Abstract
Several approaches have been taken for these in vivo studies. In many studies, the use of semi-quantitative immuno-electron microscopy is the approach of choice. Endogenous opioid receptors display differential subcellular distributions with µ opioid receptor (MOPR) being mostly present on the plasma membrane and δ- and κ-opioid receptors (DOPR and KOPR, respectively) having a significant intracellular pool. Etorphine and DAMGO cause endocytosis of the MOPR, but morphine does not, except in some dendrites. Interestingly, chronic inflammatory pain and morphine treatment promote trafficking of intracellular DOPR to the cell surface which may account for the enhanced antinociceptive effects of DOPR agonists. KOPR has been reported to be associated with secretory vesicles in the posterior pituitary and translocated to the cell surface upon salt loading along with the release of vasopressin. The study of endogenous opioid receptors using in vivo models has produced some interesting results that could not have been anticipated in vitro. In vivo studies, therefore, are essential to provide insight into the mechanisms underlying opioid receptor regulation.
Keywords: Opioid receptors, Electron microscopy, Confocal microscopy, in vivo trafficking, Internalization, Review
Introduction
Opioid receptors belong to the seven-transmembrane receptor superfamily and are coupled with Gi/o proteins. Three types of opioid receptors have been cloned, µ- , δ- and κ-opioid receptor (MOPR, DOPR and KOPR, respectively).
Opioid receptors can be activated by a variety of naturally occurring or synthetic opiates and several endogenous neuropeptides. When the opioid receptors are activated upon binding of these ligands, a common regulatory event involves internalization of the receptor from the cell surface to intracellular sites. Agonist-induced endocytosis of opioid receptors has been studied extensively in cell models. Briefly, following binding of agonists to opioid receptors on plasma membranes, receptors undergo conformational changes leading to activation of G proteins and translocation of G protein-coupled receptor kinases to the cell surface resulting in phosphorylation of the receptors. β-arrestins are recruited to the phosphorylated receptors, which are subsequently endocytosed via a clathrin-dependent pathway. The decrease in the numbers of cell surface opioid receptors may be an adaptive process to avoid over-stimulation and may account in part for tolerance to opioids. Internalized opioid receptors are either recycled back to cell surface, resulting in re-sensitization of the receptors or sorted to degradation pathways, leading to down-regulation (Liu-Chen 2004; von Zastrow et al. 2003).
Like endocytosis, trafficking of opioid receptors to the cell surface may also be regulated. In dissociated dorsal root ganglion neurons, DOPR is sorted into large dense-core vesicles through interaction with protachykinin (Guan et al. 2005). Activation of surface DOPR causes elevation of intracellular Ca2+ mostly via an inositol triphosphate-dependent mechanism that results in insertion of large dense-core vesicles-associated DOPR onto the cell surface (Bao et al. 2003). Another mechanism leading to an increase of opioid receptors on the cell surface is the pharmacological chaperone effects of opioid ligands. In cells transfected with opioid receptors, cell-permeant opioid ligands promote endoplasmic reticulum-to-Golgi trafficking of opioid receptors to enhance cell surface expression by facilitating correct folding of the newly synthesized receptors at the endoplasmic reticulum (Chen et al. 2006; Petaja-Repo et al. 2002; Wannemacher et al. 2007; Chaipatikul et al. 2003).
Most studies on opioid receptor trafficking were carried out in various in vitro cell models. The limitations of these models are obvious, including differences in cellular milieu and receptor expression levels. In this review, observations regarding in vivo trafficking of opioid receptors will be presented. While some findings are consistent with in vitro results, others are unanticipated.
Consideration of methods and approaches for subcellular localization of opioid receptors in vivo
Each type of opioid receptor has a distinct distribution in the central nervous system as revealed by receptor autoradiography studies (Mansour et al. 1988) and immunohistochemical approaches (Arvidsson et al. 1995a, b). Some regions are abundantly enriched in opioid receptors and these include the striatum, the locus coeruleus, the ventral tegmental area and the dorsal horn of the spinal cords. Therefore, these regions are commonly used for studies on endogenous opioid receptors.
The use of receptor autoradiography and electron microscopy was employed in the 1980s and 1990s (Moyse et al. 1997) where the opioid receptor ligands were labeled with 125I. The localization of opioid receptors was detected using silver grains scattered by the radioactivity of the bound radioligands. Although a useful approach at the time, this technique fell out of favor with the availability of specific antibodies that recognize each type of opioid receptors. Immunohistochemistry combined with confocal microscopy is another useful approach. Although confocal microscopes are more accessible than electron microscopes in most laboratory settings, the resolution of the former is much lower than that provided by the latter. Combining transmission electron microscopy with immunogold or immunoperoxidase labeling provides a high-resolution technique for the study of the subcellular distribution of endogenous opioid receptors in brain tissue. Although a more sensitive labeling approach, immunoperoxidase labeling is not as readily quantifiable for subcellular distribution as the labeling tends to be diffuse and has propensity to adsorb to membrane structures (Novikoff et al. 1972). In contrast, immunogold labeling is quantifiable generally by counting the sliver grains. Therefore, immunogold labeling is a major approach to quantify the subcellular localization of opioid receptors.
Importantly, it can not be over-emphasized that, with all immunohistochemical approaches, the validity of the results largely depends on the specificity and affinity of the antibodies. Specific antibodies recognizing each type of opioid receptors are available and have been characterized by different groups using complementary approaches.
Additional approaches have been used to investigate trafficking of epitope-tagged receptors artificially introduced into animals. A mouse line expressing DOPR tagged with enhanced green fluorescent protein (EGFP) at the C-terminus has been established using the gene targeting approach and allows examination of whether there is a correlation between receptor trafficking and in vivo pharmacology end points (Scherrer et al. 2006). Generation of such a knock-in mouse line is time-consuming and costly.
In another approach, exogenous opioid receptors, with epitope tags, have been introduced into and expressed in certain brain regions by use of viral vectors (Haberstock-Debic et al. 2003). Trafficking studies are carried out in a more physiological environment than in primary neurons. This review does not cover the findings from such an approach.
