Abstract
Objective
We previously demonstrated that upregulation of intermediate-conductance Ca2+-activated K+ channels (KCa3.1) is necessary for mitogen-induced phenotypic modulation in isolated porcine coronary smooth muscle cells (SMCs). The objective of the present study was to determine the role of KCa3.1 in the regulation of coronary SMC phenotypic modulation in vivo using a swine model of postangioplasty restenosis.
Methods and Results
Balloon angioplasty was performed on coronary arteries of swine using either noncoated or balloons coated with the specific KCa3.1 blocker TRAM-34. Expression of KCa3.1, c-jun, c-fos, repressor element -1 silencing transcription factor (REST), smooth muscle myosin heavy chain (SMMHC), and myocardin was measured using qRT-PCR in isolated medial cells 2 hours and 2 days postangioplasty. KCa3.1, c-jun, and c-fos mRNA levels were increased 2 hours postangioplasty, whereas REST expression decreased. SMMHC expression was unchanged at 2 hours, but decreased 2 days postangioplasty. Use of TRAM-34 coated balloons prevented KCa3.1 upregulation and REST downregulation at 2 hours, SMMHC and myocardin downregulation at 2 days, and attenuated subsequent restenosis 14 and 28 days postangioplasty. Immunohistochemical analysis demonstrated corresponding changes at the protein level.
Conclusion
Blockade of KCa3.1 by delivery of TRAM-34 via balloon catheter prevented smooth muscle phenotypic modulation and limited subsequent restenosis.
Keywords: KCa3.1, TRAM-34, coronary smooth muscle, balloon angioplasty, phenotypic modulation
Coronary smooth muscle cells (SMCs) are phenotypically identified and characterized by expression of smooth muscle specific marker genes, such as smooth muscle alpha actin (SMαA), smooth muscle myosin heavy chain (SMMHC), and smoothelin-B. Unlike other cell types, SMCs are not terminally differentiated and can alter their gene expression profile after both physiological and pathophysiological stimuli, a phenomenon defined as phenotypic modulation.1 During vasculoproliferative diseases, such as atherosclerosis and restenosis, smooth muscle cells undergo phenotypic modulation characterized by suppression of smooth muscle marker genes, increased proliferation, extracellular matrix synthesis, and migration.2–5 We previously demonstrated that upregulation of intermediate-conductance Ca2+-activated K+ channels (KCa3.1, IKCa1, encoded by the gene KCNN4), whose expression is regulated by both AP-1 (c-jun/c-fos)6 and repressor element -1 silencing transcription factor (REST),7 is necessary for mitogen-induced suppression of smooth muscle specific marker genes, as well as SMC migration in porcine coronary SMCs in vitro.6 Relevance to coronary disease was demonstrated by upregulation of KCa3.1 and decreased SMMHC in proliferating coronary SMCs in an in vivo swine model of early atherosclerosis.6 The therapeutic potential of KCa3.1 inhibitors was alluded to by the report that systemic delivery of TRAM-34 for 1 to 6 weeks decreased neointimal formation by ≈40% in the rat carotid injury model.8 Although this demonstrated a critical role for KCa3.1 in neointimal hyperplasia, it did not address the role of KCa3.1 in SMC phenotypic modulation per se. Additionally, the rat carotid injury model differs in etiology from coronary postangioplasty restenosis in humans in both vessel type (carotid versus coronary) and injury (lack of a medial tear).8–11 In contrast, the porcine coronary overstretch injury model, widely recognized as the most appropriate model for studying postangioplasty restenosis,11,12 produces a medial injury and development of a smooth muscle-rich neointima nearly identical to what is seen in humans.9–12 The purpose of the present study was to investigate the role of KCa3.1 in the regulation of coronary SMC phenotypic modulation in a swine model of postangioplasty restenosis. Furthermore, we chose to deliver TRAM-34,13,14 a specific KCa3.1 channel blocker,15 to the coronary vessel wall via coated balloon catheter, to allow site-specific delivery and potentially avoid complications of systemic TRAM-34 administration.
Materials and Methods
A detailed description of all methods is provided in the supplemental materials (available online at http://atvb.ahajournals.org).
Coronary Balloon Angioplasty
Angioplasty was performed on the left circumflex (LCX) and left anterior descending (LAD) coronary arteries of castrated male swine (27 to 47 kg; 6 to 8 months old). Animal protocols were approved by the University of Missouri Animal Care and Use Committee in accordance with the “Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research, and Training.” Either the LCX or LAD was injured with a noncoated balloon, whereas the remaining artery was injured with a TRAM-34 coated balloon (see supplemental Figure I). After angioplasty, swine recovered for either 2 hours (n=5), 2 days (n=5), 14 days (n=5), or 28 days (n=5). At the time of sacrifice, injured and noninjured segments of the LCX and LAD were isolated and either quickly frozen in liquid nitrogen (LN2) for subsequent laser capture microdissection and qRT-PCR or placed in paraformaldehyde for subsequent immunohistochemistry.
Balloon Coating
Balloons were inflated to 6 atm (nominal pressure), dipped in TRAM-34 (20 mg/mL in acetone) for 10 seconds, and dried for 1 minute. This cycle was repeated 3 times and followed by a final 5-minute drying time. The balloon was then carefully deflated and immediately guided to the coronary artery for balloon injury.
Laser Capture Microdissection and qRT-PCR
Laser capture microdissection of medial cells and qRT-PCR was performed as previously described.6,16 Target gene expression was normalized to 18S using the 2−ΔΔCT method.17
Detection of TRAM-34 in the Vessel Wall
Using high-performance liquid chromatography (HPLC)-MS, TRAM-34 was quantified by its base peak of 277 m/z (2-chlorotrityl fragment) and concentrations calculated with a 5-point calibration curve. The related compound TRAM-46 (base peak of 261 m/z, 2-fluorotrityl fragment) was used as an internal standard.
Statistics
All data are presented as mean±SE. One-way ANOVA was used for all group comparisons with posthoc comparisons where appropriate. Significance was defined as P<0.05.
