Abstract
Spectrins are tetrameric actin-cross-linking proteins that form an elastic network, termed the membrane skeleton, on the cytoplasmic surface of cellular membranes. At the plasma membrane, the membrane skeleton provides essential support, preventing loss of membrane material to environmental shear stresses. The skeleton also controls the location, abundance, and activity of membrane proteins that are critical to cell and tissue function. The ability of the skeleton to modulate membrane stability and function requires adaptor proteins that bind the skeleton to membranes. The principal adaptors are the ankyrin proteins, which bind to the β-subunit of spectrin and to the cytoplasmic domains of numerous integral membrane proteins. Here, we present the crystal structure of the ankyrin-binding domain of human β2-spectrin at 1.95Å resolution together with mutagenesis data identifying the binding surface for ankyrins on β2-spectrin.
Spectrin is the principal component of a membrane-associated cytoskeletal network termed the membrane skeleton. The membrane skeleton was first identified in human erythrocytes, where it lines the cytoplasmic surface of the plasma membrane and provides the viscoelastic support necessary for erythrocytes to withstand the shear stresses experienced during blood circulation (1). Subsequently, the membrane skeleton was characterized on the plasma membranes of most cells, where the skeleton not only supports the plasma membrane but also promotes the polarized distribution of cell adhesion molecules and ion transport proteins (2–4). In addition, the membrane skeleton is present on the cytoplasmic surface of intracellular membrane compartments; however, the function of the skeleton on most intracellular compartments is not clear.
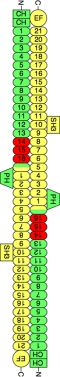
The membrane skeleton is composed of spectrins, actin filaments, and a collection of accessory proteins. Spectrins are tetrameric proteins composed of two α-spectrins and two β-spectrins that together form ∼220-nm-long elastic filaments (2). In mammals, spectrin proteins are expressed from two α-spectrin genes (α1 and α2) and five β-spectrin genes (β1–β5). The most abundant protein motifs of both α- and β-spectrins are spectrin repeats, which are present in 21 copies in α-spectrins and 16 copies in most β-spectrins (see Fig. 1). Spectrin repeats are ∼106-amino acid motifs that form three-helix coiled-coils. The principal function of the spectrin repeat is to confer elasticity to spectrin (5, 6); however, repeat 15 of most β-spectrins also has the ability to bind to the ankyrin family of accessory proteins (7). Ankyrins are adaptor proteins that bind the membrane skeleton to membranes through their ability to associate with the cytoplasmic domains of at least 12 distinct families of integral membrane proteins (3). Mammals have three ankyrin genes (Ank1, Ank2, and Ank3), each of which produces a family of proteins (ankyrin-R, -B, and -G, respectively) through alternative splicing. Defects in specific ankyrins or spectrins disrupt membrane linkages that are required for normal cell function in specific tissues and cause a variety of hereditary diseases, including hemolytic anemia, cardiac arrhythmia, premature aging, deafness, and ataxia (4, 8).
FIGURE 1.
B14–16 region of the spectrin tetramer. The diagram shows the arrangement of α2- and β2-spectrins in the spectrin tetramer along with the domain organization of each subunit. α-Spectrin is colored yellow, and β-spectrin is colored green. Spectrin repeats are indicated by number. Shown in red are the spectrin repeats of β-spectrin that were crystallized. The Src homology 3 (SH3) domain in α2-spectrin is an insert in spectrin repeat 10. EF refers to the EF-hand domains at the C terminus of α2-spectrin, which bind to calcium. In β-spectrin, the CH motifs bind to actin, whereas the pleckstrin homology (PH) domain binds to lipids. The end-to-end association of α- and β-spectrin is mediated by partial spectrin repeats that form a three-helix bundle consisting of one helix from α-spectrin and two helices from β-spectrin.
Despite the importance of the spectrin-ankyrin association, a structural basis for the interaction has not been established. Structure prediction algorithms suggest that repeat 15 of β-spectrins adopts a three-helix bundle conformation similar to other spectrin repeats; however, sequence comparisons have suggested that repeat 15 has unique features that may confer ankyrin-binding activity (7). Here, we provide a 1.95 Å resolution crystal structure of spectrin repeats 14–16 of human β2-spectrin. We also provide mutational data that identify the ankyrin-binding surface on repeat 15 of β2-spectrin.
