Abstract
Fields studying movement generation, including robotics, psychology, cognitive science, and neuroscience, utilize concepts and tools related to the pervasiveness of variability in biological systems. The concepts of variability and complexity and the nonlinear tools used to measure these concepts open new vistas for physical therapist practice and research in movement dysfunction of all types. Because mounting evidence supports the necessity of variability for health and functional movement, this perspective article argues for changes in the way therapists view variability, both in theory and in action. By providing clinical examples, as well as applying existing knowledge about complex systems, the aim of this article is to create a springboard for new directions in physical therapist research and practice.
Variability in human performance and the nonlinear manner in which skills and characteristics of movement change over time reflect the complexity of the movement system. As Bernstein1 described, multiple degrees of freedom of the body, including joints, muscles, and the nervous system, combine with external forces during movement to produce countless patterns, forms, and strategies. The redundancy of the system allows for the use of multiple strategies to accomplish any given task. Logically, there are multiple performance variants for each movement, depending on the constraints of each individual's system. How do therapists utilize these redundancies in practice? Although many movement science and neuroscience students are now well versed in the importance of variability, this information has not been integrated into many physical therapy interventions and is far from being embedded in general practice.2
Think about the last time you encouraged a patient to acquire a new or more-efficient movement. How did you go about it? Did you demonstrate the movement and ask the patient to copy your movement pattern? Did you ask the patient to experiment with various movement strategies to find success? Perhaps you asked the patient to think about the movement, the goal, a similar task, or a mental image of performance. Was some feature of the movement a focus for measurement to determine progress or change? Regardless of the practice setting, we ask patients to move, and we generally ask them to move differently than they moved before they came to us. Although our goals often do not explicitly target variability in movement, our implied expectation is that the functional movement that emerges will be adaptive and flexible enough to meet the everyday goals of our patients. To achieve this flexibility, our patients need adequate variability of the motor system. It follows that adequate variability should be a focal point of examination and intervention in order to achieve optimal function for the individual.
Clinicians trained in medical fields use linear models for prediction and problem solving.3 However, it is becoming increasingly clear that linear models are limited in many cases and are certainly not the optimal model for function. Professionals in several areas of health care, including epidemiology, infectious disease processes, and biomedicine, are turning to nonlinear models for solutions to difficult problems.4 For example, drug dosages are nonlinear; one cannot increase the dose for more effect because there is generally a threshold value at which the desired effect occurs and beyond which negative effects occur.5 In our field, therapists know that a given amount of practice cannot ensure the learning of a skill in a linear manner. Our patients usually learn sporadically, progressing in a nonlinear manner over time. In addition, rates and paths of progress vary among individuals with the same diagnoses who are similar in characteristics. Motor learning progresses nonlinearly, exhibiting nonlinear learning curves depending on the task, conditions, and characteristics of the learner.6 Linear can be defined as pertaining to a straight line, or consisting of only one dimension. Nonlinear, usually used with the term “dynamics,” as in “nonlinear dynamics,” can be defined as “not in a straight line,” or as a system whose output is not proportional to its input.* Nonlinear systems are more complex than linear systems, necessitating the use of sets of equations producing unpredictable outcomes that exhibit chaotic features. In general, biological systems, including humans, are complex, nonlinear systems with inherent variability in all healthy organisms.7
In this perspective article, we will provide an overview of the constructs of variability, complexity in human movement, and nonlinear analysis. This overview focuses on clinical relevance; therefore, the definitions and explanations herein are more conceptual than mathematical. We first describe variability of movement and its relationship to complexity and the changing view of the importance of variability for successful function. Next, we review nonlinear measures and the concept of complexity in movement. Finally, we propose principles and examples of the use of variability, complexity, and nonlinearity in examination, intervention, and research components of physical therapist practice.
The Importance of Variability and the Concepts of Nonlinearity
Variability: For Better or Worse?
Before discussing the changing perspective on variability as it pertains to clinical application, consistent interpretation requires definitions of terminology. First, the definition of variability occurs in behavioral, biological, and statistical forms. Behavioral variability describes differences in observed behavior when an entity is placed in the exact same situation.8 Webster's Dictionary defines variability in a biological sense as “the power possessed by living organisms, both animal and vegetable, of adapting themselves to modifications or changes in their environment, thus possibly giving rise to ultimate variation of structure or function.”9 Statistical variability refers to measures of centrality around a mean or an average and includes measures such the standard deviation, the range of possible values, and the variance. All of these definitions contribute to our understanding of human variability.
Human movement variability encompasses the normal variations that occur in motor performance across multiple repetitions of a task over time.10 Variability is inherent within all biological systems and reflects variation in both space and time, which can be illustrated easily in human movement. As a person walks through sand or snow, his or her footprints never repeat exactly, reflecting variability from step to step in a continuous cycle of movement. During quiet standing, we sway around a central equilibrium point without ever remaining exactly still as we maintain orientation to the world. Are these examples of variability in movement considered errors in motor performance, or are they normal output of a healthy motor system?
Traditionally, random error or noise within the system was deemed responsible for the variability measured during repetitions of a skill.11 Motor learning textbooks usually describe movement variability as error and skilled movement as movement with decreased variability.12 Generalized motor program theory (GMPT) considers variation in a given movement pattern to be the result of errors in the ability to predict the necessary parameters for using the underlying motor program.13 However, there is mounting evidence of the importance of variability in normal movement, which reveals variation not as error but as a necessary condition for function. Variability reflects multiple options for movement, providing for flexible, adaptive strategies that are not reliant on rigid programs for each task or for each changing condition encountered. Optimal variability as a central feature of normal movement is consistent with a nonlinear approach.
Counter to a therapeutic assumption that equilibrium is an indicator of health, nonlinear theories emphasize disequilibrium as healthy. This means that the system never quite settles into a stable state, and constant fluctuations characterize the healthy variability that allows adaptation to environmental change.4 A complex dynamic system is in slight disequilibrium with the environment and maintains this disequilibrium over time.14 Goldberger15 described complete equilibrium as equivalent to the death of the organism because it implies a static, nondynamic state. Therefore, health is indicated by a dynamic equilibrium that is not a static state.
Rather than being a negative feature, variability reflects important information for the maintenance of the health of the system. Reduced variability is known to cause repetitive stress injury in a mechanical sense. Although outwardly this appears to be a mechanical problem, the underlying story describes an information problem. The lack of variability in movement leads to abnormal mapping of the sensory cortex, which subsequently disturbs motor function. These neural maps (both sensory and motor) are more complex when movement variability is present and less complex when variability is reduced.16–18 Movements with an optimal amount of variability avoid this abnormal mapping and essentially contribute to the neuroplasticity needed for maintaining or achieving functional skill. Thus, variability of the movements used for the task contributes information to the nervous system, which then serves to prevent injury.
Too much variability also can be a problem, such as in an individual with an ataxic movement disorder. Movements that usually fall within a specific range of variability to accomplish a task such as gait unexpectedly fall both in and outside the acceptable range in such an individual. When one movement falls outside the expected range, the next movement is perturbed. Gait is an example of a continuous, cyclic task, within which the steps cannot be random but also are not completely repeatable or robotic. Thus, we propose that optimal movement variability lies between too much variability and complete repeatability.10 Scientists define this optimal range of variability using mathematical chaos. Furthermore, techniques from mathematical chaos describe important features of variability.19,20
How Nonlinear Tools Advance Understanding of Variability
We have established that variability is not a negative feature of functional movement or of biological systems in general. However, exactly when is variability good? Moreover, how do we determine whether the variability we see is good variability or bad variability? The answers to these questions lie in understanding dynamic models of movement.
