Abstract
To facilitate genetic studies in primary neurons, we analyzed the efficiency of cationic lipid-mediated plasmid DNA transfection using adherent and acutely dissociated neuronal suspensions derived from embryonic mouse cortical tissue. Compared to transfections using adherent cultures, the in-tube procedure enhanced the delivery of a GFP reporter plasmid between four- to eight-fold depending on the age of the harvested embryo. The procedure required relatively brief complex incubation times, and supported the transfection of cells expressing the neuronal markers NeuN and TuJ1 with improved uniformity in transfection events across the well surface. To demonstrate the utility of this approach in studying the genetic mechanisms controlling neuron development, we provide data regarding the role of the bZIP transcription factor c/EBP-β in regulating neurite outgrowth. It is anticipated that this in vitro protocol will facilitate the identification of novel genes involved in both developmental and disease-relevant signaling pathways.
Keywords: primary neuron, transfection, liposome, gene expression, high-throughput screening, in vitro assay
1.0 Introduction
It is estimated that the human genome expresses between 20,000 and 25,000 unique mRNAs that are ultimately translated into proteins. However, this estimate does not account for the range of expressed non-coding transcripts, which include tRNAs, rRNAs, snoRNAs and microRNAs (HGSC, 2004, Wright, et al., 2001). Moreover, only a fraction of the protein coding transcripts have been functionally annotated. This complexity is of particular relevance in the central nervous system given the range of neuron subtypes present in the various specialized brain regions (Muotri and Gage, 2006). Further transcript diversity is supported through alternative splicing and RNA editing, both of which can undergo dynamic regulation in response to physiologic and pathological perturbations (Blencowe, 2005). With the availability of comprehensive commercial cDNA and RNAi libraries, understanding the function of these unique transcripts in the context of disease-relevant models has become a tenable goal. However, the lack of cost-effective methods that support efficient, high-throughput gene delivery to primary neuronal cultures remains a rate limiting step in studying genetic responses within post-mitotic neurons.
Lipid mediated gene transfer is one of the most widely used techniques in basic cell biological research. In the neurosciences, transfection is often used to perturb gene function in tumor cell lines derived from neural or glial lineages. While these systems recapitulate various aspects of neuronal physiology, results obtained using transformed cell lines are an approximation of the signaling events occurring in the post-mitotic neuron. Unfortunately, the genetic manipulation of primary neuronal cultures remains a challenge given their resistance to lipid-mediated gene transfer using standard techniques. Although efficiencies as high as 25% have been reported using rat cortical neuronal cultures (Dalby, et al., 2004, Ohki, et al., 2001), most published studies report significantly lower transfection rates using adherent cultures with variability depending on the brain region analyzed (Ango, et al., 1999, Cattaneo, et al., 1996). And, since most published studies have used primary neurons from rat, it is unclear whether mouse derived primary tissue can support comparable rates of gene delivery.
Alternate approaches to lipid-based transfection have been devised to improve gene transfer rates in post-mitotic cells (Biewenga, et al., 1997, Leclere, et al., 2005). Because of their scalability and relative compatibility with in vivo disease modeling, the use of viral vector systems derived from herpes, adeno, adeno-associated and lentiviruses have become more common (Brooks, et al., 1998, Halterman, et al., 2006). However, implementation of this technique requires access to certified packaging facilities and strict adherence to the proper biosafety measures. Furthermore, packaging off-the-shelf shRNA or cDNA viral libraries for screening campaigns is beyond the physical and fiscal capacity of the typical academic research laboratory interested in performing focused genetic screens. Identification of techniques that enhance our ability to genetically modify primary neurons in vitro will accelerate the functional annotation and identification of disease-related genes.
To address this issue, we developed a novel transfection protocol for in vitro use using a standard cationic lipid preparation and cell culture techniques practiced in most research labs. Our results indicate that the in-tube transfection of acutely dissociated cortical neurons from mouse dramatically improves the efficiency of plasmid delivery. We also provide an example of how this approach may be of particular use for screening genes involved in neurite extension or in related developmental paradigms.
2.0 Materials and methods
2.1 Reagents
Optimem, Neurobasal media, B27 supplement and Lipofectamine 2000 were obtained from Invitrogen (Carlsbad, CA). The plasmid C2-eGFP containing the human CMV promoter and enhanced green fluorescent protein (eGFP) cDNA was used to track transfection efficiency (Clontech, Mountain View, CA). The full-length and dominant negative chicken c/EBP-β cDNA (DN-c/EBP) lacking the transcriptional activation domain (kindly provided by Richard M. Pope) were sub-cloned into the bi-cistronic plasmid HSVprPucCMVeGFP, which also expresses GFP under control of the human CMV promoter (Detrait, et al., 2002). DNA constructs were prepared using the Quantum midiprep kit according to the manufacturer’s recommendations (Bio-Rad, Hercules, CA).
