Abstract
Cell volume and shape are stringently regulated. This homeostasis requires the cells to sense their size and shape and to convey this information to effectors that will counteract deformations induced by osmotic or mechanical challenges. The sensors, transducers, and effectors of volume change are the subject of this review.
With few exceptions, the permeability of biological membranes to water is much greater than that to ions and other solutes. As a result, imposition of aniso-osmolar conditions across the plasma membrane causes the rapid net flow of water, and consequently, cell volume changes. When cells face osmotic challenges, they deploy regulatory mechanisms to defend their size and integrity. Moreover, even when bathed in physiological medium, cells experience isotonic volume changes when undergoing sudden gains or losses of solutes due to transport or metabolic stimulation. In addition, the shape of many cells is often altered by externally applied physical forces, by intrinsic cytoskeletal rearrangement, or by remodeling of the substratum.
The generation of osmosensitive or mechanosensitive responses can be conceptually divided into three events: sensing the shape or volume change, transmission of the information to the effectors, and execution of the regulatory response. These are discussed in turn below.
Sensors of Volume and Shape Change
In principle, three types of parameters could be sensed by cells when their volume is challenged osmotically: the concentration of solutes, the thickness or lateral tension of the bilayer, or cell shape-dependent interactions between macromolecular structures. When cells are swollen or shrunken, the osmolarity and ionic strength of the intra- and extracellular milieu are altered. The abstraction or addition of water changes the concentration of critical substrates and macromolecular crowding in general, and the changes in ionic strength alter the degree of shielding of exposed charges. Although these events have been proposed to mediate volume sensing (1), we feel that under most conditions, the changes in ionic strength and macromolecular crowding are comparatively small and contribute little to eliciting cellular responses. For example, activation of ion exchangers and cotransporters has been reported when cell volume is altered by <5% (2, 3). The accompanying change in macromolecular concentration is minute and unlikely to serve as an effective transducer of information.
Pronounced cell swelling will eventually stretch the membrane bilayer in the lateral plane. A variety of channels and transporters respond to such mechanical deformation of the bilayer, affording the cell a simple and direct means of controlling transport for the purpose of volume regulation. However, cells are not smooth spheres, and a vast amount of membrane is folded into filopodia, ruffles, and other protrusions. These can accommodate considerable increases in cell volume without incurring lateral stretching. Therefore, lateral tension of the bilayer signals the activation of volume regulatory effectors mostly in cases of extreme swelling.
Instead, we favor the notion that mechanically induced alterations in the interactions between (macro)molecular complexes are mainly responsible for the responses to volume perturbations. Two main types of mechanically induced changes can be envisaged: those resulting from altered curvature of the membrane and those caused by dislodging membrane components from their native interaction with the cytoskeleton or the extracellular matrix.
Alterations in membrane curvature can change the physical properties of lipids and potentially alter their metabolism. Inward depression of the membrane (concavity) forces greater spacing between lipid headgroups of the inner monolayer, increasing the exposure of the hydrophobic side chains. The opposite response is experienced by the outer monolayer. Conversely, outward (convex) deformation packs the headgroups of the inner monolayer and vice versa. Greater exposure of the hydrophobic tails makes them more accessible to enzymatic attack (4, 5). In addition, because some plasmalemmal lipids are anionic, altered packing of headgroups changes the density of interfacial charges.
Cell swelling and shrinking can also alter the properties of membrane proteins or lipids independently of membrane curvature. The net displacement of the membrane as the cells swell or shrink can potentially disrupt pre-existing associations between membrane components and less mobile structures, such as the cytoskeleton, the extracellular matrix, or neighboring cells. This results in dissociation of pre-existing complexes and possibly the formation of new ones. In addition, by losing their anchorage, intrinsic membrane proteins can redistribute laterally, which can promote clustering, as reported for integrins (6).
Although there are attractive theoretical options to explain how changes in cell shape and volume could be sensed, definitive mechanisms have been identified only in a handful of cases. These include the shrinkage-induced, ligand-independent activation of receptors like the epidermal growth factor receptor (7), the osmotically or mechanically provoked activation of TRP2 ion channels (8, 9), and the volume-induced activation of integrins (6).