The conversation about variability began with systems theory, a departure from linear models. In 1990 at the II STEP conference, dynamic systems theory (DST) was promoted as a model for the progression of movement skills and as a way to model change in movement skills for physical therapists.21,22 Dynamic systems theory introduced the notions of stability and nonlinearity to explain variability. Based on environmental, biomechanical, and morphological constraints, any biological system will self-organize to find the most stable solution. An increased amount of variability indicates less cooperative behavior among the components of the system, which eventually can drive the system to new attractor states or behaviorally stable solutions. This is a nonlinear system because the input does not lead to a linear change in output. Input changes the variability of the system, which may drive the emergence of a new behavior. Importantly, the measure of the variability provides a means to classify the stability of the system. Thus, in DST, small amounts of variability will indicate a highly stable behavior.
The GMPT and DST perspectives are similar in that both recognize that decreased variability results from the efficient execution of a given movement pattern. Dynamic systems theory differs, however, because it proposes that at a specific critical point, variability increases and dominates the system as new movement emerges. The system becomes highly unstable and switches to a new, stable behavioral state. An example of this process occurs when an individual learns to ride a bicycle. If a child has been using training wheels, pedaling and controlling the direction of the bicycle are stable behaviors. However, when the training wheels are removed, the system is perturbed. Pedaling and directional control are very erratic and unstable as the individual learns the interaction of balance with the speed of the bicycle and controlling movement through space. At the point where the individual understands how the parameters of speed, balance, and directional control interact, the behavioral state (independent bicycle riding) becomes stable. The system must cross a critical point, where the speed of the bicycle makes balance easier for the behavior to become less variable in its new state.
In DST, increased variability in the system reveals growing instability, which may lead to a shift to a new attractor, or a new behavior. Conversely, a lack of variability traps a behavior in a specific state or attractor. Thus, DST advances our understanding of transitions between behavioral states, with variability considered not as error but rather as a source of behavioral change. Through DST, the importance of variability attracted the attention of developmentalists, who recognize DST as a theoretical starting point to study the emergence or self-organization of developmental actions, perceptions, and cognitive skills.23 However, the variability within an existing state (as opposed to a developing state), behavior, or established movement function has not been appreciated as important to skillful movement in adults or in describing pathological conditions. The use of linear measures, such as the standard deviation, limits our understanding of variability as a window to view the nature of adaptation in functional skills. Consequently, the ideas from DST of “stable state” and “attractor” are not intrinsically accepted as part of our therapeutic world. This is partially because we have lacked the tools to see the “hidden” information in the variability of movement progressing over time. Variability and its underlying characteristics are not completely described or quantified in either GMPT or DST, even though variability has an important role in both theories.
Over time, it was recognized that neither GMPT nor DST accounts for the observation that some behaviors, which appear to be stable, paradoxically occur in quite variable ways. This is especially evident when we observe elite sports players or musicians performing skillfully. Even though they perform the same skill as others, they seem to have developed an infinite number of ways to perform it. Thus, it seems that a very stable behavioral state is supported by a very “rich” behavioral repertoire. If we consider fundamental motor skills (ie, gait, posture) and not the skills of an elite athlete, we are all skillful in our ability to walk through crowds or on diverse and challenging terrains. Therefore, it seems that variability does not decrease when we develop and refine a stable behavioral state but actually increases. The structure of variability (as opposed to amount) can be described using nonlinear tools. These nonlinear tools best capture variation in how a motor behavior emerges in time, for which the temporal organization in the distribution of values is of interest. Temporal organization, or “structure,” is quantified by the degree to which values emerge in an orderly (ie, predictable) manner, often across a range of time scales. Nonlinear tools quantify the nature or the structure of variability and provide the missing ability to quantify the concept of “stability” from DST.
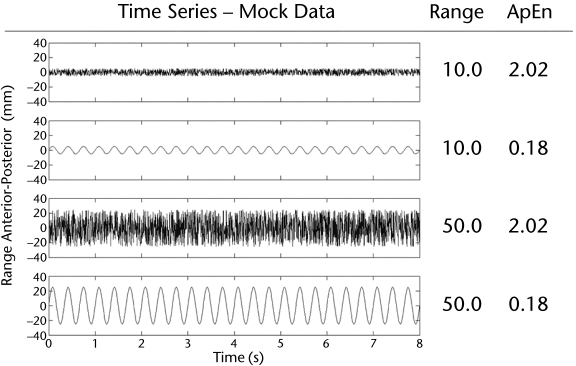
Figure 1 illustrates these concepts of variability. The figure pictures 4 different time series, with the linear measure of range and the nonlinear measure of approximate entropy (ApEn) listed beside each signal. The first and third rows show signals that look messy, seeming to be random, with one signal larger in amplitude than the other. Beside these rows, the range values reflect the varying amount of the signal between the 2 traces, with the larger number by the larger signal; however, the ApEn values are equivalent for these 2 signals. This reflects the fact that only the amplitude varies and not the structure of the time series. The second and fourth rows depict time series that are very regular, a sine wave. Again, comparison of the range shows they are different in amplitude but the same in structure, as reflected in the ApEn value. However, comparison of the first signal with the second signal (and the third signal with the fourth signal) shows that the amplitude, quantified by the range, is the same (and the standard deviation, a linear measure of variability, also would be the same) but that the structure of the series, described by the nonlinear ApEn value, is different. Therefore, the amount of variability measured by the standard deviation (linear) and the structure of variability measured by ApEn (nonlinear) are not at all the same. In fact, as we will discuss later, they often are inversely related. Moreover, these different facets of variability can reveal information that may lead to different clinical decisions, which is illustrated in a clinical example later.
Figure 1.
Comparison of linear and nonlinear measures of several signals. Four signals are displayed, with the respective values for range and approximate entropy (ApEn).
Nonlinear Measurement and Description
How Does Variability Relate to Complexity in Functional Movement?
In terms of physical therapy, variability describes the behavioral repertoire possible for a given function. We will use the example of controlling balance in a new task. If you have never walked on a tightrope, imagine your first attempt. You would likely have wide-ranging excursions of your center of pressure (COP) at the support surface and wide movements of your body segments as you try to balance. This reveals large variability according to many measures, including kinematics, COP movement, and center-of-mass movement. The performer tries many different strategies that may include stiffening or loosening various body segments in an attempt to balance on the tightrope. The speed of the performer's reactions also may be varied. However, these early attempts to accomplish the task of balancing on the tightrope would not be complex, even though they were highly variable. Complexity would arise from fine-tuned adjustments, with selected and well-practiced yet flexible strategies for balance. These strategies utilize specific information to make the optimal response, which is characteristic of a skilled and practiced tightrope walker.
The overall task is difficult to break into parts and analyze because the different components are interdependent, and there must be online adjustments calibrated to the rest of the system. The overall system is complex because the analysis of the system or function is inaccurate if examined part by part. Although describing a range of movement options quantifies variability, complexity is more difficult to measure. This is because measuring each part of the movement separately will not give us an overall measure of the complexity required for success in the function. Complexity is something that is “hidden” within the time series of a movement sequence or strategy as it emerges over time. Movements that occur at one moment affect and are affected by movements that occur either before or after the movement in the moment. Tools for measuring complexity come from nonlinear dynamics, and mathematical models incorporate time to describe this complexity.