2.2 Primary cultures
All protocols involving the use of laboratory animals received prior approval from the University of Rochester committee on animal resources (UCAR) and complied with all relevant federal guidelines. Multi-well tissue culture plates (96-well; Falcon, Cat #35-3070, Becton Dickenson, Franklin Lakes, NJ) were coated with 75 μl/well poly-L-lysine hydrobromide (Sigma P1274) prepared in borate buffer to 200 μg/ml and filter sterilized through a 0.2 μm filter. Plates were coated overnight /RT, rinsed three times with sterile ddH2O (150 μl/well) and allowed to air dry prior to use. Primary cortical cultures were prepared from timed pregnant C57Bl/6 mice (Charles River, Wilmington, MA) at either 13.5 or 15.5 days gestation (Brewer, et al., 1993). Batch processed hemispheres were incubated in 2 ml pre-warmed 0.25% trypsin/EDTA for 20 min, rinsed 3× in Ca2+/Mg2+-free Hanks balanced salt solution (HBSS) and triturated in Neurobasal medium supplemented with 0.5 mM L-glutamine, 25 uM glutamate and B27 supplement. Cells were diluted to a final concentration of 2×106 cells/ml. After transfection, cultures were maintained at 37°C in a 5% CO2 atmosphere.
2.3 Transfection procedures
Adherent monolayer cultures were transfected using Lipofectamine 2000 (0.05 –1.2 μl per well) according to the manufacturer’s instructions. The in-well procedure involved formation of the lipid:DNA mixture diluted each in 25 μl of Optimem directly in the poly-L-lysine coated 96-well plate prior to the addition of the cell suspension derived from E15.5 acutely dissociated cortices. For the in-tube modification, 100 μl of the cell suspension (2×105 cells) diluted in Neurobasal media was added to the 1.5 ml microfuge tubes (Phoenix Research Products, MAX-815) containing the preformed DNA:lipid complex diluted in OPTIMEM (50 μl). This mixture was allowed to stand at room temperature for between 10–40 min prior to plating in poly-lysine treated Falcon 96-well plates. After transfection, cultures were placed in a humidified 5% CO2 incubator for 24 h prior to analyses.
2.4 Transfection efficiency, nuclear pyknosis and neurite extension assays
Samples were imaged using an AxioObserver Z1 (Carl Zeiss, Gottingen, Germany). Fluorescent images were captured with a charged-coupled device (CCD) camera (ORCA-II, Hamamatsu, Japan). To determine transfection efficiency 96-well cultures were first fixed with 4% PFA for 15 min at room temperature, counter stained with Hoechst dye and imaged at 63× power. Eight non-overlapping fields from replicate wells (n=3) were analyzed for the number of GFP positive cells and Hoechst stained nuclei across each field. Field counts were summed and transfection ratios (# GFP positive cells/# Hoechst nuclei) were determined by averaging across replicate wells. Nuclear pyknosis was assessed under 63× magnification by counting the ratio of pyknotic to total nuclei (pyknotic/[pyknotic + heterochromatic]) from 6 non-overlapping fields across replicate wells (n=3). For the neurite assay, only those cells bearing one or more neurites greater than one cell body in the longest dimension were counted. An average of 450 cells from replicate coverslips (n=4) were analyzed.
2.5 Immunocytochemistry
Cells were fixed in 4% paraformaldehyde with 0.05% Triton X-100 for 20 min/RT and then rinsed 1× with phosphate buffered saline (PBS; 137 mM NaCl, 10 mM Phosphate, 2.7 mM KCl, and a pH of 7.4). Coverslips were processed by ICC by blocking cover slips for 1 h / RT in TBST-B (20 mM TrisHCl, pH 7.5, 0.15 M NaCl, 0.05% Triton X-100 containing 4.5% nonfat dry milk). Coverslips were transferred to a humidification chamber and placed inverted on a sheet of Parafilm holding primary antibody diluted in TBST-B for 1 h / RT: NeuN (1:100; Chemicon, Temecula, CA) and TuJ1 (1:200; Santa Cruz Biologicals, San Diego, CA; 1:200). Coverslips were washed three times in TBST-B and incubated with the appropriate fluorescent secondary antibody for 30 m / RT (1:1000; Alexa Dyes, Invitrogen). Control samples lacking the primary antibody were used to exclude non-specific staining. Samples were washed in PBS containing 10 μM Hoechst once and then twice in PBS before mounting in mowiol.