Differential subcellular localization of endogenous opioid receptors
MOPR
Several lines of evidence indicate that, irrespective of the brain region, the MOPR is mostly localized to plasma membranes (Fig. 1). For example, in the rat habenular nucleus, confocal microscopy has shown that MOPR immunoreactivity is associated primarily with plasma membranes of neurons (Keith et al. 1998). Using immunogold labeling combined with electron microscopy, Van Bockstaele and Commons (2001) showed that about 90% of MOPR immunoreactivity was located along the plasma membrane of somatodendritic processes in the rat locus coeruleus . MOPR has been shown to have a similar subcellular distribution in the striatal patches: 80% and 60% located on plasma membranes of dendritic spines and axon terminals, respectively (Wang and Pickel 2001). In the rat ventral tegmental area, immunogold labeled MOPR was seen on plasma membranes of dendrites and axon terminals (Garzon and Pickel 2001). In the dorsal horn of rat spinal cord, most of the peroxidase-labeled MOPR was associated with postsynaptic membranes of dendrites (Wang et al. 2003). Surprisingly, the majority of immunogold-labeled MOPR (> 70%) was found in cytoplasm of the dendrites of C1 adrenergic neurons in the rat rostral ventrolateral medulla (Drake et al. 2005). It may reflect the differential subcellular distribution of MOPR in brain regions.
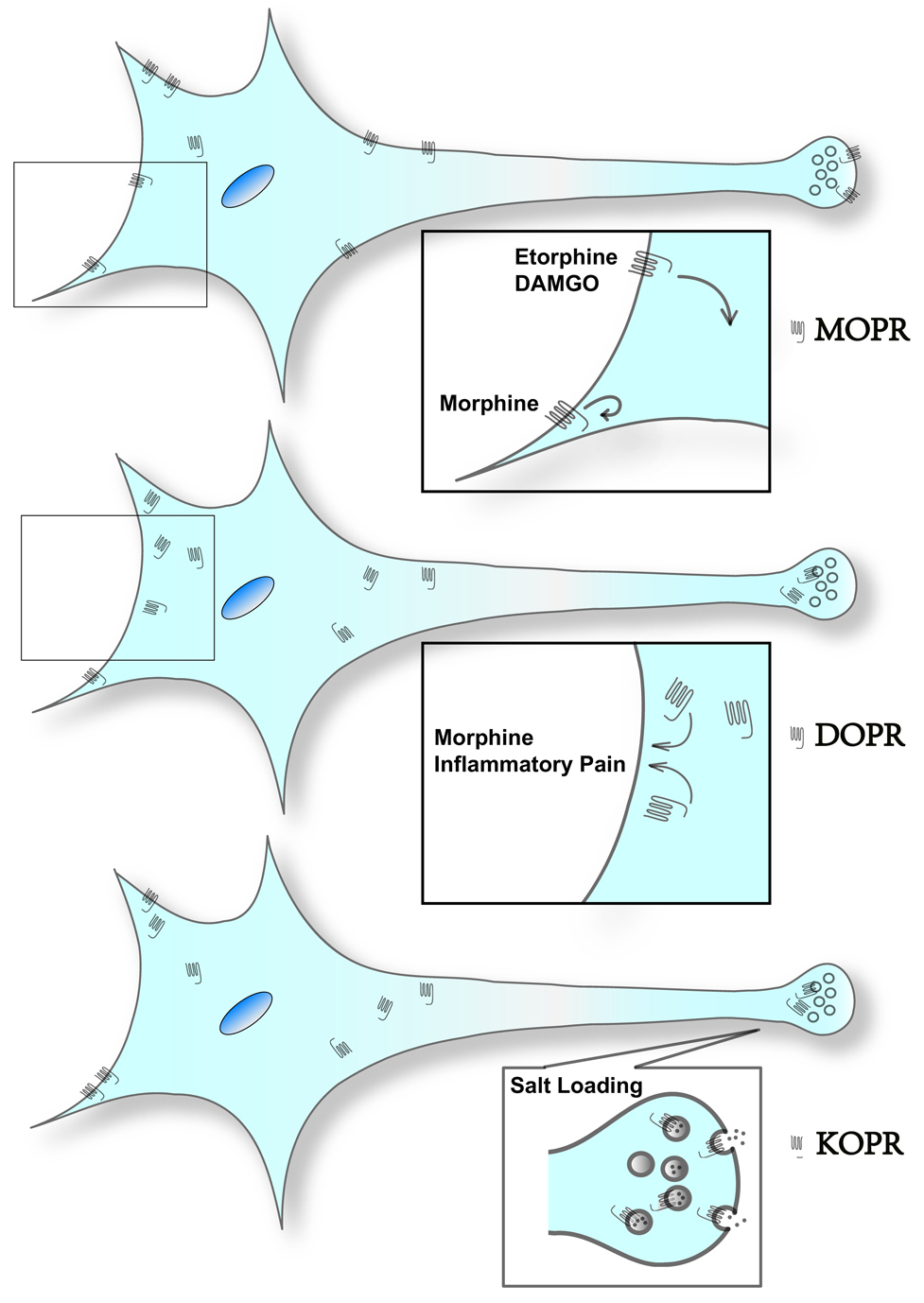
Fig. 1. Illustration on subcellular distribution of endogenous opioid receptors and their trafficking upon stimulation in vivo.
The figures are based on the observations using immuno-electron microscopy.
Upper panel, MOPR is predominantly present on cell surface. MOPR is internalized following treatment with etorphine or DAMGO, but not morphine, in spinal cord, myenteric plexus and several brain regions. Morphine causes internalization of MOPR in the dendrites, but not in the cell body, in the nucleus accumbens.
Middle panel, DOPR is mostly intracellular. Pretreatment with morphine or chronic inflammatory pain enhances trafficking of intracellular DOPR to cell surface in the spinal cord. See text for the findings on DOPR-EGFP mice.
Lower panel, KOPR has a significant intracellular pool. In the posterior pituitary, salt loading promotes the insertion of KOPR on vasopressin-containing vesicles into cell surface of axon terminals.
DOPR
In contrast to the high percentage of MOPR associated with neuronal membranes, DOPR immunolabeling is typically located intracellularly (Fig. 1). Electron microscopic analysis revealed that 80–90% of immunogold-labeled DOPR was found within the cytoplasm of rat spinal cord dorsal horn dendrites (Cahill et al. 2001a, b). Further, this pattern is similar in striatal patches (Wang and Pickel 2001) where the prevalence of the intracellular distribution is even more apparent in perikarya (Cahill et al. 2001a). In the ventral division of the reticular oral pontine nucleus of the cat, the majority of DOPR immunoreactivity was located in the cytoplasm of dendrites (79%), axons (81%) and somata (Alvira-Botero and Garzon 2006). In the rat and monkey dorsal root ganglia and dorsal horn, immunogold-labeled DOPR was frequently associated with the membranes of large dense-core vesicles (Zhang et al. 1998).
