Abstract
Activating mutations in JAK1 have been reported in acute lymphoblastic leukemias, but little is known about the mechanisms involved in their constitutive activation. Here, we studied the ability of JAK1 V658F and A634D to activate the Janus kinase (JAK)/STAT pathway upon ectopic expression in HEK293 cells alone or together with the other components of the interleukin-9 receptor complex (IL-9Rα, γc, and JAK3). Expression of JAK1 mutants alone failed to trigger STAT activation, but co-expression of the IL-9Rα chain promoted JAK1 mutant phosphorylation and STAT activation. Mutation of the FERM domain of JAK1, which is critical for cytokine receptor association, or of the single tyrosine of IL-9Rα involved in STAT recruitment abolished this activity, indicating that JAK1 mutants need to associate with a functional IL-9Rα to activate STAT factors. Several lines of evidence indicated that IL-9Rα homodimerization was involved in this process. IL-9Rα variants with mutations of the JAK-interacting BOX1 region not only failed to promote JAK1 activation but also acted as dominant negative forms reverting the effect of wild-type IL-9Rα. Coimmunoprecipitation experiments also showed the formation of IL-9Rα homodimers. Interestingly, STAT activation was partially inhibited by expression of γc, suggesting that overlapping residues are involved in IL-9Rα homodimerization and IL-9Rα/γc heterodimerization. Co-expression of wild-type JAK3 partially reverted the inhibition by γc, indicating that JAK3 cooperates with JAK1 mutants within the IL-9 receptor complex. Similar results were observed with IL-2Rβ. Taken together, our results show that IL-9Rα and IL-2Rβ homodimers efficiently mediate constitutive activation of ALL-associated JAK1 mutants.
Janus kinases (JAKs)5 represent a family of four non-receptor tyrosine kinases (JAK1, JAK2, JAK3, and TYK2) that is associated with cytokine receptors of no intrinsic kinase activity (1). During the last few years several acquired JAK mutations have been identified in different malignancies. These mutations led to a gain of kinase function and are tumorigenic. The best example is the JAK2 V617F mutation associated with myeloproliferative neoplasms (2–5). JAK2 V617F retains its ability to interact with cytokine receptors (6), and an intact FERM domain, which mediates recruitment to cytokine receptors, is required for inducing transformation of hematopoietic cells (7).
At physiological levels of expression, JAK2 V617F needs to be associated to JAK2 binding homodimeric type I cytokine receptors such as the erythropoietin receptor (EPOR) or the thrombopoietin receptor (TPOR) to allow constitutive signaling (8, 9). Because EpoR is a preformed dimer in the absence of ligand (10), a model was proposed where dimerization of JAK2 V617F via interactions with a preformed EpoR dimer promotes signaling by JAK2 V617F (8). Constitutive and increased erythropoietin or thrombopoietin signaling provide a mechanism for the erythocytosis and thrombocytosis observed in these disorders (11). The A572V mutation in JAK3 has later been identified in patients with acute megakaryoblastic leukemia (12).
Recently, mutations in JAK1, such as A634D, R724H, R879C (13), and the V658F mutation (14) have been identified in adult B and T cell-acute lymphoblastic leukemia (ALL). These mutations allow for constitutive JAK1 activation when overexpressed in JAK1-deficient cell lines (11, 13), as was shown for JAK2 V617F in JAK2-deficient cell lines (2). Moreover, these A634D and R724H mutants induce the autonomous growth of the cytokine-dependent Ba/F3 cell line, whereas the A634D and R879C mutants protect the murine ALL cell line BW5147 from dexamethasone-induced apoptosis, indicating that they represent gain of function mutations. However, the potential role of JAK1 binding receptors, which are all heterodimeric, in the mechanism of mutant JAK1-induced constitutive signaling has never been studied.
IL-9 is a multifunctional TH2 cytokine that was shown to be involved in T cell tumorigenesis in mouse and in humans (15–18). Moreover, in vitro dysregulation of the IL-9 response is associated with autonomous cell growth and malignant transformation of lymphoid cells, leading to the constitutive activation of JAK-STAT pathway (19–21). Its activities are mediated via a heterodimeric receptor complex formed by the IL-9Rα chain (IL-9Rα), which associates with JAK1, and the IL-2Rγ chain, also called γc (common γ chain), which associates with JAK3. γc is in addition involved in IL-2, -4, -7, -15, and -21 signaling, a family of cytokines involved in lymphocyte development and/or activation. IL-9Rα is sufficient to confer high affinity cytokine binding, but formation of the heterodimeric complex with γc is needed for signal transduction (21). Upon IL-9 binding, JAK1 and JAK3 are cross-activated, and IL-9Rα is phosphorylated on a single tyrosine (Tyr-116). This phosphorylated tyrosine is the only docking site for STAT1, -3, and -5, the STATS activated by IL-9 (22).
In this paper, in order to study the potential interactions between ALL-associated JAK1 mutants and the different components of IL-9 receptor complex, we co-expressed these different proteins in HEK293 cells, which lack IL-9Rα, γc, and JAK3. Our data show that JAK1 mutants alone fail to activate STAT transcriptional factors but that this process/activation is promoted by IL-9Rα homodimerization in the absence of γc and JAK3.
EXPERIMENTAL PROCEDURES
Cells and Cell Culture—HEK293 human embryonic kidney cells and COS-7 cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. These cells do not express endogenous IL-9Rα, γc, and JAK3. Human fibrosarcoma U4C cells (JAK1-deficient) and γ2A cells (JAK2-deficient) were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. Recombinant human IL-9 was produced in the baculovirus system in our laboratory and was purified as previously described (23). Recombinant human IL-2 was provided by Chiron.
Plasmid Constructions—V658F and A634D mouse JAK1 mutants were described previously (11, 13). Double JAK1 mutants V658F/Y107A and A634D/Y107A were generated using QuikChange XL II site-directed mutagenesis kit (Stratagene, La Jolla, CA). The Y107A mutation was previously described to abolish the binding of JAK1 to cytokine receptors (24). Both wild-type and mutated murine JAK1 cDNAs were subcloned into the pMX-GFP biscistronic retroviral vector upstream of the internal ribosome entry site (IRES) (11, 13). Human IL-9Rα and IL-9Rα Y116F were generated as previously described (22) and were subcloned into the pREX-IRES-CD4 vector (25) or into the pEFBos-puro vector (22). The ability of murine JAK1 to transduce the signal from human IL-9Rα has been previously described (22, 26). The IL-9Rα BOX1 mutant was generated by mutation of two proline residues to two serine residues in the BOX1 motif (PXPtoSXS) of wild-type IL-9Rα using the GeneEditor in vitro site-directed mutagenesis system (Promega, Madison, WI). Clones obtained were sequenced using DYEnamic ET Dye Terminator kit (Amersham Biosciences). The IL-9Rα IC34 construct was generated as previously described (22). Myc and HA tags were introduced after the signal sequence cleavage site in the cDNA coding for human IL-9Rα as described (10). The IL-2Rβ and IL-2Rγ (γc) were cloned to pREX-IRES-CD4 vector (25).