Linear Measures: Traditional Measures of Dispersion
Linear tools to measure variability provide information about the quantity of a signal, they but do not tell us about the time-evolving nature of the signal. Linear tools include the statistics of range, standard deviation, and coefficient of variation and are limited in their explanation of human movement variability for several reasons. One reason is that data from several averaged trials generate a “mean” picture of an individual's movement pattern. The mean removes the temporal variations of the movement and masks the true structure of variability present in the movement pattern. In addition, the valid use of linear tools to study variability assumes that variations between repetitions of a task are random and independent (of past and future repetitions), which has been shown to be false.24–26 Finally, linear tools provide different answers when compared with nonlinear tools regarding the way that they evaluate variability. For example, traditional linear measures of postural sway quantify only the magnitude of sway and not the temporally evolving dynamics (or disequilibrium) of postural control. Despite their use in many studies, it is becoming evident that linear measures, such as the range and standard deviation of the COP, do not quantify stability of the postural control system because it is possible to have a large area of the COP path while having a stable posture or an unstable posture.27,28 Therefore, linear measures of variability do not accurately define constructs important in movement, such as stability, because they only provide insight into the amount of variability (Fig. 1).
Nonlinear tools give us additional information about the structure of variability, which describes the evolution of the movement over time. This is possibly the reason why previous theoretical accounts of variability in human movement (ie, GMPT, DST) supported the notion that small amounts of variability characterize a very stable behavioral state and that improved stability links directly to decreasing variability. If we measure improved performance linearly, this conclusion is very reasonable. However, nonlinear measures provide additional information and allow understanding of complexity.
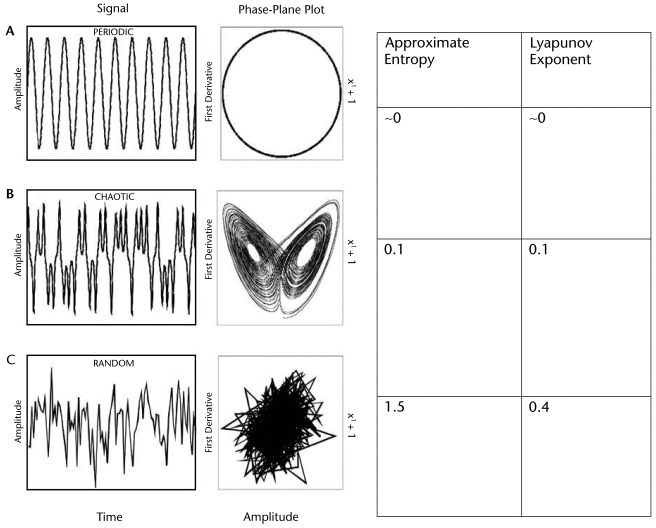
In Figure 2, it is evident that the time series signals on the left are different from each other; the first is very regular, the second seems to have some type of pattern that is difficult to describe verbally, and the third seems to have no pattern. However, when these signals are plotted versus their velocities (phase plane), it becomes clear that the first signal is completely regular, with no variation from the first cycle to the last cycle. The second signal forms a complex, yet organized, pattern, with similar paths for each cycle, but not repeating the same path and with each path dependent on a previous path and influencing the next path. The third is a random signal, where the paths are not similar and not dependent on each other. We propose that functional and healthy movement resembles chaos, the complex center picture. Nonlinear tools can determine whether a chaotic structure, or complexity, is present in movement.
Figure 2.
Time series and corresponding phase-plane plot. (A) periodic function; (B) time series from a chaotic system, the Lorenz attractor; (C) random time series.
Why Is Variability Inherent in Biological Systems?
Variability is inherent in biological systems because it ensures survival. Gerald Edelman, a Nobel laureate, described this pervasive rule as “population thinking,” and he used it to describe the complexities of the immune system and then expanded the principles to neuroscience and the way the mind works.29 The basic idea is that variability allows an organism or group of organisms to be more successful. This pertains to antibodies and viral agents, animal species, the nervous system, and the evolutionary progress of plants and animals. Variability allows choices among options, selection of strategies, and flexibility to adapt to variations in the environment. If an animal rigidly performs limited behaviors or functions only within a restricted environment, it is challenged for survival by a more-adaptive animal. This Darwinian principle describing the advantage of variability lies within many levels of organisms and is explicitly described for the growth of genetic complexity in an information-rich environment.30 Variability exists at many levels and within interacting components of a system, often operating at different time scales. Thus, variability may not be obvious at one level but can be revealed at another level. The variability inherent in biological systems from genes to behavior cannot be considered error if it is pervasive from one species to another and is linked to survival.
Why Isn't Movement Variability Just Error?
If movement variability is equivalent to error, we can reasonably assume that more-skillful individuals would have less error (or variability) at the outset of learning and then quickly drop to zero error. In fact, the opposite is true. Individuals who use a high degree of variability in cognitive strategies at the beginning of task development have greater learning and eventual success in performing the task.31 Movement researchers have started to understand the importance of variability in motor skill learning and examine performance differently. For example, a study of coordination variability in jumpers revealed a U-shaped curve in the progression of variability.32 Initial high variability occurs as different strategies are attempted. Subsequently, the learner moves toward a reduction in variability as he or she performs more successfully. Then, surprisingly, as the learner becomes an expert, the variability increases again. This skillful variability indicates increasing flexibility of skill to allow adaptation to perturbations.
Thus, the variability at the beginning of task learning may seem like error because the task is not performed efficiently or accurately. However, this initial variability also can be seen as necessary to map the possibilities of movement for the task. It then is refined into a different type of variability when the performer is skillful. Although variability typically is known to decrease as a skill is acquired, think about how our notions of the mechanisms of skill acquisition change if we consider the role of variability. If therapists consider variability to be error, it is seen as an impairment. However, if therapists consider variability necessary for skill acquisition, they will examine the structure of variability to help build skill. Nonlinear measures can unmask the hidden structure inherent in variability so that intervention can successfully address different features of variability as necessary during skill development. Although clinicians can easily understand the behavioral variability we have been discussing, such as the number of strategies for accomplishing a functional task, it is more difficult to understand what underlies that variability.
Motor skills researchers increasingly find nonlinear tools useful in revealing information through time series analysis. The ApEn measure revealed significant differences between athletes who had a concussion and controls who were healthy by analysis of the COP time series, even after other standard linear measures indicated that the athletes had fully recovered from the concussion.33,34 Moraiti et al35 used the Lyapunov exponent (LyE) measure to show that a group of patients with anterior cruciate ligament deficiencies exhibited significantly more rigid and predictable walking patterns than controls who were healthy, suggesting a decrease in system complexity and narrowed functional responsiveness. Kurz and Stergiou36 used an entropy measure to show that neurophysiological changes associated with aging may result in less certainty of the neuromuscular system in selecting a stable gait. Therefore, utilizing a nonlinear perspective to examine variability can assist in differentiating between health and nonhealth.
Nonlinear Measures: New Ways to Describe the Nature of Variability
We have established that the amount and structure of variability are 2 different things (Fig. 1). As a result, changes in measures of the amount of variability may be in a completely different direction than changes in measures that evaluate the structure of variability. Similarly, in studies of postural control, gait, and force production, researchers found that as measures of the amount of variability increased, measures of the structure or organization of variability decreased.37–39 Let us reflect on an example of postural sway in standing. As a person's range of sway increases, the standard deviation increases, indicating a greater amount of variability. However, if we use a nonlinear tool to examine the features of the variability in postural sway, we may note that sway has become more regular (with more-repeatable movement patterns). This makes sense, because the individual must have some specific strategy of control to make the appropriate adjustments for balance maintenance when the range of movement is large; otherwise, he or she would fall. The relationship between linear and nonlinear tools as described above can further our understanding of the emergence of functional, adaptive movement.