In knock-in mice expressing DOPR-EGFP, quantitative analysis of confocal images indicates that ~ 60% of DOPR-EGFP is present on the cell surface in the striatum (Scherrer et al. 2006). It is noteworthy that the Bmax of [3H]naltrindole binding to DOPR in DOPR-EGFP knock-in mice is twice as high as that in wild type mice. In addition, fusion of the DOPR at the C-terminus with EGFP may affect interactions of the DOPR with associated proteins. These two factors may affect expression, subcellular localization and trafficking of DOPR-EGFP.
KOPR
A number of neuroanatomical studies have shown that the KOPR is primarily distributed intracellularly (Fig. 1), similar to the DOPR. Harris et al. (2004) reported that ~55% of KOPR immuoreactivity was located intracellularly in the dendrites of rat spinal cords of both sexes. In axon terminals, ~55% and 70% of KOPR immunoreactivity was intracellular in male and female rats, respectively. We observed an even higher percentage (~70%) of KORP immunoreactivity located intracellularly in the dendrites of male rat spinal cord (Wang et al. submitted). Most of intracellular KOPR was not associated with any discernable organelles, but some immunoreactivity was associated with mitochondria and endosomes. In the rat posterior pituitary, ~60% of immunogold-labeled KOPR was associated with large secretory vesicles in the axon terminals and only ~11% with plasma membranes (Shuster et al. 1999).
In contrast to its localization within dendrites, KOPR was frequently associated with small synaptic vesicles in axon terminals of the rat nucleus accumbens (Svingos et al. 1999, 2001; Meshul and McGinty 2000). In addition, peroxidase-labeled KOPR immunoreactivity was detected along plasma membranes of presynaptic axon terminals, large dense-core vesicles and small vesicles of the hippocampus in guinea pigs (Drake et al. 1996). When interpreting these results, one must take into consideration the known diffusion of peroxidase reaction products and their possible absorption to membrane structures that may lead to an overestimation of the association of KOPR immunoreactivity with plasma membranes and synaptic vesicle membranes. Consistent with this notion is the finding that when KOPR was labeled with peroxidase it was predominantly associated with plasma membranes of glial cells in rat medial prefrontal cortex, but when labeled with immunogold, KOPR was mainly in the cytosol (Svingos and Colago 2002).
In summary, in vivo experimental approaches have provided valuable insight into the differential subcellular distributions of opioid receptors. The predominance of MOPR on the cell surface and the greater prevalence of DOPR and KOPR intracellularly imply that the regulation of their trafficking is likely to be different.
Trafficking of opioid receptors in vivo
The studies on trafficking of opioid receptors in vivo are summarized in Table 1.
Table 1.
Summarization of in vivo trafficking of endogenous opioid receptors
| Receptor | Method | Animal | Region | Stimulation | Findings | Reference |
|---|---|---|---|---|---|---|
| MOPR | immunohistochemistry + confocal microscopy | guinea pig | myenteric plexus | acute etorphine or morphine | internalized by etorphine, but not by morphine | (Sternini et al. 1996) |
| rat | dorsal horn of spinal cord | DAMGO, remifentanil, endomorphin-1 or morphine; noxious stimuli | internalized by DAMGO, remifentanil, endomorphin-1, but not by morphine or noxious stimuli | (Trafton et al. 2000) | ||
| morphine tolerant rat | dorsal horn of spinal cord | DAMGO | DAMGO caused similar magnitudes of internalization in control and tolerant rats | (Trafton and Basbaum 2004) | ||
| rat | dorsal horn of spinal cord | DAMGO (low dose that did not promote internalization) + Morphine | Promoted internalization | (He et al. 2002) | ||
| rat | Lateral septum | acute morphine | MOPR1C internalized | (Abbadie And Pasternak 2001) | ||
| OVX rat | medial preoptic nucleus; posterodorsal medial amygdale | estrogen | internalization | (Eckersell et al. 1998) | ||
| Immunogold-labeling + electronmicroscopy | rat | locus coeruleus | acute etorphine or morphine | internalized by etorphine, but not by morphine | (Van Bockstaele And Commons 2001) | |
| nucleus accumbens | acute morphine | internalized in dendrites, but not in cell bodies | (Haberstock-Debic et al. 2003) | |||
| rostral ventrolateral medulla | acute morphine | internalized only in the dendrites with diameter <1.4 µm | (Drake et al. 2005) | |||
| DOPR | Confocal microscopy | knock-in mouse | striatum | SNC80 | internalization correlated with tolerance | (Scherrer et al. 2006) |
| immunogold-labeling + electron microscopy | rat | dorsal horn of spinal cord | Chronic inflammatory pain | up-regulation and translocation to surface | (Cahill et al. 2003) | |
| Chronic morphine treatment | translocation to surface | (Cahill et al.2001b) | ||||
| KOPR | immunogold-labeling + electron microscopy | rat | dorsal horn of spinal cord | U50,488H or dynorphin A | internalized by dynorphin A | (Wang et al. submitted) |
| hypothalamus | salt loading | inserted into cell surface | (Shuster et al. 1999) |
MOPR
It was first demonstrated in cell models that MOPR agonists had differential effects on internalization of the receptor. MOPR was internalized by acute treatment with enkephalins, etorphine or DAMGO, but not morphine (Arden et al. 1995; Keith et al. 1996). Agonist-dependent internalization of MOPR has also been shown in tissues in vivo. Systemic injections of etorphine caused rapid internalization of MOPR in neurons in the myenteric plexus of the guinea pig as demonstrated by immunohistochemistry and confocal microscopy (Sternini et al. 1996). In contrast, acute morphine treatment (30 min) did not change localization of MOPR. Differential effects of etorphine and morphine on internalization of MOPR were also reported in neurons of the rat brain using the same approach (Keith et al. 1998). By counting the MOPR immunoreactive positive endosomes in confocal microscopy images, Trafton et al. (2000) reported similar findings for MOPR in the dorsal horn of rat spinal cord, which was internalized by DAMGO, remifentanil or endomorphin-1, but not morphine. Quantitative immunogold electron microscopy showed that acute etorphine treatment (15 min) significantly reduced the surface amount of MOPR in the dendrites in rat locus coeruleus (Fig. 1), whereas morphine, either acute (30 min) or chronic (5 days), had no effect (Van Bockstaele and Commons 2001). In the dorsal horn of rat spinal cord, the endocytosed MOPR reappeared on cell surface within 60 min (Trafton et al. 2000). The magnitude of MOPR internalization in lamina II interneurons induced by intrathecal DAMGO correlated with the extent of antinociception. However, such a correlation did not exist in morphine-tolerant rats. Although the antinociceptive effect of DAMGO was greatly decreased in morphine-tolerant rats, it promoted internalization of MOPR to a similar extent as in control rats (Trafton and Basbaum 2004), indicating the desensitized MOPR retains the capability to be internalized. Surprisingly, although endogenous opioids are expected to be released upon application of noxious stimuli, no MOPR internalization was detected in lamina II neurons in nociception models, which may be due to inadequate amount of the released endogenous opioids (Trafton et al. 2000). The findings prompted the authors to suggest that released opioid peptides may act presynaptically.
