Abstract
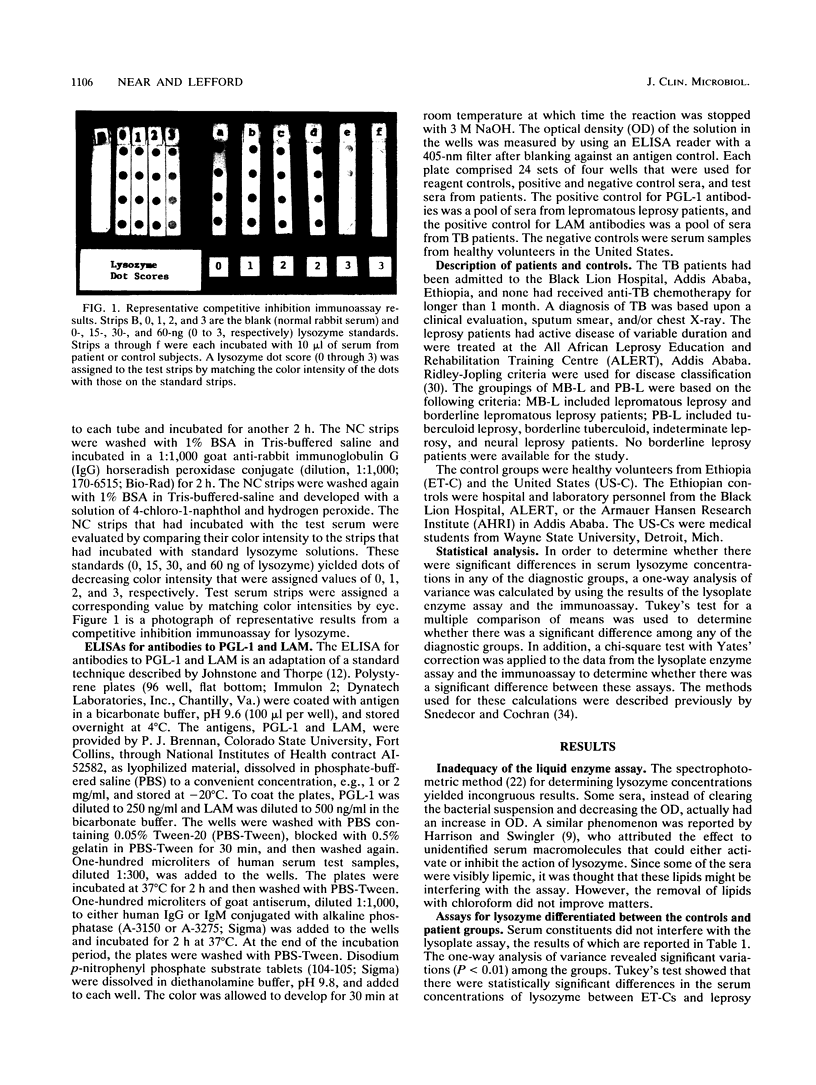
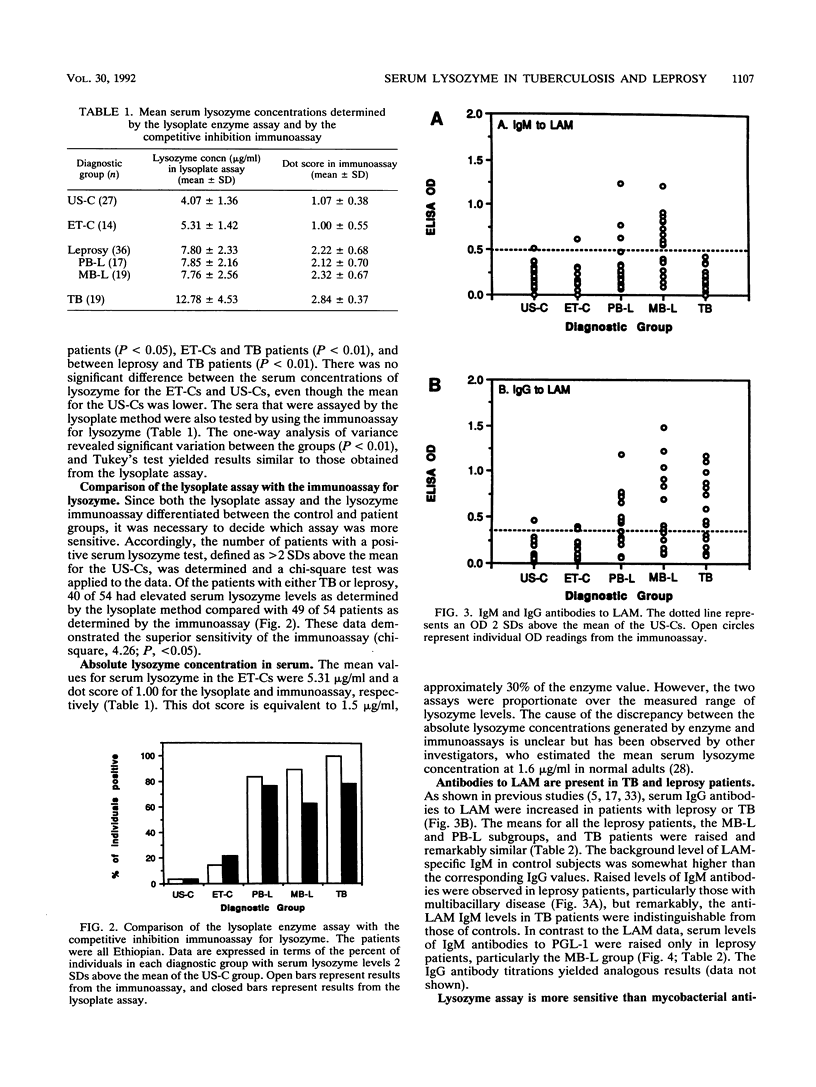
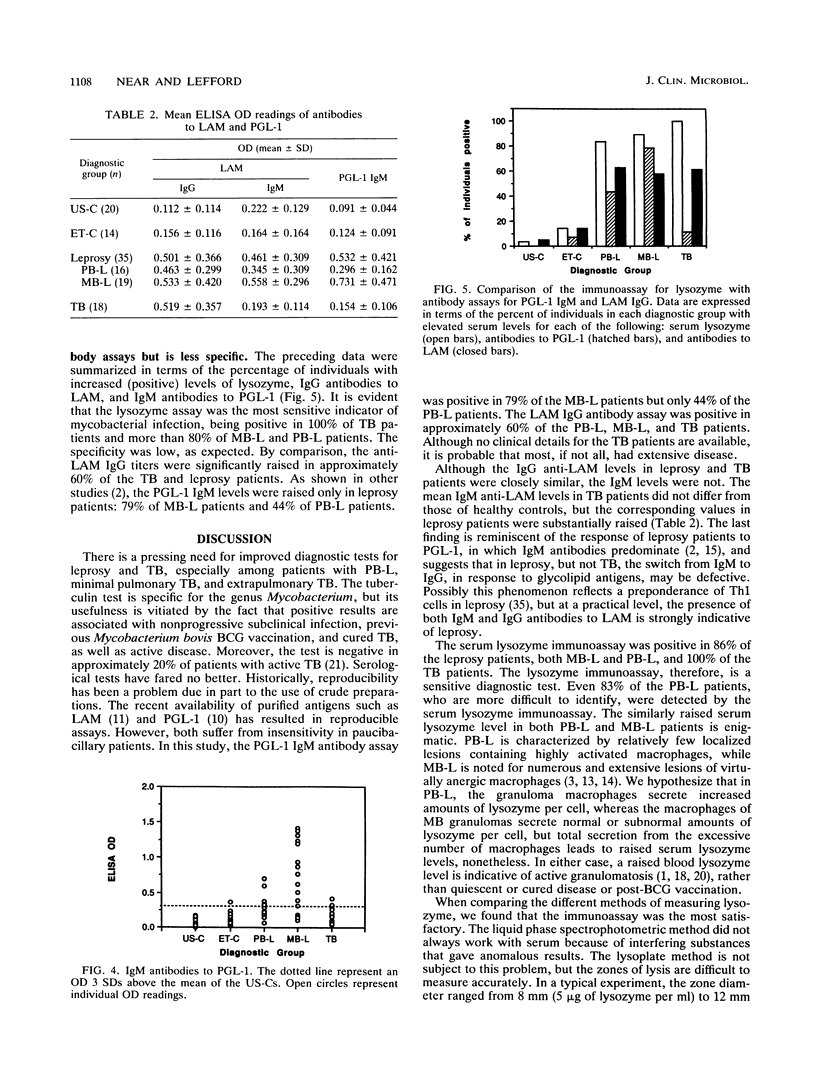
Active tuberculosis (TB) and leprosy are difficult to diagnose early because there are few organisms to detect and the specific immune response does not distinguish between active and inactive disease. We developed an immunoassay for lysozyme to see whether serum lysozyme levels could be used to identify individuals with clinical leprosy or TB. The immunoassay for lysozyme proved superior to standard enzyme assays that were less sensitive and reliable. The lysozyme assay was compared with assays for antibodies to Mycobacterium tuberculosis lipoarabinomannan (LAM) and M. leprae phenolic glycolipid-1. The sera tested were from Ethiopian leprosy (paucibacillary and multibacillary) and TB patients and from healthy Ethiopian and U.S. controls. The lysozyme assay was able to detect more of the individuals with TB (sensitivity, 100% for 19 patients) or leprosy (sensitivity, 86% for 36 patients) than either antibody assay. In particular, lysozyme levels were raised in a higher proportion of the paucibacillary leprosy patients (83% of 17), for whom the antibody assays were less sensitive; the LAM IgG and the phenolic glycolipid-1 IgM levels were raised in only 62 and 44% of 16 patients, respectively. The data suggest that lysozyme measurements may be useful in the diagnosis of mycobacterial infections and other chronic infectious granulomatoses.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carr I., Carr J., Trew J. A., Lobo A., Chattopadhyay P. K. Lysozyme production by a granuloma in vivo: output in blood and lymph in relation to ultrastructure and immunochemistry. J Pathol. 1980 Oct;132(2):105–119. doi: 10.1002/path.1711320203. [DOI] [PubMed] [Google Scholar]
