Abstract
Ca2+ signaling in mitochondria has been mainly attributed to Ca2+ entry to the matrix through the Ca2+ uniporter and activation of mitochondrial matrix dehydrogenases. However, mitochondria can also sense increases in cytosolic Ca2+ through a mechanism that involves the aspartate-glutamate carriers, extramitochondrial Ca2+ activation of the NADH malate-aspartate shuttle (MAS). Both pathways are linked through the shared substrate α-ketoglutarate (αKG). Here we have studied the interplay between the two pathways under conditions of Ca2+ activation. We show that αKG becomes limiting when Ca2+ enters in brain or heart mitochondria, but not liver mitochondria, resulting in a drop in αKG efflux through the oxoglutarate carrier and in a drop in MAS activity. Inhibition of αKG efflux and MAS activity by matrix Ca2+ in brain mitochondria was fully reversible upon Ca2+ efflux. Because of their differences in cytosolic calcium concentration requirements, the MAS and Ca2+ uniporter-mitochondrial dehydrogenase pathways are probably sequentially activated during a Ca2+ transient, and the inhibition of MAS at the center of the transient may provide an explanation for part of the increase in lactate observed in the stimulated brain in vivo.
Ca2+ signaling in mitochondria has been mainly attributed to Ca2+ entry to the matrix through the Ca2+ uniporter (CaU)2 and activation of mitochondrial dehydrogenases (mitDH) (1). However, mitochondria can also sense increases in cytosolic Ca2+ through a mechanism that involves the aspartate-glutamate carriers (AGCs) and not the CaU (2–5). Aralar (Slc25a12), also named aralar1, the AGC isoform with predominant expression in brain (6–8), is a component of the NADH malate-aspartate shuttle (MAS), which in brain is activated by extramitochondrial Ca2+ (S0.5 324 nm) (4). Immunocytochemistry and in situ hybridization data and mRNA levels in acutely isolated brain cells indicate that aralar is localized preferentially in neurons (8–12). This is consistent with a higher MAS activity in neuronal than astrocyte cultures (8) and with aralar being one of the more enriched proteins during differentiation of P19 cells to a neuronal phenotype (13). It is also consistent with the higher levels of aralar in total than in synaptosome-free mitochondrial fractions (9).
Studies in cultured neurons, which have aralar as only AGC isoform, showed that small Ca2+ signals that have limited access to mitochondria are able to activate the aralar-MAS pathway (4). However, large Ca2+ signals that induce robust mitochondrial Ca2+ transients fail to activate the pathway (4). This suggested that in neuronal mitochondria the aralar-MAS pathway is inhibited under conditions in which the CaU-mitDH pathway is activated. This surprising result indicates that Ca2+ activation of the malate aspartate shuttle and tricarboxylic acid cycle activity are somehow mutually exclusive in neurons. We have now studied the interplay between the AGG-MAS and CaU-mitDH pathways in brain mitochondria under Ca2+-stimulation conditions. Our results show that the shared metabolite αKG controls the relationship between the second transporter of MAS, the oxoglutarate carrier (OGC, Slc25a11), and αKGDH in the Krebs cycle, by virtue of the effects of Ca2+ on the kinetics of αKGDH. Interestingly, the inhibition of OGC and MAS is fully reversible, in parallel with Ca2+ egress from mitochondria.
Behaviorally evoked brain activation results in an increased cerebral blood flow and increased glucose utilization, but paradoxically, this is not accompanied with an equivalent increase in oxygen utilization (14, 15). As a consequence, the oxygen glucose index, which is close to 6 when glucose is fully oxidized in resting conditions, falls to about 5 (16). This is accompanied by an increase in brain lactate production (14, 16–19). Our results suggest that MAS inhibition during Ca2+-induced Krebs cycle activation would drive pyruvate to lactate formation and may play a role in lactate formation during brain activation.
EXPERIMENTAL PROCEDURES
Animals and Materials—3-Month-old C57BL/6xSv129 mice were housed with a 12-h light cycle and fed ad libitum on standard chow. Animals were sacrificed by cervical dislocation, and the tissue of interest was quickly dissected and kept in ice-cold media for mitochondrial isolation, which was carried out at 4 °C. All animal procedures were approved by European guidelines. All reagents were obtained from Sigma, except malate dehydrogenase (Roche Applied Science), Fura-2, Calcium-Green5N (Molecular Probes, Eugene, OR), and CGP-37157 (Tocris Biosciences, Ellisville, MO).
Mitochondrial Isolation—Mitochondria were isolated from the brain, liver, and heart of 3-month-old C57BL/6xSv129 mice, as described previously (20–22) with modifications (4, 23), and were kept on ice in MSK (in mm: 75 mannitol, 25 sucrose, 5 potassium phosphate, 20 Tris-HCl, 0.5 EDTA, 100 KCl, and 0.1% bovine serum albumin, pH 7.4). Mitochondrial protein was measured by the Bradford method, with bovine serum albumin as standard.
MAS Reconstitution and mitGPDH Measurement—MAS was reconstituted following published methods (24–26), with modifications (4, 23). Briefly, mitochondria (0.1–0.15 mg of brain and liver and 0.020–0.030 mg of heart) were resuspended in 3 ml of MSK(with 100 μm digitonin for brain preparations), and the shuttle was reconstituted in the presence of 4 units/ml aspartate aminotransferase, 6 units/ml malate dehydrogenase, 66 μm NADH, 5 mm aspartate, 5 mm malate, 0.5 mm ADP. When appropriate, 200 nm Ruthenium Red (RR) and calibrated calcium concentrations were present. mitGPDH activity was measured in a similar way in MSK, with 0.5 mm ADP, 66 μm NADH, and 2 units/ml glycerol-3-phosphate dehydrogenase (27). Once a base line is achieved, either 5 mm glutamate (MAS) or 5 mm glycerol 3-phosphate (mitGPDH) was added to trigger shuttle activity, coupled to the decrease in NADH fluorescence (excitation 340 nm, excitation 465 nm). All assays were performed at 37 °C under constant stirring.
Measurement of OGC Activity—Transport of α-KG through the OGC was measured by a modification of the MAS reconstitution system. Appropriate amounts of mitochondrial protein of the tissue of interest were resuspended in 3 ml of MSK supplemented with 66 μm NADH, 0.5 mm ADP, 3 mm (NH4)2SO4, 5 mm glutamate, 10 units/ml GDH (and 100 μm digitonin when brain mitochondria were assayed). 5 mm malate addition initiated αKG efflux from mitochondria, which was followed by a decrease in NADH fluorescence. Experiments were performed at 37 °C under constant stirring. When indicated, 200 nm RR and calibrated calcium additions were made.
Free Calcium Calibration—The free calcium concentrations in the assays obtained after the different CaCl2 additions were determined in the presence of Fura-2 (under 1 μm, Kd = 224 nm; excitation, 340 and 380 nm; emission, 510 nm; concentration 5 μm) or Calcium-Green5N (over 1 μm, Kd = 14 μm; excitation, 506 nm; emission, 532 nm; concentration 0.1 μm). Free calcium concentration was calculated as established for ratiometric and nonratiometric probes (28, 29).
Enzymatic Assays—Enzyme activities were assayed in either freshly isolated or freeze-thawed mitochondria from different tissues of 3-month-old C57BL/6xSv129 mice. Experiments were performed in a final volume of 200 μl and 20–50 μg of protein in a FLUOstar optima (BMG-Labtech) plate fluorimeter at 30 °C. Activities were calculated as the initial slope of NADH fluorescence changes per mg of protein. An NADH calibration curve was performed daily by adding known amounts of NADH to medium. The following additions were made to the medium (MME: 50 mm KCl, 10 mm HEPES, pH 7.4; 0.2% Triton, and 10 μm rotenone (32): 0.2 mm NAD+ for measurement of GDH; 0.2 mm NADH, 5 units/ml malate dehydrogenase, and 12.5 mm αKG for aspartate aminotransferase measurement; 0.2 mm NAD+, 0.3 mm thiamine pyrophosphate, 10 μm CaCl2, 0.2 mm MgCl2, 0.14 mm CoASH, and 0.5 mm ADP to assay αKGDH. After establishment of a base line, activity was triggered with the appropriate substrate as follows: 5 mm glutamate or aspartate and 12.5 mm αKG (GDH, aspartate aminotransferase, and αKGDH, respectively (30–33).
