Abstract
Little is known about the intracellular folding and trafficking of integral membrane proteins. Here we identify a hydrophobic amino acid tetrad (ILLV) close to the C terminus of the secretory Na+-K+-2Cl- cotransporter (NKCC1) that is important for the proper intracellular processing of this protein. This tetrad appears in a C-terminal sequence pattern that is conserved across species in a number of members of the NKCC1 gene family (slc12) of electroneutral salt transporters. We studied the effects of various mutations of these amino acids on NKCC1 transiently transfected into HEK-293 cells. Our results show that mutation of two of these residues to alanine leads to a >50% reduction in expression and complex glycosylation levels and that multiple mutations to alanine have cumulative effects. By contrast, scrambling of these amino acids, or mutation of other nearby conserved C-terminal residues, has little effect on these parameters. Mutation of ILLV to AAAA reduces complex glycosylation of NKCC1 by ∼90% and results in a protein that does not form stable dimers and is retained in the endoplasmic reticulum in a highly aggregated state. Our results are consistent with the hypothesis that mutation of the hydrophobic tetrad ILLV to AAAA leads to the ab initio misfolding and concomitant aggregation of this NKCC1 mutant, resulting in its retention in the endoplasmic reticulum.
Despite their abundance and obvious biological importance, relatively little is known about the processes involved in the folding and trafficking of polytopic membrane proteins. Integral membrane proteins destined for the cell surface or other cellular compartments are first inserted into the membrane of the endoplasmic reticulum (ER)3 via the translocon complex (1). In the ER, these nascent proteins acquire their mature tertiary and quaternary structures before being transferred to the Golgi complex and ultimately to their final destinations (2, 3). These processes are mediated by ER chaperone activity and by targeting signals coded into the amino acid sequence of the protein itself. Quality control mechanisms in the ER typically ensure that proteins that do not fold or oligomerize correctly are retained in the ER and eventually degraded (2, 3). A growing number of diseases have been associated with the mistargeting, misfolding, and/or intracellular accumulation and aggregation of membrane proteins (4–10). As a result, there has been increasing interest in understanding and modifying these aberrant phenomena.
The electroneutral cation-chloride-coupled cotransporter gene family (slc12) contains nine members in vertebrates. These include two Na+-K+-2Cl- cotransporters (NKCC1 and NKCC2), a Na+-Cl- cotransporter (NCC), four K+-Cl- cotransporters (KCC1, KCC2, KCC3, and KCC4), and two other homologues of unknown function. Members of the Slc12 family are known to play important roles in numerous physiological processes, including exocrine fluid secretion, renal salt and water absorption, hearing, olfaction, spermatogenesis, pain perception, visual processing, and other neuronal functions (11–14). Several studies have found evidence that residues near the end of the intracellular C terminus of Slc12 family members are required for their proper cellular processing. Thus, for example, in their investigations of mutations of NCC associated with Gitelman syndrome, Sabath et al. (8) found that several C-terminal truncation and point mutants exhibited impaired processing to the plasma membrane. In addition, in experiments from our own laboratory, we have found that truncating the C terminus of NKCC1 typically results in recombinant proteins that form intracellular aggregates (15). Studies of other integral membrane proteins have identified amino acids in their intracellular C termini that are associated with folding and/or trafficking (7, 16–26). In a number of cases, hydrophobic amino acids, particularly dileucine motifs, have been shown to be involved in these processes (16, 17, 19, 22, 23, 25).
A multiple alignment of Slc12 amino acid sequences reveals a C-terminal sequence pattern that is conserved in all vertebrate NKCCs, NCCs, and KCCs, as well as in a variety of Slc12 homologues from other nonvertebrate species, specifically xxxxhhhhRGxxxxVhThYx, where h and x represent hydrophobic and variable amino acids, respectively. In many cases, the hydrophobic tetrad hhhh includes a central dileucine motif. Here we study the effects of mutations of the above sequence pattern using the secretory Na+-K+-2Cl- cotransporter NKCC1 as a model Slc12 protein. Our results indicate that modifications of the sequence downstream of the hydrophobic tetrad (ILLV in NKCC1) have no significant effect on the trafficking of NKCC1 from the ER to the Golgi. However, mutations of the amino acids of the hydrophobic tetrad itself to alanine result in the retention of NKCC1 in the ER and its aggregation, apparently as a result of the ab initio misfolding of the protein. Interestingly, it is conservation of residue hydrophobicity rather than residue identity that appears to underlie the role of this amino acid motif.
EXPERIMENTAL PROCEDURES
Materials—Phosphate-buffered saline (PBS) was obtained from Invitrogen. Leupeptin, pepstatin, and aprotinin were from Roche Applied Science. Bovine serum albumin, ouabain, and Triton X-100 were from Sigma. 4-(2-Aminoethyl)benzenesulfonyl fluoride hydrochloride was from ICN (Aurora, OH). N-Glycosidase F and restriction enzymes were from New England BioLabs (Ipswich, MA). EZ-Link Sulfo-NHS-LCLC-Biotin, 3,3′-dithiobis(sulfosuccinimidyl propionate) (DTSSP), and immobilized Streptavidin beads were from Pierce. Brefeldin A was obtained from Biomol (Plymouth Meeting, PA). All other chemicals were from standard commercial sources and were reagent grade or the highest purity available.
The antibody α-wCT(r) was raised in rabbits against the recombinant C terminus of rat NKCC1 (27). The antibody α-wNT(r) was raised in rabbits against amino acids 3–202 of the (recombinant) N terminus of rat NKCC1 (28).
Molecular Biology—The mammalian expression vector pBK-CMVlac- (pBK-) (29) was used for expression studies. Mutants of wild-type rat NKCC1 in pBK- (15) were generated using the QuikChange II XL site-directed mutagenesis kit (Stratagene, La Jolla, CA) following the manufacturer's instructions, except that mutagenic primers were designed using the method of Zheng et al. (30). An N-terminally truncated version of NKCC1 in pBK- (nttNKCC1) (29) whose coding sequence begins at Met209, was modified (using the QuikChange kit) by inserting the sequence AYPYDVPDYAL (incorporating the HA tag) after the start codon; this HA-tagged clone is referred to as HA-nttNKCC1. All sequence modifications were confirmed by direct sequencing.
