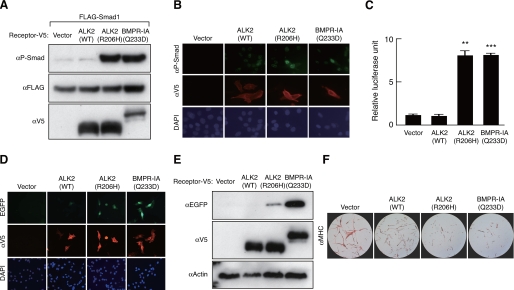
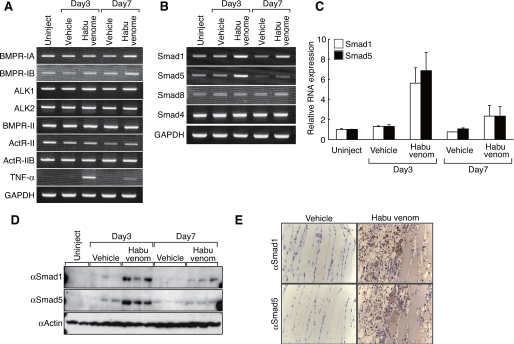
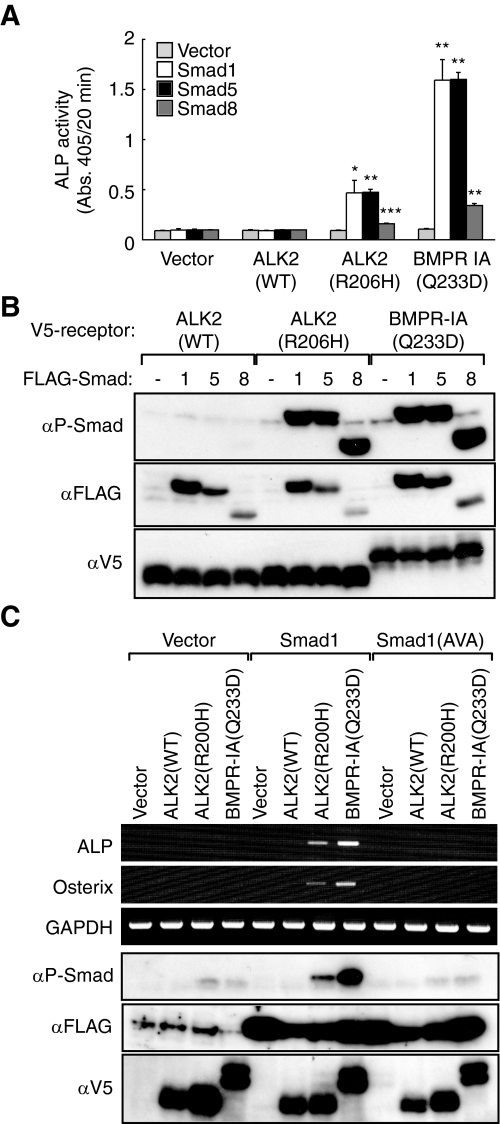
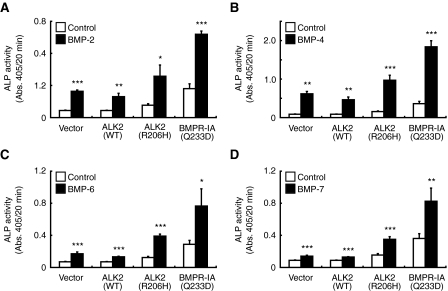
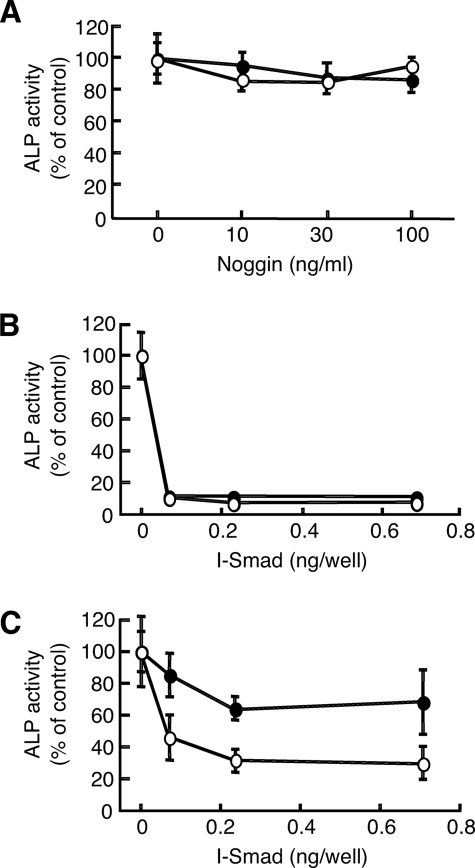
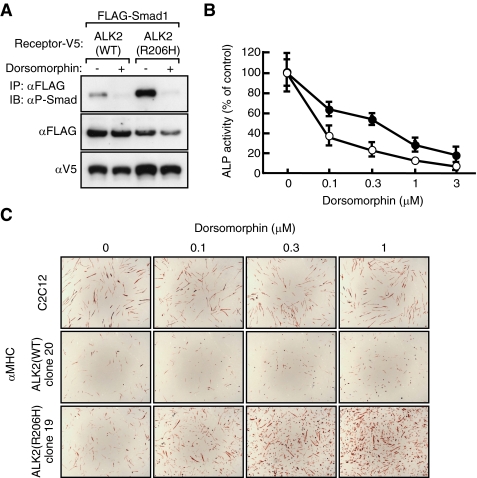
Toru Fukuda
Toru Fukuda
Divisions of aPathophysiology and
cTranslational Research, Research Center
for Genomic Medicine, Saitama Medical University, 1397-1 Yamane, Hidaka-shi,
Saitama 350-1241, Japan, bProject of
Clinical and Basic Research for FOP, Saitama Medical University, 1397-1
Yamane, Hidaka-shi, Saitama 350-1241, Japan,
dDepartment of Pediatric Emergency,
Kitakyusyu City Yahata Hospital, 4-18-1 Nishihonmachi, Yahatahigashi-ku,
Kitakyusyu-shi, Fukuoka 805-8534, Japan,
eDepartment of Orthopedic Surgery, Saga
Prefectural Hospital Koseikan, 1-12-9 Mizugae, Saga-shi, Saga 840-8571, Japan,
fNagayoshi Orthopedic Clinic, 1890
Otsukachohinokuchi, Miyazaki-shi, Miyazaki 880-0951, Japan,
gDepartment of Physical Medicine and
Rehabilitation, Tohoku University Graduate School of Medicine, 1-1
Seiryoumachi, Aoba-Ku, Sendai-shi, Miyagi 980-8474, Japan,
hDepartment of Orthopedic Surgery, Senjyudo
Hospital, 1-25 Senjyudonakamati, Gifu-shi, Gifu 500-8862, Japan,
iEndo Clinic, 4-8-11 Ogawanishimachi,
Kodaira-shi, Tokyo 187-0035, Japan,
jAwakura Clinic, 1-6-3 Satomachi,
Wajima-shi, Ishiwawa 928-0246, Japan,
kDepartment of Orthopedic Surgery, Kyushu
University Faculty of Medicine, 3-1-1 Maidashi, Higashi-ku, Fukuoka-shi,
Fukuoka 812-8582, Japan, mDepartment of
Pediatrics, Akashi Municipal Hospital, 1-33 Takashomachi, Akashi-shi, Hyogo
673-8501 Japan, nKawara Clinic, 2-12-3
Sumiyoshihonmachi, Higashinada-ku, Kobe-shi, Hyogo 658-0051, Japan,
oDepartment of Orthopaedic Surgery, Nagoya
University Graduate School and School of Medicine, 65 Tsurumaicho, Showa-ku,
Nagoya-shi, Aichi 466-8550, Japan,
pDepartment of Rehabilitation, Nagoya City
University Hospital, 1 Mizuhochoazakawasumi, Mizuho-ku, Nagoya-shi, Aichi
467-8602, Japan, qDivision of
Morphological Science, Biomedical Research Center, and Departments of
aaLaboratory Medicine,
bbPediatrics,
ccOrthopedic Surgery,
rNeurology,
sEndocrinology and Diabetes,
tObstetrics and Gynecology, and
vOral and Maxillofacial Surgery, Saitama
Medical University, 38 Moro Hongo, Moroyama-machi, Iruma-gun, Saitama350-0495,
Japan, uDepartment of Neurology, Saitama
Neuropsychiatric Institute, 6-11-1 Honchohigashi, Chuo-ku, Saitama-shi,
Saitama 338-8577, Japan, zDepartment of
Molecular Pathology, University of Tokyo Graduate School of Medicine, 7-3-1
Hongo, Bunkyo-ku, Tokyo 113-0033, Japan,
wKitasato University School of
Pharmaceutical Science, 5-9-1 Shirokane, Minato-ku, Tokyo 108-0023, Japan,
xCardiovascular Research Center and
Division of Cardiology, Department of Medicine, Massachusetts General
Hospital, Harvard Medical School, Boston, Massachusetts 02129,
yCenter for Research in Fibrodysplasia
Ossificans Progressiva and Related Disorders, Department of Orthopedic
Surgery, University of Pennsylvania School of Medicine, Philadelphia,
Pennsylvania 19104-6081, ddDivision of
Molecular Signaling and Biochemistry, Department of Biosciences, Kyushu Dental
Collage, 2-6-1 Manazuru, Kokurakita-ku, Kitakyushu-shi, Fukuoka 803-8580,
Japan, eeDepartment of Orthopedic Surgery,
University of Ryukyus Faculty of Medicine, 207 Uehara, Nishihara-cho, 903-0215
Okinawa, Japan, and lThe Research
Committee on Fibrodysplasia Ossificans Progressiva, Ministry of Health, Labour
and Welfare, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan
a,b,
Masakazu Kohda
Masakazu Kohda
Divisions of aPathophysiology and
cTranslational Research, Research Center
for Genomic Medicine, Saitama Medical University, 1397-1 Yamane, Hidaka-shi,
Saitama 350-1241, Japan, bProject of
Clinical and Basic Research for FOP, Saitama Medical University, 1397-1
Yamane, Hidaka-shi, Saitama 350-1241, Japan,
dDepartment of Pediatric Emergency,
Kitakyusyu City Yahata Hospital, 4-18-1 Nishihonmachi, Yahatahigashi-ku,
Kitakyusyu-shi, Fukuoka 805-8534, Japan,
eDepartment of Orthopedic Surgery, Saga
Prefectural Hospital Koseikan, 1-12-9 Mizugae, Saga-shi, Saga 840-8571, Japan,
fNagayoshi Orthopedic Clinic, 1890
Otsukachohinokuchi, Miyazaki-shi, Miyazaki 880-0951, Japan,
gDepartment of Physical Medicine and
Rehabilitation, Tohoku University Graduate School of Medicine, 1-1
Seiryoumachi, Aoba-Ku, Sendai-shi, Miyagi 980-8474, Japan,
hDepartment of Orthopedic Surgery, Senjyudo
Hospital, 1-25 Senjyudonakamati, Gifu-shi, Gifu 500-8862, Japan,
iEndo Clinic, 4-8-11 Ogawanishimachi,
Kodaira-shi, Tokyo 187-0035, Japan,
jAwakura Clinic, 1-6-3 Satomachi,
Wajima-shi, Ishiwawa 928-0246, Japan,
kDepartment of Orthopedic Surgery, Kyushu
University Faculty of Medicine, 3-1-1 Maidashi, Higashi-ku, Fukuoka-shi,
Fukuoka 812-8582, Japan, mDepartment of
Pediatrics, Akashi Municipal Hospital, 1-33 Takashomachi, Akashi-shi, Hyogo
673-8501 Japan, nKawara Clinic, 2-12-3
Sumiyoshihonmachi, Higashinada-ku, Kobe-shi, Hyogo 658-0051, Japan,
oDepartment of Orthopaedic Surgery, Nagoya
University Graduate School and School of Medicine, 65 Tsurumaicho, Showa-ku,
Nagoya-shi, Aichi 466-8550, Japan,
pDepartment of Rehabilitation, Nagoya City
University Hospital, 1 Mizuhochoazakawasumi, Mizuho-ku, Nagoya-shi, Aichi
467-8602, Japan, qDivision of
Morphological Science, Biomedical Research Center, and Departments of
aaLaboratory Medicine,
bbPediatrics,
ccOrthopedic Surgery,
rNeurology,
sEndocrinology and Diabetes,
tObstetrics and Gynecology, and
vOral and Maxillofacial Surgery, Saitama
Medical University, 38 Moro Hongo, Moroyama-machi, Iruma-gun, Saitama350-0495,
Japan, uDepartment of Neurology, Saitama
Neuropsychiatric Institute, 6-11-1 Honchohigashi, Chuo-ku, Saitama-shi,
Saitama 338-8577, Japan, zDepartment of
Molecular Pathology, University of Tokyo Graduate School of Medicine, 7-3-1
Hongo, Bunkyo-ku, Tokyo 113-0033, Japan,
wKitasato University School of
Pharmaceutical Science, 5-9-1 Shirokane, Minato-ku, Tokyo 108-0023, Japan,
xCardiovascular Research Center and
Division of Cardiology, Department of Medicine, Massachusetts General
Hospital, Harvard Medical School, Boston, Massachusetts 02129,
yCenter for Research in Fibrodysplasia
Ossificans Progressiva and Related Disorders, Department of Orthopedic
Surgery, University of Pennsylvania School of Medicine, Philadelphia,
Pennsylvania 19104-6081, ddDivision of
Molecular Signaling and Biochemistry, Department of Biosciences, Kyushu Dental
Collage, 2-6-1 Manazuru, Kokurakita-ku, Kitakyushu-shi, Fukuoka 803-8580,
Japan, eeDepartment of Orthopedic Surgery,
University of Ryukyus Faculty of Medicine, 207 Uehara, Nishihara-cho, 903-0215
Okinawa, Japan, and lThe Research
Committee on Fibrodysplasia Ossificans Progressiva, Ministry of Health, Labour
and Welfare, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan
c,
Kazuhiro Kanomata
Kazuhiro Kanomata
Divisions of aPathophysiology and
cTranslational Research, Research Center
for Genomic Medicine, Saitama Medical University, 1397-1 Yamane, Hidaka-shi,
Saitama 350-1241, Japan, bProject of
Clinical and Basic Research for FOP, Saitama Medical University, 1397-1
Yamane, Hidaka-shi, Saitama 350-1241, Japan,
dDepartment of Pediatric Emergency,
Kitakyusyu City Yahata Hospital, 4-18-1 Nishihonmachi, Yahatahigashi-ku,
Kitakyusyu-shi, Fukuoka 805-8534, Japan,
eDepartment of Orthopedic Surgery, Saga
Prefectural Hospital Koseikan, 1-12-9 Mizugae, Saga-shi, Saga 840-8571, Japan,
fNagayoshi Orthopedic Clinic, 1890
Otsukachohinokuchi, Miyazaki-shi, Miyazaki 880-0951, Japan,
gDepartment of Physical Medicine and
Rehabilitation, Tohoku University Graduate School of Medicine, 1-1
Seiryoumachi, Aoba-Ku, Sendai-shi, Miyagi 980-8474, Japan,
hDepartment of Orthopedic Surgery, Senjyudo
Hospital, 1-25 Senjyudonakamati, Gifu-shi, Gifu 500-8862, Japan,
iEndo Clinic, 4-8-11 Ogawanishimachi,
Kodaira-shi, Tokyo 187-0035, Japan,
jAwakura Clinic, 1-6-3 Satomachi,
Wajima-shi, Ishiwawa 928-0246, Japan,
kDepartment of Orthopedic Surgery, Kyushu
University Faculty of Medicine, 3-1-1 Maidashi, Higashi-ku, Fukuoka-shi,
Fukuoka 812-8582, Japan, mDepartment of
Pediatrics, Akashi Municipal Hospital, 1-33 Takashomachi, Akashi-shi, Hyogo
673-8501 Japan, nKawara Clinic, 2-12-3
Sumiyoshihonmachi, Higashinada-ku, Kobe-shi, Hyogo 658-0051, Japan,
oDepartment of Orthopaedic Surgery, Nagoya
University Graduate School and School of Medicine, 65 Tsurumaicho, Showa-ku,
Nagoya-shi, Aichi 466-8550, Japan,
pDepartment of Rehabilitation, Nagoya City
University Hospital, 1 Mizuhochoazakawasumi, Mizuho-ku, Nagoya-shi, Aichi
467-8602, Japan, qDivision of
Morphological Science, Biomedical Research Center, and Departments of
aaLaboratory Medicine,
bbPediatrics,
ccOrthopedic Surgery,
rNeurology,
sEndocrinology and Diabetes,
tObstetrics and Gynecology, and
vOral and Maxillofacial Surgery, Saitama
Medical University, 38 Moro Hongo, Moroyama-machi, Iruma-gun, Saitama350-0495,
Japan, uDepartment of Neurology, Saitama
Neuropsychiatric Institute, 6-11-1 Honchohigashi, Chuo-ku, Saitama-shi,
Saitama 338-8577, Japan, zDepartment of
Molecular Pathology, University of Tokyo Graduate School of Medicine, 7-3-1
Hongo, Bunkyo-ku, Tokyo 113-0033, Japan,
wKitasato University School of
Pharmaceutical Science, 5-9-1 Shirokane, Minato-ku, Tokyo 108-0023, Japan,
xCardiovascular Research Center and
Division of Cardiology, Department of Medicine, Massachusetts General
Hospital, Harvard Medical School, Boston, Massachusetts 02129,
yCenter for Research in Fibrodysplasia
Ossificans Progressiva and Related Disorders, Department of Orthopedic
Surgery, University of Pennsylvania School of Medicine, Philadelphia,
Pennsylvania 19104-6081, ddDivision of
Molecular Signaling and Biochemistry, Department of Biosciences, Kyushu Dental
Collage, 2-6-1 Manazuru, Kokurakita-ku, Kitakyushu-shi, Fukuoka 803-8580,
Japan, eeDepartment of Orthopedic Surgery,
University of Ryukyus Faculty of Medicine, 207 Uehara, Nishihara-cho, 903-0215
Okinawa, Japan, and lThe Research
Committee on Fibrodysplasia Ossificans Progressiva, Ministry of Health, Labour
and Welfare, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan
a,
Junya Nojima
Junya Nojima
Divisions of aPathophysiology and
cTranslational Research, Research Center
for Genomic Medicine, Saitama Medical University, 1397-1 Yamane, Hidaka-shi,
Saitama 350-1241, Japan, bProject of
Clinical and Basic Research for FOP, Saitama Medical University, 1397-1
Yamane, Hidaka-shi, Saitama 350-1241, Japan,
dDepartment of Pediatric Emergency,
Kitakyusyu City Yahata Hospital, 4-18-1 Nishihonmachi, Yahatahigashi-ku,
Kitakyusyu-shi, Fukuoka 805-8534, Japan,
eDepartment of Orthopedic Surgery, Saga
Prefectural Hospital Koseikan, 1-12-9 Mizugae, Saga-shi, Saga 840-8571, Japan,
fNagayoshi Orthopedic Clinic, 1890
Otsukachohinokuchi, Miyazaki-shi, Miyazaki 880-0951, Japan,
gDepartment of Physical Medicine and
Rehabilitation, Tohoku University Graduate School of Medicine, 1-1
Seiryoumachi, Aoba-Ku, Sendai-shi, Miyagi 980-8474, Japan,
hDepartment of Orthopedic Surgery, Senjyudo
Hospital, 1-25 Senjyudonakamati, Gifu-shi, Gifu 500-8862, Japan,
iEndo Clinic, 4-8-11 Ogawanishimachi,
Kodaira-shi, Tokyo 187-0035, Japan,
jAwakura Clinic, 1-6-3 Satomachi,
Wajima-shi, Ishiwawa 928-0246, Japan,
kDepartment of Orthopedic Surgery, Kyushu
University Faculty of Medicine, 3-1-1 Maidashi, Higashi-ku, Fukuoka-shi,
Fukuoka 812-8582, Japan, mDepartment of
Pediatrics, Akashi Municipal Hospital, 1-33 Takashomachi, Akashi-shi, Hyogo
673-8501 Japan, nKawara Clinic, 2-12-3
Sumiyoshihonmachi, Higashinada-ku, Kobe-shi, Hyogo 658-0051, Japan,
oDepartment of Orthopaedic Surgery, Nagoya
University Graduate School and School of Medicine, 65 Tsurumaicho, Showa-ku,
Nagoya-shi, Aichi 466-8550, Japan,
pDepartment of Rehabilitation, Nagoya City
University Hospital, 1 Mizuhochoazakawasumi, Mizuho-ku, Nagoya-shi, Aichi
467-8602, Japan, qDivision of
Morphological Science, Biomedical Research Center, and Departments of
aaLaboratory Medicine,
bbPediatrics,
ccOrthopedic Surgery,
rNeurology,
sEndocrinology and Diabetes,
tObstetrics and Gynecology, and
vOral and Maxillofacial Surgery, Saitama
Medical University, 38 Moro Hongo, Moroyama-machi, Iruma-gun, Saitama350-0495,
Japan, uDepartment of Neurology, Saitama
Neuropsychiatric Institute, 6-11-1 Honchohigashi, Chuo-ku, Saitama-shi,
Saitama 338-8577, Japan, zDepartment of
Molecular Pathology, University of Tokyo Graduate School of Medicine, 7-3-1
Hongo, Bunkyo-ku, Tokyo 113-0033, Japan,
wKitasato University School of
Pharmaceutical Science, 5-9-1 Shirokane, Minato-ku, Tokyo 108-0023, Japan,
xCardiovascular Research Center and
Division of Cardiology, Department of Medicine, Massachusetts General
Hospital, Harvard Medical School, Boston, Massachusetts 02129,
yCenter for Research in Fibrodysplasia
Ossificans Progressiva and Related Disorders, Department of Orthopedic
Surgery, University of Pennsylvania School of Medicine, Philadelphia,
Pennsylvania 19104-6081, ddDivision of
Molecular Signaling and Biochemistry, Department of Biosciences, Kyushu Dental
Collage, 2-6-1 Manazuru, Kokurakita-ku, Kitakyushu-shi, Fukuoka 803-8580,
Japan, eeDepartment of Orthopedic Surgery,
University of Ryukyus Faculty of Medicine, 207 Uehara, Nishihara-cho, 903-0215
Okinawa, Japan, and lThe Research
Committee on Fibrodysplasia Ossificans Progressiva, Ministry of Health, Labour
and Welfare, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan
a,v,
Atsushi Nakamura
Atsushi Nakamura
Divisions of aPathophysiology and
cTranslational Research, Research Center
for Genomic Medicine, Saitama Medical University, 1397-1 Yamane, Hidaka-shi,
Saitama 350-1241, Japan, bProject of
Clinical and Basic Research for FOP, Saitama Medical University, 1397-1
Yamane, Hidaka-shi, Saitama 350-1241, Japan,
dDepartment of Pediatric Emergency,
Kitakyusyu City Yahata Hospital, 4-18-1 Nishihonmachi, Yahatahigashi-ku,
Kitakyusyu-shi, Fukuoka 805-8534, Japan,
eDepartment of Orthopedic Surgery, Saga
Prefectural Hospital Koseikan, 1-12-9 Mizugae, Saga-shi, Saga 840-8571, Japan,
fNagayoshi Orthopedic Clinic, 1890
Otsukachohinokuchi, Miyazaki-shi, Miyazaki 880-0951, Japan,
gDepartment of Physical Medicine and
Rehabilitation, Tohoku University Graduate School of Medicine, 1-1
Seiryoumachi, Aoba-Ku, Sendai-shi, Miyagi 980-8474, Japan,
hDepartment of Orthopedic Surgery, Senjyudo
Hospital, 1-25 Senjyudonakamati, Gifu-shi, Gifu 500-8862, Japan,
iEndo Clinic, 4-8-11 Ogawanishimachi,
Kodaira-shi, Tokyo 187-0035, Japan,
jAwakura Clinic, 1-6-3 Satomachi,
Wajima-shi, Ishiwawa 928-0246, Japan,
kDepartment of Orthopedic Surgery, Kyushu
University Faculty of Medicine, 3-1-1 Maidashi, Higashi-ku, Fukuoka-shi,
Fukuoka 812-8582, Japan, mDepartment of
Pediatrics, Akashi Municipal Hospital, 1-33 Takashomachi, Akashi-shi, Hyogo
673-8501 Japan, nKawara Clinic, 2-12-3
Sumiyoshihonmachi, Higashinada-ku, Kobe-shi, Hyogo 658-0051, Japan,
oDepartment of Orthopaedic Surgery, Nagoya
University Graduate School and School of Medicine, 65 Tsurumaicho, Showa-ku,
Nagoya-shi, Aichi 466-8550, Japan,
pDepartment of Rehabilitation, Nagoya City
University Hospital, 1 Mizuhochoazakawasumi, Mizuho-ku, Nagoya-shi, Aichi
467-8602, Japan, qDivision of
Morphological Science, Biomedical Research Center, and Departments of
aaLaboratory Medicine,