- Cho S. N., Yanagihara D. L., Hunter S. W., Gelber R. H., Brennan P. J. Serological specificity of phenolic glycolipid I from Mycobacterium leprae and use in serodiagnosis of leprosy. Infect Immun. 1983 Sep;41(3):1077–1083. doi: 10.1128/iai.41.3.1077-1083.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper C. L., Mueller C., Sinchaisri T. A., Pirmez C., Chan J., Kaplan G., Young S. M., Weissman I. L., Bloom B. R., Rea T. H. Analysis of naturally occurring delayed-type hypersensitivity reactions in leprosy by in situ hybridization. J Exp Med. 1989 May 1;169(5):1565–1581. doi: 10.1084/jem.169.5.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falchuk K. R., Perrotto J. L., Isselbacher K. J. Serum lysozyme in Crohn's disease. A useful index of disease activity. Gastroenterology. 1975 Oct;69(4):893–896. [PubMed] [Google Scholar]
- Gelber R. H., Li F., Cho S. N., Byrd S., Rajagopalan K., Brennan P. J. Serum antibodies to defined carbohydrate antigens during the course of treated leprosy. Int J Lepr Other Mycobact Dis. 1989 Dec;57(4):744–751. [PubMed] [Google Scholar]
- Grange J. M. The rapid diagnosis of paucibacillary tuberculosis. Tubercle. 1989 Mar;70(1):1–4. doi: 10.1016/0041-3879(89)90059-7. [DOI] [PubMed] [Google Scholar]
- Grieco M. H., Reddy M. M., Kothari H. B., Lange M., Buimovici-Klein E., William D. Elevated beta 2-microglobulin and lysozyme levels in patients with acquired immune deficiency syndrome. Clin Immunol Immunopathol. 1984 Aug;32(2):174–184. doi: 10.1016/0090-1229(84)90119-3. [DOI] [PubMed] [Google Scholar]
- Hailù K., Buttò S., Bekura D., Verani P., Titti F., Sernicola L., Rapicetta M., Pasquini P., Rossi G. B. Serological survey of human immunodeficiency virus (HIV) in Ethiopia. J Med Virol. 1989 May;28(1):21–24. doi: 10.1002/jmv.1890280106. [DOI] [PubMed] [Google Scholar]
- Harrison J. F., Swingler M. The effect of serum macromolecules on the lysis of Micrococcus lysodeikticus cells by lysozyme. Clin Chim Acta. 1971 Jan;31(1):149–154. doi: 10.1016/0009-8981(71)90372-x. [DOI] [PubMed] [Google Scholar]