Cell Culture and Transient Transfection—The human embryonic kidney cell line HEK-293 was cultured in MEM (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum, 2 mm glutamine, and 100 μm each of penicillin and streptomycin. Cells were grown in 6-cm culture dishes in a humidified incubator at 37 °C and 5% CO2 and subcultured every 2–3 days. Subconfluent (70–80%) HEK-293 monolayers were transiently transfected (2 days) with the expression vectors indicated using FuGENE6 (Roche Applied Science) following the manufacturer's instructions.
Membrane Preparation, Cross-linking, and Deglycosylation—A crude membrane fraction was prepared from HEK-293 cells essentially as described previously (15, 29). The cells growing in a 6-cm culture dish were washed twice in ice-cold PBS (pH 7.4) and then suspended in 200 μl of ice-cold TEEA buffer, consisting of 20 mm Tris-HCl, pH 8.0, 1 mm EDTA, 3 mm EGTA, 300 μm 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride, 10 μm leupeptin, 10 μm pepstatin A, and 2.5 μg/ml aprotinin. The suspended cells were then homogenized by passing five times through a 25-gauge needle. This material was centrifuged at 1000 × g for 10 min, and the supernatant was saved. The pellet was resuspended in 200 μl of TEEA buffer and rehomogenized and centrifuged as before. The combined supernatants from these two homogenization steps were centrifuged at 100,000 × g for 30 min, and the resulting crude membrane fraction was resuspended in TEEA.
Cross-linking studies were performed on solubilized crude membranes prepared from transiently transfected HEK-293 cells as described previously (15). Briefly, membranes were suspended in PBS (pH 7.4) at a protein concentration of 1 mg/ml (measured using the Bio-Rad protein assay kit with bovine IgG as the standard) and solubilized in 0.3% Triton X-100. The solubilized membranes were then incubated with the homobifunctional amino acid cross-linking reagent DTSSP (1 mm) for 30 min, after which the cross-linking reaction was stopped by the addition of 100 mm glycine.
Aliquots of the crude membrane fraction were deglycosylated by treatment with N-glycosidase F as previously described (31).
Biotinylation of Intact HEK-293 Cells and Removal of Biotinylated Proteins on Streptavidin Beads—HEK-293 cells growing in a 6-cm culture dish were detached from the plate by incubation in citrate buffer containing 18.6 mm sodium citrate and 134 mm KCl at 37 °C. The suspended cells were then centrifuged at 400 × g for 1 min, washed in 10 ml of PBS (this and all subsequent steps utilized ice-cold PBS at pH 8.0), and suspended in 4–5 ml of PBS. One-ml aliquots were transferred to 1.5-ml Eppendorf tubes, centrifuged at 1000 × g for 5 s, washed again with 1 ml of PBS, and resuspended in 0.9 ml of PBS. One hundred μl of freshly prepared EZ-Link Sulfo-NHS-LCLC-Biotin (25 mm in PBS) was then added to each tube. Tubes were incubated on ice for 2 h with mixing by inversion every 10 min, and then the biotinylation reaction was stopped by the addition of 110 μl of 1 m glycine in PBS. Five min later, the cells were washed three times with 1 ml of PBS and solubilized in 0.6 ml of PBS containing 0.3% Triton X-100. After a 30-min incubation on ice, particulate matter was removed by centrifuging at 2000 × g for 10 min. A 300-μl aliquot of the supernatant was combined with 100 μl of immobilized Streptavidin beads (a 50:50 slurry in PBS) and incubated with continuous mixing for 2 h at 4 °C. The material remaining in the supernatant after removal of the beads (i.e. the unbound material) was subsequently compared with the material in the supernatant before the addition of the beads by Western blotting (see “Results”). In control experiments (not shown), we have confirmed by Western blotting with horseradish peroxidase-conjugated avidin (see below) that this treatment with immobilized Streptavidin beads is sufficient to remove virtually all biotinylated proteins from the solubilized cell supernatant.
Co-immunoprecipitation Assay—Co-immunoprecipitation of NKCC1 or the mutant AAAA (see “Results”) with HA-nttNKCC1 was carried out essentially as previously described (15). Briefly, HEK-293 cells in 6-cm dishes were transiently transfected with full-length NKCC1 (or AAAA) and HA-nttNKCC1 at a plasmid ratio of 1:8 (3 μg of total DNA). Two days later, the cells were washed with PBS, solubilized in PBS containing 0.3% Triton X-100, and immunoprecipitated with the antibody α-wNT(r), which had been preconjugated to Protein G beads (Pierce). After washing, the beads were eluted in 25 μl of PBS containing 0.3% Triton X-100 plus 0.1% SDS for 30 min on ice. The beads were then centrifuged, and the eluate was removed and mixed with 6 μl of 5× SDS-PAGE sample buffer. The material remaining on the beads was also extracted by adding 30 μl of SDS-PAGE sample buffer to the pelleted beads. Equal volumes of each of these samples were probed by Western blotting (see below).
SDS-PAGE and Western Blotting—SDS-polyacrylamide gel electrophoresis and Western blotting were carried out as previously described (15). The primary and secondary antibodies used to detect NKCC1 and its C-terminal mutants in Figs. 2, 3, 4, 5, 6 were α-wCT(r), used at a dilution of 1:5000, and horseradish peroxidase-conjugated goat anti-rabbit IgG (Pierce), used at a dilution of 1:10,000, respectively. Blots from co-immunoprecipitations of HA-nttNKCC1 with NKCC1 or AAAA were probed with the peroxidase-conjugated rat anti-HA monoclonal 3F10 (Roche Applied Science) at a dilution of 1:1000 to detect HA-nttNKCC1 (Fig. 8) or with α-wNT(r) at a dilution of 1:5000 followed by Trueblue horseradish peroxidase-conjugated anti-rabbit IgG (eBioscience) at a dilution of 1:5000 to detect NKCC1 or AAAA (not shown). Detection of horseradish peroxidase was carried out using the ECL kit from Amersham Biosciences according to the manufacturer's directions. Blots were analyzed by densitometry.
