Abstract
Pregnane x receptor is a ligand-activated transcription factor that regulates drug-inducible expression of several key cytochrome P450 enzymes and drug transporter proteins in liver and intestine in a species-specific manner. Activation of this receptor modulates several key biochemical pathways, including gluconeogenesis, β-oxidation of fatty acids, fatty acid uptake, cholesterol homeostasis, and lipogenesis. It is of current interest to determine whether the interaction between pregnane x receptor and these key biochemical pathways is evolutionarily conserved. We show here that activation of the cyclic AMP-dependent protein kinase signaling pathway synergizes with pregnane x receptor-mediated gene activation in mouse hepatocytes. Conversely, cyclic AMP-dependent protein kinase signaling has a repressive effect upon pregnane x receptor-mediated gene activation in rat and human hepatocytes. We show that the human pregnane x receptor protein can serve as an effective substrate for catalytically active cyclic AMP-dependent protein kinase in vitro. Metabolic labeling of the protein in vivo indicates that human pregnane x receptor exists as a phosphoprotein and that activation of cyclic AMP-dependent protein kinase signaling modulates the phosphorylation status of pregnane x receptor. Activation of cyclic AMP-dependent protein kinase signaling also modulates the interactions of pregnane x receptor with protein cofactors. Our results define the species-specific impact of cyclic AMP-dependent protein kinase signaling on pregnane x receptor and provide a molecular explanation of cyclic AMP-dependent protein kinase-mediated repression of human pregnane x receptor activity. Taken together, our results identify a novel mode of regulation of pregnane x receptor activity and highlight prominent functional differences in the process across species.
The nuclear hormone receptor, pregnane x receptor (PXR2; NR1I2), regulates drug-inducible gene expression in liver and intestine (1). PXR is activated by a vast array of compounds, including certain steroids and bile acids, a plethora of naturally occurring compounds, specific antibiotics, antifungal drugs, polychlorinated binphenyls, organochloride pesticides, and phenobarbital (PB) (2). The prototypical marker of PXR activation and best-characterized PXR target gene in mammals encodes certain members of the CYP3A family of cytochrome P450 (CYP) drug-metabolizing enzymes (3, 4). It is now clear that PXR-mediated gene activation coordinately regulates a group of genes that encode CYP proteins and additional drug-metabolizing enzymes, as well as drug transporter proteins in liver and intestine (5). Hence, PXR-mediated gene activation produces profound up-regulation of the metabolism, transport, and elimination of potentially toxic chemicals, including many steroids, xenobiotics, cholesterol metabolites, and other compounds, from the body.
Ligand-mediated activation of PXR occurs in a species-specific manner (6). One of the most effective activators of human PXR is the macrocyclic antibiotic, rifampicin. Interestingly, rifampicin does not appreciably activate mouse PXR. Conversely, pregnenolone 16α-carbonitrile (PCN) is an efficacious activator of mouse PXR but has only minimal effect on human PXR. The species-specific induction of CYP3A gene expression can be fully accounted for by evolution of the ligand-binding pocket of this nuclear receptor from mice to humans. We and others have demonstrated this experimentally using novel lines of PXR knock-out mice crossed with additional novel lines of transgenic mice expressing the human PXR protein (7–10). Although much is known regarding the identity of target genes and ligands for this nuclear receptor, very little is known regarding the signal transduction pathways that interface with the PXR protein.
The primary target of intracellular cAMP is cAMP-dependent protein kinase (PKA) (11). Numerous physiological stimuli, such as β-adrenergic stimulation during fasting and caloric restriction, as well as acute inflammation produce increases in the intracellular concentration of cAMP in hepatocytes. The PKA signal transduction pathway is also involved in the phosphorylation of target proteins through indirect interaction with the classical mitogen-activated protein kinase signaling pathway (12). There are conflicting reports in the literature regarding the effect of PKA signaling on drug-inducible CYP3A and CYP2B gene expression in hepatocytes. Using primary cultures of rat hepatocytes, Sidhu and Omiecinski (13) clearly demonstrated that PB-mediated induction of Cyp3A1 and Cyp2B1/2 expression is inhibited by treatment with cAMP analogues. Conversely, the same laboratory reported that forskolin treatment produces increased expression of Cyp3A1 in rat hepatocytes; however, induction was independent of cAMP and PKA signaling (14). The molecular basis for this difference was conclusively demonstrated when two groups independently found that forskolin functions as a direct agonist of both rodent and human PXR, thereby inducing ligand-dependent expression of CYP3A genes (15, 16). In mice, it has been reported that PB-mediated induction of Cyp3a11 and Cyp2b10 gene expression is inhibited by PKA activators, whereas inhibitors of PKA enhanced drug-inducible CYP gene expression (17, 18).
Studies from our laboratory using mouse models show that the PKA signal transduction pathway synergizes with ligand-dependent PXR-mediated induction of Cyp3a11 gene expression (15). These studies also revealed that the PKA-mediated synergism is a PXR-dependent phenomenon in mice. Additional studies from our laboratory using mouse models indicate that PKA signaling interfaces with constitutive androstane receptor (CAR, NR1I3) activity by modulating CAR-protein cofactor interactions and also by increasing the expression of the Car gene itself (19). During the course of these studies, we noticed significant differences in PKA-dependent alterations of drug-inducible CYP gene expression that were dependent upon the species of rodent (mouse versus rat) used to isolate primary cultures of hepatocytes. To date, there are no studies that we are aware of that have systematically investigated the effect of PKA signaling upon drug-inducible CYP gene expression in hepatocytes across multiple species. These observations led us to initiate a systematic study of the effect of PKA signaling on drug-inducible CYP3A gene expression in cultured hepatocytes across three different species: mice, rats, and humans.
Using primary cultures of hepatocytes, we show here that pharmacological activation of PKA signaling using the cyclic AMP analog, 8-bromo-cyclic AMP (8-Br-cAMP), has a species-specific effect upon PXR-mediated activation of drug-inducible CYP3A gene expression. In primary cultures of mouse hepatocytes, PKA signaling has a synergistic effect upon PXR-mediated Cyp3a11 gene activation. Conversely, in primary cultures of human and rat hepatocytes, PKA signaling dramatically represses PXR-mediated drug-inducible Cyp3A1 gene activation. We use biochemical and pharmacological methods to explore the molecular basis of the interface between the PKA signal transduction pathway and the human PXR protein. In vitro 32P-labeling experiments using recombinant human PXR protein and several catalytically active protein kinases reveal that human PXR protein serves as a comparatively effective and direct substrate for PKA. In vivo metabolic labeling experiments using [32P]orthophosphate show for the first time that human PXR exists as a phosphoprotein in cells. Moreover, Western blot analysis using antibodies directed against phosphothreonine amino acid residues reveals that activation of PKA signaling increases the phosphothreonine content of the human PXR protein. Mammalian two-hybrid analysis indicates that PKA signaling probably represses human PXR activity through increases in the strength of interaction between human PXR and the protein cofactor nuclear receptor co-repressor protein (NCoR). Elucidation of the mechanism by which PKA signaling modulates PXR activity will probably be useful in the prediction and prevention of harmful drug interactions in patients on combination therapy that also suffer from diabetes, obesity, or acute inflammation. It is of further interest to determine the extent to which the interface between PXR and key signal transduction pathways, such as the PKA signaling pathway, is evolutionarily conserved. This thrust of research will also probably be useful in helping to understand the molecular basis of altered drug metabolism pathways in patients with diabetes, obesity, and metabolic syndrome.
