Abstract
Epithelial cells expressing calprotectin, a heterodimer of S100A8 and S100A9 proteins, are more resistant to bacterial invasion. To determine structural motifs that affect resistance to bacterial invasion, mutations were constructed in S100A9 targeting the calcium-binding loops I and II (E36Q, E78Q, E36Q,E78Q) and the C terminus (S100A91–99 and S100A91–112), which contains putative antimicrobial zinc-binding and phosphorylation sites. The S100A8 and mutated S100A9 encoding plasmids were transfected into calprotectin-negative KB carcinoma cells. All transfected cells (except KB-sham) expressed 27E10-reactive heterodimers. In bacterial invasion assays with Listeria monocytogenes and Salmonella enterica serovar Typhimurium (Salmonella typhimurium), cell lines expressing S100A8 in complex with S100A9E36Q, S100A9E78Q, S100A91–99, or S100A91–112 mutants or the S100A91–114 (full-length) calprotectin resisted bacterial invasion better than KB-sham. When compared with KB-S100A8/A91–114, cells expressing truncated S100A91–99 or S100A91–112 with S100A8 also showed increased resistance to bacterial invasion. In contrast, glutamic acid residues 36 and 78 in calcium-binding loops I and II promote resistance in epithelial cells, because cells expressing S100A9E36Q,E78Q with S100A8 were unable to resist bacterial invasion. Mutations in S100A9 E36Q, E78Q were predicted to cause loss of the calcium-induced positive face in calprotectin, reducing interactions with microtubules and appearing to be crucial for keratinocyte resistance to bacterial invasion.
Mucosal keratinocytes continuously confront endogenous and exogenous invading microorganisms. Consequently the superficial keratinocytes of the oral mucosa contain a variety of indigenous bacteria (1). Yet the keratinocytes appear to resist large scale invasion and intracellular infection.
Expressed in the cytoplasm of squamous mucosal keratinocytes, calprotectin (S100A8 and S100A9, MRP8 and MRP14, calgranulin A and B, L1, cystic fibrosis antigen, and 27E10 antigen) is a heterodimeric complex of polypeptides of 10.8 and 13.2 kDa, respectively (2–4). These two subunits are members of the S100 protein family, which are involved in cell cycle progression, cell differentiation, and cytoskeleton-membrane interaction (5–7). Calprotectin is the most abundant protein found in the cytoplasm of neutrophils (8, 9) and is also found in monocytes (10), macrophages (11), and human gingival keratinocytes (2). Elevated levels of calprotectin have been observed in body fluids such as plasma, saliva, gingival crevicular fluid, stools, and synovial fluid during infections and inflammatory conditions (12). Consequently, calprotectin is broadly used as a marker for inflammatory bowel diseases (13), reactive arthritis (14), and Sjogren syndrome (15).
Functioning as an antimicrobial protein (complex), calprotectin shows broad spectrum activities against microorganisms, including Capnocytophaga sputigena (16), Candida albicans (17), Escherichia coli, Staphylococcus aureus, Staphylococcus epidermis (18), and Borrrelia burgdorferi (19). Calprotectin also inhibits bacterial invasion of epithelial cells by Listeria monocytogenes, S. typhimurium, and Porphyromonas gingivalis (20, 21). By promoting resistance to bacterial invasion, calprotectin-expressing cells, including squamous oral keratinocytes, are likely to contribute to mucosal innate immunity.
We have been studying the structural basis of calprotectin-mediated, cell-associated antimicrobial resistance. Unlike S100A8 and other members of the S100 family, S100A9 has a extended C-terminal region, which has an amino acid sequence (residues 89–108) that is identical to the N-terminal region of neutrophil immobilizing factor (22, 23) and homologous to domain 5 of high molecular weight kininogen (24). Domain 5 of high molecular weight kininogen has antimicrobial activity against E. coli, Pseudomonas aeruginosa, and Enterococcus faecalis (25). In addition, S100A9 C-terminal residues 103–105 form a polyhistidine motif (HHH), which may be involved in zinc binding (26, 27). Also suggested to be zinc-binding domains, the HXXXH motifs in S100A8 and S100A9 are commonly found in S100 proteins (4, 27, 28). Because zinc is required for bacterial growth, either the polyhistidine or HXXXH motifs have been suggested to bind and sequester zinc from microorganisms and inhibit bacterial growth (4, 27–29). In addition to zinc, calprotectin chelates other metal ions, including Mn2+, which inhibits growth of S. aureus in tissue abscesses (30).
Independent of direct antimicrobial activity, epithelial resistance to invasion may also reflect the ability of bacteria to bind and internalize. Bacterial binding and internalization could be regulated by calprotectin as an interacting partner with the cytoskeleton, although distinguishing from antimicrobial activity may not always be clear. For example, S100A8/A9 translocates across the plasma membrane and is released from the cell in a tubulin-dependent manner (31). Release from the cell is controlled by the penultimate threonine (Thr-113) residue in the C terminus of S100A9, a substrate for protein kinase C (31). Although tubulin-dependent interactions may bring calprotectin in proximity to surface bacteria, these interactions could regulate cytoskeleton-dependent internalization (32).
In epithelial cells, calprotectin exists primarily as a heterodimeric complex of S100A8 and S100A9 and the individual subunits are not readily found (2). S100A9 integrity is critical to the formation of complexes with S100A8 (33) and the calcium-binding loops within the EF-hands contribute to intermolecular stability (4). The calcium-binding loops of S100 proteins also modulate intracellular calcium signaling, which affects cell differentiation, and cell cycle and cytoskeletal interactions (5). Integrity of the S100A9 calcium-binding loops may also be critical to resistance against bacterial invasion.
We considered that keratinocyte resistance to invasion reflected the ability of the cells to bind, internalize, and host viable invaders within the cell. In this study, we hypothesized that specific structural motifs of S100A9 in the calprotectin complex regulate epithelial cell resistance to bacterial invasion. To test this hypothesis, we designed five different S100A9 mutant constructs either in the calcium-binding or C-terminal domains using in vitro site-directed mutagenesis and deletion mutagenesis, respectively. Each mutated S100A9 was then expressed in KB cells with S100A8. As we reported previously (20), calprotectin (S100A8/A9) increased the resistance of epithelial cells to bacterial invasion. In the presence of S100A8, truncation of the C-terminal domain of S100A9 made the cells more resistant to invasion than with full-length S100A9. In contrast, mutations of S100A9 calcium-binding loops resulted in complete loss of resistance to bacterial invasion. Therefore, the central core polypeptide domain of S100A9 in the calprotectin complex plays a crucial role in epithelial resistance to bacterial invasion.
EXPERIMENTAL PROCEDURES
Cells—Wild-type calprotectin-negative KB cells (American Type Culture Collection, ATCC CCL-17) were maintained in modified Eagle's media (Mediatech, Herndon, VA) supplemented with 10% fetal bovine serum (Mediatech) in 5% CO2 at 37 °C. Transfected KB cells were maintained in modified Eagle's media supplemented with 10% fetal bovine serum and 700 μg/ml G418 sulfate (Mediatech). To test the effect of calprotectin expression on viable bacteria, mutants and controls were maintained in medium without G418 sulfate for 4 days before the experiments were performed.
Bacteria—L. monocytogenes ATCC 10403S (provided by Dr. Daniel Portnoy, University of California, Berkley) and S. enterica serovar Typhimurium (S. typhimurium) ATCC 14028 (provided by Dr. Roy Curtiss III, Washington University, St. Louis) were grown in brain heart infusion medium (Difco) and on tryptic soy agar (Difco) at 37 °C. Listeria and Salmonella were harvested from log phase or stationary phase, respectively (absorbance of 0.4–0.6 at 620 nm), and used to infect KB cells.