Mitochondrial Membrane Potential—Mitochondrial membrane potential was estimated by quenching of tetramethylrhodamine methyl ester (TMRM, 549 nm excitation, 575 nm emission) fluorescence, as described previously (34). Briefly, after stabilization of TMRM (300 nm) fluorescence, 100 μg of mitochondria were added to 2 ml of MSK containing 5 mm aspartate. Changes in membrane potential were followed qualitatively by the variations of the TMRM fluorescence after addition of substrates (glutamate/malate, 5 mm), ADP (0.5 mm), and calcium (5 μm free calcium). When indicated, 50 mm Na+ replaced 50 mm K+ in MSK (MSKNa medium). A calcium unbuffered medium (MSK or MSKNa without EDTA) was also used.
Ca2+ Egress from Mitochondria—Brain mitochondria (0.25 mg/ml) were incubated in calcium-unbuffered medium (i.e. MSK or MSKNa without EDTA) in the presence of ADP (0.5 mm), aspartate (5 mm), malate (5 mm), digitonin (100 μm) and Calcium-Green5N (0.1 μm). After a stable base line was achieved, an addition of 10 μm CaCl2 was made (40 nmol/mg, giving a free calcium of 5 μm), and calcium uptake was monitored for 5 min before glutamate (5 mm) addition. Where indicated, ruthenium red (200 nm) was added to stop calcium uptake through the uniporter. In some assays, CGP-37157 (10 μm) was added to inhibit the mitochondrial Na+-Ca2+ exchanger (NCX) (35).
RESULTS
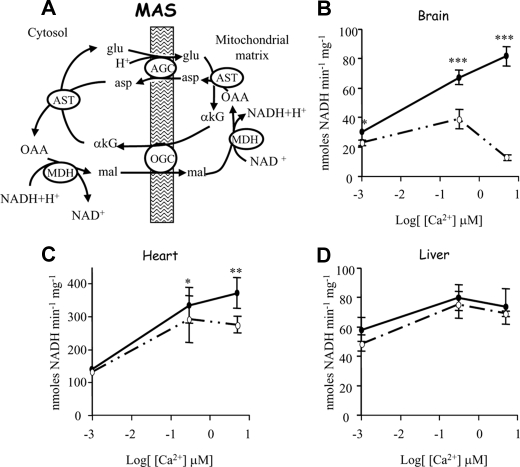
Regulation of MAS and Glycerol-3-P Dehydrogenase Activity by Extramitochondrial and Intramitochondrial Ca2+—MAS activity was shown to increase in response to extramitochondrial calcium in brain and heart mitochondria (3, 4, 36). Paradoxically, it became inhibited in a calcium-dependent manner when calcium was allowed to enter the mitochondria through the Ca2+ uniporter (4). In brain mitochondria, the remaining activity drops to 79 ± 4%, 58 ± 7%, and 17 ± 3% in the presence of 0, 0.3, and 5 μm [Ca2+]free, respectively (Fig. 1A). MAS inhibition by intramitochondrial Ca2+ is not as large in heart mitochondria, with 77 ± 6% residual activity at 5 μm free calcium (Fig. 1B). In contrast, MAS activity in liver mitochondria is not affected by intramitochondrial Ca2+, as its activity is the same in the absence or presence of RR, although it is activated by extramitochondrial calcium to a smaller extent than in brain or heart (Fig. 1C) as reported earlier (36).
FIGURE 1.
Effect of intra- and extramitochondrial calcium on the malate-aspartate NADH shuttle. A, scheme of MAS. Abbreviations used are as follows: asp, aspartate; glu, glutamate; OAA, oxalacetate; mal, malate; AST, aspartate transaminase; MDH, malate dehydrogenase. MAS activity in brain (B), heart (C), and livermitochondria(D) in the presence (solid circles) or absence (open circles) of 200 nm RR at different free calcium concentrations is shown. Data are means ± S.E. of three experiments performed in triplicate. Asterisks indicate a significant difference (Student's t test) between the presence and absence of RR at each calcium concentration (*, p < 0.05; **, p < 0.005; ***, p < 0.0005).
It is unlikely that the inhibitory effects of matrix Ca2+ on MAS activity are because of Ca2+-induced permeability transition pore opening and loss of mitochondrial metabolites (particularly NAD+) as follows: first, because assays were conducted in the presence of 0.5 mm ADP, which is a known inhibitor of permeability transition pore opening (37); second, because inhibition of MAS by Ca2+ matrix was not observed in liver mitochondria (Fig. 1C), which retains half the amount of Ca2+ than brain mitochondria before undergoing permeability transition (38); and third, because inhibition of MAS by matrix Ca2+ in brain and heart mitochondria is still observed in the presence of 5 μm cyclosporin A (results not shown).
We also investigated the effect of extra- and intramitochondrial Ca2+ on mitochondrial glycerol-3-phosphate dehydrogenase (mitGPDH), the mitochondrial component of the glycerol 3-phosphate shuttle. The activity of this enzyme is known to be activated by low concentrations of extramitochondrial calcium (≈35–100 nm (39) through a decrease in the Km value for glycerol 3-phosphate of the mitGPDH (40, 41). Unlike MAS, mitGPDH was completely Ca2+-dependent, with no detectable activity in the total absence of free Ca2+ in brain and liver mitochondria (supplemental Fig. 1). The activity of mitGPDH in heart mitochondria was not detectable under the conditions of this study, in agreement with the data of Scholz et al. (42, 43) who found a marked decrease of glycerol 3-phosphate shuttle activity in the heart with development. The enzyme requires Ca2+ in the intermembrane space, as full activation is obtained in the presence of RR in all tissues. The kinetics of activation agrees with a low S0.5 for activation, as the maximal activity was attained at 0.3 μm free Ca2+ (supplemental Fig. 1). On the other hand, Ca2+ entry in the matrix (i.e. RR not present in the assay) did not affect mitGPDH activity in either brain or liver mitochondria (supplemental Fig. 1).
Therefore, the results indicate that in brain mitochondria Ca2+ activation of MAS, but not mitGPDH, is prevented by strong Ca2+ signals that reach mitochondrial matrix. This suggests that reducing equivalents transfer to mitochondria may still proceed through mitGPDH when Ca2+ entry in mitochondria leads to an inhibition of MAS activity. This depends on whether a glycerol-P shuttle is actually present in brain, with its two enzymes within the same cell, as suggested to be the case from the astrocyte and neuronal transcriptome findings (12, 44–46).
Effect of Extra- and Intramitochondrial Calcium on OGC Activity—The activity of the OGC was studied by measuring the efflux of αKG from mitochondria, in medium containing glutamate dehydrogenase (GDH). GDH will transform αKG into glutamate in the presence of NADH and ammonium, resulting in a decrease in NADH fluorescence, when malate is added to trigger αKG efflux from mitochondria (supplemental Fig. 2). The supplemental Fig. 2B shows that the decrease of NADH fluorescence was dependent on the presence of ammonium and was prevented by the OGC inhibitor phenylsuccinate (45, 47).
To test the effects of both extra- and intramitochondrial calcium on OGC activity, αKG efflux was measured in the absence or presence of the calcium uniporter inhibitor RR, both in Ca2+-free medium or in the presence of 5 μm free Ca2+. In the presence of 200 nm RR, there was no difference in αKG efflux at the two calcium concentrations in brain, heart and liver mitochondria (Table 1), indicating that the OGC is not activated by extramitochondrial Ca2+, in accordance to the lack of calcium-binding motifs in the OGC sequence (48).
