Abstract
The hepatitis C virus (HCV) is a major cause of chronic liver disease. Here, we report a new and effective strategy for inhibiting HCV replication using 17-allylaminogeldanamycin (17-AAG), an inhibitor of heat-shock protein 90 (Hsp90). Hsp90 is a molecular chaperone with a key role in stabilizing the conformation of many oncogenic signaling proteins. We examined the inhibitory effects of 17-AAG on HCV replication in an HCV replicon cell culture system. In HCV replicon cells treated with 17-AAG, we found that HCV RNA replication was suppressed in a dose-dependent manner, and interestingly, the only HCV protein degraded in these cells was NS3 (nonstructural protein 3). Immunoprecipitation experiments showed that NS3 directly interacted with Hsp90, as did proteins expressed from ΔNS3 protease expression vectors. These results suggest that the suppression of HCV RNA replication is due to the destabilization of NS3 in disruption of the Hsp90 chaperone complex by 17-AAG.
Infection by the hepatitis C virus (HCV)2 is a major public health problem, with 170 million chronically infected people worldwide (1, 2). The current treatment by combined interferon-ribavirin therapy fails to cure the infection in 30–50% of cases (3, 4), particularly those with HCV genotypes 1 and 2. Chronic infection with HCV results in liver cirrhosis and can lead to hepatocellular carcinoma (5, 6). Although an effective combined interferon-α-ribavirin therapy is available for about 50% of the patients with HCV, better therapies are needed, and preventative vaccines have not yet been developed.
HCV is a member of the Flaviviridae family and has a positive strand RNA genome (7, 8) that encodes a large precursor polyprotein, which is cleaved by host and viral proteases to generate at least 10 functional viral proteins: core, E1 (envelope 1), E2, p7, NS2 (nonstructural protein 2), NS3, NS4A, NS4B, NS5A, and NS5B (9, 10). NS2 and the amino terminus of NS3 comprise the NS2-3 protease responsible for cleavage between NS2 and NS3 (9, 11), whereas NS3 is a multifunctional protein consisting of an amino-terminal protease domain required for processing NS3 to NS5B (12, 13). NS4A is a cofactor that activates the NS3 protease function by forming a heterodimer (14–17), and the hydrophobic protein NS4B induces the formation of a cytoplasmic vesicular structure, designated the membranous web, which is likely to contain the replication complex of HCV (18, 19). NS5A is a phosphoprotein that appears to play an important role in viral replication (20–23), and NS5B is the RNA-dependent RNA polymerase of HCV (24, 25). The 3′-untranslated region consists of a short variable sequence, a poly(U)-poly(UC) tract, and a highly conserved X region and is critical for HCV RNA replication and HCV infection (26–29).
Hsp90 (heat-shock protein 90) is a molecular chaperone that plays a key role in the conformational maturation of many cellular proteins. Hsp90 normally functions in association with other co-chaperone proteins, which together play an important role in folding newly synthesized proteins and stabilizing and refolding denatured proteins in cells subjected to stress (30–34). Its expression is induced by cellular stress and is also associated with many types of tumor. Hsp90 inhibitors are currently showing great promise as novel pharmacological agents for anticancer therapy.
Hsp90 inhibitors have two major modes of action as preferential clients for protein degradation or as Hsp70 inducers. The benzoquinone ansamycin antibiotic geldanamycin and its less toxic analogue 17-allylamino-17-demethoxygeldanamycin (17-AAG) directly bind to the ATP/ADP binding pocket of Hsp90 (34–36) and thus prevent ATP binding and the completion of client protein refolding. Recently, Waxman et al. (37) demonstrated a role for Hsp90 in promoting the cleavage of HCV NS2/3 protease, using NS2/3 translated by rabbit reticulocyte lysate. Nakagawa et al. (38) also reported that inhibition of Hsp90 is highly effective in suppressing HCV genome replication. Hsp90 may directly or indirectly interact with any of the proteins NS3 through NS5B to regulate replication of the HCV replicon. More recently, Okamoto et al. (39) reported that Hsp90 could bind to FKBP8 (FK506-binding protein 8) and form a complex with NS5A. The interaction with FKBP8 has also been shown to be the mechanism by which Hsp90 regulates HCV RNA replication, a process in which Hsp90 clearly plays an important role.