Results
KCa3.1 Is Necessary for Smooth Muscle Phenotypic Modulation Postangioplasty
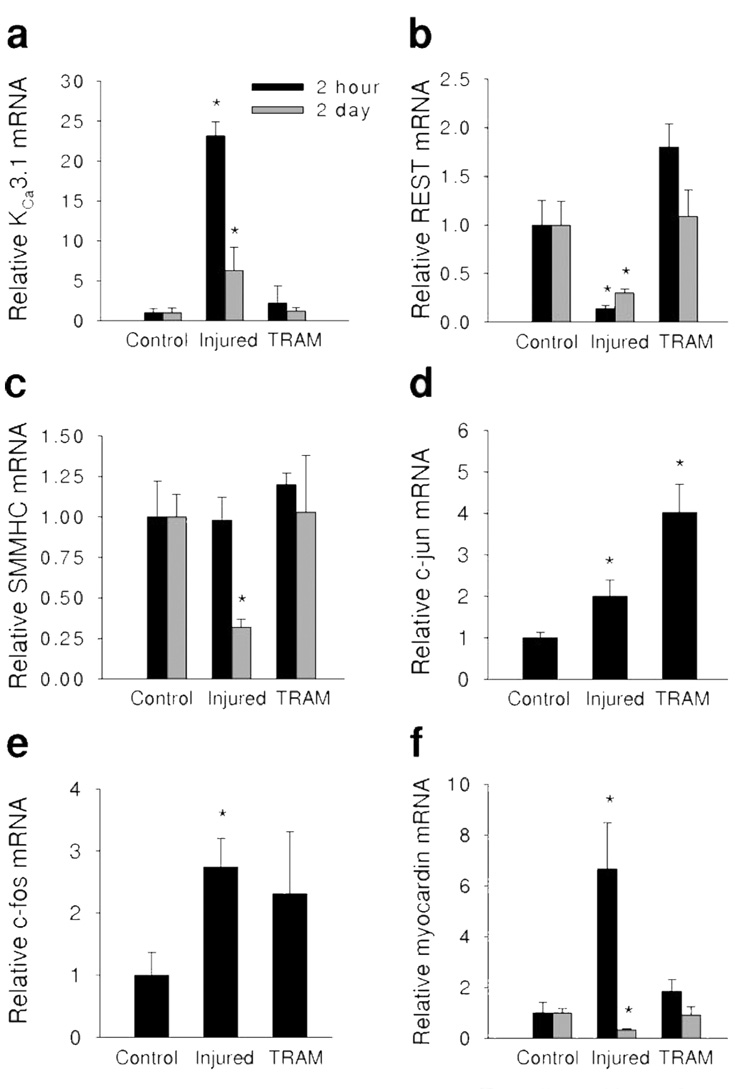
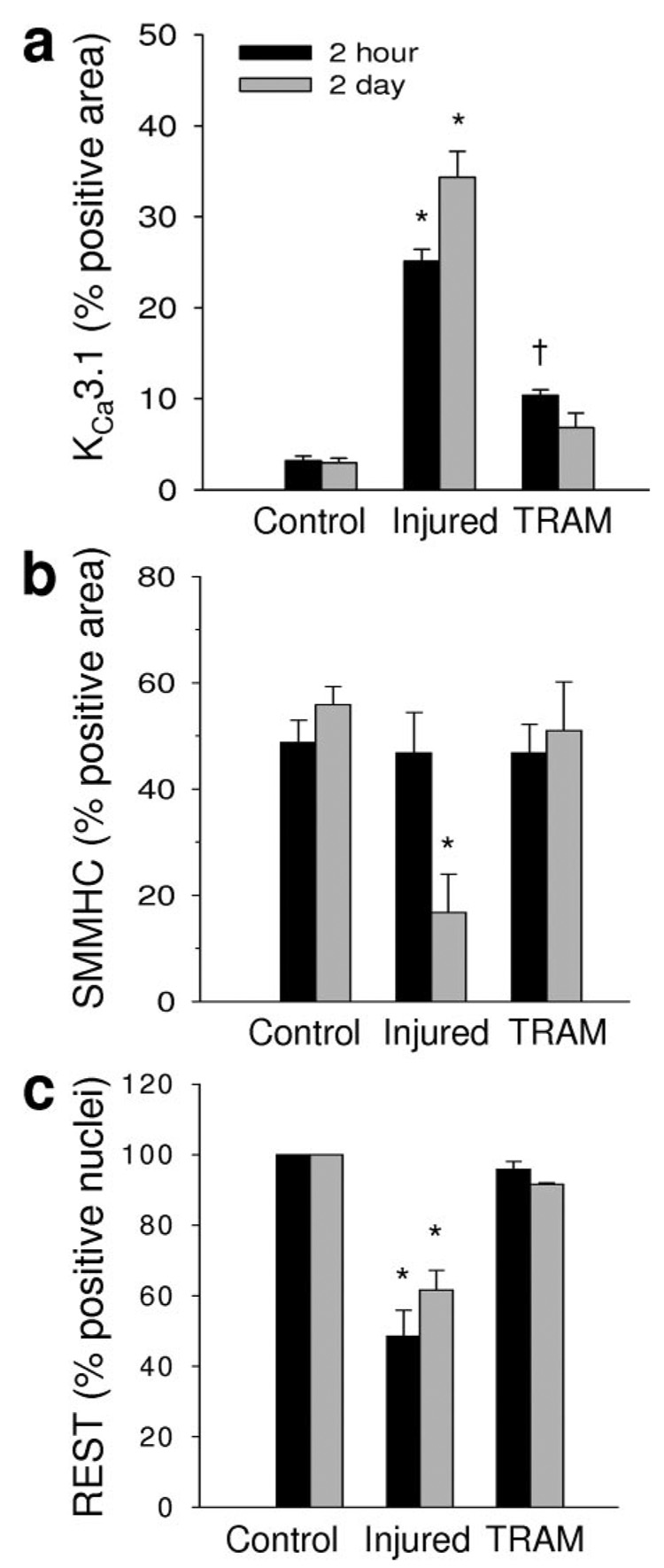
qRT-PCR was used to measure mRNA in coronary medial cells isolated by laser capture microdissection (Figure 1). Two hours postangioplasty (Figure 1 black bars), balloon injury increased KCa3.1 mRNA by ≈23 fold (Figure 1a) and decreased REST, a transcription factor which suppresses KCa3.1,7,18 by ≈5 fold (Figure 1b). TRAM-34 delivered via balloon catheter (TRAM) blocked the increase in KCa3.1 mRNA (Figure 1a) and the decrease in REST mRNA (Figure 1b) 2 hours postangioplasty. SMMHC mRNA was unchanged 2 hours after injury (Figure 1c). Two days postangioplasty (Figure 1 gray bars), KCa3.1 mRNA remained elevated (Figure 1a) while REST mRNA remained suppressed (Figure 1b), however the changes in mRNA were not as robust as 2 hours postangioplasty. In contrast to 2 hours postangioplasty, balloon injury suppressed SMMHC mRNA 2 days postangioplasty (Figure 1c). Importantly, TRAM-34 delivered via balloon catheter blocked the increase in KCa3.1 mRNA (Figure 1a), the suppression of REST (Figure 1b), and the loss of SMMHC (Figure 1c) mRNA two days postangioplasty. These data demonstrate that after balloon injury, changes in coronary smooth muscle KCa3.1 and REST precede alterations in SMMHC mRNA expression. Furthermore, TRAM-34 prevented postangioplasty-induced changes in KCa3.1, REST, and SMMHC, demonstrating that blockade of KCa3.1 can prevent phenotypic modulation in vivo.