TABLE 1.
Effect of intra- and extramitochondrial calcium on the activity of the OGC αKG efflux was measured in brain, heart, and liver mitochondria from 3-month-old C57BL/6xSv129 mice at 0 (i.e. under the Fura2 limit detection, <20 nm) and 5 μm free calcium, in the presence (RR present) or absence (No RR) of 200 nm RR. Data are mean ± S.E. of 4–6 independent experiments performed in duplicate. Asterisks denote significant difference (Student's t test) between OGC activities in presence or absence of RR at each calcium concentration (*, p < 0.05; **, p < 0.005; ***, p < 0.0005).
|
OGC activity
|
|||||||
|---|---|---|---|---|---|---|---|
|
0 μm [Ca2+]free
|
5 μm [Ca2+]free
|
||||||
| No RR | RR present | No RR | RR present | ||||
| nmol of NADH min–1 mg protein–1 | |||||||
| Brain | 116.03 ± 33.12 | 105.16 ± 17 | 46.06 ± 7*** | 127.5 ± 15 | |||
| Heart | 378.13 ± 66 | 329.2 ± 56 | 356.4 ± 66* | 447.4 ± 53 | |||
| Liver | 66.87 ± 19* | 101.2 ± 28 | 44.6 ± 8** | 103.2 ± 11 | |||
Having shown that extramitochondrial Ca2+ does not modify the OGC activity, the influence of intramitochondrial Ca2+ was studied by carrying out the same experiments in the absence of RR, when the Ca2+ uniporter is not inhibited (Table 1). Under these conditions the addition of Ca2+ results in a decrease of αKG efflux in mitochondria from brain and heart (to 37 ± 5% and 78 ± 10% residual activity, respectively (Table 1)), indicating that the OGC in these tissues is inhibited by intramitochondrial Ca2+. In liver, the absence of RR resulted in an inhibition of αKG efflux both in the absence or presence of Ca2+ (Table 1), clearly a nonspecific effect, unrelated to Ca2+ entry in mitochondria, which was not studied any further. Therefore, inhibition of αKG efflux by mitochondrial Ca2+ may explain the inhibition of MAS in brain and heart mitochondria.
Influence of Mitochondrial αKG Sources and Utilization Pathways on αKG Efflux Along the OGC—OGC and αKGDH have a common substrate, αKG. When mitochondrial Ca2+ increases, it activates αKGDH, which causes a decrease in the Km value for its substrate, αKG, and a decrease in mitochondrial αKG levels (49, 50). Our working hypothesis is that this leads to the decrease in OGC activity that we have shown above in brain and heart mitochondria. As OGC is a member of MAS, this may explain the decrease of MAS activity in brain and heart mitochondria when calcium is allowed to enter the organelle (see Fig. 1A and supplemental Fig. 2A).
We figured that a high OGC activity with respect to αKGDH would make the OGC relatively independent of αKGDH activity, and this could be a possible explanation for the lack of effect of intramitochondrial Ca2+ on liver MAS and OGC activities. However, Table 2 shows that this is not the case. Measurements of αKGDH activity in mitochondria from different tissues showed that the ratio of αKG efflux to αKGDH activity is similar in liver and brain mitochondria.
TABLE 2.
Enzyme and transporter activities involved in αKG production and utilization in mitochondria using glutamate and malate as substrates The results are mean ± S.E. of four independent experiments performed at least in duplicate. αKG production was computed as the sum of AGC-MAS and GDH, whereas αKG utilization was the sum of αKGDH and OGC. Differences (Bonferroni test) with liver (*, p < 0.05; **, p < 0.005) or heart (***, p < 0.005) are indicated.
|
Activity
|
|||||
|---|---|---|---|---|---|
| Brain | Heart | Liver | |||
| nmol of NADH min–1 mg protein–1 | |||||
| mitAST | 1798 ± 374 | 2170 ± 283 | 2569 ± 608 | ||
| αKGDH | 31.38 ± 6.78*** | 275.4 ± 28 | 21.86 ± 4.5*** | ||
| OGC | 127.5 ± 16*** | 447.4 ± 59 | 103.2 ± 12*** | ||
| AGC-MAS | 96.4 ± 8.5*** | 372 ± 35 | 99 ± 2.7*** | ||
| GDH | 11.3 ± 0.27* | 6.6 ± 0.7** | 55.26 ± 13 | ||
| Ratio production/utilization | 0.848 ± 0.0381* | 0.589 ± 0.066** | 1.38 ± 0.22 | ||
A second possibility is that liver mitochondria OGC could utilize an additional source of αKG making it independent of αKGDH. The source of αKG in the OGC assays is external glutamate, which enters mitochondria through the AGC and is transaminated with oxaloacetate by mitochondrial aspartate aminotransferase (see supplemental Fig. 2A). The transaminase can be inhibited by 5 mm aminooxyacetate (AOAA) (45), which leads to a complete block of αKG efflux in brain and heart mitochondria (supplemental Fig. 3, A and B). However, this is not the case in liver mitochondria, where AOAA only decreased the efflux to 62 ± 15% in the presence of RR and 5 μm calcium, but it had no effect in the absence of RR and 5 μm calcium (supplemental Fig. 3C).
The lack of effect of AOAA on αKG efflux in liver mitochondria points to an extra(s) source(s) of αKG in this tissue. This is probably glutamate dehydrogenase (GDH), which in liver has the highest activity among the tissues explored in this study (Table 2).
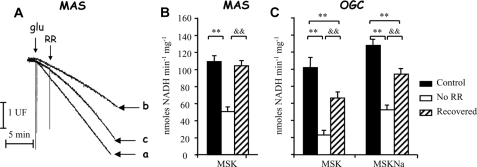
Inhibition of OGC and MAS Activity in Brain Mitochondria Is Reversible—We have shown that Ca2+ entry in heart and brain mitochondria prevents Ca2+ activation of MAS activity through an inhibition of αKG efflux along the OGC because of the Ca2+ activation of αKGDH, a situation that does not take place in liver. In these two types of mitochondria, the maximal activity of the malate-aspartate shuttle (limited by the AGC) is only slightly lower than that of the OGC (see Table 2), as has also been observed in rat brain mitochondria using an entirely different method (9). This makes MAS activity very sensitive to the decrease of the OGC activity brought about by matrix Ca2+. Because Ca2+ activation of αKGDH should last as long as Ca2+ remains in the mitochondrial matrix, we have studied whether net Ca2+ efflux from mitochondria allows reactivation of MAS.
To this end, we have first allowed mitochondria to take up Ca2+, while carrying out MAS activity during 3 min, and then Ca2+ uptake was stopped by addition of RR, and mitochondria were allowed to release the accumulated Ca2+ along RR-insensitive Ca2+ efflux pathways. The recovery of MAS and OGC activities was studied at the same time (Fig. 2). MAS activity was clearly inhibited after the incubation in the presence of Ca2+ (compare slopes of traces a and b in Fig. 2A). As observed in Fig. 2A, RR addition results in a reactivation of MAS, which is almost complete 10 min after RR addition (Fig. 2A, compare final slopes of traces a and c, and Fig. 2B). αKG efflux along the OGC was also re-activated upon Ca2+ efflux, as expected, although its activity was not fully recovered within this time period (Fig. 2C, recovery after 10 min addition of RR).
FIGURE 2.
Recovery of MAS and αKG efflux after RR addition in MSK. A, MAS activity in brain mitochondria in the presence (trace a, Control), absence (trace b, No RR), or addition (trace c, Recovered) of RR where indicated. 5 μm free calcium was present in the assay. Traces are representative of three independent experiments. B, MAS measured 10 min after RR was added to induce recovery of activity in Na+-free medium (MSK). C, αKG efflux (OGC) measured 10 min after RR was added to induce recovery of activity in Na+-free medium (MSK) or Na+ medium (MSKNa). Data are mean ± S.E. of three independent experiments performed in triplicate. (Significant difference from control, **, p < 0.005; or recovered, &&, p < 0.005).