Nonlinear measures always describe a time series, or a series of measurements taken at specific intervals over uninterrupted time. For example, the range of motion of a joint during each step as a person walks, taken with an electrogoniometer or motion analysis equipment sampled at 30 times per second over a 2-minute period of time, can be presented as a time series. Looking at a measure in the context of time because it enables us to understand the ability of the system to adapt as conditions change. The period of time may vary from seconds to days, but the important concept is that a behavior emerging from the complex system can be described over time with specific mathematical nonlinear tools that are used to quantify order, predictability, regularity, and complexity. A time series also is valuable because information that is important to understand the health or function of the individual may be revealed at different time scales.
Characterizing the nature of the complexity present in a time series is of great interest in many scientific domains, including biology. Healthy systems, whether referring to heart rate or the COP time series, correspond to a rich behavioral state with high complexity.10 This state is defined as highly variable fluctuations in physiological processes resembling mathematical chaos. This allows the system to have a relatively predictable course, which can adapt if a change in the environment occurs. Low levels of complexity correspond to states that resemble random, noisy, and erratic behavior or rigid, periodic, and regular behavior. Therefore, with low complexity, adaptability suffers. Nonlinear measures allow us to extract information hidden in a time series and to evaluate complexity. Some examples of possible tools follow.
Approximate entropy is a measure that can quantify the regularity or predictability of a time series (Fig. 1).40–44 Increasing ApEn values reveal greater irregularity. Conversely, lower values reveal a more regular or periodic behavior. Approximate entropy has been useful in the identification of differences between young and old people in the COP time series in standing,45 in revealing deficits in athletes with a concussion compared with athletes who were healthy,33,34 and in detecting developmental changes in sitting postural control.25 Approximate entropy measures the probability that the configuration of one segment of data in a time series will allow the prediction of the configuration of another segment of the time series a certain distance apart.41 Data segments with a greater likelihood of having the same configuration or pattern upon comparison will result in lower approximate entropy values, and data segments with a low likelihood of similarity between segments will result in higher values. Values closer to zero are consistent with maximum regularity, and values nearing 2 represent maximum irregularity.
Clinically, ApEn is useful for understanding the predictability of a movement. For example, Cavanaugh et al46 utilized ApEn to determine the predictability of everyday walking activities, using time series data from a pedometer. They found that inactive elderly individuals walked less and had more-predictable walking activity than active elderly individuals. This finding allows a more complete picture of the differences in walking between inactive and active individuals and allows the clinician to understand why some elderly people may have a more-difficult time responding to fluctuations in routine or adapting to different walking demands. This insight suggests intervention that can elicit greater complexity in the activity, rather than just simply increasing the amount of activity.
The largest LyE is a nonlinear measure that can measure the divergence of the movement trajectories (Fig. 2). The LyE describing purely sinusoidal and completely repeatable data with no divergence in the trajectories is zero because the trajectories overlap rather than diverging (Fig. 2A). This shows minimal change in the structure of the variability over time in the data. The LyE for random, noisy data indicates greater divergence in the data trajectories (Fig. 2C). The LyE values for the random data are larger, with values above 0.4.19 The LyE values from data that are described by mathematical chaos (ie, Lorenz attractor; Fig. 2B) are between these 2 extremes. Thus, the values between random and periodic define complexity, or highly variable fluctuations in physiological processes resembling mathematical chaos. The LyE has been used with gait time series data to characterize the underlying complexity during movement.19,47–49
Using the LyE, Yamada50 reported body sway that resembles mathematical chaos from COP data during standing in adults who were healthy, thus revealing inherent complexity. Use of the LyE in an ongoing intervention study with infants with cerebral palsy was important as a fine-grained measure to detect advancing postural control in sitting, when linear measures of the COP and clinical tools did not detect change.51 A standardized test, the sitting section of the Gross Motor Function Measure,52 did not consistently detect change. However, a variety of features of the child's movement (more attempts to stay vertical, the ability to turn the head without falling, more attempts to reach while sitting with support) were noted as changes in behavior by the parents and therapist. These small changes in movement, or attempts at new strategies, were quantified by the nonlinear measures of ApEn and LyE, but were not indicated in the linear measures. This is an example of a nonlinear tool providing “hidden” information that would not be easily measured or documented otherwise.
Surrogation is a technique used to determine whether the source of a given time series is deterministic (has order) in nature.19,53 The technique compares the actual data and a random data set that has a similar structure with the original data set in question. That is, the deterministic structure from the original data set is removed by generating a random equivalent with the same mean, variance, and power spectra as the original data. Subsequently, the LyE (or another nonlinear measure) value of the surrogate data is compared with the LyE value of the original data. Significant differences between the LyE value of the surrogate data and the LyE value of the original data indicate that the original data are not randomly derived and, therefore, may be deterministic and possibly complex in nature. Harbourne and Stergiou25 and Boker et al26 have used this technique to show that variability in the COP time series from infants during the development of independent sitting is not just noise but has a deterministic origin. This means that infants learning to sit are not just randomly “wiggling.” For the clinician, it is important to recognize that within these outwardly unorganized and noisy-looking movements are orderly patterns and the beginnings of strategies for postural control. The implication for therapists is that a movement that is just beginning to emerge will be unorganized, but necessarily so. The variability inherent in this disorganization may be necessary for ultimate successful selection of movement and postural strategies. This may reflect the system “mapping” the territory around the skill region, allowing the individual to “get back” to the successful region when perturbed.
Nonlinear analysis includes several additional tools such as detrended fluctuation analysis,54 correlation dimension, mutual information, Hurst exponent, symbolic entropy, recurrence quantification analysis, and others. These methods have a common goal: to evaluate the structure or organization of variability and uncover the underlying complexity. However, they differ in the mathematic manipulation of the available time series. Here we do not provide a complete list of all nonlinear tools but describe only a few tools to provide the basic concepts of nonlinear analysis. A more comprehensive review on the topic is available for the interested reader.19
Application of Nonlinear Concepts in Practice
Complexity in Health
Goldberger55 described the use of complexity at the bedside for physicians by providing examples of periodic behavior of pathologic systems. Disease brings about a loss of complexity, with resulting increased rigidity, such as Cheyne-Stokes breathing in patients with heart failure, tremors in patients with neurologic disease, and a sinusoidal appearance of heart rate variability in patients with congestive heart failure.56,57 The medical field is beginning to recognize the need for a nonlinear view toward complexity, particularly for problems that affect multiple systems. Ahn et al58 described a traditional reductionist approach as the antithesis of a complexity-oriented approach, with the reductionist approach being appropriate for use with acute, single-system problems, such as an acute infection. However, a disease such as diabetes requires management of a problem affecting many systems that interact in various ways. Extending the nonlinear view, it could be argued that very few problems are truly single-system problems and solvable by linear reasoning because each system interacts with other systems for optimal function. As in medicine, many clinical problems seen in physical therapy need a nonlinear approach.