In this study, we have demonstrated that NS3 also forms a complex with Hsp90, which is critical for HCV replication. On the basis of the findings that treating HCV replicon cells with the Hsp90 inhibitor, 17-AAG, suppressed HCV RNA replication, and that the only HCV protein degraded in these cells was NS3, we suggest a crucial role for Hsp90-NS3 protein complexes in the HCV life cycle.
EXPERIMENTAL PROCEDURES
Cell Culture and Reagents—The HCV replicon cell lines #50-1 (NN/1b/SG) (40), which carries a subgenomic replicon, and NNC#2 (NN/1b/FL) (41), which carries a full genome replicon, were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, nonessential amino acids, l-glutamine, penicillin/streptomycin, and 300–1,000 μg/ml G418 (Invitrogen) at 37 °C in 5% CO2. The human embryonic kidney-derived cell line 293T was grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin. 17-AAG was purchased from Sigma.
Measuring HCV RNA by Real Time PCR—HCV replicon cells were seeded at 1.5 × 105 cells in 24-well plates and cultured for 72 h. Total RNA was then isolated using TRIzol (Invitrogen) according to the manufacturer's instructions. HCV RNA was quantified by real time reverse transcription-PCR using an ABI 7700 sequence detector (PerkinElmer Life Sciences) and the following primers and TaqMan probes located in the 5′-untranslated region: forward primer (nucleotides 130–146), 5′-CGGGAGAGCCATAGTGG-3′; reverse primer (nucleotides 272–290), 5′-AGTACCACAAGGCCTTTCG-3′; and TaqMan probe (nucleotides 148–168), 5′-CTGCGGAACCGGTGAGTACAC-3′ (all purchased from Applied Biosystems). The probe sequence was labeled with the reporter dye, 6-carboxyflurorescein, at the 5′-end and with the quencher dye TAMRA at the 3′-end (42).
Western Blotting and Immunoprecipitation Analyses—Cells were lysed in 1× CAT enzyme-linked immunosorbent assay buffer (Roche Applied Sciences). Cell lysates were separated by SDS-PAGE and transferred to nitrocellulose membranes, and these were blocked with 5% skimmed milk. The primary antibodies used were monoclonal or polyclonal antibody against FLAG-M5 (Sigma), Hsp70 (Sigma), Hsp90 (Cell Signaling Technologies, Danvers, MA), Hsp90α (Calbiochem), Hsp90β (Calbiochem), and Hsf-1 (Calbiochem). Core, NS4A, and NS4B were a gift from Dr. M. Kohara (Tokyo Metropolitan Institute of Medical Science). E1, E2, NS3, NS5A, and NS5B were a gift from Prof. Y. Matsuura (Osaka University, Japan). Immunoprecipitation from cell lysates was carried out using anti-FLAG M5 antibody (Sigma) and the Protein G immunoprecipitation kit (Sigma), according to the manufacturer's instructions, and the immunoprecipitates were analyzed by Western blotting.
[3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt Assay—HCV replicon cells were seeded in 96-well plates at 3 × 104 cells/well in a final culture volume of 100 μl for 72 h before the addition of increasing concentrations of 17-AAG. After incubation for 3 days, viable cell numbers were determined using the Celltiter 96 Aqueous nonradioactive cell proliferation assay (Promega Corp., Madison, WI). The value of the background absorbance at 490 nm (A490) of wells without cells was subtracted. The percentages of viable cells were then calculated using the formula, (A490 of 17-AAG-treated sample/A490 of untreated cells) × 100.