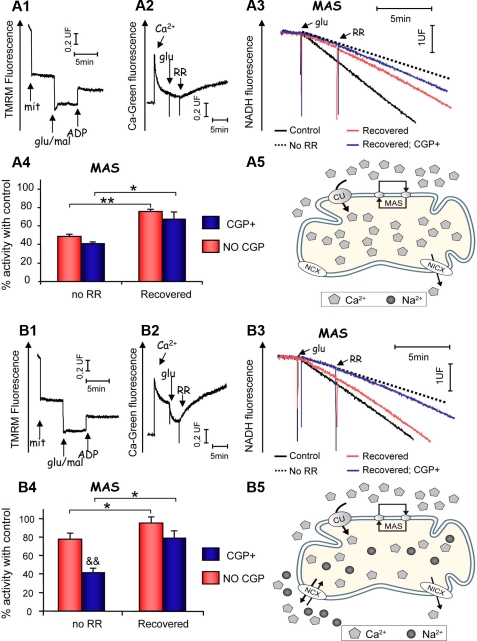
Enhanced Recovery of OGC and MAS Activity in Brain Mitochondria by NCX-mediated Ca2+ Efflux— The previous experiments were performed in a sodium-free medium (MSK), in which calcium efflux proceeds mainly through the Na+-independent Ca2+ efflux pathway, mitochondrial Na+-independent/Ca2+ exchanger (51–53), or a putative low conductance form of the permeability transition pore (54–56). As the main mitochondrial calcium efflux pathway in brain is the Na+/Ca2+ exchanger, NCX (52, 57, 58), we have studied the recovery of MAS activity in a medium with sodium (MSK with 50 mm NaCl replacing 50 mm KCl; MSKNa). Unexpectedly, we found that MAS activity was more inhibited by matrix Ca2+ in a Na+-containing than in a Na+-free medium, and recovery of MAS activity after RR addition was not complete (supplemental Fig. 4). In contrast, inhibition of αKG efflux through the OGC by intramitochondrial calcium is not so prominent in MSKNa, and its recovery induced by RR addition is much larger (Fig. 3C). These results indicate that failure to recover MAS activity in Na+ medium is not because of a failure to recover OGC activity. On the contrary, OGC activity is not as inhibited in Na+-containing as it is in Na+-free media, probably because the free calcium levels in mitochondria (and αKGDH activity) are lower when NCX is active, and Ca2+ efflux and deactivation of αKGDH are faster.
FIGURE 3.
Membrane potential, Ca2+ egress, and MAS recovery in Ca2+-unbuffered media. Brain mitochondria were incubated in Ca2+-unbuffered (without EDTA) MSK (A) or MSKNa (B). A1 and B1, membrane potential determination (representative traces), with substrates (glu+mal, 5 mm) and ADP (0.5 mm) added where indicated. A2 and B2, Ca2+ egress from mitochondria monitored from changes in Calcium-Green5N fluorescence (representative traces). Additions are as follows: Ca2+ (40 nmol/mg protein), glutamate (5 mm), RR (200 nm). Note the increased Ca2+ efflux in unbuffered MSKNa compared with unbuffered MSK media. A3 and B3, MAS activity in Ca2+-unbuffered MSK (A3) or Ca2+ unbuffered MSKNa (B3) media in control (RR present from the beginning) and recovered (RR added when indicated), in the presence or absence of CGP (10 μm) conditions. Dotted lines (No RR) indicate MAS activity before RR addition (i.e. inhibited MAS activity) for comparison purposes (representative traces are shown). A4 and B4, MAS activities in Ca2+-unbuffered MSK (A4) or MSKNa (B4). Percentages of control rates (140 ± 15 and 128 ± 12 nmoles NADH/min/mg in MSK and MSKNa, respectively) are shown for both No RR (inhibited) and recovered (RR added) conditions, in the presence (CGP+) or absence (No CGP) of the NCX inhibitor CGP37157 (10 μm). Data are means ± S.E. of two independent experiments performed in triplicate. *, p < 0.05; **, p < 0.005 significance of the difference between inhibited (No RR) and recovered conditions (Student's t test). &&, p < 0.005, significance of the difference between CGP presence (CGP+) or absence (No CGP) in each condition. The effects of the absence (A5) and presence (B5) of external Na+ in the intramitochondrial Ca2+ content are also shown.
A possible explanation for the paradoxical effects of Na+ in the recovery of MAS activity is based on the steep dependence of AGC on the proton electrochemical gradient. The transport of glutamate plus H+ against aspartate is electrogenic (3, 59–61), and the mitochondrial membrane potential may be dissipated by Ca2+ + Na+ cycling, with Ca2+ entry along the uniporter, and exit through the NCX coupled to efflux of Na+ back to the cytosol through Na+/H+ exchange (62, 63). This paradoxical effect of Na+ would not affect the OGC, which is electroneutral and thus independent upon Δp (64, 65).
Indeed, we found that mitochondria were partially depolarized when incubated in the presence of 50 mm NaCl (MSKNa; supplemental Fig. 4B). However, depolarization was not Ca2+-dependent and consequently not due to Na+ and Ca2+ cycling. It was possibly caused by Na+ influx through a channel in the mitochondrial membrane, which is inhibited by Mg2+ (66), and consequently a complete depolarization was obtained in the presence of Mg2+ (supplemental Fig. 4C). Under these conditions RR recovery of MAS activity was almost complete (supplemental Fig. 4A).
To verify the relationship between matrix Ca2+ and Ca2+ inhibition of MAS, we have next studied MAS recovery and Ca2+ efflux upon RR addition in parallel. To this end, Ca2+ fluxes were determined through the changes in extramitochondrial Ca2+ levels measured with Calcium Green in a medium with no calcium buffers and with traces of Mg2+ (i.e. MSK or MSKNa without EDTA). There were no differences in the mitochondrial membrane potential generated in the presence of glutamate + malate when mitochondria were incubated in Ca2+-unbuffered media in the absence (MSK without EDTA) or presence (MSKNa without EDTA) of Na+ (compare traces in A1 and B1 in Fig. 3). After the addition of Ca2+ (40 nmol/mg protein, giving 5 μm free Ca2+), most of it was taken up by energized mitochondria. However, the total amount of Ca2+ taken up after glutamate addition was higher in the absence than in the presence of Na+ (about 41 ± 1.6 versus 26.6 ± 3.7 nmol/mg protein in MSK and MSKNa, respectively), as observed previously with rat brain mitochondria (58, 67). Interestingly, MAS activity measured in these two conditions varied inversely with the accumulated Ca2+. It was strongly inhibited (about 50%) in the absence of Na+ (Fig. 3, A3 and A4), but much less so in the presence of Na+ (about 20%) (Fig. 3, B3 and B4).
Ca2+ efflux triggered by RR addition was strongly increased in a Na+ medium (182 ± 10 and 22.5 ± 8 nmol/min/mg initial speed in MSKNa and MSK, respectively), and after 10 min the amount of Ca2+ released was 24 or 66% of the calcium taken up in MSK and MSKNa, respectively (compare traces A2 and B2 in Fig. 3). This difference was abolished in the presence of the NCX inhibitor CGP-37157 (results not shown and see Ref. 35). Under these conditions MAS activity fully recovered control levels in Na+ media (Fig. 3B4), but recovery was not complete in the absence of Na+ (Fig. 3A4). The loss of the inhibitory effect of matrix Ca2+ on MAS was clearly because of stimulated Ca2+ efflux along the NCX because the effect of external Na+ was abolished in the presence of the NCX inhibitor (Fig. 3B4).
