Abstract
The green alga Chlamydomonas reinhardtii has a network of fermentation pathways that become active when cells acclimate to anoxia. Hydrogenase activity is an important component of this metabolism, and we have compared metabolic and regulatory responses that accompany anaerobiosis in wild-type C. reinhardtii cells and a null mutant strain for the HYDEF gene (hydEF-1 mutant), which encodes an [FeFe] hydrogenase maturation protein. This mutant has no hydrogenase activity and exhibits elevated accumulation of succinate and diminished production of CO2 relative to the parental strain during dark, anaerobic metabolism. In the absence of hydrogenase activity, increased succinate accumulation suggests that the cells activate alternative pathways for pyruvate metabolism, which contribute to NAD(P)H reoxidation, and continued glycolysis and fermentation in the absence of O2. Fermentative succinate production potentially proceeds via the formation of malate, and increases in the abundance of mRNAs encoding two malateforming enzymes, pyruvate carboxylase and malic enzyme, are observed in the mutant relative to the parental strain following transfer of cells from oxic to anoxic conditions. Although C. reinhardtii has a single gene encoding pyruvate carboxylase, it has six genes encoding putative malic enzymes. Only one of the malic enzyme genes, MME4, shows a dramatic increase in expression (mRNA abundance) in the hydEF-1 mutant during anaerobiosis. Furthermore, there are marked increases in transcripts encoding fumarase and fumarate reductase, enzymes putatively required to convert malate to succinate. These results illustrate the marked metabolic flexibility of C. reinhardtii and contribute to the development of an informed model of anaerobic metabolism in this and potentially other algae.
Chlamydomonas reinhardtii is a unicellular, soil-dwelling, photosynthetic green alga that has a diversity of fermentation pathways, inferred from the full genome sequence (1, 2). It uses these pathways for ATP production during anoxia, catabolizing starch and other intracellular carbon substrates into the predominant fermentation products formate, acetate, ethanol, CO2, and molecular hydrogen (H2) in what is classified as heterofermentation (2-7). These metabolic pathways would be active primarily at night when high rates of respiration and the absence of photosynthetic O2 evolution cause the rapid establishment of anoxia (8), especially in soil environments with high concentrations of microbes. Moreover, C. reinhardtii can balance the use of the tricarboxylic acid cycle with fermentation when the rate of respiratory O2 consumption exceeds the rate of photosynthetic O2 evolution (3, 4, 9). The catabolism of intracellular carbon stores during anoxic acclimation is a key component of C. reinhardtii metabolism as this alga does not appear to effectively assimilate extracellular sugars. Acquiring a better understanding of cellular metabolisms in algae such as C. reinhardtii under various conditions will facilitate the development of physiological models that predict metabolic circuits and the interactions among these circuits. Additionally, the secretion of fermentative metabolites by C. reinhardtii (and other algae) likely has significant impacts on the population dynamics of microbial consortia in environments inhabited by C. reinhardtii. There is also the potential to leverage the unique metabolic flexibility of C. reinhardtii for the production of valuable metabolites such as H2, organic acids, and ethanol as renewable bioenergy carriers (4, 10, 11).
Although studies of C. reinhardtii metabolism have already significantly advanced our understanding of cellular responses to anoxia (5-7, 12), additional research efforts are required to gain further insights into the proteins involved in adaptation and acclimation of the cells to anaerobiosis, the modulation of cellular metabolite levels under these conditions, and the accurate localization of fermentation pathways and proteins to specific subcellular compartments. The availability of the C. reinhardtii genome sequence, combined with high throughput “omics”-based approaches, will be critical in this effort. Moreover, the use of specific mutant strains can help establish the foundation for a more comprehensive understanding of how cells adjust metabolite fluxes when specific reactions are blocked.
A number of studies have demonstrated that C. reinhardtii can rapidly acclimate to anaerobiosis by shifting to fermentative metabolisms (5-7, 12-14). The catabolism of pyruvate to acetyl-CoA in C. reinhardtii may proceed via fermentation pathways that use either PFL1 (pyruvate formate-lyase) (5, 6, 12, 15) or PFR1 (pyruvate ferredoxin oxidoreductase) (5, 6, 12). The acetyl-CoA is further metabolized to ethanol and/or acetate, and the reduced ferredoxin generated by PFR1 activity is putatively oxidized by a number of redox proteins, including one or both of the two C. reinhardtii [FeFe] hydrogenases (HYDA1 and HYDA2) (16, 17), which are localized to the chloroplast stroma (18, 19). A recently isolated C. reinhardtii mutant, hydEF-1, is devoid of hydrogenase activity (20, 21) because of disruption of an [FeFe] hydrogenase maturase, which is required for proper enzyme assembly (20-24); consequently, neither of the hydrogenases are active in the mutant strain.
In this study we use molecular and physiological approaches to examine dark, anoxic acclimation of the C. reinhardtii hydEF-1 mutant. Interestingly, the mutant secretes much higher levels of succinate during anoxia than the parental strain. Furthermore, transcripts encoding PYC1 (pyruvate carboxylase) and MME4 (a malic enzyme) increase significantly in the mutant relative to the parental strain during anaerobiosis. Both of these proteins have the ability to carboxylate pyruvate, providing precursors for the fermentative production of succinate. Additionally, transcripts encoding fumarase (FUM1 and FUM2) and FMR1 (fumarate reductase), enzymes that catalyze the final steps of succinate synthesis, are elevated in the hydEF-1 strain. Utilization of these pathways for succinate synthesis would result in NADH reoxidation, which would sustain additional cycles of glycolysis and explain both the relative increase in succinate and decrease in CO2 production during anaerobiosis in the mutant relative to the parental strain. Microarray studies were also performed and reveal that several transcripts encoding proteins associated with cellular redox functions and other aspects of anaerobic metabolism are differentially regulated in the mutant relative to the parental strain. These findings are discussed with respect to the identification of potential new fermentation pathways and the flexibility of whole-cell metabolism in C. reinhardtii under dynamic environmental conditions.
EXPERIMENTAL PROCEDURES
Strains and Growth Conditions—C. reinhardtii CC-425 (cw15, sr-u-60, arg7-8, mt+) and hydEF-1 mutant (derived from CC-425) cells were grown in Tris/acetate/phosphate medium (TAP)2 (pH 7.2), supplemented with 200 mg·liter-1 arginine for CC-425. Algal cultures were maintained at 25 °C, vigorously bubbled with air enriched with 3% CO2, stirred using a magnetic stir bar, and illuminated with continuous light of 80 μmol photon m-2 s-1 photosynthetically active radiation at the surface of 1-liter Roux culture bottles (255 × 55 × 120 mm), in which cell densities ranged from 1 × 105 to 3 × 106 cells/ml of culture.
Chlorophyll Measurements—Chlorophyll a and b content were determined spectrophotometrically in 95% ethanol (25).
Anaerobic Induction of Liquid Cell Suspensions—C. reinhardtii cultures were grown on TAP medium to ∼16-24 μg·ml-1 total chlorophyll, centrifuged (500 ml of cells) at 3,000 × g for 1 min, and the cell pellet resuspended in one-tenth volume (50 ml) of anaerobic induction buffer containing 50 mm potassium phosphate (pH 7.2) and 3 mm MgCl2 (26). The cells were then transferred to a sealed anaerobic vial in the dark and flushed with argon for 30 min. For measuring H2 and O2 production rates, 200 μl of cell cultures were placed in a Clark-type oxygen electrode assay chamber as described previously (6, 20). Clark-type oxygen electrodes were used simultaneously to measure light-induced H2 and O2 production rates. To measure fermentative H2 production, 400 μl of head space gas was withdrawn from the sealed anaerobic vials and analyzed by gas chromatography (Hewlett Packard 5890 series II) using a Supelco column (60/80 mol sieve 5A 6 feet × 1/8 inch) coupled to a TCD detector. H2 remaining in solution was quantified using the Clark-type oxygen electrode described above without illumination of the sample chamber.