Figure 1. TRAM-34 prevents angioplasty-induced phenotypic modulation.
Relative mRNA of KCa3.1 (a), REST (b), SMMHC (c), c-jun (d), c-fos (e), and myocardin (f) 2 hours (black bars) and 2 days (gray bars) postangioplasty in non-injured (Control), balloon injured (Injured), and TRAM-34–coated balloon injured (TRAM) coronary media. *P<0.05 vs corresponding control (n=4 to 5 per group).
Balloon injury also increased the AP-1 components, c-jun (Figure 1d) and c-fos (Figure 1e), 2 hours postangioplasty, consistent with previous findings in vitro demonstrating the role of AP-1 in regulating KCa3.1 expression in smooth muscle cells and T cells.6,19 Interestingly, TRAM-34 failed to prevent the upregulation of either c-jun or c-fos 2 hours postangioplasty (Figure 1d and 1e). Lastly, we observed a transient increase in myocardin mRNA 2 hours postangioplasty, before a decrease 2 days postangioplasty (Figure 1f), both of which were blocked by TRAM-34. Myocardin has previously been demonstrated to regulate SMMHC expression6,20–25 in vitro. Therefore, decreased myocardin as well as SMMHC mRNA 2 days postangioplasty are consistent with previous data demonstrating the role of myocardin in regulating SMMHC expression.20
Delivery of TRAM-34 Into the Vessel Wall
To validate TRAM-34 transfer to the coronary wall, we measured TRAM-34 in arteries harvested 2 hours, 2 days, and 14 days postangioplasty. TRAM-34 was detectable 2 hours (8.61±3.97 ng/mg; n=2) as well as 2 days (0.52±0.28 ng/mg; n=2) postangioplasty in coronary arterial segments injured with TRAM-34 coated balloons. By 14 days postangioplasty, only 0.7% of TRAM-34 detected 2 hours postangioplasty remained in the vessel wall (0.06 ng/mg; n=1). TRAM-34 was not detectable in plasma at any point. However, low levels were detected both 2 hours and 2 days postangioplasty in the liver (0.15 and 0.12 ng/mg, respectively), and noninjured right coronary artery (0.45 and 0.12 ng/mg, respectively) demonstrating that low levels of TRAM-34 were available systemically and accumulated in nontarget tissues, consistent with the highly lipophilic nature of TRAM-34.
Immunohistochemistry 2 Hours and 2 Days Postangioplasty
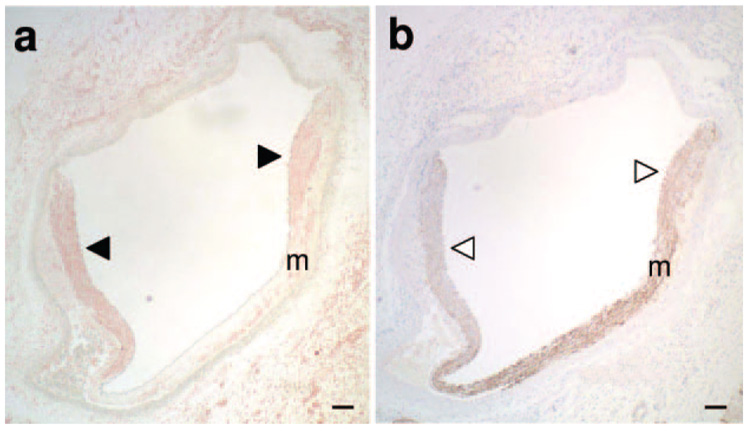
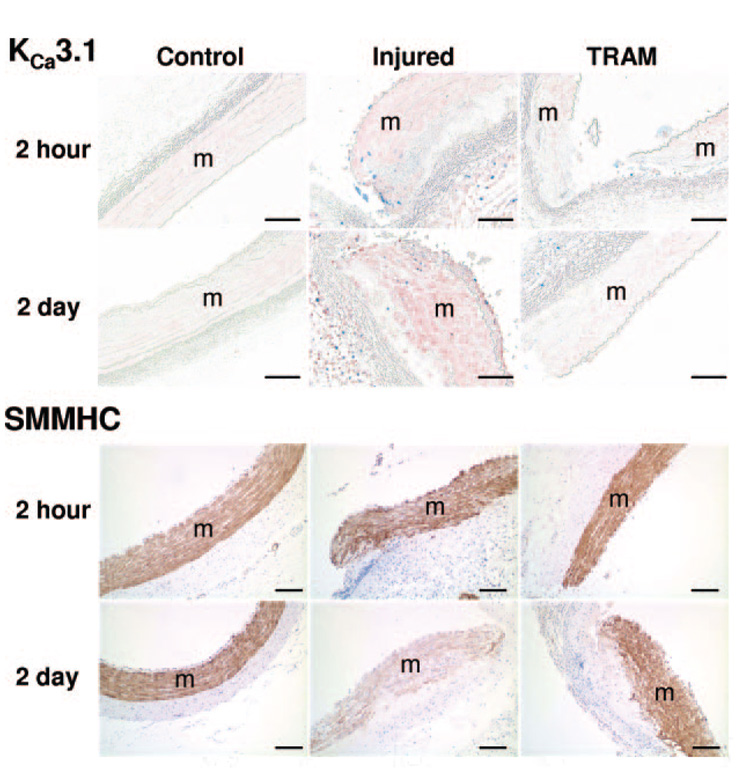