Clinical uses for nonlinear analysis appear in a variety of disciplines, including cardiology, neurology, and psychiatry. Heart rate analysis using ApEn has been used to evaluate risk factors for sudden infant death syndrome.41,42 Nonlinear analysis has been useful in verification of implantable cardiac defibrillator interventions by using entropy analysis of heart rate variability.59 In addition, postural control analysis using stabilometry has been improved by the addition of nonlinear analysis, which can serve to more accurately identify features of postural control indicating subtle problems in infants,51 developmental differences between young and elderly people,43 or changes that accompany a disease state such as parkinsonism.60,61 Gait variability also has been studied and modeled using nonlinear tools.62 Applications in the clinic for physical therapy intervention are now a realistic possibility.
Clinical Application of Nonlinear Principles and Use of Complexity in Physical Therapy
Appendix 2 lists some proposed principles for physical therapy intervention emerging from the theory and applications in other fields. An example of clinical application in intervention follows.
Two clinicians must perform an initial evaluation on an elderly man who has had a stroke. One therapist will use a traditional, linear approach, and the other therapist will use an approach based on principles of nonlinearity. As a general rule, the therapist using a linear approach assumes that decreasing the variability of movement is equivalent to improving functional skill. Therefore, the therapist has in mind the “correct” movement pattern for various functional skills, which she will guide her patient toward during intervention. Using principles of motor learning, the therapist first gives 100% feedback, and then fades subsequently to 50% feedback for various skills.63 Because the therapist wants measurable outcomes, she uses a standard walking course that has 7.6-m (25-ft) increments up to 61 m (200 ft).
To determine decreasing variability and increasing accuracy, the therapist counts errors in the set of procedures to sit-stand-walk over a standardized course. This principle of fading feedback applies as she guides the patient in transferring out of the wheelchair. She first locks the brakes of the patient's wheelchair and tells the patient to scoot to the edge of the chair. The patient receives assistance to lean forward and place his feet under his center of mass, both with physical guidance and with verbal guidance. As the patient starts to reach for the walker, the therapist tells the patient to push up from the arms of the chair. The clinician then has the patient practice the sit-to-stand activity 5 times, giving feedback as described each time. The next day, the patient makes the same errors, and the therapist provides less cueing, hoping to fade cueing over the next 2 weeks. In addition to counting errors during the sit-stand-walk practice, the therapist notices that the patient's steps are of unequal length and the affected side shuffles forward rather than exhibiting a heel-toe pattern of stepping. The therapist uses the same approach of using verbal and physical guidance to point out the errors in the patient's gait pattern, fading the feedback over time and counting errors within the measured distance of the walking track.
In contrast, the therapist using a nonlinear approach assumes that the general rule for this patient is to enhance complexity of movement in order to improve gait and functional mobility skills. This will include the concept of disequilibrium, or keeping the patient in a state of dynamic equilibrium (as described earlier) during therapy sessions. Additionally, the therapist uses the strategy of providing only information for the patient on how to do a task if the patient does not have a way to get the information; the rationale is that variability is encouraged if the patient seeks information independently, and the patient is kept in a dynamic state.
The therapist first asks the patient whether he would like to go sit in a chair 3.05 m (10 ft) away next to his wife. He agrees, and the therapist invites the patient to begin the task, assuring the patient that the task is safe while the therapist is present. The patient pushes back in the unlocked wheelchair, and the wheelchair rolls back, putting the patient further away from the targeted goal. The therapist notes that this is a point where the patient is not gathering enough information for the task and addresses this problem by having the patient do some guided exploration within the task to increase flexibility in terms of availability of options. The therapist tells the patient that he can roll the wheelchair in many different directions just using his feet and challenges him to find 10 different directions of wheelchair movement by pushing with his feet. During this exercise, the patient maps the way that foot force affects the wheelchair movement. The therapist asks whether there is a way to keep the wheelchair from rolling, and the patient remembers the brakes. The patient then makes multiple errors in his attempts to stand, including incorrect foot placement, reaching for the walker instead of the wheelchair arms, and leaning back and to one side instead of leaning forward to get up. However, the therapist does not provide guidance at this point because the patient is not making the same error but rather is exhibiting a variety of strategies, which at this stage of skill development is desirable. At several points, the therapist asks whether the strategy just used was successful, and when answered in the negative, reminds the patient to try some different strategies, just as they did by pushing the wheelchair in different directions with the feet. Occasionally, the therapist gives light touch cues to suggest an effective strategy.
At the end of the trial-and-error session, the patient stands and walks over to his wife to sit in the chair. After resting and conversing, the therapist asks the patient to walk back to the wheelchair, without giving any verbal instructions. The patient makes a few errors, self-corrects, and visibly thinks through the process of coming to a standing position but is markedly faster than on the first try. The next day the patient makes only one error, moves from a sitting position to a standing position with guarding, and elects to sit on a couch for some social contact with another patient. The therapist notes that during walking the patient had short steps and shuffled with the affected leg. The therapist sees this as a possible problem. The therapist then uses barefoot tasks, having the patient identify different textures or objects under the feet, place pressure on different parts of his feet during walking and standing, and walk with a variety of patterns through different paths and obstacle courses. The therapist is more concerned about increasing the adaptive capacity of walking by increasing variability at a functional speed than in producing a consistent heel-toe pattern.
What differs in these approaches? The basic difference is that the therapist using the linear measure seeks to reduce variability of responses within the intervention and the measurement of the task goals and come to a state of complete equilibrium, whereas the therapist using the nonlinear measure seeks to enhance complexity by encouraging slight disequilibrium, particularly at the initial stage of task learning. The therapist using the nonlinear measure builds complexity into the task, with the use of multiple systems: cognitive, social, motor, and sensory. The practice space is strategically varied, and multiple movement approaches are encouraged, as well as having an expanded environmental context in which the practice takes place. In addition, the functional task proceeds in such a way that the series of movements within the task are related to each other and dependent on each other. In the linear measure approach, each subtask is a task unto itself and separated from the other parts of the task due to the interjection of the therapist's instructions. Therefore, the series of movements and postural adjustments in the task of standing from the wheelchair are unrelated to each other and unrelated to the overriding environmental context and underlying values of the patient. Most importantly, the therapist using the linear measure prevents response flexibility from being part of the learning process by insisting that the patient avoid errors, precisely because the therapist considers them undesirable. Thus, this therapist focuses on the absolute “correct” pattern of movement, allowing little complexity in the intervention and preventing the emergence of a flexible strategy that works for this individual patient.
If these therapists had the benefit of examining the patient's initial gait by linear and nonlinear analysis, they may have noted high variability of the step length (by looking at the standard deviation) but low values on a nonlinear measure (LyE), somewhat like the signal shown in the last row of Figure 1. If the therapists wanted to decrease variability, they would work toward a time series with a signal like that shown in the second row of Figure 1 (linear approach). However, if the goal was to increase the structure of the variability for greater complexity, they would work in a nonlinear fashion toward a time series with a signal like that shown in the third row of Figure 1. You can see that looking at variability from these differing perspectives could lead toward different types of intervention, as we have just described.
Another example of the use of the principles of nonlinearity to acquire or maintain motor skill is a treatment approach for the movement problems of patients with Parkinson disease (PD). The BIG training program focuses on a single attentional parameter to drive changes in the motor system.64 This parameter is “Think BIG,” emphasizing attention to large-amplitude movements. The principles of treatment include high-intensity, multiple repetitions, saliency, and complexity, leading to neuroplastic changes and functional improvement. Although patients with PD have many movement problems, including problems with speed, smoothness, accuracy, and quality of movement patterns such as step length during gait, this approach ignores these other movement deficiencies. The focus on increasing amplitude changes the initial conditions driving the movement and shifts the system into a new state space where the movement is more skillful and complex. In this way, the BIG approach addresses complexity because amplitude serves as the avenue to provide enhanced adaptability and increased responsiveness.