MATERIALS AND METHODS
Hepatocyte Culture and Treatment—Hepatocytes were isolated from male mice or rats using a standard collagenase perfusion method, as described previously (20). Hepatocytes were plated in collagen-coated 6-well plates at a density of 8 × 105 live cells/well. Primary cultures of human hepatocytes were purchased from CellzDirect (Pittsboro, NC). Forty-eight hours after plating, hepatocytes were treated with 1 mm concentrations of 8-bromo-cyclic-AMP or 8-bromo-cyclic-GMP, 10 μm PCN, a known rodent PXR agonist, or 10 μm rifampicin, a known human PXR agonist. Mouse hepatocytes were treated for 24 h, and human hepatocytes were treated for 48 h.
RNA Isolation, Northern Blot, and Real Time Quantitative-PCR Analysis—Total RNA was isolated from cell culture using a commercially available reagent, TRIzol (Invitrogen), according to the manufacturer's directions. For Northern blot analysis, 10 μg of total RNA was resolved on a formaldehyde-agarose gel. Blots were hybridized with 32P-labeled cDNA corresponding to the cDNA sequences for mouse and rat CYP3A analogs or for 18 S RNA, as described previously (15, 21). For QPCR analysis, isolated RNA was DNase-treated (Sigma) and reverse transcribed (Promega), and quantitative PCR was performed using a Cepheid Smart Cycler™ (Sunnyvale, CA) to detect mRNA expression specific for CYP3A and CYP2B orthologues in mice, rats, and humans. Primers for CYP3A were designed as follows: mouse Cyp3a11 (5′-CAA GGA GAT GTT CCC TGT CA and 5′-CCA CGT TCA CTC CAA ATG AT), rat Cyp3A1 (5′-AAT AAG GCA CCT CCC ACC TA and 5′-GGA TCA CGG TGA AGA GCA TA), and human CYP3A4 (5′-CAG GAG GAA ATT GAT GCA GTT TT and 5′-GTC AAG ATA CTC CAT CTG TAG CAC AGT). Primers for CYP2B were designed as follows: mouse Cyp2b10 (5′-GAC TTT GGG ATG GGA AAG AG and 5′-CCA AAC ACA ATG GAG CAG AT), human CYP2B6 (5′-AAG CGG ATT TGT CTT GGT GAA and 5′-TGG AGG ATG GTG GTG AAG AAG), and rat Cyp2B1/B2 (5′-GGT ACC TGC TTC CCA AGA AC and 5′-ACA AAT GTG CTT TCC TGT GG). -Fold induction was calculated using 18 S RNA to normalize the data as described previously (22).
Generation of the Human PXR Recombinant Adenovirus— Recombinant adenoviruses were generated using the Ad-Easy™ adenoviral vector system (Stratagene). A BamHI/XhoI human PXR PCR product was inserted into the pShuttle-IRES-hrGFP1 transfer vector. The PCR primer sequences were 5′-GAC GGC CTC GAG GCT ACC TGT GAT GCC GAA CAA CTC and 5′-GAA GGC CTC GAG GCC ACC ATG GAT TAC AAG GAT GAC. These primers were designed to omit the stop codon in order to fuse the protein to a 3× FLAG epitope at the COOH terminus. Viruses were propagated in the AD-293 cell line and were purified using cesium chloride density gradient centrifugation. Cells were transduced with the purified adenovirus expressing FLAG-tagged human PXR at a multiplicity of infection of 10.
Immunopurification of FLAG-tagged Human PXR Protein—Following overnight adenoviral transduction, cells were drug-treated for 24–48 h and lysed by sonication in a buffer composed of 50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 1 mm EDTA, 1% Triton X-100, and 1× protease and phosphatase inhibitor mixtures (Thermo Scientific). Cell lysates were precleared with 20 μl of immobilized protein A (Repligen). Immunoprecipitation of the human PXR protein was accomplished using anti-FLAG M2 affinity gel (Sigma) or a polyclonal antibody directed against the human PXR protein, as indicated. Control reactions contained nonimmune IgG (Sigma) or blank virus as indicated. Free immune complexes were captured with immobilized protein A and washed three times with lysis buffer.
In Vitro Phosphorylation Analysis—Immunopurified human PXR protein was subjected to in vitro phosphorylation analysis using catalytically active purified kinases, including CDK1, CK2, GSK3 (New England Biosciences), PKA, PKC (Promega), p70S6K, AMPK, and Akt2 (Upstate). Approximately 3 μg of human PXR protein was incubated at 30 °C for 30 min with the above kinases and corresponding reaction buffers. Reaction buffer composition was as follows: CDK1 (50 mm Tris-HCl, pH 7.4, 10 mm MgCl2, 2 mm dithiothreitol, 1 mm EGTA, 200 μm ATP, 0.5 μCi of [γ-32P]ATP), CK2 (20 mm Tris-HCl, pH 7.4, 80 mm KCl, 10 mm MgCl2, 200 μm ATP, 0.5 μCi of [γ-32P]ATP), GSK3 (20 mm Tris-HCl, pH 7.4, 10 mm MgCl2, 5 mm dithiothreitol, 200 μm ATP, 0.5 μCi of [γ-32P]ATP), PKA (40 mm Tris-HCl, pH 7.4, 5 mm MgCl2, 200 μm ATP, 0.5 μCi of [γ-32P]ATP), PKC (20 mm HEPES, pH 7.4, 10 mm MgCl2, 3.4 mm CaCl2, 200 μm ATP, 0.5 μCi of [γ-32P]ATP), p70S6K (40 mm MOPS-NaOH, pH 7.0, 1 mm EDTA, 10 mm MgCl2, 0.1 mg/ml bovine serum albumin, 0.01% β-mercaptoethanol, 200 μm ATP, 0.5 μCi of [γ-32P]ATP), AMPK (30 mm HEPES, pH 7.4, 10 mm MgCl2, 0.2 mm dithiothreitol, 0.2% Nonidet P-40, 300 μm AMP, 200 μm ATP, 0.5 μCi of [γ-32P]ATP), and Akt2 (50 mm Tris-HCl, pH 7.5, 10 mm MgCl2, 0.1 mm EGTA, 0.2 mm dithiothreitol, 200 μm ATP, 0.5 μCi of [γ-32P]ATP). The samples were subjected to SDS-PAGE. The gel was dried, and autoradiography analysis was performed overnight at –80 °C.