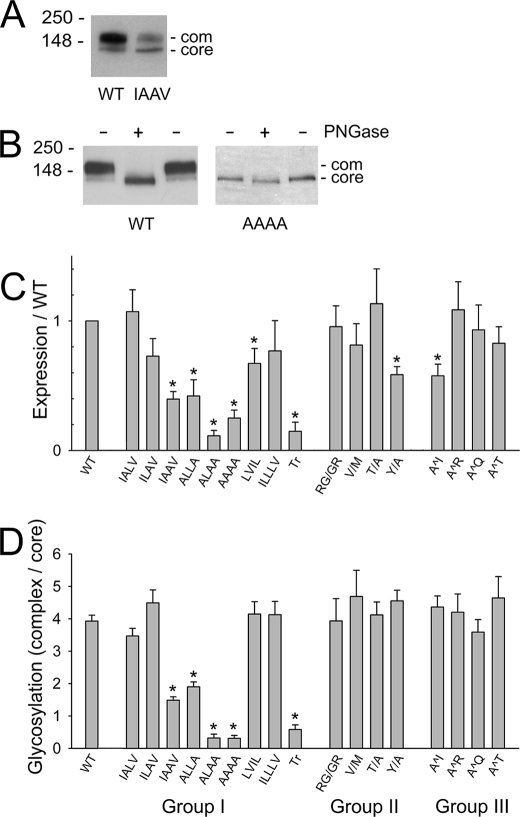
FIGURE 2.
Protein expression and complex glycosylation levels of wild-type NKCC1 and C-terminal mutants. A, Western blot of crude membrane fractions prepared from HEK-293 cells transiently transfected with WT NKCC1 or the IAAV mutant (see “Experimental Procedures” for details). The positions of the core and complex (com) glycosylated proteins are indicated. B, Western blot of crude membrane fractions from HEK-293 cells transiently transfected with WT NKCC1 or the AAAA mutant. Membranes were treated with (+) or without (-) N-glycosidase F (PNGase) as indicated (see “Experimental Procedures” for details). C, protein levels of the various C-terminal NKCC1 mutants transiently expressed in HEK-293 cells, measured relative to WT NKCC1 expression levels. In additional studies (not shown), we have established that most of the reactivity of the antibody α-wCT(r) used in these experiments is directed against the sequence between amino acids 913–998 and that the mutations studied here (after amino acid 1187) have no detectable effect its reaction with NKCC1. D, ratio of complex to core glycosylated protein levels of WT NKCC1 and the C-terminal NKCC1 mutants. Results in C and D are means ± S.E. from four or more independent determinations.
FIGURE 3.
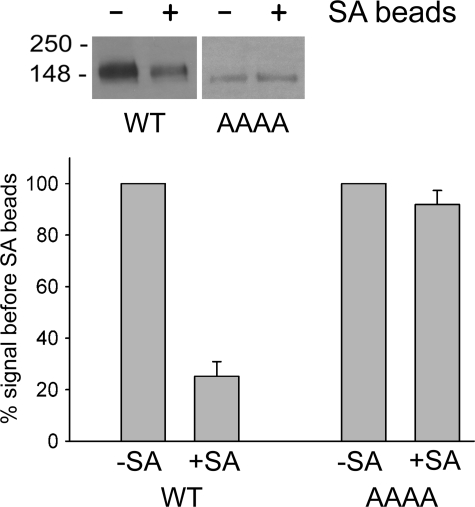
Cell surface expression of wild-type NKCC1 and the AAAA mutant. HEK-293 cells were transiently transfected with WT NKCC1 or the AAAA mutant and then incubated with EZ-Link Sulfo-NHS-LCLC-Biotin. The cells were then solubilized, and biotinylated proteins were precipitated on streptavidin (SA) beads (see “Experimental Procedures” for details). Aliquots of the cell lysate before (-) and after (+) streptavidin precipitation were probed by Western blotting (inset), and the amount of WT NKCC1 or AAAA mutant was quantitated in each case. The bar graph shows the amount of each protein found in the lysate before (-SA) and after (+SA) precipitation on the beads. Results have been normalized to the signal observed before the addition of the streptavidin beads. Means ± S.E. from three independent experiments are shown.
FIGURE 4.
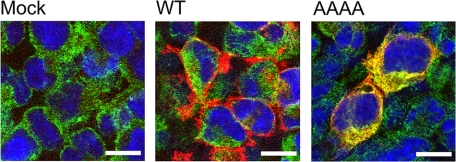
Immunofluorescent localization of wild-type NKCC1 and the AAAA mutant in HEK-293 cells. HEK-293 cells were transiently transfected with empty vector (pBK-; Mock), WT NKCC1, or the AAAA mutant and then fixed, permeabilized, and stained for NKCC1 and AAAA (red), the ER marker protein-disulfide isomerase (green), and nuclei (blue); see “Experimental Procedures” for details. The white bars represent 10 μm. Note that in these experiments, the NKCC1 antibody shows no detectable signal for the endogenous (human) NKCC1 in the HEK-293 cells. This is because the antibody used is relatively selective for the rat protein (15).
FIGURE 5.