Dual Luciferase Assay—STAT1, STAT3, and STAT5 transcriptional activity was assessed by measurements of luciferase expression in HEK293, COS-7, U4C, and γ2A cells upon transient transfection of appropriate cDNA constructs and luciferase reporter vectors, namely pGL3-pap1-luc, pLHRE-luc, or pGRR5-luc. Another reporter plasmid, Renilla luciferase (pRLTk, Promega), was co-transfected as an internal transfection control. STAT5-mediated transcription was evaluated with the pLHRE-luc reporter gene constructs harboring tandem copies of the STAT5-inducible lactogenic hormone response element (LHRE) of the rat β-casein gene promoter, inserted upstream a luciferase gene (27). STAT3-mediated transcription was evaluated with pGL3-pap1-luc plasmid containing the luciferase gene under the control of the STAT3-inducible rat Pap1 (pancreatitis-associated protein-1) promoter (28). STAT1, STAT3, and STAT5-mediated transcription was evaluated using the pGRR5-luc construct (provided by Dr. P. Brennan, Imperial Cancer Research Fund, London, UK) that contains five copies of the STAT-binding site of the FcγRI gene inserted upstream from a luciferase gene controlled by the thymidine kinase promoter. Transient transfection of HEK293, COS-7, U4C, and γ2A cells by Lipofectamine (Invitrogen) was previously described (29). Briefly, cells were seeded in 24-well plates at 2 × 105 cells/well 1 day before transfection. Transfection was carried out according to the manufacturer's recommendations using 250 ng of the appropriate constructs, 500 ng of pGL3-pap1 or pLHRE reporter plasmids, 50 ng of pRLTk control plasmid, and empty vector to normalize total transfected DNA to 1.5 μg. 4 h after transfection cells were stimulated or left unstimulated for 20 h. 24 h after transfection cells were lysed by 150 μl of Passive Lysis Buffer 1× supplied by Promega. Luciferase assays were performed using the dual luciferase reporter assay kit (Promega).
Western Blots—For Western blot analysis, 106 HEK293 cells were seeded in 6-well plates 1 day before transient transfection. In brief, HEK293 cells were transfected with 3.75 μg of different constructs depending on the experimental settings using a standard calcium phosphate transfection procedure (Promega). The day after transfection cells were collected and lysed in 250 μl of Laemmli buffer (Bio-Rad) using a 20-gauge syringe. Cellular lysates were boiled for 3 min before loading 20 μlon 12% precast Tris-glycine gels (Invitrogen) and electrophoretically transferred to nitrocellulose membranes (Hybond-C; Amersham Biosciences). After blocking the membranes in 5% milk/Tris-buffered saline-Tween (TBS-Tween), immunoblotting was performed by overnight incubations at 4 °C with primary antibodies in 0.1% TBS-Tween, using the dilution recommended by the manufacturer. After 3 × 5 min of washing in 0.1% TBS-Tween, the membranes were incubated with secondary anti-rabbit-horseradish peroxidase (HRP) or anti-mouse-HRP antibodies from Cell Signaling Technology (Beverly, MA), diluted 1/5000 in 5% phosphate-buffered saline-TBS-Tween. After 3 × 5 min of washing, a SuperSignal West Pico detection kit (Pierce) and Kodak Biomax Light Film were used for detection. The following phosphospecific antibodies were used: anti-pY1001/1002 JAK1 and anti-pY705 Stat3 (Cell Signaling Technology). Blots were re-probed with anti-JAK1, anti-STAT3 (Cell Signaling Technology), and anti-β-actin (Sigma) antibodies as control.
Immunoprecipitation—For immunoprecipitation, 6 × 106 HEK293 cells were seeded in 100-mm plates 1 day before transfection. The day after, cells were co-transfected using a standard calcium phosphate transfection procedure (Promega) with the 7 μg of Myc-tagged IL-9Rα together with 7 μg of HA-tagged IL-9Rα or HA-tagged EpoR. The day after transfection, cells were collected and lysed in 1 ml of ice-cold modified radioimmune precipitation lysis buffer (1% Nonidet P-40, 0.25% deoxycholate, 0.1% SDS, 50 mm Tris (pH 8), 300 mm NaCl, 1 mm EDTA, 2 mm Na3VO4, 1 mm NaF, and complete protease inhibitor mixture tablet (Roche Applied Science) used according to the manufacturer's recommendations) (22) using a 20-gauge syringe and kept 30 min on ice. Clarified lysates were immunoprecipitated with 2 μg of anti-c-Myc antibodies (Santa Cruz) and 20 μl of protein-G-agarose (Roche Applied Science). Immunoprecipitates were boiled, subjected to Tris-glycine gel electrophoresis, and electrotransferred onto nitrocellulose membranes followed by immunoblotting with anti-HA1.1 (Covance) and anti-c-Myc antibody (Santa Cruz). Washing and detection were performed as described above.
FACS Analysis of IL-9Rα Cell Surface Expression—The cell surface expression of the different IL-9Rα constructs was verified by FACS on an aliquot of the cells used for the luciferase assay 1 day after transient transfection. FACS analysis was performed on a BD Biosciences FACSCalibur™ flow cytometer after staining cells with biotinylated anti-human IL-9Rα antibody (22) followed with phycoerythrin-conjugated streptavidin (BD Biosciences) As a control, an aliquot of the transfected cells was stained only with phycoerythrin-conjugated streptavidin (BD Biosciences) antibody.
Reverse Transcription and Quantitative PCR—Total RNA was extracted from the aliquot of the HEK293 cells used for the luciferase assay 1 day after transient transfection. Roughly 5 × 105 cells was used to extract RNA using the TriPure isolation reagent (Roche Applied Science) according to the manufacturer's instructions. Reverse transcription was performed on 1 μg of total RNA with an oligo-(dT) primer (Roche Applied Science) and Moloney murine leukemia virus reverse transcription (Invitrogen). Quantitative PCR reactions were performed using primer sets corresponding to murine JAK1 or human JAK1 with qPCR™ Mastermix for SYBR® Green I (Eurogentec). A series of dilutions of the respective PCR product subcloned into pCR2.1-TOPO vector (Invitrogen) was used a standard for quantification. The sequences of the species-specific JAK1 primers (final concentration, 300 nm) were: mJAK1, 5′-GGAGTGCAGTATCTCTCCTCTCT-3′ (forward) and 5′-CCATGCCCAGGCACTCATTTTCA-3′ (reverse); hJAK1, 5′-TCTTGGAATCCAGTGGAGGCATAAA-3′ (forward) and 5′-CACTCTTCCCGGATCTTGTTTTTCT-3′ (reverse). Samples were first heated for 2 min at 50 °C then 10 min at 95 °C. cDNA was amplified by 40 cycles of a two-step PCR program at 95 °C for 15 s and 60 °C for 1 min. Melting point analysis was carried out by heating the amplicon from 60 to 95 °C.
