Abstract
Zinc is an essential cofactor required for the function of ∼8% of the yeast and 10% of the human proteome. All of the “small Tim” proteins of the mitochondrial intermembrane space contain a strictly conserved “twin CX3C” zinc finger motif, which can bind zinc ions in the Cys-reduced form. We have shown previously that although disulfide bond formation is essential for the function of these proteins in mitochondria, only reduced proteins can be imported into mitochondria (Lu, H., Allen, S., Wardleworth, L., Savory, P., and Tokatlidis, K. (2004) J. Biol. Chem. 279, 18952–18958 and Morgan, B., and Lu, H. (2008) Biochem. J. 411, 115–122). However, the role of zinc during the import of these proteins is unclear. This study shows that the function of zinc is complex. It can play a thiol stabilizer role preventing oxidative folding of the small Tim proteins and maintaining the proteins in an import-competent form. On the other hand, zinc-bound forms cannot be imported into mitochondria efficiently. Furthermore, our results show that zinc is a powerful inhibitor of Erv1, an essential component of the import pathway used by the small Tim proteins. We propose that zinc plays a chaperone-like role in the cytosol during biogenesis of the small Tim proteins and that the proteins are imported into mitochondria through the apo-forms.
Zinc is an essential element required for the growth and metabolism of eukaryotic cells; it plays important structural and regulatory roles in numerous zinc finger proteins (1, 2). Whereas the total cellular zinc concentration is estimated to be 0.1–0.5 mm (2), a wide range of free or labile Zn2+ concentrations (10-5–10-12) has been reported in eukaryotic cells (3–6). The majority of cellular Zn2+ is bound to proteins with widely varying binding affinities. Cysteine thiol is one of the most prominent groups for zinc binding (7). Growing evidence suggests that the switch between zinc binding and disulfide bond formation plays a key role during the function of many proteins, such as the chaperone activity of Hsp33 (8–10), the regulation of anti-σ factor RsrA (9), and the biogenesis of the mitochondrial “small Tim” proteins (11–13).
The small Tim proteins of the Saccharomyces cerevisiae yeast mitochondrial intermembrane space (IMS)2 play an essential role during the import of mitochondrial membrane proteins (14, 15), and they are themselves imported through the redox-regulated Mia40/Erv1 pathway (16, 17). All small Tim proteins contain a strictly conserved “twin CX3C” zinc finger motif, which can bind zinc in the Cys-reduced form at a molar ratio of 1:1 with an observable conformational change (18). Tim9 and Tim10, the two most abundant small Tim proteins in yeast, form a hexameric Tim9-Tim10 complex for their function in the IMS. Whereas disulfide bond formation between the four Cys residues of the zinc finger motif is essential for the complex formation, oxidized proteins cannot be imported into mitochondria; only reduced proteins can (19, 20). However, whether or how zinc binding affects the mitochondrial import of the small Tim proteins is unknown. Because zinc binding and release are highly dynamic, it is difficult to distinguish between the import of apo- and zinc-bound forms. On the other hand, the apoproteins can be oxidized by GSSG under the cytosolic redox conditions, and the oxidative folding of both Tim9 and Tim10 competes directly with their mitochondrial import (11, 20). Thus, Zn2+ may play an important role during the biogenesis of small Tim proteins. In particular, how zinc binding influences the import of the zinc finger small Tim proteins into mitochondria needs to be addressed.
In this study, the effects of zinc on the oxidative folding and mitochondrial import of Tim9 and Tim10 were investigated. We show that zinc binding can stabilize both proteins from oxidative folding. Using mitochondrial import coupled with a buffered zinc system, our results show that whereas the precursor Tim9 was stabilized in the reduced form, the level of mitochondrial import was decreased with the increase of free zinc concentration. Furthermore, an oxygen consumption assay showed that zinc can inhibit the oxidase activity of Erv1, one of the essential components of the mitochondrial import and assembly pathway used by the small Tim proteins. A model is proposed for the function of zinc during biogenesis of the zinc finger small Tim proteins. Our studies reveal a new function for zinc acting as a chemical chaperone in the cytosol.
EXPERIMENTAL PROCEDURES
Materials—Tris(2-carboxyethyl)phosphine (TCEP) and 4-acetamido-4′-maleimidylstilbene-2,2′-disulfonic acid (AMS) were obtained from Molecular Probes (Invitrogen). EDTA was from BDH, and all other chemicals were obtained from Sigma at the highest grade.
Protein Preparations—Oxidized Tim9, Tim9(F43W), and Tim10 were purified as described previously in buffer A (50 mm Tris, pH 7.4, 150 mm NaCl) (11, 21). Reduced proteins were always prepared fresh immediately before use typically by incubation of oxidized proteins with 1–2 mm TCEP for 60–90 min at room temperature, followed by gel filtration using a Superdex 75 column to remove TCEP. Erv1-His6 was expressed in Escherichia coli Rosetta-gami™ 2 (Novagen) and purified using His-tagged affinity beads as described for Erv2 (22). Then, Erv1 was dialyzed against buffer A and supplemented with 50 μm FAD before being stored at -80 °C. The protein was further purified, and free FAD was removed using a Superdex 75 gel filtration column before use.
