Abstract
Homozygous expression of Orai1 bearing the R91W mutation results in the complete abrogation of currents through the store-operated Ca2+ release-activated Ca2+ (CRAC) channels, resulting in a form of hereditary severe combined immune deficiency (SCID) syndrome (Feske, S., Gwack, Y., Prakriya, M., Srikanth, S., Puppel, S. H., Tanasa, B., Hogan, P. G., Lewis, R. S., Daly, M., and Rao, A. (2006) Nature 441, 179–185). Although heterozygous carriers of the mutation show no clinical symptoms of immunodeficiency, store-operated Ca2+ entry in their T cells is impaired, suggesting a gene-dosage effect of the mutation. We have recently demonstrated that the functional CRAC channel pore is composed of a tetrameric assembly of Orai1 subunits (Mignen, O., Thompson, J. L., and Shuttleworth, T. J. (2008) J. Physiol. 586, 419–425). Therefore, to directly quantify the effect of the SCID mutant in the heterozygous situation, we generated a series of concatenated tetramers of Orai1 that included different numbers and arrangements of the R91W Orai1 subunits. The data obtained show that inclusion of increasing numbers of mutant subunits results in a graded reduction in CRAC channel currents and that this effect is independent of the spatial arrangement or order of the mutant subunits in the tetramer. Macroscopic biophysical properties of the channels were unchanged by inclusion of the mutant subunits, although the rate at which the current activates on store depletion was slowed. We conclude that incorporation of R91W mutant Orai1 subunits in the CRAC channel pore affects the overall magnitude of its conductance and that this effect is related solely to the number of mutant subunits incorporated. Predictions based on the tetrameric channel structure indicate that the graded effect of incorporation of SCID mutant subunits into such an assembly is quantitatively consistent with the previously demonstrated impaired effects on Ca2+ entry recorded in the heterozygous carriers.
Entry of Ca2+ via the store-operated Ca2+ release-activated Ca2+ (CRAC)3 channel is known to play a key role in the response to the presentation of antigen by T lymphocytes, inducing the activation of transcription factors, such as NFAT, resulting in the production of various cytokines and the differentiation and proliferation of the cells (1, 2). Consistent with this, studies of three distinct families with members displaying severe combined immune deficiency (SCID) have demonstrated that this condition resulted from defects in CRAC channel function (3–7). Subsequent studies involving a modified genetic linkage analysis of two siblings displaying a hereditary form of SCID syndrome revealed that this condition resulted from the homozygous expression of a novel plasma membrane protein named Orai1 bearing a single point mutation (R91W) (8). Critically, expression of the wild-type Orai1 protein in cells obtained from these patients reconstituted normal store-operated Ca2+ entry and CRAC channel currents, demonstrating that Orai1 was an essential component in CRAC channel activity (8). This was further confirmed in independent genome-wide Drosophila RNA interference screens designed to identify components critical for normal store-operated Ca2+ entry, which identified dOrai, the Drosophila homolog of mammalian Orai1, as an essential factor for such entry (8–10). Finally, studies showed that mutations of conserved acidic residues in predicted transmembrane regions of the protein (specifically Glu106 and Glu190) resulted in changes in permeation and selectivity of the conductance, confirming that Orai1 constitutes the key pore-forming subunit of the CRAC channel (11–13). An alternative view has been proposed in which Orai1 is believed to act as a regulatory subunit of TRPC channels rather than forming the channel pore itself (14). However, given that the TRPC proteins typically form essentially nonselective cation channels, such a proposal is difficult to reconcile with the highly calcium-selective nature of both the endogenous store-operated channels in HEK293 cells (15) and the conductances induced in these same cells by co-expression of Orai1 and STIM1 (16–18).
Consistent with the findings in the patients displaying the hereditary form of SCID, studies demonstrated that, unlike wild-type Orai1, expression of the R91W mutant Orai1 either alone or in the presence of an excess of the essential regulatory protein STIM1 (19–21) fails to generate any detectable CRAC channel currents (8, 22, 23). Despite this, heterozygous carriers of the R91W Orai1 mutation among members of the extended family of the two SCID patients displayed no obvious clinical symptoms of immunodeficiency (8). Further examination of store-operated Ca2+ entry in T lymphocytes taken from these individuals, however, revealed that under conditions of limiting external Ca2+ concentrations, such entry was significantly impaired in the heterozygous carriers. Based on these findings, it was suggested that the R91W Orai1 mutation probably exerts a gene-dosage effect on Ca2+ entry under heterozygous conditions (8). However, to date, there has been no direct demonstration or quantification of this suggested effect.