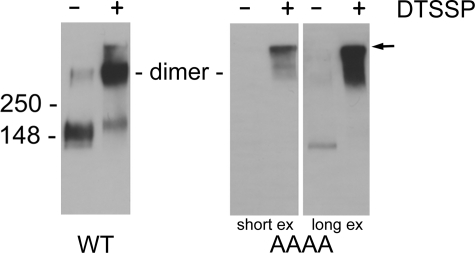
Cross-linking of wild-type NKCC1 and the AAAA mutant by DTSSP. Crude membrane fractions from HEK-293 cells transfected with wild-type NKCC1 (WT) or the AAAA mutant were solubilized in 0.3% Triton X-100, treated with cross-linker DTSSP, and analyzed by Western blotting (see “Experimental Procedures”). The middle and right-hand panels show short and long exposures, respectively, of the same blot. The arrow indicates the top of the gel.
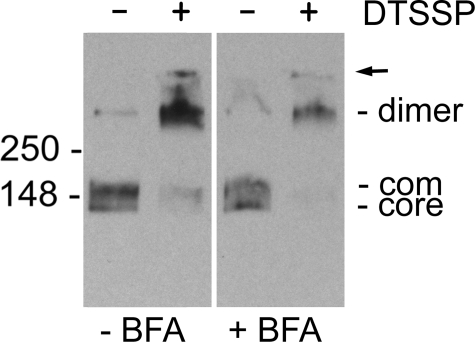
FIGURE 6.
Effect of brefeldin A treatment on NKCC1 dimerization. HEK-293 cells transiently transfected with NKCC1 were treated with (+BFA) or without (-BFA) brefeldin A (5 μg/ml) for 2 h in culture medium at 37 °C. Crude membranes were then harvested from the cells and treated with (+) or without (-) DTSSP as described under “Experimental Procedures.” The positions of the core and complex (com) glycosylated proteins are indicated. The arrow indicates the top of the gel.
FIGURE 8.
Co-immunoprecipitation assay: HA-nttNKCC1 is co-immunoprecipitated with NKCC1 but not with AAAA. HEK-293 cells were transiently transfected with HA-nttNKCC1 and either pBK-, WT NKCC1, or the AAAA mutant, as indicated. Two days later, the cells were solubilized and immunoprecipitated with Protein G beads to which the antibody α-wNT(r), raised against the N terminus of rat NKCC1, had been conjugated. The material eluted from the immunoprecipitate by 0.1% SDS (Eluate) as well the material remaining on the beads (Beads) was then probed by Western blotting with an anti-HA antibody to detect HA-nttNKCC1 (all procedures are described under “Experimental Procedures”). In additional assays in which we probed these blots with the antibodyα-wNT(r) (not shown; see “Experimental Procedures”), we have established that the amount of AAAA immunoprecipitated in these experiments is 0.52 ± 0.02 (n = 2) times that of WT NKCC1 (although, as shown in Fig. 2A, AAAA is expressed at ∼0.2 times the level of WT NKCC1, these experiments (15) are carried out with cell extract sufficient to oversaturate the Protein G-conjugated antibody when immunoprecipitating from WT NKCC1-expressing cells).
Detection of biotin-labeled proteins by Western blotting was carried out as descried above except that horseradish peroxidase-conjugated Immunopure Avidin (Pierce), used at a dilution of 1:1000, replaced the primary antibody, and no secondary antibody was used.
Immunofluorescent Staining and Confocal Microscopy—Immunofluorescent staining was performed as follows. Transiently transfected HEK-293 cells, grown on poly-l-lysine-coated circular glass coverslips, were rinsed three times with PBS (pH 7.4) and fixed with 4% paraformaldehyde in PBS for 30 min. The cells were then rinsed three times with PBS, incubated with 100 mm glycine in PBS for 20 min, permeabilized with methanol at -20 °C for 5 min, and incubated in a mixture of 5% donkey serum and 0.5% bovine serum albumin in PBS for 20 min. Next, the cells were incubated with the antibody α-wCT(r) (diluted 1:3000) for 1 h and then with an Alexa Fluor 594-conjugated anti-rabbit secondary antibody (diluted 1:200; Invitrogen) for 30 min. After three additional washes in PBS (3 min each), the cells were treated with the SelectFX Alexa Fluor 488 endoplasmic reticulum labeling kit (Invitrogen) according to the manufacturer's instructions. Finally, the cells were washed again three times with PBS before being mounted onto a slide using Vectashield (Vector, Burlingame, CA) containing 4′,6-diamidino-2-phenylinode. Fluorescence images were taken using a confocal laser-scanning microscope, Leica TCS-SP2, attached to an upright Leica DM-RE7 microscope (Leica, Wetzlar, Germany).
86Rb Influx Assays—The 86Rb influx experiments were performed as described previously (32).
Data Presentation—All experiments were carried out three or more times with similar results. All quantitative results shown are means ± S.E. from three or more independent experiments. p values ≤ 0.05 (Student's t test) were taken to represent statically significant differences.
RESULTS AND DISCUSSION
Conserved C-terminal Sequence in Slc12 Family Members
In Fig. 1A, we show the C-terminal residues of various Slc12 gene family members, illustrating that a number of these proteins from diverse species have a similar sequence pattern at their C-terminal end, namely xxxxhhhhRGxxxxVhThYx. This pattern is conserved in all vertebrate NKCCs, NCCs, and KCCs sequenced to date. Since all of these latter transporters are known to be plasma membrane proteins and since, as already discussed, the C termini of a number of integral membrane proteins have been shown to play important roles in their intracellular folding and/or trafficking, we wondered whether the conserved features of this pattern might play a similar role for these Slc12 proteins. To explore this possibility, we generated a number of C-terminal (rat) NKCC1 mutants. These mutants can be broken down into three general groups (Fig. 1B).
FIGURE 1.