Mediators and Signaling Pathways
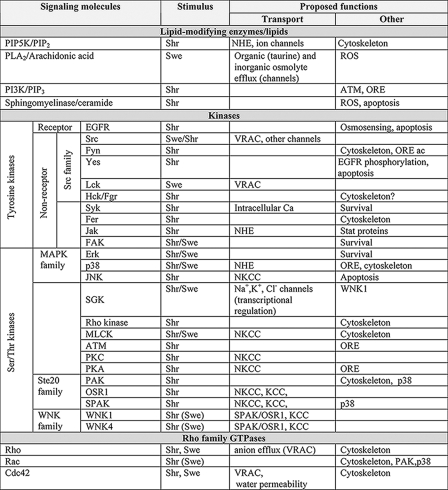
Changes in cell size trigger a plethora of signaling pathways (Table 1). In fact, a major challenge in the field is to link specific signals with the upstream sensors on one hand and the corresponding downstream effectors on the other.
TABLE 1.
Signaling pathways activated by volume changes and their proposed functions
The triggering stimulus, cell swelling (Swe) and/or shrinkage (Shr), is indicated. “(Swe)” denotes that swelling, although reported to activate the pathway, is not the predominant stimulus. “ORE” denotes the activation of the osmotic response element, whereas “Cytoskeleton” indicates actin skeleton remodeling. PIP5K, phosphatidylinositol-4-phosphate 5-kinase; PLA2, phospholipase A2; ROS, reactive oxygen species; PI3K, phosphatidylinositol 3-kinase; ATM, ataxia telangiectasia mutated; EGFR, epidermal growth factor receptor; FAK, focal adhesion kinase; MAPK, mitogen-activated protein kinase; JNK, c-Jun N-terminal kinase; SGK, serum- and glucocorticoid-inducible kinase; MLCK, myosin light chain kinase; PKC, protein kinase C; PKA, protein kinase A; PAK, p21-activated kinase.
Lipids and Lipid-modifying Enzymes—Cell shrinkage increases (10–12) and swelling decreases (12) the level of PIP2. Phosphatidylinositol-4-phosphate 5-kinase, which phosphorylates phosphatidylinositol 4-phosphate (11), is thought to mediate these responses. PIP2 is an excellent candidate as an upstream mediator of shrinkage-induced effects, as it can bind to and activate multiple volume-sensitive transporters (13, 14).
Arachidonic acid and its derivatives, generated via the cyclooxygenase and lipoxygenase pathways, have also been implicated in volume-dependent signaling (4). Arachidonic acid is liberated from glycerophospholipids by phospholipase A2, an effect attributed variously to enhanced presentation of the phospholipid, caveolar reorganization (5, 15), activation of G-proteins (16), increased cytosolic [Ca2+], and/or phosphorylation by mitogen-activated protein kinases (15). Irrespective of the mechanism, the liberated arachidonic acid or its derivatives regulate several swelling-sensitive transport systems that catalyze the efflux of inorganic and organic osmolytes (4, 17). In addition, arachidonic acid can activate the NADPH oxidase that generates reactive oxygen species, which in turn regulate certain channels (18, 19). The oxidase is also activated by ceramide released by acidic sphingomyelinase, another volume-responsive lipase (7).
Intracellular Ca2+—In many cell types, swelling produces transient increases in intracellular [Ca2+]. The underlying mechanism involves opening of stretch-dependent cation channels, Ca2+ release from internal stores, and/or swelling-stimulated release of ATP, which then acts on Ca2+-mobilizing purinergic receptors in a paracrine fashion (20). The elevated [Ca2+] can in turn activate solute transport (see below).
Protein Phosphorylation—Alterations in cell volume are associated with profound changes in protein phosphorylation. The activity of receptor and non-receptor tyrosine kinases and multiple Ser/Thr kinases is modulated by cell volume (Table 1). Volume-dependent phosphorylation is involved in (a) osmosensing (21), (b) direct regulation of channels and transporters, (c) transcription of genes encoding osmolyte-transporting systems (7, 22), and (d) cytoskeletal reorganization (23, 24). Here, we discuss only the regulation of channels and transporters.