Balloon angioplasty increased KCa3.1 protein expression (pink; Figure 2a black arrowheads), and suppressed SMMHC protein expression (brown; Figure 2b white arrowheads) near the medial tear (m=media). Therefore all comparative images in Figure 3 and 4 were taken at the edge of the medial tear. Representative images demonstrate that compared to noninjured (Control), balloon injury (Injured) increased KCa3.1 positive staining (Figure 3, top) both 2 hours and 2 days postangioplasty, an effect which was blocked by TRAM-34 coated balloons (TRAM). Immunohistochemical analysis verified an ≈8-fold and an ≈12-fold increase in KCa3.1 protein at 2 hours and 2 days postangioplasty, respectively; an effect completely prevented by TRAM (Figure 5a). Consistent with SMMHC mRNA, representative images stained for SMMHC protein demonstrated that balloon injury reduced SMMHC levels near the medial tear 2 days after angioplasty, which was also prevented by the TRAM-34–coated balloon (Figure 3, bottom), whereas SMMHC staining was unaltered 2 hours postangioplasty. Immunohistochemical analysis demonstrated a 70% decrease in SMMHC protein 2 days postangioplasty, which was completely prevented by TRAM (Figure 5b). Double labeling with Ki-67 (blue, a marker of proliferating cells) and KCa3.1 (Figure 4 pink, top), indicated that whereas all Ki-67–positive cells were also KCa3.1-positive, the converse was not true, ie, not all KCa3.1-positive cells were proliferating.
Figure 2. KCa3.1 and SMMHC protein 2 days postangioplasty.
Representative coronary sections isolated 2 days postangioplasty stained for KCa3.1 (a, HRP, pink) or SMMHC (b, DAB, brown). KCa3.1 staining was more intense (a, black arrowheads), whereas SMMHC staining was more diffuse (b white arrowheads) near the medial tear. Horizontal bar=100 µm.
Figure 3. KCa3.1 and SMMHC histology 2 hours and 2 days postangioplasty.
Representative cross-sections (8 µm; 4 to 5 per group) of control, injured, and TRAM-34–coated balloon injured (TRAM) LCX and LAD 2 hours and 2 days postangioplasty exposed to antibodies against KCa3.1 (1:600, pink, top), Ki-67 (1:200, blue, top), and SMMHC (1:800, brown, bottom). Horizontal bar=100 µm.
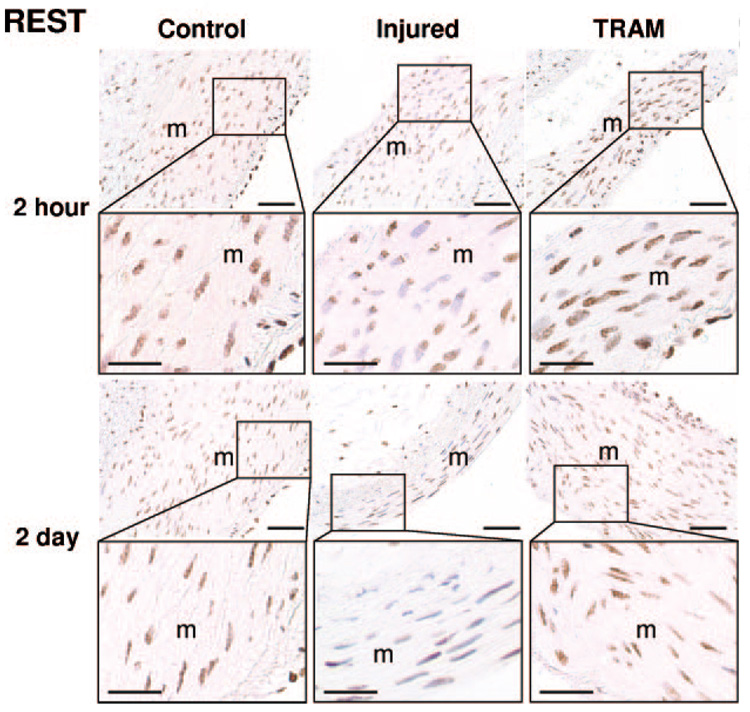
Figure 4. REST histology 2 hours and 2 days postangioplasty.
Representative cross-sections (8 µm; 4 to 5 per group) of control, injured, and TRAM-34–coated balloon injured (TRAM) LCX and LAD 2 hours and 2 days postangioplasty exposed to anti-REST (1:50,000, brown), and counterstained with hematoxylin (blue nuclei were considered REST negative). Horizontal bar=50 µm (25 µm in insets).
Figure 5. Histological analysis of KCa3.1, SMMHC, and REST.
KCa3.1 (a), SMMHC (b), and REST (c) staining were quantified using Image Pro Plus. Injury-induced changes in KCa3.1, SMMHC, and REST staining were blocked by TRAM-34 (see Figure 3 and Figure 4 for representative images). *P<0.05 vs respective control and †P<0.05 vs injured and control (n=4 to 5 per group).