Several principles of nonlinearity are inherent in this approach. First, the overall variability of movement behavior for these patients is increased as a fallout of the increase in amplitude. The individuals with PD can now make large movements as well as small movements, and all the increments in between, whereas they had previously been restricted to only small movements. Second, another principle of nonlinearity is apparent because a change in just one movement parameter, amplitude, causes a change in other movement parameters that are difficult to predict precisely. Third, the input needed for a motor system change can come from a different system (eg, cognitive, attentional, perceptual). The focus on attending to making a “bigger” movement, in this case, recalibrates the perceptual system to recognize when a movement is actually big versus the small movements common to individuals with PD. The therapist would not teach a particular movement form or strategy, but rather let the patient discover that increased complexity of various movements has an inherent value in producing success during daily tasks.
Use of Nonlinear Tools in Motor Skill Research
Setting up methodology for nonlinear analysis in a research project may seem to be a daunting task. Here are suggestions to keep in mind when designing such a project:
Carefully design your experimental setup, incorporating a matched control group of healthy participants to provide reference points for observed changes in the nonlinear parameters.
Seek partnerships with mathematicians, neuroscientists, and biomechanists who are knowledgeable about nonlinear tools. Technical expertise is needed from start to finish on the project, including sampling frequency, the length of the time series needed, and examination of the data with appropriate nonlinear tools for your questions. Knowledge of movement measurement is a benefit in interpretation of the results. Remember that you will not “speak the same language” as your collaborator, and be very patient so that ideas can be exchanged comfortably.
Measure a task, skill, or variable that may show emergence of a new level of function.
Variable and task selection must incorporate a time series; you should take as long a time series as possible within the constraints of your target population and considering the tools you are using. Many nonlinear tools need thousands of data points for accurate use.
Examine pilot data to get an idea of the nature of the fluctuations in the variable or in the behavior. Plotting position and velocity against each other and even 3-dimensionally by incorporating acceleration can help examine the organization in the data.
Replication of a previously reported project, but with the addition of nonlinear tools, can help with planning the methods and interpreting your results. This is a new area, and, consequently, it is difficult to know the “standard” approach to common issues.
If you are considering an intervention study, keep in mind that a focus on increasing or decreasing variability will need to be determined carefully, using a variety of tools to measure both linear and nonlinear factors. The 2 approaches are complementary, and they do not negate each other.
Measure more than one variable; different variables may reflect component skills or constructs differently.
Limitations of Nonlinear Measures
Because nonlinear measurement tools require the use of mathematical equations and software to evaluate time series data, nonlinear analysis is primarily done in the research setting. However, the burgeoning interest in nonlinear tools in many scientific fields bodes well for clinicians. In the future, there will likely be devices that have embedded software to calculate important measures of variability using nonlinear tools. Another limitation is the lack of understanding of variability and complexity in the field of physical therapy. Physical therapists are taught to use a reductionist approach, as in most medical fields. This lack of introduction to nonlinear principles early in the education of physical therapists biases the field against the productive use of variability and complexity.
Additional limitations of the technique itself create challenges for clinical use. Translation of nonlinear measures to clinical problems requires concurrent use of linear tools to make associations and determine clinical meaning. The lengthy time series required for analysis prohibits the use of nonlinear tools for movement that is extremely limited. Lastly, these measures require multiple repetitions or cycles of a movement.
Conclusions
Optimal variability in human movement is a characteristic of healthy functioning. Nonlinear tools reveal complexity inherent in normal variability, indicating features of motor control that are important for physical therapists to measure and implement in intervention. The application of principles based on nonlinear dynamics and use of nonlinear tools for analysis can provide innovations to guide physical therapist practice and research.
This article is the subject of an Invited Commentary and an online-only Editorial.
Appendix 1.
Working Definitions for Terms Needed to Understand Nonlinear Concepts
Attractor, attractor state:
Behaviorally, an attractor is a preferred state (ie, walk, run). Mathematically, an attractor is a set to which a dynamic system evolves after time. Points that get close enough to the attractor remain close even if slightly perturbed. Geometrically, an attractor can be a point, a curve, or even a fractal structure known as a strange attractor. Describing the attractors of chaotic dynamical systems is the focus of the mathematical theory of chaos.
Chaos:
One subject in the field of nonlinear dynamics, which is part of the broader field of dynamic systems.
Complexity:
Highly variable fluctuations in physiological processes resembling mathematical chaos.
Constraints:
Variability is introduced into the system from the constraints that dictate the system's behavior. The constraints are morphological, biomechanical, environmental, and task specific.
Deterministic:
For a given starting condition, the future state of the system is determined; randomness is not present.
Dynamic system:
A system that evolves over time.
Dynamic systems theory (DST):
An area of applied mathematics used to describe complex dynamic systems.
Linear measures:
Measures that describe the central tendency or dispersion of the values within a set of numbers, such as the mean, the range, and the standard deviation.
Mathematical chaos:
The behavior of several related systems many times seems to be erratic, with no order (ie, random). However, nonlinear measures demonstrate that such variations are not random but have a deterministic pattern, meaning that their future dynamics are fully defined by their initial conditions. This behavior is known as mathematical or deterministic chaos, or simply chaos.
Noise:
That part of a system description that is not deterministic. For simplicity, it is usually assumed to have a simple form, such as white noise.
Nonlinear measures:
Measures that quantify the relationship, or dependency, of the numbers throughout the time series. Nonlinear measures describe the patterns or structure within the time series, not simply the quantity.
Periodic:
Happening or appearing at regular intervals like a sine wave.
Phase plane:
A representation of the behavior of the dynamic system in state space. Typically, it takes the form of a 2-dimensional plot of the position X of the time series (on the horizontal-axis) versus the first derivative X′ (on the vertical axis).
Random:
Lack of pattern or order; lack of a relationship between points in a time series or parts of a system.
Regularity:
The repeatability of a pattern.
Self-organization:
The formation of moving patterns is a function of the cooperation of all of the subsystems and their interaction with the environment; it is not centrally coded or commanded.
Sinusoidal:
A regular waveform that exactly repeats itself over time.
Stability, stable state:
A rich behavioral state characterized by high complexity. The stability of an orbit in a dynamic system determines whether nearby orbits remain near or are repelled by that orbit.
State space:
The set of all possible states of a dynamic system. When modeling a dynamic system, the number of variables needed to describe the system is called the dimension of the state space.
Systems theory:
A field of science studying the nature of complex systems.
Time series:
Time series data are a specific example of an ordered list of numbers, where time is the parameter that gives order to the list.
Suggested Readings
Gleick J. Chaos: Making a New Science. New York, NY: Penguin Books; 1987.
Smith LB, Thelen E. Development as a dynamic system. Trends Cogn Sci. 2003:7;343–348.
Stergiou N, Buzzi UH, Kurz MJ, Heidel J. Nonlinear tools in human movement. In: Stergiou N, ed. Innovative Analyses for Human Movement. Champaign, IL: Human Kinetics Publishers; 2004:63–90.
Stergiou N, Harbourne R, Cavanaugh J. Optimal movement variability: a new perspective for neurologic physical therapy. J Neurol Phys Ther. 2006;30:120–129.
Strogatz S. Sync: How Order Emerges From Chaos in the Universe, Nature, and Daily Life. New York, NY: Hyperion; 2004.
Appendix 2.