Previous studies had indicated that Orai1 was capable of assembling in multimeric complexes (12, 22, 24), although the precise stoichiometry of the Orai1 assembly that forms the functional channel pore remained unclear. In a recent study, we addressed this question using the approach of expressing preassembled concatenated constructs comprising different numbers of Orai1 subunits and examining the ability of a co-expressed dominant negative (E106Q) mutant Orai1 monomer to incorporate into the assembly and inhibit the resulting current. The results of this study revealed that the functional mammalian CRAC channel pore is composed of a tetrameric assembly of Orai1 subunits (25). The same conclusion was subsequently reached in a study using an optical approach involving a single-molecule fluorescence bleaching of TIRF images (26), although another study has recently claimed that the functional tetrameric Orai1 channel structure only forms upon interaction with STIM1 (27). In the study reported here, we have used the above approach involving the generation of specifically designed preassembled concatenated tetrameric constructs to quantitatively examine the effect of the incorporation of different numbers of the R91W SCID mutant on CRAC channel currents in the heterozygote situation. In this way, we were able to overcome the problem inherent in any experiment where the mutant subunits are simply expressed as monomers, resulting in the formation of multiple channel subtypes with different subunit composition and arrangements and thereby rendering detailed quantitative analysis of the effects impossible.
EXPERIMENTAL PROCEDURES
Constructs—A HEK293 cell line stably expressing STIM1 was generated using the Flp-In™-293 system (Invitrogen), to produce an isogenic STIM1-stable line, as previously described (16). Tetrameric Orai1 constructs were prepared by deleting the stop codon and inserting a 6-amino acid linker (QLNQLE) between each subunit, as previously described (25). Subunits bearing the R91W mutation were incorporated into these tetrameric constructs as appropriate, and the orientation and number of the subunits in the final constructs were confirmed by restriction analysis. Each construct was FLAG-tagged at the C-terminal end. To enable identification of individual cells that had been successfully transfected for use in the electrophysiological experiments, cells were transfected with the relevant tetrameric construct (0.75 μg of DNA) along with an enhanced yellow fluorescent protein construct (0.25 μg).
Electrophysiogical Recording—Whole cell recordings of macroscopic CRAC channel current were obtained using sequential 250-ms voltage pulses to +60 mV, and either –40 mV or –80 mV, as indicated, delivered every 2 s from a holding potential of 0 mV. The extracellular (bath) solution contained 140 mm NaCl, 1.2 mm MgCl2, 10 mm CaCl2, 5 mm CsCl, 30 mm d-glucose, 10 mm HEPES (pH 7.4). The internal (pipette) solution contained 140 mm Cs+ acetate, 4.17 mm MgCl2, 10 mm EGTA, 10 mm HEPES (pH 7.2). Calculated free [Mg2+] in this latter solution (3 mm) was designed to inhibit the activation of MIC/MagNum currents (28–30). This was confirmed by checking for any significant changes in the outward current at +60 mV. Experiments were discarded if any such changes occurred. CRAC channels were maximally activated by use of the Ca2+-free pipette solution together with the inclusion of adenophostin A (2 μm) in this solution. All experiments were carried out at room temperature (20–22 °C).
Western Blotting—48 h after transfection with the appropriate tetrameric construct, cells were washed with phosphate-buffered saline and lysed in 500 μl of lysis buffer containing 20 mm Tris-HCl, pH 7.5, 150 mm sodium chloride, 1 mm EDTA, 1 mm EGTA, 1% Triton X-100, 2.5 mm sodium pyrophosphate, 1 mm β-glycerophosphate, 1 mm sodium orthovanadate, 1 μg/ml leupeptin plus a mini complete protease inhibitor tablet (Roche Applied Science). Cells were sonicated and spun at 16,000 × g for 10 min at 4 °C. Supernatants were added to 4× lithium dodecyl sulfate gel loading dye (NuPage; Invitrogen) plus 50 mm dithiothreitol, heated to 70 °C for 10 min, and run on a 7% SDS-polyacrylamide gel, before transfer onto nitrocellulose. Blots were probed with 1 μg ml–1 anti-FLAG M2 monoclonal antibody (Sigma), followed by 1:2000 goat anti-mouse IgG-horseradish peroxidase secondary antibody (Bio-Rad). Labeled bands were visualized by chemiluminescence (Western Lightning; Pierce) and exposure to Biomax XAR film.
Data Analysis—All data are presented as mean ± S.E. Statistical significance was determined using Student's t test, with a value of p < 0.05 taken as significant.