In Vivo Metabolic Labeling Analysis—HepG2 cells were plated in 6-well plates at a density of 1 × 106 cells/well. The cells were transduced with Ad-hPXR or Ad-GFP (multiplicity of infection = 10) overnight. After viral transduction, the cells were treated with phosphate-free Dulbecco's modified Eagle's medium containing 1% dialyzed fetal bovine serum containing 1% penicillin/streptomycin for 6 h. The culture medium was then supplemented with 300 μCi of [32P]orthophosphate/well and treated with vehicle, 1 mm 8-Br-cAMP, or 1 mm 8-bromo-cyclic GMP (8-Br-cGMP) for an additional 14 h. The cells were washed three times in 1× phosphate-buffered saline and human PXR protein was immunopurified using anti-human PXR antibody as described above. The samples were subjected to SDS-PAGE. The gel was dried, and autoradiography analysis was performed for 30 min at room temperature. For control purposes, a duplicate experiment was performed for the parallel Western blot analysis that omitted the radiolabel in order to examine the efficiency of immunopurification.
Detection of Phosphothreonine in Human PXR—The immunopurified human PXR protein was resolved using 10% SDS-PAGE and transferred to polyvinylidene difluoride membranes (Millipore). Western blot analysis was performed using monoclonal antibodies obtained from the PhosphoDetect™ phosphoserine and phosphothreonine detection kits (Calbiochem). The λ-protein phosphatase (New England Biolabs) reaction conditions were 50 mm Tris-HCl, 100 mm NaCl, 2 mm dithiothreitol, 0.1 mm EGTA, 0.01% Brij 35, 2 mm MnCl2, at pH 7.5 at 25 °C.
Transient Transfection and Reporter Gene Analysis—The XREM-Luc and mammalian two-hybrid reporter gene assays were performed as previously described (20, 21). Briefly, cells were plated in 96-well plates at a density of 7000 cells/well. After 24 h, the cells were transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. The PXR transactivation assays were transfected with 110 ng of DNA/well containing SV40-β-galactosidase (40 ng), XREM-Luc (20 ng), pGFP-hPXR (5 ng), pFC-PKA or pFC-MEK1 (10 ng), and pBluescript (35 ng). The mammalian two-hybrid assays were transfected with 110 ng of DNA/well containing SV40-β-galactosidase (40 ng), pFR-Luc (20 ng), Gal4-NCoR (20 ng), VP16-hPXR (10 ng), pFC-PKA (10 ng), and pBluescript (10 ng). The next day, the cells were drug-treated for 24 h. Luciferase activities were determined using a standard luciferase assay system (Promega). β-Galactosidase activities were determined by o-nitrophenyl-β-D-galactopyranoside assay and were read at 420 nm.
Statistical Analysis—Statistical differences between treatment groups were determined using a one-way analysis of variance followed by the Duncan's multiple range post hoc test.
RESULTS
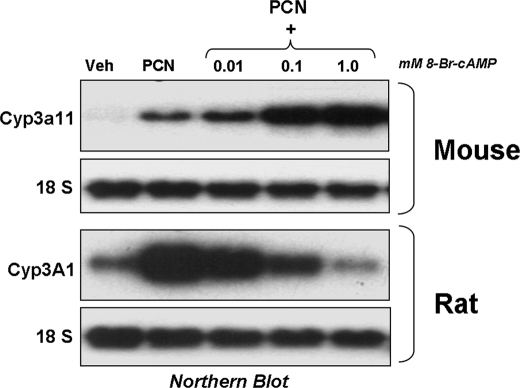
PKA Signaling has a Species-specific Effect on PXR-mediated Gene Activation in Hepatocytes—To determine if PKA signaling has a species-specific effect upon PXR-mediated gene activation, primary cultures of mouse and rat hepatocytes were isolated and treated with vehicle or 10 μm PCN for 24 h in the presence of increasing concentrations (0.01, 0.1, and 1.0 mm) of 8-Br-cAMP, and Northern blotting analysis was performed (Fig. 1). As expected, treatment with PCN produced increased expression of both Cyp3a11 and Cyp3A1 in primary cultures of mouse and rat hepatocytes, respectively. Strikingly, increasing concentrations of the PKA activator, 8-Br-cAMP, had opposite effects upon PCN-inducible CYP3A gene expression in primary cultures of mouse hepatocytes when compared with that obtained using rat hepatocytes. In mouse hepatocytes, 8-Br-cAMP synergized with PCN, producing extremely robust levels of Cyp3a11 gene expression at the highest doses, whereas in rat hepatocytes, it dramatically repressed the expression of Cyp3A1. Both the synergistic and repressive effects were concentration-dependent in both species examined, respectively. The synergistic effect of 8-Br-cAMP in mouse hepatocytes was also PXR-dependent, since there was only minimal induction of Cyp3a11 gene expression at the highest doses of 8-Br-cAMP examined in PXR knock-out mice (data not shown).
FIGURE 1.
PKA activation modulates CYP3A gene expression in primary cultures of mouse and rat hepatocytes. Primary rodent hepatocytes were treated with 10 μm PCN and increasing concentrations of 1 mm 8-Br-cAMP for 24 h before RNA isolation. Blots were probed sequentially with 32P-labeled fragments of CYP3A and 18 S.