Proposed Principles of Nonlinearity in the Acquisition and Maintenance of Motor Skill
1. An optimal amount of variability is necessary for movement to be functional and efficient; normal, efficient movement includes both deterministic and random characteristics, which can fluctuate within an optimal range.
2. Healthy motor control has characteristics of nonlinearity, including the spontaneous generation of new patterns of movement, movement possibilities that are sensitive to initial conditions, and a limited ability to precisely predict future movement based on current status.
3. If variability increases in a system without enough variability, new movement options can emerge spontaneously.
4. Because motor function is sensitive to initial conditions, each person brings a slightly different set of conditions to a motor problem, and the optimal solution to that problem may be unique to that person. Therefore, therapists cannot “prescribe” the best motor pattern or strategy that is common to all patients.
5. Input into the system can drive the system into other possibilities for movement that are not predictable. The input can come from more than one system (ie, not only motor but also sensory, cognitive, emotional, or social).
6. Measures of complexity can help predict the emergence of a new behavior or direct appropriate intervention to allow variability changes to affect function.
7. Traditional measures of variability are not equivalent to measures of complexity. For example, as the measure of standard deviation increases, the measure of approximate entropy can decrease. Measures of complexity describe the structure of variability in new ways that can help quantify subtle movement changes or characteristics.
8. Complexity is necessary for systems to adapt to changing conditions; loss of complexity means decreased ability for adaptation.
Both authors provided concept/idea/project design and writing.
This work was funded by a National Institute of Child Health and Human Development grant (NIH 1K25 HD 047194-01A1) to Dr Stergiou (primary investigator); a US Department of Education, National Institute for Disabilities and Rehabilitation Research grant (USDE FIP 84.1336-1) to Dr Stergiou (primary investigator); and the Nebraska Research Initiative.
Terms needed to understand nonlinear concepts are underlined and are listed in Appendix 1.
References
- 1.Bernstein N. The Coordination and Regulation of Movements. London, United Kingdom: Pergamon Press; 1967.
- 2.Larin H. Quantifying instructional interventions in pediatric physical therapy with the Motor Teaching Strategies Coding Instrument (MTSCI-1): a pilot study. Internet J Allied Health Sci Prac. 2007;5:1–9. Available at: http://ijahsp.nova.edu/articles/vol5num1/Larin.pdf. Accessed March 16, 2008. [Google Scholar]
- 3.Katerndahl DA. Is your practice really that predictable? Nonlinearity principles in family medicine. J Fam Prac. 2005;54:970–977. [PubMed] [Google Scholar]
- 4.Rickles D, Hawe P, Shiell A. A simple guide to chaos and complexity. J Epidemiol Community Health. 2007;61:933–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klonowski W. From conformons to human brains: an informal overview of nonlinear dynamics and its application in biomedicine. Nonlinear Biomed Phys. 2007;1:5–18. Available at: http://www.nonlinearbiomedphys.com/content/1/1/5. Accessed March 16, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newell KM, Liu YT, Mayer-Kress G. Time scales in motor learning and development. Psychol Rev. 2001;108:57–82. [DOI] [PubMed] [Google Scholar]
- 7.Walleczek J. Self-organized Biological Dynamics and Nonlinear Control: Toward Understanding Complexity, Chaos, and Emergent Function in Living Systems. Cambridge, United Kingdom: Cambridge University Press; 2000.
- 8.Wray RE, Laird JE. Variability in human behavior modeling for military simulations. Presented at: Behavior Representation in Modeling and Simulation Conference (BRIMS); 2003. Available at: http://www.speakeasy.org/∼wrayre/pubs/VariabilityinHumanBehaviorModeling_WrayLaird_BRIMS2003.pdf. Accessed March 16, 2008.
- 9.Definition of variability. Available at: http://www.webster-dictionary.net/definition/variability. Accessed March 16, 2008.
- 10.Stergiou N, Harbourne R, Cavanaugh J. Optimal movement variability: a new perspective for neurologic physical therapy. J Neurol Phys Ther. 2006;30:120–129. [DOI] [PubMed] [Google Scholar]
- 11.Glass L, Mackey MC. From Clocks to Chaos. Princeton, NJ: Princeton University Press; 1988.
- 12.Schmidt RA, Lee TD. Motor Control and Learning: A Behavioral Emphasis. 4th ed. Champaign, Ill: Human Kinetics Publishers; 2005.
- 13.Schmidt RA. Motor schema theory after 27 years: reflections and implications for a new theory. Res Q Exerc Sport. 2003;74:366–375. [DOI] [PubMed] [Google Scholar]
- 14.Price I. Complexity, complicatedness and complexity; a new science behind organizational intervention? E:Co. 2004;6:40–48. [Google Scholar]
- 15.Goldberger AL. Heart rate variability: techniques, applications and future directions. Available at: http://www.physionet.org/events/hrv-2006/goldberger-1.pdf. Accessed March 16, 2008.
- 16.Byl NN, Nagarajan SS, Merzenich MM, et al. Correlation of clinical neuromusculoskeletal and central somatosensory performance: variability in controls and patients with severe and mild focal hand dystonia. Neural Plast. 2002;9:177–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci. 1996;16:785–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merzenich MM, Jenkins WM. Reorganization of cortical representations of the hand following alterations of skin inputs induced by nerve injury, skin island transfers, and experience. J Hand Ther. 1993;6:89–104. [DOI] [PubMed] [Google Scholar]
- 19.Stergiou N, Buzzi UH, Kurz MJ, Heidel J. Nonlinear tools in human movement. In: Stergiou N, ed. Innovative Analyses for Human Movement. Champaign, IL: Human Kinetics Publishers; 2004:63–90.
- 20.Shelhamer M. Nonlinear Dynamics in Physiology: A State Space Approach. Hackensack, NJ: World Scientific Publishing Co; 2007.
- 21.Heriza C. Designing practice for motor learning: clinical implications. In: Lister MJ, ed. Contemporary Management of Motor Control Problems: Proceedings of the II STEP Conference. Alexandria, VA: Foundation for Physical Therapy; 1991.
- 22.Giuliani CA. Designing practice for motor learning: clinical implications. In: Lister MJ, ed. Contemporary Management of Motor Control Problems: Proceedings of the II STEP Conference. Alexandria, VA: Foundation for Physical Therapy; 1991.
- 23.Thelen E, Smith LB. A Dynamic Systems Approach to the Development of Cognition and Action. Cambridge, MA: MIT Press; 1999.
- 24.Hausdorff JM, Purdon PL, Peng CK, et al. Fractal dynamics of human gait: stability of long-range correlations in stride interval fluctuations. J Appl Physiol. 1996;80:1448–1457. [DOI] [PubMed] [Google Scholar]
- 25.Harbourne RT, Stergiou N. Nonlinear analysis of the development of sitting postural control. Dev Psychobiol. 2003;42:368–377. [DOI] [PubMed] [Google Scholar]
- 26.Boker SM, Schreiber T, Pompe B, Bertenthal BI. Nonlinear analysis of perceptual-motor coupling in the development of postural control. In: Kantz H, Kurths J, Mayer-Kress G, eds. Nonlinear Techniques in Physiological Time Series Analysis. Heidelberg, Germany: Springer; 1998:251–270.
- 27.Palmieri RM, Ingersoll CD, Stone MB, Krause BA. Center-of-pressure parameters used in the assessment of postural control. J Sport Rehabil. 2002;11:51–66. [Google Scholar]
- 28.Hughes MA, Duncan PW, Rose DK, et al. The relationship of postural sway to sensorimotor function, functional performance, and disability in the elderly. Arch Phys Med Rehabil. 1996;77:567–572. [DOI] [PubMed] [Google Scholar]
- 29.Edelman GM. Bright Air, Brilliant Fire: On the Matter of the Mind. New York, NY: Basic Books; 1992.