Consistent with previous reports in vascular smooth muscle,7 we observed REST localized to the nucleus of coronary smooth muscle (Figure 4). In uninjured control coronary arteries, REST was ubiquitiously expressed in medial SMCs (100% REST-positive nuclei; Figure 4 and Figure 5c). Balloon injury significantly reduced the number of REST-positive cells by ≈50% 2 hours and ≈40% 2 days postangioplasty, which was blocked by TRAM-34 coated balloons (Figure 4 and Figure 5c).
TRAM-34–Coated Balloons Reduced Restenosis
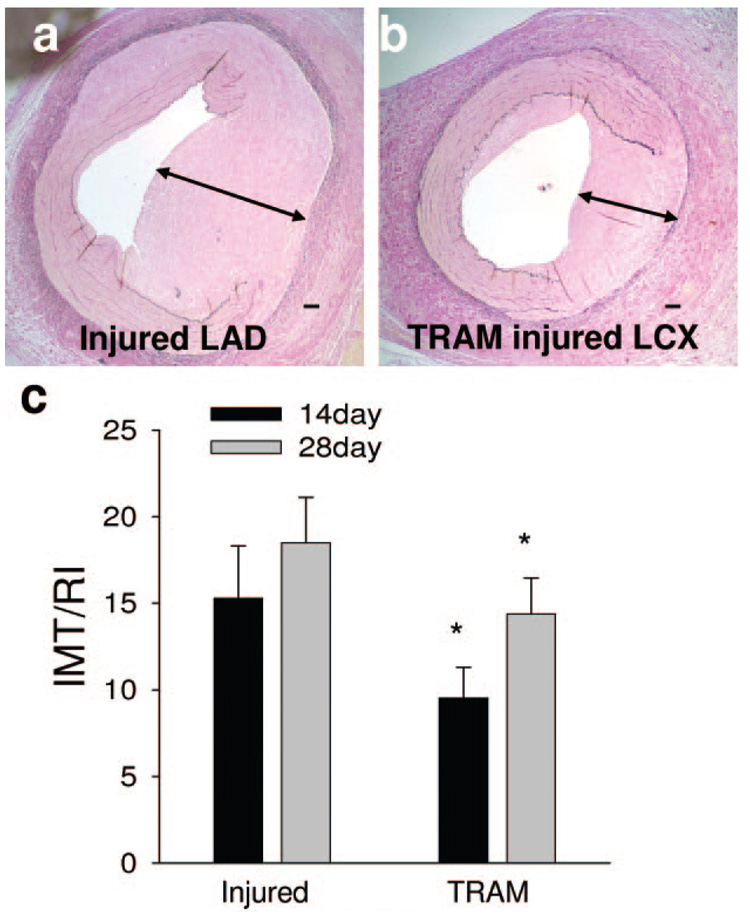
Coating balloons with TRAM-34 reduced the normalized intimal to medial thickness ratio (IMT/RI) compared to noncoated balloons within the same animal. Representative histological sections of segments injured with noncoated (Figure 6a) and TRAM-34–coated (Figure 6b) balloons taken 28 days postangioplasty demonstrate that vessels injured with TRAM-34–coated balloons had reduced neointima. Individual injury responses for TRAM-34 versus uncoated balloons are given in supplemental Figure II. Summary data demonstrate that coating balloons with TRAM-34 reduced postangioplasty restenosis by ≈38% and ≈22% at 14 and 28 days, respectively (Figure 6c), similar to what was previously published in the rat carotid injury model.8 Acetone (vehicle)-coated balloons had similar IMT/RI (14.61±3.4) to the noncoated balloons (15.59±2.7).
Figure 6. TRAM-34 prevents restenosis 14 and 28 days postangioplasty.
Representative coronary arteries injured with a noncoated (a) or TRAM-34 coated balloon (b) 28 days postangioplasty and stained with Verhoff van Giesen stain (VVG). TRAM-34 reduced normalized intimal to medial thickness ratio (IMT/RI) at both 14 (38%) and 28 (22%) days postangioplasty (c). *P<0.05 vs injured segment taken from the same animal (paired t test; n=5). Horizontal bar=100 µm.
Discussion
Recent studies have illustrated that the regulation of smooth muscle–specific marker gene expression, and thus SMC phenotypic modulation, is largely dependent on ion channel activity6,24; aka, excitation-transcription coupling.26,27 This study investigated the role of KCa3.1 and its specific blocker TRAM-34 on excitation-transcription coupling in porcine coronary SMC phenotypic modulation after balloon angioplasty. We also tested a new delivery system for TRAM-34 via coated balloon catheters demonstrating that TRAM-34 was retained in the vessel wall, effectively prevented injury induced decreases in SMMHC gene expression, and reduced subsequent restenosis.
For these studies, we chose the porcine overstretch injury model because it is the closest nonprimate model to human postangioplasty restenosis.11,12 Not only do swine have similar coronary vessel anatomy (see supplemental Figure I) and medial thickness, but the neointima that develops in response to injury is nearly identical to what is seen in humans.10–12,28–30 Furthermore, it allows use of standard balloons, catheters, and other equipment designed for human coronary angioplasty. Therefore, the results obtained using the porcine model of postangioplasty restenosis provide the best translational validation for human coronary artery disease and restenosis treatments.
This study is the first to investigate the role of KCa3.1 and smooth muscle phenotypic modulation during coronary postangioplasty restenosis. We demonstrated that balloon injury robustly increased KCa3.1 and decreased REST expression 2 hours postangioplasty (Figure 1a and 1b). Others have also demonstrated suppression of REST expression concurrent with increased KCa3.1 expression.7 Interestingly, the present study demonstrated that TRAM-34 delivered via balloon catheter not only blocked injury-induced KCa3.1 upregulation, but also the suppression of REST. It has previously been shown that delivery of the silencing transcription factor, REST, to proliferating cells represses KCa3.1 expression,7 demonstrating that REST regulates KCa3.1 expression.18 Our data suggests that expression of REST, in turn, is regulated by KCa3.1 channel activity, as inhibition of KCa3.1 channel activity by TRAM-34 prevented the downregulation of REST postangioplasty. It is plausible that the initial hyperpolarization or consequent potentiation of passive calcium entry attributable to KCa3.1 activity drives REST downregulation; however, this remains speculation, in part, because of the lack of information on regulation of REST expression.31
Suppression of REST by KCa3.1 channel activity is, however, consistent with a positive, feed forward mechanism for upregulation of KCa3.1 expression by KCa3.1 activity previously observed in coronary smooth muscle cells in culture.6 We provide further support for this model in the present study in vivo, where TRAM-34 also blocked upregulation of KCa3.1 mRNA postangioplasty.