Much of the available evidence is compiled in Table 1, but a couple of salient examples are worth highlighting. One is the Src family member Lck, which was shown to mediate the opening of swelling-activated Cl– channels (25). A second notable example is the recent realization that Ste20 (Sterile 20) family kinases (SPAK (Ste20/SPS1-related proline/alanine-rich kinase) and OSR1) and their upstream regulators, the WNK (with no K (lysine)) kinases, are the long sought-after regulators of various volume-dependent cotransporters. When activated by hyperosmolarity, WNK1 and WNK4 phosphorylate SPAK or OSR1, which in turn phosphorylates various cotransporters, thereby increasing their activity (26, 27).
Rho Family GTPases—Rho, Rac, and Cdc42 have all been shown to respond to changes in cell size (25), and this modifies the state of actin polymerization and myosin activation (28, 29). In addition, stimulation of Rac and Cdc42 by cell shrinkage may be at least partly responsible for the osmotically induced activation of p38 kinase (30), which has been implicated in the control of ion transport. Moreover, Rho and Cdc42 are important to hypotonic stress-induced stimulation of anion efflux (31, 32).
Effectors of Volume Regulation
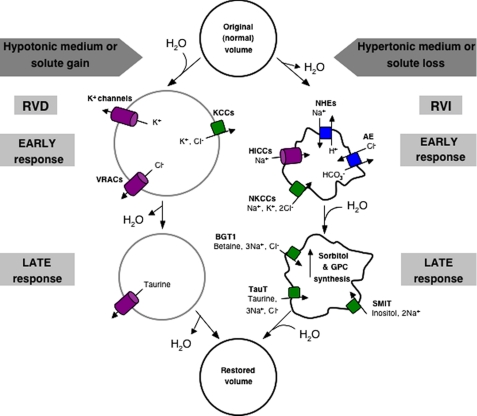
As cell volume changes, transport systems on the membrane are activated. The resulting translocation of osmolytes tends to restore the original cell size. Cell shrinkage is counteracted by gain of both inorganic (mainly Na+ and Cl–) and organic solutes and the accompanying osmotically driven influx of water. This process is known as RVI. Conversely, in swollen cells, a different set of transporters is activated, leading to loss of inorganic ions (K+ and Cl–) and organic osmolytes, followed by loss of water, a process termed RVD (Fig. 1).
FIGURE 1.
Volume regulatory responses. A schematic representation of the solute transport pathways engaged in RVD and RVI is shown. Channels/uniports are shown in purple, exchangers in blue, and cotransporters in green. GPC, glycerophosphocholine; TauT, taurine transporter; AE, anion exchanger; SMIT, sodium myo-inositol transporter.
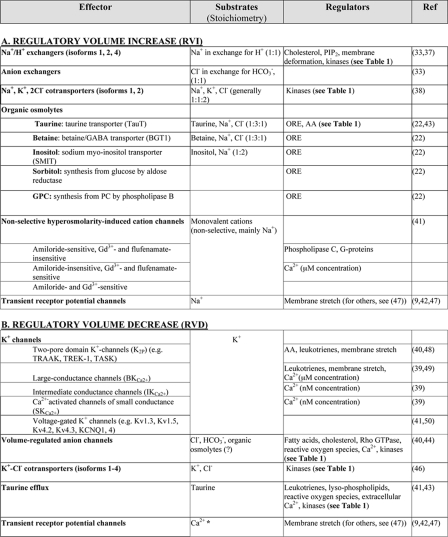
Effectors of RVI
The main solute taken up by cells to effect RVI is Na+, the major extracellular cation. Na+ is driven inwardly by the sizable concentration gradient created by the Na+/K+-ATPase (the extracellular sodium concentration is ≈140 mm, whereas the intracellular sodium concentration is <20 mm) in combination with the inward-negative membrane potential generated mainly by K+-conductive pathways. The combined electrochemical gradient is exploited by effectors to promote RVI.