- 30.Adami C, Ofria C, Collier TC. Evolution of biological complexity. Proc Natl Acad Sci U S A. 2000;97:4463–4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siegler RS. Emerging Minds: The Process of Change in Children's Thinking. New York, NY: Oxford University Press Inc; 1996.
- 32.Wilson C, Simpson SE, van Emmerik RE, Hamill J. Coordination variability and skill development in expert triple jumpers. Sports Biomech. 2008;7:2–9. [DOI] [PubMed] [Google Scholar]
- 33.Cavanaugh, JT, Guskiewicz KM, Giuliani C, et al. Recovery of postural control after cerebral concussion: new insights using approximate entropy. J Athl Train. 2006;41:305–313. [PMC free article] [PubMed] [Google Scholar]
- 34.Cavanaugh JT, Guskiewicz KM, Stergiou N. A nonlinear dynamic approach for evaluating postural control: new directions for the management of sport-related cerebral concussion. Sports Med. 2005;35:935–950. [DOI] [PubMed] [Google Scholar]
- 35.Moraiti C, Stergiou N, Ristanis S, Georgoulis AD. ACL deficiency affects stride-to-stride variability as measured using nonlinear methodology. Knee Surg Sports Traumatol Arthrosc. 2007;15:1406–1413. [DOI] [PubMed] [Google Scholar]
- 36.Kurz M, Stergiou N. The aging human neuromuscular system expresses less certainty for selecting joint kinematics during gait. Neurosci Lett. 2003;348:155–158. [DOI] [PubMed] [Google Scholar]
- 37.Slifkin AB, Newell KM. Noise, information transmission, and force variability. J Exp Psychol Hum Percept Perf. 1999;25:837–851. [DOI] [PubMed] [Google Scholar]
- 38.Riley MA. Turvey MT. Variability of determinism in motor behavior. J Mot Behav. 2002;34:99–125. [DOI] [PubMed] [Google Scholar]
- 39.Balasubramaniam R, Riley MA, Turvey MT. Specificity of postural sway to the demands of a precision task. Gait Posture. 2000;11:12–24. [DOI] [PubMed] [Google Scholar]
- 40.Oppenheim U, Kohen-Raz R, Alex D, et al. Postural characteristics of diabetic neuropathy. Diabetes Care. 1999;22:328–332. [DOI] [PubMed] [Google Scholar]
- 41.Pincus SM, Gladstone IM, Ehrenkranz RA. A regularity statistic for medical data analysis. J Clin Monit. 1991;7:335–345. [DOI] [PubMed] [Google Scholar]
- 42.Pincus SM. Approximate entropy as a measure of system complexity. Proc Natl Acad Sci U S A. 1991;88:2297–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Newell KM, Van Emmerik REA, Lee D, Sprague RL. On postural stability and variability. Gait Posture. 1993;1:225–230. [Google Scholar]
- 44.Georgoulis AD, Moraiti C, Ristanis S, Stergiou N. A novel approach to measure variability in the anterior cruciate ligament deficient knee during walking: the use of the Approximate Entropy in Orthopaedics. J Clin Monit Comput. 2006;20:11–18. [DOI] [PubMed] [Google Scholar]
- 45.Newell KM. Degrees of freedom and the development of center of pressure profiles. In: Newell KM, Molenaar PMC, eds. Applications of Nonlinear Dynamics to Developmental Process Modeling. Hillsdale, NJ: Erlbaum; 1997:63–84.
- 46.Cavanaugh JT, Coleman KL, Gaines JM, et al. Using step activity monitoring to characterize ambulatory activity in community-dwelling older adults. J Am Geriatr Soc. 2007;55:120–124. [DOI] [PubMed] [Google Scholar]
- 47.Buzzi UH, Ulrich BD. Dynamic stability of gait cycles as a function of speed and system constraints. Motor Control. 2004;8:241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kurz M, Stergiou N. Do horizontal propulsive forces influence the nonlinear structure of locomotion? J Neuroeng Rehabil. 2007;1:4–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoshino K, Motoshige T, Araki T, Matsuoka K. Effect of prolonged free-walking fatigue on gait and physiological rhythm. J Biomech. 2004;37:1271–1280. [DOI] [PubMed] [Google Scholar]
- 50.Yamada N. Chaotic swaying of the upright posture. Hum Mov Sci. 1995;14:711–726. [Google Scholar]
- 51.Harbourne RT, Deffeyes JE, DeJong SL, et al. Nonlinear variables can assist in identifying postural control deficits in infants. J Sport Exerc Psychol (Suppl). 2007;29:S9. [Google Scholar]
- 52.Russell D, Rosenbaum P, Gowland C, et al. Gross Motor Function Measure. 2nd ed. Hamilton, Ontario, Canada: McMaster University; 1993
- 53.Miller DJ, Stergiou N, Kurz MJ. An improved surrogate method for detecting the presence of chaos in gait. J Biomech. 2006;39:2873–2876. [DOI] [PubMed] [Google Scholar]
- 54.Hausdorff JM, Peng CK, Ladin Z, et al. Is walking a random walk? evidence for long-range correlations in stride interval of human gait. J Appl Physiol. 1995;78:349–358. [DOI] [PubMed] [Google Scholar]
- 55.Goldberger AL. Non-linear dynamics for clinicians: chaos theory, fractals, and complexity at the bedside. Lancet. 1996;347:1312–1314. [DOI] [PubMed] [Google Scholar]
- 56.Goldberger AL, Amaral LA, Hausdorff JM, et al. Fractal dynamics in physiology: alterations with disease and aging. Proc National Acad Sci USA. 2002;99:2466–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goldberger AL, Rigney DR, West BJ. Chaos and fractals in human physiology. Sci Am. 1990;262:42–49. [DOI] [PubMed] [Google Scholar]
- 58.Ahn AC, Tewari M, Poon C, Phillips RS. The limits of reductionism in medicine: could systems biology offer an alternative? Plos Med. 2006;3:e208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Przybylskia A, Baranowskia R, Zebrowskib JJ, Szweda H. Verification of implantable cardioverter defibrillator (ICD) interventions by nonlinear analysis of heart rate variability: preliminary results. Europace. 2004;6:617–624. [DOI] [PubMed] [Google Scholar]
- 60.Bartsch R, Plotnik M, Kantelhardt JW, et al. Fluctuation and synchronization of gait intervals and gait force profiles distinguish stages of Parkinson's disease. Physica A. 2007;383:455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schmit JM, Riley MA, Dalvi A, et al. Deterministic center of pressure patterns characterize postural instability in Parkinsons disease. Exp Brain Res. 2006;168:357–367. [DOI] [PubMed] [Google Scholar]
- 62.Hausdorff JM. Gait variability: methods, modeling, and meaning. J Neuroeng Rehabil. 2005;2:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Winstein CJ. Knowledge of results and motor learning: implications for physical therapy. Phys Ther. 1991;71:140–149. [DOI] [PubMed] [Google Scholar]
- 64.Farley BG, Koshland GF. Training BIG to move faster: the application of the speed-amplitude relation as a rehabilitation strategy for people with Parkinson's disease. Exp Brain Res. 2005;167:462–467. [DOI] [PubMed] [Google Scholar]