RESULTS
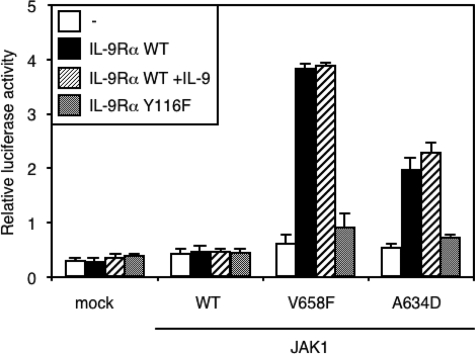
ALL-associated JAK1 Mutants (V658F and A634D) Induce Constitutive STAT Activation in the Presence of IL-9Rα—ALL-associated JAK1 mutants induce constitutive signaling after ectopic expression in Ba/F3 or BW5147 hematopoietic cells (13). These cells presumably express many receptor complexes that could bind the mutant JAKs and promote constitutive signaling. To study the role of such receptors, we co-expressed components of the IL-9 receptor complex together with JAK1 mutants in HEK293 cells, which are deficient for γc, IL-9Rα, and JAK3. Expression of two ALL-associated JAK1 mutants alone (V658F and A634D) did not induce a significant increase in the level of STAT3 transcriptional activity as assessed by a luciferase assay (Fig. 1). However, co-expression of IL-9Rα with JAK1 mutants (but not WT JAK1) in the absence of JAK3 and γc increased STAT3 activation. Similar results were obtained for STAT5 activation using pLHRE-luc reporter and with the pGRR5-luc reporter, which also responds to STAT1 (data not shown). Stimulation with IL-9 had no effect on IL-9Rα-mediated activation of STAT3 by JAK1 mutants, which is in line with the fact that, in the absence of JAK3 and γc, IL-9 receptor signaling complex cannot support IL-9-induced signal transduction. To prove that mutant JAK1-induced STAT3 activation was dependent on the presence of a functional IL-9Rα, we co-expressed the defective IL-9Rα Y116F, in which the only STAT-recruiting tyrosine residue Tyr-116 was mutated to phenylalanine. We previously showed that this mutation abolishes STAT activation by IL-9 (22). As expected, co-expression of the defective IL-9Rα Y116F did not promote mutant JAK1-induced constitutive STAT3 signaling (Fig. 1), indicating that recruitment of STATs by the IL-9Rα phosphotyrosine was necessary for mutant JAK1-induced constitutive signaling.
FIGURE 1.
ALL-associated JAK1 mutants (V658F and A634D) induce constitutive STAT3 activation in the presence of IL-9Rα. HEK293 cells were transiently transfected with different JAK1 constructs alone or in combination with IL-9Rα or the mutated IL-9Rα Y116F. The STAT3-responsive pGL3-pap1 construct was used as luciferase reporter. 4 h post-transfection, cells were incubated for 20 h with or without IL-9 before the luciferase assay. Results are the mean ± variation of duplicate samples. Similar results were obtained in three independent experiments.
These results contrast with a previous report showing that expression of JAK1 V658F in JAK1-deficient U4C fibrosarcoma cells allows for constitutive STAT3 activation (11). To address the hypothesis that this apparent discrepancy was due to the lack of expression of endogenous JAK1 in U4C cells, we measured STAT3 activation in JAK1-deficient U4C or JAK2-deficient γ2A fibrosarcoma cells transfected with wild-type or V658F JAK1. In U4C cells expression of JAK1 V658F alone allowed for constitutive STAT3 activation (8-fold increased luciferase production, as compared with wild-type JAK1). However, co-expression of IL-9Rα chain induced a further 3-fold increase in STAT3 activation (supplemental Fig. 1A). By contrast, in γ2A cells expression of JAK1 V658F only marginally increased (1.6-fold) STAT3 transcriptional activity unless the IL-9Rα chain was co-expressed (supplemental Fig. 1B). To confirm that JAK1 V658F constitutive activity in U4C cells results from the absence of expression of wild-type JAK1, we cotransfected both isoforms into U4C cells. Co-expression of increasing amounts of wild-type JAK1 together with the JAK1 V658F significantly decreased STAT3 activation (supplemental Fig. 1C).
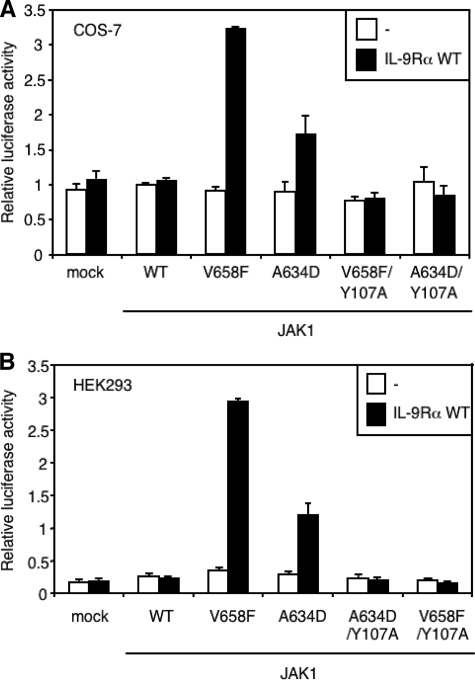
Y107A Mutation in the FERM Domain of JAK1 Mutants Abolishes Constitutive STAT3 Activation—To prove that the JAK1 mutants need to associate to IL-9Rα to constitutively activate STAT3, we mutated tyrosine residue Tyr-107 to alanine in the FERM domain of JAK1 V658F and A634D. Several residues conserved within the FERM domain of JAKs are crucial for cytokine receptor binding (30), and the Y107A mutation within the FERM domain of JAK1 has been shown to abolish JAK1 binding to the gp130 receptor chain (24). As shown in Fig. 2 for two different cell lines (HEK293 and COS-7 cells), the Y107A mutation also abolished the capacity of JAK1 mutants to activate STAT3 in the presence of IL-9Rα. These results indicate that an intact FERM domain of JAK1, essential for the binding to IL-9Rα, is needed for IL-9Rα-mediated constitutive activation of STAT3 by JAK1 mutants.
FIGURE 2.
Y107A mutation in the FERM domain of JAK1 mutants abolishes IL-9Rα-mediated constitutive STAT3 activation. COS-7 (A) or HEK293 (B) cells were transiently co-transfected with empty vector or different JAK1 constructs with intact or mutated Y107A FERM domain (V658F/Y107A or A634D/Y107A) and with or without IL-9Rα. The STAT3-responsive pGL3-pap1 construct was used as luciferase reporter. 24 h post-transfection cells were subjected to a luciferase assay. Results are the mean ± variation of duplicate samples. Similar results were obtained in three independent experiments in COS-7 and HEK293 cells.