CO2 Measurement—CO2 levels were below detection limits in the serum vial head space of anaerobically acclimated cells. Therefore, following anaerobic induction, 1 ml of anoxic cells was transferred in a gas-tight syringe to a sealed vial into which 1 ml of 1 m HCl was added. The acidified cell suspension was vigorously shaken to liberate CO2, which was quantified by gas chromatography (Hewlett Packard 5890 series II) using a Supelco column (80/100 PORAPAK N 6 feet × 1/8 inch × 2.1 mm) coupled to a TCD detector.
Metabolite Analysis—Organic acid analysis was performed by liquid chromatography (Hewlett Packard Series 1050 HPLC) using an Aminex HPX-87H (300 × 7.8 mm) ion exchange column. Dark-adapted cells were collected at the indicated time points and centrifuged (10,000 × g for 1 min), and the supernatant was transferred to a new vial and frozen in liquid N2 for subsequent analysis. Samples were thawed, centrifuged, and filtered prior to HPLC analysis. One hundred μl of cell culture supernatant was injected onto the column and eluted using 8 mm filtered sulfuric acid (J. T. Baker, Inc.) as the mobile phase at a flow rate of 0.5 ml·min-1 at 45 °C. Retention peaks were recorded using Agilent ChemStation software, and quantifications were performed by comparisons with known amounts of standard for each of the organic acids.
Ethanol was measured using a YSI 2700 SELECT electrochemical probe (YSI Inc., Yellow Springs, OH). Identical supernatants were used for metabolite and ethanol analysis; 10 μl of the supernatant was required for ethanol analysis.
Extraction of RNA—Total RNA was isolated using the Plant RNA reagent, as described by the manufacturer (Invitrogen). Approximately 40 μg of isolated RNA was treated with 5 units of RNase-free DNase (Ambion, Austin, TX) for 30 min at room temperature. The Qiagen RNeasy MinElute kit (Qiagen, Valencia, CA) was used to purify total RNA. The A260 of the eluted RNA was measured, and 4 μg of purified RNA was reserved to prepare labeled samples for microarray analysis.
Reverse Transcriptase Real Time PCR (qPCR)—The abundance of specific transcripts in total mRNA from each sample was quantified by reverse transcriptase real time PCR, designated qPCR, using the Engine Opticon system (Bio-Rad). First strand cDNA synthesis was primed on purified total RNA using specific primers for each C. reinhardtii gene of interest. The specific primers (at 0.5 μm each; supplied by IDT) were annealed to 250 ng of DNA-free total RNA and extended for 1 h at 55 °C using 200 units of the reverse transcriptase Superscript III (Invitrogen). Four μl of the single-stranded cDNA from the reverse transcriptase reaction (final volume, 20 μl) was used as the template for real time PCR amplifications, which were performed using the DyNAmo HS SYBR green reverse transcription-PCR kit according to the manufacturer's instructions (Finnzymes, Woburn, MA). Specific primers (0.3 μm) used for amplification were designed to generate amplicons of 100-200 nucleotides. Amplifications were performed using a Bio-Rad iCycler iQ detection system and the following cycling parameters: an initial single step at 95 °C for 10 min (denaturation) followed by 40 cycles of (a) 94 °C for 30 s (denaturation), (b) 56 °C for 45 s (annealing), and (c) 72 °C for 30 s (elongation). Following the 40 cycles, a final elongation/termination step was performed at 72 °C for 10 min. Primers used for qPCR are shown in supplemental Table 1, and were designed using Primer3 software (available on line). The relative expression ratio of a target gene was calculated based on the 2-ΔΔCT method (27), using the average cycle threshold (CT) calculated from triplicate measurements. Relative expression ratios from three independent experiments (different experimental replicates) are reported. The level of accumulation of the RACK1 transcript was used as a normalization control because it shows near constant expression under the conditions used in these experiments.
Microarray Fabrication—Microarrays were fabricated at the Stanford Functional Genomics Facility at Stanford University, as described previously (6).
Labeling and Purification of Reverse-transcribed cDNAs—Labeling and purification of reverse-transcribed cDNAs were performed as described previously (28). Briefly, 4 μg of purified RNA was adjusted to 4 μl with sterile MilliQ-treated water. One μl of oligo(dT)-(V) (2 μg·μl-1) was heated for 10 min at 70 °C and quickly chilled on ice. The following reagents were added sequentially to the reaction mixture: 2 μl of 5× superscript buffer; 1 μl of 0.1 m dithiothreitol; 0.2 μl of 50× dNTPs (5 mm dATP, dCTP, and dGTP and 10 mm dTTP); 1 μl of Cy3- or Cy5-dUTP; and 0.8 μl of Superscript III (200 units·μl-1). The final reaction volume was 10 μl. After allowing the reaction to proceed at 42 °C for 2 h, an additional aliquot of 0.5 μl of Superscript III was added, and the reaction was continued for an additional 1 h at 50 °C. The reaction was stopped by the addition of 0.5 μl of 500 mm EDTA and 0.5 μl of 500 mm NaOH, and the solution was incubated at 70 °C for 10 min. Neutralization of the reaction mixture was achieved by adding 0.5 μl of 500 mm HCl. The QIAquick PCR purification kit (Qiagen, Valencia, CA) was used to purify the labeled cDNA as described previously (6).
Hybridization to the Oligonucleotide Array—Pre-hybridization and hybridization of fluorescent-labeled probes and washing of the arrays were as described previously (6, 28). Detailed and updated versions of the protocols used for RNA labeling, slide pre-hybridization, hybridization, and washing can be downloaded from the Chlamydomonas Center website.
Scanning, Quantification, and Analysis of the Slides—Microarray slides were scanned for Cy5 and Cy3 fluorescent signals using a GenePix 4000B scanner (Molecular Devices) as described previously (6). Spot positions were defined using SpotReader (Niles Scientific). Analyses of the data were performed using GeneSpring 6.1 (Agilent Technologies, Foster City, CA). Images of the fluorescence at 532 nm for Cy3 and 635 nm for Cy5 were recorded and analyzed from the complete array sets (three experimental replicates for the time point 0.5 h, four experimental replicates for the time points 2 and 4 h, and three slides per time point for each experimental replicate, with two copies of each cDNA per slide). Error models were computed based on replicates. Signal ratios were considered to meet threshold criteria if they passed Student's t test for significance with a p value of ≤0.05.
RESULTS
Metabolite Analyses—The C. reinhardtii hydEF-1 mutant lacks hydrogenase activity because of disruption of the HYDEF gene, which encodes a hydrogenase maturase (20). This mutant was used to investigate the physiological responses of C. reinhardtii in the absence of H2 metabolism during anaerobic acclimation. The fermentative products that accumulated in the medium during anoxia in both cultures mutant and parental control are shown in Table 1. Four significant differences observed between mutant and parental cells were as follows: (a) the absence of H2 production in the mutant relative to the parental strain; (b) a marked difference in accumulation of CO2 between the two strains; (c) a slight reduction in the rate of accumulation of formate, acetate, and ethanol in the mutant relative to the parental strain; and (d) elevated production of succinate, only in the mutant. Rescue of the hydEF-1 mutant phenotype and restoration of the fermentation profile (along with H2 and CO2 production) observed in the parental strain was achieved by introduction of a functional copy of the HYDEF gene (data not shown). The complemented strain was not used as a reference in the microarray experiments as integration of the introduced HYDEF gene into the genome could disrupt another cellular function.
TABLE 1.
