Abstract
Introduction
2-Methoxyestradiol (2ME2) is an endogenous metabolite of the human hormone, estrogen, which has been shown to possess anti-tumor activity. 2-Fluoroethoxyestradiol (2FEE2) and 2-fluoropropanoxyestradiol (2FPE2), novel analogs of 2-methoxyestradiol, were designed and synthesized to be utilized as F-18 radiotracers for positron emission tomography (PET), with which the bio-distribution and intratumoral accumulations of 2ME2 could be measured in vivo for potential translation to human use.
Methods
2FEE2 and 2FPE2 were synthesized from 3,17β-estradiol in five steps respectively. Drug-induced microtubule depolymerization, antiproliferative activity against human cancer cell lines and HIF-1α down-regulation by 2FEE2 and 2FPE2 were investigated to examine whether these molecules possess similar anti-tumor activities as 2-methoxyestradiol. 2-[18F]Fluoroethoxyestradiol was synthesized for PET.
Results
Novel 2ME2 analogs, 2FEE2 and 2FPE2, were synthesized in 29% and 22% overall yield, respectively. 2FEE2 and 2FPE2 showed microtubule depolymerization and cytotoxicities against the human ovarian carcinoma cell line, 1A9, and the human glioma cell line, LN229. HIF-1α was down-regulated by 2FEE2 and 2FPE2 under hypoxic conditions. 2FEE2 was chosen as an F-18 radiotracer candidate, since it showed stronger antiproliferative activity than 2ME2 and 2FPE2. 2-[18F]Fluoroethoxyestradiol (2[18F]FEE2) was prepared in 8.3% decay-corrected yield in 90 min, based on a production of H[18F]F with more than 98% radiochemical purity.
Conclusions
2FEE2 and 2FPE2 showed similar activity as 2ME2. 2[18F]FEE2 was synthesized to be utilized as a PET radiotracer to measure the biological efficacy of 2ME2 and its analogs in vivo.
Keywords: 2-Fluoroethoxyestradiol, 2-Fluoropropanoxyestradiol, 2-Methoxyestradiol, PET, 2-[18F]Fluoroethoxyestradiol
1. Introduction
2-Methoxyestradiol (2ME2) (1) is an endogenous metabolite of the human estrogen, estradiol, which has drawn a lot of attention because of its anti-tumor activity against a wide variety of human tumors such as head and neck squamous cell carcinoma, glioma, prostate cancer and metastatic breast cancer [1-4]. At the cellular level, 2ME2 binds to tubulin, depolymerizes microtubules (MTs) and exerts antiangiogenic activity [5]. Mechanistically, we have previously shown that the antiangiogenic activity of 2ME2 in vivo stems from the ability of the molecule to inhibit the protein levels and transcriptional activity of the hypoxia-inducible factor 1 (HIF-1α) [6]. More importantly, we showed that inhibition of HIF-1α occurs downstream of the drug-induced MT disruption [6]. Phase I trials of 2ME2 in patients with metastatic breast cancer have been reported and showed that this orally available small molecule is well tolerated unlike other MT-targeting chemotherapy drugs [7-9]. Currently, 2ME2 is in Phase I/II oncology clinical trials.
To monitor the bio-distribution and intratumoral accumulations of 2ME2 in vivo, we have recently reported an improved synthesis of 2-[11C]methoxyestradiol (2[11C] ME2) [10]. Carbon-11 labeling does not change the chemical structure, so the pharmacokinetics and bio-distribution of 2ME2 can be investigated by positron emission tomography (PET), using 2[11C]ME2. A significant limitation in the application of carbon-11 tracers for human use is the short 20-min half-life of carbon-11. The 20-min half-life requires an on-site particle accelerator for production of carbon-11. In addition, only a single or relatively few doses can be generated from each batch production of carbon-11 tracers. Therefore, carbon-11 tracers are poor candidates for regional distribution for widespread human use.
In order to overcome the physical half-life limitation of carbon-11, we have recently focused on the development of several new analogs of 2ME2 which contain an intrinsic F-18 labeling site. These analogs were designed both as radiotracers for PET and as anti-tumor drugs. Fluorine-18 is a more desirable PET radionuclide for radiolabeling because its 110-min half-life allows substantially more time for radiochemical synthesis and for purification of the final product for human administration. Also, the 110-min half-life allows sufficient time for distribution to hospitals without on-site particle accelerators. Thus, fluorine-18 2ME2 analogs have significant advantages over 2[11C] ME2 for widespread human use.
Structure designs of F-18 analogs were based on 2-ethoxyestradiol (2), 2-(2′,2′,2′-trifluoroethoxy)estradiol (3) and 2-(2′-hydroxyethoxy)estradiol (4), published by Cushman et al. (Fig. 1) [11-13]. The substitution of 2-methoxy group with 2-ethoxy group in 2-ethoxyestradiol induced stronger inhibition of tubulin polymerization and cytotoxicities against various cancer cell lines than 2ME2. 2-(2′,2′,2′-Trifluoroethoxy)estradiol and 2-(2′-hydroxyethoxy)estradiol showed less potency as anti-tumor drugs than 2ME2; however, they retained high activity enough to show cytotoxicities against various cancer cell lines.