bbPediatrics,
ccOrthopedic Surgery,
rNeurology,
sEndocrinology and Diabetes,
tObstetrics and Gynecology, and
vOral and Maxillofacial Surgery, Saitama
Medical University, 38 Moro Hongo, Moroyama-machi, Iruma-gun, Saitama350-0495,
Japan, uDepartment of Neurology, Saitama
Neuropsychiatric Institute, 6-11-1 Honchohigashi, Chuo-ku, Saitama-shi,
Saitama 338-8577, Japan, zDepartment of
Molecular Pathology, University of Tokyo Graduate School of Medicine, 7-3-1
Hongo, Bunkyo-ku, Tokyo 113-0033, Japan,
wKitasato University School of
Pharmaceutical Science, 5-9-1 Shirokane, Minato-ku, Tokyo 108-0023, Japan,
xCardiovascular Research Center and
Division of Cardiology, Department of Medicine, Massachusetts General
Hospital, Harvard Medical School, Boston, Massachusetts 02129,
yCenter for Research in Fibrodysplasia
Ossificans Progressiva and Related Disorders, Department of Orthopedic
Surgery, University of Pennsylvania School of Medicine, Philadelphia,
Pennsylvania 19104-6081, ddDivision of
Molecular Signaling and Biochemistry, Department of Biosciences, Kyushu Dental
Collage, 2-6-1 Manazuru, Kokurakita-ku, Kitakyushu-shi, Fukuoka 803-8580,
Japan, eeDepartment of Orthopedic Surgery,
University of Ryukyus Faculty of Medicine, 207 Uehara, Nishihara-cho, 903-0215
Okinawa, Japan, and lThe Research
Committee on Fibrodysplasia Ossificans Progressiva, Ministry of Health, Labour
and Welfare, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan
a,
Jyunji Kamizono
Jyunji Kamizono
Divisions of aPathophysiology and
cTranslational Research, Research Center
for Genomic Medicine, Saitama Medical University, 1397-1 Yamane, Hidaka-shi,
Saitama 350-1241, Japan, bProject of
Clinical and Basic Research for FOP, Saitama Medical University, 1397-1
Yamane, Hidaka-shi, Saitama 350-1241, Japan,
dDepartment of Pediatric Emergency,
Kitakyusyu City Yahata Hospital, 4-18-1 Nishihonmachi, Yahatahigashi-ku,
Kitakyusyu-shi, Fukuoka 805-8534, Japan,
eDepartment of Orthopedic Surgery, Saga
Prefectural Hospital Koseikan, 1-12-9 Mizugae, Saga-shi, Saga 840-8571, Japan,
fNagayoshi Orthopedic Clinic, 1890
Otsukachohinokuchi, Miyazaki-shi, Miyazaki 880-0951, Japan,
gDepartment of Physical Medicine and
Rehabilitation, Tohoku University Graduate School of Medicine, 1-1
Seiryoumachi, Aoba-Ku, Sendai-shi, Miyagi 980-8474, Japan,
hDepartment of Orthopedic Surgery, Senjyudo
Hospital, 1-25 Senjyudonakamati, Gifu-shi, Gifu 500-8862, Japan,
iEndo Clinic, 4-8-11 Ogawanishimachi,
Kodaira-shi, Tokyo 187-0035, Japan,
jAwakura Clinic, 1-6-3 Satomachi,
Wajima-shi, Ishiwawa 928-0246, Japan,
kDepartment of Orthopedic Surgery, Kyushu
University Faculty of Medicine, 3-1-1 Maidashi, Higashi-ku, Fukuoka-shi,
Fukuoka 812-8582, Japan, mDepartment of
Pediatrics, Akashi Municipal Hospital, 1-33 Takashomachi, Akashi-shi, Hyogo
673-8501 Japan, nKawara Clinic, 2-12-3
Sumiyoshihonmachi, Higashinada-ku, Kobe-shi, Hyogo 658-0051, Japan,
oDepartment of Orthopaedic Surgery, Nagoya
University Graduate School and School of Medicine, 65 Tsurumaicho, Showa-ku,
Nagoya-shi, Aichi 466-8550, Japan,
pDepartment of Rehabilitation, Nagoya City
University Hospital, 1 Mizuhochoazakawasumi, Mizuho-ku, Nagoya-shi, Aichi
467-8602, Japan, qDivision of
Morphological Science, Biomedical Research Center, and Departments of
aaLaboratory Medicine,
bbPediatrics,
ccOrthopedic Surgery,
rNeurology,
sEndocrinology and Diabetes,
tObstetrics and Gynecology, and
vOral and Maxillofacial Surgery, Saitama
Medical University, 38 Moro Hongo, Moroyama-machi, Iruma-gun, Saitama350-0495,
Japan, uDepartment of Neurology, Saitama
Neuropsychiatric Institute, 6-11-1 Honchohigashi, Chuo-ku, Saitama-shi,
Saitama 338-8577, Japan, zDepartment of
Molecular Pathology, University of Tokyo Graduate School of Medicine, 7-3-1
Hongo, Bunkyo-ku, Tokyo 113-0033, Japan,
wKitasato University School of
Pharmaceutical Science, 5-9-1 Shirokane, Minato-ku, Tokyo 108-0023, Japan,
xCardiovascular Research Center and
Division of Cardiology, Department of Medicine, Massachusetts General
Hospital, Harvard Medical School, Boston, Massachusetts 02129,
yCenter for Research in Fibrodysplasia
Ossificans Progressiva and Related Disorders, Department of Orthopedic
Surgery, University of Pennsylvania School of Medicine, Philadelphia,
Pennsylvania 19104-6081, ddDivision of
Molecular Signaling and Biochemistry, Department of Biosciences, Kyushu Dental
Collage, 2-6-1 Manazuru, Kokurakita-ku, Kitakyushu-shi, Fukuoka 803-8580,
Japan, eeDepartment of Orthopedic Surgery,
University of Ryukyus Faculty of Medicine, 207 Uehara, Nishihara-cho, 903-0215
Okinawa, Japan, and lThe Research
Committee on Fibrodysplasia Ossificans Progressiva, Ministry of Health, Labour
and Welfare, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan
d,
Yasuo Noguchi
Yasuo Noguchi
Divisions of aPathophysiology and
cTranslational Research, Research Center
for Genomic Medicine, Saitama Medical University, 1397-1 Yamane, Hidaka-shi,
Saitama 350-1241, Japan, bProject of
Clinical and Basic Research for FOP, Saitama Medical University, 1397-1
Yamane, Hidaka-shi, Saitama 350-1241, Japan,
dDepartment of Pediatric Emergency,
Kitakyusyu City Yahata Hospital, 4-18-1 Nishihonmachi, Yahatahigashi-ku,
Kitakyusyu-shi, Fukuoka 805-8534, Japan,
eDepartment of Orthopedic Surgery, Saga
Prefectural Hospital Koseikan, 1-12-9 Mizugae, Saga-shi, Saga 840-8571, Japan,
fNagayoshi Orthopedic Clinic, 1890
Otsukachohinokuchi, Miyazaki-shi, Miyazaki 880-0951, Japan,
gDepartment of Physical Medicine and
Rehabilitation, Tohoku University Graduate School of Medicine, 1-1
Seiryoumachi, Aoba-Ku, Sendai-shi, Miyagi 980-8474, Japan,
hDepartment of Orthopedic Surgery, Senjyudo
Hospital, 1-25 Senjyudonakamati, Gifu-shi, Gifu 500-8862, Japan,
iEndo Clinic, 4-8-11 Ogawanishimachi,
Kodaira-shi, Tokyo 187-0035, Japan,
jAwakura Clinic, 1-6-3 Satomachi,
Wajima-shi, Ishiwawa 928-0246, Japan,
kDepartment of Orthopedic Surgery, Kyushu
University Faculty of Medicine, 3-1-1 Maidashi, Higashi-ku, Fukuoka-shi,
Fukuoka 812-8582, Japan, mDepartment of
Pediatrics, Akashi Municipal Hospital, 1-33 Takashomachi, Akashi-shi, Hyogo
673-8501 Japan, nKawara Clinic, 2-12-3
Sumiyoshihonmachi, Higashinada-ku, Kobe-shi, Hyogo 658-0051, Japan,
oDepartment of Orthopaedic Surgery, Nagoya
University Graduate School and School of Medicine, 65 Tsurumaicho, Showa-ku,
Nagoya-shi, Aichi 466-8550, Japan,
pDepartment of Rehabilitation, Nagoya City
University Hospital, 1 Mizuhochoazakawasumi, Mizuho-ku, Nagoya-shi, Aichi
467-8602, Japan, qDivision of
Morphological Science, Biomedical Research Center, and Departments of
aaLaboratory Medicine,
bbPediatrics,
ccOrthopedic Surgery,
rNeurology,
sEndocrinology and Diabetes,
tObstetrics and Gynecology, and
vOral and Maxillofacial Surgery, Saitama
Medical University, 38 Moro Hongo, Moroyama-machi, Iruma-gun, Saitama350-0495,
Japan, uDepartment of Neurology, Saitama
Neuropsychiatric Institute, 6-11-1 Honchohigashi, Chuo-ku, Saitama-shi,
Saitama 338-8577, Japan, zDepartment of
Molecular Pathology, University of Tokyo Graduate School of Medicine, 7-3-1
Hongo, Bunkyo-ku, Tokyo 113-0033, Japan,
wKitasato University School of
Pharmaceutical Science, 5-9-1 Shirokane, Minato-ku, Tokyo 108-0023, Japan,
xCardiovascular Research Center and
Division of Cardiology, Department of Medicine, Massachusetts General
Hospital, Harvard Medical School, Boston, Massachusetts 02129,
yCenter for Research in Fibrodysplasia
Ossificans Progressiva and Related Disorders, Department of Orthopedic
Surgery, University of Pennsylvania School of Medicine, Philadelphia,
Pennsylvania 19104-6081, ddDivision of
Molecular Signaling and Biochemistry, Department of Biosciences, Kyushu Dental
Collage, 2-6-1 Manazuru, Kokurakita-ku, Kitakyushu-shi, Fukuoka 803-8580,
Japan, eeDepartment of Orthopedic Surgery,
University of Ryukyus Faculty of Medicine, 207 Uehara, Nishihara-cho, 903-0215
Okinawa, Japan, and lThe Research
Committee on Fibrodysplasia Ossificans Progressiva, Ministry of Health, Labour
and Welfare, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan
e,
Kiyofumi Iwakiri
Kiyofumi Iwakiri
Divisions of aPathophysiology and
cTranslational Research, Research Center
for Genomic Medicine, Saitama Medical University, 1397-1 Yamane, Hidaka-shi,
Saitama 350-1241, Japan, bProject of
Clinical and Basic Research for FOP, Saitama Medical University, 1397-1
Yamane, Hidaka-shi, Saitama 350-1241, Japan,
dDepartment of Pediatric Emergency,
Kitakyusyu City Yahata Hospital, 4-18-1 Nishihonmachi, Yahatahigashi-ku,
Kitakyusyu-shi, Fukuoka 805-8534, Japan,
eDepartment of Orthopedic Surgery, Saga
Prefectural Hospital Koseikan, 1-12-9 Mizugae, Saga-shi, Saga 840-8571, Japan,
fNagayoshi Orthopedic Clinic, 1890
Otsukachohinokuchi, Miyazaki-shi, Miyazaki 880-0951, Japan,
gDepartment of Physical Medicine and
Rehabilitation, Tohoku University Graduate School of Medicine, 1-1
Seiryoumachi, Aoba-Ku, Sendai-shi, Miyagi 980-8474, Japan,
hDepartment of Orthopedic Surgery, Senjyudo
Hospital, 1-25 Senjyudonakamati, Gifu-shi, Gifu 500-8862, Japan,
iEndo Clinic, 4-8-11 Ogawanishimachi,
Kodaira-shi, Tokyo 187-0035, Japan,
jAwakura Clinic, 1-6-3 Satomachi,
Wajima-shi, Ishiwawa 928-0246, Japan,
kDepartment of Orthopedic Surgery, Kyushu
University Faculty of Medicine, 3-1-1 Maidashi, Higashi-ku, Fukuoka-shi,
Fukuoka 812-8582, Japan, mDepartment of
Pediatrics, Akashi Municipal Hospital, 1-33 Takashomachi, Akashi-shi, Hyogo
673-8501 Japan, nKawara Clinic, 2-12-3
Sumiyoshihonmachi, Higashinada-ku, Kobe-shi, Hyogo 658-0051, Japan,
oDepartment of Orthopaedic Surgery, Nagoya
University Graduate School and School of Medicine, 65 Tsurumaicho, Showa-ku,
Nagoya-shi, Aichi 466-8550, Japan,
pDepartment of Rehabilitation, Nagoya City
University Hospital, 1 Mizuhochoazakawasumi, Mizuho-ku, Nagoya-shi, Aichi
467-8602, Japan, qDivision of
Morphological Science, Biomedical Research Center, and Departments of
aaLaboratory Medicine,
bbPediatrics,
ccOrthopedic Surgery,
rNeurology,
sEndocrinology and Diabetes,
tObstetrics and Gynecology, and
vOral and Maxillofacial Surgery, Saitama
Medical University, 38 Moro Hongo, Moroyama-machi, Iruma-gun, Saitama350-0495,
Japan, uDepartment of Neurology, Saitama
Neuropsychiatric Institute, 6-11-1 Honchohigashi, Chuo-ku, Saitama-shi,
Saitama 338-8577, Japan, zDepartment of
Molecular Pathology, University of Tokyo Graduate School of Medicine, 7-3-1
Hongo, Bunkyo-ku, Tokyo 113-0033, Japan,
wKitasato University School of
Pharmaceutical Science, 5-9-1 Shirokane, Minato-ku, Tokyo 108-0023, Japan,
xCardiovascular Research Center and
Division of Cardiology, Department of Medicine, Massachusetts General
Hospital, Harvard Medical School, Boston, Massachusetts 02129,
yCenter for Research in Fibrodysplasia
Ossificans Progressiva and Related Disorders, Department of Orthopedic
Surgery, University of Pennsylvania School of Medicine, Philadelphia,
Pennsylvania 19104-6081, ddDivision of
Molecular Signaling and Biochemistry, Department of Biosciences, Kyushu Dental
Collage, 2-6-1 Manazuru, Kokurakita-ku, Kitakyushu-shi, Fukuoka 803-8580,
Japan, eeDepartment of Orthopedic Surgery,
University of Ryukyus Faculty of Medicine, 207 Uehara, Nishihara-cho, 903-0215
Okinawa, Japan, and lThe Research
Committee on Fibrodysplasia Ossificans Progressiva, Ministry of Health, Labour
and Welfare, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan
f,
Takeo Kondo
Takeo Kondo
Divisions of aPathophysiology and
cTranslational Research, Research Center
for Genomic Medicine, Saitama Medical University, 1397-1 Yamane, Hidaka-shi,
Saitama 350-1241, Japan, bProject of
Clinical and Basic Research for FOP, Saitama Medical University, 1397-1
Yamane, Hidaka-shi, Saitama 350-1241, Japan,
dDepartment of Pediatric Emergency,
Kitakyusyu City Yahata Hospital, 4-18-1 Nishihonmachi, Yahatahigashi-ku,
Kitakyusyu-shi, Fukuoka 805-8534, Japan,
eDepartment of Orthopedic Surgery, Saga
Prefectural Hospital Koseikan, 1-12-9 Mizugae, Saga-shi, Saga 840-8571, Japan,
fNagayoshi Orthopedic Clinic, 1890
Otsukachohinokuchi, Miyazaki-shi, Miyazaki 880-0951, Japan,
gDepartment of Physical Medicine and
Rehabilitation, Tohoku University Graduate School of Medicine, 1-1
Seiryoumachi, Aoba-Ku, Sendai-shi, Miyagi 980-8474, Japan,
hDepartment of Orthopedic Surgery, Senjyudo
Hospital, 1-25 Senjyudonakamati, Gifu-shi, Gifu 500-8862, Japan,
iEndo Clinic, 4-8-11 Ogawanishimachi,
Kodaira-shi, Tokyo 187-0035, Japan,
jAwakura Clinic, 1-6-3 Satomachi,
Wajima-shi, Ishiwawa 928-0246, Japan,
kDepartment of Orthopedic Surgery, Kyushu
University Faculty of Medicine, 3-1-1 Maidashi, Higashi-ku, Fukuoka-shi,
Fukuoka 812-8582, Japan, mDepartment of
Pediatrics, Akashi Municipal Hospital, 1-33 Takashomachi, Akashi-shi, Hyogo
673-8501 Japan, nKawara Clinic, 2-12-3
Sumiyoshihonmachi, Higashinada-ku, Kobe-shi, Hyogo 658-0051, Japan,
oDepartment of Orthopaedic Surgery, Nagoya
University Graduate School and School of Medicine, 65 Tsurumaicho, Showa-ku,
Nagoya-shi, Aichi 466-8550, Japan,
pDepartment of Rehabilitation, Nagoya City
University Hospital, 1 Mizuhochoazakawasumi, Mizuho-ku, Nagoya-shi, Aichi
467-8602, Japan, qDivision of
Morphological Science, Biomedical Research Center, and Departments of
aaLaboratory Medicine,
bbPediatrics,
ccOrthopedic Surgery,
rNeurology,
sEndocrinology and Diabetes,
tObstetrics and Gynecology, and
vOral and Maxillofacial Surgery, Saitama
Medical University, 38 Moro Hongo, Moroyama-machi, Iruma-gun, Saitama350-0495,
Japan, uDepartment of Neurology, Saitama
Neuropsychiatric Institute, 6-11-1 Honchohigashi, Chuo-ku, Saitama-shi,
Saitama 338-8577, Japan, zDepartment of
Molecular Pathology, University of Tokyo Graduate School of Medicine, 7-3-1
Hongo, Bunkyo-ku, Tokyo 113-0033, Japan,
wKitasato University School of
Pharmaceutical Science, 5-9-1 Shirokane, Minato-ku, Tokyo 108-0023, Japan,
xCardiovascular Research Center and
Division of Cardiology, Department of Medicine, Massachusetts General
Hospital, Harvard Medical School, Boston, Massachusetts 02129,
yCenter for Research in Fibrodysplasia
Ossificans Progressiva and Related Disorders, Department of Orthopedic
Surgery, University of Pennsylvania School of Medicine, Philadelphia,
Pennsylvania 19104-6081, ddDivision of
Molecular Signaling and Biochemistry, Department of Biosciences, Kyushu Dental
Collage, 2-6-1 Manazuru, Kokurakita-ku, Kitakyushu-shi, Fukuoka 803-8580,
Japan, eeDepartment of Orthopedic Surgery,
University of Ryukyus Faculty of Medicine, 207 Uehara, Nishihara-cho, 903-0215
Okinawa, Japan, and lThe Research
Committee on Fibrodysplasia Ossificans Progressiva, Ministry of Health, Labour
and Welfare, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan
g,
Junichi Kurose
Junichi Kurose
Divisions of aPathophysiology and
cTranslational Research, Research Center
for Genomic Medicine, Saitama Medical University, 1397-1 Yamane, Hidaka-shi,
Saitama 350-1241, Japan, bProject of
Clinical and Basic Research for FOP, Saitama Medical University, 1397-1
Yamane, Hidaka-shi, Saitama 350-1241, Japan,
dDepartment of Pediatric Emergency,
Kitakyusyu City Yahata Hospital, 4-18-1 Nishihonmachi, Yahatahigashi-ku,
Kitakyusyu-shi, Fukuoka 805-8534, Japan,
eDepartment of Orthopedic Surgery, Saga
Prefectural Hospital Koseikan, 1-12-9 Mizugae, Saga-shi, Saga 840-8571, Japan,
fNagayoshi Orthopedic Clinic, 1890