EXPERIMENTAL PROCEDURES
Protein Expression and Purification—cDNA encoding spectrin repeats 14–16 of human β2-spectrin was synthesized by PCR and ligated into the pET15b expression vector (Novagen). The resulting plasmid was then transformed into the Escherichia coli strain BL21(DE3). Expression of B14–16 protein was induced by 1 mm isopropyl β-d-thiogalactopyranoside, and expressed B14–16 was purified by sequential column chromatography using nickel-nitrilotriacetic acid-agarose, Q-Sepharose, and Superose12. The selenomethionine (SeMet)2 variant of B14–16 was expressed in the methionine auxotrophic E. coli strain B834(DE3) and grown in minimal medium supplemented with natural amino acids and SeMet. Expression and purification were unchanged.
Crystallization and X-ray Diffraction Data Collection— Crystals of B14–16 were grown using the hanging-drop vapor diffusion method from drops containing 1 μl of protein (25 mg/ml) and 1 μl of reservoir solution (14% polyethylene glycol 4000, 100 mm Tris (pH 8.5), and 300 mm MgCl2) and equilibrated over 200 μl of reservoir solution. Disphenoidal crystals appeared after 3–4 days at 4 °C and grew to their maximal extent by 2 weeks. Crystals were small (0.1-mm maximal length) and thin (0.02-mm thickness). SeMet protein crystallized in an identical fashion. Cryoprotection was performed by increasing the glycerol content in reservoir solution up to 30% in 5% steps over the course of 30 min at 4 °C. Crystals were flash-frozen using liquid propane. Crystals exhibited the symmetry of space group P212121 with unit cell parameters of a = 51.3, b = 56.1, and c = 261.6 Å and contained two molecules of B14–16 per asymmetric unit. Native crystals diffracted isotropically to a dmin of 1.95 Å and SeMet crystals to a dmin of 2.4 Å when exposed to synchrotron radiation. Data were indexed, integrated, and scaled using the HKL3000 program package (9). Data collection statistics are provided in Table 1.
TABLE 1.
Data collection, phasing, and refinement statistics for the spectrin B14–16 structure Data for the outermost shell are given in parentheses. r.m.s.d., root mean square deviation.
| Data collection | |||
| Crystal | Native | SeMeta | |
| Energy (eV) | 12,661.7 | 12,660 | |
| Resolution range (Å) | 43.60–1.95 (1.97–1.95) | 47.9–2.40 (2.42–2.40) | |
| Unique reflections | 55,069 (1,174) | 29,026 (438) | |
| Multiplicity | 4.2 (3.4) | 6.0 (2.2) | |
| Data completeness (%) | 97.3 (86.1) | 93.4 (57.5) | |
| Rmerge (%)b | 4.6 (68.2) | 7.6 (25.5) | |
| I/σ(I) | 29.4 (1.97) | 33.8 (3.47) | |
| Wilson B-value (Å2) | 30.2 | 42.7 | |
| Phase determination | |||
| Anomalous scatter | Selenium, 18 out of 18 possible sites | ||
|
Figure of merit (30-2.40 Å)
|
0.18 (0.67 after density modification)
|
||
| Refinement statistics | |||
| Resolution range (Å) | 28.6–1.95 (1.98–1.95) | ||
| No. of reflections (Rwork/Rfree) | 52,170/2788 (2241/111) | ||
| Data completeness (%) | 97.2 (85.0) | ||
| Atoms (non-H protein/solvent/other) | 5479/561/33 | ||
| Rwork (%) | 19.2 (24.0) | ||
| Rfree (%) | 25.0 (32.9) | ||
| r.m.s.d. bond length (Å) | 0.008 | ||
| r.m.s.d. bond angle | 0.845° | ||
| Mean B-value (Å; protein/solvent/other) | 39.6/44.8/64.4 | ||
| Ramachandran plot (%; favored/additional/disallowed)c | 99.7/0.3/0.0 | ||
| Maximum likelihood coordinate error | 0.31 | ||
| Missing residues | Chain A, none; chain B, Leu2015 | ||
| Alternate conformations | Chain A, 8; chain B, 8 | ||