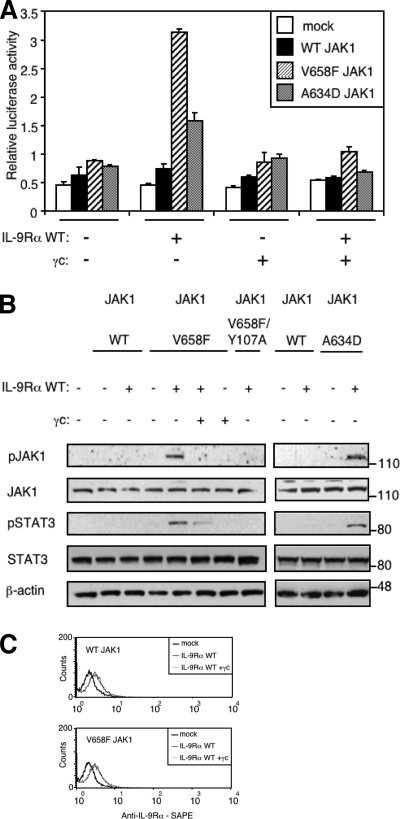
Co-expression of γc Inhibits IL-9Rα-mediated STAT3 Activation by the JAK1 V658F and A634D Mutants—The functional IL-9 receptor complex is formed by heterodimerization of IL-9Rα and γc, associated with JAK1 and JAK3, respectively. However, our results show that expression of IL-9Rα suffices for mutant JAK1-induced constitutive STAT3 activation. We, therefore, wondered how γc expression would affect this IL-9Rα-mediated constitutive STAT3 activation. Surprisingly, γc expression in the absence of JAK3 blocked IL-9Rα-mediated constitutive activation of STAT3 by JAK1 mutants, as shown by a luciferase assay (Fig. 3A). IL-9Rα-mediated activation of STAT5 by JAK1 mutants was modulated by γc in a similar manner (data not shown).
FIGURE 3.
Co-expression of γc inhibits IL-9Rα-mediated JAK1 phosphorylation and STAT3 activation. A, HEK293 cells were transiently co-transfected with empty vector, JAK1 wild-type or V658F, γc, or/and IL-9Rα in addition to the STAT3-responsive luciferase reporter pGL3-pap1. 24 h post-transfection cells were subjected to a luciferase assay. Results are the mean ± variation of duplicate samples. Similar results were obtained in three independent experiments. B, HEK293 cells were transiently co-transfected with different empty vector, JAK1 constructs, γc, or/and IL-9Rα. 24 h post-transfection, 106 cells were lysed and subjected to Western blot analysis. Phosphorylation of JAK1 and STAT3 was detected using specific anti-pJAK1 Tyr-1022/1023 and anti-pSTAT3 Tyr-705 antibodies. Membranes were reprobed with anti-JAK1, anti-STAT3 and anti-β-actin antibodies as control. Similar results were obtained in two independent experiments. C, HEK293 cells were transiently co-transfected with empty vector, JAK1 wild-type or V658F, and IL-9Rα with or without γc in addition to the STAT3-responsive luciferase reporter pGL3-pap1. One day post-transfection, an aliquot of cells was used to assess cell surface expression of IL-9Rα by FACS analysis using anti-human IL-9Rα antibody followed with phycoerythrin-conjugated streptavidin (SAPE).
To confirm our observations, we directly studied JAK1 phosphorylation by Western blot using an antibody that detects the phosphorylation of the activation loop Tyr-1022/1023, which reflects the catalytic activity of JAK1. Fig. 3B shows that neither wild-type nor JAK1 mutants were phosphorylated when expressed in HEK293 cells alone. As expected, in contrast to wild-type JAK1, co-expression of IL-9Rα with JAK1 V658F or JAK1 A634D increased JAK1 phosphorylation. This IL-9Rα-mediated JAK1 phosphorylation was blocked by co-expression of γc. Phosphorylation of JAK1 was also abolished by the Y107A mutation in the FERM domain. Phosphorylation of STAT3 paralleled JAK1 phosphorylation, confirming the results obtained using the luciferase assay.
A decrease in the expression of IL-9Rα by γc could be an obvious explanation of the γc inhibitory effect. To rule out this hypothesis, we measured the level of IL-9Rα expression by FACS. As shown in Fig. 3C, γc expression in HEK293 cells had no effect on IL-9Rα cell surface expression.
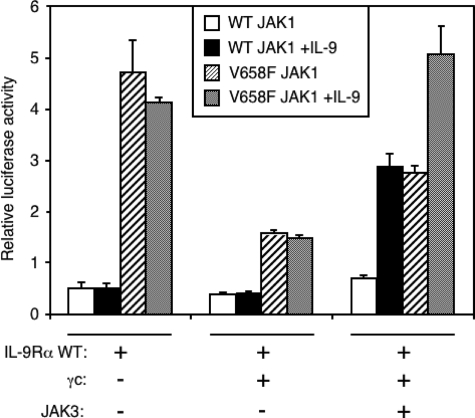
Co-expression of JAK3 Partially Reverses the γc Inhibitory Effect on IL-9Rα-mediated Activation of STAT3 by JAK1 V658F—To fully reconstitute the IL-9 receptor complex in HEK293 cells, we tested the effect of JAK3 expression on IL-9Rα-mediated STAT3 activation by JAK1 V658F. As shown in Fig. 4, co-expression of wild-type JAK1 with IL-9Rα alone or with γc did not induce STAT3 activation even after stimulation with IL-9. As expected, the co-expression of JAK3, IL-9Rα, and γc was required for IL-9-induced STAT3 activation. As previously shown, JAK1 V658F activated STAT3 in the presence of IL-9Rα alone, and this activation was inhibited by γc. In the latter situation, JAK3 co-expression partially reversed the inhibitory effect of γc on the IL-9Rα-mediated STAT3 activation by JAK1 V658F. Moreover, in the presence of a functional IL-9 receptor complex, IL-9 was able to increase STAT3 activation above the level obtained for the wild-type JAK1, suggesting that the V658F mutation also potentiates the response to IL-9. These results suggest that, when all the components of the IL-9 receptor complex are expressed, JAK1 V658F and JAK3 cross-activate themselves. Surprisingly, in the absence of IL-9, maximal constitutive activation was obtained with IL-9Rα alone.
FIGURE 4.
Co-expression of JAK3 partially abrogates the γc inhibitory effect on IL-9Rα-mediated STAT3 activation by JAK1 V658F. HEK293 cells were transiently co-transfected with JAK1 wild-type or V658F, γc, IL-9Rα, and/or with JAK3 as the last component of IL-9R complex in addition to the STAT3-responsive luciferase reporter pGL3-pap1. 4 h post-transfection cells were incubated for 20 h with or without IL-9 and subjected to a luciferase assay. Results are the mean ± variation of duplicate samples. Similar results were obtained in three independent experiments.