Although morphine alone did not induce significant internalization of MOPR, morphine plus DAMGO, at a dose that did not cause endocytosis, internalized MOPR in the dorsal horns of rat spinal cord as demonstrated in confocal images. The combination also reduced the development of tolerance to chronic morphine treatment in rats (He et al. 2002). Recently, a knock-in mice expressing mutated MOPR with DOPR C-tail has been established (Kim et al. 2008). The mutant receptor in striatal neurons cultured from the knock-in mice were internalized by morphine in vitro; however, it was not examined in vivo. The knock-in mice showed significantly reduced tolerance and dependence to morphine (Kim et al. 2008). The authors concluded that these findings supported the notion that tolerance to opioid receptors is due to sustained activation of cell surface receptors.
Interestingly, trafficking of endogenous MOPR upon acute morphine treatment appears to be compartment-specific. Haberstock-Debic et al. (2003) reported that in the rat nucleus accumbens, morphine (30 min) translocated MOPR to intracellular sites in dendrites, but not in neuronal cell bodies or axons. Drake et al. (2005) also observed that, in the rostral ventrolateral medulla, morphine induced internalization of MOPR in dendrites that had diameters <.4 µm, but not in larger dendrites. These findings imply that the abundance of molecules involved in internalization machinery may vary in different compartments of neurons. The impact from surrounding environment or neural circuitries may also play a role.
Confocal microscopy images showed that the endogenous MOPR1C, a splice variant of MOPR, in lateral septum was internalized by morphine administered intracerebroventricularly in mice (Abbadie and Pasternak 2001), but MOPR was not. The difference in the C-terminal domains is likely to account for their different abilities to be internalized.
Estrogen treatment also induced internalization of MOPR in medial preoptic nucleus and the posteriodorsal medial amygdala of ovariectomized rats when using the increase of the density of MOPR-immunoreactive fibers as an indicator for internalization (Eckersell et al. 1998). The internalization was rapid (within 30 min) and long lasting (>24 hr). The mechanisms underlying these observations are unknown.
DOPR
In DOPR-EGFP knock-in mice, acute treatment with SNC80 caused significant internalization of DOPR in caudate putamen neurons in a dose-dependent manner, concomitant with an increase in locomotor activity. In addition, DOPR internalization correlated with the occurrence of desensitization to the subsequent application of SNC80 in enhancing locomotor activity (Scherrer et al. 2006).
Since DOPR has a large intracellular pool, efforts were also devoted to investigating the stimuli that can promote cell surface expression of DOPR. Chronic inflammatory pain up-regulated mRNA and protein levels of DOPR in the dorsal horns of rat spinal cords, as demonstrated by in situ hybridization and immunoblotting (Cahill et al. 2003). Immunoelectron microscopy studies revealed that chronic inflammatory pain caused a significant increase of DOPR on the cell surface and in peripheral zones under plasma membranes, which may account for the increased antinociceptive efficacy of DOPR agonists in animals with chronic inflammatory pain (Cahill et al. 2003).
Interestingly, chronic treatment with morphine promoted movement of intracellular DOPR to the cell surface in the dorsal horn of rat spinal cord as shown by quantitative immunoelectron microscopy (Fig. 1) (Cahill et al. 2001b). The effect of morphine was mediated by MOPR which was shown by using MOPR blockade and MOPR knock-out mice (Morinville et al. 2003). Different from chronic inflammatory pain, morphine treatment regulated subcellular localization of DOPR without affecting overall expression level of DOPR (Cahill et al. 2001b).
KOPR
Intrathecal injection of dynorphin A significantly decreased cell surface KOPR in the dorsal horns of rat spinal cord, but U50,488H did not, using quantitative immunoelectron microscopy (Wang et al. submitted). The differential effects of agonists may be due to the distinct receptor conformations they induce. However, the in vivo effect of dynorphin A is more complex. It has been reported that dynorphin A(2–17), the des-Tyr derivative of dynorphin A(1–17), can activate NMDA (Vanderah et al. 1996) or bradykinin (Lai et al. 2006) receptors at high concentrations. It may also affect the trafficking of KOPR via neuronal circuitry.
KOPR in the posterior pituitary is mostly associated with vesicles containing vasopressin (Shuster et al. 1999). When salt loading causes release of vasopressin, the KOPR is translocated to cell surface along with fusion of secretory vesicles with plasma membranes (Fig. 1) (Shuster et al. 1999).
Comparisons between in vivo and in vitro studies
MOPR
By and large, the results of the in vivo studies are similar to those of in vitro studies. Most of the MOPR is present on cell membranes in transfected cells and in neurons in vivo. DAMGO and etorphine cause significant internalization of MOPR, but morphine does not, both in vitro and in vivo. However, the in vivo study revealed that morphine promoted redistribution of endogenous MOPR in certain populations of dendrites. Its physiological significance is not clear at the present time.