RESULTS AND DISCUSSION
Whole cell patch clamp determinations in untransfected HEK293 cells in which store-operated currents were activated by using a Ca2+-free pipette solution containing the potent inositol 1,4,5-trisphosphate receptor agonist adenophostin A (2 μm) revealed the development of an inward current at negative internal potentials. This current developed relatively slowly, reaching a maximum value in ∼60–100 s (data not shown). Current/voltage analysis using ramps from –100 to +60 mV demonstrated that this current was markedly inwardly rectifying, with a reversal potential >+60 mV. Along with its appearance being strictly dependent on Ca2+ store depletion, these features are characteristic of CRAC channel activity. Maximum values of this inward current averaged 0.42 ± 0.03 pA/pF (n = 5) at –80 mV, a value that is very similar to that recorded in our previous studies of endogenous CRAC channel currents in these cells (15–17) and more recently by Peinelt et al. (18).
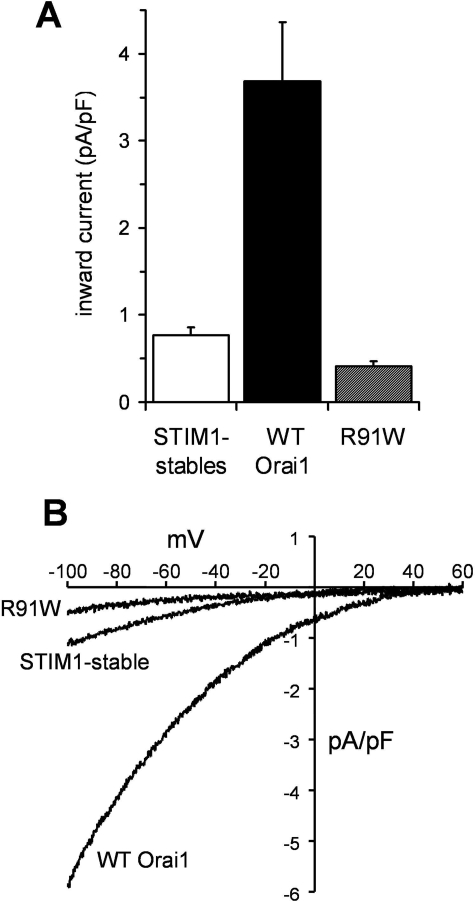
Because several studies have demonstrated an essential requirement for STIM1 in the regulation of CRAC channel activity (19, 20), all subsequent experiments used a HEK293 cell line that had been isogenically engineered to stably express a consistent level of STIM1 (STIM1-stable cells). Mean CRAC channel currents in these cells, measured as described above, were 0.77 ± 0.09 pA/pF (n = 5) at –80 mV (Fig. 1A), again similar to that recorded previously (16, 31). As has been demonstrated in various cell types, expression of wild-type Orai1 monomers in the cells stably expressing STIM1 profoundly increased CRAC channel currents (9, 13, 18, 32, 33). Under the transfection conditions used in our studies, this resulted in an approximately 4.8-fold increase in inward currents measured at –80 mV to reach a mean value of 3.68 ± 0.68 pA/pF (n = 14) at –80 mV (Fig. 1A). In contrast, under the same conditions, expression of a monomeric R91W (SCID) mutant Orai1 construct actually reduced inward CRAC channel currents in the STIM1-stable cells, to a mean value of only 0.41 ± 0.06 pA/pF (n = 13) at –80 mV (Fig. 1A). This reduction presumably reflects the association of the expressed mutant Orai1 subunits with endogenous wild-type Orai1 subunits, resulting in impaired CRAC channel activity, as has been previously reported (23). Despite the differences in magnitude, the current-voltage relationships all displayed the normal inward rectification and very positive reversal potential characteristic of CRAC channels (Fig. 1B).
FIGURE 1.
The effect of wild-type and R91W mutant Orai1 expression on CRAC channel currents. A, mean ± S.E. values for inward currents measured at –80 mV are shown for control STIM1-stable cells (n = 5) and for the same cells following transfection with either wild-type Orai1 (black column, n = 14), or Orai1 bearing the R91W mutation (hashed column, n = 13). B, representative current-voltage relationships recorded in untransfected STIM1-stable cells and the same cells transfected with either the wild-type Orai1 monomer or the R91W Orai1 monomer, as indicated.