Na+/H+ Exchangers—In many instances, Na+ influx during RVI occurs in exchange for intracellular H+. The reaction is mediated by electroneutral antiporters, members of the NHE family. Multiple NHE isoforms have been described. NHE1, NHE2, and NHE4 are activated by osmotically induced cell shrinkage and are thus potentially involved in RVI. The ubiquitously expressed NHE1 is the primary mechanism driving RVI in many cell types (33, 34). It has been shown that osmotic activation of NHE1 results from a shift in the pH sensitivity of its “set point” (35) and from enhanced affinity for extracellular Na+ (36), but the biochemical events underlying these changes are unclear. Even though the activity of numerous kinases is drastically altered by changing cell volume, shrinkage does not significantly alter the level of NHE1 phosphorylation (33, 34). Nevertheless, ancillary proteins that control its activity may be the targets of phosphorylation (Table 1). Alternatively, NHE1 may itself be mechanosensitive because it was found to be activated by lipids that alter membrane shape (37).
Na+-K+-2Cl– Cotransporters—In many cell types, NKCCs are also important contributors to Na+ influx during RVI. NKCCs belong to the cation chloride cotransporter superfamily that also features one Na+-Cl– cotransporter and four KCC isoforms (38). NKCC1 and NKCC2 are stimulated by shrinkage (38), and the mechanism of activation has been well characterized: stimulation upon shrinkage takes place through direct phosphorylation (Table 1).
Organic Osmolytes—Although the acute uptake of inorganic ions serves to restore the size of shrunken cells, it renders the ionic concentration of the normovolemic cells abnormally high. Because high ionic strength perturbs the folding and association of macromolecules, this condition is potentially harmful to the cells and not tolerable in the long run. To counteract this effect, cells develop a secondary response over a period of hours, namely the accumulation of organic osmolytes (22). At the concentrations required for volume restoration in hypertonic media, certain organic osmolytes stabilize the structure of proteins. As the organic osmolytes accumulate, cells can afford to release the excess inorganic ions, reducing their ionic strength while maintaining their volume in the face of continued hypertonic stress.
The principal compatible organic osmolytes are taurine (2-aminoethylsulfonic acid), betaine, inositol, sorbitol, and glycerophosphocholine. Two distinct mechanisms account for the accumulation of organic osmolytes: increased uptake from the extracellular medium and/or elevated rates of intrinsic biosynthesis. Taurine, as well as inositol and betaine, illustrates the first mechanism; hypertonicity increases their uptake into cells (Table 2). Cotransporters couple the uptake of the organic osmolytes with the influx of Na+ and Cl– ions (Table 2).
TABLE 2.
Effectors of volume regulation
The asterisk indicates that the generally prevailing view is that swelling-activated TRPs mediate an increase in intracellular [Ca2+], which then stimulates RVD via Ca2+-activated K+ and Cl– channels. Direct evidence linking TRPs to RVD is still insubstantial (47). ORE, osmotic response element; AA, arachidonic acid; GABA, γ-aminobutyric acid; GPC, glycerophosphocholine.
On the other hand, cellular accumulation of both sorbitol and glycerophosphocholine following hypertonic challenge is due to the second mechanism, i.e. increased synthesis. Elevated levels of the organic osmolytes in response to hypertonicity are regulated at the level of transcription of the transporters or enzymes active in their synthesis. Transcription is regulated by the osmotic response element-binding protein (22), which can be activated by several of the kinases listed in Table 1.
Hypertonicity-induced Cation Channels—Channels are effective mediators of volume regulation. HICCs are the main mediators of RVI in several cell types. When activated, HICCs allow the rather indiscriminate passage of alkali cations. As a result, Na+ enters the cells at the same time as K+ is exported. Nonetheless, the combined electrochemical gradients are such that net cation uptake ensues, accompanied by parallel conductive entry of Cl– (39). The molecular identity of HICCs is not known, but three main classes can be distinguished pharmacologically (Table 2). In addition to HICCs, TRP channels can also promote Na+ entry and volume gain.