Slc12 C-terminal sequences and associated NKCC1 mutants. A, alignment of the C-terminal amino acids of rat NKCC1 (amino acids 1184–1203) with those of other representative members of the Slc12 family. Conserved residues and the “hydrophobic tetrad” (hhhh) (ILLV in NKCC1) are shaded in gray. See “Results” for additional details. The accession numbers for the sequences illustrated are rat NKCC1 (NP_113986), rat NKCC2 (NP_062007), rat KCC1 (NP_062102), rat KCC2 (NP_599190), rat KCC3 (XP_001066756), rat KCC4 (XP_001071999), shark NKCC1 (P55013), shark NKCC2 (AAM74966), rat NCC (NP_062218), flounder NCC (AAL26926), crab (AAF05702), sea urchin (XP_783625), Fly_a (Drosophila; NP_648572), Fly_b (Drosophila; NP_726378), C_el_a (C. elegans; NP_500910), C_el_b (C. elegans; NP_001076724). B, NKCC1 mutants studied in the paper. Mutated amino acids are shaded in gray. See “Results” for details.
Group I, Mutations of the Hydrophobic Tetrad ILLV—Here we mutated 1–4 of the amino acids in the hydrophobic tetrad ILLV to alanine (mutants 1–6), scrambled their order in the NKCC1 sequence (ILLV to LVIL; mutant 7), added an additional hydrophobic amino acid (ILLV to ILLLV; mutant 8), or truncated the NKCC1 sequence at Pro1187 immediately before the hydrophobic tetrad (mutant 9).
Group II, Mutations of the Highly Conserved Amino Acids Downstream of the ILLV Hydrophobic Tetrad—Mutations were generated by switching amino acid positions (Arg1192 and Gly1193; mutant 10) or by replacement with methionine (mutant 11) or alanine (mutants 12 and 13).
Group III, Insertion Mutants—Here we inserted an extra alanine at various positions in the NKCC1 C-terminal sequence (mutants 14–17). Our rationale here was to attempt to disrupt potentially important sequence patterns by changing the relative spacing between residues.
Expression Levels and Complex Glycosylation of NKCC1 Mutants
NKCC1 contains two glycosylation consensus sites in the extracellular loop between its seventh and eighth membrane-spanning segments. In cells, NKCC1 is first core-glycosylated at one or both of these sites as it is synthesized in the ER and then subsequently complex glycosylated after it reaches the Golgi apparatus. When wild-type NKCC1 is exogenously expressed in HEK-293 cells, both the core and complex glycosylated forms are typically observed, the latter being the more abundant (15). Fig. 2A is a Western blot of wild-type (WT) NKCC1 and the IAAV mutant (Fig. 1B), illustrating the presence of the core and complex (com) glycosylated proteins. In Fig. 2B, we show the effect of N-glycosidase F on wild-type NKCC1 and on the AAAA mutant. This experiment illustrates that both the core and complex glycosylated bands are shifted downward by N-glycosidase F treatment, the former by ∼5 kDa and the latter by ∼40 kDa, confirming that both of these bands represent glycosylated forms of the protein. Note also that the AAAA mutant is predominantly expressed in the core glycosylated form (as quantitated in Fig. 2D; see below).
In Fig. 2, C and D, we illustrate the effects of our various mutations of the NKCC1 C terminus on protein expression levels (measured relative to wild-type NKCC1) and complex glycosylation (measured relative to core glycosylation), respectively. Somewhat surprisingly, the glycosylation patterns of all of the mutants in Groups II and III are not significantly different from wild-type NKCC1, and only two of these mutants (Y/A and A^I) show a decrease in protein expression levels (∼40% relative to wild type). Thus, all of these mutated proteins appear to be relatively well tolerated by the HEK-293 cells and properly processed and trafficked from the ER to the Golgi. Interestingly, although the complex glycosylation pattern of our V/M mutant does not differ from wild-type NKCC1, Sabath et al. (8) found that the corresponding NCC mutant, V995M, which has been associated with Gitelman's disease, exhibited decreased complex glycosylation relative to wild-type NCC.
In contrast to the mutants in Groups II and III, a number of the mutations of the hydrophobic tetrad ILLV (Group I) show both significantly reduced protein and complex glycosylation levels compared with wild-type NKCC1. In particular, mutations of two of these hydrophobic amino acids to alanine (mutants IAAV and ALLA) reduced both expression and complex glycosylation levels by >50%. Mutations of three or four of these amino acids to alanine (mutants ALAA and AAAA) resulted in even larger reductions in expression and complex glycosylation. Similar effects were seen by completely truncating the C terminus of the protein upstream of the hydrophobic tetrad (mutant Tr). On the other hand, mutation of one hydrophobic amino acid to alanine (mutants IALV and ILAV), scrambling of the hydrophobic tetrad (mutant LVIL), or insertion of an additional hydrophobic amino acid (mutant ILLLV) had little or no significant effect on expression or complex glycosylation levels.
Localization and Structural State of the AAAA Mutant
The above data suggest that mutation of two or more of the amino acids in the hydrophobic tetrad ILLV to alanine results in defective processing of NKCC1 from the ER to the Golgi and consequently in reduced complex glycosylation of the protein. To investigate this effect in more detail, we carried out several experiments designed to characterize the localization and structural state of the AAAA mutant. In the experiment shown in Fig. 3, we biotinylated the extracellular surface of intact HEK-293 cells transfected with either wild-type NKCC1 or the AAAA mutant and then solubilized the cells and precipitated biotinylated proteins with streptavidin (SA) beads. The bars in the figure illustrate the amount of wild-type NKCC1 and AAAA mutant in the solubilized cell lysate before (-) and after (+) the removal of biotinlyated proteins on the beads. Whereas ∼75% of the wild-type NKCC1 is bound by the beads and was therefore exposed and biotinlyated on the cell surface, no significant binding of AAAA to the beads was observed. Thus, the AAAA mutant apparently fails to reach the plasma membrane.