AMS Assays—Oxidation of purified reduced Tim9 or Tim10 was initiated by the addition of GSSG. At various time points, aliquots were removed from the folding reaction and added to nonreducing gel sample buffer containing 10 mm AMS for 30 min in the dark at room temperature. AMS interacts specifically with free thiols of the reduced protein but not the disulfide-bonded oxidized protein. Each bound AMS molecule increases the molecular mass of the protein by ∼500 Da, thus allowing efficient separation of different protein redox states by SDS-PAGE.
Fluorescence—Fluorescence measurements were acquired using a Cary Eclipse fluorescence spectrophotometer (Varian Ltd.). Tim9(F43W) and Tim10 oxidative folding was measured by recording fluorescence emission at 350 and 303 nm using excitation wavelengths of 280 and 275 nm, respectively. Slit widths for both excitation and emission were 5 nm. All experiments were performed at 25 °C.
Circular Dichroism—Analysis was performed using a JASCO J810 spectropolarimeter with a 1-mm path length quartz cuvette. Each spectrum represents an average of four scans from 200 to 260 nm at 0.2-nm intervals with the spectra for buffer alone (buffer A) subtracted.
Oxygen Consumption Assay—Oxygen consumption of Erv1 was measured at 25 °C using 2.5 mm TCEP as substrate. For all experiments, 1 μm Erv1 in buffer A, in the presence or absence of zinc was pre-equilibrated at 25 °C, followed by the addition of 2.5 mm TCEP to initiate the reaction. A Clarke-type oxygen electrode (Hansatech Instrument Ltd.) was used.
Mitochondrial Isolation—The wild type S. cerevisiae yeast D273-10B strain (MATα) was grown for 16 h at 30 °C, and the tim9ts yeast strain was grown for 30 h at 24 °C, followed by 8 h at 37 °C. Mitochondria were isolated from yeast cells as described previously (20, 23).
Import Assays—Import reactions consisted of 0.6 m sorbitol, 50 mm KCl, 0.75 mg/ml l-methionine, 1 mg/ml fatty acid-free bovine serum albumin, 50 mm Hepes-KOH, pH 7.4, 2 mm EGTA, 0.5 mg/ml mitochondria. ZnCl2 was added from 0 to 2 mm where indicated. Free Zn2+ concentrations were calculated using WEBMAXC as described previously (24). The TnT SP6-coupled transcription/translation kit (Promega) was used to synthesize 35S-labeled proteins. Typically, lysate was added to the import reaction at a final concentration of 3.3% (v/v). Import was always performed at 25 °C, and import time varied depending upon the particular experiment. Import was stopped by the addition of 5 volumes of ice-cold 0.6 m sorbitol, 20 mm Hepes, pH 7.4, containing 50 μg/ml trypsin for 20 min on ice, followed by the addition of soybean trypsin inhibitor to a final concentration of 1 mg/ml for 10 min on ice. Further post-import treatments were performed as described in the figure legends. Mitochondria were reisolated by centrifugation, followed by resuspension in gel sample buffer for Tris/Tricine/SDS-PAGE analysis and visualization by autoradiography. Import level was quantified by two-dimensional densitometry using an Aida image analyzer (Version 4.00).
For analysis of the redox states of unimported material, aliquots were removed from the import reaction, and the mitochondria were removed by centrifugation. The supernatant was added to gel sample buffer containing 10 mm AMS and incubated in the dark at room temperature for 30 min. Samples were analyzed by nonreducing Tris/Tricine/SDS-PAGE and autoradiography.
RESULTS
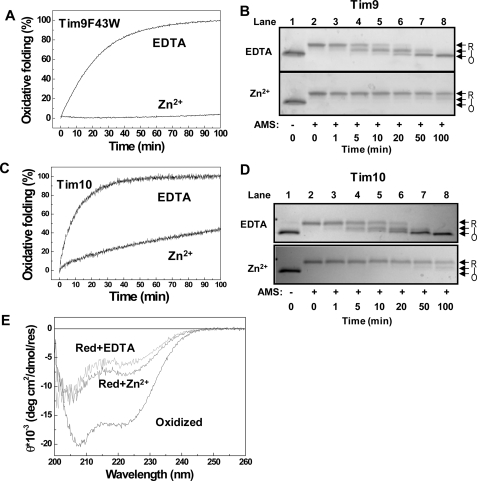
Zn2+ Binding Inhibits Oxidative Folding of Small Tim Proteins—We have previously shown that oxidized Tim9 and Tim10 are thermodynamically stable under the cytosolic glutathione conditions and that disulfide bond formation competes directly with import of both proteins (11, 20). To explore the role of zinc during the biogenesis of these proteins, we first studied the effects of zinc on the oxidative folding of Tim9 and Tim10 using purified proteins. Based on the fluorescence intensity difference between oxidized and reduced states (11, 20), oxidative folding of the reduced proteins initiated by the addition of GSSG was measured in the presence and absence of Zn2+ (Fig. 1, A and C). Because wild type Tim9 has no detectable fluorescence, the Tim9(F43W) mutant was used in this study. The results show that the apoproteins, both Tim9(F43W) and wild type Tim10, can be oxidized much faster than the proteins in the presence of zinc. Next, disulfide bond formation was confirmed by SDS-PAGE-based AMS thiol modification assay (Fig. 1, B and D). In the presence of EDTA, the reduced apoproteins became oxidized quickly to a partially oxidized intermediate and then fully oxidized state in a similar time scale as that measured by fluorescence. In the presence of Zn2+, both proteins remained reduced over the same time course. Thus, both fluorescence and AMS methods show that Zn2+ binding can inhibit oxidative folding of the small Tim proteins. Furthermore, a detectable conformational change (folding) induced by zinc binding was shown by circular dichroism, but the degree of folding was small compared with that induced by disulfide bond formation for Tim9 (Fig. 1E) and Tim10 (24). In summary, zinc binding stabilizes both Tim9 and Tim10 from oxidative folding and induces an observable conformational change in the reduced proteins.