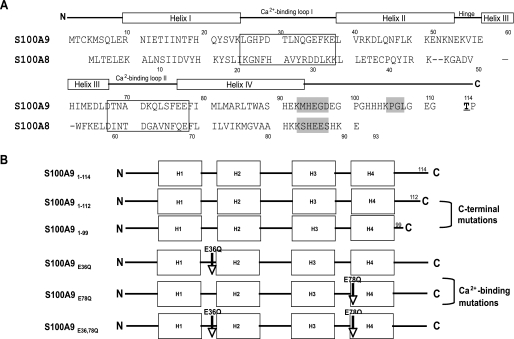
Construction of Calprotectin and S100A9 Mutant Expressing KB Cells—The structure of S100A8, S100A9, and mutant constructs in selected S100A9 functional domains are shown in Fig. 1, A and B. To construct S100A8 and S100A9 expression vectors, sequences were amplified using the following primers: S100A8, sense 5′-GGGCATCATGTTGACCGAGC-3′ and antisense 5′-GTAACTCAGCTACTCTTTGTGGCTT-3′; S100A9, sense 5′-CGATGACTTGCAAAATGTCGCAG-3′ and antisense 5′-GCCACTGTGGTCTTAGGGT-3′. To construct truncated S100A9 mutants (Fig. 1B), the sense primer was identical to S100A9 above, and the antisense primers were as follows: S100A91–112, 5′-TTAGCCCTCCCCGAGGGCTG-3′, and S100A91–99, 5′-TTACTCGTCACCCTCGTGCATCTTC-3′. S100A9 mutant sequences with point mutations in the calcium-binding loops, E36Q and E78Q (Fig. 1B), were constructed using the following oligonucleotides: S100A9E36Q, 5′-GCACCCTGAACCAGGGGCAATTCAAAGAGCTGGTGCG-3′, and 5′-CGCACCGCCTTGAATTGCCCCTGGTTCAGGGTG-3′, and S100A9E78Q, 5′-GCAGCTGAGTTCGACAGTTCATCATGCTGATGGCG-3′ and 5′-CGCCATCAGCATGATAATGCTCGAAGCCAGCTGC-3′, with the QuickChange® site-directed mutagenesis kit (Stratagene, Rockville, MD). S100A9E36Q,E78Q was constructed using all the oligonucleotides from above. PCR products were cloned and amplified using pPCR-Script® (Stratagene, La Jolla, CA). All mutants were verified by sequencing. S100A8 and mutated S100A9 sequences were then cloned into pIRES (BD Biosciences) and pKN-1 (pIRES-EGFP; BD Biosciences with the BamHI site at 1887 bp attenuated) plasmids and co-transfected into KB cells using Superfect (Qiagen, Valencia, CA). Transfectants were selected by resistance to 700 μg/ml G418 sulfate and sorted for enhanced green fluorescent protein expression using a FACSorter (BD Biosciences). Cells co-transfected with insertless pIRES and pKN-1 served as a sham-control transfectant (KB-sham). Plasmids containing S100A8 and unmodified S100A9 were co-transfected into KB cells and served as a positive calprotectin-expressing control (KB-S100A8/A91–114). Stable transfectants were confirmed by reverse transcription-PCR using PCR primers listed above.
FIGURE 1.
Structure of S100A8, S100A9, and mutations in selected functional domains. A, amino acid sequences of S100A8 and S100A9. Each subunit contains two EF-hands with helix-loop-helix motifs linked by a hinge region and flanked by N- and C-terminal domains. Calcium-binding loops are in boxes. Putative zinc-binding domains are highlighted in gray. The phosphorylation site is boldface and underlined. Source, NCBI Entrez protein P05109 (A8) and P06702 (A9) (38). B, full-length S100A9 (S100A91–114) and S100A9 mutant constructs, including C-terminal domain deletions (S100A91–112 and S100A91–99) and amino acid substitutions in the calcium-binding loops (S100A9E36Q, S100A9E78Q, and S100A9E36Q,E78Q).
Immunofluorescence—Cells were grown on coverslips overnight, washed with PBS,2 and fixed with 4% paraformaldehyde for 10 min at room temperature. Monolayers were washed three times and permeabilized with 0.2% Triton X-100 for 2 min. After washing, monolayers were then incubated with murine monoclonal antibody against the calprotectin complex (mAb 27E10, diluted 1:50; Bachem, King of Prussia, PA) for 1 h at room temperature, followed by Alexa Fluor 568-conjugated goat anti-mouse IgG (diluted 1:500; Molecular Probes, Eugene, OR) for 1 h. Both antibodies were diluted in 3% (w/v) bovine serum albumin (Sigma) in PBS. The monolayers were washed and mounted with Fluoromount G (Southern Biotechnology, Birmingham, AL). Slides were examined using a Nikon Eclipse epifluorescence microscope and photographed using a Spot digital camera (Diagnostic Instruments Inc, Sterling Heights, MI).
Sandwich ELISA—To detect calprotectin complex, cells were resuspended in Hanks' balanced salt solution (Invitrogen) and sonicated three times on ice at 50 watts for 15 s each (Sonifier Cell Disruptor W185, Heat Systems, Ultrasonics Inc., Plainview, MA). To obtain cell cytosol, sonicates were centrifuged at 10,000 × g for 20 min, and supernatants were collected, and total protein in each sample was determined by BCA protein assay kit (Pierce). Cell cytosol (50 μg) was analyzed for calprotectin using an ELISA. Briefly, 96-well plates were coated overnight at 4 °C with mAb 27E10 (diluted 1:100; Bachem), washed three times with PBS, pH 7.2, and 0.1% Tween 20, blocked for 1 h at 37 °C with blocking buffer (PBS, 0.1% Tween 20 and 0.5 mm CaCl2), and washed three more times. Cell cytosol was added, incubated for 1 h at 37 °C, and washed three times. Biotinylated murine monoclonal antibody to S100A9 (S 36.48-biotin, diluted 1:200; Bachem) was then added and incubated for 1 h at 37 °C. Extravidin-horseradish peroxidase and 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonic acid) (Sigma) were used for colorimetric detection, and the absorbance was measured at 405 nm.
Co-immunoprecipitation, Gel Electrophoresis, Silver Staining, and Western Blotting—mAb 27E10 was used for immunoprecipitation. To demonstrate co-precipitation of S100A8 and mutant S100A9 proteins, products were analyzed on silver-stained gels and Western blots. In brief, cells were treated with lysis buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1 mm EDTA, pH 8.0, 1 mm EGTA, 1% Triton X-100, 0.5% Nonidet P-40 with the proteinase inhibitors, 2 μg/ml phenylmethylsulfonyl fluoride, 1 μg/ml pepstatin A, 1 μg/ml aprotinin, 2.5 μg/ml leupeptin). Lysate protein concentration was determined using the BCA protein assay kit (Pierce).
In preparation for immunoprecipitation, cell lysates were pre-cleared (reduced nonspecific binding) using protein A/G beads (50 μl), which were previously equilibrated twice in 450 μl of cold lysis buffer and centrifuged at 7500 × g for 30–45 s. Cell lysates (1 mg of protein) were incubated with the equilibrated protein A/G beads at 4 °C for 1 h using constant mixing and then centrifuged at 7500 × g for 10 min. The supernatants (pre-cleared lysates) were collected and incubated with mAb 27E10 (5 μg) at 4 °C for 1 h with constant mixing. Next, equilibrated protein A/G beads (50 μl) were added into the mixture and incubated overnight at 4 °C with constant mixing, pelleted at 7500 × g for 30–45 s, and washed five times with lysis buffer. Immunoprecipitated protein associated with the beads was resuspended in 50 μl of 2× SDS-PAGE buffer (1.2 ml of 0.5 m Tris, pH 6.8, 2% SDS, 20% glycerol, 0.5 ml of β-mercaptoethanol, and 1.6 ml of 1% bromphenol blue) and boiled to dissociate the immunoprecipitated protein from the beads.
Immunoprecipitates (30 μl) were analyzed on 15% SDS-polyacrylamide gels, which were stained with metachromatic silver following the manufacturer's instructions (Bio-Rad). For Western blotting, KB cell lysates or the immunoprecipitated samples were separated on 15% SDS-PAGE, transferred onto a 0.2-μm nitrocellulose membrane (Bio-Rad), using a semi-dry transfer apparatus (Bio-Rad), and blocked overnight with 5% nonfat milk in TBST buffer (0.5 m NaCl, 20 mm Tris, pH 7.5, and 0.1% Tween 20). The membranes were then incubated with mouse anti-human S100A8 monoclonal antibody (C-10, Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti-human S100A9 monoclonal antibody (S 36.48, Bachem), or rabbit anti-human S100A9 polyclonal antibody (H-90, Santa Cruz Biotechnology) (each diluted 1:500) for 1 h at room temperature, washed three times with TBST buffer, and then incubated with either horseradish peroxidase-conjugated goat anti-mouse IgG or goat anti-rabbit IgG (diluted 1:1000; Santa Cruz Biotechnology), respectively, for 1 h at room temperature. After washing, immunoblots were developed using ECL Western blot detection reagents (Amersham Biosciences). Nonspecific isotype IgG was used as a negative control.