DISCUSSION
Tissue-specific Effects of Matrix Ca2+ on MAS Activity—Activation by Ca2+ of mitochondrial metabolism is mainly due to Ca2+ entry to the matrix through the calcium uniporter and activation of three matrix dehydrogenases (pyruvate dehydrogenase, αKGDH, and isocitrate dehydrogenase) (1, 5, 68). In addition, Ca2+ binding to aralar on the external side of the inner mitochondrial membrane activates MAS (4, 36) and explains the activation of respiration of brain mitochondria by extramitochondrial Ca2+ when malate plus glutamate are used as substrates (69). In this study we report that the effect of matrix Ca2+ on αKGDH results in an inhibition of MAS activity, at least in two tissues, brain and heart, but not in liver. We conclude that MAS inhibition is because of matrix Ca2+ activation of αKGDH and consequent inhibition of OGC, as suggested for heart mitochondria (70, 71). Both activities have similar Km value for αKG (1.5 and 2.1 mm for the OGC and αKGDH, respectively (49, 72)). Upon entry through the uniporter, Ca2+ stimulates αKGDH by reducing its Km value for αKG (from 2.1 to 0.16 mm, with an S0.5 ≈1.2 μm for calcium (49)). In this situation, OGC will be at a disadvantage, and thus αKG efflux will become impaired (Fig. 4C). This competition of the two pathways for a common substrate is consistent with the findings of O'Donnell et al. (71), who reported a 4-fold reduction of MAS activity in heart under high workload conditions.
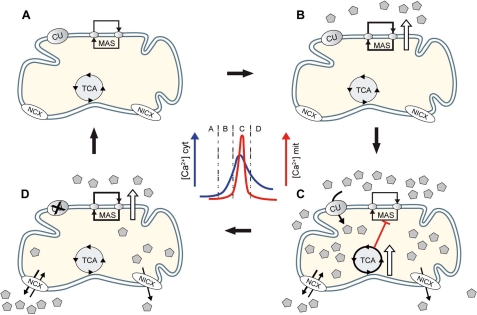
FIGURE 4.
MAS and CaU-mitDH Ca2+ sequential activation model. A, in resting conditions, both pathways function at basal rate. B, at the beginning of a Ca2+ spike, MAS is activated first because its S0.5 for Ca2+ (324 nm) is lower than the apparent affinity for Ca2+ of the CaU (1–20 μm). C, if the Ca2+ transient is high enough, uptake through the Ca2+ uniporter will take place and mitDH will be activated. Because of the competition between OGC and αKGDH, MAS activity is reduced even in the presence of elevated extramitochondrial Ca2+. D, when the transient decreases or if CaU is inhibited after a prolonged Ca2+ elevation (see “Discussion”), net efflux will lower matrix Ca2+ to basal levels, and MAS activity would recover. In the brain, this would take place faster because of the prominent presence of the more active NCX.
In liver mitochondria we have failed to observe such an inhibition of MAS by matrix Ca2+ (Fig. 1C), and a similar situation was found in beta cell mitochondria (73). As αKG efflux in liver mitochondria is only partially blocked by the transaminase inhibitor, this points to an alternative source of αKG different from transamination. This is probably GDH, which has a high activity in liver (Table 2), and beta-cell (74, 75) mitochondria. In mitochondria utilizing malate plus glutamate as substrates, αKG production can be expressed as the sum of the glutamate transported through AGC and then transaminated (aspartate aminotransferase is highly active, and thus the limiting step in this pathway is the AGC), and that generated in the GDH activity. αKG can then either be transported out of mitochondria by the OGC or serve as substrate of αKGDH (αKG consumption). If enough αKG is produced to comply with the activity of consuming pathways (αKG production/consumption ratio >1 as in liver mitochondria), no inhibition of OGC will be observed, otherwise αKG efflux (and MAS activity) will be inhibited upon calcium entry to the mitochondria and activation of αKGDH (αKG production/consumption ratio <1, as in brain and heart mitochondria; see Table 2).
Physiological Role of Reversible Matrix Ca2+-induced MAS Inhibition—The fact that the activation by extramitochondrial Ca2+ of the AGC-MAS pathway is abolished when matrix dehydrogenases are activated by intramitochondrial Ca2+ seems paradoxical, especially because MAS is required to keep a stimulated production of glucose-derived pyruvate. However, it must be borne in mind that Ca2+ signals are usually of a transient nature, and it is their frequency more than their amplitude that matters in terms of decoding and generation of an output. In every Ca2+ spike that reaches mitochondria eliciting a mitochondrial Ca2+ transient, the AGC-MAS pathway would be active at the beginning and end of the spike, but in the central part of it the CaU-mitDH pathway would take over (5). The prevalence of MAS at the beginning of a spike is based on the lower S0.5 of the system (300 nm) compared with the apparent affinity for Ca2+ of the CaU (1–20 μm) and to the lag between cytosolic and mitochondrial calcium increases (76). With a limited Ca2+ entry in mitochondria, the activation of the AGC-MAS pathway accounts for most of the increase in mitochondrial NADH production elicited by the Ca2+ spike (4), and this may prime mitochondria in the face of an immediate rise in energy expenditure (Fig. 4B) (4, 5). At the end of the spike, MAS re-activation could take place if basal levels of matrix Ca2+ are regained before cytosolic ones are reached (Fig. 4D). Indeed, MAS inhibition by intramitochondrial calcium disappears when Ca2+ efflux is enhanced over calcium uptake upon CaU inhibition. Furthermore, if the cytosolic Na+ concentration increases, as expected in depolarizing conditions, the more active NCX will reduce the extent of MAS inhibition at the center of the spike, and it will hasten the recovery of activity both of OGC and MAS activities at the end of the spike.
Our results suggest that the main NADH-producing pathway in mitochondria at the center of the spike is the Ca2+-activated tricarboxylic acid cycle or Krebs cycle, and not MAS. As MAS is the main NADH shuttle in brain, this may disrupt the cytosolic NAD+/NADH ratio, directing some of the pyruvate to lactate and limiting pyruvate supply to mitochondria. Because of the transitory nature of the calcium spikes, this imbalance should be a temporal one, and the reactivation of MAS at the end of the spike and beyond the duration of mitochondrial Ca2+ transient may be important to re-establish resting cytosolic NAD+/NADH and pyruvate/lactate ratios. Interestingly, a new mechanism to limit the duration of the mitochondrial Ca2+ elevation in response to a persistent elevation in cytosolic Ca2+, as would occur during repeated stimulation, has been described (77). According to this mechanism, CaU is inhibited by extramitochondrial calcium within a similar range of calcium concentrations as those leading to its activation (10–20 μm), but with a time constant for the uptake (≈7.9 s at 10 μm calcium) at about half that for the inhibition (≈16 s at 10 μm calcium) (77). Whether this mechanism is also present in brain mitochondria remains to be established.
Studies in heart have shown that activity-dependent inhibition of the OGC and MAS decreases the cytosolic levels of αKG and glutamate (71), and this will lead to a decrease in aspartate efflux from mitochondria and a fall in cytosolic aspartate. Brain aspartate levels are extremely dependent on the AGC activity, and they fall drastically in the aralar/AGC1 knock-out mouse (23). Although it is unlikely that total brain glutamate levels reflect MAS activity, as they are only marginally affected in the aralar knock-out mouse,3 the changes in aspartate levels could give some clues to MAS activity. Interestingly, Table 3 shows that aspartate levels consistently decrease during brain stimulation, supporting the possibility that MAS is actually inhibited under these conditions. From the above considerations it is obvious that increases in the lactate/pyruvate ratio would be predicted to occur in the brain in vivo, whether as the consequence of sporadic transient increases in cytosolic NADH/NAD+ or of trains of cytosolic NADH/NAD+ transients upon repeated depolarization, as one would expect during periods of brain activation. In agreement with this, a number of studies have shown that during task-dependent brain activation, there is an increase in brain glucose utilization larger than the increase in oxygen use and a simultaneous lactate production (14, 16–19) (see Table 3).