In Fig. 4, we compare the cellular localization of wild-type NKCC1 and the AAAA mutant immunohistochemically. From left to right, the three panels in Fig. 4 show HEK-293 cells transiently transfected with empty vector (pBK-; Mock), wild-type NKCC1, or the AAAA mutant, respectively. The cells have been fixed, permeabilized, and stained for NKCC1 or AAAA (red), the ER marker protein-disulfide isomerase (green), and nuclei (blue). In cells transfected with wild-type NKCC1 (middle panel), NKCC1 staining is mainly localized to the periphery of the cells in a region that is distinct from the ER marker. Thus, this result is consistent with that of Fig. 3, which showed that most of the wild-type protein is in the plasma membrane. On the other hand, virtually all of the AAAA mutant staining (right panel) overlaps with the ER marker. Taken together, the results of Figs. 2, 3, 4 provide strong evidence that the AAAA mutant is mainly localized to the ER and does not reach the Golgi apparatus or the plasma membrane.
Previous studies from our laboratory have demonstrated that NKCC1 exists as a homodimer in the cell membrane (15, 29). These dimers are stable after mild membrane solubilization and can be cross-linked using the amino cross-linking reagent DTSSP. This effect is illustrated in the left-hand panel of Fig. 5. Here crude membranes from HEK-293 transfected with wild-type NKCC1 were solubilized in 0.3% Triton X-100 and exposed to DTSSP. Following DTSSP treatment, we see that almost all of the wild-type NKCC1 immunoreactivity migrates at a molecular weight that is twice its monomeric size (15, 29). The two remaining panels of Fig. 5 show the results of a similar experiment carried out with crude membranes prepared from cells transfected with the AAAA mutant; the middle panel is a short exposure, and the right-hand panel is a long exposure of the same Western blot. These results show that almost all of the AAAA immunoreactivity is found near the top of the gel, indicating that most of the AAAA protein is in a highly aggregated state.
Effect of Disruption of ER to Golgi Trafficking on Wild-type NKCC1
The aggregation of the AAAA protein illustrated in Fig. 5 could be a cause or an effect of its retention in the ER. For example, if the hydrophobic tetrad were a trafficking signal, its mutation might lead to retention of NKCC1 in the ER, followed by its aggregation. To explore the possibility that retention of NKCC1-like proteins in the ER might lead to their aggregation, we treated HEK-293 cells transfected with wild-type NKCC1 with brefeldin A, a compound that blocks protein trafficking from the ER to the Golgi (33). In Fig. 6, we compare the effects of DTSSP on wild-type NKCC1 in membranes from cells treated with (+BFA) or without (-BFA) brefeldin A. In cells treated with brefeldin A, we see that complex glycosylation of NKCC1 is reduced, consistent with the effect of this drug on disrupting exit from the ER. However, cross-linking of NKCC1 from brefeldin A-treated cells demonstrates that this protein is mainly found in dimers, as seen in untreated cells. In particular, there is no evidence for the appearance of high molecular weight aggregates like those seen for AAAA after exposure to DTSSP (Fig. 5), consistent with the hypothesis that retention in the ER does not itself lead to NKCC1 aggregation.
Function of NKCC1 Mutants
Fig. 7 shows the bumetanide-sensitive (i.e. NKCC-specific (32)) component of 86Rb influx in HEK-293 cells transiently transfected with the NKCC1 mutants in Group I of Fig. 1B (viz. mutations of the hydrophobic tetrad ILLV). Transfection with NKCC1 itself yields a ∼5-fold stimulation of 86Rb uptake over the basal level observed with the empty vector pBK-; this basal bumetanide-sensitive flux is known to be due to the endogenous (human) NKCC1 found in the HEK-293 cells (32). Flux via the mutant AAAA, which shows minimal trafficking to the cell surface (Fig. 3), is similar to basal levels, as are fluxes via the mutants ALAA and Tr. All of the remaining mutants show at least some level of functional activity. Generally speaking, mutations that lead to decreased protein expression also lead to decreased flux of 86Rb. However, closer inspection of the data reveals a somewhat more complex pattern. For example, the mutants ILAV, LVIL, and ILLLV have significantly higher levels of protein expression and complex glycosylation than IAAV (p < 0.04 and p < 0.0001, respectively, in all cases), but fluxes via IAAV are comparable with those via ILAV and LVIL and significantly higher than those via ILLLV. Thus, these data suggest that the hydrophobic tetrad ILLV may also be involved in the functional properties of NKCC1. Further examination of this possibility is beyond the scope of the present paper.
FIGURE 7.
Bumetanide-sensitive 86Rb fluxes via NKCC1 mutants. 86Rb fluxes were measured as previously described (32) in HEK-293 cells transiently transfected with the NKCC1 mutants indicted. Flux measured in the presence of 250 μm bumetanide was subtracted from that measured in its absence to obtain the bumetanide-sensitive (i.e. NKCC-specific (32)) component of flux illustrated. Values are means ± S.E. from four or more independent determinations.
Co-immunoprecipitation of NKCC1 and AAAA with HA-nttNKCC1
Since AAAA-transfected HEK-293 cells exhibit bumetanide-sensitive 86Rb fluxes comparable with mock-transfected cells (Fig. 7), the endogenous HEK-293 NKCC1 in these cells is presumably fully functional and properly processed and trafficked to the plasma membrane; in particular, the endogenous NKCC1 is expected to be present in dimers (15, 29). However, very little if any of the AAAA mutant reaches the plasma membrane (Figs. 3 and 4) or appears to be in a dimerized state (Fig. 5). Since recombinant rat WT NKCC1 is expected to dimerize with the endogenous (human) protein as well as with itself (15), a simple interpretation of these results is that the AAAA mutant is unable to form dimers and thereby is without effect on the endogenous HEK-293 NKCC1. To test this hypothesis, we employed an NKCC1 co-immunoprecipitation assay previously developed in our laboratory (15). Briefly stated (see “Experimental Procedures”), we transiently co-expressed WT NKCC1 or the AAAA mutant along with HA-nttNKCC1, an HA-tagged, N-terminally truncated version of NKCC1. We then carried out an immunoprecipitation from solubilized cells with the antibody α-wNT(r) conjugated to Protein G beads. We have previously established that the cytosolic C terminus of NKCC1 is required for its dimerization and that truncation of its N terminus has no effect on dimer formation (15). Since the sequence against which the antibody α-wNT(r) was raised is missing from HA-nttNKCC1 (see “Experimental Procedures”), we expect this truncated protein to only appear in the α-wNT(r) immunoprecipitate as a component of NKCC1/HA-nttNKCC1 or AAAA/HA-nttNKCC1 dimer pairs.