FIGURE 1.
Zn2+ binding inhibits oxidative folding of the small Tim proteins. A, time course of oxidative folding of Tim9(F43W), followed by Trp fluorescence change at 350 nm. Oxidative folding of the protein was initiated by the addition of 1 mm GSSG in the presence of either 1 mm EDTA or 40 μm ZnCl2. B, oxidative folding of wild type Tim9 analyzed by AMS assay. Lane 1 is the untreated reduced Tim9. Lanes 2–8 are the AMS-treated samples after oxidative folding for the times indicated. The fully reduced (R), intermediate (I), and oxidized (O) states are indicated. C and D, oxidative folding of Tim10 measured by Tyr fluorescence intensity change at 303 nm and AMS assay, respectively. E, CD spectra of 10 μm Tim9 in buffer A before (oxidized) and after incubation with 1 mm TCEP in the presence of 1 mm EDTA or 40 μm ZnCl2. Red, reduced; deg, degrees; res, residues.
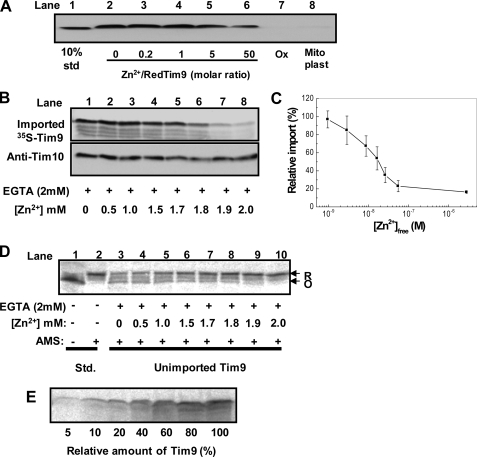
Effect of Zn2+ on Mitochondrial Import of Small Tim Proteins—First, import of purified Tim9 under various conditions was carried out using mitochondria isolated from a tim9ts yeast strain that has no detectable endogenous Tim9 and Tim10 (25). After import for 30 min, trypsin was added to degrade any unimported and mitochondria-associated precursor proteins. Furthermore, the efficiency of trypsin treatment was confirmed by mitoplasting (rupture of the mitochondrial outer membrane by osmotic swelling) performed before trypsin treatment (Fig. 2A, lane 8). Two results are shown in Fig. 2A. (i) The oxidized protein could not be imported into mitochondria, whereas reduced Tim9 could be imported in both the absence and presence of Zn2+. (ii) The apparent import level was decreased with the increase of Zn2+ concentration. Thus, it was not clear whether zinc-bound protein (ZnTim9) can be imported directly into mitochondria because apoTim9 and ZnTim9 were in equilibrium dynamically. The observed Tim9 imported in the presence of Zn2+ might still be imported via the apo-form.
FIGURE 2.
Zn2+ binding inhibits the apparent mitochondrial import of small Tim proteins. A, Western blotting of purified Tim9 (∼10 μm) imported into tim9ts mitochondria. Reduced Tim9 in the absence (lanes 2 and 8) or presence (lanes 3–6) of ZnCl2 at various molar ratios and oxidized (Ox) Tim9 (lane 7) were imported at 25 °C for 30 min, followed by trypsin treatment. As a control, mitoplasting was performed before trypsin treatment for Tim9 imported in the absence of zinc (lane 8). Lane 1, standard (std). B, Zn2+ concentration dependence of 35S-labeled Tim9 import into wild type mitochondria. 35S-Labeled Tim9 was imported in the presence of 2 mm EGTA and 0–2 mm ZnCl2 for 30 min at 25 °C. Import was analyzed by SDS-PAGE and autoradiography. The gel was also probed with anti-Tim10 antibodies as a loading control. C, quantified import level in B plotted against [Zn2+]free. Import in the absence of Zn2+ was set as 100%. Error bars represent S.E. (n = 3). D, AMS assay of the unimported Tim9 of the experiment in B. Reduced (R) and oxidized (O) states are indicated. E, concentration dependence of Tim9 on its mitochondrial import, with 3.3% (v/v) of 35S-labeled Tim9 added as 100%. Import was analyzed by SDS-PAGE and autoradiography.
To understand whether ZnTim9 can be imported efficiently, conditions at which most (if not all) proteins can be stably maintained in the zinc-bound form are required. To this end, we used a buffered zinc system (2 mm EGTA, 0–2 mm ZnCl2) to supply a sufficient amount of Zn2+ and at the same time control the concentration of free Zn2+ at μm or lower. 35S-Labeled Tim9 synthesized in rabbit reticulocyte lysate was imported into wild type mitochondria under various zinc concentrations (Fig. 2, B and C), and the redox state of the unimported Tim9 was analyzed by AMS assay in parallel (Fig. 2D) as described previously (20). As expected, the amount of Tim9 maintained in a reduced state increased with increasing Zn2+ concentration. However, zinc had an opposite effect on the import level, which decreased with the increase of free zinc concentration. To confirm that Zn2+ does not affect the integrity of the outer membrane and the susceptibility of small Tim proteins to trypsin digestion, Western blotting of endogenous Tim10 was performed. As expected, the same level of Tim10 at the full length was observed (Fig. 2B). Thus, the efficiency of Tim9 import decreased with an increase in the amount of ZnTim9 or a decrease of apoTim9. The result is consistent with the finding that the import of apoTim9 is concentration-dependent (Fig. 2E). Furthermore, a similar zinc concentration-dependent result was obtained for Tim10 (data not shown). Thus, our results suggest that zinc-bound small Tim proteins cannot be effectively imported into mitochondria.
