleeding patients. Curr Opin Hematol. 2004;11:198–204. doi: 10.1097/01.moh.0000135405.29702.3c. [DOI] [PubMed] [Google Scholar]
- 4.Franchini M, Zaffanello M, Veneri D. Recombinant factor VIIa. An update on its clinical use. Thromb Haemost. 2005;93:1027–35. doi: 10.1160/TH05-01-0032. [DOI] [PubMed] [Google Scholar]
- 5.Kessler CM. New products for managing inhibitors to coagulation factors: a focus on recombinant factor VIIa concentrate. Curr Opin Hematol. 2000;7:408–13. doi: 10.1097/00062752-200011000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Ghorashian S, Hunt BJ. “Off-license” use of recombinant activated factor VII”. Blood Rev. 2004;18:245–259. doi: 10.1016/j.blre.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Mathew P. The use of rFVIIa in non-haemophilia bleeding conditions in paediatrics. Thromb Haemost. 2004;92:738–46. doi: 10.1160/TH04-03-0163. [DOI] [PubMed] [Google Scholar]
- 8.Lisman T, De Groot PG. Mechanism of action of recombinant factor VIIa. J Thromb Haemost. 2003;1:1138–9. doi: 10.1046/j.1538-7836.2003.00225.x. [DOI] [PubMed] [Google Scholar]
- 9.Laurian Y. Treatment of bleeding in patients with platelet disorders: is there a place for recombinant factor VIIa? Pathophysiol Haemost Thromb. 2002;32:37–40. doi: 10.1159/000057300. [DOI] [PubMed] [Google Scholar]
- 10.Nurden AT. Inherited abnormalities of platelets. Thromb Haemost. 1999;82:468–80. [PubMed] [Google Scholar]
- 11.Tengborn L, Petruson B. A patient with Glanzmann thrombasthenia and epistaxis successfully treated with recombinant factor VIIa. Thromb Haemost. 1996;75:981–2. [PubMed] [Google Scholar]
- 12.Chuansumrit A, Sangkapreecha C, Hathirat P. Successful epistaxis control in a patient with Glanzmann thrombasthenia by increased bolus injection dose of recombinant factor VIIa. Thromb Haemost. 1999;82:1778. [PubMed] [Google Scholar]
- 13.Poon MC, Demers C, Jobin F, Wu JW. Recombinant factor VIIa is effective for bleeding and surgery in patients with Glanzmann thrombasthenia. Blood. 1999;94:3951–3. [PubMed] [Google Scholar]
- 14.Poon MC, d’Oiron R. Recombinant activated factor VII (NovoSeven) treatment of platelet-related bleeding disorders. International Registry on Recombinant Factor VIIa and Congenital Platelet Disorders Group. Blood Coagul Fibrinolysis. 2000;11(Suppl 1):S55–68. [PubMed] [Google Scholar]
- 15.Poon MC, d’Oiron R, Hann I, et al. Use of recombinant factor VIIa (NovoSeven) in patients with Glanzmann thrombasthenia. Semin Hematol. 2001;38:21–5. doi: 10.1016/s0037-1963(01)90143-x. [DOI] [PubMed] [Google Scholar]
- 16.Poon MC, D’Oiron R, Von Depka M, et al. International Data Collection on Recombinant Factor VIIa and Congenital Platelet Disorders Study Group. Prophylactic and therapeutic recombinant factor VIIa administration to patients with Glanzmann’s thrombasthenia: results of an international survey. J Thromb Haemost. 2004;2:1096–103. doi: 10.1111/j.1538-7836.2004.00767.x. [DOI] [PubMed] [Google Scholar]
- 17.Caglar K, Cetinkaya A, Aytac S, et al. Use of recombinant factor VIIa for bleeding in children with Glanzmann thrombasthenia. Pediatr Hematol Oncol. 2003;20:435–8. [PubMed] [Google Scholar]
- 18.Chuansumrit A. Confirmation of high dose recombinant factor VIIa in treating patients with Glanzmann thrombasthenia. J Thromb Haemost. 2003;1:396. doi: 10.1046/j.1538-7836.2003.00093.x. [DOI] [PubMed] [Google Scholar]
- 19.Chuansumrit A, Suwannuraks M, Sri-Udomporn N, et al. Recombinant activated factor VII combined with local measures in preventing bleeding from invasive dental procedures in patients with Glanzmann thrombasthenia. Blood Coagul Fibrinolysis. 2003;14:187–90. doi: 10.1097/00001721-200302000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Almeida A, Khair K, Hann I, Liesner R. The use of recombinant factor VIIa in children with inherited platelet function disorders. Br J Haematol. 2003;121:477–81. doi: 10.1046/j.1365-2141.2003.04286.x. [DOI] [PubMed] [Google Scholar]
- 21.d’Oiron R, Menart C, Trzeciak MC, et al. Use of recombinant factor VIIa in 3 patients with inherited type I Glanzmann’s thrombasthenia undergoing invasive procedures. Thromb Haemost. 2000;83:644–7. [PubMed] [Google Scholar]