DOPR and KOPR
While DOPR and KOPR expressed in cells are mostly localized on cell membranes, DOPR and KOPR in neuronal tissues in vivo are largely intracellularly located. There are several possibilities for the differences. It may be due to differences in cellular milieu between cell lines and neurons in the brain and spinal cord, including proteins involved in their trafficking and interacting proteins. In addition, immunohistochemistry for KOPR and DOPR in vitro was mostly performed with antibodies against an epitope tag added to the N-termini of the receptors, whereas in vivo studies were conducted with DOPR and KOPR antibodies against N-or C-terminal domain of the receptors. Antibodies against different epitopes may not recognize intracellular and cell surface receptors equally, thus producing different subcellular distribution patterns. Indeed, Cahill et al. (2001a) reported that antibodies directed against a C-terminal domain peptide of the DOPR recognized predominantly cell bodies and proximal dendrites, whereas those directed against an N-terminal domain peptide, labeled extensively dendritic and terminal arbors besides cell bodies. In addition, electron microscopy studies revealed that the two antibodies label differentially with antibodies against the C-terminal peptide staining twice as many DOPR-immunoreactivities on membranes compared to those against the N-terminal peptide. Moreover, when the receptor was epitope-tagged with FLAG, in most cases it contained a signal peptide to enhance endoplasmic reticulum membrane insertion and thus expression on plasma membranes (Guan et al. 1992), which may contribute to the differences.
Since DOPR has a dramatic difference in localization between in vitro and in vivo, the in vivo studies are focused on how intracellular DOPR is promoted to the cell surface, whereas the in vitro investigations have been on agonist-induced internalization and trafficking of internalized receptors.
For the KOPR, the in vivo studies are consistent with several in vitro findings that U50,488H did not internalize rat KOPR in cells (Li et al. 1999; Zhang et al. 2002).
Future studies
The in vivo studies have provided many descriptive observations. However, there is an obvious lack of mechanistic studies.
Functional consequence of receptor trafficking in vivo
Scherrer et al. (2006) have demonstrated in DOPR-EGFP mice that SNC80 enhances DOPR internalization in caudate putamen neuron, which renders the animals less sensitive to the subsequent SNC80 administration (see above). In addition, Cahill et al. (2003) reported that inflammatory pain promoted trafficking of DOPR to cell surface in dorsal horn of the rat spinal cord, leading to enhanced response to DOPR agonists. More studies are needed to address the functional significance of MOPR and KOPR trafficking in vivo. McLaughlin et al. (2004) found that chronic U50,488H administration in mice enhanced KOPR phosphorylation and caused tolerance to KOPR-mediated antinociception. Whether the tolerance is related to KOPR internalization requires further study.
Mechanisms underlying the differential subcellular distribution of endogenous opioid receptors
Although the three opioid receptors are highly homologous in their amino acid sequences, in neuronal tissues MOPR is mostly on cell surface, whereas DOPR and KOPR are predominantly intracellular. Since their sequences in the C-terminal domains are highly divergent, it is tempting to speculate that the differences in this region result in their interactions with different proteins, which play an important role in their subcellular localization. However, the majority of a mutated MOPR with the C-terminal domain replaced with that of the DOPR was still found on cell surface in primary neurons cultured from the knock-in mice (Kim et al. 2008). It will be interesting to directly examine the subcellular distribution of these mutant receptors in vivo. Another possibility that can not be ruled out is that the differential distribution may result from the differential recognition of the antibodies. Therefore, it is critical to further characterize the subcellular localization of endogenous opioid receptors using antibodies against different epitopes.
Drake et al.(2005) reported the majority of MOPR was located intracellularly in the dendrites of C1 adrenergic neurons in the rat rostral ventrolateral medulla, in contrast to other brain regions. Therefore, the differences in in vivo milieu, such as interacting proteins involved in trafficking, may lead to their differential subcellular distribution in brain regions. Identification of the interacting proteins that are involved in trafficking may help to elucidate the differences. Constitutive internalization and recycling of endogenous opioid receptors may affect their subcellular distribution. It has been reported that opioid receptors were differentially regulated in the trafficking pathways in vitro. While internalized MOPR is mostly recycled, the majority of endocytosed DOPR is sorted to lysosomes for degradation (von Zastrow et al. 2003). Antagonists can be used to stop constitutive internalization and their effects on subcellular localization of the receptors can be examined.
Most receptors in transfected cells appear to be on cell surface; therefore, the in vitro systems do not always reflect the in vivo situations. One important task is to establish an in vitro system in which subcellular distributions of opioid receptors mimic those in tissues. Kim and von Zastrow (2003) found that treatment of PC12 cells with nerve growth factor caused cell differentiation and retained the transfected DOPR intracellularly; whereas transfected MOPR is mostly on cell surface. This may be a good in vitro system that allows studies on mechanisms underlying differential subcellular distribution.
Mechanisms underlying the compartment-selective internalization of MOPR by morphine
MOPR is internalized by morphine treatment in vitro when G protein-coupled receptor kinase 2 is over-expressed (Zhang et al. 1998). It is possible that different compartments of neurons may have distinct compositions and/or abundance of internalization machinery components. In addition, we have reported previously that MOPR displayed differential glycosylation in different brain regions (Huang et al. 2008). Thus, it will be interesting to examine if the MOPR in different neuronal compartment may have distinct post-translational modifications.
Mechanisms underlying the promotion of intracellular DOPR to the cell surface
Morphine treatment enhances cell surface level of endogenous DOPR and the MOPR is required for this action. Mechanisms for this process are not clear. MOPR and DOPR have been demonstrated to form dimers in vitro (George et al. 2000; Gomes et al. 2000); however, there is no definitive evidence showing their in vivo dimerization. It will be interesting to study if MOPR-DOPR dimerization is involved. Unfortunately, there are no reagents that can promote or block dimerization of MOPR-DOPR. Alternatively, morphine may act on the MOPR via neuronal circuitry and ultimately leads to enhancement in cell surface expression of the DOPR. If this is the case, the neuronal circuitry needs to be identified.
Chronic inflammation also enhances cell surface DOPR. Biochemical processes leading to the enhancement remains to be determined. It is likely that chemical mediators of inflammation and subsequent activation of their receptors and down-stream effectors may be involved.
Functional significance of intracellular pool of KOPR
KOPR has a large intracellular pool in dorsal horns of the rat spinal cord, which was mostly dispersed in the cytosol without association with any organelles. It will be interesting to investigate whether the intracellular KOPR can be translocated to cell surface under certain physiological or pathophysiological conditions. Infusion of U69,593, a selective KOPR agonist, to rostral ventromedial medulla produced antinociceptive effects against chemical or mechanical stimuli (Schepers et al. 2007). The efficacy of U69,593 was significantly enhanced in animals that had chronic inflammatory pain induced by hind paw injection of complete Freund's adjuvant. The presence of KOPR in rostral ventromedial medulla has been reported (Drake et al. 2007). Whether the enhanced efficacy of U69,593 is due to the increase in the number of cell surface KOPR and/or down-stream signaling needs further investigation.