Concentrations of fermentation products secreted by the Chlamydomonas hydEF-1 mutant and the parental strain, CC-425, following exposure to dark anaerobiosis at the indicated times
Cells were purged for 30 min with argon in the dark and then maintained in the dark for up to 24 h. Samples were taken at 0.5, 2, 4, and 24 h, centrifuged, and filtered, and the medium was analyzed for succinic acid, ethanol, acetic acid, and formic acid using HPLC. CO2 is reported as the change in CO2 concentration relative to the concentration at the start of the anaerobic induction. H2 and CO2 accumulations were analyzed as described under “Experimental Procedures.” Data are from three independent experiments.
|
Fermentation products
|
|||||||
|---|---|---|---|---|---|---|---|
| Time | CO2 | H2 | Formic acid | Succinic acid | Acetic acid | Ethanol | |
| h | mmol/liter | ||||||
| CC-425 | |||||||
| 0.5 | −0.05 ± 0.09 | 0.02 ± 0.01 | 0.5 ± 0.2 | BDa | 0.9 ± 0.2 | 0.2 ± 0.1 | |
| 2 | 0.14 ± 0.02 | 0.03 ± 0.01 | 1.3 ± 0.1 | 0.1 ± 0.1 | 1.5 ± 0.1 | 0.7 ± 0.1 | |
| 4 | 0.64 ± 0.08 | 0.06 ± 0.01 | 2.2 ± 0.1 | 0.1 ± 0.1 | 1.8 ± 0.3 | 1.1 ± 0.2 | |
| 24 | NDb | ND | 4.8 ± 0.2 | 0.1 ± 0.1 | 4.3 ± 0.3 | 1.9 ± 0.1 | |
| hydEF-1 | |||||||
| 0.5 | −0.09 ± 0.01 | BD | 0.3 ± 0.2 | 0.1 ± 0.03 | 0.4 ± 0.2 | BD | |
| 2 | −0.09 ± 0.02 | BD | 1.0 ± 0.1 | 0.2 ± 0.1 | 0.7 ± 0.2 | 0.4 ± 0.1 | |
| 4 | −0.03 ± 0.01 | BD | 1.5 ± 0.1 | 0.4 ± 0.2 | 1.0 ± 0.2 | 0.7 ± 0.1 | |
| 24 | ND | ND | 3.5 ± 0.2 | 1.2 ± 0.1 | 2.0 ± 0.2 | 0.8 ± 0.2 | |
BD means below detection.
ND means not determined.
Succinate is not detected in significant amounts in the medium of the parental culture but does accumulate in the mutant culture, particularly after longer periods (4 and 24 h) of anaerobiosis. CO2 levels do not increase significantly during acclimation to anaerobiosis in the mutant, whereas in parental cells CO2 levels steadily rise over the acclimation period. To account for succinate production and the near constant CO2 level in the mutant, we identified genes on the draft genome encoding enzymes associated with fermentative succinate production. These encoded enzymes were placed into metabolic pathways (Fig. 1) leading to the fermentative production of succinate, which requires the carboxylation of a three-carbon substrate and is consistent with diminished CO2 accumulation in the mutant relative to the parental strain. The proteins associated with the succinate pathways depicted in Fig. 1 are given in Table 2. Because hydrogenase activity is absent in the mutant, pyruvate flux via the pyruvate ferredoxin oxidoreductase (PFR1) pathway is anticipated to be altered because hydrogenase can no longer participate in PFR1/ferredoxin reoxidation. However, it should be noted that additional metabolic pathways could potentially oxidize ferredoxin, such as sulfite reductases or NAD(P)H-ferredoxin oxidoreductases, and metabolic flux could still proceed via PFR1 provided ferredoxin was reoxidized by alternative pathways. Under our experimental conditions the parental strain accumulates 10-fold more CO2 than H2 (on a molar basis) indicating that other pathways are involved in the process under our experimental conditions (Table 1). If all of the reduced ferredoxin was oxidized by hydrogenase, this stoichiometry (CO2/H2) should be 1:1. Relative to the parental strain, the increased succinate accumulation observed in the mutant would consume both the reducing equivalents and nearly all of the CO2 generated by pyruvate ferredoxin oxidoreductase activity. In C. reinhardtii, there are several potential pathways that could lead to the production of succinate. These include the following: (a) pyruvate carboxylation by pyruvate carboxylase to yield oxaloacetate; (b) pyruvate carboxylation by malic enzyme (MME) to yield malate; and (c) phosphoenolpyruvate (PEP) carboxylation by either phosphoenolpyruvate carboxylase (PEPC) or PEP carboxykinase (PCK) to yield oxaloacetate, which can then be reduced to malate by malate dehydrogenase (MDH). The conversion of malate to succinate requires fumarase (FUM) and fumarate reductase (FMR) activities (Fig. 1).
FIGURE 1.
Anaerobic metabolic pathways in C. reinhardtii. Pathways at the upper right are proposed for succinate production and NADH reoxidation in the hydEF-1 mutant. Proteins encoded in the C. reinhardtii genome that are associated with the metabolisms depicted include the following: ACK, acetate kinase; ADH, bifunctional acetylaldehyde/alcohol dehydrogenase (alcohol dehydrogenase only in the case of the PDC pathway); FDX, ferredoxin; FMR, fumarate reductase; FUM, fumarase; HYD, hydrogenase; MDH, malate dehydrogenase; MME, malic enzyme; PAT, phosphate acetyltransferase; PDC, pyruvate decarboxylase; PEPC, phosphoenolpyruvate carboxylase; PFL, pyruvate formate-lyase; PFR, pyruvate-ferredoxin oxidoreductase; PYC, pyruvate carboxylase; PYK, pyruvate kinase. Known or putative cellular localizations of the proteins depicted are coded by color as follows: green for the chloroplast, red for the mitochondrion, orange for dual chloroplast/mitochondrion localization, blue for cytoplasm, and black for unknown. The cellular localizations of MDH1, -3, and -4, ACK2, PFL, and PAT1 were experimentally determined by Allmer et al. (46) and Atteia et al. (5), respectively. The cellular localizations of the other enzymes were determined using ChloroP, TargetP, and Predotar softwares and are highly speculative. When possible a final predicted localization was determined by doing an average of all predictions.
TABLE 2.
Chlamydomonas proteins associated with anoxic metabolism and succinate production in the hydEF-1 mutant
The sequences of the proteins are based on gene models from version 3.0 of the genome sequence. Both protein IDs and associated annotation provided on the JGI Browser are given.
| Enzyme assigned name | Annotation | Protein ID |
|---|---|---|
| FUM1 | Furmarase | 195953 |
| FUM2 | Fumarase | 196655 |
| Fumarate reductase | Fumarate reductase/succinate dehydrogenase flavoprotein | 145357 |
| MDH1 NAD-dependent | Lactate/malate, dehydrogenase | 137163 |
| MDH2 NAD-dependent | Malate dehydrogenase | 126023 |
| MDH3 NAD-dependent | Malate dehydrogenase | 158129 |
| MDH4 NAD-dependent | Malate dehydrogenase | 60444 |
| MDH5 NADP-dependent | Malate dehydrogenase | 192083 |
| MME1 NAD-dependent | Malic enzyme | 196833 |
| MME2 NADP-dependent | Malic enzyme | 147722 |
| MME3 NADP-dependent | Malic enzyme | 196832 |
| MME4 NADP-dependent | Malic enzyme | 196831 |
| MME5 NADP-dependent | Malic enzyme | 196351 |
| MME6 NADP-dependent | Malic enzyme | 126820 |
| PEPC1 isoform 1 | Phosphoenolpyruvate carboxylase | 80312 |
| PEPC2 isoform 2 | Phosphoenolpyruvate carboxylase | 182821 |
| PYC1 | Pyruvate carboxylase | 112730 |
| PYK1 | Pyruvate kinase | 136854 |
| PYK2 | Pyruvate kinase | 196263 |
| PYK3a, b, c one gene | Pyruvate kinase variants a, b, c | 196261, 107530, 122254 |
| PYK4a, b, c one gene | Pyruvate kinase variants a, b, c | 149896, 119280, 04490 |
| PYK5 | Pyruvate kinase | 118203 |
| PYK6-5/ 6-3 incomplete model but same gene | Pyruvate kinase | 196892, 181547 |
Gene Expression in the Succinate Pathway—Pyruvate is the central fermentation metabolite in Chlamydomonas, and altered metabolism of pyruvate could lead to succinate synthesis. To explore the possibility that genes associated with pyruvate metabolism are differentially regulated in the hydEF-1 mutant, we used qPCR to monitor the abundance of transcripts encoding enzymes potentially involved in metabolizing pyruvate during anaerobic acclimation of the mutant and parental strain (Fig. 1). As shown in Fig. 2, transcripts encoding PFL1 and PFR1 increase significantly in both the mutant and parental strains following the imposition of anaerobic conditions. However, the PFR1 transcript increases more in the parental strain than in the mutant, whereas the PFL1 transcript increases more in the mutant than in the parental strain. This suggests a potential readjustment of the flux of metabolites through the PFL1 and PFR1 pathways during fermentation metabolism. A slight increase in the ratio of formate produced relative to the other secreted metabolites is observed in the mutant strain; however, the total amount of formate secreted is diminished in the mutant relative to the parental strain (Table 1). This finding may reflect decreased fermentative rates in the mutant or competition from the succinate pathway, which becomes active in the mutant (see below).