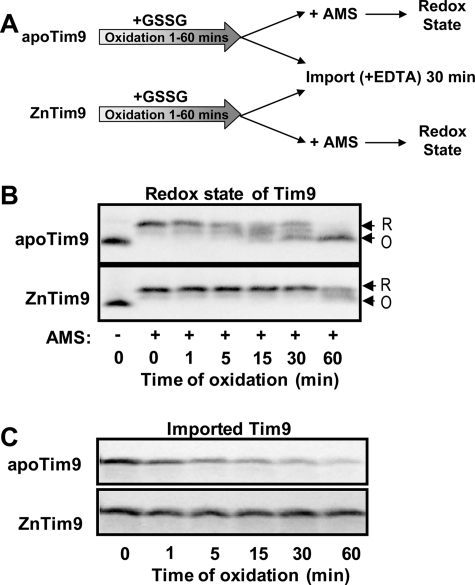
Zinc Affects Import of Small Tim Proteins Reversibly—Next, we tested whether the effect of zinc on Tim9 import is a reversible process by performing a sequential folding import experiment (Fig. 3A), in which oxidative folding of apoTim9 and ZnTim9 initiated by the addition of GSSG was followed by mitochondrial import in the presence of 5 mm EDTA. 35S-Labeled Tim9 synthesized in rabbit reticulocyte lysate was ammonium sulfate-precipitated and resuspended in buffer containing 10 mm GSH and either EGTA (for apoTim9) or buffered zinc (2 mm EGTA, 2 mm ZnCl2 for ZnTim9). Folding was initiated by the addition of 10 μm GSSG, and at the times indicated (Fig. 3, B and C), aliquots were removed and used for mitochondrial import in the presence of 5 mm EDTA (a stronger Zn2+ chelator than EGTA). Both the redox state (Fig. 3B) and mitochondrial import (Fig. 3C) of Tim9 were analyzed. In the absence of Zn2+, apoTim9 was oxidized by GSSG under cytosolic redox conditions with oxidized Tim9 observed after ∼5 min, and the oxidative folding resulted in a decreased level of mitochondrial import. On the other hand, ZnTim9 was maintained in the reduced form, which was imported into mitochondria (in the presence of EDTA) with the same efficiency as the reduced protein at time 0 through the same folding time course (Fig. 3C). Thus, zinc binding can stabilize the protein in a reduced form, and ZnTim9 can be effectively converted to the import-competent form.
FIGURE 3.
Zn2+ binding stabilizes Tim9 in the reduced form, and the effect on import is reversible. A, schematic diagram of the sequential oxidative folding and import experiment. Oxidative folding of reduced apoTim9 (2 mm EGTA) and ZnTim9 (2 mm EGTA, 2 mm ZnCl2) was initiated by the addition of 10 μm GSSG. After various times of oxidative folding, aliquots from both folding reactions were removed and subjected to analysis by AMS assay and mitochondrial import in the presence of 5 mm EDTA. B, time course of the redox state change of apo- and zinc-bound 35S-labeled Tim9 during the folding reactions. The reduced (R) and oxidized (O) states are indicated. C, analysis of the import competence of proteins removed from the folding reactions at the times indicated. Proteins were added to mitochondria immediately following removal from the folding reactions and imported in the presence of 5 mm EDTA for 30 min at 25 °C in all cases.