Bacterial Invasion Assay—Bacterial invasion was determined by the antibiotic protection assay as we described previously (20). In brief, KB transfectants (1.2 × 105 cells) were seeded overnight in 24-well plates. Cells were then incubated with L. monocytogenes or S. typhimurium at a multiplicity of infection (m.o.i.) of 100:1 and 1:1, respectively. After 2 h of incubation, the monolayers were washed with Dulbecco's PBS (Sigma) and incubated in medium containing 100 μg/ml gentamicin (Sigma) for 1.5 h to eliminate extracellular bacteria. The monolayers were then washed and incubated with sterile distilled water for 15 min to release intracellular bacteria. Released bacteria were diluted, plated with a spiral plater (Spiral Biotech, Bethesda, MD), and incubated overnight at 37 °C, and the numbers of colony-forming units (CFUs) of internalized bacteria were enumerated on a New Brunswick C-110 colony counter (New Brunswick, NJ). The invasion assay was performed in triplicate and repeated at least three times.
Immunofluorescence Analysis of Intracellular and Extracellular Listeria—Cells (1.2 × 105) were seeded on glass coverslips and grown overnight. As described previously (21), the monolayers were infected with L. monocytogenes for 2 h at an m.o.i. of 100:1, washed twice with Dulbecco's PBS, and fixed with 4% paraformaldehyde. Extracellular Listeria were stained using rabbit anti-Listeria serum (diluted 1:3000; Biodesign, Kennebunk, ME) for 1 h, washed with PBS, and then incubated with Alexa Fluor 568-conjugated goat anti-rabbit IgG (diluted 1:500; Molecular Probes) for another hour. All antibodies were diluted in 3% bovine serum albumin in PBS. Cells were then permeabilized with 0.2% Triton X-100 for 2 min and then stained for both intracellular and extracellular Listeria. Permeabilized monolayers were washed, incubated with rabbit anti-Listeria serum for 1 h, washed three times, and then incubated with Alexa Fluor 488-conjugated goat anti-rabbit IgG (1:500; Molecular Probes) for 1 h. Nuclei were stained using 4′,6′-diamidino-2-phenylindole (diluted 1:3000; Molecular Probes). To verify antibody specificity, primary antibodies were replaced by rabbit serum. To determine nonspecific binding, secondary antibodies were added without primary antibody. Cells were observed using a Nikon Eclipse fluorescence microscope at ×400 magnification, and images from 20 random fields were captured with a Spot digital camera (Diagnostic Instruments Inc.). In each field, total Listeria (Alexa 488) and extracellular Listeria (Alexa 568) were counted. The number of intracellular Listeria was determined by subtracting the number of extracellular Listeria from the total count.
Bacterial Binding Assay—Binding of Listeria to KB cells was performed as described previously (21). Cells (1.2 × 105) were seeded on glass coverslips and grown overnight. Monolayers were then incubated with L. monocytogenes at an m.o.i. of 100:1 for up to 60 min at 37 °C, washed, and fixed using 4% paraformaldehyde for 10 min. Adherent Listeria were labeled for 1 h with rabbit anti-Listeria serum (diluted 1:3000; Biodesign), washed, and incubated for 1 h with Alexa Fluor 568-conjugated goat anti-rabbit IgG. Separate coverslips were incubated with rabbit serum or secondary antibody as controls. At each time point, images from 10 random microscopic fields at ×200 magnification were captured with a Spot digital camera, and adherent bacteria were enumerated by visual counting.
Structural Analysis of Calcium-free and Calcium-bound Calprotectin—Because the structure of calcium-free calprotectin has not been determined, we generated the homology modeled structure using the program MODELLER (34). This program was chosen because of its ability to handle the alignment of a heterodimer target sequence (S100A8 and S100A9) with a homodimer structural template. Calcium-free S100A1, S100A4, and S100B structures (PDB codes 1K2H, 1M31, and 2PRU, respectively) were chosen as templates for homology modeling because these proteins have over 30% sequence identity with both S100A8 and S100A9 (35–37). The length and level of sequence identity are important factors in accurate homology model generation. These three structures were used as templates both individually and combined into a single composite template for homology model generation. There is little overall difference in the final models, but the structure used in Fig. 8 is based on the composite template to remove the bias of any individual starting structure. Additionally, the residues in the homology model generated structure were trimmed to match those described in PDB code 1XK4 (S100A8 Met-1 to His-87, S100A9 Lys-4 to Glu-92) to most closely compare changes in the molecular surface upon calcium binding. Furthermore, the absence of electron density for the C-terminal 22 residues in S100A9 in PDB code 1XK4 implies that these residues occupy multiple conformations and that there is no experimental support to favor a single prediction in our models for this tail region. The calcium-bound form of calprotectin has been experimentally determined by Skerra and co-workers, PDB code 1XK4 (4), and this information was used in our analysis. The program Swiss-PdbViewer was used to generate the ribbon diagrams, molecular surface, and the calculation of electrostatic potential with the same settings throughout Fig. 8 (38). The model structure for calcium-bound S100A8 in complex with calcium-free S100A9 was created by structural superimposition of the N- and C-terminal helices of the calcium-free calprotectin (composite model structure) onto calcium-bound calprotectin (PDB code 1XK4). Both structures were written out as one file followed by removal of the information for calcium-free S100A8 and calcium-bound S100A9.
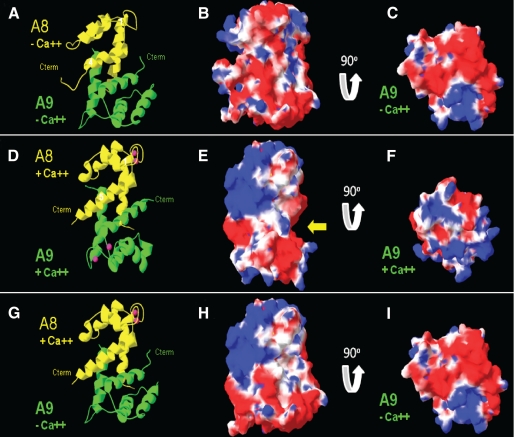
FIGURE 8.
A representation of the changes in calprotectin structure and charge resulting from S100A9E36Q,E78Q mutations. The ribbon diagram of calcium-free and calcium-bound calprotectin, both wild-type and the mutant, is presented in A, D, and G, and the corresponding calculated charged molecular surface is shown in B, E, and H. The surface of S100A9 is shown in C, F, and I; the view was obtained by rotating the charged molecular surface of calprotectin 90° on the z axis, revealing the S100A9 underside. The location of C-terminal tail of S100A9 has not been resolved by crystallography and has been omitted from this model. A–C, model structure of calcium-free calprotectin. D–F, calcium-bound form of calprotectin, based on PDB code 1XK4 (4). G–I, S100A8 calcium-bound structure combined with the calcium-free S100A9 structure based upon the E36Q,E78Q mutations. Color key: S100A8, yellow; S100A9, green; calcium, pink; positively charged surface, blue; negatively charged surface, red; and hydrophobic surface, white.
Statistical Analyses—Data are presented as the means ± S.E. Significant differences between control (KB-sham) and S100A9 mutants were determined using a two-sample Student's t test. p < 0.05 was considered to be statistically significant.
RESULTS
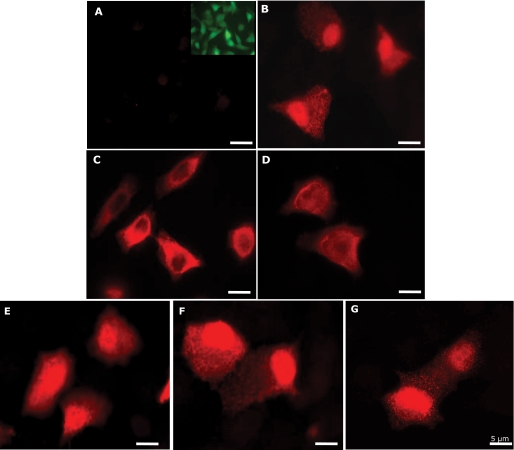
Formation of S100A8 and Mutant S100A9 Heterodimers—As shown schematically in Fig. 1, KB cells were transfected to express calprotectin (S100A8/S100A9; Fig. 2B) and S100A8 in the presence of S100A9 C-terminal deletion mutants (Fig. 2, C and D) or point mutations in the calcium-binding loops (Fig. 2, E–G). Using complex-specific mAb 27E10, S100A8 in the presence of all mutant S100A9 variants appeared to form calprotectin complexes as suggested by immunofluorescence microscopy; KB-sham, the sham-transfected control cells, was negative (Fig. 2A).
FIGURE 2.
mAb 27E10 reactivity in KB-S100A8/A9 mutants. Monolayers of KB-sham transfectant (A), KB-S100A8/A91–114 (B), KB-S100A8/A91–112 (C), KB-S100A8/A91–99 (D), KB-S100A8/A9E36Q (E), KB-S100A8/A9E78Q (F), and KB-S100A8/A9E36Q,E78Q (G) were fixed with 4% paraformaldehyde and stained as described under “Experimental Procedures.” Monolayers were washed and then permeabilized with 0.2% Triton X-100 for 2 min. Monolayers were incubated with mAb 27E10 for 1 h, followed by Alexa Fluor 568-conjugated goat anti-mouse IgG for 1 h at room temperature, which stains calprotectin red. The inset in A shows enhanced green fluorescent protein expressed in KB-sham cells. The experiments were performed three times with similar results. Scale bar, 5 μm.