Fig. 1.
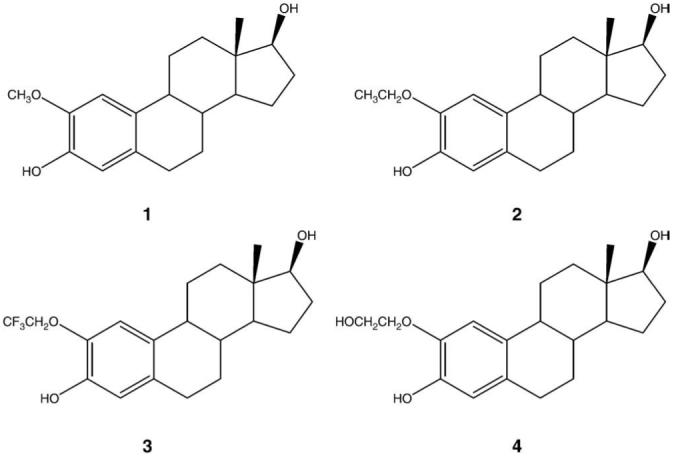
The structures of 2-methoxyestradiol and its analogs.
Based on these results, the 2-methoxy group of 2ME2 was replaced with a 2-(2′-fluoroethoxy) group and a 2-(3′-fluoropropanoxy) group, to afford F-18 radiotracers, 2-fluoroethoxyestradiol (2FEE2) (5) and 2-fluoropropanoxyestradiol (2FPE2) (6), respectively (Fig. 2).
Fig. 2.
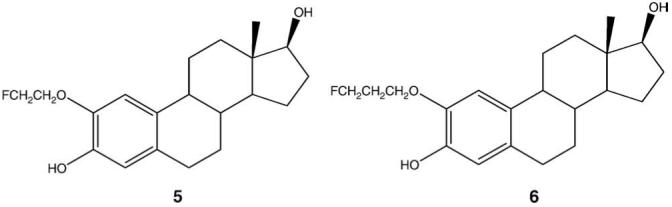
The structures of 2FEE2 and 2FPE2.
The syntheses of 2FEE2 and 2FPE2 were performed to investigate whether these newly synthesized analogs share similar biological activities as their parent compound. We performed MT depolymerization and cytotoxicity assays against human ovarian cancer cell lines and human glioma cell lines. We have further investigated the ability of 2FEE2 and 2FPE2 to down-regulate HIF-1α. A synthesis of 2-[18F]fluoroethoxyestradiol (2[18F]FEE2) is also reported.
2. Materials and methods
2.1. General
All reagents used were obtained from commercially available source. Solvents used in reactions were purchased from Sigma-Aldrich Corporation (Milwaukee, WI, USA), while solvents for chromatography were obtained from VWR Scientific Products (West Chester, PA, USA). Glassware was oven-dried at >200°C and then cooled to room temperature under argon flow. All reactions were performed under argon. F-254 silica gel, absorbed on aluminum plates in 250-μm layers, purchased from Whatman Ltd. (Clifton, NJ, USA), was utilized for thin-layer chromatography. Column chromatography was performed with silica gel 60 from EMD Chemicals Inc. (Gibbstown, NJ, USA). C-18 SepPaks were purchased from Waters, Inc. (Milford, MA, USA). 1H NMR spectra were recorded on a Varian spectrometer at 400 or 300 MHz and referenced to a NMR solvent (chemical shifts in part per million values, J values in Hertz). High-resolution mass spectrometer was obtained with an LTQ FT Ultra mass spectrometer from Thermo Scientific (Waltham, MA, USA) by using accurate mass electron ionization method. Elemental analyses were performed by Atlantic Microlabs, Inc. (Norcross, GA, USA) and within ±0.4% of the theoretical values.
2-Hydroxy-3,17β-O-bis(methoxymethyl)estradiol was synthesized from 3,17β-estradiol in three steps with 65% overall yield by a previously published method [9].
2.2. Chemistry
2.2.1. 2-Fluoroethoxy-3,17β-O-bis(methoxymethyl) estradiol and 2-fluoropropanoxy-3,17β-O-bis (methoxymethyl)estradiol
2-Hydroxy-3,17β-O-bis(methoxy)methylestradiol (52 mg, 0.14mmol) was dissolved in 2 ml of dimethylformamide (DMF). 0.3 g of K2CO3·1/2H2O and 5mg of Bu4NI were added to the solution, followed by the addition of 0.1 ml of 1-bromo-2-fluoroethane. The reaction mixture was stirred overnight at 100°C. The reaction mixture was cooled to room temperature, followed by water (10 ml) addition. The product was extracted with diethyl ether (20 ml×3). The combined organic layer was washed with brine (20 ml), dried with MgSO4 and concentrated in vacuo. The crude product was yellowish viscous oil. 2-Fluoroethoxy-3,17β-O-bis(methoxymethyl)estradiol was purified by column chromatography (eluent: 1:4=EtOAc/hexane) to yield 51 mg (0.12 mmol, 86%) of the pure compound. 1H NMR (CDCl3) δ 0.8 (s, 3H), 1.2-2.2 (m, 13H), 2.8 (dd, 2H), 3.4 (s, 3H), 3.5 (s, 3H), 3.6-3.7 (t, 1H), 4.2 (dd, 1H), 4.3 (dd, 1H), 4.6-4.7 (m, 3H), 4.8 (dd, 1H), 5.2 (s, 2H), 6.86 (s, 1H), 6.90 (s, 1H). Accurate mass EI (m/z) 421 (100%), 422 (M+, 14%). Anal. calcd for C24H35FO5: C, 68.22; H, 8.35. Found: C, 68.27; H, 8.35.