Otsukachohinokuchi, Miyazaki-shi, Miyazaki 880-0951, Japan,
gDepartment of Physical Medicine and
Rehabilitation, Tohoku University Graduate School of Medicine, 1-1
Seiryoumachi, Aoba-Ku, Sendai-shi, Miyagi 980-8474, Japan,
hDepartment of Orthopedic Surgery, Senjyudo
Hospital, 1-25 Senjyudonakamati, Gifu-shi, Gifu 500-8862, Japan,
iEndo Clinic, 4-8-11 Ogawanishimachi,
Kodaira-shi, Tokyo 187-0035, Japan,
jAwakura Clinic, 1-6-3 Satomachi,
Wajima-shi, Ishiwawa 928-0246, Japan,
kDepartment of Orthopedic Surgery, Kyushu
University Faculty of Medicine, 3-1-1 Maidashi, Higashi-ku, Fukuoka-shi,
Fukuoka 812-8582, Japan, mDepartment of
Pediatrics, Akashi Municipal Hospital, 1-33 Takashomachi, Akashi-shi, Hyogo
673-8501 Japan, nKawara Clinic, 2-12-3
Sumiyoshihonmachi, Higashinada-ku, Kobe-shi, Hyogo 658-0051, Japan,
oDepartment of Orthopaedic Surgery, Nagoya
University Graduate School and School of Medicine, 65 Tsurumaicho, Showa-ku,
Nagoya-shi, Aichi 466-8550, Japan,
pDepartment of Rehabilitation, Nagoya City
University Hospital, 1 Mizuhochoazakawasumi, Mizuho-ku, Nagoya-shi, Aichi
467-8602, Japan, qDivision of
Morphological Science, Biomedical Research Center, and Departments of
aaLaboratory Medicine,
bbPediatrics,
ccOrthopedic Surgery,
rNeurology,
sEndocrinology and Diabetes,
tObstetrics and Gynecology, and
vOral and Maxillofacial Surgery, Saitama
Medical University, 38 Moro Hongo, Moroyama-machi, Iruma-gun, Saitama350-0495,
Japan, uDepartment of Neurology, Saitama
Neuropsychiatric Institute, 6-11-1 Honchohigashi, Chuo-ku, Saitama-shi,
Saitama 338-8577, Japan, zDepartment of
Molecular Pathology, University of Tokyo Graduate School of Medicine, 7-3-1
Hongo, Bunkyo-ku, Tokyo 113-0033, Japan,
wKitasato University School of
Pharmaceutical Science, 5-9-1 Shirokane, Minato-ku, Tokyo 108-0023, Japan,
xCardiovascular Research Center and
Division of Cardiology, Department of Medicine, Massachusetts General
Hospital, Harvard Medical School, Boston, Massachusetts 02129,
yCenter for Research in Fibrodysplasia
Ossificans Progressiva and Related Disorders, Department of Orthopedic
Surgery, University of Pennsylvania School of Medicine, Philadelphia,
Pennsylvania 19104-6081, ddDivision of
Molecular Signaling and Biochemistry, Department of Biosciences, Kyushu Dental
Collage, 2-6-1 Manazuru, Kokurakita-ku, Kitakyushu-shi, Fukuoka 803-8580,
Japan, eeDepartment of Orthopedic Surgery,
University of Ryukyus Faculty of Medicine, 207 Uehara, Nishihara-cho, 903-0215
Okinawa, Japan, and lThe Research
Committee on Fibrodysplasia Ossificans Progressiva, Ministry of Health, Labour
and Welfare, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan
h,
Ken-ichi Endo
Ken-ichi Endo
Divisions of aPathophysiology and
cTranslational Research, Research Center
for Genomic Medicine, Saitama Medical University, 1397-1 Yamane, Hidaka-shi,
Saitama 350-1241, Japan, bProject of
Clinical and Basic Research for FOP, Saitama Medical University, 1397-1
Yamane, Hidaka-shi, Saitama 350-1241, Japan,
dDepartment of Pediatric Emergency,
Kitakyusyu City Yahata Hospital, 4-18-1 Nishihonmachi, Yahatahigashi-ku,
Kitakyusyu-shi, Fukuoka 805-8534, Japan,
eDepartment of Orthopedic Surgery, Saga
Prefectural Hospital Koseikan, 1-12-9 Mizugae, Saga-shi, Saga 840-8571, Japan,
fNagayoshi Orthopedic Clinic, 1890
Otsukachohinokuchi, Miyazaki-shi, Miyazaki 880-0951, Japan,
gDepartment of Physical Medicine and
Rehabilitation, Tohoku University Graduate School of Medicine, 1-1
Seiryoumachi, Aoba-Ku, Sendai-shi, Miyagi 980-8474, Japan,
hDepartment of Orthopedic Surgery, Senjyudo
Hospital, 1-25 Senjyudonakamati, Gifu-shi, Gifu 500-8862, Japan,
iEndo Clinic, 4-8-11 Ogawanishimachi,
Kodaira-shi, Tokyo 187-0035, Japan,
jAwakura Clinic, 1-6-3 Satomachi,
Wajima-shi, Ishiwawa 928-0246, Japan,
kDepartment of Orthopedic Surgery, Kyushu
University Faculty of Medicine, 3-1-1 Maidashi, Higashi-ku, Fukuoka-shi,
Fukuoka 812-8582, Japan, mDepartment of
Pediatrics, Akashi Municipal Hospital, 1-33 Takashomachi, Akashi-shi, Hyogo
673-8501 Japan, nKawara Clinic, 2-12-3
Sumiyoshihonmachi, Higashinada-ku, Kobe-shi, Hyogo 658-0051, Japan,
oDepartment of Orthopaedic Surgery, Nagoya
University Graduate School and School of Medicine, 65 Tsurumaicho, Showa-ku,
Nagoya-shi, Aichi 466-8550, Japan,
pDepartment of Rehabilitation, Nagoya City
University Hospital, 1 Mizuhochoazakawasumi, Mizuho-ku, Nagoya-shi, Aichi
467-8602, Japan, qDivision of
Morphological Science, Biomedical Research Center, and Departments of
aaLaboratory Medicine,
bbPediatrics,
ccOrthopedic Surgery,
rNeurology,
sEndocrinology and Diabetes,
tObstetrics and Gynecology, and
vOral and Maxillofacial Surgery, Saitama
Medical University, 38 Moro Hongo, Moroyama-machi, Iruma-gun, Saitama350-0495,
Japan, uDepartment of Neurology, Saitama
Neuropsychiatric Institute, 6-11-1 Honchohigashi, Chuo-ku, Saitama-shi,
Saitama 338-8577, Japan, zDepartment of
Molecular Pathology, University of Tokyo Graduate School of Medicine, 7-3-1
Hongo, Bunkyo-ku, Tokyo 113-0033, Japan,
wKitasato University School of
Pharmaceutical Science, 5-9-1 Shirokane, Minato-ku, Tokyo 108-0023, Japan,
xCardiovascular Research Center and
Division of Cardiology, Department of Medicine, Massachusetts General
Hospital, Harvard Medical School, Boston, Massachusetts 02129,
yCenter for Research in Fibrodysplasia
Ossificans Progressiva and Related Disorders, Department of Orthopedic
Surgery, University of Pennsylvania School of Medicine, Philadelphia,
Pennsylvania 19104-6081, ddDivision of
Molecular Signaling and Biochemistry, Department of Biosciences, Kyushu Dental
Collage, 2-6-1 Manazuru, Kokurakita-ku, Kitakyushu-shi, Fukuoka 803-8580,
Japan, eeDepartment of Orthopedic Surgery,
University of Ryukyus Faculty of Medicine, 207 Uehara, Nishihara-cho, 903-0215
Okinawa, Japan, and lThe Research
Committee on Fibrodysplasia Ossificans Progressiva, Ministry of Health, Labour
and Welfare, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan
i,
Takeshi Awakura
Takeshi Awakura
Divisions of aPathophysiology and
cTranslational Research, Research Center
for Genomic Medicine, Saitama Medical University, 1397-1 Yamane, Hidaka-shi,
Saitama 350-1241, Japan, bProject of
Clinical and Basic Research for FOP, Saitama Medical University, 1397-1
Yamane, Hidaka-shi, Saitama 350-1241, Japan,
dDepartment of Pediatric Emergency,
Kitakyusyu City Yahata Hospital, 4-18-1 Nishihonmachi, Yahatahigashi-ku,
Kitakyusyu-shi, Fukuoka 805-8534, Japan,
eDepartment of Orthopedic Surgery, Saga
Prefectural Hospital Koseikan, 1-12-9 Mizugae, Saga-shi, Saga 840-8571, Japan,
fNagayoshi Orthopedic Clinic, 1890
Otsukachohinokuchi, Miyazaki-shi, Miyazaki 880-0951, Japan,
gDepartment of Physical Medicine and
Rehabilitation, Tohoku University Graduate School of Medicine, 1-1
Seiryoumachi, Aoba-Ku, Sendai-shi, Miyagi 980-8474, Japan,
hDepartment of Orthopedic Surgery, Senjyudo
Hospital, 1-25 Senjyudonakamati, Gifu-shi, Gifu 500-8862, Japan,
iEndo Clinic, 4-8-11 Ogawanishimachi,
Kodaira-shi, Tokyo 187-0035, Japan,
jAwakura Clinic, 1-6-3 Satomachi,
Wajima-shi, Ishiwawa 928-0246, Japan,
kDepartment of Orthopedic Surgery, Kyushu
University Faculty of Medicine, 3-1-1 Maidashi, Higashi-ku, Fukuoka-shi,
Fukuoka 812-8582, Japan, mDepartment of
Pediatrics, Akashi Municipal Hospital, 1-33 Takashomachi, Akashi-shi, Hyogo
673-8501 Japan, nKawara Clinic, 2-12-3
Sumiyoshihonmachi, Higashinada-ku, Kobe-shi, Hyogo 658-0051, Japan,
oDepartment of Orthopaedic Surgery, Nagoya
University Graduate School and School of Medicine, 65 Tsurumaicho, Showa-ku,
Nagoya-shi, Aichi 466-8550, Japan,
pDepartment of Rehabilitation, Nagoya City
University Hospital, 1 Mizuhochoazakawasumi, Mizuho-ku, Nagoya-shi, Aichi
467-8602, Japan, qDivision of
Morphological Science, Biomedical Research Center, and Departments of
aaLaboratory Medicine,
bbPediatrics,
ccOrthopedic Surgery,
rNeurology,
sEndocrinology and Diabetes,
tObstetrics and Gynecology, and
vOral and Maxillofacial Surgery, Saitama
Medical University, 38 Moro Hongo, Moroyama-machi, Iruma-gun, Saitama350-0495,
Japan, uDepartment of Neurology, Saitama
Neuropsychiatric Institute, 6-11-1 Honchohigashi, Chuo-ku, Saitama-shi,
Saitama 338-8577, Japan, zDepartment of
Molecular Pathology, University of Tokyo Graduate School of Medicine, 7-3-1
Hongo, Bunkyo-ku, Tokyo 113-0033, Japan,
wKitasato University School of
Pharmaceutical Science, 5-9-1 Shirokane, Minato-ku, Tokyo 108-0023, Japan,
xCardiovascular Research Center and
Division of Cardiology, Department of Medicine, Massachusetts General
Hospital, Harvard Medical School, Boston, Massachusetts 02129,
yCenter for Research in Fibrodysplasia
Ossificans Progressiva and Related Disorders, Department of Orthopedic
Surgery, University of Pennsylvania School of Medicine, Philadelphia,
Pennsylvania 19104-6081, ddDivision of
Molecular Signaling and Biochemistry, Department of Biosciences, Kyushu Dental
Collage, 2-6-1 Manazuru, Kokurakita-ku, Kitakyushu-shi, Fukuoka 803-8580,
Japan, eeDepartment of Orthopedic Surgery,
University of Ryukyus Faculty of Medicine, 207 Uehara, Nishihara-cho, 903-0215
Okinawa, Japan, and lThe Research
Committee on Fibrodysplasia Ossificans Progressiva, Ministry of Health, Labour
and Welfare, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan
j,
Junichi Fukushi
Junichi Fukushi
Divisions of aPathophysiology and
cTranslational Research, Research Center
for Genomic Medicine, Saitama Medical University, 1397-1 Yamane, Hidaka-shi,
Saitama 350-1241, Japan, bProject of
Clinical and Basic Research for FOP, Saitama Medical University, 1397-1
Yamane, Hidaka-shi, Saitama 350-1241, Japan,
dDepartment of Pediatric Emergency,
Kitakyusyu City Yahata Hospital, 4-18-1 Nishihonmachi, Yahatahigashi-ku,
Kitakyusyu-shi, Fukuoka 805-8534, Japan,
eDepartment of Orthopedic Surgery, Saga
Prefectural Hospital Koseikan, 1-12-9 Mizugae, Saga-shi, Saga 840-8571, Japan,
fNagayoshi Orthopedic Clinic, 1890
Otsukachohinokuchi, Miyazaki-shi, Miyazaki 880-0951, Japan,
gDepartment of Physical Medicine and
Rehabilitation, Tohoku University Graduate School of Medicine, 1-1
Seiryoumachi, Aoba-Ku, Sendai-shi, Miyagi 980-8474, Japan,
hDepartment of Orthopedic Surgery, Senjyudo
Hospital, 1-25 Senjyudonakamati, Gifu-shi, Gifu 500-8862, Japan,
iEndo Clinic, 4-8-11 Ogawanishimachi,
Kodaira-shi, Tokyo 187-0035, Japan,
jAwakura Clinic, 1-6-3 Satomachi,
Wajima-shi, Ishiwawa 928-0246, Japan,
kDepartment of Orthopedic Surgery, Kyushu
University Faculty of Medicine, 3-1-1 Maidashi, Higashi-ku, Fukuoka-shi,
Fukuoka 812-8582, Japan, mDepartment of
Pediatrics, Akashi Municipal Hospital, 1-33 Takashomachi, Akashi-shi, Hyogo
673-8501 Japan, nKawara Clinic, 2-12-3
Sumiyoshihonmachi, Higashinada-ku, Kobe-shi, Hyogo 658-0051, Japan,
oDepartment of Orthopaedic Surgery, Nagoya
University Graduate School and School of Medicine, 65 Tsurumaicho, Showa-ku,
Nagoya-shi, Aichi 466-8550, Japan,
pDepartment of Rehabilitation, Nagoya City
University Hospital, 1 Mizuhochoazakawasumi, Mizuho-ku, Nagoya-shi, Aichi
467-8602, Japan, qDivision of
Morphological Science, Biomedical Research Center, and Departments of
aaLaboratory Medicine,
bbPediatrics,
ccOrthopedic Surgery,
rNeurology,
sEndocrinology and Diabetes,
tObstetrics and Gynecology, and
vOral and Maxillofacial Surgery, Saitama
Medical University, 38 Moro Hongo, Moroyama-machi, Iruma-gun, Saitama350-0495,
Japan, uDepartment of Neurology, Saitama
Neuropsychiatric Institute, 6-11-1 Honchohigashi, Chuo-ku, Saitama-shi,
Saitama 338-8577, Japan, zDepartment of
Molecular Pathology, University of Tokyo Graduate School of Medicine, 7-3-1
Hongo, Bunkyo-ku, Tokyo 113-0033, Japan,
wKitasato University School of
Pharmaceutical Science, 5-9-1 Shirokane, Minato-ku, Tokyo 108-0023, Japan,
xCardiovascular Research Center and
Division of Cardiology, Department of Medicine, Massachusetts General
Hospital, Harvard Medical School, Boston, Massachusetts 02129,
yCenter for Research in Fibrodysplasia
Ossificans Progressiva and Related Disorders, Department of Orthopedic
Surgery, University of Pennsylvania School of Medicine, Philadelphia,
Pennsylvania 19104-6081, ddDivision of
Molecular Signaling and Biochemistry, Department of Biosciences, Kyushu Dental
Collage, 2-6-1 Manazuru, Kokurakita-ku, Kitakyushu-shi, Fukuoka 803-8580,
Japan, eeDepartment of Orthopedic Surgery,
University of Ryukyus Faculty of Medicine, 207 Uehara, Nishihara-cho, 903-0215
Okinawa, Japan, and lThe Research
Committee on Fibrodysplasia Ossificans Progressiva, Ministry of Health, Labour
and Welfare, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan
k,
Yasuharu Nakashima
Yasuharu Nakashima
Divisions of aPathophysiology and
cTranslational Research, Research Center
for Genomic Medicine, Saitama Medical University, 1397-1 Yamane, Hidaka-shi,
Saitama 350-1241, Japan, bProject of
Clinical and Basic Research for FOP, Saitama Medical University, 1397-1
Yamane, Hidaka-shi, Saitama 350-1241, Japan,
dDepartment of Pediatric Emergency,
Kitakyusyu City Yahata Hospital, 4-18-1 Nishihonmachi, Yahatahigashi-ku,
Kitakyusyu-shi, Fukuoka 805-8534, Japan,
eDepartment of Orthopedic Surgery, Saga
Prefectural Hospital Koseikan, 1-12-9 Mizugae, Saga-shi, Saga 840-8571, Japan,
fNagayoshi Orthopedic Clinic, 1890
Otsukachohinokuchi, Miyazaki-shi, Miyazaki 880-0951, Japan,
gDepartment of Physical Medicine and
Rehabilitation, Tohoku University Graduate School of Medicine, 1-1
Seiryoumachi, Aoba-Ku, Sendai-shi, Miyagi 980-8474, Japan,
hDepartment of Orthopedic Surgery, Senjyudo
Hospital, 1-25 Senjyudonakamati, Gifu-shi, Gifu 500-8862, Japan,
iEndo Clinic, 4-8-11 Ogawanishimachi,
Kodaira-shi, Tokyo 187-0035, Japan,
jAwakura Clinic, 1-6-3 Satomachi,
Wajima-shi, Ishiwawa 928-0246, Japan,
kDepartment of Orthopedic Surgery, Kyushu
University Faculty of Medicine, 3-1-1 Maidashi, Higashi-ku, Fukuoka-shi,
Fukuoka 812-8582, Japan, mDepartment of
Pediatrics, Akashi Municipal Hospital, 1-33 Takashomachi, Akashi-shi, Hyogo
673-8501 Japan, nKawara Clinic, 2-12-3
Sumiyoshihonmachi, Higashinada-ku, Kobe-shi, Hyogo 658-0051, Japan,
oDepartment of Orthopaedic Surgery, Nagoya
University Graduate School and School of Medicine, 65 Tsurumaicho, Showa-ku,
Nagoya-shi, Aichi 466-8550, Japan,
pDepartment of Rehabilitation, Nagoya City
University Hospital, 1 Mizuhochoazakawasumi, Mizuho-ku, Nagoya-shi, Aichi
467-8602, Japan, qDivision of
Morphological Science, Biomedical Research Center, and Departments of
aaLaboratory Medicine,
bbPediatrics,
ccOrthopedic Surgery,
rNeurology,
sEndocrinology and Diabetes,
tObstetrics and Gynecology, and
vOral and Maxillofacial Surgery, Saitama
Medical University, 38 Moro Hongo, Moroyama-machi, Iruma-gun, Saitama350-0495,
Japan, uDepartment of Neurology, Saitama
Neuropsychiatric Institute, 6-11-1 Honchohigashi, Chuo-ku, Saitama-shi,
Saitama 338-8577, Japan, zDepartment of
Molecular Pathology, University of Tokyo Graduate School of Medicine, 7-3-1
Hongo, Bunkyo-ku, Tokyo 113-0033, Japan,
wKitasato University School of
Pharmaceutical Science, 5-9-1 Shirokane, Minato-ku, Tokyo 108-0023, Japan,
xCardiovascular Research Center and
Division of Cardiology, Department of Medicine, Massachusetts General
Hospital, Harvard Medical School, Boston, Massachusetts 02129,
yCenter for Research in Fibrodysplasia
Ossificans Progressiva and Related Disorders, Department of Orthopedic