3.0 Results
3.1 Standard and in-well transfection of primary cortical neurons
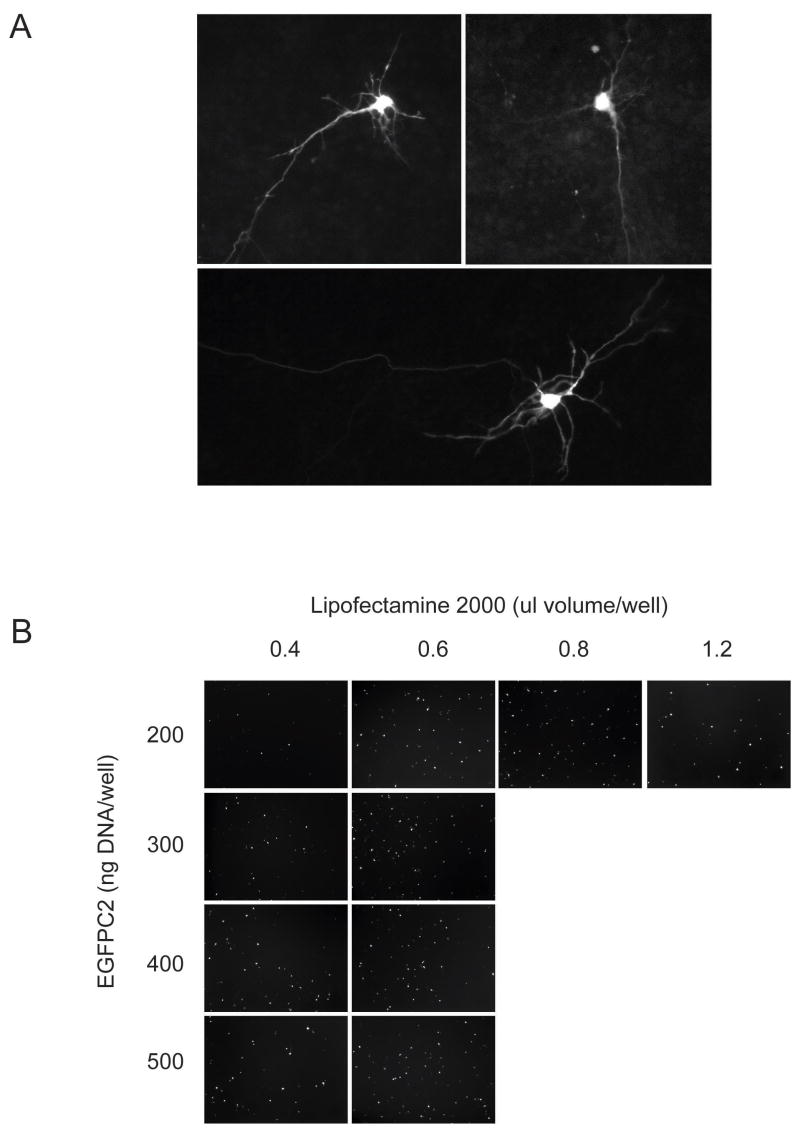
In our review of the literature, all reports describing lipid-based DNA transfection rates in dissociated neuronal cultures used rat-derived tissue. To determine whether mouse cultures would produce comparable results, we transfected DIV 4 adherent cortical mouse neuronal cultures using the manufacturers recommended protocol. Despite the relative inefficiency of gene delivery after 24 h in culture (1.0 ± 0.3%), many transfected cells exhibited features consistent with a neuronal phenotype including branched dendritic arbors and long axonal projections (Fig. 1A). Transfection of adherent cultures also resulted in a uniform distribution of targeted cells across the well surface.
Figure 1. Transfection of primary cortical neuronal cultures using standard approaches.
(A) Transfection results obtained using adherent DIV 4 mouse cortical cultures exposed to lipid:DNA complexes. Fluorescence microscopic images were obtained 24 h after transfection (40× power). (B) Optimization of the in-well transfection technique using acutely dissociated cortical neurons. After the DNA:lipid were allowed to complex in the poly-L-lysine treated microtiter plate, acutely dissociated neuron suspensions were added, and imaged after 24 h later for transfection efficiency. The images shown (20× power) represent those fields containing the highest density of transfection events.
Since transfection rates appear proportional to the rate of cellular division in immortalized lines (Pelisek, et al., 2002, Pickering, et al., 1994), we hypothesized that substitution of adherent post-mitotic neuronal cultures with acutely dissociated cortical hemispheric suspensions enriched in the fraction of dividing neural precursor cells would boost transfection rates. To test this we performed an in-well transfection wherein the DNA and lipid solutions were allowed to complex directly in the microtiter plate prior to the addition of the cell suspension. Under low-power magnification the in-well procedure produced a clustering of GFP+ cells at the well edge resulting in significant variability between field-to-field counts (data not shown). However the frequency of gene transfer within this region provided insight regarding optimal lipid:DNA ratios used in subsequent trials (Fig. 1B). While transfection efficiencies plateaued at 400 ng per well, the addition of increasing lipid volumes above 0.6 μl produced observable cytotoxicity (data not shown).