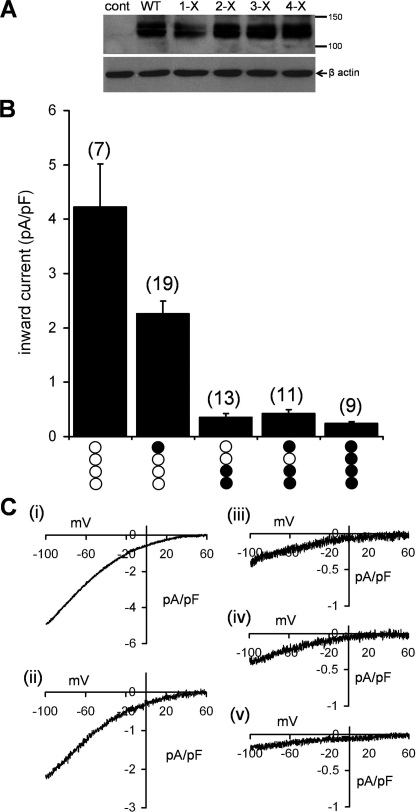
Given that the functional CRAC channel pore is comprised of a tetrameric assembly of Orai1 subunits (25, 26), the expression of both wild-type and mutant Orai1 in heterozygous carriers of the R91W mutation can result in channels displaying a variety of different combinations of these subunits. Therefore, to quantitatively examine the effect of the R91W SCID mutant in the heterozygous situation, we generated a series of FLAG-tagged concatenated tetramers of Orai1 that included different numbers and arrangements of the (R91W) mutant Orai1 subunits. These subunits were each linked together in the tetrameric construct by insertion of a short, 6-amino acid linker sequence between each subunit. Initially, five different constructs were assembled, as follows: the wild-type tetramer (WT-WT-WT-WT) and tetramers containing a single mutant subunit (WT-WT-WT-R91W), two mutant subunits (R91W-R91W-WT-WT), three mutant subunits (R91W-R91W-WT-R91W), and the fully mutant tetramer (R91W-R91W-R91W-R91W). Western blots of cell lysates obtained from populations of cells transfected with the FLAG-tagged tetramers showed that all constructs expressed as intact full-length tetrameric proteins running at an appropriate apparent molecular mass of ∼130–140 kDa (Fig. 2A).
FIGURE 2.
Expression of concatenated Orai1 tetramers incorporating various numbers of the R91W Orai1 subunits and their effects on CRAC channel currents. A, representative Western blot of the FLAG-tagged tetramers expressed in the STIM1-stable cells. The bands corresponding to the various tetrameric constructs each run at approximately the anticipated molecular mass of the tetrameric protein (∼130–140 kDa). The presence of a double band in each case probably reflects differentially glycosylated forms of the tetramer (22). Gels were stripped and reprobed with β-actin as a loading control. B, macroscopic inward CRAC channel currents measured in STIM1-stable cells expressing each of the five different tetrameric constructs. The composition of each construct is represented below each value (open circles, wild-type subunit; filled circles, R91W mutant subunits). Inward currents were measured at –80 mV. Values are mean ± S.E.; n values are indicated in brackets for each data set. C, representative current-voltage relationships obtained from the wild-type tetramer (i) and tetramers containing one R91W mutant subunit (ii), two mutant subunits (iii), three mutant subunits (iv), and four mutant subunits (v). Note the different scales for the current densities.
Analysis of the magnitude of macroscopic CRAC channel currents in cells expressing the wild-type tetrameric construct averaged 4.23 ± 0.79 pA/pF (n = 7) at –80 mV (Fig. 2B), a value very similar to that obtained in our earlier study (25). Substitution of a single wild-type subunit with the R91W mutant subunit in such a tetramer resulted in an approximately 50% reduction in the recorded currents (Fig. 2, B and C). Incorporating an additional mutant subunit into the tetramer further reduced the recorded current to essentially negligible values (<0.4 pA/pF at –80 mV), and inclusion of additional mutant subunits in the tetrameric constructs failed to further significantly affect these very small currents (Fig. 2, B and C).
It should be noted that this graded effect of the incorporation of R91W SCID mutant subunits in the tetrameric Orai1 channel pore assemblies differs markedly from that seen with the E106Q Orai1 pore mutant. This latter mutation has been reported to act as a dominant negative for Ca2+ conductance in the CRAC channel (11, 12, 22, 31), and, as we have previously reported (25), the inclusion of only a single E106Q mutant subunit in a preassembled tetrameric Orai1 construct effectively eliminates the resulting CRAC channel currents.
As noted above, we found that the inclusion of only two R91W SCID mutant subunits in the Orai1 tetrameric constructs was sufficient to effectively reduce currents through the CRAC channel to negligible values. However, in the experiments described above, the two mutant subunits were incorporated into the tetramer adjacent to each other in a tandem pairing. We therefore wondered whether the observed effect may be altered if the two mutant subunits were separated in the linear tetrameric sequence by wild-type subunits. We therefore designed an R91W-WT-R91W-WT tetrameric construct. Expression of this in the STIM1-stable cells resulted in inward CRAC channel currents of 0.34 ± 0.04 pA/pF (n = 7) at –80 mV, a value that was not significantly different from that recorded with the R91W-R91W-WT-WT construct (0.36 ± 0.07 pA/pF, n = 13). We conclude that the magnitude of the resulting CRAC channel current is unaffected by the placement or spatial arrangement of these mutant subunits around the channel pore.