TABLE 3.
Metabolite levels during brain stimulation conditions in vivo and in vitro Data for glutamate (Glu), aspartate (Asp), and Lactate (Lact) content both at control (rest) and during brain work (stimulated) conditions from indicated Tables of the selected references are shown. ND, not determined in the original study; NMDA, N-methyl-d-aspartic acid.
| System | Stimulation | Rest | Stimulated | Ref. | |
|---|---|---|---|---|---|
| Rat dorsal cerebral cortex | 5-min sensory stimulation | Glu | 12.5 ± 0.6 | 13.3 ± 0.3 | μmol/g wet weight (Ref. 18, see Tables 3 and 4) |
| Asp | 4.0 ± 0.3 | 3.3 ± 0.3 | |||
| Lact | 0.6 ± 0.1 | 1.7 ± 0.4 | |||
| Rat, microdialysis | Acoustic stimulation | Glu | ND | ND | mm (Ref 17, see Table V) |
| Asp | ND | ND | |||
| Lact | 0.09 ± 0.02 | 0.2 ± 0.05 | |||
| Rat dorsal cerebral cortex | Sensory stimulation | Glu | ND | ND | μmol/g wet weight (Ref. 16, see Table 2) |
| Asp | ND | ND | |||
| Lact | 1 ± 0.5 | 1.9 ± 0.4 | |||
| Mouse cerebral cortical slices | Repetitive K+ depolarization | Glu | 48.6 ± 6.6 | 37.2 ± 7.6 | nmol/mg protein (Ref. 85, see Table 1) |
| Asp | 20.7 ± 3.5 | 11.4 ± 3.7 | |||
| Lact | ND | ND | |||
| Cerebellar neuron culture | Repetitive NMDA and K+ depolarization | Glu | 51 ± 17.2 | 42.9 ± 0.9 | nmol/mg protein; lactate % labeling (Ref. 86, see Table 1) |
| Asp | 14.3 ± 21 | 10.1 ± 1.3 | |||
| Lact | 5.6 ± 1.8 | 11.5 ± 0.9 | |||
| In vivo human brain NMR spectroscopy | Visual cortex stimulation | Glu | +0.23 ± 0.01% | % change versus rest (Ref. 19, see Table 1) | |
| Asp | –14 ± 0.02% | ||||
| Lact | +24 ± 0.01% | ||||
| Rat cortex | Bicuculline-induced seizure | Glu | 11.8 ± 1.8 | 11.5 ± 1.1 | μmol/g (Ref. 87, see Table 4) |
| Asp | 3.6 ± 0.8 | 2.8 ± 0.3 | |||
| Lact | 3.2 ± 07 | 13 ± 4.9 |
It has been proposed that most of the lactate produced upon brain activation arises in astrocytes, which increase the glycolytic rate to energize the uptake of glutamate along with Na+ in excitatory synapses (78, 79). Indeed, it is likely that aerobic glycolysis (i.e. the production of lactate from glucose in the presence of oxygen) takes place preferentially in astrocytes because, compared with neurons, these cells have reduced MAS activity and reduced aralar/AGC1 levels (8, 9, 11), and freshly isolated astrocytes release more lactate than neurons (see Refs. 80, 81 and reviewed in Ref. 82). However, our results show that even neurons, which have a robust malate-aspartate shuttle, can provide an alternative source of lactate, which would specifically arise under stimulation conditions. Interestingly, a recent study of evoked glucose and oxygen metabolism in rat cerebellum in vivo has shown that increases in evoked lactate production required a time threshold to start and were not dependent on glutamate reuptake (83), a result that may point to neurons, and not only astrocytes, as lactate producers (84). It is conceivable that the prolonged stimulation required to increase lactate release could lead to repeated mitochondrial calcium transients causing persistent Ca2+ activation of mitochondrial dehydrogenases, inhibition of the OGC and MAS, and lactate production.
Conclusions—In this work, we show that MAS activation by extramitochondrial calcium is abolished in the event of αKGDH activation by intramitochondrial calcium, in brain and heart mitochondria. This inhibition is based on the competition of both pathways for the shared substrate αKG. When matrix calcium concentrations regain a basal level, MAS activity is recovered, and this may extend the mitochondrial energization beyond the end of the cytosolic Ca2+ signal. This inhibition of MAS activity may contribute, in part, to explain the “lactate paradox” observed upon brain stimulation in vivo, as it would lead to increased aerobic glycolysis and lactate formation, although enough oxygen is available and the Krebs cycle is activated.
Supplementary Material
Acknowledgments
We thank Barbara Sesé, Inmaculada Ocaña, Juliana Sánchez García, and Isabel Manso for excellent technical assistance. The CIBER de Enfermedades Raras is an initiative of the ISCIII.
This work was supported by Ministerio de Educación y Ciencia Grants BFU2005-C02-01 and BFU2008-04084/BMC, Comunidad de Madrid Grant S-GEN-0269-2006 MITOLAB-CM, and European Union Grant LSHM-CT-2006-518153. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–4.
Footnotes
The abbreviations used are: CaU, calcium uniporter; αKG, αketoglutarate; αKGDH, αketoglutarate dehydrogenase; AGC, aspartate-glutamate carrier; AOAA, aminooxyacetate; GDH, glutamate dehydrogenase; MAS, malate-aspartate shuttle; mitDH, mitochondrial dehydrogenases; mitGPDH, mitochondrial glycerol-3-phosphate dehydrogenase; NCX, mitochondrial Na+/Ca2+ exchanger; OGC, oxoglutarate carrier; RR, ruthenium red; TMRM, tetramethylrhodamine methyl ester.