Bijvoet pairs were kept separate for data processing
Rmerge = 100 ΣhΣi|Ih,i – 〈Ih〉|/ΣhΣhIh,i, where the outer sum (h) is over the unique reflections, and the inner sum (i) is over the set of independent observations of each unique reflection
This was as defined by the validation suite MolProbity (17)
Phase Determination and Structure Refinement—Phases were obtained from a single-wavelength anomalous dispersion experiment using a SeMet-B14–16 crystal with data to a resolution of 2.4 Å. Eighteen selenium sites were located using the program SHELXD (10); this represented five single-occupancy sites and two double-conformation sites per B14–16 monomer. Phases were refined with the program MLPHARE (11), resulting in an overall figure of merit of 0.18 for data between 50.0 and 2.4 Å. Phases were further improved by density modification and 2-fold non-crystallographic averaging with the program DM (12), resulting in a figure of merit of 0.67. An initial model containing ∼95% of all residues was automatically generated by alternating cycles of the program ARP/wARP (13).
Additional residues were manually modeled in the programs O (14) and Coot (15). Refinement was performed with native data to a resolution of 1.95 Å using the programs PHENIX and REFMAC (16) with a random 5.1% of all data set aside for an Rfree calculation. The current model contains two B14–16 monomers (molecule A with residues 1697–2015 and molecule B with residues 1697–2014), 561 waters, one molecule of Tris, four molecules of glycerol, and one magnesium ion. Two and three residues of vector sequence were also modeled at the N terminus of molecules A and B, respectively, of B14–16. Sixteen residues were modeled in alternate conformations. The Rwork is 0.198, and the Rfree is 0.255. A Ramachandran plot generated with MolProbity (17) indicated that 99.7% of all protein residues are in the most favored regions with the remaining 0.3% in additionally allowed regions. Phasing and model refinement statistics are provided in Table 1.
Yeast Two-hybrid Analysis—Nucleotides 4689–6279 of the human β2-spectrin cDNA were fused to the cDNA of the Gal4 activation domain in the pACT2 vector. Mutations in the repeat 15 region of β2-spectrin were introduced using QuikChange (Stratagene). Nucleotides 2584–4329 of the human ankyrin-B cDNA or nucleotides 2620–4428 of the human ankyrin-G cDNA (encoding the spectrin-binding domains of the respective ankyrins) were fused to the cDNA of the Gal4 DNA-binding domain in the pAS2-1 vector. Yeast were cotransformed with recombinant pACT2 and pAS2-1 vectors and grown for 6 days on agar plates either lacking leucine and tryptophan or lacking adenine, histidine, leucine, and tryptophan. All yeast strains grew on agar lacking leucine and tryptophan. Growth on agar lacking adenine, histidine, leucine, and tryptophan was scored as ++ for strong growth, + for moderate growth, and - for no growth.
Figure Preparation—Structure figures were rendered using the MegaPOV extension of POV-Ray with primitive files produced using Coot (Fig. 2) (15), PyMOL (Figs. 3 and 6C) (DeLano Scientific LLC), or Swiss-PdbViewer (Figs. 4 and 5) (18).
FIGURE 2.
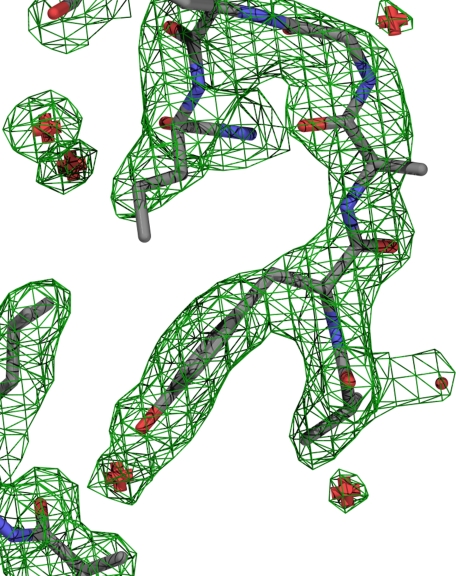
Representative electron density. Shown is the representative electron density near Tyr1874 with the final model shown as sticks. Carbons are colored gray; nitrogens, blue; and oxygens, red. Waters are shown as red pluses. Electron density at 1σ is shown as a green wire network.