FIGURE 2.
Relative level of selected transcripts associated with pyruvate metabolism determined by qPCR at the indicated time of dark, anaerobic acclimation in hydEF-1 and the parental strain CC-425. Changes in indicated transcript levels following exposure of cells to dark, anaerobic conditions (0.5, 2, and 4 h) are presented as an n-fold change (arbitrary units, a.u.) relative (rel.) to RNA levels at time 0 (just prior to transfer and vigorously oxygenated). The results are normalized to RACK1 transcript levels, which remained constant over the course of the experiment. The results show the mean ± S.D. (error bars) for data from three biological (two technical repetitions for each biological replicate) qPCR replicates.
Although the transcripts level of encoding PFR1 are reduced in the mutant, flux through this pathway may still be required to generate CO2 for the carboxylation reactions leading to succinate production. This would require ferredoxin to be reoxidized by cellular pathways other than the [FeFe] hydrogenases (e.g. formation of NAD(P)H and/or reduction of sulfite). The fermentative metabolite data indicate that succinate production in the mutant attained a level that is 60-70% of the level that would be produced if all of the CO2 generated during anaerobic acclimation of the parental strain were used for the synthesis of succinate. These data are suggestive of reduced metabolic flux through the pyruvate ferredoxin oxidoreductase pathway in the mutant; however, pyruvate ferredoxin oxidoreductase activity (at attenuated levels) may still serve to generate the reducing equivalents and CO2 used to convert the three-carbon metabolites pyruvate and/or phosphenolpyruvate to four-carbon fermentation products.
Transcripts encoding PEPC1, PYC1, and MME4 increase significantly only in the hydEF-1 mutant during anaerobiosis (Fig. 2 and supplemental Fig. 1), which suggests that PEPC1 and/or pyruvate carboxylation via PYC1 and/or MME4 may stimulate succinate formation in this strain. Although PFL1, PFR1, PCK1, and PYC1 are encoded by single genes and PEPC by two genes, there are six genes encoding putative malic enzymes. However, only the level of the MME4 transcript increases dramatically (some of the other transcripts increase to a much lesser extent) during anaerobiosis in the mutant strain, with only a small increase in the transcript level in the parental strain (note the differences in the scale for the qPCR results in Fig. 2). These results suggest that MME4 may be predominantly responsible for MME-dependent pyruvate carboxylation during anaerobiosis in the mutant strain. The levels of transcripts encoding PEPC2 and PCK1, enzymes that could also be involved in succinate formation, are not significantly different in the mutant and parental strains (data not shown). Overall, these results suggest that in the absence of active [FeFe] hydrogenases, fermentation metabolites are rerouted away from pyruvate catabolism toward the carboxylation of pyruvate (and perhaps PEP) through PYC1 and MME4 (and perhaps PEPC1). Succinate formation is proposed to proceed via the pathways shown in Fig. 1. This is in accord with the findings that transcripts encoding one of the two C. reinhardtii fumarases (FUM1) and the fumarate reductase increase specifically in the mutant but not in the parental strain during anoxia (Fig. 3). Transcripts encoding the MDH enzymes increase to a small extent (particularly MDH2) in response to anaerobiosis, but these increases are similar in the mutant and parental strains (Fig. 3). Together, the qPCR data indicate that the three pathways shown in Fig. 1 for succinate production increase in the hydEF-1 mutant relative to the parental strain during anaerobiosis.
FIGURE 3.
Relative level of selected transcripts associated with succinate accumulation determined by qPCR at the indicated time of dark, anaerobic acclimation in hydEF-1 and the parental strain CC-425. Changes in transcript levels following exposure of the cells to dark, anaerobic conditions (0.5, 2, and 4 h) are presented as an n-fold change (arbitrary units, a.u.) relative (rel.) to RNA levels at time 0 (just prior to transfer and vigorously oxygenated). The results are normalized to RACK1 transcript levels, which remained constant over the course of the experiment. The results show the mean ± S.D. (error bars) for data from three biological (two technical repetitions for each biological replicate) qPCR replicates.
Attempts to demonstrate in vitro succinate formation using cellular extracts of parental and mutant cells were inconclusive. Succinate production was higher in hydEF-1 extracts relative to the parental strain (data not shown) when supplemented with pyruvate, bicarbonate, and NADH. However, the results were not statistically unequivocal, and we are refining the assay to optimize both cell breakage and the specific reaction conditions.
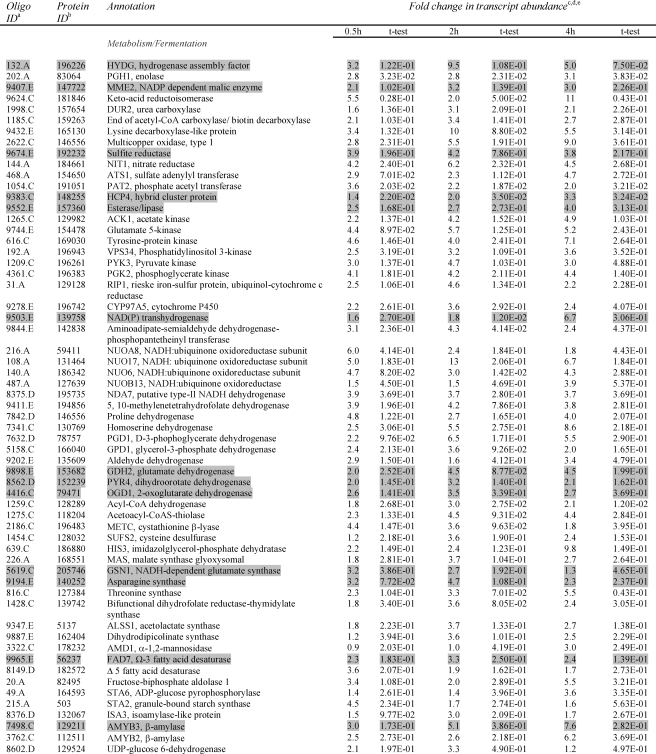
Microarray Analyses—Microarray analyses (28, 29) were performed to examine genome-wide differences between the parental strain and the hydEF-1 mutant during dark, anaerobic acclimation. The previous array data (6) for acclimation of the parental CC-425 strain to dark, anoxic conditions were compared with the current array results obtained for the hydEF-1 mutant. The relative levels of ∼10,000 different transcripts (28) were analyzed at various times following exposure of cells to dark, anaerobic conditions. Transcript levels were filtered to capture those for which there was an elevation greater than 1 or a diminution of less than 0.5-fold in relative abundance at 0.5, 2, or 4 h following the imposition of anaerobic conditions. Genes encoding putative fermentative/metabolism proteins with transcripts that accumulated to over 3.0-fold following transfer of mutant cells to anoxic conditions (relative to aerobic control values) are presented in Table 3.
TABLE 3.
Transcripts associated with metabolism/fermentation showing at least a ≥3.0-fold change in abundance in at least one time point following the transfer of hydEF-1 mutant cells from light, aerobic conditions to dark, anaerobic conditions
Also shown is the identification number of the oligonucleotide, the protein ID, and the associated annotation from the JGI browser. Shaded transcripts are similarly upregulated in the parental strain.
a Identification number of the oligonucleotide sequence used to make the array element is shown.
b Identification number of protein generated from the corresponding gene model (JGI C. reinhardtii version 3.0 is shown).
c Change in transcript abundance at 0.5, 2, and 4 h after transferring cells to dark, anaerobic conditions relative to time 0 (just prior to transfer) is shown.
d Average of three experimental replicates for time point 0.5 h, and four experimental replicates for time points 2 and 4 h. Two technical repetitions were done for each experimental repetition (each slide contained each array element in duplicate).
e Student's t test is shown.