Surgery, University of Pennsylvania School of Medicine, Philadelphia,
Pennsylvania 19104-6081, ddDivision of
Molecular Signaling and Biochemistry, Department of Biosciences, Kyushu Dental
Collage, 2-6-1 Manazuru, Kokurakita-ku, Kitakyushu-shi, Fukuoka 803-8580,
Japan, eeDepartment of Orthopedic Surgery,
University of Ryukyus Faculty of Medicine, 207 Uehara, Nishihara-cho, 903-0215
Okinawa, Japan, and lThe Research
Committee on Fibrodysplasia Ossificans Progressiva, Ministry of Health, Labour
and Welfare, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan
k,l,
Tomohiro Chiyonobu
Tomohiro Chiyonobu
Divisions of aPathophysiology and
cTranslational Research, Research Center
for Genomic Medicine, Saitama Medical University, 1397-1 Yamane, Hidaka-shi,
Saitama 350-1241, Japan, bProject of
Clinical and Basic Research for FOP, Saitama Medical University, 1397-1
Yamane, Hidaka-shi, Saitama 350-1241, Japan,
dDepartment of Pediatric Emergency,
Kitakyusyu City Yahata Hospital, 4-18-1 Nishihonmachi, Yahatahigashi-ku,
Kitakyusyu-shi, Fukuoka 805-8534, Japan,
eDepartment of Orthopedic Surgery, Saga
Prefectural Hospital Koseikan, 1-12-9 Mizugae, Saga-shi, Saga 840-8571, Japan,
fNagayoshi Orthopedic Clinic, 1890
Otsukachohinokuchi, Miyazaki-shi, Miyazaki 880-0951, Japan,
gDepartment of Physical Medicine and
Rehabilitation, Tohoku University Graduate School of Medicine, 1-1
Seiryoumachi, Aoba-Ku, Sendai-shi, Miyagi 980-8474, Japan,
hDepartment of Orthopedic Surgery, Senjyudo
Hospital, 1-25 Senjyudonakamati, Gifu-shi, Gifu 500-8862, Japan,
iEndo Clinic, 4-8-11 Ogawanishimachi,
Kodaira-shi, Tokyo 187-0035, Japan,
jAwakura Clinic, 1-6-3 Satomachi,
Wajima-shi, Ishiwawa 928-0246, Japan,
kDepartment of Orthopedic Surgery, Kyushu
University Faculty of Medicine, 3-1-1 Maidashi, Higashi-ku, Fukuoka-shi,
Fukuoka 812-8582, Japan, mDepartment of
Pediatrics, Akashi Municipal Hospital, 1-33 Takashomachi, Akashi-shi, Hyogo
673-8501 Japan, nKawara Clinic, 2-12-3
Sumiyoshihonmachi, Higashinada-ku, Kobe-shi, Hyogo 658-0051, Japan,
oDepartment of Orthopaedic Surgery, Nagoya
University Graduate School and School of Medicine, 65 Tsurumaicho, Showa-ku,
Nagoya-shi, Aichi 466-8550, Japan,
pDepartment of Rehabilitation, Nagoya City
University Hospital, 1 Mizuhochoazakawasumi, Mizuho-ku, Nagoya-shi, Aichi
467-8602, Japan, qDivision of
Morphological Science, Biomedical Research Center, and Departments of
aaLaboratory Medicine,
bbPediatrics,
ccOrthopedic Surgery,
rNeurology,
sEndocrinology and Diabetes,
tObstetrics and Gynecology, and
vOral and Maxillofacial Surgery, Saitama
Medical University, 38 Moro Hongo, Moroyama-machi, Iruma-gun, Saitama350-0495,
Japan, uDepartment of Neurology, Saitama
Neuropsychiatric Institute, 6-11-1 Honchohigashi, Chuo-ku, Saitama-shi,
Saitama 338-8577, Japan, zDepartment of
Molecular Pathology, University of Tokyo Graduate School of Medicine, 7-3-1
Hongo, Bunkyo-ku, Tokyo 113-0033, Japan,
wKitasato University School of
Pharmaceutical Science, 5-9-1 Shirokane, Minato-ku, Tokyo 108-0023, Japan,
xCardiovascular Research Center and
Division of Cardiology, Department of Medicine, Massachusetts General
Hospital, Harvard Medical School, Boston, Massachusetts 02129,
yCenter for Research in Fibrodysplasia
Ossificans Progressiva and Related Disorders, Department of Orthopedic
Surgery, University of Pennsylvania School of Medicine, Philadelphia,
Pennsylvania 19104-6081, ddDivision of
Molecular Signaling and Biochemistry, Department of Biosciences, Kyushu Dental
Collage, 2-6-1 Manazuru, Kokurakita-ku, Kitakyushu-shi, Fukuoka 803-8580,
Japan, eeDepartment of Orthopedic Surgery,
University of Ryukyus Faculty of Medicine, 207 Uehara, Nishihara-cho, 903-0215
Okinawa, Japan, and lThe Research
Committee on Fibrodysplasia Ossificans Progressiva, Ministry of Health, Labour
and Welfare, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan
m,
Akira Kawara
Akira Kawara
Divisions of aPathophysiology and
cTranslational Research, Research Center
for Genomic Medicine, Saitama Medical University, 1397-1 Yamane, Hidaka-shi,
Saitama 350-1241, Japan, bProject of
Clinical and Basic Research for FOP, Saitama Medical University, 1397-1
Yamane, Hidaka-shi, Saitama 350-1241, Japan,
dDepartment of Pediatric Emergency,
Kitakyusyu City Yahata Hospital, 4-18-1 Nishihonmachi, Yahatahigashi-ku,
Kitakyusyu-shi, Fukuoka 805-8534, Japan,
eDepartment of Orthopedic Surgery, Saga
Prefectural Hospital Koseikan, 1-12-9 Mizugae, Saga-shi, Saga 840-8571, Japan,
fNagayoshi Orthopedic Clinic, 1890
Otsukachohinokuchi, Miyazaki-shi, Miyazaki 880-0951, Japan,
gDepartment of Physical Medicine and
Rehabilitation, Tohoku University Graduate School of Medicine, 1-1
Seiryoumachi, Aoba-Ku, Sendai-shi, Miyagi 980-8474, Japan,
hDepartment of Orthopedic Surgery, Senjyudo
Hospital, 1-25 Senjyudonakamati, Gifu-shi, Gifu 500-8862, Japan,
iEndo Clinic, 4-8-11 Ogawanishimachi,
Kodaira-shi, Tokyo 187-0035, Japan,
jAwakura Clinic, 1-6-3 Satomachi,
Wajima-shi, Ishiwawa 928-0246, Japan,
kDepartment of Orthopedic Surgery, Kyushu
University Faculty of Medicine, 3-1-1 Maidashi, Higashi-ku, Fukuoka-shi,
Fukuoka 812-8582, Japan, mDepartment of
Pediatrics, Akashi Municipal Hospital, 1-33 Takashomachi, Akashi-shi, Hyogo
673-8501 Japan, nKawara Clinic, 2-12-3
Sumiyoshihonmachi, Higashinada-ku, Kobe-shi, Hyogo 658-0051, Japan,
oDepartment of Orthopaedic Surgery, Nagoya
University Graduate School and School of Medicine, 65 Tsurumaicho, Showa-ku,
Nagoya-shi, Aichi 466-8550, Japan,
pDepartment of Rehabilitation, Nagoya City
University Hospital, 1 Mizuhochoazakawasumi, Mizuho-ku, Nagoya-shi, Aichi
467-8602, Japan, qDivision of
Morphological Science, Biomedical Research Center, and Departments of
aaLaboratory Medicine,
bbPediatrics,
ccOrthopedic Surgery,
rNeurology,
sEndocrinology and Diabetes,
tObstetrics and Gynecology, and
vOral and Maxillofacial Surgery, Saitama
Medical University, 38 Moro Hongo, Moroyama-machi, Iruma-gun, Saitama350-0495,
Japan, uDepartment of Neurology, Saitama
Neuropsychiatric Institute, 6-11-1 Honchohigashi, Chuo-ku, Saitama-shi,
Saitama 338-8577, Japan, zDepartment of
Molecular Pathology, University of Tokyo Graduate School of Medicine, 7-3-1
Hongo, Bunkyo-ku, Tokyo 113-0033, Japan,
wKitasato University School of
Pharmaceutical Science, 5-9-1 Shirokane, Minato-ku, Tokyo 108-0023, Japan,
xCardiovascular Research Center and
Division of Cardiology, Department of Medicine, Massachusetts General
Hospital, Harvard Medical School, Boston, Massachusetts 02129,
yCenter for Research in Fibrodysplasia
Ossificans Progressiva and Related Disorders, Department of Orthopedic
Surgery, University of Pennsylvania School of Medicine, Philadelphia,
Pennsylvania 19104-6081, ddDivision of
Molecular Signaling and Biochemistry, Department of Biosciences, Kyushu Dental
Collage, 2-6-1 Manazuru, Kokurakita-ku, Kitakyushu-shi, Fukuoka 803-8580,
Japan, eeDepartment of Orthopedic Surgery,
University of Ryukyus Faculty of Medicine, 207 Uehara, Nishihara-cho, 903-0215
Okinawa, Japan, and lThe Research
Committee on Fibrodysplasia Ossificans Progressiva, Ministry of Health, Labour
and Welfare, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan
n,
Yoshihiro Nishida
Yoshihiro Nishida
Divisions of aPathophysiology and
cTranslational Research, Research Center
for Genomic Medicine, Saitama Medical University, 1397-1 Yamane, Hidaka-shi,
Saitama 350-1241, Japan, bProject of
Clinical and Basic Research for FOP, Saitama Medical University, 1397-1
Yamane, Hidaka-shi, Saitama 350-1241, Japan,
dDepartment of Pediatric Emergency,
Kitakyusyu City Yahata Hospital, 4-18-1 Nishihonmachi, Yahatahigashi-ku,
Kitakyusyu-shi, Fukuoka 805-8534, Japan,
eDepartment of Orthopedic Surgery, Saga
Prefectural Hospital Koseikan, 1-12-9 Mizugae, Saga-shi, Saga 840-8571, Japan,
fNagayoshi Orthopedic Clinic, 1890
Otsukachohinokuchi, Miyazaki-shi, Miyazaki 880-0951, Japan,
gDepartment of Physical Medicine and
Rehabilitation, Tohoku University Graduate School of Medicine, 1-1
Seiryoumachi, Aoba-Ku, Sendai-shi, Miyagi 980-8474, Japan,
hDepartment of Orthopedic Surgery, Senjyudo
Hospital, 1-25 Senjyudonakamati, Gifu-shi, Gifu 500-8862, Japan,
iEndo Clinic, 4-8-11 Ogawanishimachi,
Kodaira-shi, Tokyo 187-0035, Japan,
jAwakura Clinic, 1-6-3 Satomachi,
Wajima-shi, Ishiwawa 928-0246, Japan,
kDepartment of Orthopedic Surgery, Kyushu
University Faculty of Medicine, 3-1-1 Maidashi, Higashi-ku, Fukuoka-shi,
Fukuoka 812-8582, Japan, mDepartment of
Pediatrics, Akashi Municipal Hospital, 1-33 Takashomachi, Akashi-shi, Hyogo
673-8501 Japan, nKawara Clinic, 2-12-3
Sumiyoshihonmachi, Higashinada-ku, Kobe-shi, Hyogo 658-0051, Japan,
oDepartment of Orthopaedic Surgery, Nagoya
University Graduate School and School of Medicine, 65 Tsurumaicho, Showa-ku,
Nagoya-shi, Aichi 466-8550, Japan,
pDepartment of Rehabilitation, Nagoya City
University Hospital, 1 Mizuhochoazakawasumi, Mizuho-ku, Nagoya-shi, Aichi
467-8602, Japan, qDivision of
Morphological Science, Biomedical Research Center, and Departments of
aaLaboratory Medicine,
bbPediatrics,
ccOrthopedic Surgery,
rNeurology,
sEndocrinology and Diabetes,
tObstetrics and Gynecology, and
vOral and Maxillofacial Surgery, Saitama
Medical University, 38 Moro Hongo, Moroyama-machi, Iruma-gun, Saitama350-0495,
Japan, uDepartment of Neurology, Saitama
Neuropsychiatric Institute, 6-11-1 Honchohigashi, Chuo-ku, Saitama-shi,
Saitama 338-8577, Japan, zDepartment of
Molecular Pathology, University of Tokyo Graduate School of Medicine, 7-3-1
Hongo, Bunkyo-ku, Tokyo 113-0033, Japan,
wKitasato University School of
Pharmaceutical Science, 5-9-1 Shirokane, Minato-ku, Tokyo 108-0023, Japan,
xCardiovascular Research Center and
Division of Cardiology, Department of Medicine, Massachusetts General
Hospital, Harvard Medical School, Boston, Massachusetts 02129,
yCenter for Research in Fibrodysplasia
Ossificans Progressiva and Related Disorders, Department of Orthopedic
Surgery, University of Pennsylvania School of Medicine, Philadelphia,
Pennsylvania 19104-6081, ddDivision of
Molecular Signaling and Biochemistry, Department of Biosciences, Kyushu Dental
Collage, 2-6-1 Manazuru, Kokurakita-ku, Kitakyushu-shi, Fukuoka 803-8580,
Japan, eeDepartment of Orthopedic Surgery,
University of Ryukyus Faculty of Medicine, 207 Uehara, Nishihara-cho, 903-0215
Okinawa, Japan, and lThe Research
Committee on Fibrodysplasia Ossificans Progressiva, Ministry of Health, Labour
and Welfare, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan
o,
Ikuo Wada
Ikuo Wada
Divisions of aPathophysiology and
cTranslational Research, Research Center
for Genomic Medicine, Saitama Medical University, 1397-1 Yamane, Hidaka-shi,
Saitama 350-1241, Japan, bProject of
Clinical and Basic Research for FOP, Saitama Medical University, 1397-1
Yamane, Hidaka-shi, Saitama 350-1241, Japan,
dDepartment of Pediatric Emergency,
Kitakyusyu City Yahata Hospital, 4-18-1 Nishihonmachi, Yahatahigashi-ku,
Kitakyusyu-shi, Fukuoka 805-8534, Japan,
eDepartment of Orthopedic Surgery, Saga
Prefectural Hospital Koseikan, 1-12-9 Mizugae, Saga-shi, Saga 840-8571, Japan,
fNagayoshi Orthopedic Clinic, 1890
Otsukachohinokuchi, Miyazaki-shi, Miyazaki 880-0951, Japan,
gDepartment of Physical Medicine and
Rehabilitation, Tohoku University Graduate School of Medicine, 1-1
Seiryoumachi, Aoba-Ku, Sendai-shi, Miyagi 980-8474, Japan,
hDepartment of Orthopedic Surgery, Senjyudo
Hospital, 1-25 Senjyudonakamati, Gifu-shi, Gifu 500-8862, Japan,
iEndo Clinic, 4-8-11 Ogawanishimachi,
Kodaira-shi, Tokyo 187-0035, Japan,
jAwakura Clinic, 1-6-3 Satomachi,
Wajima-shi, Ishiwawa 928-0246, Japan,
kDepartment of Orthopedic Surgery, Kyushu
University Faculty of Medicine, 3-1-1 Maidashi, Higashi-ku, Fukuoka-shi,
Fukuoka 812-8582, Japan, mDepartment of
Pediatrics, Akashi Municipal Hospital, 1-33 Takashomachi, Akashi-shi, Hyogo
673-8501 Japan, nKawara Clinic, 2-12-3
Sumiyoshihonmachi, Higashinada-ku, Kobe-shi, Hyogo 658-0051, Japan,
oDepartment of Orthopaedic Surgery, Nagoya
University Graduate School and School of Medicine, 65 Tsurumaicho, Showa-ku,
Nagoya-shi, Aichi 466-8550, Japan,
pDepartment of Rehabilitation, Nagoya City
University Hospital, 1 Mizuhochoazakawasumi, Mizuho-ku, Nagoya-shi, Aichi
467-8602, Japan, qDivision of
Morphological Science, Biomedical Research Center, and Departments of
aaLaboratory Medicine,
bbPediatrics,
ccOrthopedic Surgery,
rNeurology,
sEndocrinology and Diabetes,
tObstetrics and Gynecology, and
vOral and Maxillofacial Surgery, Saitama
Medical University, 38 Moro Hongo, Moroyama-machi, Iruma-gun, Saitama350-0495,
Japan, uDepartment of Neurology, Saitama
Neuropsychiatric Institute, 6-11-1 Honchohigashi, Chuo-ku, Saitama-shi,
Saitama 338-8577, Japan, zDepartment of
Molecular Pathology, University of Tokyo Graduate School of Medicine, 7-3-1
Hongo, Bunkyo-ku, Tokyo 113-0033, Japan,
wKitasato University School of
Pharmaceutical Science, 5-9-1 Shirokane, Minato-ku, Tokyo 108-0023, Japan,
xCardiovascular Research Center and
Division of Cardiology, Department of Medicine, Massachusetts General
Hospital, Harvard Medical School, Boston, Massachusetts 02129,
yCenter for Research in Fibrodysplasia
Ossificans Progressiva and Related Disorders, Department of Orthopedic
Surgery, University of Pennsylvania School of Medicine, Philadelphia,
Pennsylvania 19104-6081, ddDivision of
Molecular Signaling and Biochemistry, Department of Biosciences, Kyushu Dental
Collage, 2-6-1 Manazuru, Kokurakita-ku, Kitakyushu-shi, Fukuoka 803-8580,
Japan, eeDepartment of Orthopedic Surgery,
University of Ryukyus Faculty of Medicine, 207 Uehara, Nishihara-cho, 903-0215
Okinawa, Japan, and lThe Research
Committee on Fibrodysplasia Ossificans Progressiva, Ministry of Health, Labour
and Welfare, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan
p,
Masumi Akita
Masumi Akita
Divisions of aPathophysiology and
cTranslational Research, Research Center
for Genomic Medicine, Saitama Medical University, 1397-1 Yamane, Hidaka-shi,
Saitama 350-1241, Japan, bProject of
Clinical and Basic Research for FOP, Saitama Medical University, 1397-1
Yamane, Hidaka-shi, Saitama 350-1241, Japan,
dDepartment of Pediatric Emergency,
Kitakyusyu City Yahata Hospital, 4-18-1 Nishihonmachi, Yahatahigashi-ku,
Kitakyusyu-shi, Fukuoka 805-8534, Japan,
eDepartment of Orthopedic Surgery, Saga
Prefectural Hospital Koseikan, 1-12-9 Mizugae, Saga-shi, Saga 840-8571, Japan,
fNagayoshi Orthopedic Clinic, 1890
Otsukachohinokuchi, Miyazaki-shi, Miyazaki 880-0951, Japan,
gDepartment of Physical Medicine and
Rehabilitation, Tohoku University Graduate School of Medicine, 1-1
Seiryoumachi, Aoba-Ku, Sendai-shi, Miyagi 980-8474, Japan,
hDepartment of Orthopedic Surgery, Senjyudo
Hospital, 1-25 Senjyudonakamati, Gifu-shi, Gifu 500-8862, Japan,
iEndo Clinic, 4-8-11 Ogawanishimachi,
Kodaira-shi, Tokyo 187-0035, Japan,
jAwakura Clinic, 1-6-3 Satomachi,
Wajima-shi, Ishiwawa 928-0246, Japan,
kDepartment of Orthopedic Surgery, Kyushu
University Faculty of Medicine, 3-1-1 Maidashi, Higashi-ku, Fukuoka-shi,
Fukuoka 812-8582, Japan, mDepartment of
Pediatrics, Akashi Municipal Hospital, 1-33 Takashomachi, Akashi-shi, Hyogo
673-8501 Japan, nKawara Clinic, 2-12-3
Sumiyoshihonmachi, Higashinada-ku, Kobe-shi, Hyogo 658-0051, Japan,
oDepartment of Orthopaedic Surgery, Nagoya