FIGURE 3.
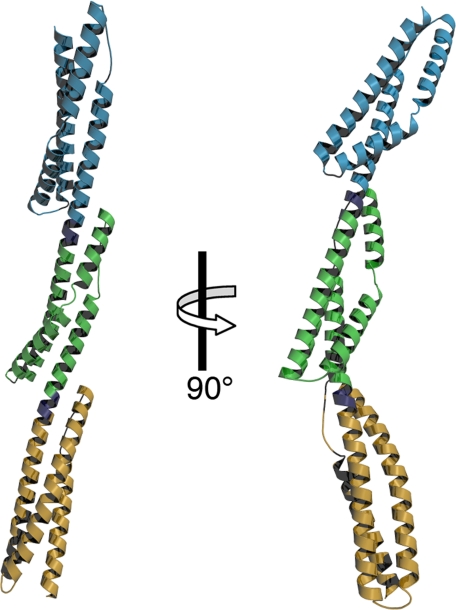
Crystal structure of B14–16. Shown are orthogonal ribbon representations of the crystal structure of B14–16. Repeat 14 is colored tan; repeat 15, green; and repeat 16, blue. The linkers connecting repeats are colored purple.
FIGURE 6.
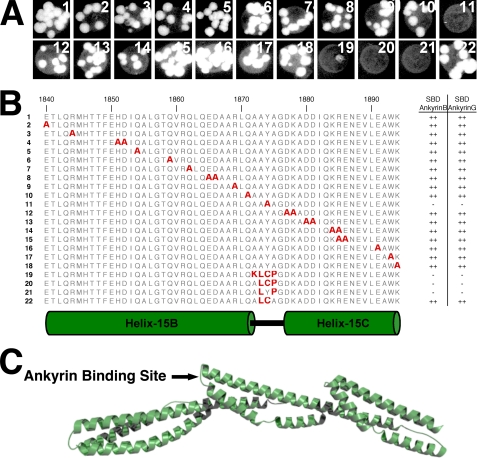
Tyr1874 of loop-15BC is required for ankyrin binding to repeats 14–16 of β2-spectrin. β2-Spectrin variants with alanine mutations at 23 surface-exposed residues from helix-15B, loop-15BC, and helix-15C were screened for the ability to bind to the spectrin-binding domain (SBD) of ankyrin-B and ankyrin-G by yeast two-hybrid analyses. Shown in A is growth of yeast strains expressing the indicated spectrin variants fused to the Gal4 activation domain and the ankyrin-B spectrin-binding domain fused to the Gal4 DNA-binding domain on plates deficient for adenosine, histidine, leucine, and tryptophan. Similar results were observed in yeast strains expressing the spectrin variants together with the ankyrin-G spectrin-binding domain (yeast growth not shown). The location of individual mutations relative to secondary structure elements is shown in B along-side relative growth ability as indicated by ++ for strong growth and - for no growth. The location of the proposed ankyrin-binding site is indicated on the B14–16 structure in C.
FIGURE 4.
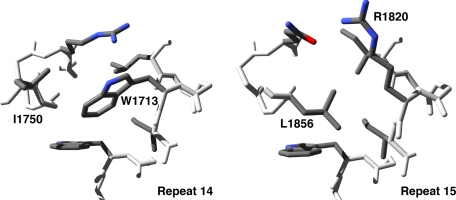
Repeat 15 has a unique hydrophobic core near Arg1820. Most spectrin repeats have a characteristic tryptophan at position 17 of the A-helix that forms part of the hydrophobic core as exemplified in repeat 14 by Trp1713 (A). In repeat 15, Arg1820 resides at position 17, and the hydrophobic core is centered on Leu1856 from helix-15B (B).