The results of such an experiment are shown in Fig. 8. Here we have eluted the immunoprecipitated material (on Protein G beads) with 0.1% SDS, which, we have previously shown, disrupts most WT NKCC1 dimer pairs (15, 29), and performed Western blots using an anti-HA antibody on this eluate as well as on the material remaining on the eluted beads to detect HA-nttNKCC1. A strong HA-nttNKCC1 signal is seen in the eluate from cells cotransfected with WT NKCC1 and HA-nttNKCC1, indicating the dimerization of these proteins. A much weaker HA-nttNKCC1 signal is seen in the eluate from cells cotransfected with empty vector (pBK-) and HA-nttNKCC1, presumably reflecting dimerization of HA-nttNKCC1 with the endogenous NKCC1 of the HEK-293 cells, as previously suggested (15). However, no evidence for an increased signal relative to that seen with pBK- is evident in either the eluate or the beads from cells cotransfected with AAAA and HA-nttNKCC1. We quantitated these results by expressing the total amount of immunoprecipitated HA-nttNKCC1 (eluate plus beads) relative to that measured in cells transfected with pBK-. These analyses show that the amounts of immunoprecipitated HA-nttNKCC1 from cells expressing WT NKCC1 and AAAA were 4.8 ± 1.2 times and 1.2 ± 0.4 times, respectively, that found with pBK-. Although the amount of AAAA immunoprecipitated in these assays is ∼50% of that of WT NKCC1 (see the legend to Fig. 8), these experiments nevertheless provide no evidence for an HA-nttNKCC1 signal representing dimerization or coimmunoprecipitation of HA-nttNKCC1 with AAAA. Thus, these results indicate that, in contrast to WT NKCC1, there is no detectable stable interaction between AAAA and HA-nttNKCC1, consistent with the above conjecture that AAAA is unable to form dimers.
Conclusions
Here we have identified a hydrophobic amino acid tetrad ILLV close to the C terminus of NKCC1 that is important for the proper intracellular processing of this protein and its appearance in the plasma membrane. This hydrophobic tetrad is a feature of a number of vertebrate and nonvertebrate Slc12 family members (Fig. 1A). Although other C-terminal amino acids are conserved in these same Slc12 proteins (Fig. 1A), our results indicate that these residues do not play a large role in the intracellular processing of NKCC1 (Fig. 2). Our experiments show that mutations of two of the amino acids in the hydrophobic tetrad to alanine (our mutants ALLA and IAAV) lead to a >50% reduction in complex glycosylation and expression levels of the mutant proteins relative to wild-type NKCC1, whereas additional mutations to alanine (our mutants ALAA and AAAA) lead to even more dramatic effects (Fig. 2). In experiments where we explored the fate of the AAAA mutant, we found that it failed to reach the cell surface (Fig. 3) and co-localized with an ER marker (Fig. 4). These results provide strong evidence that this mutant is trapped in the ER. When we investigated the structural state of AAAA, we found that this protein was predominantly in a highly aggregated state (Fig. 5).
Several observations indicate that mutation of the hydrophobic tetrad ILLV to AAAA leads to the misfolding and concomitant aggregation of this NKCC1 mutant and that this is the reason for its retention in the ER. First, it is well established that misfolded and/or aggregated proteins are typically retained in the ER (2, 3), consistent with the localization of AAAA in this compartment. Second, our experiments with brefeldin A demonstrate that preventing wild-type NKCC1 from exiting from the ER does not lead to its aggregation (Fig. 6); thus, the aggregation of AAAA does not appear to be a result of its retention in the ER but rather a cause. Finally, we find no evidence that AAAA forms stable dimers (Fig. 8). Taken together, these results indicate that the AAAA mutant misfolds soon after its synthesis, making it incapable of stable dimer formation and resulting in its aggregation and retention in the ER.
Our results do not address the nature of the physical role of the hydrophobic tetrad identified here in the folding and processing of the Slc12 proteins. We anticipate that structural studies beyond the scope of the present paper will be required to give a definitive understanding of its significance. Interestingly, the data presented here as well as comparison with other Slc12 sequences (Fig. 1A) suggest that it is conservation of residue hydrophobicity rather than residue identity that underlies the role of this amino acid motif.
Acknowledgments
We thank Dr. William D. Swaim for assistance with immunostaining and confocal microscopy and Drs. Roberto Weigert and Jennifer Lippincott-Schwartz for several helpful discussions.
This work was supported, in whole or in part, by the National Institutes of Health Intramural Research Program, NIDCR. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: ER, endoplasmic reticulum; NKCC, Na+-K+-2Cl- cotransporter; NCC, Na+-Cl- cotransporter; KCC, K+-Cl- cotransporter; PBS, phosphate-buffered saline; DTSSP, 3,3′-dithiobis(sulfosuccinimidyl propionate); pBK-, pBK-CMVlac-; WT, wild type; HA, hemagglutinin.