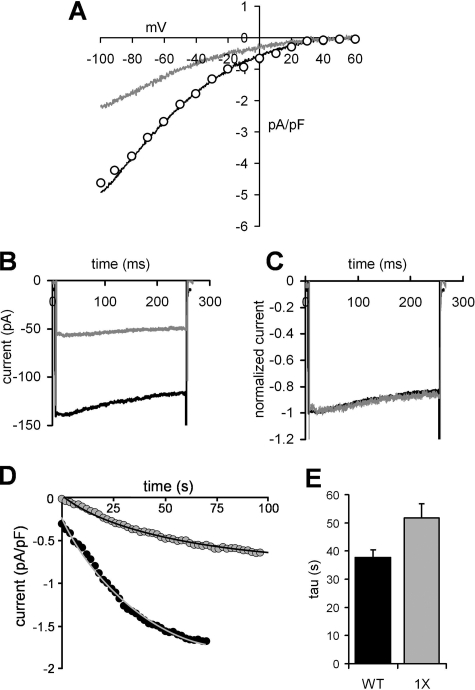
As yet, it is unclear exactly how the R91W mutation acts to affect the behavior of the CRAC channel. Previous studies have indicated that the mutant subunits are able to co-assemble with the wild-type Orai1 and that resulting channels appear to traffic to the plasma membrane normally (8, 23, 34). Moreover, the physical interaction between STIM1 and expressed R91W Orai1 monomers, at least as assessed by an increase in fluorescence resonance energy transfer following store depletion, is indistinguishable from that observed with the wild-type Orai monomers (23, 34). Of course, although these data indicate that the R91W mutation does not affect the physical association of STIM1 and Orai1, it may still impair the functional interaction between these two proteins. Consistent with this is the finding that although the apparent physical interactions between STIM1 and Orai1 are unaffected by deletions of the entire N terminus of Orai1 (amino acids 1–88 or 1–90), such deletions do result in the complete loss of measurable CRAC channel currents (23, 24). Of course, such large scale deletions reveal little as to the underlying basis of the observed effects. Moreover, with specific regard to the SCID mutation, neither of the reported deletions actually removed the critical Arg91 residue. Based on this, we chose to examine whether the inclusion of mutant subunits in the Orai1 tetramers might result in changes to the overall biophysical characteristics of the channel conductance, particularly those features of the macroscopic current that might result in an apparent reduced current magnitude, namely shifts in the current-voltage relationship and changes in the magnitude of fast inactivation. Simple visual comparison of representative current-voltage relationships obtained with the wild-type tetramer and those seen in the experiments with the tetramer incorporating a single R91W mutant subunit indicated that both displayed similar marked inward rectification and very positive reversal potentials (Fig. 3A). To more accurately evaluate the two curves, the values for the single R91W mutant tetramer were scaled by an appropriate factor to allow direct comparison of the two curves (Fig. 3A). The results demonstrate that the fundamental current-voltage relationships of the two currents are identical, with the only difference being in their respective magnitudes. We next turned to an examination of the extent of fast inactivation of the recorded currents. Fast inactivation is a characteristic feature of CRAC channel currents and is observed as a decay in the current magnitude during brief pulses to negative potentials. Studies have shown that the extent of this inactivation is dependent on the amount of Ca2+ entering via the channel, and it is believed to involve Ca2+-sensitive sites positioned within a few nanometers of the cytosolic face of the channel pore (35). Examination of the extent of fast inactivation of the CRAC channel current measured in cells expressing the wild-type tetramer during brief pulses to negative potentials were compared with those recorded under the same conditions in cells expressing the single R91W mutant tetramer (Fig. 3B). The data obtained demonstrate that the percent inactivation of the current during brief pulses to –120 mV was identical in the two groups. This is best seen when the two currents are normalized to the same maximum value (Fig. 3C). Analyses from several such experiments resulted in a mean fast inactivation of 17.8 ± 1.6% (n = 5) at –120 mV for the cells expressing the wild-type tetramer versus 15.0 ± 2.6% (n = 5) for cells expressing the single R91W mutant tetramer. Clearly, the data obtained indicate that the observed graded inhibition of currents in Orai1 tetramers incorporating the R91W mutation does not involve changes in either of these characteristic features of the macroscopic CRAC channel current.
FIGURE 3.