Acknowledgment
The authors acknowledge the grant support from National Institutes of Health DA17302 to LYLC and DA09082 to EJVB.
Abbreviations
- DOPR
δ opioid receptor
- EGFP
enhanced green fluorescent protein
- KOPR
κ opioid receptor
- MOPR
µ opioid receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication.As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbadie C, Pasternak GW. Differential in vivo internalization of MOR-1 and MOR-1C by morphine. Neuroreport. 2001;12(14):3069–3072. doi: 10.1097/00001756-200110080-00017. [DOI] [PubMed] [Google Scholar]
- Alvira-Botero MX, Garzon M. Cellular and subcellular distributions of delta opioid receptor activation sites in the ventral oral pontine tegmentum of the cat. Brain Research. 2006;1123(1):101–111. doi: 10.1016/j.brainres.2006.09.030. [DOI] [PubMed] [Google Scholar]
- Arden JR, Segredo V, Wang Z, Lameh J, Sadee W. Phosphorylation and agonist-specific intracellular trafficking of an epitope-tagged mu-opioid receptor expressed in HEK 293 cells. Journal of Neurochemistry. 1995;65:1636–1645. doi: 10.1046/j.1471-4159.1995.65041636.x. [DOI] [PubMed] [Google Scholar]
- Arvidsson U, Dado RJ, Riedl M, Lee JH, Law P-Y, Loh HH, Elde R, Wessendorf MW. Delta-opioid receptor immunoreactivity: distribution in brainstem and spinal cord, and relationship to biogenic amines and enkephalin. Journal of Neuroscience. 1995a;15:1215–1235. doi: 10.1523/JNEUROSCI.15-02-01215.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson U, Riedl M, Chakrabarti S, Lee JH, Nakano AH, Dado RJ, Loh HH, Law P-Y, Wessendorf MW, Elde R. Distribution and targeting of a mu-opioid receptor (MOR1) in brain and spinal cord. Journal of Neuroscience. 1995b;15:3328–3341. doi: 10.1523/JNEUROSCI.15-05-03328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L, Jin SX, Zhang C, Wang LH, Xu ZZ, Zhang FX, Wang LC, Ning FS, Cai HJ, Guan JS, Xiao HS, Xu ZQ, He C, Hokfelt T, Zhou Z, Zhang X. Activation of delta opioid receptors induces receptor insertion and neuropeptide secretion. Neuron. 2003;37(1):121–133. doi: 10.1016/s0896-6273(02)01103-0. [DOI] [PubMed] [Google Scholar]
- Cahill CM, McClellan KA, Morinville A, Hoffert C, Hubatsch D, O'Donnell D, Beaudet A. Immunohistochemical distribution of delta opioid receptors in the rat central nervous system: evidence for somatodendritic labeling and antigen-specific cellular compartmentalization. Journal of Comparative Neurology. 2001a;440(1):65–84. doi: 10.1002/cne.1370. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Morinville A, Hoffert C, O'Donnell D, Beaudet A. Up-regulation and trafficking of delta opioid receptor in a model of chronic inflammation: implications for pain control. Pain. 2003;101(1–2):199–208. doi: 10.1016/s0304-3959(02)00333-0. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Morinville A, Lee MC, Vincent JP, Collier B, Beaudet A. Prolonged morphine treatment targets delta opioid receptors to neuronal plasma membranes and enhances delta-mediated antinociception. Journal of Neuroscience. 2001b;21(19):7598–7607. doi: 10.1523/JNEUROSCI.21-19-07598.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaipatikul V, Erickson-Herbrandson LJ, Loh HH, Law PY. Rescuing the traffic-deficient mutants of rat mu-opioid receptors with hydrophobic ligands. Molecular Pharmacology. 2003;64(1):32–41. doi: 10.1124/mol.64.1.32. [DOI] [PubMed] [Google Scholar]
- Chen Y, Chen C, Wang Y, Liu-Chen LY. Ligands regulate cell surface level of the human {kappa} opioid receptor by activation-induced down-regulation and pharmacological chaperone-mediated enhancement: Differential effects of nonpeptide and peptide agonists. Journal of Pharmacology & Experimental Therapeutics. 2006;319(2):765–775. doi: 10.1124/jpet.106.107987. [DOI] [PubMed] [Google Scholar]
- Drake CT, Aicher SA, Montalmant FL, Milner TA. Redistribution of mu-opioid receptors in C1 adrenergic neurons following chronic administration of morphine. Experimental Neurology. 2005;196(2):365–372. doi: 10.1016/j.expneurol.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Drake CT, De Oliveira AX, Harris JA, Connor DM, Winkler CW, Aicher SA. Kappa opioid receptors in the rostral ventromedial medulla of male and female rats. Journal of Comparative Neurology. 2007;500(3):465–476. doi: 10.1002/cne.21184. [DOI] [PubMed] [Google Scholar]
- Drake CT, Patterson TA, Simmons ML, Chavkin C, Milner TA. Kappa opioid receptor-like immunoreactivity in guinea pig brain: ultrastructural localization in presynaptic terminals in hippocampal formation. Journal of Comparative Neurology. 1996;370(3):377–395. doi: 10.1002/(SICI)1096-9861(19960701)370:3<377::AID-CNE8>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Eckersell CB, Popper P, Micevych PE. Estrogen-induced alteration of mu-opioid receptor immunoreactivity in the medial preoptic nucleus and medial amygdala. Journal of Neuroscience. 1998;18(10):3967–3976. doi: 10.1523/JNEUROSCI.18-10-03967.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzon M, Pickel VM. Plasmalemmal mu-opioid receptor distribution mainly in nondopaminergic neurons in the rat ventral tegmental area. Synapse. 2001;41(4):311–328. doi: 10.1002/syn.1088. [DOI] [PubMed] [Google Scholar]
- George SR, Fan T, Xie Z, Tse R, Tam V, Varghese G, O'owd BF. Oligomerization of mu- and delta-opioid receptors. Generation of novel functional properties. Journal of Biological Chemistry. 2000;275(34):26128–26135. doi: 10.1074/jbc.M000345200. [DOI] [PubMed] [Google Scholar]