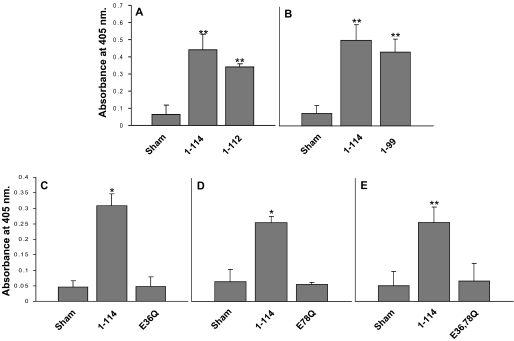
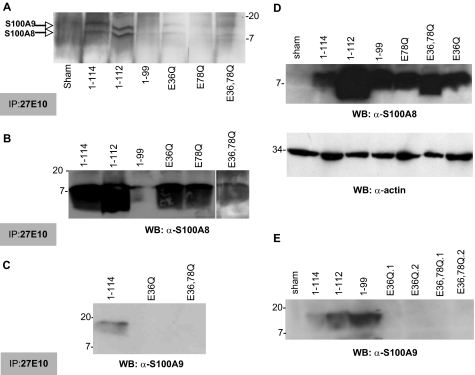
Antigen(s) precipitated by mAb 27E10 were recovered from cytosol of clones expressing calprotectin or S100A8 co-expressed with mutant S100A9 and detected with biotinylated S100A9 mAb (S 36.48-biotin; Bachem) in sandwich ELISA (Fig. 3). Truncated variants of S100A9 co-expressed with S100A8 and calprotectin appeared to form similar amounts of calprotectin complex in KB cell cytosol and significantly more than the sham control (*, p < 0.05; **, p < 0.001; Fig. 3, A and B). In the same conditions, cells expressing S100A9 point mutations in calcium-binding loops did not appear to contain cytosolic heterodimers with S100A8 based on reaction with the anti-S100A9 mAb (Fig. 3, C–E). To learn whether anti-S100A9 immunoreactivity was lost when point mutated S100A9 formed heterodimers with S100A8, immunoprecipitates were analyzed by SDS-PAGE and Western blotting (Fig. 4). On silver-stained gels, S100A8 and S100A9 (S100A91–114) proteins were visualized at 10.8 and 13.2 kDa, respectively (Fig. 4A). Recombinant proteins with similar molecular weights were detected in immunoprecipitates of S100A8 co-expressed with S100A91–112, S100A9E36Q, S100A9E78Q, and S100A9E36Q,E78Q; S100A8 with S100A91–99 was not well resolved. After immunoprecipitation with mAb 27E10, S100A8/A91–114, S100A8/A91–112, S100A8/A91–99, S100A8/A9E36Q, S100A8/A9E78Q, and S100A8/A9E36Q,E78Q resolved on Western blots in reaction with anti-S100A8 (Fig. 4B). In contrast, anti-S100A9 antibodies failed to detect 27E10 immunoprecipitated S100A9E36Q or S100A9E36Q,E78Q (Fig. 4C) or E78Q (data not shown). To confirm that the anti-S100A9 antibody did not react with S100A9 calcium-binding loop mutants, cell lysates were analyzed directly by Western blotting. Lysates from all clones except the sham control reacted with anti-S100A8 (Fig. 4D). Anti-S100A9 reacted only with S100A9 and the C-terminal deletion mutants of S100A9 (S100A91–99 and S100A91–112), and failed to react when the calcium-binding loops were mutated (Fig. 4E). As expected, the calprotectin complex-specific mAb 27E10 did not react with either subunit of calprotectin in Western blots (data not shown). In general, clones producing the most heterodimers were chosen for further study. Clones with S100A9 calcium-binding loop point mutations were selected based on strong signals with 27E10 in immunofluorescence staining and anti-S100A8 after 27E10 immunoprecipitation.
FIGURE 3.
Calprotectin production in S100A9 mutants. Calprotectin complex in KB-S100A8/A91–112 (A), KB-S100A8/A91–99 (B), KB-S100A8/A9E36Q (C), KB-S100A8/A9E78Q (D), and KB-S100A8/A9E36Q,E78Q (E) were estimated using a sandwich ELISA as described under “Experimental Procedures.” KB-sham and KB-S100A8/A91–114 cells were used as negative and positive controls. Values are means ± S.E. (n ≥ 3; *, p < 0.05; **, p < 0.001). For each mutant, at least 10 clones were tested. Each experiment was performed in four replicates. The results are representative clones from each mutant.
FIGURE 4.
Analysis of S100A8 and mutated S100A9 in KB transfectants. To analyze the heterodimeric complexes, the cell lysates (1 mg of protein) from KB-sham, KB-S100A8/A91–114, KB-S100A8/A91–112, KB-S100A8/A91–199, KB-S100A8/A9E36Q, KB-S100A8/A9E78Q, and KB-S100A8/A9E36Q,E78Q were co-immunoprecipitated (IP) using mAb 27E10. The immunoprecipitated proteins were then separated by 15% SDS-PAGE and either silver-stained or electroblotted onto nitrocellulose paper (A) and detected with anti-S100A8 (B) and anti-S100A9 antibodies (C), as described under “Experimental Procedures.” Cell lysates were also directly analyzed for S100A8 (D) and S100A9 (E) by Western blots (WB) as described under “Experimental Procedures.” Actin expression was used as protein loading control (lower panel in D).
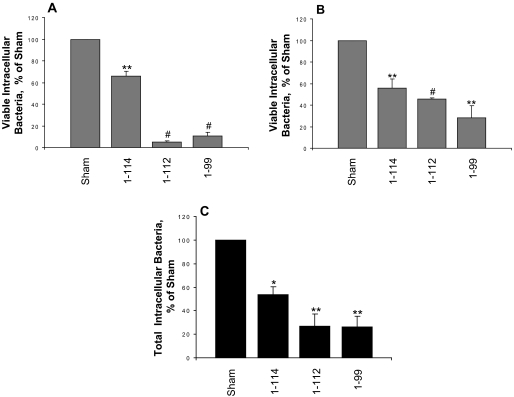
S100A9 C-terminal Deletion Increases Resistance to Bacterial Invasion—To determine whether the C-terminal domain of S100A9 is crucial for cellular resistance to bacterial invasion, KB-S100A8/A91–112, KB-S100A8/A91–99, and control cells were incubated for 2 h with either L. monocytogenes ATCC 10403S (Fig. 5A) or S. typhimurium ATCC 14028 (Fig. 5B) at m.o.i. 100:1 or 1:1, respectively. When compared with the sham control, KB-S100A8/A91–114, KB-S100A8/A91–112, and KB-S100A8/A91–99 permitted significantly fewer viable intracellular Listeria (4.9 and 10.7% invasion, respectively) and Salmonella (45.5 and 28% invasion, respectively) (p < 0.01). The numbers of internalized Listeria and Salmonella in calprotectin-negative cells (KB-sham) ranged from 1 × 106 to 3 × 107 CFU/well (1 ml) and from 2 × 104 to 4 × 105 CFU/well (1 ml), respectively. KB-S100A8/A91–99 cells showed the greatest resistance to invasion with viable intracellular Listeria and Salmonella, showing 7- and 5-fold fewer intracellular CFUs than in KB-sham. Remarkably, KB-S100A8/A91–112 and KB-S100A8/A91–99 resisted invasion by Listeria more effectively than KB-S100A8/A91–114 (p = 0.002 and p = 0.02, respectively; Fig. 5A). KB-S100A8/A91–112 and KB-S100A8/A91–99 also appeared to resist Salmonella invasion more effectively than KB-S100A8/A91–114 (p = 0.03 and not significant, respectively; Fig. 5B).
FIGURE 5.
C-terminal deletion of S100A9 increases resistance to Listeria and Salmonella invasion. KB-sham, KB-S100A8/A91–114, KB-S100A8/A91–112, and KB-S100A8/A91–99 were analyzed for bacterial invasion using an antibiotic protection assay as described under “Experimental Procedures.” Monolayers were incubated with L. monocytogenes ATCC 10403S (A) or S. typhimurium ATCC 14028 (B) at an m.o.i. of 100:1 and 1:1, respectively, for 2 h. Each experiment was performed in triplicate wells. Values are means ± S.E. of viable intracellular bacteria, relative to KB-sham (100%) from at least three independent experiments. C, immunofluorescence staining for intracellular and extracellular Listeria in KB-sham, KB-S100A8/A91–114, KB-S100A8/A91–112, and KB-S100A8/A91–99 transfectants. Monolayers were incubated with L. monocytogenes for 2 h. The intracellular bacteria were enumerated and reported as means ± S.E. relative to KB-sham (100%). The results shown are from three independent experiments (*, p < 0.05; **, p < 0.01; #, p < 0.001).