Plasmids and Transfection—The pFLAG-CMV-NS3 vector was constructed by subcloning a DNA fragment encoding full-length NS3, Δhelicase, Δprotease, ΔPH 1, ΔPH 2, and ΔH 1 into the EcoRI and XbaI sites of the pFLAG-CMV™-2 expression vector (Sigma), so that the amino-terminal FLAG epitope was fused in frame with NS3. The core expression vector was a gift from Dr. M. Kohara. The vector was transfected into 293T cells using the FuGENE 6 transfection reagent (Roche Applied Science) according to the manufacturer's instructions.
RESULTS
Hsp90 Inhibitor 17-AAG Suppresses HCV RNA Replication—To investigate the effect of 17-AAG on HCV replication, cells containing a full HCV genome replicon (NNC#2) or a subgenomic replicon (#50-1) were treated with 17-AAG (Fig. 1, A and B). Both of the HCV replicon cell lines were treated for 72 h with different concentrations of 17-AAG or with DMSO as a control. In cells treated with 50 nm 17-AAG, HCV RNA replication was suppressed by 99% in both of the HCV replicon cell lines, and the inhibition of RNA replication occurred in a dose-dependent manner (Fig. 2A). The half-maximal inhibitory concentration (IC50) values of 17-AAG for HCV replication were 0.3 nm in NNC#2 cells and 0.1 nm in #50-1 cells. Furthermore, we used a tetrazolium-based [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt assay to determine the viability of NNC#2 and #50-1 cells in the presence of 17-AAG. 17-AAG showed no toxicity to NNC#2 and #50-1 cells at 50 nm, (Fig. 2B). These results suggested that 17-AAG had a greater inhibitory effect on HCV RNA replication than 100 units/ml interferon-α.
FIGURE 1.
Schematic representation of HCV replicon and structure of 17-AAG. A, structure of the HCV replicon RNAs, comprising the HCV 5′-untranslated region, including the HCV internal ribosome entry site (IRES), the neomycin phosphotransferase gene (Neor), the encephalomyocarditis virus (EMCV) IRES or HCV IRES, and the coding region for HCV proteins NS3 to NS5B (in the HCV subgenomic replicon) or core to NS5B (in the HCV full-length replicon). B, structures of the Hsp90 inhibitor, 17-AAG.
FIGURE 2.
Hsp90 inhibits HCV RNA replication in HCV replicon cells. A, inhibition of HCV replication by 17-AAG in NNC#2 (white squares) and #50-1 cells (black squares) measured by real time reverse transcription-PCR after 72 h. Interferon-α was used as a positive control. The data are means ± S.D. from triplicate experiments. B, cytotoxic effects of 17-AAG in NNC#2 (white squares) and #50-1 (black squares), shown as the percentage reduction in viable cell numbers in an [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (inner salt)] assay. The data are means ± S.D. from triplicate experiments.
Long Term Suppression of HCV RNA Replication—We next examined the effect of 17-AAG on HCV replication over time. When NNC#2 cells were cultured with 50 nm 17-AAG only on day0(white squares), the level of HCV RNA was reduced by 2 log on day 3 but had increased to control levels by day 12 (Fig. 3B). However, when 50 nm 17-AAG was added to the cells at 3-day intervals for 15 days (black squares), the observed significant reduction in HCV RNA (by 3 log) was sustained from day 3 to day 15. We used trypan blue staining to check that long term treatment with 17-AAG did not induce cellular toxicity (Fig. 3A). Our results suggested that 17-AAG has the potential to safely induce long term suppression in HCV replication.
FIGURE 3.