3.2 In-tube transfection using E13.5 cortical neurons
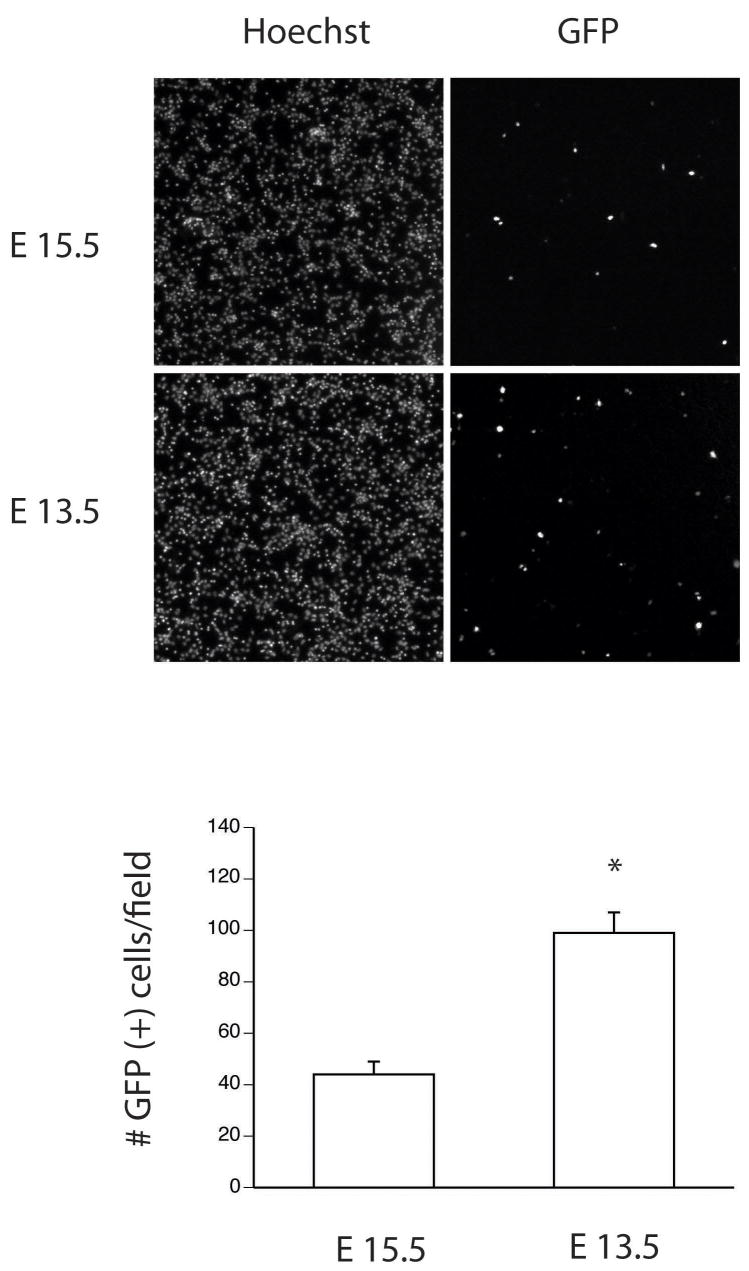
Batch transfection of immortalized lines is commonly used in multi-well assays and high-throughput screening campaigns. Although less important for enzyme or reporter based assays, inter-sample field-to-field variability can pose a challenge for certain image-based analyses. To address this issue, we tested whether mixing the preformed lipid-DNA complex and cell suspension prior to plating (referred to hereafter as “in-tube” transfection) would support efficient plasmid delivery and improve the uniformity of cell dispersal. The in-tube protocol modification was superior in both regards. Delivery rates of 4.0 ± 0.2% were achieved using 400 ng of reporter DNA and 0.5 ml lipid co-incubated with 200,000 suspension neurons (data not shown). Given these results, we hypothesized that dissociated tissue from younger embryos may support higher rates of transfection. To test this, we compared lipid mediated gene transfer in suspension cultures derived from E13.5 and E15.5 embryos. Results demonstrate that while the total cell yield obtained from E13.5 embryos was lower, transfection rates increased 2.2-fold with an absolute efficiency of 8.0 ± 1.7 % (Fig. 2).
Figure 2. In-tube transfection of E13.5 cortical cultures improves transfection rates.
Low power micrograph (4×) of transfection rates obtained using the in-tube transfection technique. Acutely dissociated primary cultures from E15.5 and E13.5 mice were transfected using the in-tube method and analyzed for the number GFP+ cells 24 h post-transfection. Hoechst 33342 staining was used to calculate the total cells per field counts. The data represent the average number of GFP+ cells obtained under 40× power from eight non-overlapping fields and significance was determined by Student’s t- testing (avg ± stdv; * = p < 0.02).
3.3 Effects of incubation time and poly-L-lysine coating
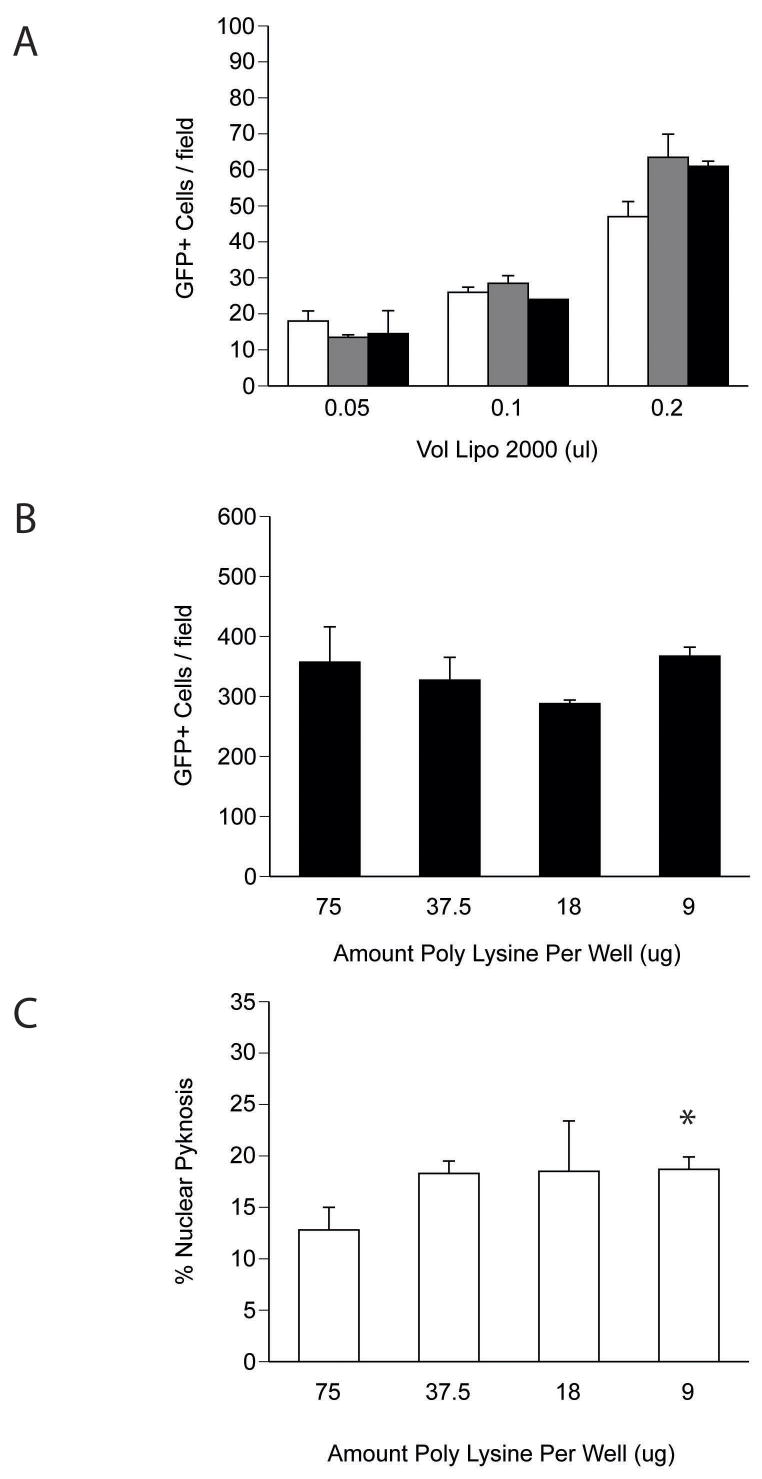