The microarray data (Table 3 and supplemental Table 2) indicate that the majority of metabolic pathways activated during dark, anoxic acclimation in the parental strain (6) are also activated in the mutant. These include transcripts encoding the [FeFe] hydrogenase maturase (HYDG), sulfite reductase, hybrid cluster protein, amylase, and NADH transhydrogenase. Several transcripts encoding enzymes involved in amino acid synthesis and catabolism are also observed to increase, which is consistent with the turnover of proteins involved in aerobic metabolism and the synthesis of proteins required for fermentation metabolism. Increased accumulation of transcripts associated with lipid metabolism was also observed, suggesting that lipid catabolism or reorganization occurs during acclimation to anoxia. Additionally, transcripts encoding fermentative enzymes, such as ACK1 (acetate kinase), PAT2 (phosphate acetyltransferase), pyruvate dehydrogenase kinase, pyruvate decarboxylase, HYDA1, and HYDA2 are observed to increase in both the parental control (6) and the mutant, but only at levels lower than 3-fold in the mutant, and therefore these genes do not appear in Table 3. There are also several transcripts encoding proteins of both known and unknown functions that were observed to increase in the mutant during anoxic acclimation but were not detected in previous microarray analyses of anoxia in the parental strain (6). Included among these are several transcripts encoding components of complex I, sulfate adenylyltransferase, Rieske FeS protein, and cytochrome P450.
A second analysis of the microarray data compared transcript abundance in the hydEF-1 mutant and the parental strain after 4 h of exposure to anaerobic conditions. Table 4 shows the 15 most up-regulated and 10 most significantly down-regulated transcripts encoding putative proteins associated with defined metabolic processes. Among the most significantly up-regulated genes are those encoding nitrate reductase and the hydrogenase maturase HYDG (20). The increase in HYDG is likely a cellular response that reflects the inability of the cell to make an active hydrogenase enzyme. Alternatively, it may be a consequence of the insertional mutation in the adjacent gene, HYDEF (20). Nitrate reduction represents an alternative terminal electron acceptor to proton reduction and may be increased in the mutant during anoxia as a mechanism to oxidize reduced ferredoxin that is generated by PFR1. Likewise, sulfate activation and reduction would be a way of eliminating intracellular reducing equivalents. As shown in Table 4, transcripts encoding sulfate adenylyltransferase, which catalyzes the first step of both the assimilatory and dissimilatory sulfate reduction pathways, accumulates to significantly higher levels in the mutant relative to the parental control. The conversion of sulfite to sulfide is catalyzed by sulfite reductase, whose transcripts increase during anoxia in both the mutant and parental strains. Additional fermentation transcripts encoding proteins associated with NADH oxidation/reduction and ATP synthesis/transport are differentially regulated, which is consistent with hydrogenase activity influencing the relative levels of NADH and ATP during anoxia. Interestingly, components of both mitochondrial complex I and cytochrome b6f are differentially up-regulated in the mutant, suggesting that the redox status of these electron transport chains has been altered in the hydEF-1 mutant. Several genes, encoding some MMEs, FMR1, and PCK1, were not represented on the array.
TABLE 4.
The 15 most highly up-regulated and 10 most significantly down-regulated transcripts associated with metabolism in the hydEF-1 mutant relative to the CC-425 parental strain after 4 h of dark, anaerobic acclimation
Both the protein ID and associated annotation from the JGI browser are given. Shaded transcripts are significantly upregulated in the parental strain.
a Identification number of the oligonucleotide sequence used to make the array element is shown.
b Identification number of protein generated from the corresponding gene model (JGI C. reinhardtii version 3.0 is shown).
c Change in transcript abundance at 4 h after transferring cells to dark, anaerobic conditions relative to time 0 h (just prior to transfer) is shown.
d Average of three experimental repetitions (two technical repetitions for each experimental repetition) is shown.
e Student's t test is shown.
* Genes differentially regulated in the hydEF-1 mutant versus CC-425 in standard culture conditions (TAP, light, aerobic conditions); 428.A (0 h, 5.21; t test, 2.79E-02); 3762.C (0 h, 7.45; t test, 5.52E-02) are shown.
DISCUSSION
The flexibility of algal metabolism is of interest for developing an informed understanding of the following: (a) metabolite exchange in natural microbial communities; (b) the role of unicellular algae in global carbon cycling; and (c) strategies for engineering phototrophic microorganisms for renewable energy production (11, 30-33). The C. reinhardtii genome sequence has provided gene models for populating previously documented metabolic pathways, revealed a diversity of predicted metabolisms, provided insights into the evolution of the green algal lineage, and indicated that parallel processes may occur in multiple cellular compartments (5, 6). An intriguing aspect of algal physiology is the ways in which these organisms acclimate to anoxia (8, 9, 34, 35), which likely occurs as a consequence of microbial respiration at night in environments that are not well oxygenated (e.g. soil environment from which C. reinhardtii was originally isolated). Several research efforts are currently focused on developing a more comprehensive understanding of anoxic metabolism in this alga to support future efforts to generate H2, organic acids, and/or ethanol. Hydrogenase activity in C. reinhardtii has been the focus of extensive research because it catalyzes the production of the renewable energy carrier H2 (4, 33, 36-41). To further study the role of hydrogenases in algal physiology and to define the metabolic adjustments induced by the loss of hydrogenase activity, physiological responses observed during anoxic acclimation of a C. reinhardtii mutant lacking hydrogenase activity were examined relative to the parental strain. Interestingly, rather than causing an increased metabolic flux into alternative pathways operating at significant levels in the parental strain, activation of new metabolic pathways leading to succinate accumulation were observed. The ability of C. reinhardtii to switch to a pathway that is minimally active in the parental strain, continues to demonstrate the metabolic versatility of this alga and its ability to alter its metabolism in response to environmental change.
Succinate Accumulation—The increased production of succinate, the absence of H2 production, and the decreased evolution of CO2 were the most significant metabolic differences observed in the hydEF-1 mutant relative to the parental strain during dark, anoxic acclimation. In the mutant, decreased CO2 evolution and increased abundance of transcripts encoding proteins able to carboxylate pyruvate, congruent with succinate production, suggest that in the absence of hydrogenase activity, pyruvate is carboxylated to generate either malate and/or oxaloacetate. These metabolites are putatively converted into succinate as depicted in Fig. 1. Both malic enzyme and pyruvate carboxylase use CO2 as a substrate to carboxylate pyruvate, and the activity of either or both may be responsible for the nearly steady state levels of CO2 observed in the mutant. Transcripts encoding both of these enzymes increase significantly in the mutant strain during anoxic acclimation. Although the malic enzyme typically decarboxylates malate generating pyruvate, examples of malic enzymes functioning in the reverse direction have been reported (42-44). Overexpression of the gene (scfA) encoding the malic enzyme in Escherichia coli leads to succinate accumulation in a mutant background with elevated pyruvate levels resulting from disruptions of the genes encoding both lactate dehydrogenase and PFL (43, 44). Of the C. reinhardtii malic enzymes, SfcA is most similar to MME4.
Pyruvate carboxylation can also proceed via pyruvate carboxylase to form oxaloacetate, and transcripts encoding PYC1 increase during anoxia in the mutant. However, the transcripts of five putative MDH enzymes, required for the conversion of oxaloacetate into malate and subsequently succinate, do not increase dramatically in the mutant relative to the parental strain. Furthermore, the level of the PYC1 transcript increases ∼20-fold, whereas the MME4 transcript increases in abundance by over 500-fold in the mutant. Based on the qPCR Ct values for the six MME genes, MME4 transcripts are least abundant under oxic conditions in both the parental strain and the hydEF-1 mutant. The relatively strong induction of MME4 compared with PYC1 and MDH transcripts suggests that MME4 may be responsible for the majority of increased metabolic flux toward succinate production. An alternative pathway to succinate via PEPC1 may be modestly activated, based on transcript levels. In addition to PEPC, the conversion of phosphoenolpyruvate to oxaloacetate could also potentially proceed via PCK (43); however, this pathway does not appear to contribute to fermentative succinate formation in E. coli (45), and we do not observe significant differential regulation of the PCK1 transcript in C. reinhardtii (data not shown). Transcripts encoding fumarase and fumarate reductase, required for the conversion of malate to succinate, increase significantly only in the mutant during anoxic acclimation, which is consistent with activation of the pathway shown in Fig. 1; MME4 would carboxylate pyruvate to generate malate, which could then be metabolized to succinate by the activities of FUM and FMR. Elevated succinate production in the mutant provides a means to reoxidize cellular reductant to sustain additional rounds of anaerobic glucose catabolism. Moreover, ATP production is maintained by the conversion of PEP to pyruvate and subsequent carboxylation by malic enzyme. The pathways shown in Fig. 1 reflect whole-cell metabolic networks. To better understand the fermentative metabolism of C. reinhardtii, it will be important to experimentally establish the compartmentalization of the individual enzymes. We have used existing proteomic data (5, 46) and in silico analysis of peptide localization sequences to derive putative localizations, as shown in Fig. 1. However, it should be cautioned that the in silico predictions are highly speculative.