A common downstream mechanism for increased KCa3.1 expression is likely via AP-1. In vitro PDGF-BB induced upregulation of KCa3.1 mRNA, as well as binding of c-jun to the 5′ AP-1 binding site of the KCa3.1 promoter were blocked by TRAM-34.6 Both c-jun and c-fos are components of the cis-binding element, AP-1,32 and the KCa3.1 promoter contains several AP-1 binding sites.19,33 In the present study in vivo, both c-jun and c-fos were increased 2 hours postangioplasty, in association with the increase in KCa3.1 expression. However, although TRAM-34 blocked injury-induced upregulation of KCa3.1 mRNA, it did not prevent increases in c-fos or c-jun mRNA. The reason for this apparent dissociation of c-fos/c-jun from KCa3.1 is not certain. However, our previous observations that TRAM-34 prevented PDGF-BB–induced enrichment of the KCa3.1 promoter with c-jun and acetylated histone-4 (H4Ac) indicate that TRAM-34 may prevent activation of the KCa3.1 promoter at the epigenetic level, ie, by preventing histone aceytlation and promoter availablity, rather than preventing increases in c-fos/c-jun mRNA. Further investigation will be required to fully delineate this mechanism.
A primary goal of the present study was to determine whether KCa3.1 regulates coronary smooth muscle phenotypic modulation in an in vivo model of postangioplasty restenosis. To this end we clearly demonstrated that blockade of KCa3.1 could prevent postangioplasty phenotypic modulation of coronary SMCs, as indicated by SMMHC gene expression. Balloon injury decreased SMMHC gene expression 2 days postangioplasty, which was associated with a simultaneous decrease in myocardin expression. This result was expected because of previously published data demonstrating the role of myocardin in regulating SMMHC transcription,20–26 and is consistent with reports in the mouse carotid injury model where myocardin expression was not significantly decreased until 3 days after injury.34 Interestingly, the decrease in myocardin mRNA seen at 2 days postangioplasty was preceded by a transient increase 2 hours postangioplasty, a time when SMMHC mRNA is unchanged. This “uncoupling” of myocardin from SMMHC expression has been reported previously and proposed to result from the opposing interaction of myocardin with repressor trans-acting factors. For example, Doi et al observed an elevation in HERP1 and myocardin in cultured SMCs as well as in neointimal cells after rat aortic balloon injury, and overexpression of HERP1 prevented myocardin-induced smooth muscle gene expression.35 Furthermore, NOTCH signaling suppresses myocardin-induced smooth muscle gene expression36 by targeting HERP1.35 Therefore, it is plausible that repressor trans-acting factors, eg, HERP1, were also increased 2 hours postangioplasty, and prevented myocardin-induced SMMHC expression.
We also examined protein levels associated with coronary balloon angioplasty to determine whether they were consistent with changes in mRNA. Alterations in medial KCa3.1, REST, and SMMHC protein as assessed by immunohistochemistry were consistent with mRNA levels determined by qRT-PCR. Additionally, it is important to note that although many of the cells near the medial tear of injured vessels stained positive for KCa3.1 (Figure 3, top), not all of those cells were positive for Ki-67, a marker of proliferation. Thus, not all cells expressing KCa3.1 are concurrently proliferating, although the majority of proliferating medial cells express KCa3.1. These data are consistent with a model whereby coronary SMCs upregulate KCa3.1 before entering the cell cycle, evidenced by inhibition of SMC proliferation by TRAM-34.8
Although the focus of the present study was to determine the role of KCa3.1 in early gene expression changes postangioplasty, we also determined whether acute, localized balloon catheter delivery of TRAM-34 was effective at inhibiting subsequent neointimal lesion formation. Local balloon catheter delivery of TRAM-34 inhibited neointimal formation by 38% at 14 days and 22% at 28 days postangioplasty, similar to the 40% reduction in intimal thickening reported with daily systemic administration of TRAM-34 in a rat carotid injury model.8 Thus, the present study provides translational validation by demonstrating similar effects in the swine model of postangioplasty coronary restenosis, the industry standard for preclinical coronary intervention. Although the development of drug eluting stents has revolutionized interventional cardiology and effectively eliminated early restenosis, recent evidence has demonstrated that patients with drug-eluting stents have higher rates of late restenosis as well as late thrombosis,37 leading some38 cardiologists to advocate use of bare metal stents or balloon angioplasty coupled with alternative drug delivery methods.37,39 Sheller et al demonstrated that paclitaxel delivered via coated balloon catheter significantly reduced restenosis,39 prompting a call to pursue drug delivery by coated balloon catheter.37,38 The present study is the first to report that catheter-based delivery of TRAM-34,14 a specific KCa3.1 channel blocker,13,15 prevents phenotypic modulation of SMCs immediately after coronary angioplasty and limits subsequent restenosis, thus implicating KCa3.1 as a therapeutic target in coronary vasculoproliferative disease.
Supplementary Material
Acknowledgments
We thank Jan Ivey, Rebecca Shaw, and Jennifer Casati for their substantial technical assistance and David Beech and Ian Wood for critical reading of the manuscript.
Sources of Funding
This work was funded by NIH HL 52490 and NASA.
Footnotes
The online version of this article, along with updated information and services, is located on the World Wide Web at: http://atvb.ahajournals.org
This is an un-copyedited author manuscript that was accepted for publication in Arteriosclerosis, Thrombosis, and Vascular Biology, copyright The American Heart Association. This may not be duplicated or reproduced, other than for personal use or within the “Fair Use of Copyrighted Materials” (section 107, title 17, U.S. Code) without prior permission of the copyright owner, The American Heart Association. The final copyedited article, which is the version of record, can be found at Artiosclerosis, Thrombosis, and Vascular Biology. The American Heart Association disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties.