To learn if the recovered intracellular CFUs reflected the total number of intracellular bacteria, Listeria was stained using a double immunofluorescence antibody approach. As expected, KB-sham cells contained more intracellular Listeria than the other clones. The number of intracellular bacteria in KB-sham cells was normalized to 100% invasion for each day's experiment. When compared with KB-sham cells, the KB-S100A8/A91–114 cells contained 50% fewer intracellular Listeria; the C-terminal mutants, KB-S100A8/A91–112 and KB-S100A8/A91–99, each contained about 75% fewer intracellular Listeria (Fig. 5C).
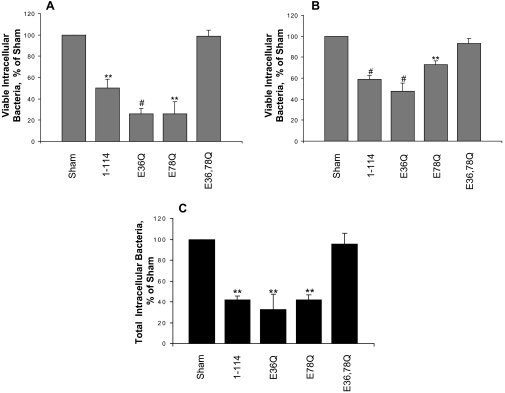
S100A9E36Q,E78Q Mutations Ablate Epithelial Resistance to Bacterial Invasion—To determine whether S100A9 calcium-binding loops I and II contribute to resistance to bacterial invasion conferred by calprotectin heterodimer, we quantified viable intracellular Listeria after invasion into KB-S100A8/A9E36Q, KB-S100A8/A9E78Q, and KB-S100A8/A9E36Q,E78Q cells. Consistent with the data above, KB-S100A8/A91–114 showed greater resistance to invasion by Listeria (50.3% invasion; p < 0.01; Fig. 6A) and Salmonella (59.1% invasion p < 0.001; Fig. 6B) than the KB-sham transfectant control. In identical conditions, calprotectin-negative KB-sham cells contained 1 × 106 and 3 × 107 CFU/well (1 ml) internalized Listeria and Salmonella, respectively. Not markedly different from KB-S100A8/A91–114, KB-S100A8/A9E36Q and KB-S100A8/A9E78Q hosted similar levels of invasion, showing 4-fold fewer viable intracellular Listeria (Fig. 6A) and 1.5–2-fold fewer Salmonella (Fig. 6B) than KB-sham. In contrast, KB-S100A8/A9E36Q,E78Q cells, which have mutations in both S100A9 calcium-binding loops, fail to resist invasion by Listeria (99% invasion; Fig. 6A) and Salmonella (93% invasion; Fig. 6B) relative to KB-sham.
FIGURE 6.
Calcium-binding loops of S100A9 and epithelial cell resistance to Listeria and Salmonella invasion. KB-sham, KB-S100A8/A91–114, KB-S100A8/A9E36Q, KB-S100A8/A9E378Q, and KB-S100A8/A9E36Q,E78Q were analyzed for bacterial invasion using an antibiotic protection assay. Monolayers were incubated with L. monocytogenes ATCC 10403S (A) or S. typhimurium ATCC 14028 (B) at an m.o.i. of 100:1 and 1:1, respectively, for 2 h. Each experiment was performed in triplicate wells. Values are means ± S.E. of viable intracellular bacteria, relative to KB-sham (100%), from at least three independent experiments. C, immunofluorescence staining for intracellular and extracellular Listeria in KB-sham, KB-S100A8/A91–114, KB-S100A8/A9E36Q, KB-S100A8/A9E78Q, and KB-S100A8/A9E36Q,E78Q transfectants. Monolayers were incubated with L. monocytogenes for 2 h. The intracellular bacteria were enumerated and reported as means ± S.E. relative to KB-sham (100%) as in the legend of Fig. 5. The results shown are from three independent experiments (**, p < 0.01; #, p < 0.001).
KB-S100A8/A91–114, KB-S100A8/A9E36Q, and KB-S100A8/A9E78Q showed similar percentages of total intracellular Listeria (ranges from 32.9 to 42%), when compared with KB-sham (Fig. 6C). Cells expressing calprotectin or single mutations in the calcium-binding loops of S100A9 co-expressed with S100A8 showed significant resistance to invasion (p < 0.01). Conversely, KB-S100A8/A9E36Q,E78Q, which expressed S100A9 with mutations in both calcium-binding loops, showed a high level of intracellular Listeria, similar to KB-sham (Fig. 6C).
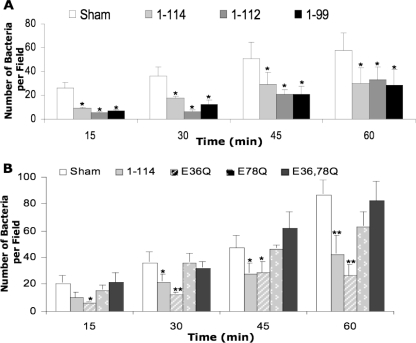
We next determined whether calprotectin-dependent resistance to invasion could be explained by differences in bacterial binding to the cells. Monolayers were incubated for 15–60 min with L. monocytogenes at an m.o.i. of 100. Cell-associated Listeria in nonpermeabilized cells were stained and counted as bound. For all tested KB cell lines, the numbers of bound bacteria increased with time. At all time points, significantly fewer Listeria bound to KB-S100A8/A91–114 and C-terminal mutants (KB-S100A8/A91–112 and KB-S100A8/A91–99; p < 0.05; Fig. 7A) or KB-S100A8/A9E36Q (p < 0.05; Fig. 7B) than KB-sham. Listeria bound in similar numbers to KB-S100A8/A91–114 and C-terminal mutants (Fig. 7A). The number of Listeria bound to KB-sham and either KB-S100A8/A9E78Q or KB-S100A8/A9E36Q,E78Q was similar at all time points (Fig. 7B).
FIGURE 7.
Amino acid substitutions in the first and second calcium-binding loops of S100A9 decrease epithelial resistance to Listeria binding. S100A9 C-terminal deletion mutants (KB-S100A8/A91–112 and KB-S100A8/A91–99) (A) and S100A9 calcium-binding loop mutants (KB-S100A8/A9E36Q, KB-S100A8/A9E78Q, KB-S100A8/A9E36Q,E78Q) (B) were incubated with L. monocytogenes ATCC 10403S for up to 1 h. Nonadherent bacteria were washed out, and the monolayers were fixed with 4% paraformaldehyde. KB-sham and KB-S100A8/A91–114 were used as negative and positive controls, respectively. Adherent bacteria were stained with specific antibodies and counted as described under “Experimental Procedures.” Values are means ± S.E. from three independent experiments. (*, p < 0.05; **, p < 0.01).
Calcium-induced Conformational Changes in Calprotectin— The predicted changes in calprotectin structure and charge upon calcium binding are shown in Fig. 8. This orientation was chosen to display the calcium-binding sites for both S100A8 and S100A9. The ribbon diagram of calcium-free calprotectin, generated by homology modeling, is shown in Fig. 8A. Calcium-free S100A8 (Fig. 8A, shown in yellow) and A9 (shown in green) contains four helices with the majority of the heterodimer interface formed by the interactions between the N- and C-terminal helices. The corresponding molecular surface for calcium-free calprotectin and the overall negative charge potential (represented in red) is shown in Fig. 8B. By rotating this view 90° on the z axis, the S100A9 distal end of calprotectin and the overall negative charge are displayed in Fig. 8C. The ribbon diagram of calcium-bound calprotectin was generated based on PDB code 1XK4 and is shown in Fig. 8D. The heterodimer interface is conserved and consists primarily of the N- and C-terminal helices. Calcium binding, however, has induced the formation of an additional helix in the middle of the primary sequence, and these three helices are rotated relative to their position in calcium-free calprotectin. Additionally, the C-terminal helix expands by several turns upon calcium binding. Binding calcium changes the overall shape of calprotectin (Fig. 8E) from approximating a cube to cylindrical with the creation of cleft (indicated by the yellow arrow in Fig. 8E). Furthermore, the calprotectin surface shows more positive potential (represented in blue) with both the creation of a positive patch on S100A8 (Fig. 8E) and an increase in the positive areas of S100A9 (Fig. 8F). The S100A8 calcium-bound structure combined with the E36Q,E78Q-mutated calcium-free S100A9 structure is shown as a ribbon diagram in Fig. 8G. The predicted effects of these mutations on the molecular surface are shown in Fig. 8H. When calcium is bound to calprotectin, the positively charged face of S100A8 appears unaffected by the E36Q,E78Q mutations in S100A9. However, the mutations in S100A9 result in the loss of the cleft in calprotectin as well as loss of a positively charged face on S100A9 (Fig. 8I).