2-Fluoropropanoxy-3,17β-O-bis(methoxymethyl)estradiol was synthesized by the same method as 2-fluoroethoxy-3,17β-O-bis(methoxymethyl)estradiol, using 1-bromo-3-fluoropropane instead of 1-bromo-2-fluoroethane as an alkylating agent. A yield was 43% after purification by column chromatography (eluent: 1:4=EtOAc/hexane). 1H NMR (CDCl3) δ 0.8 (s, 3H), 1.2-2.3 (m, 15H), 2.8 (dd, 2H), 3.4 (s, 3H), 3.6 (s, 3H), 3.7 (t, 1H), 4.1 (t, 2H), 4.6 (t, 1H), 4.7 (m, 2H), 4.8 (t, 1H), 5.2 (s, 2H), 6.8 (s, 1H), 6.9 (s, 1H). Accurate mass EI (m/z) 435 (100%), 436 (M+, 16%). Anal. calcd for C25H37FO5: C, 68.78; H, 8.54. Found: C, 68.78; H, 8.55.
2.2.2. 2FEE2 and 2FPE2
2-Fluoroethoxy-3,17β-O-bis(methoxymethyl)estradiol (51 mg, 0.12 mmol) was dissolved in 1 ml of tetrahydrofuran (THF) and then 1 ml of 6N aqueous HCl was added to the solution. The reaction mixture was stirred vigorously for 1 h, to which 10 ml of water was added. The desired product was extracted with ethyl acetate (20 ml×3). The organic phase was washed with brine, dried with MgSO4 and concentrated in vacuo. 2FEE2 was purified by column chromatography (eluent: EtOAc/CH2Cl2=1:9) to yield 21 mg (0.065 mmol, 52%). 1H NMR (CDCl3) δ 0.8 (s, 3H), 1.2-2.3 (m, 13H), 2.8 (dd, 2H), 3.7 (t, 1H), 4.2 (dd, 1H), 4.3 (dd, 1H), 4.7 (m, 1H), 4.8 (m, 1H), 6.7 (s, 1H), 6.8 (s, 1H). Accurate mass EI (m/z) 334 (M+, 14%). Anal. calcd for C20H27FO3: C, 71.83; H, 8.14. Found: C, 69.71; H, 8.02.
2FPE2 was synthesized by the same method as 2FEE2 and purified by column chromatography (eluent: EtOAc/CH2Cl2=1:9) in 52% yield. 1H NMR (CDCl3) δ 0.8 (s, 3H), 1.2-2.3 (m, 15H), 2.8 (dd, 2H), 3.8 (t, 1H), 4.2 (t, 2H), 4.6 (t, 1H), 4.8 (t, 1H), 6.6 (s, 1H), 6.8 (s, 1H). Accurate mass EI (m/z) 349 (100%), 348 (M+, 32%). Anal. calcd for C21H29FO3: C, 72.38; H, 8.39. Found: C, 71.93; H, 8.58.
2.3. Biological activities of 2FEE2 and 2FPE2
2ME2 was purchased from Tetrionics (Madison, WI, USA). The stock solutions of 2ME2, 2FEE2 and 2FPE2 (0.1 mM) were made in DMSO and diluted in incubation media. LN229, U87 and 1A9 cells were cultured as described previously [6]. For generating hypoxic conditions, cells were placed in Modular Incubator Chambers (Billups-Rothenberg, Del Mar, CA, USA), which was flushed with 94% N2, 5% CO2 and 1% O2, and sealed.
2.3.1. Microtubule depolymerization
The human glioma cell lines, LN229 and U87, were treated with 5 μM of 2ME2, 2FEE2 and 2FPE2 overnight. Cells were fixed and processed for double-labeling immunofluorescence as previously described [6]. A Zeiss LSM 510 laser scanning confocal microscope, with a Zeiss X 100 1.3 oil-immersion objective, was used for image acquisition.
2.3.2. Cytotoxicity in cancer cell lines
The antiproliferative effects of 2FEE2 and 2FPE2 against the human ovarian carcinoma cell line, 1A9, and the human glioma cell line, LN229, were assessed in a 72-h growth inhibition assay with sulforhodamine-B as described in Refs. [14,15]. The 1A9/2MRC 2ME2-resistant clone, established in Dr. Paraskevi Giannakakou’s lab as a drug-resistant clone derivative of the 1A9 parent cell line, was also used [16]. Relative resistance for each compound is calculated as the IC50 value obtained for the 1A9/2MRC cells divided by that obtained for the parental, drug-sensitive 1A9.