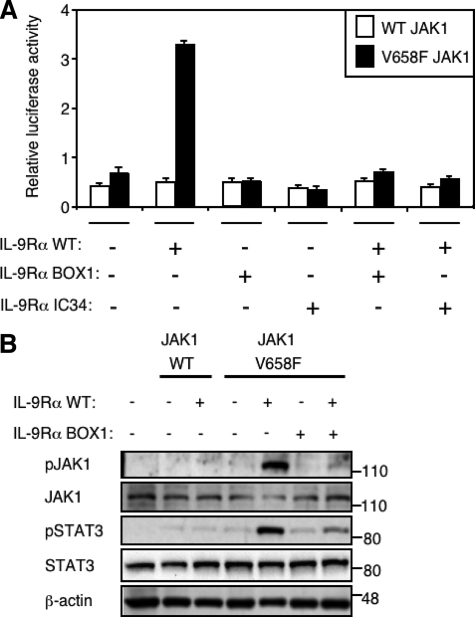
Dominant Negative Effect of IL-9Rα BOX1 Mutant on Constitutive JAK1 V658F Phosphorylation and STAT3 Activation Mediated by IL-9Rα—We hypothesized that the constitutive STAT activation observed when JAK1 mutants are co-expressed with the IL-9Rα could be explained by the formation of IL-9Rα homodimers. Indeed, this receptor conformation would allow the cross-phosphorylation of two JAK1 mutants with maximal constitutive activation. In this situation the γc inhibitory effect on IL-9Rα-mediated constitutive signaling could be explained by shifting IL-9Rα homodimeric conformation toward classical heterodimers. To test this hypothesis, we co-expressed WT IL-9Rα with IL-9Rα BOX1 or IL-9Rα IC34 mutants together with JAK1 V658F and looked at the level of STAT3 activation in HEK293 cells by luciferase assay. IL-9Rα BOX1 was obtained by the mutation of two proline residues to serine in the BOX1 motif of IL-9Rα, generating a BOX1-defective IL-9Rα mutant that fails to bind JAK1. IL-9Rα IC34 was obtained after the truncation of the intracellular part of IL-9Rα close to BOX1 residues and was also unable to bind JAK1 (22). Fig. 5A shows that these receptors are defective, as their co-expression with JAK1 V658F did not induce constitutive STAT3 activation. Moreover, when co-expressed with IL-9Rα WT, these BOX1-defective receptors abolished IL-9Rα-mediated STAT3 activation by JAK1 V658F, exerting a dominant negative effect. This effect is best explained by the disruption of WT IL-9Rα homodimers and the formation of heterodimers between WT and BOX1-defective IL-9Rα. Such heterodimers would not allow cross-activation of JAK1 mutants. We confirmed these results by Western blot analysis of the JAK1 V658F phosphorylation. Fig. 5B shows that phosphorylation of JAK1 was present only when JAK1 V658F mutant was co-expressed with IL-9Rα WT but not with IL-9Rα BOX1. Furthermore, co-expression of IL-9Rα BOX1 showed a dominant negative effect on wild-type IL-9Rα-mediated phosphorylation of JAK1 V658F. As expected, STAT3 phosphorylation detected by Western blot paralleled JAK1 phosphorylation (Fig. 5B). The expression of wild-type IL-9Rα and different IL-9Rα mutants was measured by FACS and showed similar levels of cell surface expression 24 h after transient transfection of HEK293 cells (supplemental Fig. 2).
FIGURE 5.
Dominant negative effect of IL-9Rα BOX1 mutant on constitutive JAK1 V658F phosphorylation and STAT3 activation mediated by IL-9Rα WT. A, HEK293 cells were transiently co-transfected with JAK1 wild-type or V658F and different IL-9Rα constructs in addition to the STAT3-responsive luciferase reporter pGL3-pap1. 24 h post-transfection cells were subjected to a luciferase assay. Results are the mean ± variation of duplicate samples. Similar results were obtained in three independent experiments. B, HEK293 cells were transiently co-transfected with JAK1 wild-type or V658F and different IL-9Rα constructs. 24 h post-transfection 106 cells were lysed and subjected to Western blot analysis. Phosphorylation of JAK1 and STAT3 was detected using specific anti-pJAK1 Tyr-1022/1023 and anti-pSTAT3 Tyr-705 antibodies. Membranes were reprobed with anti-JAK1, anti-STAT3, and anti-β-actin antibodies as control. Similar results were obtained in three independent experiments.
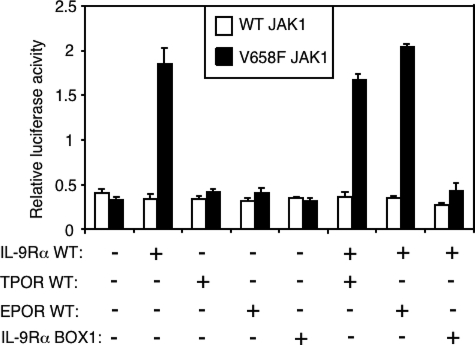
In the following experiments we focused on the specificity of the dominant negative effect of IL-9Rα BOX1 on wild-type IL-9Rα-mediated STAT3 constitutive activation by JAK1 V658F. Measuring luciferase activity regulated by a STAT3-specific promoter, we compared the effect of TPOR, EPOR, and IL-9Rα BOX1 mutant expression on this constitutive STAT3 activation in HEK293 cells. As shown in Fig. 6, the dominant negative effect was restricted to IL-9Rα BOX1 mutant, as neither TPOR nor EPOR showed any inhibitory effect on wild-type IL-9Rα-mediated STAT3 activation by JAK1 V658F. Also, in contrast to wild-type IL-9Rα, TPOR and EPOR, which are coupled to JAK2 and not to JAK1, were not able to mediate activation of STAT3 by the JAK1 V658F.
FIGURE 6.
IL-9Rα BOX1 mutant, but not TPOR or EPOR, inhibits IL-9Rα-mediated activation of STAT3 by JAK1 V658F. HEK293 cells were transiently co-transfected with JAK1 wild-type or V658F, IL-9Rα wild-type, and/or BOX1 mutant or/and with the thrombopoietin or erythropoietin receptors in addition to the STAT3-responsive luciferase reporter. 24 h post-transfection cells were subjected to a luciferase assay. Results are the mean ± variation of duplicate samples. Similar results were obtained in two independent experiments.
Altogether, these data confirmed the specific dominant negative effect of IL-9Rα BOX1 on IL-9Rα WT-mediated activation of STAT3 by JAK1 V658F supporting our hypothesis of IL-9Rα homodimers. When these homodimers are associated with JAK1 V658F, they promote JAK1 mutant cross-phosphorylation and activation of downstream signaling pathways.
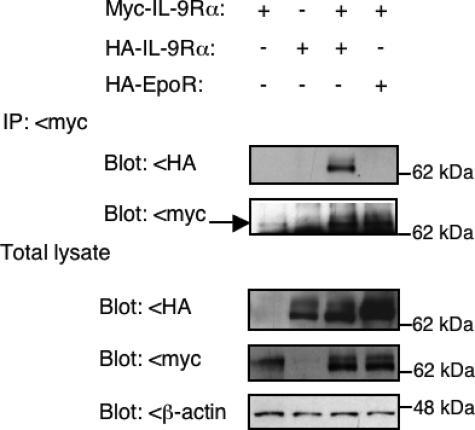
Coimmunoprecipitation Shows Evidence of IL-9Rα Homodimerization—To further confirm IL-9Rα homodimerization, Myc-tagged IL-9Rα and HA-tagged IL-9Rα or EpoR were expressed in HEK293 cells. Cellular extracts were immunoprecipitated with an anti-Myc antibody and analyzed by Western blot with anti-HA antibodies to detect co-immunoprecipitation. As shown in Fig. 7, HA-tagged IL-9Rα, but not HA-tagged EpoR, co-immunoprecipitated with Myc-tagged IL-9Rα when both were expressed in HEK293 cells. HA-tagged IL-9Rα did not co-immunoprecipitate in the absence of Myc-tagged IL-9Rα, confirming specificity of the immunoprecipitation assay and used antibodies. Co-expression of wild-type or V658F JAK1 did not change the pattern of homodimerization (data not shown).