References
- 1.White, S. H., and von Heijne G. (2004) Curr. Opin. Struct. Biol. 14 397-404 [DOI] [PubMed] [Google Scholar]
- 2.Kopito, R. R. (1997) Cell 88 427-430 [DOI] [PubMed] [Google Scholar]
- 3.Ellgaard, L., and Helenius, A. (2003) Nat. Rev. Mol. Cell Biol. 4 181-191 [DOI] [PubMed] [Google Scholar]
- 4.Cheung, J. C., and Deber, C. M. (2008) Biochemistry 47 1465-1473 [DOI] [PubMed] [Google Scholar]
- 5.Sorensen, S., Ranheim, T., Bakken, K. S., Leren, T. P., and Kulseth, M. A. (2006) J. Biol. Chem. 281 468-476 [DOI] [PubMed] [Google Scholar]
- 6.Robben, J. H., Knoers, N. V., and Deen, P. M. (2005) Am. J. Physiol. 289 F265-F272 [DOI] [PubMed] [Google Scholar]
- 7.Robben, J. H., Knoers, N. V., and Deen, P. M. (2006) Am. J. Physiol. 291 F257-F270 [DOI] [PubMed] [Google Scholar]
- 8.Sabath, E., Meade, P., Berkman, J., de los Heros, P., Moreno, E., Bobadilla, N. A., Vazquez, N., Ellison, D. H., and Gamba, G. (2004) Am. J. Physiol. 287 F195-F203 [DOI] [PubMed] [Google Scholar]
- 9.de Jong, J. C., Van der Vliet, W. A., Van Den Heuvel, L. P., Willems, P. H., Knoers, N. V., and Bindels, R. J. (2002) J. Am. Soc. Nephrol. 13 1442-1448 [DOI] [PubMed] [Google Scholar]
- 10.Illing, M. E., Rajan, R. S., Bence, N. F., and Kopito, R. R. (2002) J. Biol. Chem. 277 34150-34160 [DOI] [PubMed] [Google Scholar]
- 11.Gamba, G. (2005) Physiol. Rev. 85 423-493 [DOI] [PubMed] [Google Scholar]
- 12.Delpire, E., and Mount, D. B. (2002) Annu. Rev. Physiol. 64 803-843 [DOI] [PubMed] [Google Scholar]
- 13.Kaneko, H., Putzier, I., Frings, S., Kaupp, U. B., and Gensch, T. (2004) J. Neurosci. 24 7931-7938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gavrikov, K. E., Nilson, J. E., Dmitriev, A. V., Zucker, C. L., and Mangel, S. C. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 18793-18798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parvin, M. N., Gerelsaikhan, T., and Turner, R. J. (2007) Biochemistry 46 9630-9637 [DOI] [PubMed] [Google Scholar]
- 16.Pena-Munzenmayer, G., Catalan, M., Cornejo, I., Figueroa, C. D., Melvin, J. E., Niemeyer, M. I., Cid, L. P., and Sepulveda, F. V. (2005) J. Cell Sci. 118 4243-4252 [DOI] [PubMed] [Google Scholar]
- 17.Krause, G., Hermosilla, R., Oksche, A., Rutz, C., Rosenthal, W., and Schulein, R. (2000) Mol. Pharmacol. 57 232-242 [PubMed] [Google Scholar]
- 18.Miranda, M., Sorkina, T., Grammatopoulos, T. N., Zawada, W. M., and Sorkin, A. (2004) J. Biol. Chem. 279 30760-30770 [DOI] [PubMed] [Google Scholar]
- 19.Duvernay, M. T., Zhou, F., and Wu, G. (2004) J. Biol. Chem. 279 30741-30750 [DOI] [PubMed] [Google Scholar]
- 20.Larsen, M. B., Fjorback, A. W., and Wiborg, O. (2006) Biochemistry 45 1331-1337 [DOI] [PubMed] [Google Scholar]
- 21.Kasir, J., Ren, X., Furman, I., and Rahamimoff, H. (1999) J. Biol. Chem. 274 24873-24880 [DOI] [PubMed] [Google Scholar]
- 22.Sharma, N., Crane, A., Clement, J. P., Gonzalez, G., Babenko, A. P., Bryan, J., and Guilar-Bryan, L. (1999) J. Biol. Chem. 274 20628-20632 [DOI] [PubMed] [Google Scholar]
- 23.Loo, T. W., Bartlett, M. C., and Clarke, D. M. (2005) J. Biol. Chem. 280 2522-2528 [DOI] [PubMed] [Google Scholar]
- 24.Pankevych, H., Korkhov, V., Freissmuth, M., and Nanoff, C. (2003) J. Biol. Chem. 278 30283-30293 [DOI] [PubMed] [Google Scholar]
- 25.Iodice, L., Sarnataro, S., and Bonatti, S. (2001) J. Biol. Chem. 276 28920-28926 [DOI] [PubMed] [Google Scholar]
- 26.Mueller, G. M., Kashlan, O. B., Bruns, J. B., Maarouf, A. B., Aridor, M., Kleyman, T. R., and Hughey, R. P. (2007) J. Biol. Chem. 282 33475-33483 [DOI] [PubMed] [Google Scholar]
- 27.Kurihara, K., Moore-Hoon, M. L., Saitoh, M., and Turner, R. J. (1999) Am. J. Physiol. 277 C1184-C1193 [DOI] [PubMed] [Google Scholar]
- 28.Evans, R. L., Park, K., Turner, R. J., Watson, G. E., Nguyen, H. V., Dennett, M. R., Hand, A. R., Flagella, M., Shull, G. E., and Melvin, J. E. (2000) J. Biol. Chem. 275 26720-26726 [DOI] [PubMed] [Google Scholar]
- 29.Moore-Hoon, M. L., and Turner, R. J. (2000) Biochemistry 39 3718-3724 [DOI] [PubMed] [Google Scholar]
- 30.Zheng, L., Baumann, U., and Reymond, J. L. (2004) Nucleic Acids Res. 32 e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dohke, Y., and Turner, R. J. (2002) J. Biol. Chem. 277 15215-15219 [DOI] [PubMed] [Google Scholar]
- 32.Dehaye, J. P., Nagy, A., Premkumar, A., and Turner, R. J. (2003) J. Biol. Chem. 278 11811-11817 [DOI] [PubMed] [Google Scholar]
- 33.Lippincott-Schwartz, J., and Liu, W. (2006) Trends Cell Biol. 16 e1-e4 [DOI] [PubMed] [Google Scholar]