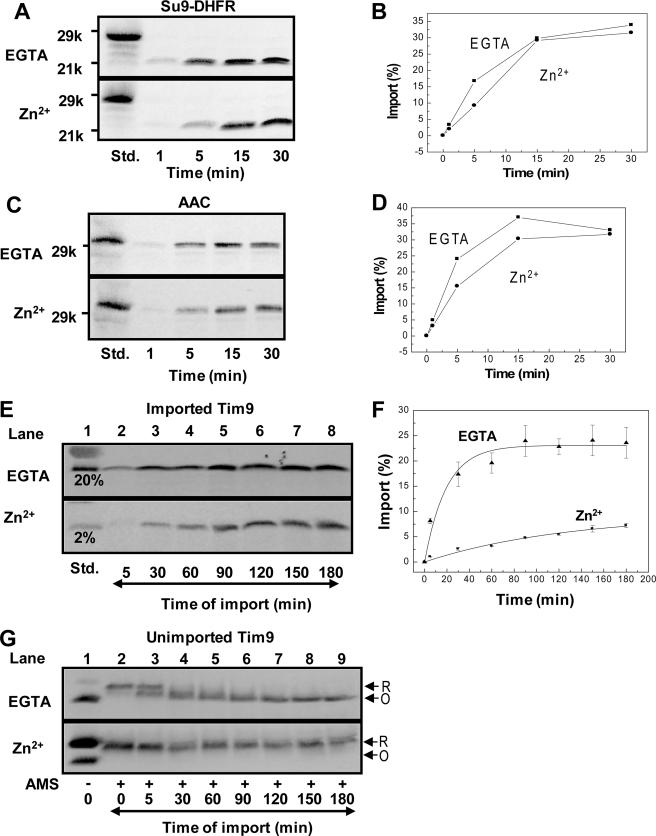
Zinc Ions Inhibit Import of Small Tim Proteins but Not the TOM or TIM Translocase Complexes—One possibility for the inhibitory effect of Zn2+ on the observed import of Tim9 and Tim10 is that zinc inhibits part of the general mitochondrial import machinery. Thus, the effects of Zn2+ on the import of the matrix-localized protein Su9-DHFR (a hybrid protein containing the presequence of subunit 9 of the mitochondrial ATP synthase coupled to dihydrofolate reductase) and the inner membrane-localized protein ADP/ATP carrier (AAC) were examined. Both precursors enter mitochondria through the TOM40 (translocase of the outer membrane) complex, after which they follow distinct import pathways of TIM23 (translocase of the inner membrane) and TIM22, respectively. Time courses of import of 35S-labeled Su9-DHFR (Fig. 4, A and B) and 35S-labeled AAC (Fig. 4, C and D) were performed in the absence or presence of Zn2+. The results show that Zn2+ had no obvious effect on the import of either protein and thus no effect on the function of the TOM40, TIM22, and TIM23 translocase. In contrast, both the rate and the level of Tim9 import were significantly decreased by the addition of the same concentration of Zn2+ (Fig. 4, E and F), although proteins were maintained in a reduced state by zinc ions (Fig. 4G). The apparent initial rate of import in the presence of zinc was ∼8-fold slower than that in the absence of zinc. Thus, the presence of zinc selectively inhibits the import of the small Tim proteins, not the TOM and TIM translocase complexes. We anticipate that the inhibition of Tim9 import may be a result of inhibition of the mitochondrial import and assembly pathway by zinc, and/or ZnTim9 cannot be imported directly into mitochondria.
FIGURE 4.
Zinc does not inhibit the mitochondrial import of Su9-DHFR and AAC. A, time course of import of 35S-labeled Su9-DHFR in the absence (EGTA) or presence of buffered Zn2+. B, quantification of import levels in A based on a standard (Std.), which represents 40% of the material in the import reaction. C and D, time course of import of 35S-labeled AAC as described for Su9-DHFR. E and F, time course of import of apoTim9 (EGTA) or ZnTim9 (Zn2+). Import level was determined based on a standard, which represents 20 and 2% of the material in the apoTim9 and ZnTim9 reactions, respectively. Errors bars represent S.E. (n = 3). G, AMS assay of the unimported Tim9 from the same import assay in E. All import assays were analyzed by SDS-PAGE and autoradiography.
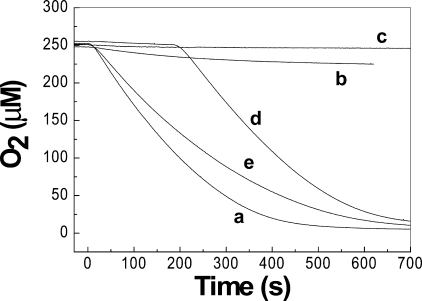
Zinc Inhibits Oxidase Activity of Erv1—To determine whether zinc ions can inhibit the import pathway used by the small Tim proteins, the oxidase activity of Erv1, an essential component of the Mia40/Erv1 import machinery, was studied in the presence and absence of zinc ions. For this purpose, TCEP was used as the electron donor because TCEP is a strong reductant and not a chelator of zinc, unlike dithiothreitol (26). Time courses of oxygen consumption showed that Erv1 catalyzed the reaction most effectively in the presence of EDTA, with a turnover number of ∼1.0 μm/s (Fig. 5, curve a). However, Erv1 activity was strongly impaired by the addition of 1 μm Zn2+ (Fig. 5, curve b), which gave a molar ratio of Erv1 to zinc of 1:1. Furthermore, Erv1 activity was completely inhibited by the presence of 10 μm Zn2+ (Fig. 5, curve c), and the activity could be recovered by the addition of EDTA (Fig. 5, curve d). Interestingly, even in the absence of added zinc and EDTA, Erv1 activity was clearly lower than in the presence of EDTA (Fig. 5, compare curves e and a); only ∼80% activity was obtained in the absence of added zinc and EDTA compared with in the presence of 1 mm EDTA. Taken together, these results show that zinc has a high affinity for Erv1 and can inhibit the oxidase activity of Erv1 and hence the Mia40/Erv1 import pathway.
FIGURE 5.
Zinc ions inhibit the oxidase activity of Erv1. Time courses of oxygen consumption were done with 2.5 mm TCEP as electron donor in the presence of 1 μm Erv1 in all conditions plus 1 mm EDTA (curve a), 1 μm ZnCl2 (curve b), and 10 μm ZnCl2 (curves c and d); 1 mm EDTA was added to curve d at 180 s to chelate zinc ions. No EDTA or ZnCl2 was added to curve e. For all measurements, the solutions without TCEP were pre-equilibrated at 25 °C, followed by the addition of 2.5 mm TCEP at time 0.