FIGURE 7.
Co-immunoprecipitation shows evidence of IL-9Rα homodimerization. HEK293 cells were transiently co-transfected with Myc- and/or HA-tagged IL-9Rα or HA-tagged EPOR. 24 h post-transfection, cellular extracts were immunoprecipitated (IP) with an anti-Myc antibody and analyzed by Western blot with anti-HA antibodies to detect co-immunoprecipitation of IL-9Rα. Anti-Myc antibodies were used as the control, and total lysates were analyzed with the same antibodies. Similar results were obtained in three independent experiments.
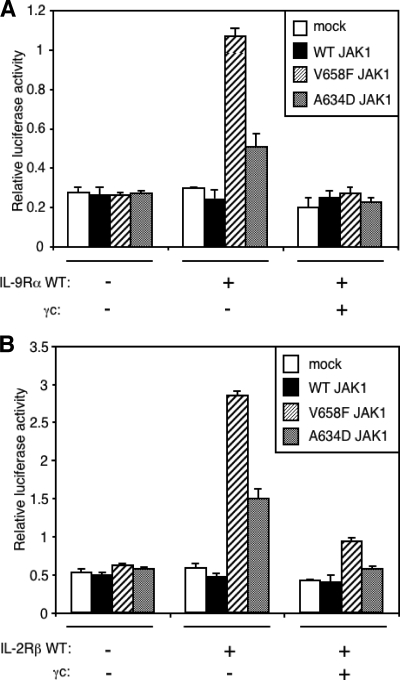
Both IL-2Rβ and IL-9Rα Allow for JAK1 V658F- and JAK1 A634D-induced Constitutive Activation of STAT5—To verify whether the results obtained with IL-9Rα could be extended to other receptors of the same family, we studied the effect of IL-2Rβ expression on JAK1 mutant-induced STAT5 activation. IL-2 and IL-9 receptor complexes share many similarities. They both require γc, JAK3, and JAK1 for their cytokine-induced signal transduction. However, in contrast to IL-9, IL-2 activates only STAT5. We co-expressed IL-9Rα or IL-2Rβ with JAK1 V658F or A634D in HEK293 cells and assessed STAT5 activity by a luciferase assay. As shown in Fig. 8, IL-2Rβ had the same effect as IL-9Rα and was able to mediate mutant JAK1-induced constitutive STAT5 activation. The formation of IL-2Rß homodimers was strongly suggested by the inhibitory effect of γc co-expression. Altogether, these results show that the mechanism of IL-9Rα homodimerization and mutant JAK1-mediated constitutive activation of STATs can be applied to other cytokine receptor chains from the same cytokine receptor family.
FIGURE 8.
Both IL-9Rα and IL-2Rß allows for JAK1 V658F- and JAK1 A634D-induced constitutive activation of STAT5. A, HEK293 cells were transiently co-transfected with empty vector, JAK1 wild-type, V658F or A634D, IL-9Rα and/or γc in addition to the STAT5-responsive luciferase reporter pLHRE. 24 h post-transfection cells were subjected to a luciferase assay. Results are the mean ± variation of duplicate samples. Similar results were obtained in three independent experiments. B, the same experiment was performed with IL-2Rβ. Results are the mean ± variation of duplicate samples. Similar results were obtained in three independent experiments.
DISCUSSION
In this paper we made two important observations. First, we demonstrate that expression of ALL-associated JAK1 mutants (V658F and A634D) alone does not suffice for constitutive activation of the JAK-STAT pathway but that this process is promoted by JAK1 binding receptors like IL-9Rα or IL-2Rβ. Secondly, we show that IL-9Rα can form homodimers, which mediate JAK1 mutant constitutive activation even better than the functional IL-9 receptor complex.
Recently, the discovery of several mutations in JAK1 in ALL expanded the list of oncogenic JAK mutants, a list led by the JAK2 V617F mutation associated to myeloproliferative neoplasm. In contrast to other oncogenic tyrosine kinases, like BCR-ABL, TEL-JAK2, or FLT3-ITD, JAKs are cytoplasmic tyrosine kinases that cannot form dimers by themselves. For JAK2 V617F, it was demonstrated that association with a homodimeric type I cytokine receptor such as EPOR or TPOR was necessary for constitutive signaling. Preformed dimerization of receptors such as EPOR was proposed to mediate activation or signaling by JAK2 V617F (8). The increased signal mediated by these receptors provides a molecular explanation for the erythrocytosis and thrombocytosis observed in these patients. Therefore, we speculated that JAK1 mutants would also need to associate to receptors to mediate constitutive signaling. Among the different JAK1 binding receptors, we studied the common γ chain family of receptors (IL-2R, -4R, -7R, -9R, -15R, -21R) because they are expressed in B and T cell precursors and because their activation would favor B or T cell proliferation, the phenotype observed in the disease. Indeed, we showed that two of these receptors, IL-9Rα and IL-2Rβ, were able to scaffold mutant JAK1 to mediate constitutive signaling. Moreover, for the IL-9Rα, we demonstrated that the STATs need to be recruited by a phosphotyrosine of the receptor, showing that the complex of the receptor and its associated JAK mutants has to be considered as the functional oncogenic entity.
In T cell ALL (T-ALL), the IL-2Rβ·JAK1 mutant complex could certainly be oncogenic and explain part of the phenotype. Indeed, IL-2Rβ is expressed in T-ALL (31), and its activation by IL-2 promotes T-cell proliferation and protects T-cells from corticoid-induced apoptosis (32). The expression of IL-9Rα in human T-ALL is controversial, but IL-9 clearly promotes murine T-ALL growth (15, 33). In addition, other complexes of the same family associating mutants JAK1 with IL-4Rα or IL-7Rα, both expressed in most T-ALL (33), are also oncogenic entity candidates.
A particularly intriguing observation was that the IL-9Rα-mediated mutant JAK1 constitutive activation was observed in cells lacking γc and JAK3, necessary for the formation of a functional IL-9 receptor complex. This could be explained by the existence of IL-9Rα monomers able to scaffold JAK1 mutants for activation and able to recruit the STAT factors. However, the dominant negative effect of BOX1 mutants and the co-immunoprecipitation experiments showing the existence of IL-9Rα homodimers argue against that hypothesis. Precedent for preformed dimeric complexes exists for EPOR (10) and for growth hormone receptor (34). Recent experiments from the Henis laboratory (35) using the immunofluorescence co-patching technology also showed that IL-9Rα and IL-2Rβ expressed either transiently in HEK293 cells or stably in BaF3 cells (IL-3-dependent pro B cell line) can form ligand-independent homomeric complexes, but this technology does not allow for identification of the stoichiometry of the complexes. Our data provide direct evidence for dimerization of IL-9Rα, as the dominant negative effect we detect with the IL-9Rα BOX1 mutant would only be consistent for dimers and not higher order complexes (i.e. tetramers). Presumably, the dimeric conformation favors the cross-phosphorylation of the two JAK1 mutants associated with IL-9Rα followed by IL-9Rα phosphorylation and STATs activation. One of the JAK1 mutants, V658F, represents the homologous mutation of JAK2 V617F (11), and indeed, such a mechanism was demonstrated for JAK2 V617F, where the homodimeric EPOR conformation was necessary for the constitutive activation (9).