DISCUSSION
We have previously reported that calprotectin can confer resistance to bacterial growth in the cytoplasm of intact cells (20), reduced bacterial binding to the cells, and significantly decreased invasion (21). How calprotectin protects and confers innate immunity to cells against invading microorganisms is not known. In vitro, calprotectin antagonizes the growth of various microorganisms (16–18, 39). The mechanism of antimicrobial activity in vitro is not well understood, but calprotectin has been suggested to inhibit microbial growth by chelating Zn2+ using HXXXH motifs commonly found in S100 proteins and HHH motif of residues 103–105 found in the C-terminal domain of S100A9 (27, 29). In this study, we characterized structural domains of S100A9 in association with S100A8 that are necessary to regulate epithelial cell resistance against invasion by L. monocytogenes and S. typhimurium.
Bacterial invasion depends on bacterial binding to the plasma membrane, subsequent cytoskeletal rearrangements to facilitate internalization, and intracellular survival. After invasion, keratinocytes are likely to harbor a mixture of live and dead intracellular bacteria. The antibiotic protection assay was used to estimate only viable intracellular bacteria that could be enumerated on agar, whereas immunofluorescence staining was used to directly visualize and count the total viable and nonviable bacteria within the keratinocytes. If a mutation abrogated antibacterial activity, a greater proportion of total intracellular Listeria (direct counts) was expected to be viable (estimated as CFUs). Indeed, with some exceptions, the CFUs recovered from the S100A9 mutants in this study mirrored the amount of visualized internal Listeria, suggesting that the ablated domains confer resistance to invasion through functions other than antibacterial activity (see Fig. 6). When the percentage reduction in viable Listeria exceeded the reduction in total bacteria, resistance to invasion may be attributable to intracellular antimicrobial activity and fewer Listeria entering the KB cells (see Fig. 5). These data confirmed that deletion of the C-terminal domain of S100A9 when co-expressed with S100A8 increased cellular resistance to bacterial invasion when compared with both KB-S100A8/A91–114 and KB-sham. Other work from our group suggests that intracellular anti-Listeria activity might not be apparent until 5–7 h post-invasion (40). Consequently, the shorter term experiments (2 h post-invasion) we report here largely identify the role of calprotectin in regulating the binding and internalization of Listeria and Salmonella. The life cycles of Listeria and Salmonella differ, and the 2-h incubation time used to study internalization may not be sufficient to observe the direct intracellular antibacterial effects of calprotectin. Nonetheless, when compared with KB-sham, calprotectin-expressing cells are more resistant to invasion by Listeria (4-fold greater) than Salmonella (2-fold greater). After invasion, Salmonella remains in vacuoles, whereas Listeria escapes from endosomes and resides in the cytoplasm (41) where cytoplasmic calprotectin would be encountered. Short term intracellular calprotectin-mediated anti-Listeria activity could contribute to the resistance to invasion seen in our assays.
We first tested KB cells that had been co-transfected with truncated C-terminal S100A9 constructs and full-length S100A8. The extended C terminus of S100A9 may actually enable invasion by Listeria and Salmonella because loss of residues 113–114 or 100–114 resulted in significantly fewer internalized Listeria and Salmonella than in calprotectin-expressing cells. We had expected that any perturbation of calprotectin would increase the susceptibility of the cells to invasion. This unexpected finding suggests that the C-terminal residues 113 and 114 regulate mechanisms that facilitate internalization. Because internalization also reflects the number of bacteria bound, it is important to note that the S100A9 C-terminal mutants also showed about 3-fold fewer bound Listeria over time than KB-sham (Fig. 7A). S100A9 residue Thr-113 is the only phosphorylation site in calprotectin, regulating microtubule-dependent translocation to the plasma membrane (33), binding (42, 43) and releasing arachidonic acid (44). Therefore, deletion of phosphorylation and arachidonic acid-binding sites in S100A9 could disrupt calprotectin localization in the plasma membrane and dysregulate tubulin cytoskeletal events needed to bind and internalize bacteria.
The C terminus also includes a portion of a potential zinc-binding motif, 103HHH105 with potential antimicrobial activity (29). When co-expressed with S100A8, S100A91–112 and S100A91–99 appear to reduce invasion similarly, and differences in presumptive intracellular anti-Listeria activity could not be detected. S100A9 has another potential component of a zinc-binding motif, 91HEXXH95, which could also contribute to antimicrobial activity (29). In vitro, these domains alone are not sufficient for antimicrobial activity because synthetic peptides containing HEXXH and HHH motifs did not inhibit Candida growth (29). Antibacterial activity attributable to the zinc-binding motifs could require the presence of the calprotectin complex. Yet S100 family members other than calprotectin show antimicrobial activity, including S100A7, S100A12, and S100A15 (45–47). These S100 protein family members do not possess an extended C-terminal domain with an HHH motif, nor do they appear to form heterodimers. Pretreatment of S100A7 with zinc did not impact antibacterial activity, and truncation of the C terminus of S100A7 to delete the zinc-binding HEXXH motif slightly reduced antibacterial activity in vitro (48). The central core domain of the S100A7 protein, which includes a functional EF-hand motif, showed full antibacterial activity, suggesting that the zinc-binding site in the C terminus of S100A7 may be necessary but not sufficient for antimicrobial activity. As recently reported, the antimicrobial activity of calprotectin may also depend on chelation of other metal ions such as Mn2+ (30), but other metal ion-binding motifs in the complex have not yet been determined. Hence, it is possible that other antimicrobial mechanisms may have been altered by the S100A9 mutations we report.
Complex formation by S100A8 and S100A9 could be necessary for antimicrobial activity and cellular resistance to invasion. Calprotectin complex formed with S100A91–99 co-expressed with full-length S100A8 (Figs. 2, 3, 4 and Table 1). Similarly, murine S100A91–101 (49), human S100A91–101 (50) and human S100A91–93 (24) each formed heterodimers with S100A8. Hence, heterodimerization into the calprotectin complex is independent of the extended C-terminal domain of S100A9.
TABLE 1.
Verification of calprotectin complex formation in S100A9 mutants
| Cell lines | ELISAa | IFb | WBc(anti-S100A8) | WBc(anti-S100A9) | IPdthen WB (anti-S100A8) | IPdthen silver staining |
|---|---|---|---|---|---|---|
| KB-S100A8/A91–114 | + | + | + | + | + | + |
| KB-S100A8/A91–112 | + | + | + | + | + | + |
| KB-S100A8/A91–99 | + | + | + | + | + | + |
| KB-S100A8/A9E36Q | – | + | + | – | + | + |
| KB-S100A8/A9E78Q | – | + | + | – | + | + |
| KB-S100A8/A9E36Q,E78Q | – | + | + | – | + | + |
ELISA for calprotectin using mAb 27E10 as capture antibody is shown
IF indicates immunofluorescence staining for calprotectin using mAb 27E10
WB indicates Western blotting using anti-S100A8 and anti-S100A9, respectively
IP indicates immunoprecipitation using mAb 27E10 and then reacted with anti-S100A8 in Western blot or stained with metachromatic silver
Calprotectin complex formation with S100A8 appears to be stabilized by S100A9 through the C-terminal half of helix IV (adjacent to the C-terminal domain) or the hydrophobic amino acids in helix I (see Fig. 1) (9, 49, 51). Each S100 protein has two EF-hands. The canonical C-terminal EF-hand is formed by helices III and IV with an intervening calcium-binding loop; the N-terminal EF-hand contains a calcium-binding loop between helices I and II. Like other S100 proteins, calprotectin C-terminal EF-hands have higher affinity for calcium ions than N-terminal EF-hands (6). To study the role of the calcium-binding loops within the EF-hands, we designed two different point mutations at amino acid residues, Glu-36 and Glu-78, which coordinate calcium ions (52–54). We substituted glutamic acid with glutamine to reduce the calcium-binding affinity of S100A9 (55, 56).