- Gomes I, Jordan BA, Gupta A, Trapaidze N, Nagy V, Devi LA. Heterodimerization of mu and delta opioid receptors: A role in opiate synergy. Journal of Neuroscience. 2000;20(22):RC110. doi: 10.1523/JNEUROSCI.20-22-j0007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan JS, Xu ZZ, Gao H, He SQ, Ma GQ, Sun T, Wang LH, Zhang ZN, Lena I, Kitchen I, Elde R, Zimmer A, He C, Pei G, Bao L, Zhang X. Interaction with vesicle luminal protachykinin regulates surface expression of delta-opioid receptors and opioid analgesia. Cell. 2005;122(4):619–631. doi: 10.1016/j.cell.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Guan XM, Kobilka TS, Kobilka BK. Enhancement of membrane insertion and function in a type IIIb membrane protein following introduction of a cleavable signal peptide. Journal of Biological Chemistry. 1992;267(31):21995–21998. [PubMed] [Google Scholar]
- Haberstock-Debic H, Wein M, Barrot M, Colago EE, Rahman Z, Neve RL, Pickel VM, Nestler EJ, von Zastrow M, Svingos AL. Morphine acutely regulates opioid receptor trafficking selectively in dendrites of nucleus accumbens neurons. Journal of Neuroscience. 2003;23(10):4324–4332. doi: 10.1523/JNEUROSCI.23-10-04324.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA, Chang PC, Drake CT. Kappa opioid receptors in rat spinal cord: sex-linked distribution differences. Neuroscience. 2004;124(4):879–890. doi: 10.1016/j.neuroscience.2003.12.042. [DOI] [PubMed] [Google Scholar]
- He L, Fong J, von Zastrow M, Whistler JL. Regulation of opioid receptor trafficking and morphine tolerance by receptor oligomerization. Cell. 2002;108(2):271–282. doi: 10.1016/s0092-8674(02)00613-x. [DOI] [PubMed] [Google Scholar]
- Huang P, Chen C, Xu W, Yoon SI, Unterwald EM, Pintar JE, Wang Y, Chong PL, Liu-Chen LY. Brain region-specific N-glycosylation and lipid rafts association of the rat mu opioid receptor. Biochemical and Biophysical Research Communications. 2008;365(1):82–88. doi: 10.1016/j.bbrc.2007.10.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith DE, Anton B, Murray SR, Zaki PA, Chu PC, Lissin DV, Monteillet-Agius G, Stewart PL, Evans CJ, von Zastrow M. mu-Opioid receptor internalization: opiate drugs have differential effects on a conserved endocytic mechanism in vitro and in the mammalian brain. Molecular Pharmacology. 1998;53(3):377–384. [PubMed] [Google Scholar]
- Keith DE, Murray SR, Zaki PA, Chu PC, Lissin DV, Kang L, Evans CJ, von Zastrow M. Morphine activates opioid receptors without causing their rapid internalization. Journal of Biological Chemistry. 1996;271:19021–19024. doi: 10.1074/jbc.271.32.19021. [DOI] [PubMed] [Google Scholar]
- Kim JA, Bartlett S, He L, Nielsen CK, Chang AM, Kharazia V, Waldhoer M, Ou CJ, Taylor S, Ferwerda M, Cado D, Whistler JL. Morphine-induced receptor endocytosis in a novel knockin mouse reduces tolerance and dependence. Current Biology. 2008;18(2):129–135. doi: 10.1016/j.cub.2007.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KA, von Zastrow M. Neurotrophin-regulated sorting of opioid receptors in the biosynthetic pathway of neurosecretory cells. Journal of Neuroscience. 2003;23(6):2075–2085. doi: 10.1523/JNEUROSCI.23-06-02075.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J, Luo MC, Chen Q, Ma S, Gardell LR, Ossipov MH, Porreca F. Dynorphin A activates bradykinin receptors to maintain neuropathic pain. Nature Neuroscience. 2006;9(12):1534–1540. doi: 10.1038/nn1804. [DOI] [PubMed] [Google Scholar]
- Li J-G, Luo LY, Krupnick JG, Benovic JL, Liu-Chen L-Y. U50,488H-induced internalization of the human kappa opioid receptor involves a beta-arrestin- and dynamin-dependent mechanism. Kappa receptor internalization is not required for mitogen-activated protein kinase activation. Journal of Biological Chemistry. 1999;274(17):12087–12094. doi: 10.1074/jbc.274.17.12087. [DOI] [PubMed] [Google Scholar]
- Liu-Chen L-Y. Agonist-induced regulation and trafficking of kappa opioid receptors. Life Sciences. 2004;75(5):511–536. doi: 10.1016/j.lfs.2003.10.041. [DOI] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Anatomy of CNS opioid receptors. Trends in Neurosciences. 1988;11:308–314. doi: 10.1016/0166-2236(88)90093-8. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Myers LC, Zarek PE, Caron MG, Lefkowitz RJ, Czyzyk TA, Pintar JE, Chavkin C. Prolonged kappa opioid receptor phosphorylation mediated by G-protein receptor kinase underlies sustained analgesic tolerance. Journal of Biological Chemistry. 2004;279:1810–1818. doi: 10.1074/jbc.M305796200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshul CK, McGinty JF. Kappa opioid receptor immunoreactivity in the nucleus accumbens and caudate-putamen is primarily associated with synaptic vesicles in axons. Neuroscience. 2000;96(1):91–99. doi: 10.1016/s0306-4522(99)90481-5. [DOI] [PubMed] [Google Scholar]
- Morinville A, Cahill CM, Esdaile MJ, Aibak H, Collier B, Kieffer BL, Beaudet A. Regulation of delta-opioid receptor trafficking via mu-opioid receptor stimulation: evidence from mu-opioid receptor knock-out mice. Journal of Neuroscience. 2003;23(12):4888–4898. doi: 10.1523/JNEUROSCI.23-12-04888.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyse E, Marcel D, Leonard K, Beaudet A. Electron microscopic distribution of mu opioid receptors on noradrenergic neurons of the locus coeruleus. European Journal of Neuroscience. 1997;9(1):128–139. doi: 10.1111/j.1460-9568.1997.tb01361.x. [DOI] [PubMed] [Google Scholar]
- Novikoff AB, Novikoff PM, Quintana N, Davis C. Diffusion artificats in 3,3'-diaminobenzidine cytochemistry. Journal of Histochemistry and Cytochemistry. 1972;20(9):745–749. doi: 10.1177/20.9.745. [DOI] [PubMed] [Google Scholar]