University Graduate School and School of Medicine, 65 Tsurumaicho, Showa-ku,
Nagoya-shi, Aichi 466-8550, Japan,
pDepartment of Rehabilitation, Nagoya City
University Hospital, 1 Mizuhochoazakawasumi, Mizuho-ku, Nagoya-shi, Aichi
467-8602, Japan, qDivision of
Morphological Science, Biomedical Research Center, and Departments of
aaLaboratory Medicine,
bbPediatrics,
ccOrthopedic Surgery,
rNeurology,
sEndocrinology and Diabetes,
tObstetrics and Gynecology, and
vOral and Maxillofacial Surgery, Saitama
Medical University, 38 Moro Hongo, Moroyama-machi, Iruma-gun, Saitama350-0495,
Japan, uDepartment of Neurology, Saitama
Neuropsychiatric Institute, 6-11-1 Honchohigashi, Chuo-ku, Saitama-shi,
Saitama 338-8577, Japan, zDepartment of
Molecular Pathology, University of Tokyo Graduate School of Medicine, 7-3-1
Hongo, Bunkyo-ku, Tokyo 113-0033, Japan,
wKitasato University School of
Pharmaceutical Science, 5-9-1 Shirokane, Minato-ku, Tokyo 108-0023, Japan,
xCardiovascular Research Center and
Division of Cardiology, Department of Medicine, Massachusetts General
Hospital, Harvard Medical School, Boston, Massachusetts 02129,
yCenter for Research in Fibrodysplasia
Ossificans Progressiva and Related Disorders, Department of Orthopedic
Surgery, University of Pennsylvania School of Medicine, Philadelphia,
Pennsylvania 19104-6081, ddDivision of
Molecular Signaling and Biochemistry, Department of Biosciences, Kyushu Dental
Collage, 2-6-1 Manazuru, Kokurakita-ku, Kitakyushu-shi, Fukuoka 803-8580,
Japan, eeDepartment of Orthopedic Surgery,
University of Ryukyus Faculty of Medicine, 207 Uehara, Nishihara-cho, 903-0215
Okinawa, Japan, and lThe Research
Committee on Fibrodysplasia Ossificans Progressiva, Ministry of Health, Labour
and Welfare, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan
b,q,
Tetsuo Komori
Tetsuo Komori
Divisions of aPathophysiology and
cTranslational Research, Research Center
for Genomic Medicine, Saitama Medical University, 1397-1 Yamane, Hidaka-shi,
Saitama 350-1241, Japan, bProject of
Clinical and Basic Research for FOP, Saitama Medical University, 1397-1
Yamane, Hidaka-shi, Saitama 350-1241, Japan,
dDepartment of Pediatric Emergency,
Kitakyusyu City Yahata Hospital, 4-18-1 Nishihonmachi, Yahatahigashi-ku,
Kitakyusyu-shi, Fukuoka 805-8534, Japan,
eDepartment of Orthopedic Surgery, Saga
Prefectural Hospital Koseikan, 1-12-9 Mizugae, Saga-shi, Saga 840-8571, Japan,
fNagayoshi Orthopedic Clinic, 1890
Otsukachohinokuchi, Miyazaki-shi, Miyazaki 880-0951, Japan,
gDepartment of Physical Medicine and
Rehabilitation, Tohoku University Graduate School of Medicine, 1-1
Seiryoumachi, Aoba-Ku, Sendai-shi, Miyagi 980-8474, Japan,
hDepartment of Orthopedic Surgery, Senjyudo
Hospital, 1-25 Senjyudonakamati, Gifu-shi, Gifu 500-8862, Japan,
iEndo Clinic, 4-8-11 Ogawanishimachi,
Kodaira-shi, Tokyo 187-0035, Japan,
jAwakura Clinic, 1-6-3 Satomachi,
Wajima-shi, Ishiwawa 928-0246, Japan,
kDepartment of Orthopedic Surgery, Kyushu
University Faculty of Medicine, 3-1-1 Maidashi, Higashi-ku, Fukuoka-shi,
Fukuoka 812-8582, Japan, mDepartment of
Pediatrics, Akashi Municipal Hospital, 1-33 Takashomachi, Akashi-shi, Hyogo
673-8501 Japan, nKawara Clinic, 2-12-3
Sumiyoshihonmachi, Higashinada-ku, Kobe-shi, Hyogo 658-0051, Japan,
oDepartment of Orthopaedic Surgery, Nagoya
University Graduate School and School of Medicine, 65 Tsurumaicho, Showa-ku,
Nagoya-shi, Aichi 466-8550, Japan,
pDepartment of Rehabilitation, Nagoya City
University Hospital, 1 Mizuhochoazakawasumi, Mizuho-ku, Nagoya-shi, Aichi
467-8602, Japan, qDivision of
Morphological Science, Biomedical Research Center, and Departments of
aaLaboratory Medicine,
bbPediatrics,
ccOrthopedic Surgery,
rNeurology,
sEndocrinology and Diabetes,
tObstetrics and Gynecology, and
vOral and Maxillofacial Surgery, Saitama
Medical University, 38 Moro Hongo, Moroyama-machi, Iruma-gun, Saitama350-0495,
Japan, uDepartment of Neurology, Saitama
Neuropsychiatric Institute, 6-11-1 Honchohigashi, Chuo-ku, Saitama-shi,
Saitama 338-8577, Japan, zDepartment of
Molecular Pathology, University of Tokyo Graduate School of Medicine, 7-3-1
Hongo, Bunkyo-ku, Tokyo 113-0033, Japan,
wKitasato University School of
Pharmaceutical Science, 5-9-1 Shirokane, Minato-ku, Tokyo 108-0023, Japan,
xCardiovascular Research Center and
Division of Cardiology, Department of Medicine, Massachusetts General
Hospital, Harvard Medical School, Boston, Massachusetts 02129,
yCenter for Research in Fibrodysplasia
Ossificans Progressiva and Related Disorders, Department of Orthopedic
Surgery, University of Pennsylvania School of Medicine, Philadelphia,
Pennsylvania 19104-6081, ddDivision of
Molecular Signaling and Biochemistry, Department of Biosciences, Kyushu Dental
Collage, 2-6-1 Manazuru, Kokurakita-ku, Kitakyushu-shi, Fukuoka 803-8580,
Japan, eeDepartment of Orthopedic Surgery,
University of Ryukyus Faculty of Medicine, 207 Uehara, Nishihara-cho, 903-0215
Okinawa, Japan, and lThe Research
Committee on Fibrodysplasia Ossificans Progressiva, Ministry of Health, Labour
and Welfare, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan
b,r,
Konosuke Nakayama
Konosuke Nakayama
Divisions of aPathophysiology and
cTranslational Research, Research Center
for Genomic Medicine, Saitama Medical University, 1397-1 Yamane, Hidaka-shi,
Saitama 350-1241, Japan, bProject of
Clinical and Basic Research for FOP, Saitama Medical University, 1397-1
Yamane, Hidaka-shi, Saitama 350-1241, Japan,
dDepartment of Pediatric Emergency,
Kitakyusyu City Yahata Hospital, 4-18-1 Nishihonmachi, Yahatahigashi-ku,
Kitakyusyu-shi, Fukuoka 805-8534, Japan,
eDepartment of Orthopedic Surgery, Saga
Prefectural Hospital Koseikan, 1-12-9 Mizugae, Saga-shi, Saga 840-8571, Japan,
fNagayoshi Orthopedic Clinic, 1890
Otsukachohinokuchi, Miyazaki-shi, Miyazaki 880-0951, Japan,
gDepartment of Physical Medicine and
Rehabilitation, Tohoku University Graduate School of Medicine, 1-1
Seiryoumachi, Aoba-Ku, Sendai-shi, Miyagi 980-8474, Japan,
hDepartment of Orthopedic Surgery, Senjyudo
Hospital, 1-25 Senjyudonakamati, Gifu-shi, Gifu 500-8862, Japan,
iEndo Clinic, 4-8-11 Ogawanishimachi,
Kodaira-shi, Tokyo 187-0035, Japan,
jAwakura Clinic, 1-6-3 Satomachi,
Wajima-shi, Ishiwawa 928-0246, Japan,
kDepartment of Orthopedic Surgery, Kyushu
University Faculty of Medicine, 3-1-1 Maidashi, Higashi-ku, Fukuoka-shi,
Fukuoka 812-8582, Japan, mDepartment of
Pediatrics, Akashi Municipal Hospital, 1-33 Takashomachi, Akashi-shi, Hyogo
673-8501 Japan, nKawara Clinic, 2-12-3
Sumiyoshihonmachi, Higashinada-ku, Kobe-shi, Hyogo 658-0051, Japan,
oDepartment of Orthopaedic Surgery, Nagoya
University Graduate School and School of Medicine, 65 Tsurumaicho, Showa-ku,
Nagoya-shi, Aichi 466-8550, Japan,
pDepartment of Rehabilitation, Nagoya City
University Hospital, 1 Mizuhochoazakawasumi, Mizuho-ku, Nagoya-shi, Aichi
467-8602, Japan, qDivision of
Morphological Science, Biomedical Research Center, and Departments of
aaLaboratory Medicine,
bbPediatrics,
ccOrthopedic Surgery,
rNeurology,
sEndocrinology and Diabetes,
tObstetrics and Gynecology, and
vOral and Maxillofacial Surgery, Saitama
Medical University, 38 Moro Hongo, Moroyama-machi, Iruma-gun, Saitama350-0495,
Japan, uDepartment of Neurology, Saitama
Neuropsychiatric Institute, 6-11-1 Honchohigashi, Chuo-ku, Saitama-shi,
Saitama 338-8577, Japan, zDepartment of
Molecular Pathology, University of Tokyo Graduate School of Medicine, 7-3-1
Hongo, Bunkyo-ku, Tokyo 113-0033, Japan,
wKitasato University School of
Pharmaceutical Science, 5-9-1 Shirokane, Minato-ku, Tokyo 108-0023, Japan,
xCardiovascular Research Center and
Division of Cardiology, Department of Medicine, Massachusetts General
Hospital, Harvard Medical School, Boston, Massachusetts 02129,
yCenter for Research in Fibrodysplasia
Ossificans Progressiva and Related Disorders, Department of Orthopedic
Surgery, University of Pennsylvania School of Medicine, Philadelphia,
Pennsylvania 19104-6081, ddDivision of
Molecular Signaling and Biochemistry, Department of Biosciences, Kyushu Dental
Collage, 2-6-1 Manazuru, Kokurakita-ku, Kitakyushu-shi, Fukuoka 803-8580,
Japan, eeDepartment of Orthopedic Surgery,
University of Ryukyus Faculty of Medicine, 207 Uehara, Nishihara-cho, 903-0215
Okinawa, Japan, and lThe Research
Committee on Fibrodysplasia Ossificans Progressiva, Ministry of Health, Labour
and Welfare, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan
b,s,
Akira Nanba
Akira Nanba
Divisions of aPathophysiology and
cTranslational Research, Research Center
for Genomic Medicine, Saitama Medical University, 1397-1 Yamane, Hidaka-shi,
Saitama 350-1241, Japan, bProject of
Clinical and Basic Research for FOP, Saitama Medical University, 1397-1
Yamane, Hidaka-shi, Saitama 350-1241, Japan,
dDepartment of Pediatric Emergency,
Kitakyusyu City Yahata Hospital, 4-18-1 Nishihonmachi, Yahatahigashi-ku,
Kitakyusyu-shi, Fukuoka 805-8534, Japan,
eDepartment of Orthopedic Surgery, Saga
Prefectural Hospital Koseikan, 1-12-9 Mizugae, Saga-shi, Saga 840-8571, Japan,
fNagayoshi Orthopedic Clinic, 1890
Otsukachohinokuchi, Miyazaki-shi, Miyazaki 880-0951, Japan,
gDepartment of Physical Medicine and
Rehabilitation, Tohoku University Graduate School of Medicine, 1-1
Seiryoumachi, Aoba-Ku, Sendai-shi, Miyagi 980-8474, Japan,
hDepartment of Orthopedic Surgery, Senjyudo
Hospital, 1-25 Senjyudonakamati, Gifu-shi, Gifu 500-8862, Japan,
iEndo Clinic, 4-8-11 Ogawanishimachi,
Kodaira-shi, Tokyo 187-0035, Japan,
jAwakura Clinic, 1-6-3 Satomachi,
Wajima-shi, Ishiwawa 928-0246, Japan,
kDepartment of Orthopedic Surgery, Kyushu
University Faculty of Medicine, 3-1-1 Maidashi, Higashi-ku, Fukuoka-shi,
Fukuoka 812-8582, Japan, mDepartment of
Pediatrics, Akashi Municipal Hospital, 1-33 Takashomachi, Akashi-shi, Hyogo
673-8501 Japan, nKawara Clinic, 2-12-3
Sumiyoshihonmachi, Higashinada-ku, Kobe-shi, Hyogo 658-0051, Japan,
oDepartment of Orthopaedic Surgery, Nagoya
University Graduate School and School of Medicine, 65 Tsurumaicho, Showa-ku,
Nagoya-shi, Aichi 466-8550, Japan,
pDepartment of Rehabilitation, Nagoya City
University Hospital, 1 Mizuhochoazakawasumi, Mizuho-ku, Nagoya-shi, Aichi
467-8602, Japan, qDivision of
Morphological Science, Biomedical Research Center, and Departments of
aaLaboratory Medicine,
bbPediatrics,
ccOrthopedic Surgery,
rNeurology,
sEndocrinology and Diabetes,
tObstetrics and Gynecology, and
vOral and Maxillofacial Surgery, Saitama
Medical University, 38 Moro Hongo, Moroyama-machi, Iruma-gun, Saitama350-0495,
Japan, uDepartment of Neurology, Saitama
Neuropsychiatric Institute, 6-11-1 Honchohigashi, Chuo-ku, Saitama-shi,
Saitama 338-8577, Japan, zDepartment of
Molecular Pathology, University of Tokyo Graduate School of Medicine, 7-3-1
Hongo, Bunkyo-ku, Tokyo 113-0033, Japan,
wKitasato University School of
Pharmaceutical Science, 5-9-1 Shirokane, Minato-ku, Tokyo 108-0023, Japan,
xCardiovascular Research Center and
Division of Cardiology, Department of Medicine, Massachusetts General
Hospital, Harvard Medical School, Boston, Massachusetts 02129,
yCenter for Research in Fibrodysplasia
Ossificans Progressiva and Related Disorders, Department of Orthopedic
Surgery, University of Pennsylvania School of Medicine, Philadelphia,
Pennsylvania 19104-6081, ddDivision of
Molecular Signaling and Biochemistry, Department of Biosciences, Kyushu Dental
Collage, 2-6-1 Manazuru, Kokurakita-ku, Kitakyushu-shi, Fukuoka 803-8580,
Japan, eeDepartment of Orthopedic Surgery,
University of Ryukyus Faculty of Medicine, 207 Uehara, Nishihara-cho, 903-0215
Okinawa, Japan, and lThe Research
Committee on Fibrodysplasia Ossificans Progressiva, Ministry of Health, Labour
and Welfare, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan
b,t,
Yuichi Maruki
Yuichi Maruki
Divisions of aPathophysiology and
cTranslational Research, Research Center
for Genomic Medicine, Saitama Medical University, 1397-1 Yamane, Hidaka-shi,
Saitama 350-1241, Japan, bProject of
Clinical and Basic Research for FOP, Saitama Medical University, 1397-1
Yamane, Hidaka-shi, Saitama 350-1241, Japan,
dDepartment of Pediatric Emergency,
Kitakyusyu City Yahata Hospital, 4-18-1 Nishihonmachi, Yahatahigashi-ku,
Kitakyusyu-shi, Fukuoka 805-8534, Japan,
eDepartment of Orthopedic Surgery, Saga
Prefectural Hospital Koseikan, 1-12-9 Mizugae, Saga-shi, Saga 840-8571, Japan,
fNagayoshi Orthopedic Clinic, 1890
Otsukachohinokuchi, Miyazaki-shi, Miyazaki 880-0951, Japan,
gDepartment of Physical Medicine and
Rehabilitation, Tohoku University Graduate School of Medicine, 1-1
Seiryoumachi, Aoba-Ku, Sendai-shi, Miyagi 980-8474, Japan,
hDepartment of Orthopedic Surgery, Senjyudo
Hospital, 1-25 Senjyudonakamati, Gifu-shi, Gifu 500-8862, Japan,
iEndo Clinic, 4-8-11 Ogawanishimachi,
Kodaira-shi, Tokyo 187-0035, Japan,
jAwakura Clinic, 1-6-3 Satomachi,
Wajima-shi, Ishiwawa 928-0246, Japan,
kDepartment of Orthopedic Surgery, Kyushu
University Faculty of Medicine, 3-1-1 Maidashi, Higashi-ku, Fukuoka-shi,
Fukuoka 812-8582, Japan, mDepartment of
Pediatrics, Akashi Municipal Hospital, 1-33 Takashomachi, Akashi-shi, Hyogo
673-8501 Japan, nKawara Clinic, 2-12-3
Sumiyoshihonmachi, Higashinada-ku, Kobe-shi, Hyogo 658-0051, Japan,
oDepartment of Orthopaedic Surgery, Nagoya
University Graduate School and School of Medicine, 65 Tsurumaicho, Showa-ku,
Nagoya-shi, Aichi 466-8550, Japan,
pDepartment of Rehabilitation, Nagoya City
University Hospital, 1 Mizuhochoazakawasumi, Mizuho-ku, Nagoya-shi, Aichi
467-8602, Japan, qDivision of
Morphological Science, Biomedical Research Center, and Departments of
aaLaboratory Medicine,
bbPediatrics,
ccOrthopedic Surgery,
rNeurology,
sEndocrinology and Diabetes,
tObstetrics and Gynecology, and
vOral and Maxillofacial Surgery, Saitama
Medical University, 38 Moro Hongo, Moroyama-machi, Iruma-gun, Saitama350-0495,
Japan, uDepartment of Neurology, Saitama
Neuropsychiatric Institute, 6-11-1 Honchohigashi, Chuo-ku, Saitama-shi,
Saitama 338-8577, Japan, zDepartment of
Molecular Pathology, University of Tokyo Graduate School of Medicine, 7-3-1
Hongo, Bunkyo-ku, Tokyo 113-0033, Japan,
wKitasato University School of
Pharmaceutical Science, 5-9-1 Shirokane, Minato-ku, Tokyo 108-0023, Japan,
xCardiovascular Research Center and
Division of Cardiology, Department of Medicine, Massachusetts General
Hospital, Harvard Medical School, Boston, Massachusetts 02129,
yCenter for Research in Fibrodysplasia
Ossificans Progressiva and Related Disorders, Department of Orthopedic
Surgery, University of Pennsylvania School of Medicine, Philadelphia,
Pennsylvania 19104-6081, ddDivision of
Molecular Signaling and Biochemistry, Department of Biosciences, Kyushu Dental
Collage, 2-6-1 Manazuru, Kokurakita-ku, Kitakyushu-shi, Fukuoka 803-8580,
Japan, eeDepartment of Orthopedic Surgery,
University of Ryukyus Faculty of Medicine, 207 Uehara, Nishihara-cho, 903-0215
Okinawa, Japan, and lThe Research
Committee on Fibrodysplasia Ossificans Progressiva, Ministry of Health, Labour
and Welfare, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan
b,u,
Tetsuya Yoda
Tetsuya Yoda
Divisions of aPathophysiology and
cTranslational Research, Research Center
for Genomic Medicine, Saitama Medical University, 1397-1 Yamane, Hidaka-shi,
Saitama 350-1241, Japan, bProject of
Clinical and Basic Research for FOP, Saitama Medical University, 1397-1
Yamane, Hidaka-shi, Saitama 350-1241, Japan,