Disclosures
None.
References
- 1.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 2.Layne MD, Yet SF, Maemura K, Hsieh CM, Liu X, Ith B, Lee ME, Perrella MA. Characterization of the mouse aortic carboxypeptidase-like protein promoter reveals activity in differentiated and dedifferentiated vascular smooth muscle cells. Circ Res. 2002;90:728–736. doi: 10.1161/01.res.0000013289.97650.c8. [DOI] [PubMed] [Google Scholar]
- 3.Regan CP, Adam PJ, Madsen CS, Owens GK. Molecular mechanisms of decreased smooth muscle differentiation marker expression after vascular injury. J Clin Invest. 2000;106:1139–1147. doi: 10.1172/JCI10522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bar H, Wende P, Watson L, Denger S, van Eys G, Kreuzer J, Jahn L. Smoothelin is an indicator of reversible phenotype modulation of smooth muscle cells in balloon-injured rat carotid arteries. Basic Res Cardiol. 2002;97:9–16. doi: 10.1007/s395-002-8382-z. [DOI] [PubMed] [Google Scholar]
- 5.Nagai R, Suzuki T, Aizawa K, Miyamoto S, Amaki T, Kawai-Kowase K, Sekiguchi KI, Kurabayashi M. Phenotypic modulation of vascular smooth muscle cells: dissection of transcriptional regulatory mechanisms. Ann N Y Acad Sci. 2001;947:56–67. doi: 10.1111/j.1749-6632.2001.tb03930.x. [DOI] [PubMed] [Google Scholar]
- 6.Tharp DL, Wamhoff BR, Turk JR, Bowles DK. Upregulation of intermediate-conductance Ca2+-activated K+ channel (IKCa1) mediates phenotypic modulation of coronary smooth muscle. Am J Physiol Heart Circ Physiol. 2006;291:H2493–H2503. doi: 10.1152/ajpheart.01254.2005. [DOI] [PubMed] [Google Scholar]
- 7.Cheong A, Bingham AJ, Li J, Kumar B, Sukumar P, Munsch C, Buckley NJ, Neylon CB, Porter KE, Beech DJ, Wood IC. Downregulated REST transcription factor is a switch enabling critical potassium channel expression and cell proliferation. Molecular Cell. 2005;20:45–52. doi: 10.1016/j.molcel.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 8.Kohler R, Wulff H, Eichler I, Kneifel M, Neumann D, Knorr A, Grgic I, Kampfe D, Si H, Wibawa J, Real R, Borner K, Brakemeier S, Orzechowski HD, Reusch HP, Paul M, Chandy KG, Hoyer J. Blockade of the intermediate-conductance calcium-activated potassium channel as a new therapeutic strategy for restenosis. Circulation. 2003;108:1119–1125. doi: 10.1161/01.CIR.0000086464.04719.DD. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz RS, Murphy JG, Edwards WD, Camrud AR, Vlietsra RE, Holmes DR. Restenosis after balloon angioplasty - a practical proliferative model in porcine coronary arteries. Circulation. 1990;82:2190–2200. doi: 10.1161/01.cir.82.6.2190. [DOI] [PubMed] [Google Scholar]
- 10.Carter AJ, Laird J, Farb A, Kufs W, Wortham DC, Virmani R. Morphologic characteristics of lesion formation and time course of smooth muscle cell proliferation in a porcine proliferative restenosis model. J Am Coll Cardiol. 1994;24:1398–1405. doi: 10.1016/0735-1097(94)90126-0. [DOI] [PubMed] [Google Scholar]
- 11.Kantor B, Ashai K, David R, Schwartz RS. The experimental animal models for assessing treatment of restenosis. Cardiovasc Rad Med. 1999;1:48–54. doi: 10.1016/s1522-1865(98)00005-5. [DOI] [PubMed] [Google Scholar]
- 12.Touchard AG, Schwartz RS. Preclinical restenosis models: challenges and successes. Toxicol Pathol. 2006;34:11–18. doi: 10.1080/01926230500499407. [DOI] [PubMed] [Google Scholar]
- 13.Wulff H, Gutman GA, Cahalan MD, Chandy KG. Delineation of the Clotrimazole/TRAM-34 binding site on the intermediate conductance calcium-activated potassium channel, IKCa1. J Biol Chem. 2001;276:32040–32045. doi: 10.1074/jbc.M105231200. [DOI] [PubMed] [Google Scholar]
- 14.Wulff H, Kolski-Andreaco A, Sankaranarayanan A, Sabatier JM, Shakkottai V. Modulators of small- and intermediate-conductance calcium-activated potassium channels and their therapeutic indications. Curr Med Chem. 2007;14:1437–1457. doi: 10.2174/092986707780831186. [DOI] [PubMed] [Google Scholar]
- 15.Wulff H, Miller MJ, Hansel W, Grissmer S, Cahalan MD, Chandy KG. Design of a potent and selective inhibitor of the intermediate-conductance Ca2+-activated K+ channel, IKCa1: A potential immunosuppressant. Proc Natl Acad Sci U S A. 2000;97:8151–8156. doi: 10.1073/pnas.97.14.8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowles DK, Maddali KK, Ganjam VK, Rubin LJ, Tharp DL, Turk JR, Heaps CL. Endogenous testosterone increases L-type Ca2+ channel expression in porcine coronary smooth muscle. Am J Physiol Heart Circ Physiol. 2004;287:H2091–H2098. doi: 10.1152/ajpheart.00258.2004. [DOI] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Cheong A, Wood IC, Beech DJ. Less REST, more vascular disease? Regulation of cell cycle and migration of vascular smooth muscle cells. Cell Cycle. 2006;5:129–131. doi: 10.4161/cc.5.2.2310. [DOI] [PubMed] [Google Scholar]
- 19.Ghanshani S, Wulff H, Miller MJ, Rohm H, Neben A, Gutman GA, Cahalan MD, Chandy KG. Up-regulation of the IKCa1 Potassium channel during T-cell activation: molecular mechanism and functional consequences. J Biol Chem. 2000;275:37137–37149. doi: 10.1074/jbc.M003941200. [DOI] [PubMed] [Google Scholar]