B. Padro, L. Contreras, and J. Satrústegui, unpublished results.
References
- 1.Nichols, B. J., and Denton, R. M. (1995) Mol. Cell. Biochem. 149–150, 203-212 [DOI] [PubMed] [Google Scholar]
- 2.Lasorsa, F. M., Pinton, P., Palmieri, L., Fiermonte, G., Rizzuto, R., and Palmieri, F. (2003) J. Biol. Chem. 278 38686-38692 [DOI] [PubMed] [Google Scholar]
- 3.Palmieri, L., Pardo, B., Lasorsa, F. M., del Arco, A., Kobayashi, K., Iijima, M., Runswick, M. J., Walker, J. E., Saheki, T., Satrustegui, J., and Palmieri, F. (2001) EMBO J. 20 5060-5069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pardo, B., Contreras, L., Serrano, A., Ramos, M., Kobayashi, K., Iijima, M., Saheki, T., and Satrustegui, J. (2006) J. Biol. Chem. 281 1039-1047 [DOI] [PubMed] [Google Scholar]
- 5.Satrústegui, J., Pardo, B., and del Arco, A. (2007) Physiol. Rev. 87 29-67 [DOI] [PubMed] [Google Scholar]
- 6.Begum, L., Jalil, M. A., Kobayashi, K., Iijima, M., Li, M. X., Yasuda, T., Horiuchi, M., del Arco, A., Satrustegui, J., and Saheki, T. (2002) Biochim. Biophys. Acta 1574 283-292 [DOI] [PubMed] [Google Scholar]
- 7.del Arco, A., Morcillo, J., Martinez-Morales, J. R., Galian, C., Martos, V., Bovolenta, P., and Satrustegui, J. (2002) Eur. J. Biochem. 269 3313-3320 [DOI] [PubMed] [Google Scholar]
- 8.Ramos, M., del Arco, A., Pardo, B., Martinez-Serrano, A., Martinez-Morales, J. R., Kobayashi, K., Yasuda, T., Bogonez, E., Bovolenta, P., Saheki, T., and Satrustegui, J. (2003) Brain Res. Dev. Brain Res. 143 33-46 [DOI] [PubMed] [Google Scholar]
- 9.Berkich, D. A., Ola, M. S., Cole, J., Sweatt, A. J., Hutson, S. M., and LaNoue, K. F. (2007) J. Neurosci. Res. 85 3367-3377 [DOI] [PubMed] [Google Scholar]
- 10.Cahoy, J. D., Emery, B., Kaushal, A., Foo, L. C., Zamanian, J. L., Christopherson, K. S., Xing, Y., Lubischer, J. L., Krieg, P. A., Krupenko, S. A., Thompson, W. J., and Barres, B. A. (2008) J. Neurosci. 28 264-278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu, Y., Ola, M. S., Berkich, D. A., Gardner, T. W., Barber, A. J., Palmieri, F., Hutson, S. M., and LaNoue, K. F. (2007) J. Neurochem. 101 120-131 [DOI] [PubMed] [Google Scholar]
- 12.Lovatt, D., Sonnewald, U., Waagepetersen, H. S., Schousboe, A., He, W., Lin, J. H., Han, X., Takano, T., Wang, S., Sim, F. J., Goldman, S. A., and Nedergaard, M. (2007) J. Neurosci. 27 12255-12266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watkins, J., Basu, S., and Bogenhagen, D. F. (2008) J. Proteome Res. 7 328-338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox, P. T., Raichle, M. E., Mintun, M. A., and Dence, C. (1988) Science 241 462-464 [DOI] [PubMed] [Google Scholar]
- 15.Raichle, M. E., and Mintun, M. A. (2006) Annu. Rev. Neurosci. 29 449-476 [DOI] [PubMed] [Google Scholar]
- 16.Madsen, P. L., Cruz, N. F., Sokoloff, L., and Dienel, G. A. (1999) J. Cereb. Blood Flow Metab. 19 393-400 [DOI] [PubMed] [Google Scholar]
- 17.Cruz, N. F., Ball, K. K., and Dienel, G. A. (2007) J. Neurosci. Res. 85 3254-3266 [DOI] [PubMed] [Google Scholar]
- 18.Dienel, G. A., Wang, R. Y., and Cruz, N. F. (2002) J. Cereb. Blood Flow Metab. 22 1490-1502 [DOI] [PubMed] [Google Scholar]
- 19.Mangia, S., Tkac, I., Gruetter, R., Van de Moortele, P. F., Maraviglia, B., and Ugurbil, K. (2007) J. Cereb. Blood Flow Metab. 27 1055-1063 [DOI] [PubMed] [Google Scholar]
- 20.Han, D., Antunes, F., Canali, R., Rettori, D., and Cadenas, E. (2003) J. Biol. Chem. 278 5557-5563 [DOI] [PubMed] [Google Scholar]
- 21.Martinez-Serrano, A., and Satrustegui, J. (1992) Mol. Biol. Cell 3 235-248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rolfe, D. F., Hulbert, A. J., and Brand, M. D. (1994) Biochim. Biophys. Acta 1188 405-416 [DOI] [PubMed] [Google Scholar]
- 23.Jalil, M. A., Begum, L., Contreras, L., Pardo, B., Iijima, M., Li, M. X., Ramos, M., Marmol, P., Horiuchi, M., Shimotsu, K., Nakagawa, S., Okubo, A., Sameshima, M., Isashiki, Y., Del Arco, A., Kobayashi, K., Satrustegui, J., and Saheki, T. (2005) J. Biol. Chem. 280 31333-31339 [DOI] [PubMed] [Google Scholar]
- 24.Atlante, A., Gagliardi, S., Marra, E., Calissano, P., and Passarella, S. (1999) J. Neurochem. 73 237-246 [DOI] [PubMed] [Google Scholar]
- 25.Cederbaum, A. I., Lieber, C. S., Beattie, D. S., and Rubin, E. (1973) Arch. Biochem. Biophys. 158 763-781 [DOI] [PubMed] [Google Scholar]
- 26.Cheeseman, A. J., and Clark, J. B. (1988) J. Neurochem. 50 1559-1565 [DOI] [PubMed] [Google Scholar]
- 27.Dawson, A. G., and Cooney, G. J. (1978) FEBS Lett. 91 169-172 [DOI] [PubMed] [Google Scholar]
- 28.Grynkiewicz, G., Poenie, M., and Tsien, R. Y. (1985) J. Biol. Chem. 260 3440-3450 [PubMed] [Google Scholar]
- 29.Martinez, A., Vitorica, J., and Satrustegui, J. (1988) Neurosci. Lett. 88 336-342 [DOI] [PubMed] [Google Scholar]
- 30.Robert, R., and Horder, M. (1983) in Methods of Enzymatic Analysis. Enzymes 1: Oxidoreductases, Transferases (Bergmeyer, H. U., Bergmeyer, J., and Grassl, M., eds) Vol. III, 3rd Ed., Verlag Chemie, Weinheim, Germany
- 31.Erakovic, V., Zupan, G., Varljen, J., Laginja, J., and Simonic, A. (2001) Epilepsia 42 181-189 [DOI] [PubMed] [Google Scholar]
- 32.Starkov, A. A., Fiskum, G., Chinopoulos, C., Lorenzo, B. J., Browne, S. E., Patel, M. S., and Beal, M. F. (2004) J. Neurosci. 24 7779-7788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tretter, L., and Adam-Vizi, V. (2000) J. Neurosci. 20 8972-8979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chalmers, S., and Nicholls, D. G. (2003) J. Biol. Chem. 278 19062-19070 [DOI] [PubMed] [Google Scholar]
- 35.Hernandez-SanMiguel, E., Vay, L., Santo-Domingo, J., Lobaton, C. D., Moreno, A., Montero, M., and Alvarez, J. (2006) Cell Calcium 40 53-61 [DOI] [PubMed] [Google Scholar]
- 36.Contreras, L., Gomez-Puertas, P., Iijima, M., Kobayashi, K., Saheki, T., and Satrustegui, J. (2007) J. Biol. Chem. 282 7098-7106 [DOI] [PubMed] [Google Scholar]
- 37.Bernardi, P., Krauskopf, A., Basso, E., Petronilli, V., Blachly-Dyson, E., Di Lisa, F., and Forte, M. A. (2006) FEBS J. 273 2077-2099 [DOI] [PubMed] [Google Scholar]
- 38.Panov, A., Dikalov, S., Shalbuyeva, N., Hemendinger, R., Greenamyre, J. T., and Rosenfeld, J. (2007) Am. J. Physiol. 292 C708-C718 [DOI] [PubMed] [Google Scholar]
- 39.Rutter, G., Pralong, W., and Wollheim, C. (1992) Biochim. Biophys. Acta 1175 107-113 [DOI] [PubMed] [Google Scholar]
- 40.MacDonald, M. J., and Brown, L. J. (1996) Arch. Biochem. Biophys. 326 79-84 [DOI] [PubMed] [Google Scholar]
- 41.Wernette, M. E., Ochs, R. S., and Lardy, H. A. (1981) J. Biol. Chem. 256 12767-12771 [PubMed] [Google Scholar]