FIGURE 5.
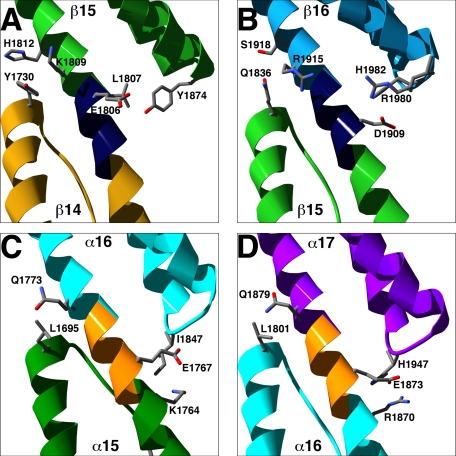
Inter-repeat contacts control orientation between repeats. Shown are ribbon representations of the inter-repeat junctions between repeats 14/15 (A) and repeats 15/16 (B) of β2-spectrin and the inter-repeat junctions between repeats 15/16 (C) and repeats 16/17 (D) of α2-spectrin. Linkers are colored dark blue in β2-spectrin and orange in α2-spectrin. Terminal residues of the inter-repeat contacts are labeled and shown as sticks.
RESULTS
Structure Determination and Description—A recombinant protein containing spectrin repeats 14–16 (Fig. 1) of human β2-spectrin (B14–16) was purified from E. coli and crystallized. The crystal structure was solved by the single-wavelength anomalous dispersion method using SeMet-substituted B14–16 protein crystals. The Se-Met crystals diffracted to 2.4 Å, and single-wavelength anomalous dispersion phasing of these data generated an electron density map that was used to produce an initial model. Native crystals diffracted to 1.95 Å, and phases were extended to this limit using the 2.4 Å structure as an initial model. The asymmetric unit contained two molecules of B14–16, 561 waters, one molecule of Tris, four molecules of glycerol and one magnesium ion. The final model had an Rwork of 19.8% and an Rfree of 25.5% at 1.95 Å resolution. Statistics for the data collection and representative electron density are shown in Table 1 and Fig. 2.
The structure of B14–16 consists of three spectrin repeats connected by two linkers (Fig. 3). Each spectrin repeat forms an α-helical bundle consisting of three helices (A, B, and C) and two loops (AB and BC). For the purposes of this study, the helices and loops have been designated by repeat number and structural element (e.g. helix-14A refers to the A-helix of repeat 14, loop-15BC refers to the BC-loop of repeat 15). All helices show typical helical hydrogen bond networks with the exception of helix-15B and helix-16B. These helices are kinked near their midpoints and have ordered waters that form hydrogen bonds with backbone amide and carbonyl oxygens at the kink. The linkers between repeats are helical and are contiguous with the C-helix of the preceding repeats and the A-helix of the succeeding repeats. The overall structure of B14–16 is elongated with axial dimensions of 156 × 45 Å.
Each helix has a heptad pattern of hydrophobic residues that is typical of α-helical coiled-coils (19). These hydrophobic residues form an interlocking network of van der Waals contacts at the core of each spectrin repeat. As part of this heptad pattern, spectrin repeats commonly have tryptophan at position 17 in the A-helix and leucine near the end of the C-helix (20). Repeats 14 and 16 have both conserved residues; however, repeat 15 has an arginine (Arg1820) in place of the tryptophan at position 17. Arg1820 is not part of the hydrophobic core: instead, the side chain of Leu1856 of helix-15B lies in the space normally occupied by tryptophans at position 17 (Fig. 4). Leu1856 can occupy space in the core because the B-helix of repeat 15 is rotated ∼45° relative to the B-helices of repeats 14 and 16. This rotation points the side chain of Leu1856 toward the core, unlike the corresponding residues in repeats 14 and 16, which are oriented along the edge of the core.