The mAb 27E10 specific epitope (85RLTW88) on S100A9 is clearly intact in all of our mutants (24), suggesting that this antibody serves as a molecule probe for complex formation. S100A9 with mutated calcium-binding loops complexed with S100A8, because transfected KB cells reacted 27E10 as shown by immunofluorescence microscopy (Fig. 2) and immunoprecipitation of cell lysates (Fig. 4, A and B). Although calprotectin with mutated S100A9 calcium-binding Glu-36 and Glu-78 was not detectable by a sandwich ELISA (Fig. 3, C–E), we show clearly that these mutations rendered S100A9 unable to react with anti-S100A9 antibody used for detection of calprotectin in this assay or S100A9 in Western blots (Fig. 4, C and E). Indeed, we screened several polyclonal and monoclonal anti-S100A9 antibodies, and all failed to detect S100A9 with mutated calcium-binding loops (Table 1). Nonetheless, anti-S100A8 antibody can detect S100A8 in the presence of S100A9 calcium-binding mutants (Fig. 3D and Table 1). Anti-S100A8 was not useful as a detection antibody for ELISA. In ELISA, this antibody was unable to detect calprotectin complex in KB-S100A8/A91–114 cells or in C-terminal mutants (data not shown). Using the yeast two-hybrid system, S100A9E78Q also appeared to form heterodimers with S100A8, although losing affinity for calcium (54). Consistent with data obtained using other approaches, our calcium-binding loop mutations in S100A9 co-expressed with S100A8 in KB epithelial cells form heterodimeric complexes.
Mutations in both calcium-binding loops of S100A9 in KB-S100A8/A9E36Q,E78Q were sufficient to ablate epithelial resistance to bacterial invasion. The calcium-binding loops of S100A9 were critical to the ability of calprotectin to resist bacterial invasion. Although KB-S100A8/A9E36Q and KB-S100A8/A9E78Q with individual calcium-binding loop mutations sustained resistance to invasion conferred by calprotectin, mutation of both calcium-binding loops of S100A9 (KB-S100A8/A9E36Q,E78Q) made the KB cells permissive to invasion by Listeria and Salmonella at levels comparable with the sham transfectant (Fig. 6). Hence, native calcium-binding motifs function together to promote resistance to bacterial invasion in KB cells.
Calprotectin also affected bacteria at the surface of the KB cells in a manner dependent on the fidelity of the calcium-binding loops. The pattern of Listeria binding to the KB cell membrane generally paralleled the pattern of susceptibility or resistance to invasion conferred by the mutant constructs. The number of bacteria bound to KB-S100A8/A9E36Q,E78Q was similar to KB-sham but greater than KB-S100A8/A91–114 and S100A8/A9E36Q (Fig. 7B). Although calprotectin generally affects invasion by controlling interactions at the cell surface, S100A8/A9E78Q showed comparatively high binding of Listeria (Fig. 7B) but low invasion (Fig. 6). The calprotectin S100A9 calcium-binding loops therefore appear to partially regulate epithelial resistance to bacterial invasion by controlling the number of bound bacteria that could ultimately be internalized. Although we have previously shown that cellular binding and intracellular growth of invasive bacteria are distinct functions of calprotectin (21), it remains to be determined whether the calcium-binding loops contribute to both functions and whether the interactions are direct or indirect.
The binding of calcium by the S100 proteins induces movement and alterations of secondary structural elements, which generate new molecular surfaces. This is illustrated by comparing Fig. 8, A–C (calcium-free) with D–F (calcium-bound). Changing the length and number of helices as well as their orientations relative to each other brings about this conversion in functional form. The alteration of form is continued as residues are converted from a random coil into a helix, modifying the associations between side chains and establishing a new molecular surface. One example of the new surface is the creation of a cleft (Fig. 8E, arrow) formed in response to binding calcium. The adjustment of side chains to a new conformation alters the electrostatic potential of the molecular surface. In the case of calprotectin, the binding of calcium is responsible for creating a more positively charged surface.
Upon binding calcium, the molecular surface of S100A9 changes potential from negative to positive (Fig. 8, C and F), and the surface of calprotectin mirrors similar changes (B and E). The positively charged face of S100A8 in calprotectin that forms when calcium is bound may not be involved in bacterial invasion because this conformation is maintained upon E36Q,E78Q mutation (Fig. 8H).
The positive face of S100A9, however, may be critical to bacterial invasion (Fig. 8, C and F). The E36Q,E78Q mutations in S100A9 eliminate calcium binding and appears to lock S100A9 in a calcium-free conformation. S100A8 should still bind calcium. The alteration of the molecular surface and the electrostatic potential provides several mechanistic explanations for how the calcium-binding domain could be involved in mediating resistance to bacterial invasion. The S100A9 E36Q,E78Q mutant loses interactions with any partner that requires a positively charged S100A9 functional surface. For example, negatively charged tubulin complexes with calprotectin and is involved in bacterial invasion (40). Because the E36Q,E78Q mutations in S100A9 result in loss of this interaction,3 we speculate that the positively charged surface of S100A9 contributes to interactions with tubulin cytoskeleton. Consistent with our structural predictions, calprotectin has been shown to contribute to cytoskeletal re-organization and microtubule polymerization in a calcium-dependent manner (4, 54, 57, 58).
Ca2+-, Zn2+-, and Cu2+-binding motifs of S100 proteins generally regulate functional binding to effector molecules (5, 6). Calcium binding by calprotectin would be expected to affect concentrations of intracellular divalent cations, which can alter phosphorylation of specific molecules in downstream signaling cascades. Calprotectin may also interact with bacteria at the cell surface in a manner mimicking S100A12, which shows Ca2+-dependent chaperone/anti-chaperone-like function (59). Calprotectin resistance to bacterial invasion therefore can involve complex downstream responses to calcium-dependent changes in structural motifs in S100A9 in oral keratinocytes.
This work was supported, in whole or in part, by National Institutes of Health Grant R01DE11831 (NIDCR). This work was also supported by a scholarship from the Royal Thai Government. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: PBS, phosphate-buffered saline; mAb, monoclonal antibody; CFU, colony-forming unit; PDB, Protein Data Bank; m.o.i., multiplicity of infection; ELISA, enzyme-linked immunosorbent assay.