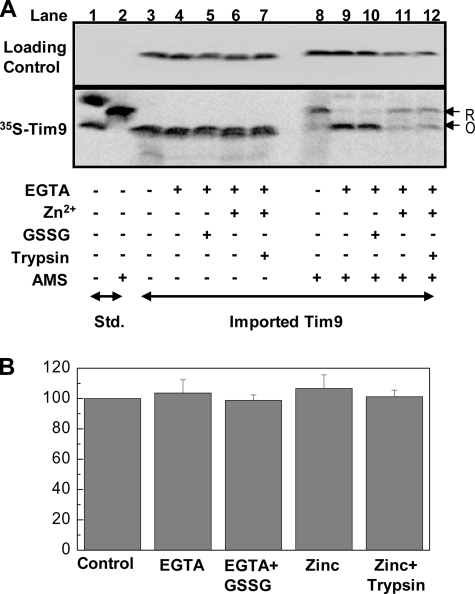
ZnTim9 Can Be Stably Retained in the Mitochondrial IMS—Next, we asked whether the poor Tim9 import in the presence of zinc is purely due to the inactivation of the Mia40/Erv1 pathway or because ZnTim9 itself cannot be imported directly or efficiently. To address this issue, we investigated whether the imported ZnTim9 can leak out of the mitochondrial IMS. 35S-Labeled Tim9 was imported into mitochondria in the presence of Zn2+, followed by trypsin treatment to remove unimported proteins. Then, the mitochondria were split into five equal aliquots and subjected to five post-import treatments: (i) untreated control and (ii–v) mitochondria resuspended in import buffer containing (ii) EGTA, (iii) EGTA + GSSG, (iv) buffered Zn2+, or (v) buffered Zn2+, followed by another trypsin treatment. After the treatments, each sample was further subdivided in two for analysis of import level (Fig. 6A, lanes 3–7) and redox state (lanes 8–12) of the imported Tim9 in parallel. The results show that the levels of imported Tim9 remained the same after all five post-import treatments (Fig. 6, A and B). Furthermore, the redox state of untreated ZnTim9 was mainly reduced (Fig. 6A, lane 8), and ZnTim9 became oxidized after EGTA treatment with or without the addition of GSSG (lanes 9 and 10). Interestingly, >60% of the protein remained in the reduced form when resuspended in Zn2+-containing buffers (Fig. 6A, lanes 11 and 12). Taken together, these results show that ZnTim9 was trapped inside mitochondria in a reduced form and could not leak out. Thus, ZnTim9 cannot pass freely across the outer membrane, unlike the small Tim apoproteins (27). Therefore, ZnTim9 probably cannot be imported directly or at least not as efficiently as apoproteins.
FIGURE 6.
ZnTim9 is stably trapped inside the IMS. A, SDS-PAGE analysis of imported ZnTim9. 35S-Labeled Tim9 was imported for 30 min at 25 °C under buffered Zn2+ conditions. Unimported material was removed by trypsin treatment, and the import reaction was subsequently split into five equal aliquots and resuspended in buffers under five different conditions as indicated (lanes 3–7) for post-import treatments. After a 30-min incubation at 25 °C, the samples were further split into sets for SDS-PAGE analysis without (lanes 3–7) and with (lanes 8–12) AMS treatment. The gel was also probed with anti-Tim10 antibodies as a loading control. Lanes 1 and 2, standards (Std.). R, reduced; O, oxidized. B, quantification of import levels in A (lanes 3–7). Error bars represent S.E. (n = 3).
DISCUSSION
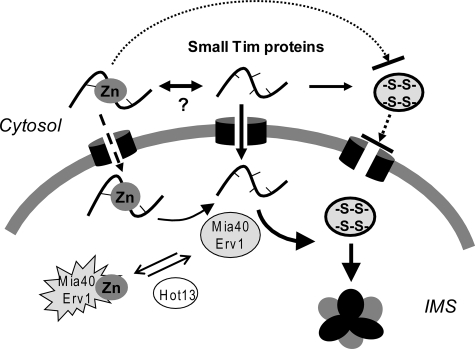
In this study, we have shown that zinc can play dual roles during the biogenesis of the zinc finger small Tim proteins. The reduced small Tim apoproteins can be oxidized under the cytosolic redox conditions, which inhibit their import into mitochondria. Zinc can act as a chaperone-like thiol stabilizer preventing the proteins from oxidative folding in the cytosol and as an inhibitor of the Mia40/Erv1 import pathway used by the small Tim and many other mitochondrial IMS proteins. We propose a model for the role of zinc during biogenesis of the mitochondrial small Tim proteins as depicted in Fig. 7. Following the synthesis of the proteins in the cytosol, zinc binds to the reduced proteins to protect them from oxidative folding. Most of the proteins, if not all, are imported into mitochondria through the apo-form. It is likely that the zinc-bound form cannot be imported into mitochondria directly, or at least not as efficiently as the apoprotein, as a significantly low level of import was observed for ZnTim9, whereas imported ZnTim9 cannot leak out of the IMS. Biogenesis of the small Tim proteins will then be ensured by oxidative folding catalyzed by Mia40/Erv1 and complex formation in the IMS as shown previously.
FIGURE 7.