dDepartment of Pediatric Emergency,
Kitakyusyu City Yahata Hospital, 4-18-1 Nishihonmachi, Yahatahigashi-ku,
Kitakyusyu-shi, Fukuoka 805-8534, Japan,
eDepartment of Orthopedic Surgery, Saga
Prefectural Hospital Koseikan, 1-12-9 Mizugae, Saga-shi, Saga 840-8571, Japan,
fNagayoshi Orthopedic Clinic, 1890
Otsukachohinokuchi, Miyazaki-shi, Miyazaki 880-0951, Japan,
gDepartment of Physical Medicine and
Rehabilitation, Tohoku University Graduate School of Medicine, 1-1
Seiryoumachi, Aoba-Ku, Sendai-shi, Miyagi 980-8474, Japan,
hDepartment of Orthopedic Surgery, Senjyudo
Hospital, 1-25 Senjyudonakamati, Gifu-shi, Gifu 500-8862, Japan,
iEndo Clinic, 4-8-11 Ogawanishimachi,
Kodaira-shi, Tokyo 187-0035, Japan,
jAwakura Clinic, 1-6-3 Satomachi,
Wajima-shi, Ishiwawa 928-0246, Japan,
kDepartment of Orthopedic Surgery, Kyushu
University Faculty of Medicine, 3-1-1 Maidashi, Higashi-ku, Fukuoka-shi,
Fukuoka 812-8582, Japan, mDepartment of
Pediatrics, Akashi Municipal Hospital, 1-33 Takashomachi, Akashi-shi, Hyogo
673-8501 Japan, nKawara Clinic, 2-12-3
Sumiyoshihonmachi, Higashinada-ku, Kobe-shi, Hyogo 658-0051, Japan,
oDepartment of Orthopaedic Surgery, Nagoya
University Graduate School and School of Medicine, 65 Tsurumaicho, Showa-ku,
Nagoya-shi, Aichi 466-8550, Japan,
pDepartment of Rehabilitation, Nagoya City
University Hospital, 1 Mizuhochoazakawasumi, Mizuho-ku, Nagoya-shi, Aichi
467-8602, Japan, qDivision of
Morphological Science, Biomedical Research Center, and Departments of
aaLaboratory Medicine,
bbPediatrics,
ccOrthopedic Surgery,
rNeurology,
sEndocrinology and Diabetes,
tObstetrics and Gynecology, and
vOral and Maxillofacial Surgery, Saitama
Medical University, 38 Moro Hongo, Moroyama-machi, Iruma-gun, Saitama350-0495,
Japan, uDepartment of Neurology, Saitama
Neuropsychiatric Institute, 6-11-1 Honchohigashi, Chuo-ku, Saitama-shi,
Saitama 338-8577, Japan, zDepartment of
Molecular Pathology, University of Tokyo Graduate School of Medicine, 7-3-1
Hongo, Bunkyo-ku, Tokyo 113-0033, Japan,
wKitasato University School of
Pharmaceutical Science, 5-9-1 Shirokane, Minato-ku, Tokyo 108-0023, Japan,
xCardiovascular Research Center and
Division of Cardiology, Department of Medicine, Massachusetts General
Hospital, Harvard Medical School, Boston, Massachusetts 02129,
yCenter for Research in Fibrodysplasia
Ossificans Progressiva and Related Disorders, Department of Orthopedic
Surgery, University of Pennsylvania School of Medicine, Philadelphia,
Pennsylvania 19104-6081, ddDivision of
Molecular Signaling and Biochemistry, Department of Biosciences, Kyushu Dental
Collage, 2-6-1 Manazuru, Kokurakita-ku, Kitakyushu-shi, Fukuoka 803-8580,
Japan, eeDepartment of Orthopedic Surgery,
University of Ryukyus Faculty of Medicine, 207 Uehara, Nishihara-cho, 903-0215
Okinawa, Japan, and lThe Research
Committee on Fibrodysplasia Ossificans Progressiva, Ministry of Health, Labour
and Welfare, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan
b,v,
Hiroshi Tomoda
Hiroshi Tomoda
Divisions of aPathophysiology and
cTranslational Research, Research Center
for Genomic Medicine, Saitama Medical University, 1397-1 Yamane, Hidaka-shi,
Saitama 350-1241, Japan, bProject of
Clinical and Basic Research for FOP, Saitama Medical University, 1397-1
Yamane, Hidaka-shi, Saitama 350-1241, Japan,
dDepartment of Pediatric Emergency,
Kitakyusyu City Yahata Hospital, 4-18-1 Nishihonmachi, Yahatahigashi-ku,
Kitakyusyu-shi, Fukuoka 805-8534, Japan,
eDepartment of Orthopedic Surgery, Saga
Prefectural Hospital Koseikan, 1-12-9 Mizugae, Saga-shi, Saga 840-8571, Japan,
fNagayoshi Orthopedic Clinic, 1890
Otsukachohinokuchi, Miyazaki-shi, Miyazaki 880-0951, Japan,
gDepartment of Physical Medicine and
Rehabilitation, Tohoku University Graduate School of Medicine, 1-1
Seiryoumachi, Aoba-Ku, Sendai-shi, Miyagi 980-8474, Japan,
hDepartment of Orthopedic Surgery, Senjyudo
Hospital, 1-25 Senjyudonakamati, Gifu-shi, Gifu 500-8862, Japan,
iEndo Clinic, 4-8-11 Ogawanishimachi,
Kodaira-shi, Tokyo 187-0035, Japan,
jAwakura Clinic, 1-6-3 Satomachi,
Wajima-shi, Ishiwawa 928-0246, Japan,
kDepartment of Orthopedic Surgery, Kyushu
University Faculty of Medicine, 3-1-1 Maidashi, Higashi-ku, Fukuoka-shi,
Fukuoka 812-8582, Japan, mDepartment of
Pediatrics, Akashi Municipal Hospital, 1-33 Takashomachi, Akashi-shi, Hyogo
673-8501 Japan, nKawara Clinic, 2-12-3
Sumiyoshihonmachi, Higashinada-ku, Kobe-shi, Hyogo 658-0051, Japan,
oDepartment of Orthopaedic Surgery, Nagoya
University Graduate School and School of Medicine, 65 Tsurumaicho, Showa-ku,
Nagoya-shi, Aichi 466-8550, Japan,
pDepartment of Rehabilitation, Nagoya City
University Hospital, 1 Mizuhochoazakawasumi, Mizuho-ku, Nagoya-shi, Aichi
467-8602, Japan, qDivision of
Morphological Science, Biomedical Research Center, and Departments of
aaLaboratory Medicine,
bbPediatrics,
ccOrthopedic Surgery,
rNeurology,
sEndocrinology and Diabetes,
tObstetrics and Gynecology, and
vOral and Maxillofacial Surgery, Saitama
Medical University, 38 Moro Hongo, Moroyama-machi, Iruma-gun, Saitama350-0495,
Japan, uDepartment of Neurology, Saitama
Neuropsychiatric Institute, 6-11-1 Honchohigashi, Chuo-ku, Saitama-shi,
Saitama 338-8577, Japan, zDepartment of
Molecular Pathology, University of Tokyo Graduate School of Medicine, 7-3-1
Hongo, Bunkyo-ku, Tokyo 113-0033, Japan,
wKitasato University School of
Pharmaceutical Science, 5-9-1 Shirokane, Minato-ku, Tokyo 108-0023, Japan,
xCardiovascular Research Center and
Division of Cardiology, Department of Medicine, Massachusetts General
Hospital, Harvard Medical School, Boston, Massachusetts 02129,
yCenter for Research in Fibrodysplasia
Ossificans Progressiva and Related Disorders, Department of Orthopedic
Surgery, University of Pennsylvania School of Medicine, Philadelphia,
Pennsylvania 19104-6081, ddDivision of
Molecular Signaling and Biochemistry, Department of Biosciences, Kyushu Dental
Collage, 2-6-1 Manazuru, Kokurakita-ku, Kitakyushu-shi, Fukuoka 803-8580,
Japan, eeDepartment of Orthopedic Surgery,
University of Ryukyus Faculty of Medicine, 207 Uehara, Nishihara-cho, 903-0215
Okinawa, Japan, and lThe Research
Committee on Fibrodysplasia Ossificans Progressiva, Ministry of Health, Labour
and Welfare, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan
b,w,
Paul B Yu
Paul B Yu
Divisions of aPathophysiology and
cTranslational Research, Research Center
for Genomic Medicine, Saitama Medical University, 1397-1 Yamane, Hidaka-shi,
Saitama 350-1241, Japan, bProject of
Clinical and Basic Research for FOP, Saitama Medical University, 1397-1
Yamane, Hidaka-shi, Saitama 350-1241, Japan,
dDepartment of Pediatric Emergency,
Kitakyusyu City Yahata Hospital, 4-18-1 Nishihonmachi, Yahatahigashi-ku,
Kitakyusyu-shi, Fukuoka 805-8534, Japan,
eDepartment of Orthopedic Surgery, Saga
Prefectural Hospital Koseikan, 1-12-9 Mizugae, Saga-shi, Saga 840-8571, Japan,
fNagayoshi Orthopedic Clinic, 1890
Otsukachohinokuchi, Miyazaki-shi, Miyazaki 880-0951, Japan,
gDepartment of Physical Medicine and
Rehabilitation, Tohoku University Graduate School of Medicine, 1-1
Seiryoumachi, Aoba-Ku, Sendai-shi, Miyagi 980-8474, Japan,
hDepartment of Orthopedic Surgery, Senjyudo
Hospital, 1-25 Senjyudonakamati, Gifu-shi, Gifu 500-8862, Japan,
iEndo Clinic, 4-8-11 Ogawanishimachi,
Kodaira-shi, Tokyo 187-0035, Japan,
jAwakura Clinic, 1-6-3 Satomachi,
Wajima-shi, Ishiwawa 928-0246, Japan,
kDepartment of Orthopedic Surgery, Kyushu
University Faculty of Medicine, 3-1-1 Maidashi, Higashi-ku, Fukuoka-shi,
Fukuoka 812-8582, Japan, mDepartment of
Pediatrics, Akashi Municipal Hospital, 1-33 Takashomachi, Akashi-shi, Hyogo
673-8501 Japan, nKawara Clinic, 2-12-3
Sumiyoshihonmachi, Higashinada-ku, Kobe-shi, Hyogo 658-0051, Japan,
oDepartment of Orthopaedic Surgery, Nagoya
University Graduate School and School of Medicine, 65 Tsurumaicho, Showa-ku,
Nagoya-shi, Aichi 466-8550, Japan,
pDepartment of Rehabilitation, Nagoya City
University Hospital, 1 Mizuhochoazakawasumi, Mizuho-ku, Nagoya-shi, Aichi
467-8602, Japan, qDivision of
Morphological Science, Biomedical Research Center, and Departments of
aaLaboratory Medicine,
bbPediatrics,
ccOrthopedic Surgery,
rNeurology,
sEndocrinology and Diabetes,
tObstetrics and Gynecology, and
vOral and Maxillofacial Surgery, Saitama
Medical University, 38 Moro Hongo, Moroyama-machi, Iruma-gun, Saitama350-0495,
Japan, uDepartment of Neurology, Saitama
Neuropsychiatric Institute, 6-11-1 Honchohigashi, Chuo-ku, Saitama-shi,
Saitama 338-8577, Japan, zDepartment of
Molecular Pathology, University of Tokyo Graduate School of Medicine, 7-3-1
Hongo, Bunkyo-ku, Tokyo 113-0033, Japan,
wKitasato University School of
Pharmaceutical Science, 5-9-1 Shirokane, Minato-ku, Tokyo 108-0023, Japan,
xCardiovascular Research Center and
Division of Cardiology, Department of Medicine, Massachusetts General
Hospital, Harvard Medical School, Boston, Massachusetts 02129,
yCenter for Research in Fibrodysplasia
Ossificans Progressiva and Related Disorders, Department of Orthopedic
Surgery, University of Pennsylvania School of Medicine, Philadelphia,
Pennsylvania 19104-6081, ddDivision of
Molecular Signaling and Biochemistry, Department of Biosciences, Kyushu Dental
Collage, 2-6-1 Manazuru, Kokurakita-ku, Kitakyushu-shi, Fukuoka 803-8580,
Japan, eeDepartment of Orthopedic Surgery,
University of Ryukyus Faculty of Medicine, 207 Uehara, Nishihara-cho, 903-0215
Okinawa, Japan, and lThe Research
Committee on Fibrodysplasia Ossificans Progressiva, Ministry of Health, Labour
and Welfare, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan
x,
Eileen M Shore
Eileen M Shore
Divisions of aPathophysiology and
cTranslational Research, Research Center
for Genomic Medicine, Saitama Medical University, 1397-1 Yamane, Hidaka-shi,
Saitama 350-1241, Japan, bProject of
Clinical and Basic Research for FOP, Saitama Medical University, 1397-1
Yamane, Hidaka-shi, Saitama 350-1241, Japan,
dDepartment of Pediatric Emergency,
Kitakyusyu City Yahata Hospital, 4-18-1 Nishihonmachi, Yahatahigashi-ku,
Kitakyusyu-shi, Fukuoka 805-8534, Japan,
eDepartment of Orthopedic Surgery, Saga
Prefectural Hospital Koseikan, 1-12-9 Mizugae, Saga-shi, Saga 840-8571, Japan,
fNagayoshi Orthopedic Clinic, 1890
Otsukachohinokuchi, Miyazaki-shi, Miyazaki 880-0951, Japan,
gDepartment of Physical Medicine and
Rehabilitation, Tohoku University Graduate School of Medicine, 1-1
Seiryoumachi, Aoba-Ku, Sendai-shi, Miyagi 980-8474, Japan,
hDepartment of Orthopedic Surgery, Senjyudo
Hospital, 1-25 Senjyudonakamati, Gifu-shi, Gifu 500-8862, Japan,
iEndo Clinic, 4-8-11 Ogawanishimachi,
Kodaira-shi, Tokyo 187-0035, Japan,
jAwakura Clinic, 1-6-3 Satomachi,
Wajima-shi, Ishiwawa 928-0246, Japan,
kDepartment of Orthopedic Surgery, Kyushu
University Faculty of Medicine, 3-1-1 Maidashi, Higashi-ku, Fukuoka-shi,
Fukuoka 812-8582, Japan, mDepartment of
Pediatrics, Akashi Municipal Hospital, 1-33 Takashomachi, Akashi-shi, Hyogo
673-8501 Japan, nKawara Clinic, 2-12-3
Sumiyoshihonmachi, Higashinada-ku, Kobe-shi, Hyogo 658-0051, Japan,
oDepartment of Orthopaedic Surgery, Nagoya
University Graduate School and School of Medicine, 65 Tsurumaicho, Showa-ku,
Nagoya-shi, Aichi 466-8550, Japan,
pDepartment of Rehabilitation, Nagoya City
University Hospital, 1 Mizuhochoazakawasumi, Mizuho-ku, Nagoya-shi, Aichi
467-8602, Japan, qDivision of
Morphological Science, Biomedical Research Center, and Departments of
aaLaboratory Medicine,
bbPediatrics,
ccOrthopedic Surgery,
rNeurology,
sEndocrinology and Diabetes,
tObstetrics and Gynecology, and
vOral and Maxillofacial Surgery, Saitama
Medical University, 38 Moro Hongo, Moroyama-machi, Iruma-gun, Saitama350-0495,
Japan, uDepartment of Neurology, Saitama
Neuropsychiatric Institute, 6-11-1 Honchohigashi, Chuo-ku, Saitama-shi,
Saitama 338-8577, Japan, zDepartment of
Molecular Pathology, University of Tokyo Graduate School of Medicine, 7-3-1
Hongo, Bunkyo-ku, Tokyo 113-0033, Japan,
wKitasato University School of
Pharmaceutical Science, 5-9-1 Shirokane, Minato-ku, Tokyo 108-0023, Japan,
xCardiovascular Research Center and
Division of Cardiology, Department of Medicine, Massachusetts General
Hospital, Harvard Medical School, Boston, Massachusetts 02129,
yCenter for Research in Fibrodysplasia
Ossificans Progressiva and Related Disorders, Department of Orthopedic
Surgery, University of Pennsylvania School of Medicine, Philadelphia,
Pennsylvania 19104-6081, ddDivision of
Molecular Signaling and Biochemistry, Department of Biosciences, Kyushu Dental
Collage, 2-6-1 Manazuru, Kokurakita-ku, Kitakyushu-shi, Fukuoka 803-8580,
Japan, eeDepartment of Orthopedic Surgery,
University of Ryukyus Faculty of Medicine, 207 Uehara, Nishihara-cho, 903-0215
Okinawa, Japan, and lThe Research
Committee on Fibrodysplasia Ossificans Progressiva, Ministry of Health, Labour
and Welfare, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan
y,
Frederick S Kaplan
Frederick S Kaplan
Divisions of aPathophysiology and
cTranslational Research, Research Center
for Genomic Medicine, Saitama Medical University, 1397-1 Yamane, Hidaka-shi,
Saitama 350-1241, Japan, bProject of
Clinical and Basic Research for FOP, Saitama Medical University, 1397-1
Yamane, Hidaka-shi, Saitama 350-1241, Japan,
dDepartment of Pediatric Emergency,
Kitakyusyu City Yahata Hospital, 4-18-1 Nishihonmachi, Yahatahigashi-ku,
Kitakyusyu-shi, Fukuoka 805-8534, Japan,
eDepartment of Orthopedic Surgery, Saga
Prefectural Hospital Koseikan, 1-12-9 Mizugae, Saga-shi, Saga 840-8571, Japan,
fNagayoshi Orthopedic Clinic, 1890
Otsukachohinokuchi, Miyazaki-shi, Miyazaki 880-0951, Japan,
gDepartment of Physical Medicine and
Rehabilitation, Tohoku University Graduate School of Medicine, 1-1
Seiryoumachi, Aoba-Ku, Sendai-shi, Miyagi 980-8474, Japan,
hDepartment of Orthopedic Surgery, Senjyudo
Hospital, 1-25 Senjyudonakamati, Gifu-shi, Gifu 500-8862, Japan,
iEndo Clinic, 4-8-11 Ogawanishimachi,
Kodaira-shi, Tokyo 187-0035, Japan,
jAwakura Clinic, 1-6-3 Satomachi,
Wajima-shi, Ishiwawa 928-0246, Japan,
kDepartment of Orthopedic Surgery, Kyushu
University Faculty of Medicine, 3-1-1 Maidashi, Higashi-ku, Fukuoka-shi,
Fukuoka 812-8582, Japan, mDepartment of
Pediatrics, Akashi Municipal Hospital, 1-33 Takashomachi, Akashi-shi, Hyogo
673-8501 Japan, nKawara Clinic, 2-12-3
Sumiyoshihonmachi, Higashinada-ku, Kobe-shi, Hyogo 658-0051, Japan,
oDepartment of Orthopaedic Surgery, Nagoya
University Graduate School and School of Medicine, 65 Tsurumaicho, Showa-ku,
Nagoya-shi, Aichi 466-8550, Japan,
pDepartment of Rehabilitation, Nagoya City
University Hospital, 1 Mizuhochoazakawasumi, Mizuho-ku, Nagoya-shi, Aichi
467-8602, Japan, qDivision of
Morphological Science, Biomedical Research Center, and Departments of
aaLaboratory Medicine,
bbPediatrics,
ccOrthopedic Surgery,
rNeurology,
sEndocrinology and Diabetes,
tObstetrics and Gynecology, and
vOral and Maxillofacial Surgery, Saitama
Medical University, 38 Moro Hongo, Moroyama-machi, Iruma-gun, Saitama350-0495,
Japan, uDepartment of Neurology, Saitama
Neuropsychiatric Institute, 6-11-1 Honchohigashi, Chuo-ku, Saitama-shi,
Saitama 338-8577, Japan, zDepartment of
Molecular Pathology, University of Tokyo Graduate School of Medicine, 7-3-1
Hongo, Bunkyo-ku, Tokyo 113-0033, Japan,
wKitasato University School of