2.3.3. HIF-1α down-regulation
LN229 cells and 1A9 cells were treated with three different concentrations of 2ME2, 2FEE2 or 2FPE2 overnight and then subjected either to hypoxia or normoxia for an additional 6 h. Whole-cell extracts were analyzed by SDS-PAGE and immunoblotted with an antibody against HIF-1α as previously described [6].
2.4. Radiochemistry
2[18F]FEE2 was synthesized from a precursor, 2-hydroxy-3,17β-O-bis(methoxy)methylestradiol, and a prosthetic group, [18F]fluoroethyl 4-bromobenzenesulfonate ([18F] fluoroethylbrosylate). H[18F]F was produced at Emory University with an 11-MeV Siemens RDS 112 negative-ion cyclotron (Knoxville, TN, USA) by the 18O (p,n) 18F reaction, using [18O]H2O. A chemistry process control unit (CPCU) from CTI, Inc. (Knoxville, TN, USA) was used for an automated synthesis of [18F]fluoroethylbrosylate. All the equipment for CPCU was prepared as previously described [17]. A Bioscan system 200 detector (Bioscan, Washington, DC, USA) was used for radiometric thin-layer chromatography.
2.4.1. Automated synthesis of [18F]fluoroethyl 4-bromobenzenesulfonate ([18F]fluoroethylbrosylate)
A schematic of the CPCU used to synthesize [18F] fluoroethylbrosylate is shown in Fig. 3 [16]. The kryptofix solution was added to the reaction vessel and 37 to 56 GBq (1-1.5 Ci) of H[18F]F in about 1 ml of [18O]H2O was delivered to a T/R cartridge. The activity was released to the vessel by passing potassium carbonate solution through a T/R cartridge. The vessel was heated at 110°C for 7 min with nitrogen flow to evaporate water. Three milliliters of acetonitrile was added to the vessel and evaporated for 8 min to dry K[18F]F completely. The vessel was cooled at room temperature for 2.5 min, to which the 1,2-ethanediol bis-(4-bromobenzenesulfonate) (dibrosylate) solution was added. The reaction mixture was heated at 90°C for 10 min. Following incorporation of F-18, 10 ml of ether was added to the vessel and the whole reaction mixture was passed through a silica SepPak and transferred to a 15-ml v-tube in a hot cell by nitrogen pressure.
Fig. 3.
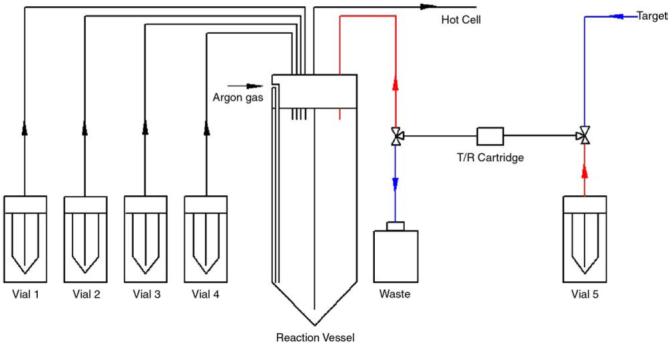
Diagram of a CPCU setup for a synthesis of [18F]fluoroethylbrosylate. Vial 1: 5 mg of Kryptofix2,2,2 in 1 ml of acetonitrile; a 1-ml v-vial. Vial 2: 3 ml of acetonitrile; a 5-ml v-vial. Vial 3: 7 mg of 1,2-ethanediol bis-(4-bromobenzenesulfonate) in 1 ml of acetonitrile; a 1-ml v-vial. Vial 4: 10 ml of diethyl ether; a 10-ml vial. Vial 5: 1 mg of potassium carbonate in 0.6 ml of low F-19 water; a 1-ml vial.
2.4.2. Radiolabeling of 2FEE2
[18F]Fluoroethylbrosylate was concentrated in an open 15-ml v-tube by evaporation of diethyl ether with nitrogen flow at room temperature. A 2-hydroxy-3,17β-O-bis(methoxymethyl)estradiol (3 mg) solution in DMF (300 μl), which contained 1 mg of NaH as a base, was added to the v-tube with a polyethylene syringe. The reaction mixture was heated at 140°C for 10 min in a silicone oil bath. One hundred microliters of 6N aqueous HCl was added to the reaction mixture and then heated at 125°C for 10 min. The reaction v-tube was cooled in a water bath at room temperature. One hundred microliters of 6N aqueous NaOH was added to the reaction tube at room temperature for neutralization. Three hundred microliters of HPLC eluent (50:50:0.1=CH3CN/H2O/Et3N) was added to the tube and the whole mixture was loaded onto a preparative HPLC loop. An X-Terra Prep RP18 HPLC column (19×100 mm, Waters) was used with 9 ml/min rate. Each minute fraction was collected, while radioactivity was monitored with a probe (Carrol-Ramsey Model 101-S-DC-P single-channel detector with preamplifier and standard probe, Carrol-Ramsey Associates, Berkley, CA, USA). Fraction 9 contained the desired 2[18F]FEE2.