- Hunter S. W., Brennan P. J. A novel phenolic glycolipid from Mycobacterium leprae possibly involved in immunogenicity and pathogenicity. J Bacteriol. 1981 Sep;147(3):728–735. doi: 10.1128/jb.147.3.728-735.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter S. W., Gaylord H., Brennan P. J. Structure and antigenicity of the phosphorylated lipopolysaccharide antigens from the leprosy and tubercle bacilli. J Biol Chem. 1986 Sep 15;261(26):12345–12351. [PubMed] [Google Scholar]
- Kaplan G., Kiessling R., Teklemariam S., Hancock G., Sheftel G., Job C. K., Converse P., Ottenhoff T. H., Becx-Bleumink M., Dietz M. The reconstitution of cell-mediated immunity in the cutaneous lesions of lepromatous leprosy by recombinant interleukin 2. J Exp Med. 1989 Mar 1;169(3):893–907. doi: 10.1084/jem.169.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan G., Nusrat A., Sarno E. N., Job C. K., McElrath J., Porto J. A., Nathan C. F., Cohn Z. A. Cellular responses to the intradermal injection of recombinant human gamma-interferon in lepromatous leprosy patients. Am J Pathol. 1987 Aug;128(2):345–353. [PMC free article] [PubMed] [Google Scholar]
- Koster F. T., Scollard D. M., Umland E. T., Fishbein D. B., Hanly W. C., Brennan P. J., Nelson K. E. Cellular and humoral immune response to a phenolic glycolipid antigen (PhenGL-I) in patients with leprosy. J Clin Microbiol. 1987 Mar;25(3):551–556. doi: 10.1128/jcm.25.3.551-556.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefford M. J., Hunegnaw M., Siwik E. The value of IgM antibodies to PGL-I in the diagnosis of leprosy. Int J Lepr Other Mycobact Dis. 1991 Sep;59(3):432–440. [PubMed] [Google Scholar]
- Levis W. R., Meeker H. C., Schuller-Levis G., Sersen E., Brennan P. J., Fried P. Mycobacterial carbohydrate antigens for serological testing of patients with leprosy. J Infect Dis. 1987 Nov;156(5):763–769. doi: 10.1093/infdis/156.5.763. [DOI] [PubMed] [Google Scholar]