The effect of inclusion of a single R91W mutant subunit in the concatenated Orai1 tetramer on the biophysical properties of the CRAC channel currents. A, comparison of representative current-voltage relationships recorded in cells transfected with the wild-type Orai1 tetramer (black line) or the Orai1 tetramer incorporating a single R91W mutant subunit (gray line). Also shown are values from this latter trace scaled by a factor of 2.1 (open circles). B, representative current traces recorded during a brief pulse to –120 mV taken from a cell expressing the wild-type Orai1 tetramer (black line) or a cell expressing the Orai1 tetramer incorporating a single R91W mutant subunit (gray line). C, the same individual traces shown in B normalized to their respective maximum values. D, representative traces showing the rate of adenophostin A-induced activation of CRAC channel currents measured at –40 mV upon achieving the whole cell configuration in a cell expressing the wild-type tetramer (black circles) and a cell expressing the Orai1 tetramer incorporating a single R91W mutant subunit (gray circles). The lines represent the fitted exponential curves to the respective current values. E, mean ± S.E. values for the calculated time constant for activation (tau) determined from fits of the activation traces to simple single exponential curves for cells expressing the wild-type Orai1 tetramer (black column, n = 22) and the Orai1 tetramer containing a single R91W mutant subunit (gray column, n = 18).
Recently, Muik et al. (23) have reported that the co-expression of wild-type Orai1 monomers along with R91W monomers resulted in an activation of CRAC channel currents following passive store depletion (using a Ca2+-free, EGTA-buffered internal solution) that was significantly slower than that observed in cells expressing the wild-type Orai monomer alone. However, the magnitude of this effect was not quantified. We therefore examined whether the same effect could be observed in our experiments with the defined tetrameric Orai1 assemblies. To more precisely control the response, we used the more rapid method of depleting stores involving the inclusion of the potent inositol 1,4,5-trisphosphate receptor agonist adenophostin A in the Ca2+-free intracellular (pipette) solution. The data obtained using this approach showed that the rate of increase in CRAC channel currents following store depletion could be adequately described by a simple single exponential curve (Fig. 3D). Analysis of these curves revealed that the current in cells expressing the wild-type Orai1 tetramer developed with a time constant (tau) equal to 37.7 ± 2.7 s (n = 22) (Fig. 3E). This was increased to a value of 51.7 ± 5.1 s (n = 18) in the cells expressing the tetramer containing a single R91W subunit, an increase of some 37% (p = 0.03), confirming the earlier findings of Muik et al. (23). However, the underlying basis for the observed slower activation remains unclear.
In summary, the data presented here show that the only factor affected by incorporation of the R91W (SCID) mutation into the CRAC channel appears to be the overall magnitude of the macroscopic channel conductance, and this effect is related solely to the number of mutant subunits incorporated, not on their position or order in the tetramer. How this affect is achieved, however, remains unclear. Potential actions fall into two groups: effects on permeation and effects on channel gating. A recent study noted that the changes in fluorescence resonance energy transfer between adjacent expressed Orai1 subunits that were recorded upon activation of the channel were still observed in cells expressing the R91W mutant Orai (34), leading the authors to suggest that that gating of the channel was unlikely to have been affected and that a defect in permeation was the more likely cause. However, detailed evaluation of these possibilities would probably require analysis at the single channel level. Unfortunately, with existing estimates of the single channel conductance of the CRAC channel of only ∼20 femtosiemens, or less than 1% of that of voltage-gated Ca2+ channels (29), such analyses are currently not feasible.
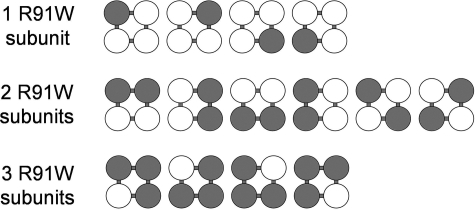
Our data quantifying the graded effect of incorporation of the R91W mutation on macroscopic CRAC channel currents enables us to estimate the possible effects in heterozygous carriers of this mutation. For this, it is necessary to consider all the possible combinations of channel structures that could be generated in the heterozygous condition (Fig. 4). Thus, a single mutant monomer can incorporate into a tetrameric structure in a total of four different ways; two mutants can incorporate in six different ways; and three can incorporate mutants in four different ways. Together with the WT homotetramer and the R91W homotetramer, this gives a total of 16 different possibilities. Of these possible combinations, only WT homotetramers (full current) and the single mutant tetramers (52% of full current) result in significant currents. Given this, the predicted total CRAC channel current in heterozygotes would be (1 × 100) + (4 × 52)/16 = 19.25% of the normal full current. It is interesting to compare this theoretical value obtained from experiments involving the incorporation of R91W mutant subunits in the preassembled Orai1 tetramers with the effects actually recorded in heterozygous carriers of this mutation. As noted above, studies of members of the extended family of the two siblings bearing the R91W mutation indicated a reduction in the rate of overall CRAC-mediated Ca2+ entry in T cells obtained from the heterozygous carriers of the mutation (8). Although showing significant individual variability, the data indicate that, under limiting external Ca2+ concentrations (0.5-0.2 mm), mean store-operated T cell Ca2+ entry in the heterozygote mutant-carrying relatives was equivalent to ∼33% of that recorded in homozygous wild-type family relatives.4 This value is slightly larger than that predicted above. However, it is important to note that our analysis assumes, among other things, that the wild-type and R91W mutant proteins express at equivalent levels, that their processing and trafficking to the plasma membrane is similar, and that they assemble and incorporate into the channel pore in an equivalent manner. Thus, the small apparent difference in percentage inhibition in the theoretical analysis compared with those actually recorded in heterozygous relatives could easily be explained by some fraction of the nonfunctional pool of channels (i.e. any channel containing two or more R91W subunits) being effectively eliminated (e.g. as a result of an impaired ability to successfully assemble as a tetramer). Nevertheless, it is clear that our predicted overall currents based on measurements of preassembled channels expressed in a cell line are, at least superficially, broadly consistent with the observed rates of Ca2+ entry in cells obtained from the relevant heterozygous carriers of the mutation.