Long term inhibition of HCV replication in NNC#2 cells. A, cytotoxic effect of 17-AAG in NNC#2 cells, shown as the percentage reduction of viable cell numbers assessed by trypan blue staining. NNC#2 cells were treated with 50 nm 17-AAG on day 0 only (white squares) or at 3-day intervals for 15 days (black squares). The data are means ± S.D. from triplicate experiments. B, measurement of HCV replication by real time reverse transcription-PCR. Inhibition of HCV RNA replication in NNC#2 cells treated with 50 nm 17-AAG on day 0 only (white squares) or at 3-day intervals for 15 days (black squares). Day 0, mock. The data are means ± S.D. from triplicate experiments.
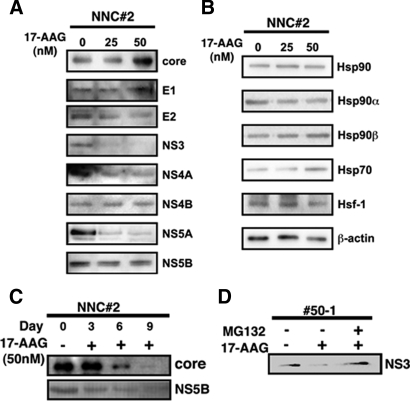
Reduced Expression of NS3 Protein in 17-AAG-treated HCV Replicon Cells—To investigate the mechanism by which 17-AAG inhibited HCV replication, we analyzed the expression of HCV core, E1, E2, NS3, NS4A, NS4B, NS5A, and NS5B proteins by Western blotting. NNC#2 cells treated with increasing doses of 17-AAG showed a marked reduction in the expression of NS3 (Fig. 4A) after 3 days, in common with the level of HCV RNA (Fig. 2A). However, levels of the other proteins were unchanged. This dose-dependent inhibition suggested that NS3 was more sensitive to 17-AAG than the other proteins. Similar effects on NS3 expression and RNA replication were seen in #50-1 cells treated with 17-AAG (Fig. 4A).
FIGURE 4.
Effect of 17-AAG on HCV NS3 protein levels. A, Western blot analysis of HCV protein expression in NNC#2 or #50-1 cells treated with 17-AAG. NNC#2 or #50-1 cells were treated with 25 and 50 nm 17-AAG for 3 days. Cell lysates were separated by SDS-PAGE, immunoblotted, and probed with antibodies specific for HCV core, E1, E2, NS3, NS4A, NS4B, NS5A, and NS5B. B, Western blot analysis of Hsp90, Hsp70, and other chaperone expression in NNC#2 cells treated with 17-AAG (25 and 50 nm, as indicated) for 3 days. C, expression of HCV core and NS5B protein in cells treated with 50 nm 17-AAG for 9 days. D, effect of 50 nm 17-AAG on NS3 expression in #50-1 cells simultaneously treated with 100 nm MG132.
Another effect of 17-AAG treatment seen in these cells was an increase in Hsp70 expression and a slight increase in Hsp90 expression (Fig. 4B). The induction of Hsp70 expression suggested that Hsp90 inhibition by 17-AAG strongly activated HSF-1 (heat-shock transcription factor 1) (43). We also examined the levels of HCV core and NS5B protein expression in NNC#2 cells treated with 50 nm 17-AAG. Reduced levels of these proteins were seen in NNC#2 cells on day 6, and both HCV core and NS5B protein were undetectable on day 9 (Fig. 4C). To determine whether 17-AAG promoted the degradation of NS3, we next looked at the effect of 17-AAG on #50-1 cells in which proteasomal degradation was also inhibited. Although 17-AAG treatment still induced a reduction in the NS3 protein level in #50-1 cells (Fig. 4D), the degradation of NS3 was completely blocked in the presence of the proteasome inhibitor, MG132. This suggested that the pharmacological effect of 17-AAG was dependent on the proteasome system (44, 45).