Effectors of RVD
Because the electrochemical K+ gradient is directed outward, this ion is thermodynamically poised to drive net efflux of salt and water, leading to volume loss. This requires concomitant efflux of a counterion, generally Cl–. Coupling between K+ and Cl– can be tight, enforced by electroneutral cotransporters, or can take place via parallel conductive pathways.
Cation Channels—Activation of conductive K+ efflux by swelling has been reported in a variety of cells (40, 41). Remarkably, not one but multiple well characterized K+ channels are stimulated by swelling (Table 2). These often coexist in the same cell type, and their individual contribution to volume regulation has rarely been established.
Of note, TRP channels, which were mentioned in the context of RVI, have also been implicated in RVD (9, 42). Ca2+ entering cells through TRP channels is thought to serve as a signal for activation of other channels that mediate net loss of ions.
Anion Channels—The substantive loss of osmolytes required for RVD can take place only if the conductive efflux of K+ is accompanied by a parallel anion efflux, which takes place via VRACs. Cl– is thought to be the preferred substrate of VRACs, but the same pathway may also account for at least part of the hypotonicity-induced efflux of organic osmolytes (40, 43).
Despite intensive study over many years, the molecular identity of VRACs remains unknown (44). A reduction in intracellular ionic strength, rather than cell swelling, appears to be the parameter sensed by VRACs (45).
K+-Cl– Cotransporters—KCCs mediate electroneutral coupled exit of K+ and Cl– from swollen cells. Four isoforms (KCC1–4) have been cloned to date, all of which are activated by swelling (46). KCCs are regulated by cycles of serine/threonine phosphorylation and dephosphorylation. Unlike NKCCs, which are stimulated by phosphorylation following cell shrinkage, KCCs activate in response to swelling, and the activation is caused by dephosphorylation. This raises the tantalizing possibility that a common set of volume-sensitive kinases/phosphatases regulates both RVI and RVD.
Taurine Efflux—Taurine efflux, which is normally modest, increases exponentially when cells are swollen (43). The consequences are impressive: 30–90% of the intracellular taurine (39, 43) leaves the cell following swelling. The efflux pathway is distinct from the Na+-dependent taurine transporter described earlier and, in fact, behaves more like a channel than a transporter (39, 43). The molecular identity of the pathway is unknown, but VRAC was suggested to mediate also the efflux of organic osmolytes, including taurine (40, 43). As was the case for RVI, activation of taurine transport during RVD takes place after a distinct lag time, suggesting that release of organic osmolytes acts a second, more chronic line of defense against swelling.
Supplementary Material
This work was supported by the Canadian Cystic Fibrosis Foundation, the Heart and Stroke Foundation of Ontario, the Canadian Institutes of Health Research, and the National Science and Engineering Research Council. This minireview will be reprinted in the 2009 Minireview Compendium, which will be available in January, 2010.
Footnotes
The abbreviations used are: TRP, transient receptor potential; PIP2, phosphatidylinositol 4,5-bisphosphate; RVI, regulatory volume increase; RVD, regulatory volume decrease; NHE, Na+/H+ exchanger; NKCC, Na+-K+-2Cl– cotransporter; KCC, K+-Cl– cotransporter; HICC, hypertonicity-induced cation channel; VRAC, volume-regulated anion channel.