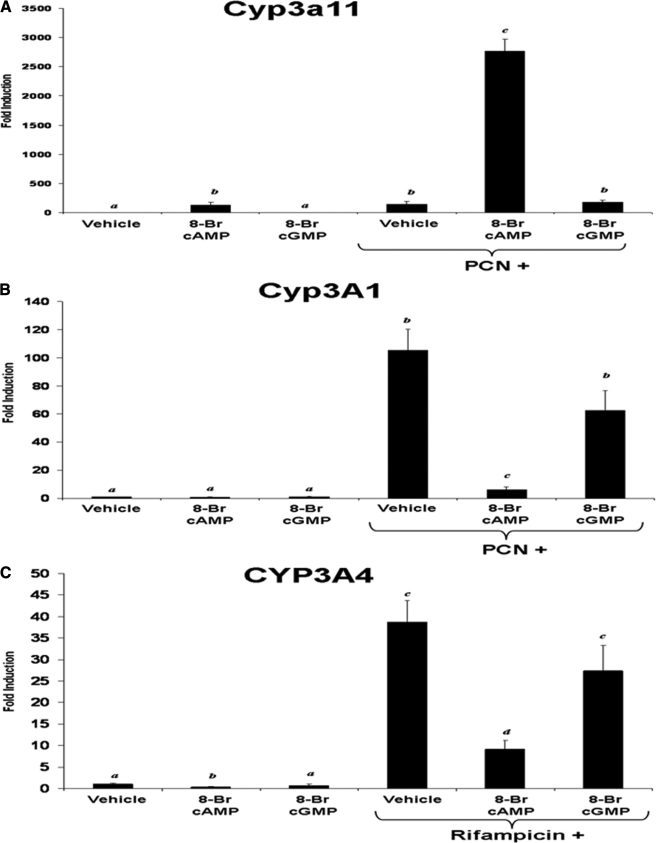
To further examine the specificity of the effect of the effect of PKA signaling across species, we treated primary cultures of mouse, rat, and human hepatocytes for 24 h with either 8-Br-cAMP or 8-Br-cGMP as a control compound, in the presence and absence of 10 μm PCN or rifampicin and performed real time quantitative PCR to analyze the expression levels of CYP3A. Fig. 2A shows that treatment of primary cultures of mouse hepatocytes with 1 mm 8-Br-cAMP or 10 μm PCN for 24 h induced expression of Cyp3a11 to approximately equivalent levels, whereas co-treatment with 8-Br-cAMP and PCN produced a synergistic level of PXR target gene expression (>2000-fold induction). Treatment with 1 mm 8-Br-cGMP alone had no significant effect upon Cyp3a11 gene expression levels, and co-treatment with 8-Br-cGMP and PCN produced very similar effects when compared with PCN treatment alone. Primary cultures of rat and human hepatocytes were also treated using identical experimental conditions. Treatment of cultured hepatocytes with 1 mm 8-Br-cAMP isolated from these two species produced the exact opposite effect of that observed with mouse hepatocytes. Fig. 2B shows that treatment of primary cultures of rat hepatocytes with either 8-Br-cAMP or 8-Br-cGMP produced little or no effect upon Cyp3A1 gene expression levels, whereas treatment with 10 μm PCN induced expression as expected. However, treatment of rat hepatocytes with 8-Br-cAMP together with PCN reduced the -fold induction of Cyp3A1 expression to less than 10% of that observed when compared with PCN treatment alone. In contrast, treatment of rat hepatocytes with 8-Br-cGMP together with PCN induced expression of Cyp3A1 comparable with that observed with PCN treatment alone. When primary cultures of human hepatocytes were used, 10 μm rifampicin was substituted for PCN treatment; other than that, the experimental conditions were identical. Fig. 2C reveals that treatment with 8-Br-cAMP produced dramatic repression of rifampicin-inducible CYP3A4 gene expression. Treatment with 8-Br-cGMP had very little or no effect upon either the basal or rifampicin-inducible expression of the CYP3A4 gene.
FIGURE 2.
PKA activation has a species-specific effect on CYP3A gene expression in primary cultures of hepatocytes. Primary cultures of mouse (A), rat (B), and human (C) hepatocytes were treated with either 10 μm PCN or 10 μm rifampicin and with 1 mm 8-Br-cAMP or 1 mm 8-Br-cGMP for 24 h and monitored for the induction of CYP3A orthologues. Data are expressed as -fold induction over the vehicle control and are normalized to 18 S expression and represent the mean of the replicates ± S.D. (n = 3). Letters different from each other indicate a statistical difference between treatment groups (p < 0.05).
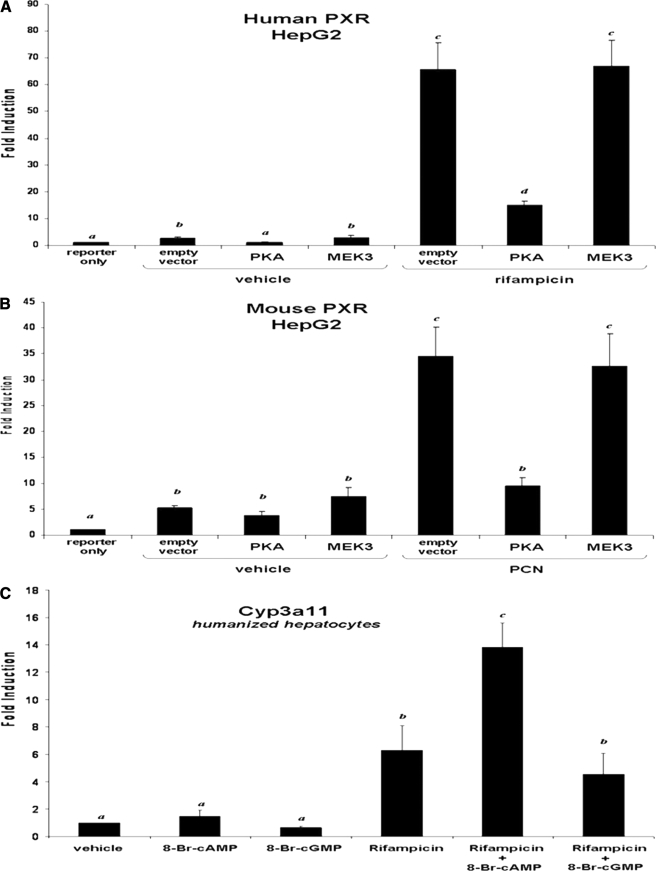
Species Specificity Resides in the Cyclic AMP Signaling Pathway—We next sought to determine whether the species-specific response to cAMP resides in the PXR protein or is a function of how PKA signaling interfaces with PXR activity. The XREM-Luc reporter gene was used in HepG2 cells to determine if overexpression of PKA altered activity toward the CYP3A4 promoter in a species-specific manner. A plasmid encoding human PXR was co-transfected with the XREM-Luc reporter gene in the presence and absence of an additional expression vector encoding the catalytic domain of PKA (Clontech). A plasmid encoding MEK3 (Clontech) was used as a control for kinase overexpression. Twenty-four hours post-transfection, selected wells were treated with rifampicin for an additional 24 h (Fig. 3A). As expected, rifampicin activated XREM-Luc reporter gene activity ∼65-fold in the presence of human PXR. Overexpression of PKA repressed reporter gene activity by ∼70%, whereas overexpression of MEK3 did not. Identical experiments using a plasmid encoding mouse PXR yielded similar results in that overexpression of PKA significantly repressed XREM-Luc reporter gene activity, whereas overexpression of MEK3 did not (Fig. 3B). These data suggest that PKA signaling is a repressive signal in the human genetic background and that repression of PXR activity is independent of the species of PXR protein.
FIGURE 3.
Species-specific modulation of PXR activity resides in the PKA signaling pathway. HepG2 cells were transfected with expression vectors for human PXR (A) or mouse PXR (B), together with the XREM-Luc reporter gene, in the presence or absence of an expression vector encoding constitutively active PKA. Cells were treated with 10 μm rifampicin, 10 μm PCN, 1 mm 8-Br-cAMP, and 1 mm 8-Br-cGMP for 24 h. Data are expressed as the mean of replicates ± S.D. (n = 8) and are normalized to β-galactosidase activity. C, primary cultures of humanized mouse hepatocytes were treated with 10 μm rifampicin in the presence and absence of 1 mm 8-Br-cAMP and 1 mm 8-Br-cGMP for 24 h. Data are expressed as the mean of replicates ± S.D. (n = 3). Letters different from each other indicate a statistical difference between treatment groups (p < 0.05).