Between individual repeats are helical linkers that join the C-helices of preceding repeats with the A-helices of succeeding repeats into one long continuous helix. In the B14–16 structure, van der Waals contacts between repeats dictate the orientation of neighboring repeats about the helical linker. At the N-terminal end of the interface, residues in the linker contact residues in the BC-loop of the succeeding repeat, whereas at the C-terminal end of the interface, residues of the AB-loop of the preceding repeat contact residues from two turns of the A-helix of the succeeding repeat (Fig. 5, A and B). Comparison of the inter-repeat contacts of B14–16 with the previously described contacts between repeats 15–17 of α2-spectrin (21) suggests that differences in the inter-repeat contacts contribute to the curvature differences in the two structures. In contrast to the B14–16 inter-repeat contacts, the interfaces in the α2-spectrin structure have less extensive contacts between the AB-loop and the A-helix and have additional contacts between the C-helix and the BC-loop (Fig. 5, C and D). These differences specify a different orientation between repeats in the α2- and β2-spectrin structures. In repeats 15–17 of α2-spectrin, the inter-repeat contacts specify a nearly straight concatomer of repeats, whereas in repeats 14–16 of β2-spectrin, the contacts specify a substantial bend (Fig. 3). Consistent with the observed differences in curvature, electron micrographs of purified spectrin tetramers frequently show greater curvature near their midpoints (22, 23), where repeats 14–16 of β-spectrins reside (Fig. 1).
Ankyrin-binding Site—Kennedy et al. (7) used deletion analysis to localize the ankyrin-binding site to repeat 15 and suggested, based upon sequence comparisons, that helix-15B played a role in ankyrin binding. We used yeast two-hybrid analyses to test whether exposed residues of helix-15B are required for ankyrin binding. Alanine mutations were made at 11 surface-exposed residues of helix-15B and tested for their effect on ankyrin binding using the spectrin-binding domains of ankyrin-B and ankyrin-G. None of the 11 mutations prevented the yeast two-hybrid reaction between repeats 13–16 of β2-spectrin and the spectrin-binding domain of either ankyrin-B or ankyrin-G (Fig. 6), suggesting that helix-15B does not play a significant role in ankyrin binding.
We next examined helix-15C because the point mutation A1884V in helix-15C of β1-spectrin is associated with hereditary spherocytosis (24). Ten surface-exposed residues of helix-15C were mutated to alanine and tested for ankyrin-binding ability using yeast two-hybrid analyses. As with mutations on helix-15B, none of the mutations in helix-15C prevented ankyrin binding (Fig. 6). Ala1884 of β1-spectrin corresponds to Ala1892 of β2-spectrin, which resides in a hydrophobic pocket formed by Phe1818, Ile1821, and Gln1822. This pocket does not have sufficient space to accommodate a valine, and the A1884V mutation in β1-spectrin may disrupt the structure of repeat 15.
Finally, we tested whether loop-15BC plays a role in ankyrin binding. Loop-15BC consists of the sequence AAYA1875, and in contrast to the mutations in the B- and C-helices, mutation of the surface-exposed Tyr1874 to alanine abrogated the yeast two-hybrid interaction with ankyrin (Fig. 6). The AAYA sequence is well conserved between different β-spectrins with the exception of β5-spectrin. β5-Spectrin does not bind to ankyrins (25), and replacement of the BC-loop sequence of β2-spectrin (AAYA1875) with the corresponding residues of spectrin repeat 15 of β5-spectrin (KLCP1907) also abrogated ankyrin binding (Fig. 6). Together, these observations show that ankyrins bind to loop-15BC of β-spectrins.
DISCUSSION
The spectrin-ankyrin linkage provides the principal mechanism by which the mechanical properties of spectrin are coupled to membranes. Ankyrin binds to spectrin repeat 15 of β-spectrins, and sequence comparisons suggested that repeat 15 has unique features that confer ankyrin binding (7). The crystal structure of B14–16 shows that the sequence differences in repeat 15 produce only minor structural differences (Fig. 3). The most pronounced structural difference is a small rotation in helix-15B, which is associated with a unique feature in the hydrophobic core in which Leu1856 of the B-helix fills space normally occupied in other spectrin repeats by tryptophans from the A-helix (Fig. 4). Consequences of the rotation in helix-15B partially explain the reported sequence differences of repeat 15 (7) but do not explain why ankyrin binds specifically to repeat 15 because alanine mutagenesis experiments suggest that helix-15B does not play a major role in ankyrin binding (Fig. 6).