Protein Folding in Hsp90-NS3 Interaction—To investigate the role of Hsp90 in HCV NS3 activation, the FLAG-NS3 protein was transfected into 293T cells, with or without 17-AAG, and the cell lysates were analyzed by Western blotting. The expression of NS3 from FLAG-NS3 was reduced in the presence of 17-AAG (Fig. 5A), suggesting that Hsp90 is involved in HCV NS3 degradation, possibly through a physical interaction. We confirmed this specific interaction by immunoprecipitating 293T cell lysates with anti-FLAG antibody. This clearly showed that FLAG and Hsp90 co-precipitated, suggesting that NS3 was bound to the chaperone complex formed with Hsp90 (Fig. 5B). NS3 mutants lacking the protease and helicase regions were generated in order to identify the region responsible for the interaction with Hsp90 (Fig. 5C). FLAG-NS3, FLAG-NS3-Δhelicase, or FLAG-NS3-Δprotease were transfected into 293T cells, and anti-FLAG antibody immunoprecipitates were analyzed by Western blotting (Fig. 5D). Although FLAG-NS3-Δprotease was clearly co-immunoprecipitated with Hsp90, no protein band corresponding to FLAG-NS3-Δhelicase was detected (Fig. 5D), suggesting that the NS3 helicase region mediates binding to Hsp90. To confirm this finding, plasmids expressing different NS3 helicase mutants fused with FLAG (ΔPH 1, ΔPH 2, and ΔH 1) were constructed (Fig. 5E). Expressing these NS3 helicase mutants in 293T cells and analyzing their immunoprecipitates with anti-FLAG antibody by Western blotting showed that, although all of the NS3 helicase mutant proteins were immunoprecipitated by anti-FLAG-antibody, no Hsp90 was co-precipitated (Fig. 5F).
FIGURE 5.
Hsp90 regulates HCV NS3 protein stability. A, Western blot showing the inhibition of NS3 protein expression in 293T cells caused by 17-AAG. Cells were transfected with pFLAG-NS3 in the presence of 250 nm 17-AAG for 48 h. B, FLAG-NS3 was expressed in 293T cells and immunoprecipitated (IP) from cell lysates with anti-FLAG antibody. Proteins immunoprecipitated were analyzed by Western blotting using anti-Hsp90, anti-Hsp90α, anti-Hsp90β, and anti-FLAG antibodies. The data shown in each panel are representative of three independent experiments. FLAG-CMV, empty plasmid vector. C, schematic representations of HCV NS3 protein and its deletion mutants. D, FLAG-NS3, FLAG-Δprotease, and FLAG-Δhelicase were expressed in 293T cells and immunoprecipitated from cell lysates with anti-FLAG antibody. Proteins immunoprecipitated with anti-Hsp90, Hsp90α, Hsp90β, and FLAG antibodies were analyzed by Western blotting. The data shown in each panel are representative of three independent experiments. E, schematic representations of HCV NS3 protein and further deletion mutants. F, FLAG-ΔPH 1, FLAG-ΔPH 2, and FLAG-ΔH 1 were expressed in 293T cells and immunoprecipitated from cell lysates with anti-FLAG antibody. Proteins immunoprecipitated with anti-Hsp90, Hsp90α, Hsp90β, and FLAG antibodies were analyzed by Western blotting. The data shown in each panel are representative of three independent experiments. G, FLAG-NS3, pEF-Core, FLAG-Δprotease, FLAG-ΔPH 2, and FLAG-ΔH 1 were expressed in 293T cells treated with 17-AAG. Proteins immunoprecipitated with anti-core, Hsp90 antibody were analyzed by Western blotting. 17-AAG-treated cell lysates were analyzed on Western blots, using the specific antibodies shown to the right of the panels. Lane 1, control; lane 2, 17-AAG (1 μm).