- Petaja-Repo UE, Hogue M, Bhalla S, Laperriere A, Morello JP, Bouvier M. Ligands act as pharmacological chaperones and increase the efficiency of delta opioid receptor maturation. EMBO Journal. 2002;21(7):1628–1637. doi: 10.1093/emboj/21.7.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepers RJ, Mahoney JL, Shippenberg TS. Inflammation-induced changes in rostral ventromedial medulla mu and kappa opioid receptor mediated antinociception. Pain. 2007;136(3):320–330. doi: 10.1016/j.pain.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Scherrer G, Tryoen-Toth P, Filliol D, Matifas A, Laustriat D, Cao YQ, Basbaum AI, Dierich A, Vonesh JL, Gaveriaux-Ruff C, Kieffer BL. Knockin mice expressing fluorescent delta-opioid receptors uncover G protein-coupled receptor dynamics in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(25):9691–9696. doi: 10.1073/pnas.0603359103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuster SJ, Riedl M, Li X, Vulchanova L, Elde R. Stimulus-dependent translocation of kappa opioid receptors to the plasma membrane. Journal of Neuroscience. 1999;19(7):2658–2664. doi: 10.1523/JNEUROSCI.19-07-02658.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternini C, Spann M, Anton B, Keith DE, Bunnett NW, von Zastrow M, Evans C, Brecha NC. Agonist-selective endocytosis of mu opioid receptor by neurons in vivo. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:9241–9246. doi: 10.1073/pnas.93.17.9241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svingos AL, Chavkin C, Colago EE, Pickel VM. Major coexpression of kappa-opioid receptors and the dopamine transporter in nucleus accumbens axonal profiles. Synapse. 2001;42(3):185–192. doi: 10.1002/syn.10005. [DOI] [PubMed] [Google Scholar]
- Svingos AL, Colago EE. Kappa-Opioid and NMDA glutamate receptors are differentially targeted within rat medial prefrontal cortex. Brain Research. 2002;946(2):262–271. doi: 10.1016/s0006-8993(02)02894-9. [DOI] [PubMed] [Google Scholar]
- Svingos AL, Colago EE, Pickel VM. Cellular sites for dynorphin activation of kappa-opioid receptors in the rat nucleus accumbens shell. Journal of Neuroscience. 1999;19(5):1804–1813. doi: 10.1523/JNEUROSCI.19-05-01804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trafton JA, Abbadie C, Marek K, Basbaum AI. Postsynaptic signaling via the [mu]-opioid receptor: responses of dorsal horn neurons to exogenous opioids and noxious stimulation. Journal of Neuroscience. 2000;20(23):8578–8584. doi: 10.1523/JNEUROSCI.20-23-08578.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trafton JA, Basbaum AI. [d-Ala2,N-MePhe4,Gly-ol5]enkephalin-induced internalization of the micro opioid receptor in the spinal cord of morphine tolerant rats. Neuroscience. 2004;125(3):541–543. doi: 10.1016/j.neuroscience.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Commons KG. Internalization of mu-opioid receptors produced by etorphine in the rat locus coeruleus. Neuroscience. 2001;108(3):467–477. doi: 10.1016/s0306-4522(01)00426-2. [DOI] [PubMed] [Google Scholar]
- Vanderah TW, Laughlin T, Lashbrook JM, Nichols ML, Wilcox GL, Ossipov MH, Malan TP, Jr, Porreca F. Single intrathecal injections of dynorphin A or des-Tyr-dynorphins produce long-lasting allodynia in rats: blockade by MK-801 but not naloxone. Pain. 1996;68(2–3):275–281. doi: 10.1016/s0304-3959(96)03225-3. [DOI] [PubMed] [Google Scholar]
- von Zastrow M, Svingos A, Haberstock-Debic H, Evans C. Regulated endocytosis of opioid receptors: cellular mechanisms and proposed roles in physiological adaptation to opiate drugs. Current Opinion in Neurobiology. 2003;13(3):348–353. doi: 10.1016/s0959-4388(03)00069-2. [DOI] [PubMed] [Google Scholar]
- Wang H, Pickel VM. Preferential cytoplasmic localization of delta-opioid receptors in rat striatal patches: comparison with plasmalemmal mu-opioid receptors. Journal of Neuroscience. 2001;21(9):3242–3250. doi: 10.1523/JNEUROSCI.21-09-03242.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QP, Zadina JE, Guan JL, Shioda S. Morphological evidence of endomorphin as an agonist for the mu-opioid receptor in the rat spinal cord. Neuroscience Letters. 2003;341(2):107–110. doi: 10.1016/s0304-3940(03)00182-4. [DOI] [PubMed] [Google Scholar]
- Wannemacher KM, Yadav PN, Howells RD. A select set of opioid ligands induce up-regulation by promoting the maturation and stability of the rat kappa-opioid receptor in human embryonic kidney 293 cells. Journal of Pharmacology &Experimental Therapeutics. 2007;323(2):614–625. doi: 10.1124/jpet.107.125500. [DOI] [PubMed] [Google Scholar]
- Zhang F, Li J, Li J-G, Liu-Chen L-Y. (−)U50,488H [(trans)-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidinyl)-cyclohexyl]benzeneace tamide] induces internalization and down-regulation of the human, but not the rat, kappa-opioid receptor: Structural basis for the differential regulation. Journal of Pharmacology & Experimental Therapeutics. 2002;302(3):1184–1192. doi: 10.1124/jpet.302.3.1184. [DOI] [PubMed] [Google Scholar]
- Zhang J, Ferguson SS, Barak LS, Bodduluri SR, Laporte SA, Law P-Y, Caron MG. Role for G protein-coupled receptor kinase in agonist-specific regulation of mu-opioid receptor responsiveness. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:7157–7162. doi: 10.1073/pnas.95.12.7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Bao L, Arvidsson U, Elde R, Hokfelt T. Localization and regulation of the delta-opioid receptor in dorsal root ganglia and spinal cord of the rat and monkey: evidence for association with the membrane of large dense-core vesicles. Neuroscience. 1998;82(4):1225–1242. doi: 10.1016/s0306-4522(97)00341-2. [DOI] [PubMed] [Google Scholar]