2.4.3. Dose formulation of 2FEE2
The preparative HPLC fraction, which contained 2[18F] FEE2, was diluted with 30 ml of sterile water. The whole liquid was loaded onto a C-18 SepPak, which was preconditioned with 5 ml of ethanol, followed by 10 ml of sterile water. The C-18 SepPak was washed with 50 ml of saline, followed by 0.5 ml of ethanol. 2[18F]FEE2 was eluted with 1 ml of ethanol into a vial containing 9 ml of saline. The solution was filtered through two Gelman Teflon filters (1.0 and 0.22 μm pore size) by argon flow. Gelman filters were preconditioned with 3 ml of ethanol and dried with 3 ml of air.
2.4.4. Quality control of 2FEE2
An aliquot from a dose solution was tested for pH, radiochemical purity and chemical purity. pH was measured with a universal pH paper. Ten microliters of a dose solution was analyzed by HPLC. (C18, 5 μm, 3.9×150 mm, Waters; eluent: 50:50:0.1=CH3CN/H2O/Et3N; rate: 1 ml/min.) UV absorbance and radiometric detection were monitored at the same time, and each peak area was used for chemical purity and radiochemical purity measurement. A pure 2FEE2 solution was mixed with a dose solution, and the same retention time of hot and cold 2FEE2 confirmed the identity of 2[18F]FEE2.
A decay-corrected radiochemical yield at the end of synthesis, RCY, was based on an activity of H[18F]F at the end of bombardment. The specific activity of 2[18F]FEE2 at the end of synthesis was determined by measuring the radioactivity of 10 μl of a dose solution with a dose calibrator (Capintec CRC-712M, Ramsey, NJ, USA), which was decay corrected, and by measuring the UV peak area of an analytical HPLC. UV peak area was converted to an amount of 2-fluoroethoxyestradiol by using a standard curve.
2.5. Measurement of distribution coefficient (log P or log D)
Distribution coefficient of 2[18F]FEE2 was determined by a previously reported method [10,18].
3. Results and discussion
3.1. Chemistry
The syntheses of 2FEE2 and 2FPE2 were carried out as shown in Scheme 1. 2-Hydroxy-3,17β-O-bis(methoxymethyl) estradiol was synthesized in three steps from 3,17β-estradiol, purchased from Sigma-Aldrich, in 61% overall yield.
Scheme 1.
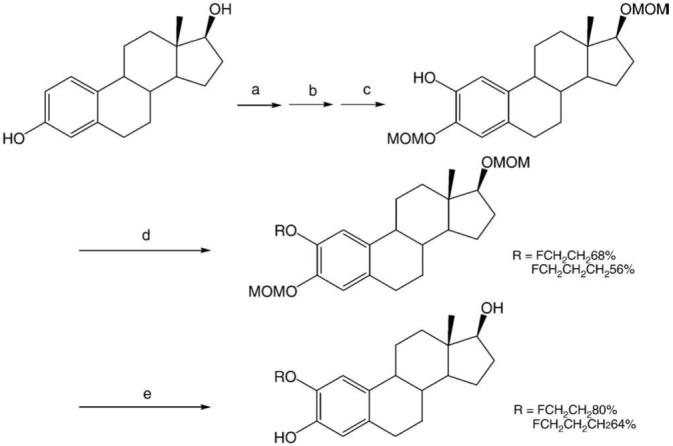
Syntheses of 2FEE2 and 2FPE2. Reagents and conditions: (a) MOM*Cl, N,N-diisopropylethylamine, THF. (b) (1) sec-BuLi, THF, 78°C; (2) trimethylborate, 0°C to rt, >30 min; (3) aqueous NH4Cl. (c) Aqueous sodium perborate tetrahydrate, EtOAc. (d) 1-Bromo-2-fluoroethane (or 1-bromo-3-fluoropropane), K2CO3, Bu4NI, DMF, 100°C, 24 h. (e) 6N aqueous HCl, THF, 1 h. *MOM=methoxymethyl.
2-Fluoroethoxy-O-methoxymethylestradiol was synthesized from 2-hydroxy-3,17β-O-bis(methoxymethyl)estradiol and an excess amount of 1-bromo-2-fluoroethane, using K2CO3 as a base in DMF at around 100°C in 24 h. Tetrabutylammonium iodide was used as a phase transfer catalyst. The same synthetic method was used for a synthesis of 2ME2 from 2-hydroxy-3,17β-O-bis(methoxymethyl)estradiol and methyl iodide [19,20].
The methoxymethyl group was removed by 6N aqueous hydrochloric acid at room temperature in 1 h. 2FEE2 was synthesized in 29% overall yield, starting from 3,17β-estradiol in five steps. 2FPE2 was synthesized by the same method in 22% overall yield.
3.2. Biological activities
LN229 or U87 cells were treated overnight with 5 μM of each of the indicated compounds. At the end of drug treatment, cells were fixed and stained with an antibody against tubulin (red). DNA was counterstained with SYTOX Green. Images were obtained with the Zeiss LSM 510 confocal microscope. Scale bar is at 10 μm.