To optimize delivery rates further, we tested the influence of complex incubation time and poly-lysine concentration on culture transfection rates. Time-course analyses demonstrated that a 10 min incubation of the cell-lipid-DNA mixture was comparable to longer times and did not exhibit dependence on the volume of lipid used (Fig. 3A). Next, to determine whether charge interactions between the substrate and nucleic acid influenced gene transfer rates, we varied the amount of poly-lysine used to coat the plate while keeping the cell density (200,000 neurons/100 μl), DNA amount (400 ng) and lipid volume (0.5 μl) constant. Twenty-four hours after treatment, cultures were fixed and assessed for rates of transfection and survival. While varying poly-L-lysine at the time of plate coating had no effect on the number of GFP expressing cells per field, the use of higher doses of poly-L-lysine was associated with a trend toward improved cell survival (Fig. 3B–C).
Figure 3. Optimization of transfection parameters using dissociated suspension cortical neurons.
(A) Effect of incubation time on in-tube transfection rates. Cell-lipid-DNA complexes were incubated in 1.5 ml microtubes at RT for the times incubated before plating (10 min - open bars, 20 min - grey bars, 40 min - black bars). (b) Poly-L-lysine concentration does not influence transfection rates using the in-tube technique. The number of GFP+ cells per 20× field was analyzed across the range of poly-L-lysine doses used during the coating procedure. (C) Effect of poly-L-lysine on survival. DIV 1 transfected cultures were fixed and stained with Hoechst stain and the fraction of pyknotic to total nuclei was assessed as an indicator of cell viability. The data represent the percent of pyknotic nuclei observed under high power (63×) from five non-overlapping fields consisting of between 200–400 cells. Significance was determined by Student’s t-testing (avg ± stdv; * = p < 0.02, n=3 wells).