We also confirmed that the NS3 helicase region mediated the specific interaction with Hsp90 by transfecting FLAG-NS3 and FLAG-NS3 deletion mutants into 293T cells pretreated with 17-AAG (Fig. 5G). The proteins expressed by FLAG-NS3 and FLAG-NS3-Δprotease were degraded in cells pretreated with 17-AAG, whereas no degradation of the ΔPH 2 and ΔH 1 NS3 mutants lacking helicase regions was seen (Fig. 5G). Further, when pEF-core was expressed in 293T cells, core was unable to co-immunoprecipitate Hsp90, and no degradation of core protein was observed (Fig. 5G). Our data demonstrate that 17-AGG destabilizes several binding proteins (NS3 and NS3-Δprotease) to Hsp90 but stablilizes some nonbinding proteins (the ΔPH 2 and ΔH 1 NS3 mutants lacking helicase regions and core) to Hsp90. In previous reports (46), similar effects were observed when wild-type and mutated p53 were translated in the presence of geldanamycin. These results further supported the hypothesis that Hsp90 has a role in folding the NS3 helicase domain and that this has an important role in stabilizing the full-length NS3 protein. A protein complex that includes NS3 and Hsp90 is therefore implicated in the control of HCV replication.
DISCUSSION
The Hsp90 inhibitor, 17-AAG, is known to have highly selective effects on tumor cells that are a result of its high affinity for Hsp90 client oncoproteins, which are incorporated into the Hsp90-dependent multichaperone complex, thereby increasing their binding affinity for 17-AAG more than 100-fold (47). This high selectivity effectively minimizes the toxic side effects of 17-AAG so that it is a good candidate for clinical application, especially in treating neurodegenerative diseases. In this study, we observed the inhibitory effects of 17-AAG on the replication of an HCV subgenomic replicon that lacked NS2. On the other hand, Waxman et al. (37) demonstrated a role for Hsp90 in promoting the cleavage of HCV NS2/3 protein using NS2/3 translated in rabbit reticulocyte lysate and expressed in Jurkat cells. Because the replicon cells used in our study genetically lacked NS2, our results suggest that Hsp90 may directly interact with the NS3 protein in the HCV replicon.
In cell lines in which 17-AAG was a potent inhibitor of HCV replication, with IC50 values of 3–10 nm, we also found strong evidence that the association between HCV Hsp90 and NS3, but not other NS proteins, was the essential mechanism controlling the preferential degradation of NS3 after 17-AAG treatments. Furthermore, we showed that NS3 interacted with Hsp90 through the NS3 helicase domain. It was also clear that the expression of NS3 protein with helicase activity in 293T cells pretreated with 17-AAG was reduced, but the expression of NS3 mutants lacking the helicase regions (ΔPH 2 and ΔH1) was not. The role of Hsp90 in achieving and/or stabilizing the NS3 protein was suggested by the fact that only 17-AGG bound to Hsp90 was capable of affecting NS3. The use of Hsp90 inhibitors represents a novel strategy for the development of anti-HCV therapies.
Acknowledgments
We are grateful to M. Sato, R. Tobita, and Y. Katumura for excellent technical assistance.
This work was supported by a grant-in-aid for HCV research from the Ministry of Health, Labor, and Welfare of Japan and by a grant-in-aid for high technology research from the Ministry of Education, Science, Sports, and Culture of Japan. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: HCV, hepatitis C virus; 17-AAG, 17-allylamino-17-demethoxygeldanamycin.