- 22.Valentino LA. Use of rFVIIa in 4 children with Glanzmann thromboasthenia. J Pediatr Hematol Oncol. 2004;28:653–8. doi: 10.1097/01.mph.0000212993.49188.73. [DOI] [PubMed] [Google Scholar]
- 23.Peters M, Heijboer H. Treatment of a patient with Bernard-Soulier syndrome and recurrent nosebleeds with recombinant factor VIIa. Thromb Haemost. 1998;80:352. [PubMed] [Google Scholar]
- 24.Fressinaud E, Sigaud-Fiks M, Le Boterff C, Piot B. Use of recombinant factor VIIa (NovoSeven®) for dental extraction in a patient affected by platelet-type (pseudo) von Willebrand disease. Haemophilia; XXIIIth Congress of the World Federation of Hemophilia (abstract).1998. [Google Scholar]
- 25.Poon MC. Management of thrombocytopenic bleeding: is there a role for recombinant coagulation factor VIIa? Curr Hematol Rep. 2003;2:139–47. [PubMed] [Google Scholar]
- 26.Goodnough LT. Experiences with recombinant human factor VIIa in patients with thrombocytopenia. Semin Hematol. 2004;41(suppl 1):25–9. doi: 10.1053/j.seminhematol.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 27.Culic S. Recombinant factor VIIa for refractive haemorrhage in autoimmune idiopathic thrombocytopenic purpura. Br J Haematol. 2003;120:909–10. doi: 10.1046/j.1365-2141.2003.04151_2.x. [DOI] [PubMed] [Google Scholar]
- 28.Wrobel G, Dobaczewski G, Patkowski D, et al. Experiences with recombinant activated factor VII in the treatment of severe refractory thrombocytopenia. Pediatr Blood Cancer. 2006;47:729–30. doi: 10.1002/pbc.21013. [DOI] [PubMed] [Google Scholar]
- 29.Kristensen J, Killander A, Hippe E, et al. Clinical experience with recombinant factor VIIa in patients with thrombocytopenia. Haemostasis. 1996;26(suppl 1):159–64. doi: 10.1159/000217260. [DOI] [PubMed] [Google Scholar]
- 30.Blatt J, Gold SH, Wiley JM, et al. Off-label use of recombinant factor VIIa in patients following bone marrow transplantation. Bone Marrow Transplant. 2001;28:405–7. doi: 10.1038/sj.bmt.1703157. [DOI] [PubMed] [Google Scholar]
- 31.de Fabritiis P, Dentamaro T, Picardi A, et al. Recombinant factor VIIa for the management of severe hemorrhages in patients with hematologic malignancies. Haematologica. 2004;89:243–5. [PubMed] [Google Scholar]
- 32.Hicks K, Peng D, Gajewski JL. Treatment of diffuse alveolar hemorrhage after allogeneic bone marrow transplant with recombinant factor VIIa. Bone Marrow Transplant. 2002;30:975–8. doi: 10.1038/sj.bmt.1703731. [DOI] [PubMed] [Google Scholar]
- 33.Vidarsson B, Onundarson PT. Recombinant factor VIIa for bleeding in refractory thrombocytopenia. Thromb Haemost. 2000;83:634–5. [PubMed] [Google Scholar]
- 34.Gerotziafas GT, Zervas C, Gavrielidis G, et al. Effective hemostasis with rFVIIa treatment in two patients with severe thrombocytopenia and life-threatening hemorrhage. Am J Hematol. 2002;69:219–22. doi: 10.1002/ajh.10056. [DOI] [PubMed] [Google Scholar]
- 35.Brenner B, Hoffman R, Balashov D, et al. Control of bleeding caused by thrombocytopenia associated with hematologic malignancy: an audit of the clinical use of recombinant activated factor VII. Clin Appl Thromb Hemost. 2005;11:401–10. doi: 10.1177/107602960501100406. [DOI] [PubMed] [Google Scholar]
- 36.Mantzios G, Tsirigotis P, Pappa V, et al. Massive pulmonary embolism after treatment with rFVIIa in a thrombocytopenic patient with acute myelogenous leukemia and intractable bleeding. Eur J Haematol. 2007;78:173–4. doi: 10.1111/j.1600-0609.2006.00799.x. [DOI] [PubMed] [Google Scholar]
- 37.Savani BN, Dunbar CE, Rick ME. Combination therapy with rFVIIa and platelets for hemorrhage in patients with severe thrombocytopenia and alloimmunization. Am J Hematol. 2006;81:218–9. doi: 10.1002/ajh.20506. [DOI] [PubMed] [Google Scholar]
- 38.Revesz T, Arets B, Bierings M, et al. Recombinant factor VIIa in severe uremic bleeding. Thromb Haemost. 1998;80:353. [PubMed] [Google Scholar]
- 39.Moisescu E, Ardelean L, Simion I, et al. Recombinant factor VIIa treatment of bleeding associated with acute renal failure. Blood Coagul Fibrinolysis. 2000;11:575–7. doi: 10.1097/00001721-200009000-00010. [DOI] [PubMed] [Google Scholar]
- 40.Stepinska J, Banaszewski M, Konopka A, Szajewski T. Activated recombinant factor VII (rFVIIa) in bleeding management after therapy with IIb/IIIa-inhibitor tirofiban. Thromb Haemost. 2002;87:355–6. [PubMed] [Google Scholar]