We also wanted to determine whether human PXR would be positively regulated in the context of a murine hepatic genetic background. We therefore utilized our line of PXR knock-out mice that have been engineered to express a human PXR transgene in liver (8). Following liver perfusion, primary cultures of “PXR-humanized” mouse hepatocytes were treated with 8-Br-cAMP in the presence and absence of the human PXR agonist rifampicin (Fig. 3C). As expected, treatment with rifampicin produced a 6-fold increase in Cyp3a11 gene expression. Co-treatment of hepatocytes with 8-Br-cAMP and rifampicin had a positive effect, producing ∼14-fold increase in Cyp3a11 gene expression. As a negative control, treatment with 8-Br-cGMP had no effect on rifampicin-inducible gene expression. Taken together, these data further support the hypothesis that the observed species-specific interaction between cAMP and PXR activation in hepatocytes is a function of how PKA signaling interfaces with CYP3A gene expression across species and is not due to differences in primary amino acid sequences in the human and mouse PXR proteins.
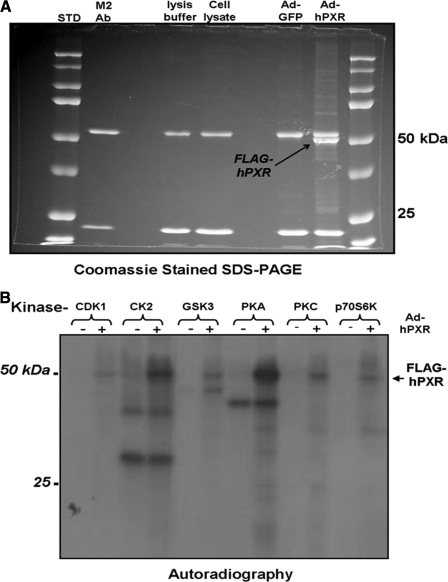
Human PXR Is a Phosphoprotein in Vitro and in Vivo—The extent to which down-regulation of human PXR activity is associated with alterations in the phosphorylation status of the PXR protein is unknown. We therefore created an adenoviral expression vector encoding a FLAG-tagged version of the human PXR protein in order to facilitate immunopurification of the recombinant protein from cultured cells. Analysis of protein isolated from adenovirus-infected CV-1 cells using SDS-PAGE and Coomassie Blue staining shows that the FLAG-hPXR protein is ∼52 kDa (Fig. 4A). Western blot analysis using either anti-FLAG antibodies or anti-hPXR antibodies confirms that the recombinant protein is indeed FLAG-tagged human PXR (data not shown). When incubated in vitro with a series of catalytically active protein kinases, PXR served as the most effective substrate for PKA, followed by casein kinase II, glycogen synthase kinase, and protein kinase C (Fig. 4B). Catalytically active AMP kinase and AKT2 were unable to directly phosphorylate the human PXR protein in vitro (data not shown).
FIGURE 4.
hPXR is phosphorylated by protein kinases in vitro. HepG2 cells were transduced with adenovirus expressing FLAG-tagged human PXR. A, human PXR was isolated from cellular extracts using immunoprecipitation with the M2 FLAG monoclonal antibody (Ab) and detected using SDS-PAGE and Coomassie staining. B, the recombinant human PXR protein was phosphorylated with γ-32P and myriad kinases in vitro. Radiolabeled proteins were visualized by autoradiography.
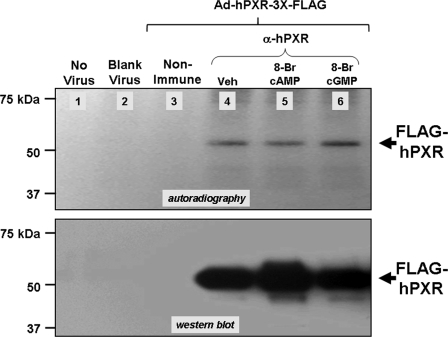
To determine whether PKA can directly affect phosphorylation status of human PXR, CV-1 or HepG2 cells expressing recombinant FLAG-tagged human PXR protein were subjected to in vivo labeling using [32P]orthophosphate and treated with either 8-Br-cAMP or 8-Br-cGMP in the presence and absence of rifampicin. The CV-1 cell line was chosen due to its ease of culturing as a confluent monolayer and uniform infection with the adenoviral vector; however, identical results were obtained using either cell line. The human PXR protein was immunoprecipitated and resolved using SDS-PAGE, the gels were dried, and subsequent autoradiography revealed that the human PXR exists as a phosphoprotein (Fig. 5, lane 4). Immunoprecipitates from noninfected cells, blank virus-infected cells, and nonimmune serum were included as negative controls (Fig. 5, lanes 1-3). The addition of rifampicin also had no effect upon the overall phosphorylation level of the human PXR protein (data not shown). A duplicate “cold” experiment was performed and used for Western blot analysis with anti-hPXR antibodies to ensure specificity of immunoprecipitation and roughly equivalent loading (Fig. 5, bottom).
FIGURE 5.
The human PXR protein exists as a phosphoprotein in vivo. HepG2 cells were transduced with adenoviral expression vector encoding FLAG-tagged human PXR. Cells were treated with phosphate-free medium for 6 h and then treated with medium containing 300 μCi/well [32P]orthophosphate together with vehicle, 1 mm 8-Br-cAMP, or 1 mm 8-Br-cGMP for 14 h. Phosphorylated PXR protein was visualized by autoradiography (top). Total PXR protein was visualized by Western blot using anti-HPXR antibodies in a duplicate cold experiment (bottom).
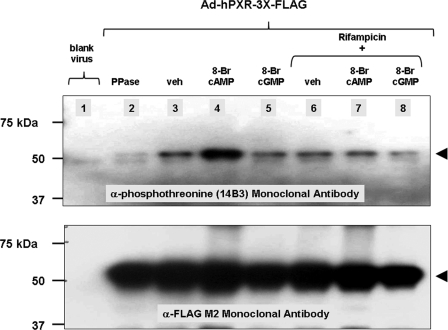
We next screened a panel of seven commercially available monoclonal antibodies against the immunopurified human PXR protein, which potentially recognize phosphoserine (four) and phosphothreonine (three) in the context of differing surrounding amino acid residues. Three of these antibodies, 1C8 (anti-phosphoserine), 14B3, and 1E11 (anti-phosphothreonine), effectively and specifically recognized immunopurified human PXR phosphoprotein in Western blot analysis. To more closely examine whether activation of the PKA signaling pathway could alter the phosphorylation status of specific serine/threonine residues on the human PXR protein, we treated adenovirus-infected cells with either 8-Br-cAMP or 8-Br-cGMP in the presence or absence of rifampicin. Western blotting analysis revealed that treatment with 8-Br-cyclic AMP specifically up-regulated the recognized phosphothreonine content of the immunopurified human PXR protein, whereas treatment with 8-Br-cGMP did not (Fig. 6, lanes 4 and 5). Treatment with rifampicin alone did not alter phosphothreonine content, whereas co-treatment with rifampicin prevented the up-regulation of 8-Br-cAMP-mediated phosphorylation of the human PXR protein (Fig. 6, lane 7). Immunoprecipitates from cells infected with blank virus (Fig. 6, lane 1) and immunopurified recombinant human PXR protein treated with λ-protein phosphatase (Fig. 6, lane 2) were included as negative controls. The blot was stripped and reprobed with anti-FLAG monoclonal antibody to ensure equal loading (Fig. 6, bottom). Similar results were obtained with another monoclonal antibody anti-phosphothreonine 1E11 (Calbiochem) (data not shown).