References
- 1.Alter, H. J., Purcell, R. H., Shih, J. W., Melpolder, J. C., Houghton, M., Choo, Q. L., and Kuo, G. (1989) N. Engl. J. Med. 321 1494-1500 [DOI] [PubMed] [Google Scholar]
- 2.Choo, Q. L., Kuo, G., Weiner, A. J, Overby, L. R., Bradley, D. W., and Houghton, M. (1989) Science 244 359-362 [DOI] [PubMed] [Google Scholar]
- 3.McHutchison, J. G., Gordon, S. C., Schiff, E. R., Shiffman, M. L., Lee, W. M., Rustgi, V. K., Goodman, Z. D., Ling, M. H., Cort. S., and Albrecht, J. K. (1998) N. Engl. J. Med. 339 1485-1492 [DOI] [PubMed] [Google Scholar]
- 4.Glue, P., Rouzier-Panis, R., Raffanel, C., Sabo, R., Gupta, S. K., Salfi, M., Jacobs, S., and Clement, R. P. (2000) Hepatology 32 647-653 [DOI] [PubMed] [Google Scholar]
- 5.Saito, I., Miyamura, T., Ohbayashi, A., Harada, H., Katayama, T., Kikuchi, S., Watanabe, Y., Koi, S., Onji, M., and Ohtaet, Y. (1990) Proc. Natl. Acad. Sci. U. S. A. 87 6547-6549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seeff, L. B. (1997) Hepatology 26 21S-28S [DOI] [PubMed] [Google Scholar]
- 7.Bartenschlager, R., and Lohmann, V. (2001) Antiviral Res. 52 1-17 [DOI] [PubMed] [Google Scholar]
- 8.Taylor, D. R., Shi, S. T., Romano, P. R., Barber, G. N., and Lai, M. M. (1999) Science 285 107-110 [DOI] [PubMed] [Google Scholar]
- 9.Grakoui, A., Wychowski, C., Lin. C., Feinstone, S. M., and Rice, C. M. (1993) J. Virol. 67 1385-1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hijikata, M., Mizushima, H., Akagi, T., Mori, S., Kakiuchi, N., Kato, N., Tanaka, T., Kimura, K., and Shimotohno, K. (1993) J. Virol. 67 4665-4675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grakoui, A., McCourt, D. W., Wychowski, C., Feinstone, S. M., and Rice, C. M. (1993) Proc. Natl. Acad. Sci. U. S. A. 90 10583-10587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartenschlager, R., Ahlborn-Laake, L., Mous, J., and Jacobsen, H. (1993) J. Virol. 67 3835-3844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grakoui, A., McCourt, D. W., Wychowski, C., Feinstone, S. M., and Rice, C. M. (1993) J. Virol. 67 2832-2843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartenschlager, R., Lohmann, V., Wilkinson, T., and Koch, J. O. (1995) J. Virol. 69 7519-7528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Failla, C., Tomei, L., and De Francesco, F. (1995) J. Virol. 69 1769-1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin, C., Thomson, J. A., and Rice, C. M. (1995) J. Virol. 69 4373-4380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanji, Y., Hijikata, M., Satoh, S., Kaneko, T., and Shimotohno, K. (1995) J. Virol. 69 1575-1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egger, D., Wolk, B., Gosert, R., Bianchi, L., Blum, H. E., Moradpour, D., and Bienz, K. (2002) J. Virol. 76 5974-5984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gosert, R., Egger, D., Lohmann, V., Bartenschlager, R., Blum, H. E., Bienz, K., and Moradpour, D. (2003) J. Virol. 77 5487-5492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blight, K. J., Kolykhalov, A. A., and Rice, C. M. (2000) Science 290 1972-1974 [DOI] [PubMed] [Google Scholar]
- 21.Guo, J. T., Bichko, V. V., and Seeger, C. (2001) J. Virol. 75 8516-8523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krieger, N., Lohmann, V., and Bartenschlager, R. (2001) J. Virol. 75 4614-4624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lohmann, V., Hoffmann, S., Herian, U., Penin, F., and Bartenschlager, R. (2003) J. Virol. 77 3007-3019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Behrens, S. E., Tomei, L., and De Francesco, R. (1996) EMBO J. 15 12-22 [PMC free article] [PubMed] [Google Scholar]