Anoxic Metabolism—Although transcripts encoding PFR1 are observed to increase in the mutant during anoxic acclimation, their levels are significantly reduced relative to the parental strain. This may be a consequence of the inability of hydrogenase to oxidize ferredoxin and restricted metabolic flux through the PFR1 pathway. Intriguingly, in parental cells, the amount of H2 detected is significantly less (∼10 times less) than the amount of CO2 released under our experimental conditions. If PFR1 were solely responsible for the production of CO2, and if ferredoxin were reoxidized exclusively by hydrogenase to produce H2, then dark, fermentative levels of H2 and CO2 should be equal. The significantly reduced H2 levels relative to CO2 indicate that other enzymes such as ferredoxin-NAD(P)H oxidoreductases or perhaps sulfite or nitrite reductases may also be responsible for oxidizing ferredoxin. Additional enzymes such as pyruvate decarboxylase may also contribute to CO2 levels. Redox calculations based on the assumption that all secreted metabolites quantified in Table 1 are derived from starch/glucose indicate that ∼1.6 mm of electron reducing equivalents are not reoxidized by the observed metabolites in the parental strain. These could be reoxidized if 0.2 mm sulfate, which is present in TAP medium, were reduced to sulfide. Moreover, anabolic pathways such as lipid synthesis could also play a role in oxidizing these reducing equivalents. In the case of the hydEF-1 mutant, there is an excess of metabolites relative to the amount of NADH available; however, this can be accounted for if reducing equivalents (and CO2) from PFR1 activity are used in the synthesis of succinate.
It is also perplexing that in parental cells, the ratio of acetate to ethanol is not equal (Table 1). If these fermentation metabolites were all derived from acetyl-CoA, these ratios should be approximately equal, or other mechanisms to oxidize NADH must be triggered that establish a NAD+/NADH stoichiometry that sustains glycolysis (Fig. 1). Because the cells were cultured in TAP medium, which contains acetate (some of which remained in the medium at the time of cell harvesting), it is likely that some of the measured acetate originated from the medium rather than from pyruvate metabolism. Secretion of medium-derived acetate by cells at the onset of anaerobiosis is consistent with high levels of this organic acid at the 0.5-h time point (Table 1) relative to other fermentative metabolites. If the majority of acetate in the medium at 0.5 h is not derived from starch degradation, then the fermentation stoichiometry more closely approximates the formate/acetate/ethanol stoichiometry of 2:1:1 reported by others (7, 13). However, even if we adjust our calculations and assume that most of the acetate at 0.5 h is medium-derived, the sum of acetate and ethanol still remains slightly in excess of the detected levels of formate. This finding is consistent with CO2 production coming from pyruvate decarboxylation which, if mediated by PFR1, could contribute to the production of fermentative H2 in the parental strain.
The pathways leading to succinate production require CO2 input, which likely originates from the following: (a) PFR1; (b) PDC; (c) pyruvate dehydrogenase during anoxic acclimation when cells are metabolizing residual O2; or (d) reserves of bicarbonate that accumulate within cells during aerobic culturing. It is likely that PFR1 is still active to an extent and that CO2 produced by this pathway is used to carboxylate pyruvate or PEP. Ferredoxin could then potentially be used to generate NAD(P)H for succinate production. Together, the data suggest that although hydrogenase activity does not appear to be exclusively responsible for ferredoxin oxidation in the parental strain, its absence in cells that have acclimated to dark, anoxic conditions causes the activation of metabolic pathways leading to succinate synthesis. As the amount of succinate produced in the mutant exceeds the H2 produced by the parental strain by 5-10-fold, the rerouting of metabolites does not simply compensate at a stoichiometric level for the loss of the electron valve activity of the hydrogenase. Regulatory processes must become active that generate a new cellular homeostasis. The regulators that control this process have not been identified, although it is conceivable that the loss of hydrogenase activity results in increased cellular reductant that signals changes in gene expression via reduced NAD(P) or redox sensors such as thioredoxins (47, 48).
Transcriptome—The microarray data are consistent with the development of fermentation metabolism during dark anaerobiosis in both the mutant and parental strains. Transcripts encoding the hydrogenase maturation factor HYDG, and starch and pyruvate-catabolizing enzymes, including amylases, acetate kinase, pyruvate kinase, and phosphate acetyltransferase, all increase in the mutant and parental strains. Moreover, transcripts for pyruvate dehydrogenase kinase, which phosphorylates and inhibits pyruvate dehydrogenase, also increases in the mutant relative to the parental strain. The pyruvate dehydrogenase complex oxidizes pyruvate-forming NADH, acetyl-CoA, and CO2 during aerobic metabolism, and inhibition of pyruvate dehydrogenase redirects pyruvate metabolism into fermentation pathways.
Dark anaerobiosis also elicits an increase in the abundance of several transcripts encoding enzymes involved in amino acid catabolism or synthesis, such as lysine decarboxylase, proline dehydrogenase, glutamate kinase, threonine synthase, cystathionine β-lyase, and asparagine synthase. This is likely a function of proteome reorganization as enzymes used in aerobic metabolism are degraded, and fermentation enzymes are synthesized. Transcripts also increase for cysteine desulfurase, an enzyme that mobilizes sulfide for the formation of FeS clusters that would be required by several fermentative FeS proteins, including ADH1, PFLA1, and PFR1.
The microarray data also indicate that dark anaerobiosis leads to increases in transcripts encoding subunits of the mitochondrial NADH-oxidizing complex I, including NUOA8, which appears to be the most differentially up-regulated gene in the mutant relative to the parental strain after 4 h of anoxic acclimation. Other significant differences between the mutant and the parental strain include increases in levels of transcripts in the mutant for nitrate reductase (NIT1) and the sulfate adenylyltransferase. ATS1 activates sulfate for subsequent reduction. Sulfite reductase transcripts also increase during anaerobiosis. Reductions of nitrate, sulfate, and fumarate have all been linked to anaerobic respiration, where these substrates replace O2 as alternative electron acceptors (49-51) and the reductive process can be electrogenically coupled to the production of ATP in some instances (52-54). This method of ATP production, in addition to substrate level phosphorylation, can provide a substantial source of energy for certain organisms during anaerobiosis. Although it is tempting to conclude that increases in transcripts encoding components of complex I, NIT1, ATS1, sulfite reductase, and fumarate reductase reflect activation of components of anaerobic respiration induced in response to anoxia, it should be noted that most of these C. reinhardtii enzymes are not typically associated with anaerobic respiration. In the case of nitrate and sulfite reductases, each enzyme is most similar to assimilatory enzymes used in biosynthetic pathways. Moreover, in the case of NIT1, the cells were cultured in TAP medium, which does not contain nitrite/nitrate, and the C. reinhardtii genome does not appear to encode the enzyme ammonia monooxygenase, the first step in ammonia oxidation (55). It is therefore difficult to envision, under our experimental conditions, the origin of nitrite/nitrate. However, the conditions used in these studies neither reflect the variety of nutrient and temperature conditions experienced in the environment nor the multitude of direct and indirect interactions among the organisms that populate soil environments. Interestingly, the addition of nitrate to the C. reinhardtii culture medium suppresses H2 production (56), suggesting that the cells have the capacity to use substrates such as nitrate and perhaps sulfate as terminal electron acceptors during anaerobiosis, and thiosulfate formation has been reported in anaerobic extracts of C. reinhardtii (57).