FIGURE 6.
PKA signaling modulates the phosphorylation status of human PXR in vivo. CV-1 cells were transduced with an adenovirus expressing FLAG-tagged human PXR. Cells were treated with 10 μm rifampicin, 1 mm 8-Br-cAMP, and 1 mm 8-Br-cGMP for 24 h. The PXR protein was immunoprecipitated using anti-hPXR antibody and subjected to Western blot analysis using anti-phosphothreonine 14B3 antibody (top). The blot was stripped and reprobed with anti-FLAG monoclonal antibody to ensure equal loading (bottom). PPase, protein phosphatase; veh, vehicle.
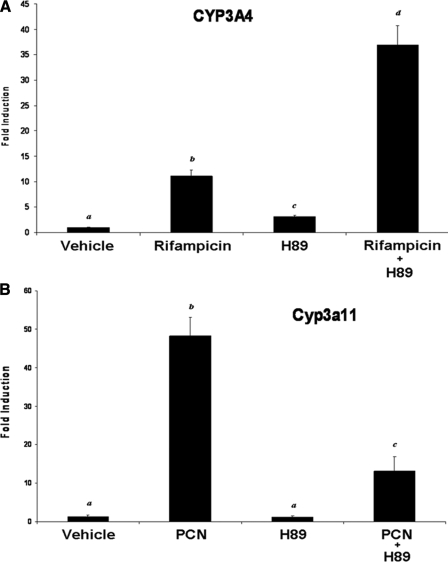
Physiological Levels of PKA Signaling Modulate PXR Target Gene Activation in a Species-specific Manner—Using pharmacological and biochemical methods to elevate PKA signaling, the experiments presented thus far are consistent with the hypothesis that PKA signaling interfaces with PXR activity in a species-specific manner. It was therefore of interest to determine whether endogenous PKA signaling modulates PXR target gene activation across species. We therefore used the PKA-selective inhibitor H89 to examine the effect of inhibition of PKA on human and mouse PXR activity. HepG2 cells were transduced with the adenoviral expression vector encoding human PXR and treated with rifampicin in the presence and absence of H89, and expression levels of the CYP3A4 gene were determined using quantitative PCR (Fig. 7A). Treatment of cells with rifampicin produced significant increases in CYP3A4 gene expression. Treatment of cells with H89 alone produced a significant increase in the level of CYP3A4, although the levels were less than that produced by rifampicin treatment alone. Co-treatment of cells with rifampicin and H89 produced significantly increased levels of CYP3A4 gene expression when compared with either treatment alone. The opposite trend was observed when similar experiments were conducted using primary cultures of mouse hepatocytes (Fig. 7B). As expected, treatment with PCN alone produced significant increases in Cyp3a11 gene expression levels, whereas treatment with H89 alone did not. Co-treatment of hepatocytes with PCN and H89 produced significantly lower levels of Cyp3a11 gene expression when compared with that observed with PCN treatment alone. Taken together, these data indicate that physiological levels of cyclic AMP and PKA signaling interface with PXR activity in a species-specific manner.
FIGURE 7.
Endogenous levels of PKA signaling modulate PXR activity in a species-specific manner. A, HepG2 cells were transduced with the adenoviral expression vector encoding FLAG-tagged human PXR. Cells were treated with vehicle or 10 μm rifampicin, 10 μm H89, or 10 μm rifampicin plus 10 μm H89. B, primary cultures of mouse hepatocytes were treated with vehicle or 10 μm rifampicin, 10 μm H89, or 10 μm rifampicin plus 10 μm H89. Endogenous levels of CYP3A were determined using quantitative PCR analysis. Data are expressed as the mean of replicates ± S.D. (n = 3). Letters different from each other indicate a statistical difference between treatment groups (p < 0.05).
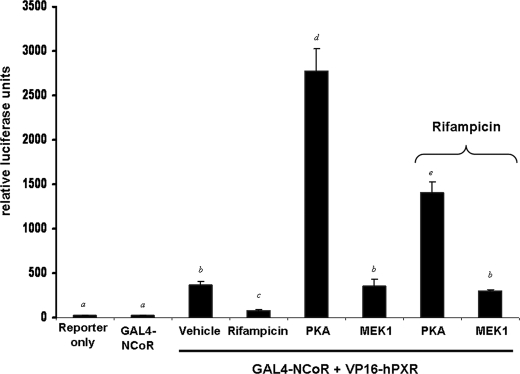
Activation of PKA Signaling Promotes Association of Human PXR with NCoR—Because rifampicin-activated human PXR localized in part to the nucleus and was repressed by both treatment with 8-Br-cAMP and co-expression of the catalytic subunit of PKA, we reasoned that PKA signaling might modulate the ability of PXR to interact with NCoR. To investigate this possibility, we used the nuclear receptor interaction domain (Arg2065–Gly2287) from NCoR (GAL4-NCoR) and full-length human PXR fused to VP16 (VP16-hPXR) in the mammalian two-hybrid system. Transfection of CV-1 cells with GAL4-NCoR together with VP16-human PXR produced increased reporter gene activity, whereas transfection of GAL4-NCoR alone did not (Fig. 8). As expected, treatment of transfected cells with 10 μm rifampicin decreased the strength of interaction between these two fusion proteins. Co-transfection of the catalytic subunit of PKA increased the strength of association between PXR and NCoR by ∼5-fold, whereas co-transfection of the constitutively active kinase MEK1 had no effect. Interestingly, although administration of rifampicin in the presence of PKA weakened the association between NCoR and human PXR, the relative strength of association was well above that obtained in the absence of PKA. Similar results were obtained when using 8-Br-cAMP to activate PKA signaling and 8-Br-cGMP as a negative control in HepG2 cells (data not shown).
FIGURE 8.