C. Champaiboon, B. D. Guenther, K. F. Ross, and M. C. Herzberg, unpublished observations.
References
- 1.Rudney, J. D., and Chen, R. (2006) Arch. Oral. Biol. 51 291-298 [DOI] [PubMed] [Google Scholar]
- 2.Ross, K. F., and Herzberg, M. C. (2001) Infect. Immun. 69 3248-3254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Striz, I., and Trebichavsky, I. (2004) Physiol. Res. 53 245-253 [PubMed] [Google Scholar]
- 4.Korndorfer, I. P., Brueckner, F., and Skerra, A. (2007) J. Mol. Biol. 370 887-898 [DOI] [PubMed] [Google Scholar]
- 5.Kligman, D., and Hilt, D. C. (1988) Trends Biochem. Sci. 13 437-443 [DOI] [PubMed] [Google Scholar]
- 6.Marenholz, I., Heizmann, C. W., and Fritz, G. (2004) Biochem. Biophys. Res. Commun. 322 1111-1122 [DOI] [PubMed] [Google Scholar]
- 7.Zimmer, D. B., Wright Sadosky, P., and Weber, D. J. (2003) Microsc. Res. Tech. 60 552-559 [DOI] [PubMed] [Google Scholar]
- 8.Dale, I., Fagerhol, M. K., and Naesgaard, I. (1983) Eur. J. Biochem. 134 1-6 [DOI] [PubMed] [Google Scholar]
- 9.Hessian, P. A., Edgeworth, J., and Hogg, N. (1993) J. Leukocyte Biol. 53 197-204 [PubMed] [Google Scholar]
- 10.Dale, I., Brandtzaeg, P., Fagerhol, M. K., and Scott, H. (1985) Am. J. Clin. Pathol. 84 24-34 [DOI] [PubMed] [Google Scholar]
- 11.Odink, K., Cerletti, N., Bruggen, J., Clerc, R. G., Tarcsay, L., Zwadlo, G., Gerhards, G., Schlegel, R., and Sorg, C. (1987) Nature 330 80-82 [DOI] [PubMed] [Google Scholar]
- 12.Johne, B., Fagerhol, M. K., Lyberg, T., Prydz, H., Brandtzaeg, P., Naess-Andresen, C. F., and Dale, I. (1997) Mol. Pathol. 50 113-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roseth, A. G., Schmidt, P. N., and Fagerhol, M. K. (1999) Scand. J. Gastroenterol. 34 50-54 [DOI] [PubMed] [Google Scholar]
- 14.Hammer, H. B., Kvien, T. K., Glennas, A., and Melby, K. (1995) Clin. Exp. Rheumatol. 13 59-64 [PubMed] [Google Scholar]
- 15.Cuida, M., Halse, A. K., Johannessen, A. C., Tynning, T., and Jonsson, R. (1997) Eur. J. Oral. Sci. 105 228-233 [DOI] [PubMed] [Google Scholar]
- 16.Miyasaki, K. T., Bodeau, A. L., Murthy, A. R., and Lehrer, R. I. (1993) J. Dent. Res. 72 517-523 [DOI] [PubMed] [Google Scholar]
- 17.Murthy, A. R., Lehrer, R. I., Harwig, S. S., and Miyasaki, K. T. (1993) J. Immunol. 151 6291-6301 [PubMed] [Google Scholar]
- 18.Sohnle, P. G., Collins-Lech, C., and Wiessner, J. H. (1991) J. Infect. Dis. 163 187-192 [DOI] [PubMed] [Google Scholar]
- 19.Lusitani, D., Malawista, S. E., and Montgomery, R. R. (2003) Infect. Immun. 71 4711-4716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nisapakultorn, K., Ross, K. F., and Herzberg, M. C. (2001) Infect. Immun. 69 4242-4247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nisapakultorn, K., Ross, K. F., and Herzberg, M. C. (2001) Infect. Immun. 69 3692-3696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edgeworth, J., Freemont, P., and Hogg, N. (1989) Nature 342 189-192 [DOI] [PubMed] [Google Scholar]
- 23.Watt, K. W., Brightman, I. L., and Goetzl, E. J. (1983) Immunology 48 79-86 [PMC free article] [PubMed] [Google Scholar]
- 24.Hessian, P. A., and Fisher, L. (2001) Eur. J. Biochem. 268 353-363 [DOI] [PubMed] [Google Scholar]
- 25.Nordahl, E. A., Rydengard, V., Morgelin, M., and Schmidtchen, A. (2005) J. Biol. Chem. 280 34832-34839 [DOI] [PubMed] [Google Scholar]
- 26.Sohnle, P. G., Collins-Lech, C., and Wiessner, J. H. (1991) J. Infect. Dis. 164 137-142 [DOI] [PubMed] [Google Scholar]
- 27.Loomans, H. J., Hahn, B. L., Li, Q. Q., Phadnis, S. H., and Sohnle, P. G. (1998) J. Infect. Dis. 177 812-814 [DOI] [PubMed] [Google Scholar]
- 28.Clohessy, P. A., and Golden, B. E. (1996) J. Leukocyte Biol. 60 674. [DOI] [PubMed] [Google Scholar]
- 29.Sohnle, P. G., and Hahn, B. L. (2000) Antimicrob. Agents Chemother. 44 139-142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corbin, B. D., Seeley, E. H., Raab, A., Feldmann, J., Miller, M. R., Torres, V. J., Anderson, K. L., Dattilo, B. M., Dunman, P. M., Gerads, R., Caprioli, R. M., Nacken, W., Chazin, W. J., and Skaar, E. P. (2008) Science 319 962-965 [DOI] [PubMed] [Google Scholar]
- 31.Rammes, A., Roth, J., Goebeler, M., Klempt, M., Hartmann, M., and Sorg, C. (1997) J. Biol. Chem. 272 9496-9502 [DOI] [PubMed] [Google Scholar]
- 32.Steele-Mortimer, O. (2008) Curr. Opin. Microbiol. 11 38-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van den Bos, C., Roth, J., Koch, H. G., Hartmann, M., and Sorg, C. (1996) J. Immunol. 156 1247-1254 [PubMed] [Google Scholar]
- 34.Sali, A., and Blundell, T. L. (1993) J. Mol. Biol. 234 779-815 [DOI] [PubMed] [Google Scholar]
- 35.Rustandi, R. R., Baldisseri, D. M., Inman, K. G., Nizner, P., Hamilton, S. M., Landar, A., Landar, A., Zimmer, D. B., and Weber, D. J. (2002) Biochemistry 41 788-796 [DOI] [PubMed] [Google Scholar]
- 36.Vallely, K. M., Rustandi, R. R., Ellis, K. C., Varlamova, O., Bresnick, A. R., and Weber, D. J. (2002) Biochemistry 41 12670-12680 [DOI] [PubMed] [Google Scholar]
- 37.Malik, S., Revington, M., Smith, S. P., and Shaw, G. S. (2008) Proteins 73 28-42 [DOI] [PubMed] [Google Scholar]
- 38.Guex, N., and Peitsch, M. C. (1997) Electrophoresis 18 2714-2723 [DOI] [PubMed] [Google Scholar]
- 39.Sohnle, P. G., Hahn, B. L., and Santhanagopalan, V. (1996) J. Infect. Dis. 174 1369-1372 [DOI] [PubMed] [Google Scholar]
- 40.Zaia, A. A., Sappington, K. J., Nisapakultorn, K., Chazin, W. J., Dietrich, E. A., Ross, K. F., and Herzberg, M. C. (2009) Mucosal Immunol. 2 43-53 [DOI] [PubMed] [Google Scholar]
- 41.Cossart, P., and Sansonetti, P. J. (2004) Science 304 242-248 [DOI] [PubMed] [Google Scholar]
- 42.Kerkhoff, C., Klempt, M., Kaever, V., and Sorg, C. (1999) J. Biol. Chem. 274 32672-32679 [DOI] [PubMed] [Google Scholar]
- 43.Sopalla, C., Leukert, N., Sorg, C., and Kerkhoff, C. (2002) Biol. Chem. 383 1895-1905 [DOI] [PubMed] [Google Scholar]
- 44.Leslie, C. C. (2004) Biochem. Cell Biol. 82 1-17 [DOI] [PubMed] [Google Scholar]
- 45.Glaser, R., Harder, J., Lange, H., Bartels, J., Christophers, E., and Schroder, J. M. (2005) Nat. Immunol. 6 57-64 [DOI] [PubMed] [Google Scholar]
- 46.Gottsch, J. D., Eisinger, S. W., Liu, S. H., and Scott, A. L. (1999) Infect. Immun. 67 6631-6636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buchau, A. S., Hassan, M., Kukova, G., Lewerenz, V., Kellermann, S., Wurthner, J. U., Wolf, R., Walz, M., Gallo, R. L., and Ruzicka, T. (2007) J. Investig. Dermatol. 127 2596-2604 [DOI] [PubMed] [Google Scholar]
- 48.Lee, K. C., and Eckert, R. L. (2007) J. Investig. Dermatol. 127 945-957 [DOI] [PubMed] [Google Scholar]
- 49.Propper, C., Huang, X., Roth, J., Sorg, C., and Nacken, W. (1999) J. Biol. Chem. 274 183-188 [DOI] [PubMed] [Google Scholar]
- 50.Sohnle, P. G., Hunter, M. J., Hahn, B., and Chazin, W. J. (2000) J. Infect. Dis. 182 1272-1275 [DOI] [PubMed] [Google Scholar]
- 51.Leukert, N., Sorg, C., and Roth, J. (2005) Biol. Chem. 386 429-434 [DOI] [PubMed] [Google Scholar]
- 52.Itou, H., Yao, M., Fujita, I., Watanabe, N., Suzuki, M., Nishihira, J., and Tanaka, I. (2002) J. Mol. Biol. 316 265-276 [DOI] [PubMed] [Google Scholar]
- 53.Ishikawa, K., Nakagawa, A., Tanaka, I., Suzuki, M., and Nishihira, J. (2000) Acta Crystallogr. Sect. D Biol. Crystallogr. 56 559-566 [DOI] [PubMed] [Google Scholar]
- 54.Leukert, N., Vogl, T., Strupat, K., Reichelt, R., Sorg, C., and Roth, J. (2006) J. Mol. Biol. 359 961-972 [DOI] [PubMed] [Google Scholar]
- 55.Maune, J. F., Beckingham, K., Martin, S. R., and Bayley, P. M. (1992) Biochemistry 31 7779-7786 [DOI] [PubMed] [Google Scholar]
- 56.Carlstrom, G., and Chazin, W. J. (1993) J. Mol. Biol. 231 415-430 [DOI] [PubMed] [Google Scholar]
- 57.Roth, J., Burwinkel, F., van den Bos, C., Goebeler, M., Vollmer, E., and Sorg, C. (1993) Blood 82 1875-1883 [PubMed] [Google Scholar]
- 58.Goebeler, M., Roth, J., van den Bos, C., Ader, G., and Sorg, C. (1995) Biochem. J. 309 419-424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hatakeyama, T., Okada, M., Shimamoto, S., Kubota, Y., and Kobayashi, R. (2004) Eur. J. Biochem. 271 3765-3775 [DOI] [PubMed] [Google Scholar]