It is also possible that differences in the levels of specific transcripts in the mutant relative to the parental strain reflect more general regulatory and signaling mechanisms. These differences may be elicited in response to the overall increase in abundance of intracellular reducing equivalents that result from both a loss of hydrogenase activity and anaerobiosis. This physiological state may mirror cells experiencing specific conditions, which are often dynamic, in the natural environment. Experimental conditions used in the laboratory are less dynamic and often artificial relative to what C. reinhardtii would experience in the environment. For these studies, aerobic cultures were illuminated, stirred, and vigorously aerated by bubbling with a mixture of CO2-enriched air, which favors high photosynthetic activity and aerobic respiration. Furthermore, anaerobic samples were rigorously controlled to exclude all O2. In natural settings, the transition from aerobic conditions to anaerobiosis is likely more gradual, and increased expression of some genes encoding polypeptides, such as those of complex I, may reflect regulatory responses that have evolved to oxidize cellular reductant during a transition first to hypoxia, rather than to complete anoxia. A much more comprehensive understanding of anoxic cellular processes, accurate subcellular localization of relevant enzymes, and a detailed understanding of metabolite exchange between organelles is required for the following: (a) to develop an improved understanding of hydrogenase activity in cellular metabolism during dark anaerobiosis; (b) to determine whether other potential terminal electron acceptors, such as nitrate and sulfate, function to alleviate intracellular redox stress; and (c) to examine whether enhanced expression of some genes, as revealed by microarray analyses, translates into increased enzymatic activity.
Summary—In summary, the fermentative metabolism of C. reinhardtii has been shown to readily accommodate the loss of hydrogenase activity by synthesizing succinate, which would contribute to the reoxidization of NADH and sustain glycolysis for the production of ATP during anaerobiosis. Activation of this pathway, which is minimally active in the parental strain (based on transcript and metabolite analyses), further illustrates the metabolic flexibility of C. reinhardtii, an alga that appears to be well adapted to withstand a variety of environmental challenges, including anoxia. It is possible that the ability to synthesize succinate in the absence of hydrogenase activity reflects an adaptation used in natural settings in which fermentation metabolism is elicited under conditions in which hydrogenase activity is inhibited (e.g. brief exposures to gases such as CO or O2, which are likely not strictly excluded in certain environments). The transcriptome data indicate that the loss of hydrogenase activity results in the increased expression of several transcripts associated with a variety of cellular redox processes. Some of these processes have been associated with aerobic respiration, indicating that there are still several aspects of anoxic acclimation that are not fully understood and warrant further investigation. The metabolic capacity of C. reinhardtii, particularly during anaerobiosis, is extremely versatile and complicated. This alga readily responds to variations in culturing and assay conditions in ways that we are just beginning to elucidate. The availability of the C. reinhardtii genome sequence, high throughput omics-based approaches, and mutants disrupted in a variety of cellular process will provide the foundation to further examine and understand the metabolic flexibility of this fascinating phototroph.
Supplementary Material
This work was supported by the Office of Biological and Environmental Research, GTL Program, Office of Science, United States Department of Energy (to A. R. G., M. C. P., and M. S.), by National Science Foundation Grant MCB 0235878 (to A. R. G.), and by Air Force Office of Scientific Research Grant FA9550-05-1-0365 (to M. C. P.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables I and II and Fig. 1.
Footnotes
The abbreviations used are: TAP, Tris/acetate/phosphate medium; qPCR, quantified by reverse transcriptase real time PCR; HPLC, high pressure liquid chromatography; PEP, phosphoenolpyruvate; PEPC, phosphoenolpyruvate carboxylase; PCK, phosphoenolpyruvate carboxylase; MDH, malate dehydrogenase; FUM, fumarase; FMR, fumarate reductase; MME, malic enzyme.
References
- 1.Merchant, S. S., Prochnik, S. E., Vallon, O., Harris, E. H., Karpowicz, S. J., Witman, G. B., Terry, A., Salamov, A., Fritz-Laylin, L. K., Marechal-Drouard, L., Marshall, W. F., Qu, L. H., Nelson, D. R., Sanderfoot, A. A., Spalding, M. H., Kapitonov, V. V., Ren, Q., Ferris, P., Lindquist, E., Shapiro, H., Lucas, S. M., Grimwood, J., Schmutz, J., Cardol, P., Cerutti, H., Chanfreau, G., Chen, C. L., Cognat, V., Croft, M. T., Dent, R., Dutcher, S., Fernandez, E., Fukuzawa, H., Gonzalez-Ballester, D., Gonzalez-Halphen, D., Hallmann, A., Hanikenne, M., Hippler, M., Inwood, W., Jabbari, K., Kalanon, M., Kuras, R., Lefebvre, P. A., Lemaire, S. D., Lobanov, A. V., Lohr, M., Manuell, A., Meier, I., Mets, L., Mittag, M., Mittelmeier, T., Moroney, J. V., Moseley, J., Napoli, C., Nedelcu, A. M., Niyogi, K., Novoselov, S. V., Paulsen, I. T., Pazour, G., Purton, S., Ral, J. P., Riano-Pachon, D. M., Riekhof, W., Rymarquis, L., Schroda, M., Stern, D., Umen, J., Willows, R., Wilson, N., Zimmer, S. L., Allmer, J., Balk, J., Bisova, K., Chen, C. J., Elias, M., Gendler, K., Hauser, C., Lamb, M. R., Ledford, H., Long, J. C., Minagawa, J., Page, M. D., Pan, J., Pootakham, W., Roje, S., Rose, A., Stahlberg, E., Terauchi, A. M., Yang, P., Ball, S., Bowler, C., Dieckmann, C. L., Gladyshev, V. N., Green, P., Jorgensen, R., Mayfield, S., Mueller-Roeber, B., Rajamani, S., Sayre, R. T., Brokstein, P., Dubchak, I., Goodstein, D., Hornick, L., Huang, Y. W., Jhaveri, J., Luo, Y., Martinez, D., Ngau, W. C., Otillar, B., Poliakov, A., Porter, A., Szajkowski, L., Werner, G., Zhou, K., Grigoriev, I. V., Rokhsar, D. S., and Grossman, A. R. (2007) Science 318 245-25017932292 [Google Scholar]
- 2.Grossman, A. R., Croft, M., Gladyshev, V. N., Merchant, S. S., Posewitz, M. C., Prochnik, S., and Spalding, M. H. (2007) Curr. Opin. Plant Biol. 10 190-198 [DOI] [PubMed] [Google Scholar]
- 3.Kosourov, S., Seibert, M., and Ghirardi, M. L. (2003) Plant Cell Physiol. 44 146-155 [DOI] [PubMed] [Google Scholar]
- 4.Ghirardi, M. L., Posewitz, M. C., Maness, P.-C., Dubini, A., Yu, J., and Seibert, M. (2007) Annu. Rev. Plant Biol. 58 71-91 [DOI] [PubMed] [Google Scholar]
- 5.Atteia, A., van Lis, R., Gelius-Dietrich, G., Adrait, A., Garin, J., Joyard, J., Rolland, N., and Martin, W. (2006) J. Biol. Chem. 281 9909-9918 [DOI] [PubMed] [Google Scholar]
- 6.Mus, F., Dubini, A., Seibert, M., Posewitz, M. C., and Grossman, A. R. (2007) J. Biol. Chem. 282 25475-25486 [DOI] [PubMed] [Google Scholar]