Further mutagenesis experiments also failed to detect a role for helix-15C; however, substitution of alanine at Tyr1874 in loop-15BC did impair ankyrin binding (Fig. 6). Loop-15BC consists of the sequence AAYA1875, which forms a relatively flat, uncharged surface (Fig. 2) that has the potential to form van der Waals and hydrogen bond contacts. These types of contacts are strengthened by moderate ionic strength, and the ankyrin-dependent interaction of spectrin with membranes is most stable at moderate ionic strength (23, 26), consistent with a role for loop-15BC in ankyrin binding. Interestingly, the AAYA1875 → ALCA variant also supported ankyrin binding, and modeling of the ALCA variant suggests that the ALCA sequence forms a flat uncharged surface similar to the AAYA sequence (data not shown). By contrast, the A1875P mutation likely disrupts the structure of loop-15BC because Ala1875 does not have phi-psi angles compatible with proline.
A key question regarding the spectrin-ankyrin interaction is how ankyrin maintains its interaction with spectrin during extension of spectrin under mechanical load. Spectrin repeats undergo reversible forced unfolding as spectrin tetramers are stretched in response to shear stresses impingent on the plasma membrane (5, 6). Despite this unfolding, the attachment of spectrin to membranes remains intact over a broad range of shear stresses (27, 28), suggesting that the ankyrin linkage to repeat 15 of β-spectrin is maintained even while spectrin repeats are unfolding in response to extension force. Our data, together with previous molecular dynamics simulations of forced unfolding of spectrin repeats, suggest a possible mechanism for how ankyrins maintain their spectrin association. Forced unfolding using atomic force microscopy shows that spectrin repeats unfold through a series of stable intermediates as the ends of spectrin repeats are pulled apart (29). Molecular dynamics simulations of these extensions suggest that a segment centered on the BC-loop is remarkably well preserved in these intermediates (29). The ability of ankyrin to bind to loop-15BC may thus allow ankyrin to maintain its interaction with spectrin even when repeat 15 exists in non-native conformations.
Acknowledgments
We thank Jan Hoffman for assistance with the yeast two-hybrid experiments and Brenda Pallares for administrative support.
The atomic coordinates and structure factors (code 3EDV) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
This work was supported, in whole or in part, by National Institutes of Health Grant HL085218 from NHLBI. The structure shown in this study was derived from work performed on beamline 19-ID at the Argonne National Laboratory Structural Biology Center at the Advanced Photon Source, which is operated by UChicago Argonne, LLC, for the United States Department of Energy, Office of Biological and Environmental Research, under Contract DE-AC02-06CH11357. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviation used is: SeMet, selenomethionine.
References
- 1.Mohandas, N., Chasis, J. A., and Shohet, S. B. (1983) Semin. Hematol. 20 225-242 [PubMed] [Google Scholar]
- 2.Bennett, V., and Baines, A. J. (2001) Physiol. Rev. 81 1353-1392 [DOI] [PubMed] [Google Scholar]
- 3.Mohler, P. J., and Bennett, V. (2005) Front. Biosci. 10 2832-2840 [DOI] [PubMed] [Google Scholar]
- 4.Bennett, V., and Healy, J. (2008) Trends Mol. Med. 14 28-36 [DOI] [PubMed] [Google Scholar]