PKA increases the strength of interaction between hPXR and NCoR in mammalian-2-hybrid reporter gene assays. CV-1 cells were transfected with expression vectors for Gal4-NCoR receptor-interacting domain, VP16-human PXR, pFR-Luc reporter gene, and constitutively active kinases. Cells were treated with 10 μm rifampicin for 24 h. Data are expressed as the mean of replicates ± S.D. (n = 8) and are normalized to β-galactosidase activity. Letters different from each other indicate a statistical difference between treatment groups (p < 0.05).
DISCUSSION
Diabetes, fasting, obesity, protein-calorie malnutrition, and long term alcohol consumption all produce alterations in the expression and activity of hepatic drug-metabolizing enzymes (23). Moreover, the expression and activity of CYP3A4, the primary drug-metabolizing enzyme found in liver and intestine, is rapidly and dramatically repressed in response to acute inflammatory states (24). Because PXR is a master regulator of drug-inducible transcription of the CYP3A4 gene, there is a high level of interest in understanding the potential role of this transcription factor in mediating transcriptional suppression during these specific pathological conditions. Although much is known regarding the identity of target genes and ligands for this important nuclear receptor protein, comparatively little is known regarding the post-translational modification of the human PXR protein. Therefore, we and others have sought to understand the molecular mechanisms that comprise the potential interface between human PXR and important cellular signal transduction cascades that mediate repression of drug metabolism, energy metabolism, and glucose production in liver.
The species-specific nature of nuclear receptor signaling is a well documented and highly relevant area of scientific inquiry. For example, although peroxisome proliferators have carcinogenic consequences in the livers of rodents, epidemiological studies have revealed that similar effects are unlikely to occur in humans (25). Additionally, PB has been used for decades as the prototypical nongenotoxic tumor-promoting agent in numerous rodent studies of hepatocarcinogenesis; however, epidemiological studies indicate that PB does not cause liver tumors in humans (26). Although the primary event governing activation of nuclear receptors is ligand binding, increasing amounts of evidence suggest that cell signaling pathways and modulation of nuclear receptor-cofactor-phosphorylation status also determines overall responsiveness to environmental stimuli (27, 28). Phosphorylation has been implicated in regulation of 1) nuclear receptor transactivation capacity, 2) DNA binding, 3) subcellular localization, 4) protein cofactor interaction profile, and 5) protein stability.
It is a longstanding observation that treatment of patients with rifampicin tends to suppress immunological responses in liver cells. The precise molecular basis for the repression of the inflammatory response following PXR activation is not currently known, although it probably involves a kinase-mediated signaling cascade. Symmetrically, activation of the inflammatory response by treatment with lipopolysaccharide or tumor necrosis factor α decreases PXR-mediated gene activation. Recent studies suggest that activation of NF-κB interferes with the formation of the PXR-retinoid X receptor heterodimeric complex on the CYP3A4 promoter (29). Additional studies in rodents suggest that down-regulation of PXR target genes by inflammatory cytokines is PXR-dependent (30). Our results provide additional evidence for a key interface between kinase-mediated signal transduction pathways and PXR activity. Moreover, our results provide compelling evidence for pronounced species-specific differences in the coupling of pivotal kinase cascades and PXR activity. Moreover, since PKA signaling is up-regulated during acute inflammation, our results describe a potential molecular mechanism for the observed repression of PXR target gene expression during this pathophysiological condition.
Recent research indicates that some metabolic signal transduction pathways interface with PXR. The extent to which this interaction is dependent on kinase signaling and the phosphorylation status of PXR is unknown and requires further investigation. Activation of PXR has recently been shown to decrease energy metabolism and increase hepatic triglyceride levels through down-regulation of gluconeogenesis, fatty acid oxidation, and ketogenesis and by up-regulating hepatic lipogenesis (31–35). The cross-talk of PXR with these fundamental biological processes is thought to be due to the ability of PXR to interact directly with FoxO1, FoxA2, CREB, and PGC-1α. In addition, a recent study indicates that human PXR can be phosphorylated at more than one site by the serine-threonine protein kinase CDK2 in vitro (36). The same study showed that PXR activity toward the CYP3A4 promoter is inhibited during S-phase of the cell cycle.
In the current study, we show that activity of the PXR protein is modulated in hepatocytes by treatment with 8-Br-cAMP, a well characterized and specific activator of PKA in a species-specific manner. We also show that the phosphorylation status of threonine residues is altered following activation of PKA signaling in cultured cell lines. There are several important implications of these findings. First, this is the first demonstration that we are aware of that PXR exists in cells as a phosphoprotein. Second, the demonstrated alterations in the threonine phosphorylation status of PXR and modulation of NCoR corepressor protein recruitment following activation of PKA signaling impart a new level of understanding regarding the potential molecular basis of repression of PXR activity by various signal transduction pathways. Specifically, our data are consistent with the model that phosphorylation of the human PXR protein or PXR-associated protein favors recruitment of corepressor multiprotein complexes, thereby producing repression of drug-inducible PXR target gene expression. However, it should be noted here that our data do not exclude the possibility that the molecular basis of PKA-mediated repression of PXR activity is multifactorial in nature. For example, alterations in the phosphorylation status of the PXR protein could in principle inhibit the DNA binding capacity of PXR, alter its subcellular distribution, reduce PXR protein stability, and prevent PXR association with coactivator proteins. Whether repression of PXR activity by PKA signaling is regulated by direct phosphorylation of the human PXR protein is not currently known; however, extensive mutagenesis of the human PXR protein failed to identify a single residue that was responsible for PKA-dependent repression in the reporter gene assay (data not shown). Although our Western blotting data generated using an anti-phosphothreonine antibody suggest that the level of threonine phosphorylation is altered by cAMP in the recombinant human PXR, the question remains as to how PKA signaling represses PXR activity in the presence of rifampicin. Our Western blot data should be interpreted carefully, since the anti-phosphoantibodies used here recognize only a subset of phosphothreonine residues in any given protein due to the nature of the surrounding amino acid sequences and the accessibility of the given epitope. Finally, our description of the species-specific effects of PKA signaling raises the possibility that preclinical testing of novel drug candidates in “humanized” PXR and CAR mice poses more of a problem than previously realized. Future experiments should be focused upon determining which serine and/or threonine residues are subject to regulated phosphorylation and which kinases and phosphatases alter the activity of the human PXR protein.
Acknowledgments
We thank Dr. Xunshan Ding for helpful discussions.
This work was supported, in whole or in part, by National Institutes of Health Grant R01-DK068443. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: PXR, pregnane x receptor; CAR, constitutive androstane receptor; hPXR, human PXR; PB, phenobarbital; CYP, cytochrome P450; PCN, pregnenolone 16α-carbonitrile; PKA, cAMP-dependent protein kinase; 8-Br-cAMP, 8-bromo-cyclic cAMP; 8-Br-cGMP, 8-bromo-cyclic cGMP; NCoR, nuclear receptor co-repressor protein; MOPS, 4-morpholinepropanesulfonic acid.