3.4 In-tube transfection facilitates genetic screening in vitro
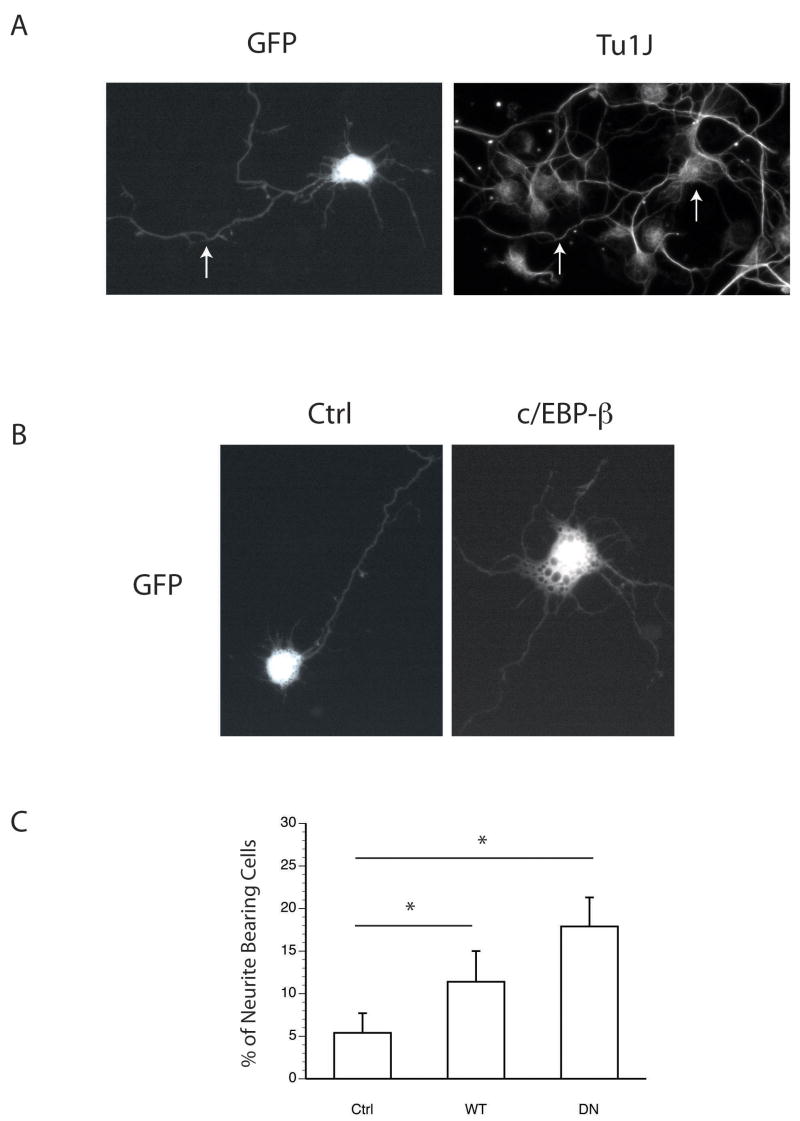
The bZIP transcription factor CAAT/enhancer-binding protein beta (c/EBP-β) supports the commitment of precursor stem cells to a neuronal fate and promotes neurite formation (Cortes-Canteli, et al., 2002, Nadeau, et al., 2005, Paquin, et al., 2005). c/EBP-β is translated in both a full-length (LAP) as well as an internally translated (LIP) form, and thus, can function both as an activator and repressor of transcription (Descombes and Schibler, 1991). It remains unclear whether one, or both of these divergent activities are required for neurite formation. To demonstrate the utility of the intube technique in facilitating functional genetic analyses, we tested the ability of either full-length c/EBP-β or its dominant negative form (DN-c/EBP), which lacks the transcriptional activation domain, to stimulate neurite formation in vitro following in-tube transfection. Although primary cultures prepared from E13.5–15.5 hemispheres contain few glial constituents, we first performed ICC using the post-mitotic neuronal marker NeuN (or TuJ1, as shown in Fig. 4) to confirm the identity of the GFP expressing cells. Results indicate that on average 52% of cells were strongly positive for both NeuN and GFP across control, c/EBP-β and DN-c/EBP constructs. Interestingly, delivery of c/EBP-β and DN-c/EBP both increased the number of GFP-positive cells bearing neurites (Fig.4B–C). These data indicate that in-tube technique targets neurons for transfection in vitro, and that both the trans-dominant inhibitory function and transcriptional activity of c/EBP-β were capable of stimulating neuritogenesis in nascent cortical neurons. In the larger context, these data indicate that in-tube transfection can be used to study the genetic determinants governing neurite in developing cortical cultures.
Figure 4. In-tube transfection facilitates genetic analyses in primary neuronal cultures.
(A) In-tube transfection targets cultured cells expressing the neuronal marker TuJ1. The arrows highlight the overlap between GFP signal and TuJ1 immunoreactivity in the cell soma and axon. Acute cultures were in-tube transfected and fixed at DIV 1 before immunostaining. (B–C) E15.5 cortical neurons were transfected with control, and c/EBP test plasmids as shown, and analyzed for the percent of cells bearing neurites > 1× the somal diameter. Data are presented as the average ± stdv and significance was determined by Student’s t-testing. (* = p < 0.05; n=4).
4.0 Discussion
In the current study we describe a rapid and convenient approach to conduct genetic screens in dissociated primary cortical neurons. We compared rates of transfection using DIV4 adherent cultures against those obtained with the in-well and intube techniques. While transfection of established monolayer cultures produced GFP+ cells with striking axonal and dendritic components, this technique was hampered by low transfection rates. Although more convenient, incubation of the lipid-DNA complex directly within the culture well prior to addition of the dissociated cell mass, resulted in a low-efficiency and non-homogeneous distribution of transfection events at the edge position where the DNA was initially delivered. This effect may be related to electrostatic interactions between the DNA, lipid and the charged lysine used to coat the culture plate. We addressed this issue by allowing the lipid:DNA complex and cell suspension to associate prior to plating. Increases in the available cell surface area for transfection and higher relative concentrations of DNA-complexed liposomes could also explain the observed improvement in overall transfection rates. Our results indicate optimum plasmid delivery was achieved using 400ng DNA, 0.5 μl lipid and 200,000 acutely dissociated E13.5 cortical neurons. This combination resulted in an eight-fold increase in transfection rates (1% to 8%) when compared with those obtained under standard conditions.