- Lyberg T., Closs O., Prydz H. Effect of purified protein derivative and sonicates of Mycobacterium leprae and Mycobacterium bovis BCG on thromboplastin response in human monocytes in vitro. Infect Immun. 1982 Dec;38(3):855–859. doi: 10.1128/iai.38.3.855-859.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon A. C., Nurlign A., Kebede B., Becx-Bleumink M., Lefford M. J. Urinary phenolic glycolipid 1 in the diagnosis and management of leprosy. J Infect Dis. 1991 Mar;163(3):653–656. doi: 10.1093/infdis/163.3.653. [DOI] [PubMed] [Google Scholar]
- Musson R. A., Shafran H., Henson P. M. Intracellular levels and stimulated release of lysosomal enzymes from human peripheral blood monocytes and monocyte-derived macrophages. J Reticuloendothel Soc. 1980 Sep;28(3):249–264. [PubMed] [Google Scholar]
- Nash D. R., Douglass J. E. Anergy in active pulmonary tuberculosis. A comparison between positive and negative reactors and an evaluation of 5 TU and 250 TU skin test doses. Chest. 1980 Jan;77(1):32–37. doi: 10.1378/chest.77.1.32. [DOI] [PubMed] [Google Scholar]
- Osserman E. F., Lawlor D. P. Serum and urinary lysozyme (muramidase) in monocytic and monomyelocytic leukemia. J Exp Med. 1966 Nov 1;124(5):921–952. doi: 10.1084/jem.124.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual R. S., Gee J. B., Finch S. C. Usefulness of serum lysozyme measurement in diagnosis and evaluation of sarcoidosis. N Engl J Med. 1973 Nov 15;289(20):1074–1076. doi: 10.1056/NEJM197311152892007. [DOI] [PubMed] [Google Scholar]
- Perillie P. E., Khan K., Finch S. C. Serum lysozyme in pulmonary tuberculosis. Am J Med Sci. 1973 Apr;265(4):297–302. doi: 10.1097/00000441-197304000-00005. [DOI] [PubMed] [Google Scholar]