References
- 1.Burg, M. B. (2000) Cell Physiol. Biochem. 10 251–256 [DOI] [PubMed] [Google Scholar]
- 2.Parker, J. C., Dunham, P. B., and Minton, A. P. (1995) J. Gen. Physiol. 105 677–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russell, J. M. (2000) Physiol. Rev. 80 211–276 [DOI] [PubMed] [Google Scholar]
- 4.Lambert, I. H., Pedersen, S. F., and Poulsen, K. A. (2006) Acta Physiol. (Oxf.) 187 75–85 [DOI] [PubMed] [Google Scholar]
- 5.Lehtonen, J. Y., and Kinnunen, P. K. (1995) Biophys. J. 68 1888–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schliess, F., and Haussinger, D. (2007) Methods Enzymol. 428 129–144 [DOI] [PubMed] [Google Scholar]
- 7.Sheikh-Hamad, D., and Gustin, M. C. (2004) Am. J. Physiol. 287 F1102–F1110 [DOI] [PubMed] [Google Scholar]
- 8.Liedtke, C. M., and Cole, T. S. (2002) Biochim. Biophys. Acta 1589 77–88 [DOI] [PubMed] [Google Scholar]
- 9.Pedersen, S. F., and Nilius, B. (2007) Methods Enzymol. 428 183–207 [DOI] [PubMed] [Google Scholar]
- 10.Nasuhoglu, C., Feng, S., Mao, Y., Shammat, I., Yamamato, M., Earnest, S., Lemmon, M., and Hilgemann, D. W. (2002) Am. J. Physiol. 283 C223–C234 [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto, M., Chen, M. Z., Wang, Y. J., Sun, H. Q., Wei, Y., Martinez, M., and Yin, H. L. (2006) J. Biol. Chem. 281 32630–32638 [DOI] [PubMed] [Google Scholar]
- 12.Nielsen, D. K., Jensen, A. K., Harbak, H., Christensen, S. C., and Simonsen, L. O. (2007) J. Physiol. (Lond.) 582 1027–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aharonovitz, O., Zaun, H. C., Balla, T., York, J. D., Orlowski, J., and Grinstein, S. (2000) J. Cell Biol. 150 213–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rohacs, T. (2007) Pfluegers Arch. 453 753–762 [DOI] [PubMed] [Google Scholar]
- 15.Kudo, I., and Murakami, M. (2002) Prostaglandins Other Lipid Mediat. 68–69 3–58 [DOI] [PubMed] [Google Scholar]
- 16.Thoroed, S. M., Lauritzen, L., Lambert, I. H., Hansen, H. S., and Hoffmann, E. K. (1997) J. Membr. Biol. 160 47–58 [DOI] [PubMed] [Google Scholar]
- 17.Meves, H. (2008) Br. J. Pharmacol. 155 4–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friis, M. B., Vorum, K. G., and Lambert, I. H. (2008) Am. J. Physiol. 294 C1552–C1565 [DOI] [PubMed] [Google Scholar]
- 19.Varela, D., Simon, F., Riveros, A., Jorgensen, F., and Stutzin, A. (2004) J. Biol. Chem. 279 13301–13304 [DOI] [PubMed] [Google Scholar]
- 20.Okada, Y., Maeno, E., Shimizu, T., Dezaki, K., Wang, J., and Morishima, S. (2001) J. Physiol. (Lond.) 532 3–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosette, C., and Karin, M. (1996) Science 274 1194–1197 [DOI] [PubMed] [Google Scholar]
- 22.Burg, M. B., Ferraris, J. D., and Dmitrieva, N. I. (2007) Physiol. Rev. 87 1441–1474 [DOI] [PubMed] [Google Scholar]
- 23.Di Ciano, C., Nie, Z., Szaszi, K., Lewis, A., Uruno, T., Zhan, X., Rotstein, O. D., Mak, A., and Kapus, A. (2002) Am. J. Physiol. 283 C850–C865 [DOI] [PubMed] [Google Scholar]
- 24.Pedersen, S. F., Hoffmann, E. K., and Mills, J. W. (2001) Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 130 385–399 [DOI] [PubMed] [Google Scholar]
- 25.Lepple-Wienhues, A., Szabo, I., Laun, T., Kaba, N. K., Gulbins, E., and Lang, F. (1998) J. Cell Biol. 141 281–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delpire, E., and Gagnon, K. B. (2008) Biochem. J. 409 321–331 [DOI] [PubMed] [Google Scholar]