Early in cortical development, the subventricular zone supports the expansion of neural stem cells (NSC), neural progenitor cells (NPCs) and intermediate progenitor cells that migrate throughout the cortex and other brain regions before undergoing terminal differentiation (Pontious, et al., 2008). The proportion of these progenitor cells declines as the cortical mantle reaches maturity, and although neurogenesis continues into the adult period, it does so at a much slower rate (Alvarez-Buylla and Lois, 1995). Protocols used to prepare dissociated cortical cultures typically recommend harvesting tissue relatively late into the corticogenic period to enhance neuron yield (Finlay and Darlington, 1995). While cells in S-phase, exhibit higher competence for DNA transfer (Brunner, et al., 2000, Pelisek, et al., 2002), post-mitotic primary neurons, which contain an intact nuclear membrane, are particularly difficult to transfect. When we compared transfection rates between cortical preparations derived from E13.5 and E15.5 mice, we found that the younger embryos were superior in this regard. Although not directly tested, we speculate that this observation was due to enrichment of the set of transfection-competent progenitor cells.
While transfection rates were dramatically lower in established cultures, the morphological detail in terms of synapse length and dendrite complexity of GFP+ cells was impressive. Such morphological detail may be of value to those studying the genetic underpinnings of processes such as synaptogenesis and axoplasmic transport. Conversely, manipulation of nascent cortical neurons by acute transfection using the intube procedure would theoretically be better suited for developmentally oriented inquiry including the genetic mechanisms controlling neuritogenesis, cell fate and survival. While we found that transfected cells also express the neuronal markers NeuN and TuJ1, it remains to be seen whether particular neuron sub-types are more or less susceptible to in-tube mediated transfection. It will also be interesting to see whether alternative lipid formulations can improve upon transfection rates while reducing lipid-mediated toxicity.
Image-based, high-content functional genomic screens have been employed to dissect the genetic mechanisms regulating neurite outgrowth, cell proliferation and apoptotic signaling (Bailey, et al., 2006, Laketa, et al., 2007). Due to their scalability and the relatively low cost of maintenance, investigators often opt to use immortalized cell lines rather than primary cultures in these cell-based assays. Our protocol modifications have several practical implications for those interested in performing high-content screens in primary neuron cultures. First, improved transfection rates and well-to-well uniformity effectively reduce the number of wells needed to reach statistical significance in population studies. Second, these procedures should be easily scalable to accommodate assays across multiple conditions and time points. Determining whether this technique will permit the long-term study of acutely transfected, genetically modified neurons in vitro will require further study.
In summary, we describe a novel modification to standard transfection protocols that dramatically improves upon the low rates of transfection typically obtained using established in vitro cultured primary neuronal tissue. We believe that the methods outlined herein will enable others to address important biological questions regarding gene function in the developing and diseased post-mitotic neuron.
Acknowledgments
The authors would like to thank Jennifer Anstey for her assistance in the review and preparation of the manuscript.
This work was supported by grants from the National Institutes of Health [K99-NS060764 to MWH] and [R01-NS038569 to NFS].
References
- Alvarez-Buylla A, Lois C. Neuronal stem cells in the brain of adult vertebrates. Stem Cells. 1995;13:263–272. doi: 10.1002/stem.5530130307. [DOI] [PubMed] [Google Scholar]
- Ango F, Albani-Torregrossa S, Joly C, Robbe D, Michel JM, Pin JP, Bockaert J, Fagni L. A simple method to transfer plasmid DNA into neuronal primary cultures: functional expression of the mGlu5 receptor in cerebellar granule cells. Neuropharmacology. 1999;38:793–803. doi: 10.1016/s0028-3908(99)00005-2. [DOI] [PubMed] [Google Scholar]
- Bailey SN, Ali SM, Carpenter AE, Higgins CO, Sabatini DM. Microarrays of lentiviruses for gene function screens in immortalized and primary cells. Nat Methods. 2006;3:117–122. doi: 10.1038/nmeth848. [DOI] [PubMed] [Google Scholar]
- Biewenga JE, Destree OH, Schrama LH. Plasmid-mediated gene transfer in neurons using the biolistics technique. J Neurosci Methods. 1997;71:67–75. doi: 10.1016/s0165-0270(96)00127-6. [DOI] [PubMed] [Google Scholar]
- Blencowe BJ. Splicing on the brain. Nat Genet. 2005;37:796–797. doi: 10.1038/ng0805-796. [DOI] [PubMed] [Google Scholar]
- Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- Brooks AI, Halterman MW, Chadwick CA, Davidson BL, Haak-Frendscho M, Radel C, Porter C, Federoff HJ. Reproducible and efficient murine CNS gene delivery using a microprocessor-controlled injector. J Neurosci Methods. 1998;80:137–147. doi: 10.1016/s0165-0270(97)00207-0. [DOI] [PubMed] [Google Scholar]
- Brunner S, Sauer T, Carotta S, Cotten M, Saltik M, Wagner E. Cell cycle dependence of gene transfer by lipoplex, polyplex and recombinant adenovirus. Gene Ther. 2000;7:401–407. doi: 10.1038/sj.gt.3301102. [DOI] [PubMed] [Google Scholar]
- Cattaneo E, Conti L, Gritti A, Frolichsthal P, Govoni S, Vescovi A. Non-virally mediated gene transfer into human central nervous system precursor cells. Brain Res Mol Brain Res. 1996;42:161–166. doi: 10.1016/s0169-328x(96)00159-3. [DOI] [PubMed] [Google Scholar]
- Cortes-Canteli M, Pignatelli M, Santos A, Perez-Castillo A. CCAAT/enhancer-binding protein beta plays a regulatory role in differentiation and apoptosis of neuroblastoma cells. J Biol Chem. 2002;277:5460–5467. doi: 10.1074/jbc.M108761200. [DOI] [PubMed] [Google Scholar]
- Dalby B, Cates S, Harris A, Ohki EC, Tilkins ML, Price PJ, Ciccarone VC. Advanced transfection with Lipofectamine 2000 reagent: primary neurons, siRNA, and high-throughput applications. Methods. 2004;33:95–103. doi: 10.1016/j.ymeth.2003.11.023. [DOI] [PubMed] [Google Scholar]
- Descombes P, Schibler U. A liver-enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein, LIP, are translated from the same mRNA. Cell. 1991;67:569–579. doi: 10.1016/0092-8674(91)90531-3. [DOI] [PubMed] [Google Scholar]
- Detrait ER, Bowers WJ, Halterman MW, Giuliano RE, Bennice L, Federoff HJ, Richfield EK. Reporter gene transfer induces apoptosis in primary cortical neurons. Mol Ther. 2002;5:723–730. doi: 10.1006/mthe.2002.0609. [DOI] [PubMed] [Google Scholar]
- Finlay BL, Darlington RB. Linked regularities in the development and evolution of mammalian brains. Science. 1995;268:1578–1584. doi: 10.1126/science.7777856. [DOI] [PubMed] [Google Scholar]
- Halterman MW, Giuliano RE, Bowers WJ, Federoff HJ. Improved HSV-1 amplicon packaging using virion host shutoff mutants lacking mRNAse activity. J Gene Med. 2006;8:1320–1328. doi: 10.1002/jgm.972. [DOI] [PubMed] [Google Scholar]
- HGSC. Finishing the euchromatic sequence of the human genome. Nature. 2004;431:931–945. doi: 10.1038/nature03001. [DOI] [PubMed] [Google Scholar]
- Laketa V, Simpson JC, Bechtel S, Wiemann S, Pepperkok R. High-content microscopy identifies new neurite outgrowth regulators. Mol Biol Cell. 2007;18:242–252. doi: 10.1091/mbc.E06-08-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclere PG, Panjwani A, Docherty R, Berry M, Pizzey J, Tonge DA. Effective gene delivery to adult neurons by a modified form of electroporation. J Neurosci Methods. 2005;142:137–143. doi: 10.1016/j.jneumeth.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Muotri AR, Gage FH. Generation of neuronal variability and complexity. Nature. 2006;441:1087–1093. doi: 10.1038/nature04959. [DOI] [PubMed] [Google Scholar]
- Nadeau S, Hein P, Fernandes KJ, Peterson AC, Miller FD. A transcriptional role for C/EBP beta in the neuronal response to axonal injury. Mol Cell Neurosci. 2005;29:525–535. doi: 10.1016/j.mcn.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Ohki EC, Tilkins ML, Ciccarone VC, Price PJ. Improving the transfection efficiency of post-mitotic neurons. J Neurosci Methods. 2001;112:95–99. doi: 10.1016/s0165-0270(01)00441-1. [DOI] [PubMed] [Google Scholar]
- Paquin A, Barnabe-Heider F, Kageyama R, Miller FD. CCAAT/enhancer-binding protein phosphorylation biases cortical precursors to generate neurons rather than astrocytes in vivo. J Neurosci. 2005;25:10747–10758. doi: 10.1523/JNEUROSCI.2662-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelisek J, Engelmann MG, Golda A, Fuchs A, Armeanu S, Shimizu M, Mekkaoui C, Rolland PH, Nikol S. Optimization of nonviral transfection: variables influencing liposome-mediated gene transfer in proliferating vs. quiescent cells in culture and in vivo using a porcine restenosis model. J Mol Med. 2002;80:724–736. doi: 10.1007/s00109-002-0368-9. [DOI] [PubMed] [Google Scholar]
- Pickering JG, Jekanowski J, Weir L, Takeshita S, Losordo DW, Isner JM. Liposome-mediated gene transfer into human vascular smooth muscle cells. Circulation. 1994;89:13–21. doi: 10.1161/01.cir.89.1.13. [DOI] [PubMed] [Google Scholar]
- Pontious A, Kowalczyk T, Englund C, Hevner RF. Role of intermediate progenitor cells in cerebral cortex development. Dev Neurosci. 2008;30:24–32. doi: 10.1159/000109848. [DOI] [PubMed] [Google Scholar]
- Wright FA, Lemon WJ, Zhao WD, Sears R, Zhuo D, Wang JP, Yang HY, Baer T, Stredney D, Spitzner J, Stutz A, Krahe R, Yuan B. A draft annotation and overview of the human genome. Genome Biol. 2001;2:RESEARCH0025. doi: 10.1186/gb-2001-2-7-research0025. [DOI] [PMC free article] [PubMed] [Google Scholar]