- Plikaytis B. B., Gelber R. H., Shinnick T. M. Rapid and sensitive detection of Mycobacterium leprae using a nested-primer gene amplification assay. J Clin Microbiol. 1990 Sep;28(9):1913–1917. doi: 10.1128/jcm.28.9.1913-1917.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porstmann B., Jung K., Schmechta H., Evers U., Pergande M., Porstmann T., Kramm H. J., Krause H. Measurement of lysozyme in human body fluids: comparison of various enzyme immunoassay techniques and their diagnostic application. Clin Biochem. 1989 Oct;22(5):349–355. doi: 10.1016/s0009-9120(89)80031-1. [DOI] [PubMed] [Google Scholar]
- Rea T. H., Taylor C. R. Serum and tissue lysozyme in leprosy. Infect Immun. 1977 Dec;18(3):847–856. doi: 10.1128/iai.18.3.847-856.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley D. S., Jopling W. H. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966 Jul-Sep;34(3):255–273. [PubMed] [Google Scholar]
- Roche P. W., Britton W. J., Failbus S. S., Ludwig H., Theuvenet W. J., Adiga R. B. Heterogeneity of serological responses in paucibacillary leprosy--differential responses to protein and carbohydrate antigens and correlation with clinical parameters. Int J Lepr Other Mycobact Dis. 1990 Jun;58(2):319–327. [PubMed] [Google Scholar]
- Roche P. W., Britton W. J., Failbus S. S., Williams D., Pradhan H. M., Theuvenet W. J. Operational value of serological measurements in multibacillary leprosy patients: clinical and bacteriological correlates of antibody responses. Int J Lepr Other Mycobact Dis. 1990 Sep;58(3):480–490. [PubMed] [Google Scholar]
- Sada E., Brennan P. J., Herrera T., Torres M. Evaluation of lipoarabinomannan for the serological diagnosis of tuberculosis. J Clin Microbiol. 1990 Dec;28(12):2587–2590. doi: 10.1128/jcm.28.12.2587-2590.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street N. E., Mosmann T. R. Functional diversity of T lymphocytes due to secretion of different cytokine patterns. FASEB J. 1991 Feb;5(2):171–177. doi: 10.1096/fasebj.5.2.1825981. [DOI] [PubMed] [Google Scholar]
- Zager R. A. Urinary protein markers of tubulointerstitial nephritis. Invest Urol. 1980 Nov;18(3):197–202. [PubMed] [Google Scholar]