Model for the function of zinc during biogenesis of the small Tim proteins. Following the synthesis of the proteins in the cytosol, zinc binds to the reduced proteins to protect them from oxidative folding, as the oxidized proteins cannot be imported. The equilibrium between the apo- and zinc-bound forms may be mediated by a currently unknown cytosolic factor. Most of the proteins are imported into mitochondria through the apo-form, followed by oxidative folding catalyzed by the Mia40/Erv1 system and complex formation in the IMS. A small fraction of the proteins may be imported into the IMS in a zinc-bound form, which may inhibit the activity of the Mia40/Erv1 system and thus may require the help of Hot13.
Our import results suggest that Zn2+ is removed from the zinc finger small Tim proteins upon their import into the mitochondrial IMS, which is further supported by the result that zinc is detrimental to the activity of Erv1 and thus the Mia40/Erv1 import machinery located in the IMS. Consistent with our results, a similar inhibitory effect of zinc on the yeast sulfhydryl oxidase Erv2 was reported previously (22), suggesting that zinc may be an inhibitor for the activity of sulfhydryl oxidases in general. Furthermore, our results are consistent with the findings that different cell compartments are biased toward different elements (2, 28–30), and whereas the cytosol has a high level of zinc storage, the zinc level in mitochondria is low and regulated independently (31). However, how the equilibrium between the apo- and zinc-bound forms of the small Tim proteins is mediated in the cytosol is unknown. It may be mediated by another currently unknown factor or through protein-protein interactions, because zinc binding is highly regulated in the cell.
We cannot exclude that a small fraction of the proteins may be imported in a zinc-bound form, followed by zinc removal or transfer to Mia40/Erv1 to allow oxidative folding of the small Tim proteins (Fig. 7). A recent study suggested that Hot13 (helper of Tim proteins), a Cys-rich IMS protein, can promote oxidation of Mia40 by removing zinc, which otherwise inhibits the activity of Mia40 (32). Although both Mia40 and Erv1 are essential proteins for the viability of yeast cells, Hot13 is not (33). Thus, the function of Hot13 as a zinc chelator may be as a second line of security in case zinc is imported into the IMS along with the zinc finger small Tim proteins to ensure that the Mia40/Erv1 pathway works efficiently. Thus, the Hot13 studies also indicate that it is important to remove zinc from the small Tim proteins before they are imported into the mitochondrial IMS.
Unlike the oxidized small Tim proteins that are completely import-incompetent, zinc-bound forms can be reversibly converted into the import-efficient state, the apo-forms. Thus, zinc binding can stabilize the small Tim proteins in a reduced and mitochondrial import-competent form. Interestingly, unlike the structural roles played by zinc in many other zinc finger proteins (2, 34, 35), zinc binding to the small Tim proteins results in only a moderate degree of protein folding or conformational change, which is much smaller than that induced by disulfide bond formation (18, 24). This suggests that zinc binding to the small Tim proteins is highly dynamic and cannot induce or stabilize the proteins in a stable conformation, whereas the formation of two pairs of disulfide bonds in juxtaposition can bridge the two parts of each protein together covalently and more closely. The difference between the small Tim and other proteins may reflect the special role of zinc that has to balance between stabilizing the proteins in reduced states and keeping them in unfolded states ready for mitochondrial import. Our results suggest that zinc plays a new function as a small chemical chaperone in the cytosol during biogenesis of the small Tim proteins. The chaperone-like thiol stabilizer role may be an important function of zinc in many other zinc finger proteins.
In summary, this study shows that zinc has dual roles as a chaperone-like thiol stabilizer and inhibitor of the Mia40/Erv1 pathway during biogenesis of the small Tim proteins. Zinc-bound proteins cannot be imported efficiently into the mitochondrial IMS. A new biological function for zinc as a small chemical chaperone in the cytosol is revealed. It will be challenging and exciting to understand the molecular basis for the chaperone-like function of zinc in the future.
Acknowledgments
We thank Kostas Tokatlidis (Institute of Molecular Biology and Biotechnology, Crete) for Tim9, Thomas Lisowsky (Botanisches Institute) for Erv1, Nikolaus Pfanner (University Freiburg) for AAC, Kai Hell (University München) for Su9-DHFR constructs, and Carla Koehler (UCLA) for the tim9ts yeast strain. We thank Martin Pool and Neil Bullied (Manchester University) for comments on the manuscript.
This work was supported by Biotechnology and Biological Sciences Research Council Grant BB/C514323 and the Royal Society. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: IMS, intermembrane space; AAC, ADP/ATP carrier; AMS, 4-acetamido-4′-maleimidylstilbene-2,2′-disulfonic acid; TCEP, tris(2-carboxyethyl)phosphine; Tricine, N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine.