2ME2 binds to tubulin at the colchicine binding site and promotes depolymerization of MT polymers into soluble αβ-tubulin dimers (Fig. 4). As a result, the dynamic equilibrium between MT polymers and αβ-tubulin dimers that normally exists in cells is shifted in favor of the soluble form of tubulin [6,11]. To examine the ability of 2FEE2 and 2FPE2 to depolymerize cellular MTs, we assessed the effects of each small molecule on the MT cytoskeleton in two human glioma cell lines, using indirect immunofluorescence followed by confocal microscopy. As shown in Fig. 4, the human LN229 (top panel) or the U87 (bottom panel) glioma cell lines were treated overnight with 5 μM of each compound. All three compounds exhibited comparable MT depolymerizing activities, following overnight treatment of the glioma cell lines.
Fig. 4.
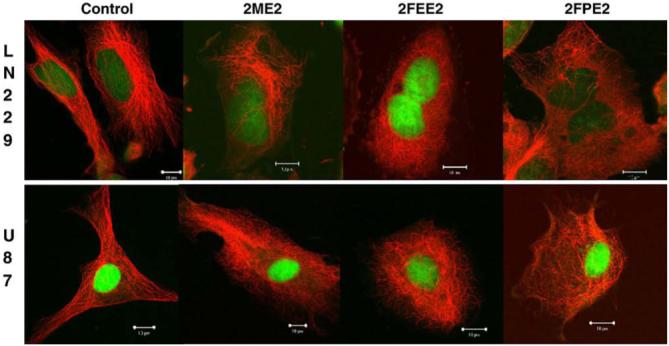
Effects of 2ME2 analogs on microtubule depolymerization.
Cells were plated in a 96-well plate and treated with the indicated drugs for 72 h. At the end of drug treatment, cells were fixed and total-cell protein was stained with sulforhodamine-B. IC50 values were determined at the drug concentration causing 50% cell growth inhibition, as compared to the control untreated cells. Relative resistance is calculated as the ratio of IC50 of the drug-resistant clone over that of the parental 1A9. Values stand for IC50 in micromolar.
Then, we examined the antiproliferative activities of 2ME2 and its analogs against the LN229, the 1A9 and the 1A9/2MRC cell lines as shown in Table 1. Using a 72-h cell growth-inhibition assay, we observed that 2FEE2 was about twofold more potent than 2ME2 against the LN 229, 1A9 cell lines. Interestingly, this compound exhibited a 77-fold loss in activity against the β-tubulin mutant, 2ME2-resistant 1A9/2MRC clone. This result suggests that the Vβ236I tubulin mutation that was acquired by the 1A9/2MRC cells as a result of drug-selection pressure (insert in Ref. [16]) is an important residue critically involved in the binding of both 2ME2 and 2FEE2 to tubulin. Finally, of the three compounds tested 2FPE2 was significantly (10-fold) less potent than 2ME2 for all three tumor cell lines, suggesting that the structural modifications introduced in this compound do not favor the drug’s interaction with tubulin.
Table 1.
Cytotoxicity profiles of 2ME2 analogs
| Compounds | Cell lines |
Relative resistance | ||
|---|---|---|---|---|
| LN 229 | 1A9 parental | 1A9/2ME2 Resistant (Vβ236I) | ||
| 2ME2 | 0.85 | 0.25 | 22.3 | 89 |
| 2FEE2 | 0.49 | 0.11 | 8.44 | 77 |
| 2FPE2 | 8.65 | 1.75 | 28 | 16 |
The LN229 (top panel) or 1A9 cells (bottom panel) were treated overnight with the respective drug concentrations (Fig. 5). Western blot analysis for HIF-1α is shown. N stands for normoxia, H stands for hypoxia.
Fig. 5.
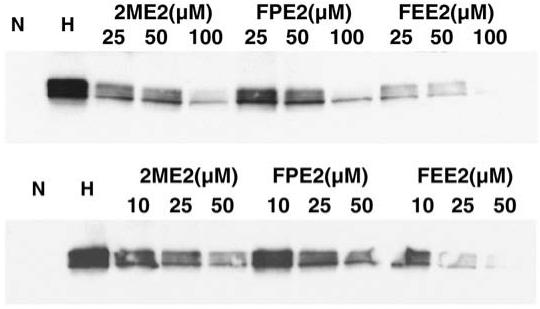
Effects of 2ME2 analogs on HIF-1α down-regulation.
Our previous work showed that 2ME2 treatment of a human prostate cancer cell line, PC-3, and a human breast cancer cell line, MDA-MB-231, reduced HIF-1α protein levels under hypoxia in a dose-dependent manner [6]. To explore the ability of 2ME2 or its analogs to inhibit HIF-1α, we treated the LN229 human glioma cell line or the 1A9 human ovarian carcinoma with three different concentrations of 2ME2, 2FEE2 or 2FPE2 as shown in Fig. 5. All three compounds were able to inhibit HIF-1α in a dose-dependent manner. Among the three compounds, 2FEE2 seemed somewhat more efficient than 2ME2, in down-regulating HIF-1α, consistent with this compound’s enhanced antiproliferative activity (Table 1).