- 7.Gfeller, R. P., and Gibbs, M. (1984) Plant Physiol. 75 212-218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steunou, A. S., Bhaya, D., Bateson, M. M., Melendrez, M. C., Ward, D. M., Brecht, E., Peters, J. W., Kuhl, M., and Grossman, A. R. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 2398-2403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melis, A., Zhang, L., Forestier, M., Ghirardi, M. L., and Seibert, M. (2000) Plant Physiol. 122 127-136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kruse, O., Rupprecht, J., Mussgnug, J. H., Dismukes, G. C., and Hankamer, B. (2005) Photochem. Photobiol. Sci. 4 957-970 [DOI] [PubMed] [Google Scholar]
- 11.Hankamer, B., Lehr, F., Rupprecht, J., Mussgnug, J. H., Posten, C., and Kruse, O. (2007) Physiol. Plant 131 10-21 [DOI] [PubMed] [Google Scholar]
- 12.Hemschemeier, A., and Happe, T. (2005) Biochem. Soc. Trans. 33 39-41 [DOI] [PubMed] [Google Scholar]
- 13.Kreutzberg, K. (1984) Physiol. Plant. 61 87-94 [Google Scholar]
- 14.Ohta, S., Miyamoto, K., and Miura, Y. (1987) Plant Physiol. 83 1022-1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hemschemeier, A., Jacobs, J., and Happe, T. (2008) Eukaryot. Cell 7 518-526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forestier, M., King, P., Zhang, L., Posewitz, M., Schwarzer, S., Happe, T., Ghirardi, M. L., and Seibert, M. (2003) Eur. J. Biochem. 270 2750-2758 [DOI] [PubMed] [Google Scholar]
- 17.Happe, T., and Kaminski, A. (2002) Eur. J. Biochem. 269 1022-1032 [DOI] [PubMed] [Google Scholar]
- 18.Happe, T., Mosler, B., and Naber, J. D. (1994) Eur. J. Biochem. 222 769-774 [DOI] [PubMed] [Google Scholar]
- 19.Roessler, P. G., and Lien, S. (1984) Plant Physiol. 75 705-709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Posewitz, M. C., King, P. W., Smolinski, S. L., Zhang, L., Seibert, M., and Ghirardi, M. L. (2004) J. Biol. Chem. 279 25711-25720 [DOI] [PubMed] [Google Scholar]
- 21.Posewitz, M. C., King, P. W., Smolinski, S. L., Smith, R. D., Ginley, A. R., Ghirardi, M. L., and Seibert, M. (2005) Biochem. Soc. Trans. 33 102-104 [DOI] [PubMed] [Google Scholar]
- 22.Posewitz, M. C., Mulder, D. W., and Peters, J. W. (2008) Curr. Chem. Biol. 2 178-199 [Google Scholar]
- 23.McGlynn, S. E., Ruebush, S. S., Naumov, A., Nagy, L. E., Dubini, A., King, P. W., Broderick, J. B., Posewitz, M. C., and Peters, J. W. (2007) J. Biol. Inorg. Chem. 12 443-447 [DOI] [PubMed] [Google Scholar]
- 24.Bock, A., King, P. W., Blokesch, M., and Posewitz, M. C. (2006) Adv. Microb. Physiol. 51 1-71 [DOI] [PubMed] [Google Scholar]
- 25.Harris, E. H. (1989) The Chlamydomonas Sourcebook. A Comprehensive Guide to Biology and Laboratory Use, pp. 780-785, Academic Press, San Diego [DOI] [PubMed]
- 26.Ghirardi, M., Togasaki, R., and Seibert, M. (1997) Appl. Biochem. Biotech. 63-5, 141-151 [DOI] [PubMed] [Google Scholar]
- 27.Livak, K. J., and Schmittgen, T. D. (2001) Methods (San Diego) 25 402-408 [DOI] [PubMed] [Google Scholar]
- 28.Eberhard, S., Jain, M., Im, C. S., Pollock, S., Shrager, J., Lin, Y., Peek, A. S., and Grossman, A. R. (2006) Curr. Genet. 49 106-124 [DOI] [PubMed] [Google Scholar]
- 29.Im, C. S., Zhang, Z., Shrager, J., Chang, C. W., and Grossman, A. R. (2003) Photosynth. Res. 75 111-125 [DOI] [PubMed] [Google Scholar]
- 30.Li, Y., Horsman, M., Wu, N., Lan, C. Q., and Dubois-Calero, N. (2008) Biotechnol. Prog. 24 815-820 [DOI] [PubMed] [Google Scholar]
- 31.Melis, A., Seibert, M., and Ghirardi, M. L. (2007) Adv. Exp. Med. Biol. 616 110-121 [DOI] [PubMed] [Google Scholar]
- 32.Nagy, L. E., Meuser, J. E., Plummer, S., Seibert, M., Ghirardi, M. L., King, P. W., Ahmann, D., and Posewitz, M. C. (2007) Biotechnol. Lett. 29 421-430 [DOI] [PubMed] [Google Scholar]
- 33.Ghirardi, M. L., King, P. W., Posewitz, M. C., Maness, P. C., Fedorov, A., Kim, K., Cohen, J., Schulten, K., and Seibert, M. (2005) Biochem. Soc. Trans. 33 70-72 [DOI] [PubMed] [Google Scholar]
- 34.Quinn, J. M., Eriksson, M., Moseley, J. L., and Merchant, S. (2002) Plant Physiol. 128 463-471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moseley, J., Quinn, J., Eriksson, M., and Merchant, S. (2000) EMBO J. 19 2139-2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rochaix, J. D. (2002) FEBS Lett. 529 34-38 [DOI] [PubMed] [Google Scholar]
- 37.Melis, A., and Happe, T. (2001) Plant Physiol. 127 740-748 [PMC free article] [PubMed] [Google Scholar]
- 38.Kruse, O., Rupprecht, J., Bader, K. P., Thomas-Hall, S., Schenk, P. M., Finazzi, G., and Hankamer, B. (2005) J. Biol. Chem. 280 34170-34177 [DOI] [PubMed] [Google Scholar]
- 39.Harris, E. H. (2001) Annu. Rev. Plant Physiol. Plant Mol. Biol. 52 363-406 [DOI] [PubMed] [Google Scholar]
- 40.Weaver, P. F., Lien, S., and Seibert, M. (l980) Solar Energy 24 3-45 [Google Scholar]
- 41.Seibert, M., King, P., Posewitz, M. C., Melis, A., and Ghirardi, M. L. (2008) Bioenergy (Wall, J., Demain, A., and Harwood, C., eds) pp. 273-291, American Society for Microbiology, Washington, D. C.
- 42.Bologna, F. P., Andreo, C. S., and Drincovich, M. F. (2007) J. Bacteriol. 189 5937-5946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee, S. J., Song, H., and Lee, S. Y. (2006) Appl. Environ. Microbiol. 72 1939-1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stols, L., and Donnelly, M. I. (1997) Appl. Environ. Microbiol. 63 2695-2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Millard, C. S., Chao, Y. P., Liao, J. C., and Donnelly, M. I. (1996) Appl. Environ. Microbiol. 62 1808-1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Allmer, J., Naumann, B., Markert, C., Zhang, M., and Hippler, M. (2006) Proteomics 6 6207-6220 [DOI] [PubMed] [Google Scholar]
- 47.Wagner, V., Gessner, G., Heiland, I., Kaminski, M., Hawat, S., Scheffler, K., and Mittag, M. (2006) Eukaryot. Cell 5 457-468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lemaire, S. D., Guillon, B., Le Marechal, P., Keryer, E., Miginiac-Maslow, M., and Decottignies, P. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 7475-7480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Unden, G., and Bongaerts, J. (1997) Biochim. Biophys. Acta 1320 217-234 [DOI] [PubMed] [Google Scholar]
- 50.Richardson, D. J., Berks, B. C., Russell, D. A., Spiro, S., and Taylor, C. J. (2001) Cell. Mol. Life Sci. 58 165-178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matias, P. M., Pereira, I. A., Soares, C. M., and Carrondo, M. A. (2005) Prog. Biophys. Mol. Biol. 89 292-329 [DOI] [PubMed] [Google Scholar]
- 52.Stoimenova, M., Igamberdiev, A. U., Gupta, K. J., and Hill, R. D. (2007) Planta 226 465-474 [DOI] [PubMed] [Google Scholar]
- 53.Ozawa, K., Meikari, T., Motohashi, K., Yoshida, M., and Akutsu, H. (2000) J. Bacteriol. 182 2200-2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhandari, B., Naik, M. S., and Nicholas, D. J. D. (1984) FEBS Lett. 168 321-326 [Google Scholar]
- 55.Arp, D. J., Chain, P. S., and Klotz, M. G. (2007) Annu. Rev. Microbiol. 61 503-528 [DOI] [PubMed] [Google Scholar]
- 56.Aparicio, P. J., Azuara, M. P., Ballesteros, A., and Fernandez, V. M. (1985) Plant Physiol. 78 803-806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hodson, R. C., and Schiff, J. A. (1971) Plant Physiol. 47 296-299 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.