Pharmaceutical Science, 5-9-1 Shirokane, Minato-ku, Tokyo 108-0023, Japan,
xCardiovascular Research Center and
Division of Cardiology, Department of Medicine, Massachusetts General
Hospital, Harvard Medical School, Boston, Massachusetts 02129,
yCenter for Research in Fibrodysplasia
Ossificans Progressiva and Related Disorders, Department of Orthopedic
Surgery, University of Pennsylvania School of Medicine, Philadelphia,
Pennsylvania 19104-6081, ddDivision of
Molecular Signaling and Biochemistry, Department of Biosciences, Kyushu Dental
Collage, 2-6-1 Manazuru, Kokurakita-ku, Kitakyushu-shi, Fukuoka 803-8580,
Japan, eeDepartment of Orthopedic Surgery,
University of Ryukyus Faculty of Medicine, 207 Uehara, Nishihara-cho, 903-0215
Okinawa, Japan, and lThe Research
Committee on Fibrodysplasia Ossificans Progressiva, Ministry of Health, Labour
and Welfare, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan
y,
Kohei Miyazono
Kohei Miyazono
Divisions of aPathophysiology and
cTranslational Research, Research Center
for Genomic Medicine, Saitama Medical University, 1397-1 Yamane, Hidaka-shi,
Saitama 350-1241, Japan, bProject of
Clinical and Basic Research for FOP, Saitama Medical University, 1397-1
Yamane, Hidaka-shi, Saitama 350-1241, Japan,
dDepartment of Pediatric Emergency,
Kitakyusyu City Yahata Hospital, 4-18-1 Nishihonmachi, Yahatahigashi-ku,
Kitakyusyu-shi, Fukuoka 805-8534, Japan,
eDepartment of Orthopedic Surgery, Saga
Prefectural Hospital Koseikan, 1-12-9 Mizugae, Saga-shi, Saga 840-8571, Japan,
fNagayoshi Orthopedic Clinic, 1890
Otsukachohinokuchi, Miyazaki-shi, Miyazaki 880-0951, Japan,
gDepartment of Physical Medicine and
Rehabilitation, Tohoku University Graduate School of Medicine, 1-1
Seiryoumachi, Aoba-Ku, Sendai-shi, Miyagi 980-8474, Japan,
hDepartment of Orthopedic Surgery, Senjyudo
Hospital, 1-25 Senjyudonakamati, Gifu-shi, Gifu 500-8862, Japan,
iEndo Clinic, 4-8-11 Ogawanishimachi,
Kodaira-shi, Tokyo 187-0035, Japan,
jAwakura Clinic, 1-6-3 Satomachi,
Wajima-shi, Ishiwawa 928-0246, Japan,
kDepartment of Orthopedic Surgery, Kyushu
University Faculty of Medicine, 3-1-1 Maidashi, Higashi-ku, Fukuoka-shi,
Fukuoka 812-8582, Japan, mDepartment of
Pediatrics, Akashi Municipal Hospital, 1-33 Takashomachi, Akashi-shi, Hyogo
673-8501 Japan, nKawara Clinic, 2-12-3
Sumiyoshihonmachi, Higashinada-ku, Kobe-shi, Hyogo 658-0051, Japan,
oDepartment of Orthopaedic Surgery, Nagoya
University Graduate School and School of Medicine, 65 Tsurumaicho, Showa-ku,
Nagoya-shi, Aichi 466-8550, Japan,
pDepartment of Rehabilitation, Nagoya City
University Hospital, 1 Mizuhochoazakawasumi, Mizuho-ku, Nagoya-shi, Aichi
467-8602, Japan, qDivision of
Morphological Science, Biomedical Research Center, and Departments of
aaLaboratory Medicine,
bbPediatrics,
ccOrthopedic Surgery,
rNeurology,
sEndocrinology and Diabetes,
tObstetrics and Gynecology, and
vOral and Maxillofacial Surgery, Saitama
Medical University, 38 Moro Hongo, Moroyama-machi, Iruma-gun, Saitama350-0495,
Japan, uDepartment of Neurology, Saitama
Neuropsychiatric Institute, 6-11-1 Honchohigashi, Chuo-ku, Saitama-shi,
Saitama 338-8577, Japan, zDepartment of
Molecular Pathology, University of Tokyo Graduate School of Medicine, 7-3-1
Hongo, Bunkyo-ku, Tokyo 113-0033, Japan,
wKitasato University School of
Pharmaceutical Science, 5-9-1 Shirokane, Minato-ku, Tokyo 108-0023, Japan,
xCardiovascular Research Center and
Division of Cardiology, Department of Medicine, Massachusetts General
Hospital, Harvard Medical School, Boston, Massachusetts 02129,
yCenter for Research in Fibrodysplasia
Ossificans Progressiva and Related Disorders, Department of Orthopedic
Surgery, University of Pennsylvania School of Medicine, Philadelphia,
Pennsylvania 19104-6081, ddDivision of
Molecular Signaling and Biochemistry, Department of Biosciences, Kyushu Dental
Collage, 2-6-1 Manazuru, Kokurakita-ku, Kitakyushu-shi, Fukuoka 803-8580,
Japan, eeDepartment of Orthopedic Surgery,
University of Ryukyus Faculty of Medicine, 207 Uehara, Nishihara-cho, 903-0215
Okinawa, Japan, and lThe Research
Committee on Fibrodysplasia Ossificans Progressiva, Ministry of Health, Labour
and Welfare, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan
b,z,
Masaru Matsuoka
Masaru Matsuoka
Divisions of aPathophysiology and
cTranslational Research, Research Center
for Genomic Medicine, Saitama Medical University, 1397-1 Yamane, Hidaka-shi,
Saitama 350-1241, Japan, bProject of
Clinical and Basic Research for FOP, Saitama Medical University, 1397-1
Yamane, Hidaka-shi, Saitama 350-1241, Japan,
dDepartment of Pediatric Emergency,
Kitakyusyu City Yahata Hospital, 4-18-1 Nishihonmachi, Yahatahigashi-ku,
Kitakyusyu-shi, Fukuoka 805-8534, Japan,
eDepartment of Orthopedic Surgery, Saga
Prefectural Hospital Koseikan, 1-12-9 Mizugae, Saga-shi, Saga 840-8571, Japan,
fNagayoshi Orthopedic Clinic, 1890
Otsukachohinokuchi, Miyazaki-shi, Miyazaki 880-0951, Japan,
gDepartment of Physical Medicine and
Rehabilitation, Tohoku University Graduate School of Medicine, 1-1
Seiryoumachi, Aoba-Ku, Sendai-shi, Miyagi 980-8474, Japan,
hDepartment of Orthopedic Surgery, Senjyudo
Hospital, 1-25 Senjyudonakamati, Gifu-shi, Gifu 500-8862, Japan,
iEndo Clinic, 4-8-11 Ogawanishimachi,
Kodaira-shi, Tokyo 187-0035, Japan,
jAwakura Clinic, 1-6-3 Satomachi,
Wajima-shi, Ishiwawa 928-0246, Japan,
kDepartment of Orthopedic Surgery, Kyushu
University Faculty of Medicine, 3-1-1 Maidashi, Higashi-ku, Fukuoka-shi,
Fukuoka 812-8582, Japan, mDepartment of
Pediatrics, Akashi Municipal Hospital, 1-33 Takashomachi, Akashi-shi, Hyogo
673-8501 Japan, nKawara Clinic, 2-12-3
Sumiyoshihonmachi, Higashinada-ku, Kobe-shi, Hyogo 658-0051, Japan,
oDepartment of Orthopaedic Surgery, Nagoya
University Graduate School and School of Medicine, 65 Tsurumaicho, Showa-ku,
Nagoya-shi, Aichi 466-8550, Japan,
pDepartment of Rehabilitation, Nagoya City
University Hospital, 1 Mizuhochoazakawasumi, Mizuho-ku, Nagoya-shi, Aichi
467-8602, Japan, qDivision of
Morphological Science, Biomedical Research Center, and Departments of
aaLaboratory Medicine,
bbPediatrics,
ccOrthopedic Surgery,
rNeurology,
sEndocrinology and Diabetes,
tObstetrics and Gynecology, and
vOral and Maxillofacial Surgery, Saitama
Medical University, 38 Moro Hongo, Moroyama-machi, Iruma-gun, Saitama350-0495,
Japan, uDepartment of Neurology, Saitama
Neuropsychiatric Institute, 6-11-1 Honchohigashi, Chuo-ku, Saitama-shi,
Saitama 338-8577, Japan, zDepartment of
Molecular Pathology, University of Tokyo Graduate School of Medicine, 7-3-1
Hongo, Bunkyo-ku, Tokyo 113-0033, Japan,
wKitasato University School of
Pharmaceutical Science, 5-9-1 Shirokane, Minato-ku, Tokyo 108-0023, Japan,
xCardiovascular Research Center and
Division of Cardiology, Department of Medicine, Massachusetts General
Hospital, Harvard Medical School, Boston, Massachusetts 02129,
yCenter for Research in Fibrodysplasia
Ossificans Progressiva and Related Disorders, Department of Orthopedic
Surgery, University of Pennsylvania School of Medicine, Philadelphia,
Pennsylvania 19104-6081, ddDivision of
Molecular Signaling and Biochemistry, Department of Biosciences, Kyushu Dental
Collage, 2-6-1 Manazuru, Kokurakita-ku, Kitakyushu-shi, Fukuoka 803-8580,
Japan, eeDepartment of Orthopedic Surgery,
University of Ryukyus Faculty of Medicine, 207 Uehara, Nishihara-cho, 903-0215
Okinawa, Japan, and lThe Research
Committee on Fibrodysplasia Ossificans Progressiva, Ministry of Health, Labour
and Welfare, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan
aa,
Kenji Ikebuchi
Kenji Ikebuchi
Divisions of aPathophysiology and
cTranslational Research, Research Center
for Genomic Medicine, Saitama Medical University, 1397-1 Yamane, Hidaka-shi,
Saitama 350-1241, Japan, bProject of
Clinical and Basic Research for FOP, Saitama Medical University, 1397-1
Yamane, Hidaka-shi, Saitama 350-1241, Japan,
dDepartment of Pediatric Emergency,
Kitakyusyu City Yahata Hospital, 4-18-1 Nishihonmachi, Yahatahigashi-ku,
Kitakyusyu-shi, Fukuoka 805-8534, Japan,
eDepartment of Orthopedic Surgery, Saga
Prefectural Hospital Koseikan, 1-12-9 Mizugae, Saga-shi, Saga 840-8571, Japan,
fNagayoshi Orthopedic Clinic, 1890
Otsukachohinokuchi, Miyazaki-shi, Miyazaki 880-0951, Japan,
gDepartment of Physical Medicine and
Rehabilitation, Tohoku University Graduate School of Medicine, 1-1
Seiryoumachi, Aoba-Ku, Sendai-shi, Miyagi 980-8474, Japan,
hDepartment of Orthopedic Surgery, Senjyudo
Hospital, 1-25 Senjyudonakamati, Gifu-shi, Gifu 500-8862, Japan,
iEndo Clinic, 4-8-11 Ogawanishimachi,
Kodaira-shi, Tokyo 187-0035, Japan,
jAwakura Clinic, 1-6-3 Satomachi,
Wajima-shi, Ishiwawa 928-0246, Japan,
kDepartment of Orthopedic Surgery, Kyushu
University Faculty of Medicine, 3-1-1 Maidashi, Higashi-ku, Fukuoka-shi,
Fukuoka 812-8582, Japan, mDepartment of
Pediatrics, Akashi Municipal Hospital, 1-33 Takashomachi, Akashi-shi, Hyogo
673-8501 Japan, nKawara Clinic, 2-12-3
Sumiyoshihonmachi, Higashinada-ku, Kobe-shi, Hyogo 658-0051, Japan,
oDepartment of Orthopaedic Surgery, Nagoya
University Graduate School and School of Medicine, 65 Tsurumaicho, Showa-ku,
Nagoya-shi, Aichi 466-8550, Japan,
pDepartment of Rehabilitation, Nagoya City
University Hospital, 1 Mizuhochoazakawasumi, Mizuho-ku, Nagoya-shi, Aichi
467-8602, Japan, qDivision of
Morphological Science, Biomedical Research Center, and Departments of
aaLaboratory Medicine,
bbPediatrics,
ccOrthopedic Surgery,
rNeurology,
sEndocrinology and Diabetes,
tObstetrics and Gynecology, and
vOral and Maxillofacial Surgery, Saitama
Medical University, 38 Moro Hongo, Moroyama-machi, Iruma-gun, Saitama350-0495,
Japan, uDepartment of Neurology, Saitama
Neuropsychiatric Institute, 6-11-1 Honchohigashi, Chuo-ku, Saitama-shi,
Saitama 338-8577, Japan, zDepartment of
Molecular Pathology, University of Tokyo Graduate School of Medicine, 7-3-1
Hongo, Bunkyo-ku, Tokyo 113-0033, Japan,
wKitasato University School of
Pharmaceutical Science, 5-9-1 Shirokane, Minato-ku, Tokyo 108-0023, Japan,
xCardiovascular Research Center and
Division of Cardiology, Department of Medicine, Massachusetts General
Hospital, Harvard Medical School, Boston, Massachusetts 02129,
yCenter for Research in Fibrodysplasia
Ossificans Progressiva and Related Disorders, Department of Orthopedic
Surgery, University of Pennsylvania School of Medicine, Philadelphia,
Pennsylvania 19104-6081, ddDivision of
Molecular Signaling and Biochemistry, Department of Biosciences, Kyushu Dental
Collage, 2-6-1 Manazuru, Kokurakita-ku, Kitakyushu-shi, Fukuoka 803-8580,
Japan, eeDepartment of Orthopedic Surgery,
University of Ryukyus Faculty of Medicine, 207 Uehara, Nishihara-cho, 903-0215
Okinawa, Japan, and lThe Research
Committee on Fibrodysplasia Ossificans Progressiva, Ministry of Health, Labour
and Welfare, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan
b,aa,
Akira Ohtake
Akira Ohtake
Divisions of aPathophysiology and
cTranslational Research, Research Center
for Genomic Medicine, Saitama Medical University, 1397-1 Yamane, Hidaka-shi,
Saitama 350-1241, Japan, bProject of
Clinical and Basic Research for FOP, Saitama Medical University, 1397-1
Yamane, Hidaka-shi, Saitama 350-1241, Japan,
dDepartment of Pediatric Emergency,
Kitakyusyu City Yahata Hospital, 4-18-1 Nishihonmachi, Yahatahigashi-ku,
Kitakyusyu-shi, Fukuoka 805-8534, Japan,
eDepartment of Orthopedic Surgery, Saga
Prefectural Hospital Koseikan, 1-12-9 Mizugae, Saga-shi, Saga 840-8571, Japan,
fNagayoshi Orthopedic Clinic, 1890
Otsukachohinokuchi, Miyazaki-shi, Miyazaki 880-0951, Japan,
gDepartment of Physical Medicine and
Rehabilitation, Tohoku University Graduate School of Medicine, 1-1
Seiryoumachi, Aoba-Ku, Sendai-shi, Miyagi 980-8474, Japan,
hDepartment of Orthopedic Surgery, Senjyudo
Hospital, 1-25 Senjyudonakamati, Gifu-shi, Gifu 500-8862, Japan,
iEndo Clinic, 4-8-11 Ogawanishimachi,
Kodaira-shi, Tokyo 187-0035, Japan,
jAwakura Clinic, 1-6-3 Satomachi,
Wajima-shi, Ishiwawa 928-0246, Japan,
kDepartment of Orthopedic Surgery, Kyushu
University Faculty of Medicine, 3-1-1 Maidashi, Higashi-ku, Fukuoka-shi,
Fukuoka 812-8582, Japan, mDepartment of
Pediatrics, Akashi Municipal Hospital, 1-33 Takashomachi, Akashi-shi, Hyogo
673-8501 Japan, nKawara Clinic, 2-12-3
Sumiyoshihonmachi, Higashinada-ku, Kobe-shi, Hyogo 658-0051, Japan,
oDepartment of Orthopaedic Surgery, Nagoya
University Graduate School and School of Medicine, 65 Tsurumaicho, Showa-ku,
Nagoya-shi, Aichi 466-8550, Japan,
pDepartment of Rehabilitation, Nagoya City
University Hospital, 1 Mizuhochoazakawasumi, Mizuho-ku, Nagoya-shi, Aichi
467-8602, Japan, qDivision of
Morphological Science, Biomedical Research Center, and Departments of
aaLaboratory Medicine,
bbPediatrics,
ccOrthopedic Surgery,
rNeurology,
sEndocrinology and Diabetes,
tObstetrics and Gynecology, and
vOral and Maxillofacial Surgery, Saitama
Medical University, 38 Moro Hongo, Moroyama-machi, Iruma-gun, Saitama350-0495,
Japan, uDepartment of Neurology, Saitama
Neuropsychiatric Institute, 6-11-1 Honchohigashi, Chuo-ku, Saitama-shi,
Saitama 338-8577, Japan, zDepartment of
Molecular Pathology, University of Tokyo Graduate School of Medicine, 7-3-1
Hongo, Bunkyo-ku, Tokyo 113-0033, Japan,
wKitasato University School of
Pharmaceutical Science, 5-9-1 Shirokane, Minato-ku, Tokyo 108-0023, Japan,
xCardiovascular Research Center and
Division of Cardiology, Department of Medicine, Massachusetts General
Hospital, Harvard Medical School, Boston, Massachusetts 02129,
yCenter for Research in Fibrodysplasia
Ossificans Progressiva and Related Disorders, Department of Orthopedic
Surgery, University of Pennsylvania School of Medicine, Philadelphia,
Pennsylvania 19104-6081, ddDivision of
Molecular Signaling and Biochemistry, Department of Biosciences, Kyushu Dental
Collage, 2-6-1 Manazuru, Kokurakita-ku, Kitakyushu-shi, Fukuoka 803-8580,
Japan, eeDepartment of Orthopedic Surgery,
University of Ryukyus Faculty of Medicine, 207 Uehara, Nishihara-cho, 903-0215
Okinawa, Japan, and lThe Research
Committee on Fibrodysplasia Ossificans Progressiva, Ministry of Health, Labour
and Welfare, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan
b,bb,
Hiromi Oda
Hiromi Oda
Divisions of aPathophysiology and
cTranslational Research, Research Center
for Genomic Medicine, Saitama Medical University, 1397-1 Yamane, Hidaka-shi,
Saitama 350-1241, Japan, bProject of
Clinical and Basic Research for FOP, Saitama Medical University, 1397-1
Yamane, Hidaka-shi, Saitama 350-1241, Japan,
dDepartment of Pediatric Emergency,
Kitakyusyu City Yahata Hospital, 4-18-1 Nishihonmachi, Yahatahigashi-ku,
Kitakyusyu-shi, Fukuoka 805-8534, Japan,
eDepartment of Orthopedic Surgery, Saga
Prefectural Hospital Koseikan, 1-12-9 Mizugae, Saga-shi, Saga 840-8571, Japan,
fNagayoshi Orthopedic Clinic, 1890
Otsukachohinokuchi, Miyazaki-shi, Miyazaki 880-0951, Japan,
gDepartment of Physical Medicine and
Rehabilitation, Tohoku University Graduate School of Medicine, 1-1
Seiryoumachi, Aoba-Ku, Sendai-shi, Miyagi 980-8474, Japan,
hDepartment of Orthopedic Surgery, Senjyudo
Hospital, 1-25 Senjyudonakamati, Gifu-shi, Gifu 500-8862, Japan,
iEndo Clinic, 4-8-11 Ogawanishimachi,
Kodaira-shi, Tokyo 187-0035, Japan,
jAwakura Clinic, 1-6-3 Satomachi,
Wajima-shi, Ishiwawa 928-0246, Japan,
kDepartment of Orthopedic Surgery, Kyushu
University Faculty of Medicine, 3-1-1 Maidashi, Higashi-ku, Fukuoka-shi,