- 5.Rief, M., Pascual, J., Saraste, M., and Gaub, H. E. (1999) J. Mol. Biol. 286 553-561 [DOI] [PubMed] [Google Scholar]
- 6.Johnson, C. P., Tang, H. Y., Carag, C., Speicher, D. W., and Discher, D. E. (2007) Science 317 663-666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kennedy, S. P., Warren, S. L., Forget, B. G., and Morrow, J. S. (1991) J. Cell Biol. 115 267-277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubreuil, R. R. (2006) J. Membr. Biol. 211 151-161 [DOI] [PubMed] [Google Scholar]
- 9.Minor, W., Cymborowski, M., Otwinowski, Z., and Chruszcz, M. (2006) Acta Crystallogr. Sect. D Biol. Crystallogr. 62 859-866 [DOI] [PubMed] [Google Scholar]
- 10.Schneider, T. R., and Sheldrick, G. M. (2002) Acta Crystallogr. Sect. D Biol. Crystallogr 58 1772-1779 [DOI] [PubMed] [Google Scholar]
- 11.Otwinowski, Z. (1991) in Isomorphous Replacement and Anomalous Scattering (Wolf, W., Evans, P. R., and Leslie, A. G. W., eds) pp. 80-86, Science & Engineering Research Council, Cambridge
- 12.Cowtan, K., and Main, P. (1998) Acta Crystallogr. Sect. D Biol. Crystallogr 54 487-493 [DOI] [PubMed] [Google Scholar]
- 13.Morris, R. J., Zwart, P. H., Cohen, S., Fernandez, F. J., Kakaris, M., Kirillova, O., Vonrhein, C., Perrakis, A., and Lamzin, V. S. (2004) J. Synchrotron Radiat. 11 56-59 [DOI] [PubMed] [Google Scholar]
- 14.Jones, T. A., Zou, J. Y., Cowan, S. W., and Kjeldgaard, M. (1991) Acta Crystallogr. Sect. A 47 110-119 [DOI] [PubMed] [Google Scholar]
- 15.Emsley, P., and Cowtan, K. (2004) Acta Crystallogr. Sect. D Biol. Crystallogr. 60 2126-2132 [DOI] [PubMed] [Google Scholar]
- 16.Murshudov, G. N., Vagin, A. A., and Dodson, E. J. (1997) Acta Crystallogr. Sect. D Biol. Crystallogr. 53 240-255 [DOI] [PubMed] [Google Scholar]
- 17.Davis, I. W., Leaver-Fay, A., Chen, V. B., Block, J. N., Kapral, G. J., Wang, X., Murray, L. W., Arendall, W. B., III, Snoeyink, J., Richardson, J. S., and Richardson, D. C. (2007) Nucleic Acids Res. 35 W375-W383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guex, N., and Peitsch, M. C. (1997) Electrophoresis 18 2714-2723 [DOI] [PubMed] [Google Scholar]
- 19.Beck, K., and Brodsky, B. (1998) J. Struct. Biol. 122 17-29 [DOI] [PubMed] [Google Scholar]
- 20.Hartwig, J. H. (1995) Protein Profile 2 703-800 [PubMed] [Google Scholar]
- 21.Kusunoki, H., Minasov, G., Macdonald, R. I., and Mondragon, A. (2004) J. Mol. Biol. 344 495-511 [DOI] [PubMed] [Google Scholar]
- 22.Shotton, D. M., Burke, B. E., and Branton, D. (1979) J. Mol. Biol. 131 303-329 [DOI] [PubMed] [Google Scholar]
- 23.Bennett, V., Davis, J., and Fowler, W. E. (1982) Nature 299 126-131 [DOI] [PubMed] [Google Scholar]
- 24.Gallagher, P. G., and Forget, B. G. (1998) Blood Cells Mol. Dis. 24 539-543 [DOI] [PubMed] [Google Scholar]
- 25.Stabach, P. R., and Morrow, J. S. (2000) J. Biol. Chem. 275 21385-21395 [DOI] [PubMed] [Google Scholar]
- 26.Burridge, K., Kelly, T., and Mangeat, P. (1982) J. Cell Biol. 95 478-486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Discher, D. E., Mohandas, N., and Evans, E. A. (1994) Science 266 1032-1035 [DOI] [PubMed] [Google Scholar]
- 28.Mohandas, N., and Evans, E. (1994) Annu. Rev. Biophys. Biomol. Struct. 23 787-818 [DOI] [PubMed] [Google Scholar]
- 29.Altmann, S. M., Grunberg, R. G., Lenne, P. F., Ylanne, J., Raae, A., Herbert, K., Saraste, M., Nilges, M., and Horber, J. K. (2002) Structure (Lond.) 10 1085-1096 [DOI] [PubMed] [Google Scholar]