3.3. Radiochemistry
2[18F]FEE2 was synthesized from 2-hyroxy-3,17β-O-bis (methoxymethyl)estradiol and a prosthetic group, [18F] fluoroethylbrosylate, in 8.3% decay-corrected yield in around 90 min (Scheme 2). The radiochemical purity and chemical purity of 2[18F]FEE2 were more than 98% based on radiometric peak areas and UV peak areas in analytical HPLC. The standard 2FEE2 and 2[18F]FEE2 were eluted at the same retention time (3.4 min), which confirmed the identity of 2[18F]FEE2. The identity of the material was also confirmed with radiometric TLC. The Rf values for two eluent systems (1:1=EtOAc/hexane, 2:1=EtOAc/hexane) were 0.5 and 0.8, respectively. These values were confirmed by cold TLC with phosphomolybdic acid (10% in ethanol) staining method. The specific activity of 2[18F]FEE2 was 1 Ci/μmol at the end of synthesis (n=5). pH of an aliquot from a dose vial of 2[18F]FEE2 was around 7. Log P of 2 [18F]FEE2 was 2.5 (n=4) at pH 7.
Scheme 2.
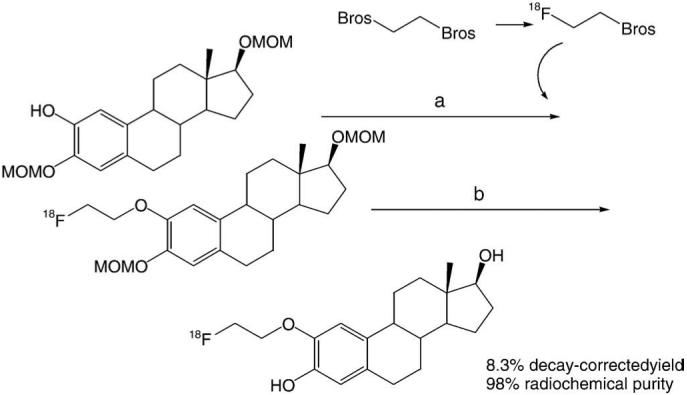
Synthesis of 2[18F]FEE2. Reagents and conditions: (a) (1) K18F, K222, CH3CN, 90°C, 10 min; (2) NaH, DMF, 140°C, 10 min. (b) (1) 6N aqueous HCl, 125°C, 10 min; (2) 6N aqueous NaOH, rt.
4. Conclusion
2FEE2 and 2FPE2 were synthesized as analogs of 2ME2, an endogenous antitumor molecule. These molecules contain an intrinsic site for F-18 labeling for positron emission tomographic measurement of pharmacokinetics and pharmacodynamics. 2FEE2 and 2FPE2 showed similar biological activities to 2ME2 regarding the drug-induced MT depolymerization and down-regulation of HIF-1α. Evaluation of their antiproliferative activities in a 72-h cell growth-inhibition assay showed that 2FEE2 was twofold more potent than 2ME2, whereas 2FPE2 was an order of magnitude less potent. Radiochemically pure 2[18F]FEE2 was synthesized by coupling a precursor, 2-hydroxy-3,17β-O-bis(methoxymethyl)estradiol, with a prosthetic group, 2-[18F]fluoroethylbrosylate, in 8.3% decay-corrected yield, based on a production of H[18F]F.
Acknowledgment
This research was supported in part by NIH RO1 (7 R01 CA100202-04), NIH RO1 (7 R01 CA114335) and Entremed awards made to Dr. Paraskevi Giannakakou.
We would like to acknowledge the use of Shared Instrumentation provided by grants from the NIH and the NSF for the mass spectroscopy data.
References
- [1].Ricker JL, Chen Z, Yang XP, Pribluda VS, Swartz GM, Van Waes C. 2-Methoxyestradiol inhibits hypoxia-inducible factor 1a, tumor growth, and angiogenesis and augments paclitaxel efficacy in head and neck squamous cell carcinoma. Clin Cancer Res. 2004;10:8665–73. doi: 10.1158/1078-0432.CCR-04-1393. [DOI] [PubMed] [Google Scholar]
- [2].Kang SH, Cho HT, Devi S, Zhang Z, Escuin D, Liang Z, et al. Antitumor effect of 2-methoxyestradiol in a rat orthotopic brain tumor model. Cancer Res. 2006;66:11991–7. doi: 10.1158/0008-5472.CAN-06-1320. [DOI] [PubMed] [Google Scholar]
- [3].Ray G, Dhar G, Van Veldhuizen PJ, Banerjee S, Saxena NK, Sengupta K, et al. Modulation of cell-cycle regulatory signaling network by 2-methoxyestradiol in prostate cancer cells is mediated through multiple signal transduction pathways. Biochemistry. 2006;45:3703–13. doi: 10.1021/bi051570k. [DOI] [PubMed] [Google Scholar]
- [4].Kimbro KS, Simons JW. Hypoxia-inducible factor-1 in human breast and prostate cancer. Endocr Relat Cancer. 2006;13:739–49. doi: 10.1677/erc.1.00728. [DOI] [PubMed] [Google Scholar]
- [5].D’Amato RJ, Lin CM, Flynn E, Folkman J, Hamel E. 2-Methoxyestradiol, and endogenous mammalian metabolite, inhibits tubulin polymerization by interacting at the colchicine site. Proc Natl Acad Sci U S A. 1994;91:3964–8. doi: 10.1073/pnas.91.9.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mabjeesh NJ, Escuin D, LaVallee TM, Pribluda VS, Swartz GM, Johnson MS, et al. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell. 2003;3:363–75. doi: 10.1016/s1535-6108(03)00077-1. [DOI] [PubMed] [Google Scholar]