Fukuoka 812-8582, Japan, mDepartment of
Pediatrics, Akashi Municipal Hospital, 1-33 Takashomachi, Akashi-shi, Hyogo
673-8501 Japan, nKawara Clinic, 2-12-3
Sumiyoshihonmachi, Higashinada-ku, Kobe-shi, Hyogo 658-0051, Japan,
oDepartment of Orthopaedic Surgery, Nagoya
University Graduate School and School of Medicine, 65 Tsurumaicho, Showa-ku,
Nagoya-shi, Aichi 466-8550, Japan,
pDepartment of Rehabilitation, Nagoya City
University Hospital, 1 Mizuhochoazakawasumi, Mizuho-ku, Nagoya-shi, Aichi
467-8602, Japan, qDivision of
Morphological Science, Biomedical Research Center, and Departments of
aaLaboratory Medicine,
bbPediatrics,
ccOrthopedic Surgery,
rNeurology,
sEndocrinology and Diabetes,
tObstetrics and Gynecology, and
vOral and Maxillofacial Surgery, Saitama
Medical University, 38 Moro Hongo, Moroyama-machi, Iruma-gun, Saitama350-0495,
Japan, uDepartment of Neurology, Saitama
Neuropsychiatric Institute, 6-11-1 Honchohigashi, Chuo-ku, Saitama-shi,
Saitama 338-8577, Japan, zDepartment of
Molecular Pathology, University of Tokyo Graduate School of Medicine, 7-3-1
Hongo, Bunkyo-ku, Tokyo 113-0033, Japan,
wKitasato University School of
Pharmaceutical Science, 5-9-1 Shirokane, Minato-ku, Tokyo 108-0023, Japan,
xCardiovascular Research Center and
Division of Cardiology, Department of Medicine, Massachusetts General
Hospital, Harvard Medical School, Boston, Massachusetts 02129,
yCenter for Research in Fibrodysplasia
Ossificans Progressiva and Related Disorders, Department of Orthopedic
Surgery, University of Pennsylvania School of Medicine, Philadelphia,
Pennsylvania 19104-6081, ddDivision of
Molecular Signaling and Biochemistry, Department of Biosciences, Kyushu Dental
Collage, 2-6-1 Manazuru, Kokurakita-ku, Kitakyushu-shi, Fukuoka 803-8580,
Japan, eeDepartment of Orthopedic Surgery,
University of Ryukyus Faculty of Medicine, 207 Uehara, Nishihara-cho, 903-0215
Okinawa, Japan, and lThe Research
Committee on Fibrodysplasia Ossificans Progressiva, Ministry of Health, Labour
and Welfare, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan
b,cc,
Eijiro Jimi
Eijiro Jimi
Divisions of aPathophysiology and
cTranslational Research, Research Center
for Genomic Medicine, Saitama Medical University, 1397-1 Yamane, Hidaka-shi,
Saitama 350-1241, Japan, bProject of
Clinical and Basic Research for FOP, Saitama Medical University, 1397-1
Yamane, Hidaka-shi, Saitama 350-1241, Japan,
dDepartment of Pediatric Emergency,
Kitakyusyu City Yahata Hospital, 4-18-1 Nishihonmachi, Yahatahigashi-ku,
Kitakyusyu-shi, Fukuoka 805-8534, Japan,
eDepartment of Orthopedic Surgery, Saga
Prefectural Hospital Koseikan, 1-12-9 Mizugae, Saga-shi, Saga 840-8571, Japan,
fNagayoshi Orthopedic Clinic, 1890
Otsukachohinokuchi, Miyazaki-shi, Miyazaki 880-0951, Japan,
gDepartment of Physical Medicine and
Rehabilitation, Tohoku University Graduate School of Medicine, 1-1
Seiryoumachi, Aoba-Ku, Sendai-shi, Miyagi 980-8474, Japan,
hDepartment of Orthopedic Surgery, Senjyudo
Hospital, 1-25 Senjyudonakamati, Gifu-shi, Gifu 500-8862, Japan,
iEndo Clinic, 4-8-11 Ogawanishimachi,
Kodaira-shi, Tokyo 187-0035, Japan,
jAwakura Clinic, 1-6-3 Satomachi,
Wajima-shi, Ishiwawa 928-0246, Japan,
kDepartment of Orthopedic Surgery, Kyushu
University Faculty of Medicine, 3-1-1 Maidashi, Higashi-ku, Fukuoka-shi,
Fukuoka 812-8582, Japan, mDepartment of
Pediatrics, Akashi Municipal Hospital, 1-33 Takashomachi, Akashi-shi, Hyogo
673-8501 Japan, nKawara Clinic, 2-12-3
Sumiyoshihonmachi, Higashinada-ku, Kobe-shi, Hyogo 658-0051, Japan,
oDepartment of Orthopaedic Surgery, Nagoya
University Graduate School and School of Medicine, 65 Tsurumaicho, Showa-ku,
Nagoya-shi, Aichi 466-8550, Japan,
pDepartment of Rehabilitation, Nagoya City
University Hospital, 1 Mizuhochoazakawasumi, Mizuho-ku, Nagoya-shi, Aichi
467-8602, Japan, qDivision of
Morphological Science, Biomedical Research Center, and Departments of
aaLaboratory Medicine,
bbPediatrics,
ccOrthopedic Surgery,
rNeurology,
sEndocrinology and Diabetes,
tObstetrics and Gynecology, and
vOral and Maxillofacial Surgery, Saitama
Medical University, 38 Moro Hongo, Moroyama-machi, Iruma-gun, Saitama350-0495,
Japan, uDepartment of Neurology, Saitama
Neuropsychiatric Institute, 6-11-1 Honchohigashi, Chuo-ku, Saitama-shi,
Saitama 338-8577, Japan, zDepartment of
Molecular Pathology, University of Tokyo Graduate School of Medicine, 7-3-1
Hongo, Bunkyo-ku, Tokyo 113-0033, Japan,
wKitasato University School of
Pharmaceutical Science, 5-9-1 Shirokane, Minato-ku, Tokyo 108-0023, Japan,
xCardiovascular Research Center and
Division of Cardiology, Department of Medicine, Massachusetts General
Hospital, Harvard Medical School, Boston, Massachusetts 02129,
yCenter for Research in Fibrodysplasia
Ossificans Progressiva and Related Disorders, Department of Orthopedic
Surgery, University of Pennsylvania School of Medicine, Philadelphia,
Pennsylvania 19104-6081, ddDivision of
Molecular Signaling and Biochemistry, Department of Biosciences, Kyushu Dental
Collage, 2-6-1 Manazuru, Kokurakita-ku, Kitakyushu-shi, Fukuoka 803-8580,
Japan, eeDepartment of Orthopedic Surgery,
University of Ryukyus Faculty of Medicine, 207 Uehara, Nishihara-cho, 903-0215
Okinawa, Japan, and lThe Research
Committee on Fibrodysplasia Ossificans Progressiva, Ministry of Health, Labour
and Welfare, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan
b,dd,
Ichiro Owan
Ichiro Owan
Divisions of aPathophysiology and
cTranslational Research, Research Center
for Genomic Medicine, Saitama Medical University, 1397-1 Yamane, Hidaka-shi,
Saitama 350-1241, Japan, bProject of
Clinical and Basic Research for FOP, Saitama Medical University, 1397-1
Yamane, Hidaka-shi, Saitama 350-1241, Japan,
dDepartment of Pediatric Emergency,
Kitakyusyu City Yahata Hospital, 4-18-1 Nishihonmachi, Yahatahigashi-ku,
Kitakyusyu-shi, Fukuoka 805-8534, Japan,
eDepartment of Orthopedic Surgery, Saga
Prefectural Hospital Koseikan, 1-12-9 Mizugae, Saga-shi, Saga 840-8571, Japan,
fNagayoshi Orthopedic Clinic, 1890
Otsukachohinokuchi, Miyazaki-shi, Miyazaki 880-0951, Japan,
gDepartment of Physical Medicine and
Rehabilitation, Tohoku University Graduate School of Medicine, 1-1
Seiryoumachi, Aoba-Ku, Sendai-shi, Miyagi 980-8474, Japan,
hDepartment of Orthopedic Surgery, Senjyudo
Hospital, 1-25 Senjyudonakamati, Gifu-shi, Gifu 500-8862, Japan,
iEndo Clinic, 4-8-11 Ogawanishimachi,
Kodaira-shi, Tokyo 187-0035, Japan,
jAwakura Clinic, 1-6-3 Satomachi,
Wajima-shi, Ishiwawa 928-0246, Japan,
kDepartment of Orthopedic Surgery, Kyushu
University Faculty of Medicine, 3-1-1 Maidashi, Higashi-ku, Fukuoka-shi,
Fukuoka 812-8582, Japan, mDepartment of
Pediatrics, Akashi Municipal Hospital, 1-33 Takashomachi, Akashi-shi, Hyogo
673-8501 Japan, nKawara Clinic, 2-12-3
Sumiyoshihonmachi, Higashinada-ku, Kobe-shi, Hyogo 658-0051, Japan,
oDepartment of Orthopaedic Surgery, Nagoya
University Graduate School and School of Medicine, 65 Tsurumaicho, Showa-ku,
Nagoya-shi, Aichi 466-8550, Japan,
pDepartment of Rehabilitation, Nagoya City
University Hospital, 1 Mizuhochoazakawasumi, Mizuho-ku, Nagoya-shi, Aichi
467-8602, Japan, qDivision of
Morphological Science, Biomedical Research Center, and Departments of
aaLaboratory Medicine,
bbPediatrics,
ccOrthopedic Surgery,
rNeurology,
sEndocrinology and Diabetes,
tObstetrics and Gynecology, and
vOral and Maxillofacial Surgery, Saitama
Medical University, 38 Moro Hongo, Moroyama-machi, Iruma-gun, Saitama350-0495,
Japan, uDepartment of Neurology, Saitama
Neuropsychiatric Institute, 6-11-1 Honchohigashi, Chuo-ku, Saitama-shi,
Saitama 338-8577, Japan, zDepartment of
Molecular Pathology, University of Tokyo Graduate School of Medicine, 7-3-1
Hongo, Bunkyo-ku, Tokyo 113-0033, Japan,
wKitasato University School of
Pharmaceutical Science, 5-9-1 Shirokane, Minato-ku, Tokyo 108-0023, Japan,
xCardiovascular Research Center and
Division of Cardiology, Department of Medicine, Massachusetts General
Hospital, Harvard Medical School, Boston, Massachusetts 02129,
yCenter for Research in Fibrodysplasia
Ossificans Progressiva and Related Disorders, Department of Orthopedic
Surgery, University of Pennsylvania School of Medicine, Philadelphia,
Pennsylvania 19104-6081, ddDivision of
Molecular Signaling and Biochemistry, Department of Biosciences, Kyushu Dental
Collage, 2-6-1 Manazuru, Kokurakita-ku, Kitakyushu-shi, Fukuoka 803-8580,
Japan, eeDepartment of Orthopedic Surgery,
University of Ryukyus Faculty of Medicine, 207 Uehara, Nishihara-cho, 903-0215
Okinawa, Japan, and lThe Research
Committee on Fibrodysplasia Ossificans Progressiva, Ministry of Health, Labour
and Welfare, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan
ee,
Yasushi Okazaki
Yasushi Okazaki
Divisions of aPathophysiology and
cTranslational Research, Research Center
for Genomic Medicine, Saitama Medical University, 1397-1 Yamane, Hidaka-shi,
Saitama 350-1241, Japan, bProject of
Clinical and Basic Research for FOP, Saitama Medical University, 1397-1
Yamane, Hidaka-shi, Saitama 350-1241, Japan,
dDepartment of Pediatric Emergency,
Kitakyusyu City Yahata Hospital, 4-18-1 Nishihonmachi, Yahatahigashi-ku,
Kitakyusyu-shi, Fukuoka 805-8534, Japan,
eDepartment of Orthopedic Surgery, Saga
Prefectural Hospital Koseikan, 1-12-9 Mizugae, Saga-shi, Saga 840-8571, Japan,
fNagayoshi Orthopedic Clinic, 1890
Otsukachohinokuchi, Miyazaki-shi, Miyazaki 880-0951, Japan,
gDepartment of Physical Medicine and
Rehabilitation, Tohoku University Graduate School of Medicine, 1-1
Seiryoumachi, Aoba-Ku, Sendai-shi, Miyagi 980-8474, Japan,
hDepartment of Orthopedic Surgery, Senjyudo
Hospital, 1-25 Senjyudonakamati, Gifu-shi, Gifu 500-8862, Japan,
iEndo Clinic, 4-8-11 Ogawanishimachi,
Kodaira-shi, Tokyo 187-0035, Japan,
jAwakura Clinic, 1-6-3 Satomachi,
Wajima-shi, Ishiwawa 928-0246, Japan,
kDepartment of Orthopedic Surgery, Kyushu
University Faculty of Medicine, 3-1-1 Maidashi, Higashi-ku, Fukuoka-shi,
Fukuoka 812-8582, Japan, mDepartment of
Pediatrics, Akashi Municipal Hospital, 1-33 Takashomachi, Akashi-shi, Hyogo
673-8501 Japan, nKawara Clinic, 2-12-3
Sumiyoshihonmachi, Higashinada-ku, Kobe-shi, Hyogo 658-0051, Japan,
oDepartment of Orthopaedic Surgery, Nagoya
University Graduate School and School of Medicine, 65 Tsurumaicho, Showa-ku,
Nagoya-shi, Aichi 466-8550, Japan,
pDepartment of Rehabilitation, Nagoya City
University Hospital, 1 Mizuhochoazakawasumi, Mizuho-ku, Nagoya-shi, Aichi
467-8602, Japan, qDivision of
Morphological Science, Biomedical Research Center, and Departments of
aaLaboratory Medicine,
bbPediatrics,
ccOrthopedic Surgery,
rNeurology,
sEndocrinology and Diabetes,
tObstetrics and Gynecology, and
vOral and Maxillofacial Surgery, Saitama
Medical University, 38 Moro Hongo, Moroyama-machi, Iruma-gun, Saitama350-0495,
Japan, uDepartment of Neurology, Saitama
Neuropsychiatric Institute, 6-11-1 Honchohigashi, Chuo-ku, Saitama-shi,
Saitama 338-8577, Japan, zDepartment of
Molecular Pathology, University of Tokyo Graduate School of Medicine, 7-3-1
Hongo, Bunkyo-ku, Tokyo 113-0033, Japan,
wKitasato University School of
Pharmaceutical Science, 5-9-1 Shirokane, Minato-ku, Tokyo 108-0023, Japan,
xCardiovascular Research Center and
Division of Cardiology, Department of Medicine, Massachusetts General
Hospital, Harvard Medical School, Boston, Massachusetts 02129,
yCenter for Research in Fibrodysplasia
Ossificans Progressiva and Related Disorders, Department of Orthopedic
Surgery, University of Pennsylvania School of Medicine, Philadelphia,
Pennsylvania 19104-6081, ddDivision of
Molecular Signaling and Biochemistry, Department of Biosciences, Kyushu Dental
Collage, 2-6-1 Manazuru, Kokurakita-ku, Kitakyushu-shi, Fukuoka 803-8580,
Japan, eeDepartment of Orthopedic Surgery,
University of Ryukyus Faculty of Medicine, 207 Uehara, Nishihara-cho, 903-0215
Okinawa, Japan, and lThe Research
Committee on Fibrodysplasia Ossificans Progressiva, Ministry of Health, Labour
and Welfare, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan
b,c,
Takenobu Katagiri
Takenobu Katagiri
Divisions of aPathophysiology and
cTranslational Research, Research Center
for Genomic Medicine, Saitama Medical University, 1397-1 Yamane, Hidaka-shi,
Saitama 350-1241, Japan, bProject of
Clinical and Basic Research for FOP, Saitama Medical University, 1397-1
Yamane, Hidaka-shi, Saitama 350-1241, Japan,
dDepartment of Pediatric Emergency,
Kitakyusyu City Yahata Hospital, 4-18-1 Nishihonmachi, Yahatahigashi-ku,
Kitakyusyu-shi, Fukuoka 805-8534, Japan,
eDepartment of Orthopedic Surgery, Saga
Prefectural Hospital Koseikan, 1-12-9 Mizugae, Saga-shi, Saga 840-8571, Japan,
fNagayoshi Orthopedic Clinic, 1890
Otsukachohinokuchi, Miyazaki-shi, Miyazaki 880-0951, Japan,
gDepartment of Physical Medicine and
Rehabilitation, Tohoku University Graduate School of Medicine, 1-1
Seiryoumachi, Aoba-Ku, Sendai-shi, Miyagi 980-8474, Japan,
hDepartment of Orthopedic Surgery, Senjyudo
Hospital, 1-25 Senjyudonakamati, Gifu-shi, Gifu 500-8862, Japan,
iEndo Clinic, 4-8-11 Ogawanishimachi,
Kodaira-shi, Tokyo 187-0035, Japan,
jAwakura Clinic, 1-6-3 Satomachi,
Wajima-shi, Ishiwawa 928-0246, Japan,
kDepartment of Orthopedic Surgery, Kyushu
University Faculty of Medicine, 3-1-1 Maidashi, Higashi-ku, Fukuoka-shi,
Fukuoka 812-8582, Japan, mDepartment of
Pediatrics, Akashi Municipal Hospital, 1-33 Takashomachi, Akashi-shi, Hyogo
673-8501 Japan, nKawara Clinic, 2-12-3
Sumiyoshihonmachi, Higashinada-ku, Kobe-shi, Hyogo 658-0051, Japan,
oDepartment of Orthopaedic Surgery, Nagoya
University Graduate School and School of Medicine, 65 Tsurumaicho, Showa-ku,
Nagoya-shi, Aichi 466-8550, Japan,
pDepartment of Rehabilitation, Nagoya City
University Hospital, 1 Mizuhochoazakawasumi, Mizuho-ku, Nagoya-shi, Aichi
467-8602, Japan, qDivision of
Morphological Science, Biomedical Research Center, and Departments of
aaLaboratory Medicine,
bbPediatrics,
ccOrthopedic Surgery,
rNeurology,
sEndocrinology and Diabetes,
tObstetrics and Gynecology, and
vOral and Maxillofacial Surgery, Saitama
Medical University, 38 Moro Hongo, Moroyama-machi, Iruma-gun, Saitama350-0495,
Japan, uDepartment of Neurology, Saitama
Neuropsychiatric Institute, 6-11-1 Honchohigashi, Chuo-ku, Saitama-shi,
Saitama 338-8577, Japan, zDepartment of
Molecular Pathology, University of Tokyo Graduate School of Medicine, 7-3-1
Hongo, Bunkyo-ku, Tokyo 113-0033, Japan,
wKitasato University School of
Pharmaceutical Science, 5-9-1 Shirokane, Minato-ku, Tokyo 108-0023, Japan,
xCardiovascular Research Center and
Division of Cardiology, Department of Medicine, Massachusetts General
Hospital, Harvard Medical School, Boston, Massachusetts 02129,
yCenter for Research in Fibrodysplasia
Ossificans Progressiva and Related Disorders, Department of Orthopedic
Surgery, University of Pennsylvania School of Medicine, Philadelphia,
Pennsylvania 19104-6081, ddDivision of
Molecular Signaling and Biochemistry, Department of Biosciences, Kyushu Dental
Collage, 2-6-1 Manazuru, Kokurakita-ku, Kitakyushu-shi, Fukuoka 803-8580,
Japan, eeDepartment of Orthopedic Surgery,
University of Ryukyus Faculty of Medicine, 207 Uehara, Nishihara-cho, 903-0215
Okinawa, Japan, and lThe Research
Committee on Fibrodysplasia Ossificans Progressiva, Ministry of Health, Labour
and Welfare, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan
a,b,1