References
- 1.Berg, J. M., and Shi, Y. (1996) Science 271 1081-1085 [DOI] [PubMed] [Google Scholar]
- 2.Frausto, J. J. R., and Williams, R. J. P. (2001) The Biological Chemistry of the Elements, pp. 315-339, Oxford University Press, Oxford
- 3.Sensi, S. L., Ton-That, D., Sullivan, P. G., Jonas, E. A., Gee, K. R., Kaczmarek, L. K., and Weiss, J. H. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 6157-6162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sensi, S. L., Canzoniero, L. M., Yu, S. P., Ying, H. S., Koh, J. Y., Kerchner, G. A., and Choi, D. W. (1997) J. Neurosci. 17 9554-9564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haase, H., Mocchegiani, E., and Rink, L. (2006) Biogerontology 28 1-4 [DOI] [PubMed] [Google Scholar]
- 6.Eide, D. J. (2006) Biochim. Biophys. Acta 1763 711-722 [DOI] [PubMed] [Google Scholar]
- 7.Maret, W. (2004) Biochemistry 43 3301-3309 [DOI] [PubMed] [Google Scholar]
- 8.Jakob, U., Eser, M., and Bardwell, J. C. (2000) J. Biol. Chem. 275 38302-38310 [DOI] [PubMed] [Google Scholar]
- 9.Li, W., Bottrill, A. R., Bibb, M. J., Buttner, M. J., Paget, M. S., and Kleanthous, C. (2003) J. Mol. Biol. 333 461-472 [DOI] [PubMed] [Google Scholar]
- 10.Tang, W., and Wang, C. C. (2001) Biochemistry 40 14985-14994 [DOI] [PubMed] [Google Scholar]
- 11.Lu, H., and Woodburn, J. (2005) J. Mol. Biol. 353 897-910 [DOI] [PubMed] [Google Scholar]
- 12.Chacinska, A., Pfannschmidt, S., Wiedemann, N., Kozjak, V., Sanjuan Szklarz, L. K., Schulze-Specking, A., Truscott, K. N., Guiard, B., Meisinger, C., and Pfanner, N. (2004) EMBO J. 23 3735-3746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terziyska, N., Lutz, T., Kozany, C., Mokranjac, D., Mesecke, N., Neupert, W., Herrmann, J. M., and Hell, K. (2005) FEBS Lett. 579 179-184 [DOI] [PubMed] [Google Scholar]
- 14.Koehler, C. M. (2004) Trends Biochem. Sci. 29 1-4 [DOI] [PubMed] [Google Scholar]
- 15.Pfanner, N., Wiedemann, N., Meisinger, C., and Lithgow, T. (2004) Nat. Struct. Mol. Biol. 11 1044-1048 [DOI] [PubMed] [Google Scholar]
- 16.Tokatlidis, K. (2005) Cell 121 965-967 [DOI] [PubMed] [Google Scholar]
- 17.Hell, K. (2008) Biochim. Biophys. Acta 1783 601-609 [DOI] [PubMed] [Google Scholar]
- 18.Lu, H., Golovanov, A. P., Alcock, F., Grossmann, J. G., Allen, S., Lian, L. Y., and Tokatlidis, K. (2004) J. Biol. Chem. 279 18959-18966 [DOI] [PubMed] [Google Scholar]
- 19.Lu, H., Allen, S., Wardleworth, L., Savory, P., and Tokatlidis, K. (2004) J. Biol. Chem. 279 18952-18958 [DOI] [PubMed] [Google Scholar]
- 20.Morgan, B., and Lu, H. (2008) Biochem. J. 411 115-122 [DOI] [PubMed] [Google Scholar]
- 21.Ivanova, E., Jowitt, T. A., and Lu, H. (2008) J. Mol. Biol. 375 229-239 [DOI] [PubMed] [Google Scholar]
- 22.Wang, W., Winther, J. R., and Thorpe, C. (2007) Biochemistry 46 3246-3254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meisinger, C., Pfanner, N., and Truscott, K. N. (2006) Methods Mol. Biol. 313 33-39 [DOI] [PubMed] [Google Scholar]
- 24.Ivanova, E., Ball, M., and Lu, H. (2008) Proteins Struct. Funct. Bioinformat. 71 467-475 [DOI] [PubMed] [Google Scholar]
- 25.Leuenberger, D., Curran, S. P., Wong, D., and Koehler, C. M. (2003) Traffic 4 144-152 [DOI] [PubMed] [Google Scholar]
- 26.Krezel, A., Latajka, R., Bujacz, G. D., and Bal, W. (2003) Inorg. Chem. 42 1994-2003 [DOI] [PubMed] [Google Scholar]
- 27.Muller, J. M., Milenkovic, D., Guiard, B., Pfanner, N., and Chacinska, A. (2008) Mol. Biol. Cell 19 226-236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams, R. J. (1984) Endeavour 8 65-70 [DOI] [PubMed] [Google Scholar]
- 29.Tottey, S., Waldron, K. J., Firbank, S. J., Reale, B., Bessant, C., Sato, K., Cheek, T. R., Gray, J., Banfield, M. J., Dennison, C., and Robinson, N. J. (2008) Nature 455 1138-1142 [DOI] [PubMed] [Google Scholar]
- 30.Vallee, B. L., and Auld, D. S. (1995) EXS (Basel) 73 259-277 [DOI] [PubMed] [Google Scholar]
- 31.Simm, C., Lahner, B., Salt, D., LeFurgey, A., Ingram, P., Yandell, B., and Eide, D. J. (2007) Eukaryot. Cell 6 1166-1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mesecke, N., Bihlmaier, K., Grumbt, B., Longen, S., Terziyska, N., Hell, K., and Herrmann, J. M. (2008) EMBO Rep. 9 1107-1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Curran, S. P., Leuenberger, D., Leverich, E. P., Hwang, D. K., Beverly, K. N., and Koehler, C. M. (2004) J. Biol. Chem. 279 43744-43751 [DOI] [PubMed] [Google Scholar]
- 34.Maret, W. (2005) J. Trace Elem. Med. Biol. 19 7-12 [DOI] [PubMed] [Google Scholar]
- 35.Matthews, J. M., and Sunde, M. (2002) IUBMB Life 54 351-355 [DOI] [PubMed] [Google Scholar]