FIGURE 4.
Determining the number of possible combinations of wild-type and mutant subunits in tetrameric assemblies. Illustrated are the different ways in which various numbers (1–3) of mutant subunits could incorporate into a tetramer (open circles, wild-type subunits; filled circles, mutant subunits). For example, a single mutant subunit can insert at four different possible positions in a tetrameric construct. Note that although all four possibilities are spatially identical, they are sequentially distinct. Similarly, two mutant subunits can incorporate in six different ways, and three mutant subunits can incorporate in four different ways. See “Results and Discussion” for details.
Finally, it should be noted that the data we have obtained serve to emphasize the utility of such preassembled constructs of the channel pore and the additional insights that can be obtained from the expression of appropriately modified “designer constructs” in elucidating the detailed effects of molecular and pharmacological manipulations on such specifically defined assemblies. As has already proven to be the case for a variety of channels and receptors, the use of such concatenated constructs will likely prove to be a powerful tool in further examination of the properties and behavior of both CRAC channels and other members of the Orai-based family of channel proteins.
Acknowledgments
We thank Dr. Stefan Feske (New York University) for helpful comments on an early draft of the manuscript and for providing original data on the Ca2+ entry rates in heterozygous members of the extended family of the two SCID patients. We also thank Pauline Leakey for excellent technical assistance.
This work was supported by National Institutes of Health Grant GM040457 (to T. J. S.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: CRAC, calcium release-activated Ca2+; SCID, severe combined immune deficiency; pF, picofarads; WT, wild type.
S. Feske, personal communication.
References
- 1.Lewis, R. S. (2001) Annu. Rev. Immunol. 19 497–521 [DOI] [PubMed] [Google Scholar]
- 2.Feske, S. (2007) Nat. Rev. Immunol. 7 690–702 [DOI] [PubMed] [Google Scholar]
- 3.Partiseti, M., Le Deist, F., Hivroz, C., Fischer, A., Korn, H., and Choquet, D. (1994) J. Biol. Chem. 269 32327–32335 [PubMed] [Google Scholar]
- 4.Le Deist, F., Hivroz, C., Partiseti, M., Thomas, C., Buc, H. A., Oleastro, M., Belohradsky, B., Choquet, D., and Fischer, A. (1995) Blood 85 1053–1062 [PubMed] [Google Scholar]
- 5.Feske, S., Muller, J. M., Graf, D., Kroczek, R. A., Drager, R., Niemeyer, C., Baeuerle, P. A., Peter, H. H., and Schlesier, M. (1996) Eur. J. Immunol. 26 2119–2126 [DOI] [PubMed] [Google Scholar]
- 6.Feske, S., Giltnane, J., Dolmetsch, R., Staudt, L. M., and Rao, A. (2001) Nat. Immunol. 2 316–324 [DOI] [PubMed] [Google Scholar]
- 7.Feske, S., Prakriya, M., Rao, A., and Lewis, R. S. (2005) J. Exp. Med. 202 651–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feske, S., Gwack, Y., Prakriya, M., Srikanth, S., Puppel, S. H., Tanasa, B., Hogan, P. G., Lewis, R. S., Daly, M., and Rao, A. (2006) Nature 441 179–185 [DOI] [PubMed] [Google Scholar]
- 9.Zhang, S. L., Yeromin, A. V., Zhang, X. H., Yu, Y., Safrina, O., Penna, A., Roos, J., Stauderman, K. A., and Cahalan, M. D. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 9357–9362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vig, M., Peinelt, C., Beck, A., Koomoa, D. L., Rabah, D., Koblan-Huberson, M., Kraft, S., Turner, H., Fleig, A., Penner, R., and Kinet, J. P. (2006) Science 312 1220–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeromin, A. V., Zhang, S. L., Jiang, W., Yu, Y., Safrina, O., and Cahalan, M. D. (2006) Nature 443 226–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vig, M., Beck, A., Billingsley, J. M., Lis, A., Parvez, S., Peinelt, C., Koomoa, D. L., Soboloff, J., Gill, D. L., Fleig, A., Kinet, J. P., and Penner, R. (2006) Curr. Biol. 16 2073–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prakriya, M., Feske, S., Gwack, Y., Srikanth, S., Rao, A., and Hogan, P. G. (2006) Nature 443 230–233 [DOI] [PubMed] [Google Scholar]