- 20.Chen J, Kitchen CM, Streb JW, Miano JM. Myocardin: a component of a molecular switch for smooth muscle differentiation. J Mol Cell Cardiol. 2002;34:1345–1356. doi: 10.1006/jmcc.2002.2086. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida T, Sinha S, Dandre F, Wamhoff BR, Hoofnagle MH, Kremer BE, Wang DZ, Olson EN, Owens GK. Myocardin is a key regulator of CArG-dependent transcription of multiple smooth muscle marker genes. Circ Res. 2003;92:856–864. doi: 10.1161/01.RES.0000068405.49081.09. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z, Wang DZ, Pipes GCT, Olson EN. Myocardin is a master regulator of smooth muscle gene expression. Proc Natl Acad Sci U S A. 2003;100:7129–7134. doi: 10.1073/pnas.1232341100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li S, Wang DZ, Wang Z, Richardson JA, Olson EN. The serum response factor coactivator myocardin is required for vascular smooth muscle development. Proc Natl Acad Sci U S A. 2003;100:9366–9370. doi: 10.1073/pnas.1233635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wamhoff BR, Bowles DK, McDonald OG, Sinha S, Somlyo AP, Somlyo AV, Owens GK. L-type voltage-gated Ca2+ channels modulate expression of smooth muscle differentiation marker genes via a Rho kinase/myocardin/SRF-dependent mechanism. Circ Res. 2004;95:406–414. doi: 10.1161/01.RES.0000138582.36921.9e. [DOI] [PubMed] [Google Scholar]
- 25.Du KL, Ip HS, Li J, Chen M, Dandre F, Yu W, Lu MM, Owens GK, Parmacek MS. Myocardin Is a critical serum response factor cofactor in the transcriptional program regulating smooth muscle cell differentiation. Mol Cell Biol. 2003;23:2425–2437. doi: 10.1128/MCB.23.7.2425-2437.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wamhoff BR, Bowles DK, Owens GK. Excitation-transcription coupling in arterial smooth muscle. Circ Res. 2006;98:868–878. doi: 10.1161/01.RES.0000216596.73005.3c. [DOI] [PubMed] [Google Scholar]
- 27.Barlow CA, Rose P, Pulver-Kaste RA, Lounsbury KM. Excitation-transcription coupling in smooth muscle. J Physiol (Lond) 2006;570:59–64. doi: 10.1113/jphysiol.2005.098426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwartz RS, Topol EJ, Serruys PW, Sangiorgi G, Holmes DR. Artery size, neointima, and remodeling: time for some standards. J Am Coll Cardiol. 1998;32:2087–2094. doi: 10.1016/s0735-1097(98)00500-2. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz RS, Holder DJ, Holmes DR, Jr, Veinot JP, Camrud AR, Jorgenson MA, Johnson RG., Jr Neointimal thickening after severe coronary artery injury is limited by short-term administration of a factor Xa Inhibitor: results in a porcine model. Circulation. 1996;93:1542–1548. doi: 10.1161/01.cir.93.8.1542. [DOI] [PubMed] [Google Scholar]
- 30.Turk JR, Laughlin MH. Physical activity and atherosclerosis: which animal model? Can J Appl Physiol. 2004;29:657–683. doi: 10.1139/h04-042. [DOI] [PubMed] [Google Scholar]
- 31.Ooi L, Wood IC. Chromatin crosstalk in development and disease: lessons from REST. Nat Rev Genet. 2007;8:544–554. doi: 10.1038/nrg2100. [DOI] [PubMed] [Google Scholar]
- 32.Curran T, Franza BR., Jr Fos and Jun: the AP-1 connection. Cell. 1988;55:395–397. doi: 10.1016/0092-8674(88)90024-4. [DOI] [PubMed] [Google Scholar]
- 33.Neylon CB, Lang RJ, Fu Y, Bobik A, Reinhart PH. Molecular cloning and characterization of the intermediate-conductance Ca2+-activated K+ channel in vascular smooth muscle: relationship between KCa channel diversity and smooth muscle cell function. Circ Res. 1999;85:e33–e343. doi: 10.1161/01.res.85.9.e33. [DOI] [PubMed] [Google Scholar]
- 34.Hendrix JA, Wamhoff BR, McDonald OG, Sinha S, Yoshida T, Owens GK. 5′ CArG degeneracy in smooth muscle α-actin is required for injury-induced gene suppression in vivo. J Clin Invest. 2005;115:418–427. doi: 10.1172/JCI22648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doi H, Iso T, Yamazaki M, Akiyama H, Kanai H, Sato H, Kawai-Kowase K, Tanaka T, Maeno T, Okamoto Ei, Arai M, Kedes L, Kurabayashi M. HERP1 inhibits myocardin-induced vascular smooth muscle cell differentiation by interfering with SRF binding to CArG box. Arterioscler Thromb Vasc Biol. 2005;25:2328–2334. doi: 10.1161/01.ATV.0000185829.47163.32. [DOI] [PubMed] [Google Scholar]
- 36.Proweller A, Pear WS, Parmacek MS. Notch signaling represses myocardin-induced smooth muscle cell differentiation. J Biol Chem. 2005;280:8994–9004. doi: 10.1074/jbc.M413316200. [DOI] [PubMed] [Google Scholar]
- 37.Camenzind E. Treatment of in-stent restenosis - back to the future? N Engl J Med. 2006;355:2149–2151. doi: 10.1056/NEJMe068215. [DOI] [PubMed] [Google Scholar]
- 38.Scheller B, Speck U, Bohm M. Prevention of restenosis: is angioplasty the answer? Heart. 2007;93:539–541. doi: 10.1136/hrt.2007.118059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scheller B, Hehrlein C, Bocksch W, Rutsch W, Haghi D, Dietz U, Bohm M, Speck U. Treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. N Engl J Med. 2006;355:2113–2124. doi: 10.1056/NEJMoa061254. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.