- 25.Lohmann, V., Korner, F., Herian, U., and Bartenschlager, R. (1997) J. Virol. 71 8416-8428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friebe, P., and Bartenschlager, R. (2002) J. Virol. 76 5326-5338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kolykhalov, A. A., Mihalik, K., Feinstone, S. M., and Rice, C. M. (2000) J. Virol. 74 2046-2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yanagi, M., St. Claire, M., Emerson, S. U., and Purcell Bukh, J. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 2291-2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yi, M., and Lemon, S. M. (2003) J. Virol. 77 3557-3568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Picard, D. (2002) Cell Mol. Life Sci. 59 1640-1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wegele, H., Muller, L., and Buchner, J. (2004) Rev. Physiol. Biochem. Pharmacol. 151 1-44 [DOI] [PubMed] [Google Scholar]
- 32.Pratt, W. B., and Toft, D. O. (2003) Exp. Biol. Med. 228 111-133 [DOI] [PubMed] [Google Scholar]
- 33.Smith, D. F., Whitesell, L., and Katsanis, E. (1998) Pharmacol. Rev. 50 493-514 [PubMed] [Google Scholar]
- 34.McClellan, A. J., and Frydaman, J (2001) Nat. Cell Biol. 3 E1-E311146635 [Google Scholar]
- 35.Grenert, J. P., Sullivan, W. P., Fadden, P., Haystead, T. A., Clark, J., Mimnaugh, E., Krutzsch, H., Ochel, H. J., Schulte, T. W., Sausville, E., Neckers, L. M., and Toft, D. O. (1997) J. Biol. Chem. 272 23843-23850 [DOI] [PubMed] [Google Scholar]
- 36.Supko, J. G., Hickman, R. L., Grever, M. R., and Malspeis, L. (1995) Cancer Chemother. Pharmacol. 36 305-315 [DOI] [PubMed] [Google Scholar]
- 37.Waxman, L., Whitney, M., Pollok, B. A., Kuo, L. C., and Darke, P. L. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 13931-13935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakagawa, S., Umehara, T., Matsuda, C., Kuge, S., Sudoh, M., and Kohara, M. (2007) Biochem. Biophys. Res. Commun. 353 882-888 [DOI] [PubMed] [Google Scholar]
- 39.Okamoto, T., Nishimura, Y., Ichimura, T., Suzuki, K., Miyamura, T., Suzuki, T., Moriishi, K., and Matsuura, Y. (2006) EMBO J. 25 5015-5025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kishine, H., Sugiyama, K., Hijikata, M., Kato, N., Takahashi, H., Noshi, T., Nio, Y., Hosaka, M., Miyanari, Y., and Shimotohno, K. (2002) Biochem. Biophys. Res. Commun. 290 993-999 [DOI] [PubMed] [Google Scholar]
- 41.Ishii, N., Watashi, K., Hishiki, T., Goto, K., Inoue, D., Hijikata, M., Wakita, T., Kato, N., and Shimotohno, K. (2006) J. Virol. 80 4510-4520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takeuchi, T., Katsume, A., Tanaka, T., Abe, A., Inoue, K., Tsukiyamakohara, K., Kawaguchi, R., Tanaka, S., and Kohara, M. (1999) Gastroenterology 11 636-642 [DOI] [PubMed] [Google Scholar]
- 43.Sittler, A., Lurz, R., Ueder, G., Priller, J., Lehrach, H., Hayer-Hartl, M. K., Hartl, F. U., and Wanker, E. E. (2001) Hum. Mol. Genet. 10 1307-1315 [DOI] [PubMed] [Google Scholar]
- 44.Bonvini, P., Dalla Rosa, H., Vignes, N., and Rosolen, A. (2004) Cancer Res. 64 3256-3264 [DOI] [PubMed] [Google Scholar]
- 45.Mimnaugh, E. G., Chavany, C., and Neckers, L. (1996) J. Biol. Chem. 271 22796-22801 [DOI] [PubMed] [Google Scholar]
- 46.Blagosklonny, M. V., Toretsky, J., Bohen, S., and Neckers L. (1996) Proc. Natl. Acad. Sci. U. S. A. 93 8379-8383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kamal, A., Thao, L., Sensintaffar, J., Zhang, L., Boehm, M. F., Fritz, L. C., and Burrows, F. J. (2003) Nature 425 357-359 [DOI] [PubMed] [Google Scholar]