- 14.Liao, Y., Erxleben, C., Yildirim, E., Abramowitz, J., Armstrong, D. L., and Birnbaumer, L. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 4682–4687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mignen, O., and Shuttleworth, T. J. (2000) J. Biol. Chem. 275 9114–9119 [DOI] [PubMed] [Google Scholar]
- 16.Mignen, O., Thompson, J. L., and Shuttleworth, T. J. (2007) J. Physiol. 579 703–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mignen, O., Thompson, J. L., and Shuttleworth, T. J. (2008) J. Physiol. 586 185–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peinelt, C., Vig, M., Koomoa, D. L., Beck, A., Nadler, M. J., Koblan-Huberson, M., Lis, A., Fleig, A., Penner, R., and Kinet, J. P. (2006) Nat. Cell Biol. 8 771–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roos, J., DiGregorio, P. J., Yeromin, A. V., Ohlsen, K., Lioudyno, M., Zhang, S., Safrina, O., Kozak, J. A., Wagner, S. L., Cahalan, M. D., Velicelebi, G., and Stauderman, K. A. (2005) J. Cell Biol. 169 435–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang, S. L., Yu, Y., Roos, J., Kozak, J. A., Deerinck, T. J., Ellisman, M. H., Stauderman, K. A., and Cahalan, M. D. (2005) Nature 437 902–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liou, J., Kim, M. L., Heo, W. D., Jones, J. T., Myers, J. W., Ferrell, J. E., Jr., and Meyer, T. (2005) Curr. Biol. 15 1235–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gwack, Y., Srikanth, S., Feske, S., Cruz-Guilloty, F., Oh-Hora, M., Neems, D. S., Hogan, P. G., and Rao, A. (2007) J. Biol. Chem. 282 16232–16243 [DOI] [PubMed] [Google Scholar]
- 23.Muik, M., Frischauf, I., Derler, I., Fahrner, M., Bergsmann, J., Eder, P., Schindl, R., Hesch, C., Polzinger, B., Fritsch, R., Kahr, H., Madl, J., Gruber, H., Groschner, K., and Romanin, C. (2008) J. Biol. Chem. 283 8014–8022 [DOI] [PubMed] [Google Scholar]
- 24.Li, Z., Lu, J., Xu, P., Xie, X., Chen, L., and Xu, T. (2007) J. Biol. Chem. 282 29448–29456 [DOI] [PubMed] [Google Scholar]
- 25.Mignen, O., Thompson, J. L., and Shuttleworth, T. J. (2008) J. Physiol. 586 419–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ji, W., Xu, P., Li, Z., Lu, J., Liu, L., Zhan, Y., Chen, Y., Hille, B., Xu, T., and Chen, L. (2008) Proc. Natl. Acad. Sci. U. S. A. 105 13668–13673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Penna, A., Demuro, A., Yeromin, A. V., Zhang, S. L., Safrina, O., Parker, I., and Cahalan, M. D. (2008) Nature 456 116–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kozak, J. A., Kerschbaum, H. H., and Cahalan, M. D. (2002) J. Gen. Physiol. 120 221–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prakriya, M., and Lewis, R. S. (2002) J. Gen. Physiol. 119 487–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hermosura, M. C., Monteilh-Zoller, M. K., Scharenberg, A. M., Penner, R., and Fleig, A. (2002) J. Physiol. 539 445–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lis, A., Peinelt, C., Beck, A., Parvez, S., Monteilh-Zoller, M., Fleig, A., and Penner, R. (2007) Curr. Biol. 17 794–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soboloff, J., Spassova, M. A., Tang, X. D., Hewavitharana, T., Xu, W., and Gill, D. L. (2006) J. Biol. Chem. 281 20661–20665 [DOI] [PubMed] [Google Scholar]
- 33.Mercer, J. C., Dehaven, W. I., Smyth, J. T., Wedel, B., Boyles, R. R., Bird, G. S., and Putney, J. W., Jr. (2006) J. Biol. Chem. 281 24979–24990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Navarro-Borelly, L., Somasundaram, A., Yamashita, M., Ren, D., Miller, R. J., and Prakriya, M. (2008) J. Physiol. 586 5383–5401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zweifach, A., and Lewis, R. S. (1995) J. Gen. Physiol. 105 209–226 [DOI] [PMC free article] [PubMed] [Google Scholar]