- 42.Scholz, T. D., and Koppenhafer, S. L. (1995) Pediatr. Res. 38 221-227 [DOI] [PubMed] [Google Scholar]
- 43.Scholz, T. D., Koppenhafer, S. L., TenEyck, C. J., and Schutte, B. C. (1997) J. Mol. Cell. Cardiol. 29 1605-1613 [DOI] [PubMed] [Google Scholar]
- 44.Leveille, P. J., McGinnis, J. F., Maxwell, D. S., and de Vellis, J. (1980) Brain Res. 196 287-305 [DOI] [PubMed] [Google Scholar]
- 45.McKenna, M. C., Waagepetersen, H. S., Schousboe, A., and Sonnewald, U. (2006) Biochem. Pharmacol. 71 399-407 [DOI] [PubMed] [Google Scholar]
- 46.Nguyen, N. H., Brathe, A., and Hassel, B. (2003) J. Neurochem. 85 831-842 [DOI] [PubMed] [Google Scholar]
- 47.de Bari, L., Atlante, A., Guaragnella, N., Principato, G., and Passarella, S. (2002) Biochem. J. 365 391-403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Runswick, M., Walker, J., Bisaccia, F., Iacobazzi, V., and Palmieri, F. (1990) Biochemistry 29 11033-11040 [DOI] [PubMed] [Google Scholar]
- 49.McCormack, J. G., and Denton, R. M. (1979) Biochem. J. 180 533-544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wan, B., LaNoue, K. F., Cheung, J. Y., and Scaduto, R. C., Jr. (1989) J. Biol. Chem. 264 13430-13439 [PubMed] [Google Scholar]
- 51.Gunter, K. K., and Gunter, T. E. (1994) J. Bioenerg. Biomembr. 26 471-485 [DOI] [PubMed] [Google Scholar]
- 52.Gunter, T. E., Yule, D. I., Gunter, K. K., Eliseev, R. A., and Salter, J. D. (2004) FEBS Lett. 567 96-102 [DOI] [PubMed] [Google Scholar]
- 53.Villa, A., Garcia-Simon, M. I., Blanco, P., Sese, B., Bogonez, E., and Satrustegui, J. (1998) Biochim. Biophys. Acta 1373 347-359 [DOI] [PubMed] [Google Scholar]
- 54.Ichas, F., Jouaville, L. S., and Mazat, J. P. (1997) Cell 89 1145-1153 [DOI] [PubMed] [Google Scholar]
- 55.Ichas, F., and Mazat, J. P. (1998) Biochim. Biophys. Acta 1366 33-50 [DOI] [PubMed] [Google Scholar]
- 56.Wang, W., Fang, H., Groom, L., Cheng, A., Zhang, W., Liu, J., Wang, X., Li, K., Han, P., Zheng, M., Yin, J., Wang, W., Mattson, M. P., Kao, J. P., Lakatta, E. G., Sheu, S. S., Ouyang, K., Chen, J., Dirksen, R. T., and Cheng, H. (2008) Cell 134 279-290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carafoli, E. (2002) Proc. Natl. Acad. Sci. U. S. A. 99 1115-1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vitorica, J., and Satrustegui, J. (1985) Biochem. J. 225 41-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.LaNoue, K. F., Bryla, J., and Bassett, D. J. (1974) J. Biol. Chem. 249 7514-7521 [PubMed] [Google Scholar]
- 60.LaNoue, K. F., Meijer, A. J., and Brouwer, A. (1974) Arch. Biochem. Biophys. 161 544-550 [DOI] [PubMed] [Google Scholar]
- 61.LaNoue, K. F., and Tischler, M. E. (1974) J. Biol. Chem. 249 7522-7528 [PubMed] [Google Scholar]
- 62.Bernardinelli, Y., Azarias, G., and Chatton, J. Y. (2006) Glia 54 460-470 [DOI] [PubMed] [Google Scholar]
- 63.Nicholls, D. G. (1978) Biochem. J. 170 511-522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gunter, T. E., and Pfeiffer, D. R. (1990) Am. J. Physiol. 258 C755-C786 [DOI] [PubMed] [Google Scholar]
- 65.McCormack, J. G., Halestrap, A. P., and Denton, R. M. (1990) Physiol. Rev. 70 391-425 [DOI] [PubMed] [Google Scholar]
- 66.Bernardi, P., Angrilli, A., and Azzone, G. F. (1990) Eur. J. Biochem. 188 91-97 [DOI] [PubMed] [Google Scholar]
- 67.Nicholls, D. G., and Scott, I. D. (1980) Biochem. J. 186 833-839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McCormack, J., and Denton, R. (1999) in Calcium as a Cellular Regulator (Carafoli, E., and Klee, C., eds) pp. 529-544, Oxford University Press, New York
- 69.Gellerich, F. N., Gizatullina, Z., Nguyen, H. P., Trumbeckaite, S., Vielhaber, S., Seppet, E., Zierz, S., Landwehrmeyer, B., Riess, O., von Horsten, S., and Striggow, F. (2008) J. Biol. Chem. 283 30715-30724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.O'Donnell, J. M., Doumen, C., LaNoue, K. F., White, L. T., Yu, X., Alpert, N. M., and Lewandowski, E. D. (1998) Am. J. Physiol. 274 H467-H476 [DOI] [PubMed] [Google Scholar]
- 71.O'Donnell, J. M., Kudej, R. K., LaNoue, K. F., Vatner, S. F., and Lewandowski, E. D. (2004) Am. J. Physiol. 286 H2237-H2242 [DOI] [PubMed] [Google Scholar]
- 72.Sluse, F. E., Goffart, G., and Liebecq, C. (1973) Eur. J. Biochem. 32 283-291 [DOI] [PubMed] [Google Scholar]
- 73.Marmol, P., Pardo, B., Wiederkehr, A., Del Arco, A., Wollheim, C. B., and Satrustegui, J. (2009) J. Biol. Chem. 284 515-524 [DOI] [PubMed] [Google Scholar]
- 74.Carobbio, S., Frigerio, F., Rubi, B., Vetterli, L., Bloksgaard, M., Gjinovci, A., Pournourmohammadi, S., Herrera, P. L., Reith, W., Mandrup, S., and Maechler, P. (2008) J. Biol. Chem. 284 921-929 [DOI] [PubMed] [Google Scholar]
- 75.Yang, S. J., Huh, J. W., Kim, M. J., Lee, W. J., Kim, T. U., Choi, S. Y., and Cho, S. W. (2003) Biochimie (Paris) 85 581-586 [DOI] [PubMed] [Google Scholar]
- 76.Monteith, G. R., and Blaustein, M. P. (1999) Am. J. Physiol. 276 C1193-C1204 [DOI] [PubMed] [Google Scholar]
- 77.Moreau, B., Nelson, C., and Parekh, A. B. (2006) Curr. Biol. 16 1672-1677 [DOI] [PubMed] [Google Scholar]
- 78.Kasischke, K. A., Vishwasrao, H. D., Fisher, P. J., Zipfel, W. R., and Webb, W. W. (2004) Science 305 99-103 [DOI] [PubMed] [Google Scholar]
- 79.Magistretti, P. J., Pellerin, L., Rothman, D. L., and Shulman, R. G. (1999) Science 283 496-497 [DOI] [PubMed] [Google Scholar]
- 80.Pellerin, L., and Magistretti, P. J. (1994) Proc. Natl. Acad. Sci. U. S. A. 91 10625-10629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Poitry-Yamate, C. L., Poitry, S., and Tsacopoulos, M. (1995) J. Neurosci. 15 5179-5191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nehlig, A., and Coles, J. A. (2007) Glia 55 1238-1250 [DOI] [PubMed] [Google Scholar]
- 83.Caesar, K., Hashemi, P., Douhou, A., Bonvento, G., Boutelle, M. G., Walls, A. B., and Lauritzen, M. (2008) J. Physiol. (Lond.) 586 1337-1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kasischke, K. A. (2008) J. Physiol. (Lond.) 586 1207-1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Waagepetersen, H. S., Doring, S., and Schousboe, A. (2008) Neurochem. Res. 33 1610-1617 [DOI] [PubMed] [Google Scholar]
- 86.Bak, L. K., Schousboe, A., Sonnewald, U., and Waagepetersen, H. S. (2006) J. Cereb. Blood Flow Metab. 26 1285-1297 [DOI] [PubMed] [Google Scholar]
- 87.Patel, A. B., Chowdhury, G. M., de Graaf, R. A., Rothman, D. L., Shulman, R. G., and Behar, K. L. (2005) J. Neurosci. Res. 79 128-138 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.