- 27.Kahle, K. T., Ring, A. M., and Lifton, R. P. (2008) Annu. Rev. Physiol. 70 329–355 [DOI] [PubMed] [Google Scholar]
- 28.Di Ciano-Oliveira, C., Sirokmany, G., Szaszi, K., Arthur, W. T., Masszi, A., Peterson, M., Rotstein, O. D., and Kapus, A. (2003) Am. J. Physiol. 285 C555–C566 [DOI] [PubMed] [Google Scholar]
- 29.Di Ciano-Oliveira, C., Thirone, A. C., Szaszi, K., and Kapus, A. (2006) Acta Physiol. (Oxf.) 187 257–272 [DOI] [PubMed] [Google Scholar]
- 30.Gagnon, K. B., England, R., and Delpire, E. (2007) Cell Physiol. Biochem. 20 131–142 [DOI] [PubMed] [Google Scholar]
- 31.Tamma, G., Procino, G., Strafino, A., Bononi, E., Meyer, G., Paulmichl, M., Formoso, V., Svelto, M., and Valenti, G. (2007) Endocrinology 148 1118–1130 [DOI] [PubMed] [Google Scholar]
- 32.Tilly, B. C., Edixhoven, M. J., Tertoolen, L. G., Morii, N., Saitoh, Y., Narumiya, S., and de Jonge, H. R. (1996) Mol. Biol. Cell 7 1419–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alexander, R. T., and Grinstein, S. (2006) Acta Physiol. (Oxf.) 187 159–167 [DOI] [PubMed] [Google Scholar]
- 34.Grinstein, S., Woodside, M., Sardet, C., Pouyssegur, J., and Rotin, D. (1992) J. Biol. Chem. 267 23823–23828 [PubMed] [Google Scholar]
- 35.Grinstein, S., Rothstein, A., and Cohen, S. (1985) J. Gen. Physiol. 85 765–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dunham, P. B., Kelley, S. J., and Logue, P. J. (2004) Am. J. Physiol. 287 C336–C344 [DOI] [PubMed] [Google Scholar]
- 37.Fuster, D., Moe, O. W., and Hilgemann, D. W. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 10482–10487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haas, M., and Forbush, B., 3rd (2000) Annu. Rev. Physiol. 62 515–534 [DOI] [PubMed] [Google Scholar]
- 39.Wehner, F., Olsen, H., Tinel, H., Kinne-Saffran, E., and Kinne, R. K. (2003) Rev. Physiol. Biochem. Pharmacol. 148 1–80 [DOI] [PubMed] [Google Scholar]
- 40.Stutzin, A., and Hoffmann, E. K. (2006) Acta Physiol. (Oxf.) 187 27–42 [DOI] [PubMed] [Google Scholar]
- 41.Wehner, F., Bondarava, M., ter Veld, F., Endl, E., Nurnberger, H. R., and Li, T. (2006) Acta Physiol. (Oxf.) 187 21–25 [DOI] [PubMed] [Google Scholar]
- 42.Liedtke, W. (2007) Exp. Physiol. 92 507–512 [DOI] [PubMed] [Google Scholar]
- 43.Lambert, I. H. (2004) Neurochem. Res. 29 27–63 [DOI] [PubMed] [Google Scholar]
- 44.Nilius, B., and Droogmans, G. (2003) Acta Physiol. Scand. 177 119–147 [DOI] [PubMed] [Google Scholar]
- 45.Nilius, B., Prenen, J., Voets, T., Eggermont, J., and Droogmans, G. (1998) J. Physiol. (Lond.) 506 353–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adragna, N. C., Di Fulvio, M., and Lauf, P. K. (2004) J. Membr. Biol. 201 109–137 [DOI] [PubMed] [Google Scholar]
- 47.Pedersen, S. F., Owsianik, G., and Nilius, B. (2005) Cell Calcium 38 233–252 [DOI] [PubMed] [Google Scholar]
- 48.Lesage, F., and Lazdunski, M. (2000) Am. J. Physiol. 279 F793–F801 [DOI] [PubMed] [Google Scholar]
- 49.Gasull, X., Ferrer, E., Llobet, A., Castellano, A., Nicolas, J. M., Pales, J., and Gual, A. (2003) Investig. Ophthalmol. Vis. Sci. 44 706–714 [DOI] [PubMed] [Google Scholar]
- 50.Grunnet, M., Jespersen, T., MacAulay, N., Jorgensen, N. K., Schmitt, N., Pongs, O., Olesen, S. P., and Klaerke, D. A. (2003) J. Physiol. (Lond.) 549 419–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.