References
- 1.Willson, T. M., and Kliewer, S. A. (2002), Nat. Rev. Drug Discov. 1 259–266 [DOI] [PubMed] [Google Scholar]
- 2.Goodwin, B., Redinbo, M. R., and Kliewer, S. A. (2002) Annu. Rev. Pharmacol. Toxicol. 42 1–23 [DOI] [PubMed] [Google Scholar]
- 3.Kliewer, S. A., Moore, J. T., Wade, L., Staudinger, J. L., Watson, M. A., Jones, S. A., McKee, D. D., Oliver, B. B., Willson, T. M., Zetterstrom, R. H., Perlmann, T., and Lehmann, J. M. (1998) Cell 92 73–82 [DOI] [PubMed] [Google Scholar]
- 4.Lehmann, J. M., McKee, D. D., Watson, M. A., Willson, T. M., Moore, J. T., and Kliewer, S. A. (1998) J. Clin. Invest. 102 1016–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maglich, J. M., Stoltz, C. M., Goodwin, B., Hawkins-Brown, D., Moore, J. T., and Kliewer, S. A. (2002) Mol. Pharmacol. 62 638–646 [DOI] [PubMed] [Google Scholar]
- 6.Moore, L. B., Maglich, J. M., McKee, D. D., Wisely, B., Willson, T. M., Kliewer, S. A., Lambert, M. H., and Moore, J. T. (2002) Mol. Endocrinol. 16 977–986 [DOI] [PubMed] [Google Scholar]
- 7.Scheer, N., Ross, J., Rode, A., Zevnik, B., Niehaves, S., Faust, N., and Wolf, C. R. (2008) J. Clin. Invest. 118 3228–3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lichti-Kaiser, K., and Staudinger, J. L. (2008) Drug Metab. Dispos. 36 1538–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma, X., Shah, Y., Cheung, C., Guo, G. L., Feigenbaum, L., Krausz, K. W., Idle, J. R., and Gonzalez, F. J. (2007) Drug Metab. Dispos. 35 194–200 [DOI] [PubMed] [Google Scholar]
- 10.Xie, W., Barwick, J. L., Downes, M., Blumberg, B., Simon, C. M., Nelson, M. C., Neuschwander-Tetri, B. A., Brunt, E. M., Guzelian, P. S., and Evans, R. M. (2000) Nature 406 435–439 [DOI] [PubMed] [Google Scholar]
- 11.Beavo, J. A., Bechtel, P. J., and Krebs, E. G. (1974) Proc. Natl. Acad. Sci. U. S. A. 71 3580–3583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dumaz, N., and Marais, R. (2005) FEBS J. 272 3491–3504 [DOI] [PubMed] [Google Scholar]
- 13.Sidhu, J. S., and Omiecinski, C. J. (1995) J. Biol. Chem. 270 12762–12773 [DOI] [PubMed] [Google Scholar]
- 14.Sidhu, J. S., and Omiecinski, C. J. (1996) J. Pharmacol. Exp. Ther. 276 238–245 [PubMed] [Google Scholar]
- 15.Ding, X., and Staudinger, J. L. (2005) J. Pharmacol. Exp. Ther. 312 849–856 [DOI] [PubMed] [Google Scholar]
- 16.Dowless, M. S., Barbee, J. L., Borchert, K. M., Bocchinfuso, W. P., and Houck, K. A. (2005) Eur. J. Pharmacol. 512 9–13 [DOI] [PubMed] [Google Scholar]
- 17.Galisteo, M., Marc, N., Fautrel, A., Guillouzo, A., Corcos, L., and Lagadic-Gossmann, D. (1999) J. Pharmacol. Exp. Ther. 290 1270–1277 [PubMed] [Google Scholar]
- 18.Marc, N., Galisteo, M., Lagadic-Gossmann, D., Fautrel, A., Joannard, F., Guillouzo, A., and Corcos, L. (2000) Eur. J. Biochem. 267 963–970 [DOI] [PubMed] [Google Scholar]
- 19.Ding, X., Lichti, K., Kim, I., Gonzalez, F. J., and Staudinger, J. L. (2006) J. Biol. Chem. 281 26540–26551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brobst, D. E., Ding, X., Creech, K. L., Goodwin, B., Kelley, B., and Staudinger, J. L. (2004) J. Pharmacol. Exp. Ther. 310 528–535 [DOI] [PubMed] [Google Scholar]
- 21.Ding, X., and Staudinger, J. L. (2005) Biochem. Pharmacol. 69 867–873 [DOI] [PubMed] [Google Scholar]
- 22.Staudinger, J. L., Madan, A., Carol, K. M., and Parkinson, A. (2003) Drug Metab. Dispos. 31 523–527 [DOI] [PubMed] [Google Scholar]
- 23.Kim, S. K., and Novak, R. F. (2007) Pharmacol. Ther. 113 88–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aitken, A. E., Richardson, T. A., and Morgan, E. T. (2006) Annu. Rev. Pharmacol. Toxicol. 46 123–149 [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez, F. J., and Shah, Y. M. (2008) Toxicology 246 2–8 [DOI] [PubMed] [Google Scholar]
- 26.Kodama, S., and Negishi, M. (2006) Drug. Metab. Rev. 38 75–87 [DOI] [PubMed] [Google Scholar]
- 27.Staudinger, J. L., and Lichti, K. (2008) Mol. Pharmacol. 5 17–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rochette-Egly, C. (2003) Cell. Signal. 15 355–366 [DOI] [PubMed] [Google Scholar]
- 29.Zhou, C., Tabb, M. M., Nelson, E. L., Grun, F., Verma, S., Sadatrafiei, A., Lin, M., Mallick, S., Forman, B. M., Thummel, K. E., and Blumberg, B. (2006) J. Clin. Invest. 116 2280–2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teng, S., and Piquette-Miller, M. (2005) J. Pharmacol. Exp. Ther. 312 841–848 [DOI] [PubMed] [Google Scholar]
- 31.Nakamura, K., Moore, R., Negishi, M., and Sueyoshi, T. (2007) J. Biol. Chem. 282 9768–9776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou, J., Zhai, Y., Mu, Y., Gong, H., Uppal, H., Toma, D., Ren, S., Evans, R. M., and Xie, W. (2006) J. Biol. Chem. 281 15013–15020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kodama, S., Koike, C., Negishi, M., and Yamamoto, Y. (2004) Mol. Cell. Biol. 24 7931–7940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miao, J., Fang, S., Bae, Y., and Kemper, J. K. (2006) J. Biol. Chem. 281 14537–14546 [DOI] [PubMed] [Google Scholar]
- 35.Kodama, S., Moore, R., Yamamoto, Y., and Negishi, M. (2007) Biochem. J. 407 373–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin, W., Wu, J., Dong, H., Bouck, D., Zeng, F. Y., and Chen, T. (2008) J. Biol. Chem. 283 30650–30657 [DOI] [PMC free article] [PubMed] [Google Scholar]