- [7].James J, Murry DJ, Treston AM, Storniolo AM, Sledge GW, Sidor CM, et al. Phase I safety, pharmacokinetic and pharmacodynamic studies of 2-methoxyestradiol alone or in combination with docetaxel in patients with locally recurrent or metastatic breast cancer. Invest New Drugs. 2007;25:41–8. doi: 10.1007/s10637-006-9008-5. [DOI] [PubMed] [Google Scholar]
- [8].Mooberry SL. Mechanism of action of 2-methoxyestradiol: new developments. Drug Resist Updat. 2003;6:355–61. doi: 10.1016/j.drup.2003.10.001. [DOI] [PubMed] [Google Scholar]
- [9].Hamel E, Lin CM, Flynn E, D’Amato RJ. Interactions of 2-methoxyestradiol, an endogenous mammalian metabolite, with unpolymerized tubulin and with tubulin polymers. Biochemistry. 1996;35:1304–10. doi: 10.1021/bi951559s. [DOI] [PubMed] [Google Scholar]
- [10].Mun J, Voll RJ, Goodman MM. Synthesis of 2-[11C]methoxy-3,17β-estradiol to measure the pharmacokinetics of an antitumor drug candidate, 2-methoxy-3,17β-estradiol. J Labelled Comp Radioparm. 2006;49:1117–24. [Google Scholar]
- [11].Cushman M, He HM, Katzenellenbogen JA, Lin CM, Hamel E. Synthesis, antitubulin and antimitotic activity, and cytotoxicity of analogs of 2-methoxyestradiol, an endogenous mammalian metabolite of estradiol that Inhibits tubulin polymerization by binding the colchicine binding site. J Med Chem. 1995;38:2041–9. doi: 10.1021/jm00012a003. [DOI] [PubMed] [Google Scholar]
- [12].Cushman M, He HM, Katzenellenbogen JA, Varma RK, Hamel E, Lin CM, et al. Synthesis of analogs of 2-methoxyestradiol with enhanced inhibitory effects on tubulin polymerization and cancer cell growth. J Med Chem. 1997;40:2323–34. doi: 10.1021/jm9700833. [DOI] [PubMed] [Google Scholar]
- [13].Cushman M, Mohanakrishnan AK, Hollingshead M, Hamel E. The effect of exchanging various substituents at the 2-position of 2-methoxyestradiol on cytotoxicity in human cancer cell cultures and inhibition of tubulin polymerization. J Med Chem. 2002;45:4748–54. doi: 10.1021/jm020218r. [DOI] [PubMed] [Google Scholar]
- [14].Nicolaou KC, Namoto KR, Andreas UT, Shoji M, Li J, D’Amico G, et al. Chemical synthesis and biological evaluation of cis- and trans-12,13-cyclopropyl and 12,13-cyclobutyl epothilones and related pyridine side chain analogues. J Am Chem Soc. 2001;123:9313–23. doi: 10.1021/ja011338b. [DOI] [PubMed] [Google Scholar]
- [15].Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82:1107–12. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- [16].Escuin D, Wang Y, Lavallee T, Nettles JH, Snyder JP, Giannakakou P. Development and molecular characterization of a human ovarian cancer cell line resistant to 2-Methoxyestradiol (1A9-2ME2R) [abstract]; Proceedings of the 96th Annual Meeting of the American Association for Cancer Research; Anaheim, California (CA). 2005 Apr 16-20; 2005. p. 1202. Abstract nr 5092. [Google Scholar]
- [17].Voll RJ, McConathy J, Waldrep MS, Crowe RJ, Goodman MM. Semi-automated preparation of the dopamine transporter ligand [18F]FECNT for human PET imaging studies. Appl Radiat Isot. 2005;63:353–61. doi: 10.1016/j.apradiso.2005.04.008. [DOI] [PubMed] [Google Scholar]
- [18].Wilson AA, Houle S. Radiosynthesis of carbon-11 labeled N-methyl-2-(arylthio)benzylamines: potential radiotracers for the serotonin reuptake receptor. J Labelled Comp Radiopharm. 1999;42:1277–88. [Google Scholar]
- [19].He HM, Cushman M. A versatile synthesis of 2-methoxyestradiol, an endogenous metabolite of estradiol which inhibits tubulin polymerization by binding to the colchicine binding site. Bioorg Med Chem Lett. 1994;4:1725–8. doi: 10.1021/jm00012a003. [DOI] [PubMed] [Google Scholar]
- [20].Wang Z, Cushman M. An optimized synthesis of 2-methoxyestradiol, a naturally occurring human metabolite with anticancer activity. Syn Commun. 1998;28:4431–7. [Google Scholar]