In this model the receptor has a dual role to play; that is, as a scaffold for cross-activation of JAK mutants and as a docking site for the recruitment of the STAT transcription factors. The absence of any potential STAT recruitment site in the cytoplasmic domain of γc implies that this receptor chain is a weak contributor to the oncogenic activity of JAK mutants. Indeed, although γc has the ability to form homodimers, this chain failed to promote STAT activation when associated with the JAK3 A572V mutant unless IL-9Rα or IL-2Rβ were co-expressed (35). According to this model, which STAT is constitutively activated does not depend on the JAK mutant itself but depends on the cytokine receptor associated with a particular JAK mutant. Here, we show constitutive activation of STAT5 by JAK1 mutants associated with IL-9Rα or IL-2Rβ chains, whereas STAT3 activation was detected only in the presence of IL-9Rα. Activation of STAT5 is strongly associated with proliferation and tumoral transformation of hematopoietic progenitors. In contrast, STAT3 activation outcome varies depending on the cellular context and was shown to promote either cell proliferation (36) or differentiation (37). It would be, therefore, important to pay attention to the respective activation of different STATs in ALL expressing JAK1 mutants. Activation of different STAT factors might indeed reflect the involvement of distinct cytokine receptor complexes leading to different oncogenic evolutions.
The fact that signaling by complexes of IL-9Rα and JAK1 mutant without γc and JAK3 was higher than that mediated by the physiological IL-9 receptor complex can be explained by two non-exclusive hypotheses. The first one is that homodimers allow for cross-activation of two JAK1 mutants and increase signaling compared with the physiological JAK1/JAK3 cross-activation. The second one is that homodimers possess a STAT-recruiting phosphotyrosine on each of the IL-9Rα chains, whereas there is only one phosphotyrosine in the functional IL-9 receptor complex. Therefore, in ALL, the level of γc and JAK3 expression, which varies according to the level of B or T cell differentiation and activation, will certainly impact the level of constitutive signaling. Situations might exist when chains like the IL-9Rα or IL-2Rβ might be expressed in excess when compared with γc or to JAK3. Antigen stimulation of naïve T cells results in a strong increase in JAK3 mRNA and protein levels (38, 39) which is followed by an increase in IL-2Rβ and γc levels (40). Future experiments will have to address the possibility that some JAK1 mutant ALL subclones would acquire a proliferative advantage after alteration of the γc gene, which is located on Xq13.1, and therefore expressed from a single allele (41).
Supplementary Material
This work was supported in part by the Belgian Programme on Interuniversity Poles of Attraction initiated by the Belgian State, Prime Minister's Office, Science Policy Programming, by the Actions de Recherche Concertées of the Communauté Française de Belgique, by Foundation Salus Sanguinis, Atlantic Philanthropies/Ludwig Institute for Cancer Research Ltd. Clinical Discovery Program, and the opération Télévie. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement”in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–3.
Footnotes
The abbreviations used are: JAK, Janus kinase; IL, interleukin; IL-9Rα, IL-9 receptor complex; EPOR, erythropoietin receptor; TPOR, thrombopoietin receptor; ALL, acute lymphoblastic leukemia; T-ALL, T cell ALL; STAT, signal transducers and activators of transcription; HA, hemagglutinin; FACS, fluorescence-activated cell sorter; WT, wild type.
References
- 1.Schindler, C., Levy, D. E., and Decker, T. (2007) J. Biol. Chem. 282 20059–20063 [DOI] [PubMed] [Google Scholar]
- 2.James, C., Ugo, V., Le Couedic, J. P., Staerk, J., Delhommeau, F., Lacout, C., Garcon, L., Raslova, H., Berger, R., Bennaceur-Griscelli, A., Villeval, J. L., Constantinescu, S. N., Casadevall, N., and Vainchenker, W. (2005) Nature 434 1144–1148 [DOI] [PubMed] [Google Scholar]
- 3.Kralovics, R., Passamonti, F., Buser, A. S., Teo, S. S., Tiedt, R., Passweg, J. R., Tichelli, A., Cazzola, M., and Skoda, R. C. (2005) N. Engl. J. Med. 352 1779–1790 [DOI] [PubMed] [Google Scholar]
- 4.Levine, R. L., Wadleigh, M., Cools, J., Ebert, B. L., Wernig, G., Huntly, B. J., Boggon, T. J., Wlodarska, I., Clark, J. J., Moore, S., Adelsperger, J., Koo, S., Lee, J. C., Gabriel, S., Mercher, T., D'Andrea, A., Frohling, S., Dohner, K., Marynen, P., Vandenberghe, P., Mesa, R. A., Tefferi, A., Griffin, J. D., Eck, M. J., Sellers, W. R., Meyerson, M., Golub, T. R., Lee, S. J., and Gilliland, D. G. (2005) Cancer Cell 7 387–397 [DOI] [PubMed] [Google Scholar]
- 5.Baxter, E. J., Scott, L. M., Campbell, P. J., East, C., Fourouclas, N., Swanton, S., Vassiliou, G. S., Bench, A. J., Boyd, E. M., Curtin, N., Scott, M. A., Erber, W. N., and Green, A. R. (2005) Lancet 365 1054–1061 [DOI] [PubMed] [Google Scholar]
- 6.Vainchenker, W., and Constantinescu, S. N. (2005) Hematology Am. Soc. Hematol. Educ. Program, 2005 195–200 [DOI] [PubMed] [Google Scholar]
- 7.Wernig, G., Gonneville, J. R., Crowley, B. J., Rodrigues, M. S., Reddy, M. M., Hudon, H. E., Walz, C., Reiter, A., Podar, K., Royer, Y., Constantinescu, S. N., Tomasson, M. H., Griffin, J. D., Gilliland, D. G., and Sattler, M. (2008) Blood 111 3751–3759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu, X., Levine, R., Tong, W., Wernig, G., Pikman, Y., Zarnegar, S., Gilliland, D. G., and Lodish, H. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 18962–18967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu, X., Huang, L. J., and Lodish, H. F. (2008) J. Biol. Chem. 283 5258–5266 [DOI] [PubMed] [Google Scholar]
- 10.Constantinescu, S. N., Keren, T., Socolovsky, M., Nam, H., Henis, Y. I., and Lodish, H. F. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 4379–4384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Staerk, J., Kallin, A., Demoulin, J. B., Vainchenker, W., and Constantinescu, S. N. (2005) J. Biol. Chem. 280 41893–41899 [DOI] [PubMed] [Google Scholar]
- 12.Walters, D. K., Mercher, T., Gu, T. L., O'Hare, T., Tyner, J. W., Loriaux, M., Goss, V. L., Lee, K. A., Eide, C. A., Wong, M. J., Stoffregen, E. P., McGreevey, L., Nardone, J., Moore, S. A., Crispino, J., Boggon, T. J., Heinrich, M. C., Deininger, M. W., Polakiewicz, R. D., Gilliland, D. G., and Druker, B. J. (2006) Cancer Cell 10 65–75 [DOI] [PubMed] [Google Scholar]
- 13.Flex, E., Petrangeli, V., Stella, L., Chiaretti, S., Hornakova, T., Knoops, L., Ariola, C., Fodale, V., Clappier, E., Paoloni, F., Martinelli, S., Fragale, A., Sanchez, M., Tavolaro, S., Messina, M., Cazzaniga, G., Camera, A., Pizzolo, G., Tornesello, A., Vignetti, M., Battistini, A., Cave, H., Gelb, B. D., Renauld, J. C., Biondi, A., Constantinescu, S. N., Foa, R., and Tartaglia, M. (2008) J. Exp. Med. 205 751–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeong, E. G., Kim, M. S., Nam, H. K., Min, C. K., Lee, S., Chung, Y. J., Yoo, N. J., and Lee, S. H. (2008) Clin. Cancer Res. 14 3716–3721 [DOI] [PubMed] [Google Scholar]
- 15.Renauld, J. C., van der Lugt, N., Vink, A., van Roon, M., Godfraind, C., Warnier, G., Merz, H., Feller, A., Berns, A., and Van Snick, J. (1994) Oncogene 9 1327–1332 [PubMed] [Google Scholar]
- 16.Merz, H., Houssiau, F. A., Orscheschek, K., Renauld, J. C., Fliedner, A., Herin, M., Noel, H., Kadin, M., Mueller-Hermelink, H. K., Van Snick, J., and Feller, A. C. (1991) Blood 78 1311–1317 [PubMed] [Google Scholar]
- 17.Fischer, M., Bijman, M., Molin, D., Cormont, F., Uyttenhove, C., van Snick, J., Sundstrom, C., Enblad, G., and Nilsson, G. (2003) Leukemia 17 2513–2516 [DOI] [PubMed] [Google Scholar]
- 18.Kelleher, K., Bean, K., Clark, S. C., Leung, W. Y., Yang-Feng, T. L., Chen, J. W., Lin, P. F., Luo, W., and Yang, Y. C. (1991) Blood 77 1436–1441 [PubMed] [Google Scholar]
- 19.Uyttenhove, C., Druez, C., Renauld, J. C., Herin, M., Noel, H., and Van Snick, J. (1991) J. Exp. Med. 173 519–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demoulin, J. B., Uyttenhove, C., Lejeune, D., Mui, A., Groner, B., and Renauld, J. C. (2000) Cancer Res. 60 3971–3977 [PubMed] [Google Scholar]
- 21.Knoops, L., and Renauld, J. C. (2004) Growth Factors 22 207–215 [DOI] [PubMed] [Google Scholar]
- 22.Demoulin, J. B., Uyttenhove, C., Van Roost, E., DeLestre, B., Donckers, D., Van Snick, J., and Renauld, J. C. (1996) Mol. Cell. Biol. 16 4710–4716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Druez, C., Coulie, P., Uyttenhove, C., and Van Snick, J. (1990) J. Immunol. 145 2494–2499 [PubMed] [Google Scholar]
- 24.Haan, C., Is'harc, H., Hermanns, H. M., Schmitz-Van De Leur, H., Kerr, I. M., Heinrich, P. C., Grotzinger, J., and Behrmann, I. (2001) J. Biol. Chem. 276 37451–37458 [DOI] [PubMed] [Google Scholar]
- 25.Liu, X., Constantinescu, S. N., Sun, Y., Bogan, J. S., Hirsch, D., Weinberg, R. A., and Lodish, H. F. (2000) Anal. Biochem. 280 20–28 [DOI] [PubMed] [Google Scholar]
- 26.Renauld, J. C., Druez, C., Kermouni, A., Houssiau, F., Uyttenhove, C., Van Roost, E., and Van Snick, J. (1992) Proc. Natl. Acad. Sci. U. S. A. 89 5690–5694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerland, K., Bataille-Simoneau, N., Basle, M., Fourcin, M., Gascan, H., and Mercier, L. (2000) Mol. Cell. Endocrinol. 168 1–9 [DOI] [PubMed] [Google Scholar]
- 28.Peelman, F., Iserentant, H., De Smet, A. S., Vandekerckhove, J., Zabeau, L., and Tavernier, J. (2006) J. Biol. Chem. 281 15496–15504 [DOI] [PubMed] [Google Scholar]
- 29.Dumoutier, L., Van Roost, E., Colau, D., and Renauld, J. C. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 10144–10149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saharinen, P., Takaluoma, K., and Silvennoinen, O. (2000) Mol. Cell. Biol. 20 3387–3395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baba, H., Yamada, Y., Mori, N., Hayashibara, T., Harasawa, H., Tsuruda, K., Sugahara, K., Soda, H., Takasaki, Y., Tawara, M., Hirakata, Y., Tomonaga, M., and Kamihira, S. (2002) Eur. J. Haematol. 68 362–369 [DOI] [PubMed] [Google Scholar]
- 32.Renauld, J. C., Vink, A., Louahed, J., and Van Snick, J. (1995) Blood 85 1300–1305 [PubMed] [Google Scholar]
- 33.Vink, A., Renauld, J. C., Warnier, G., and Van Snick, J. (1993) Eur. J. Immunol. 23 1134–1138 [DOI] [PubMed] [Google Scholar]
- 34.Brown, R. J., Adams, J. J., Pelekanos, R. A., Wan, Y., McKinstry, W. J., Palethorpe, K., Seeber, R. M., Monks, T. A., Eidne, K. A., Parker, M. W., and Waters, M. J. (2005) Nat. Struct. Mol. Biol. 12 814–821 [DOI] [PubMed] [Google Scholar]
- 35.Malka, Y., Hornakova, T., Royer, Y., Knoops, L., Renauld, J. C., Constantinescu, S. N., and Henis, Y. I. (2008) J. Biol. Chem. 283 33569–33577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hideshima, T., Bergsagel, P. L., Kuehl, W. M., and Anderson, K. C. (2004) Blood 104 607–618 [DOI] [PubMed] [Google Scholar]
- 37.Bromberg, J., and Darnell, J. E., Jr. (2000) Oncogene 19 2468–2473 [DOI] [PubMed] [Google Scholar]
- 38.Kawamura, M., McVicar, D. W., Johnston, J. A., Blake, T. B., Chen, Y. Q., Lal, B. K., Lloyd, A. R., Kelvin, D. J., Staples, J. E., Ortaldo, J. R., and O'Shea, J. J. (1994) Proc. Natl. Acad. Sci. U. S. A. 91 6374–6378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnston, J. A., Kawamura, M., Kirken, R. A., Chen, Y. Q., Blake, T. B., Shibuya, K., Ortaldo, J. R., McVicar, D. W., and O'Shea, J. J. (1994) Nature 370 151–153 [DOI] [PubMed] [Google Scholar]
- 40.Nakarai, T., Robertson, M. J., Streuli, M., Wu, Z., Ciardelli, T. L., Smith, K. A., and Ritz, J. (1994) J. Exp. Med. 180 241–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noguchi, M., Yi, H., Rosenblatt, H. M., Filipovich, A. H., Adelstein, S., Modi, W. S., McBride, O. W., and Leonard, W. J. (1993) Cell 73 147–157 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.