Abstract
During development, differentiating oligodendrocytes progress in distinct maturation steps from premyelinating to myelinating cells. Such maturing oligodendrocytes express both receptors mediating signaling via extracellular lysophosphatidic acid (LPA) and the major enzyme generating extracellular LPA, namely phosphodiesterase-Iα/autotaxin (PD-Iα/ATX). However, the biological role of extracellular LPA during the maturation of differentiating oligodendrocytes is currently unclear. Here, we demonstrate that application of exogenous LPA induced an increase in the area occupied by the oligodendrocytes’ process network, but only when PD-Iα/ATX expression was down-regulated. This increase in network area was caused primarily by the formation of membranous structures. In addition, LPA increased the number of cells positive for myelin basic protein (MBP). This effect was associated by an increase in the mRNA levels coding for MBP but not myelin oligodendrocyte glycoprotein (MOG). Taken together, these data suggest that LPA may play a crucial role in regulating the later stages of oligodendrocyte maturation.
Keywords: LPA receptor, MBP expression, myelination, oligodendrocyte, PD-Iα/ATX
Introduction
Oligodendrocytes are the myelin-forming cells of the central nervous system (CNS). During development, they originate from progenitor cells that are generated in discrete areas of the CNS and migrate to their final destination to differentiate in a cell autonomous fashion into post-migratory, premyelinating oligodendrocytes [1, 2]. At the prospective sites of myelination such post-migratory, premyelinating oligodendrocytes undergo distinct stages of maturation, which are defined by changes in morphology and gene expression pattern (see Fig. 1A and [3–7]). In particular, cells first survey the environment for axonal segments to be myelinated by extending a large and complex network of fine processes. This step of extensive process outgrowth is followed by the transformation of cellular protrusions into membranous structures ultimately forming the myelin sheath and by the expression of proteins involved in the regulation of myelination such as myelin basic protein (MBP) and myelin oligodendrocyte glycoprotein (MOG). Despite the well described characterization of these maturation stages, however, little is known about the signals that regulate their well coordinated progression [8].
Fig. 1.
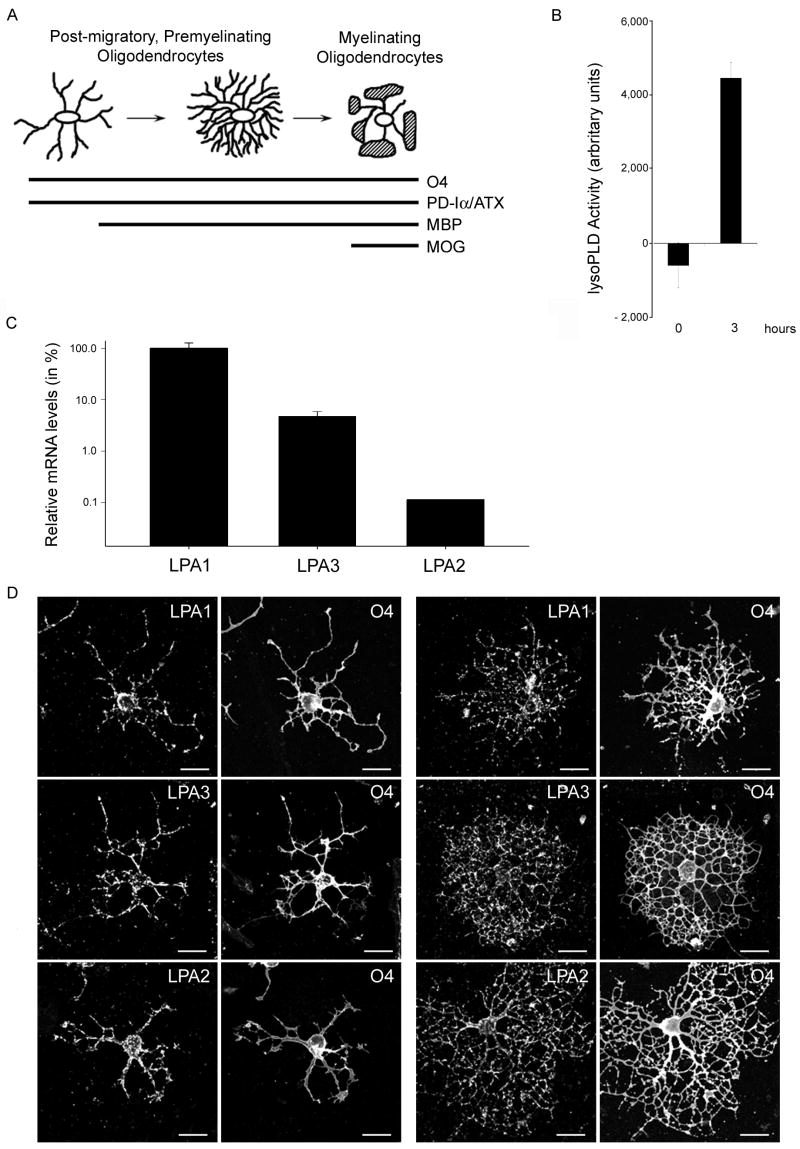
Differentiating oligodendrocytes secrete enzymatically active PD-Iα/ATX and express all three classical LPA receptors. A. Scheme of morphological and gene expression characteristics of differentiating oligodendrocytes. At all stages oligodendrocytes express the cell surface antigen O4 and the secreted protein phosphodiesterase-Iα/autotaxin (PD-Iα/ATX). The myelin proteins myelin basic protein (MBP) and myelin oligodendrocyte glycoprotein (MOG) are expressed with increasing stages of maturation. B. PD-Iα/ATX’s lysoPLD activity was determined in tissue culture supernatants from O4 immunopanned oligodendrocytes (4 days after plating) using the fluorogenic assay described by Ferguson et al. (2006). Means ± SEM of three independent experiments done in triplicates are shown. Numbers on the Y-axis represent fluorescence increase as a measure for lysoPLD activity in arbitrary units. C. Relative LPA receptor mRNA levels present in differentiating oligodendrocytes. mRNA levels were determined by real-time qRT-PCR [40] and are presented in % on a logarithmic scale. LPA1 mRNA levels were set to 100% and the levels for LPA3 and LPA2 calculated accordingly. Means ± SEM of three pooled samples run in triplicate are shown. D. Immunocytochemistry of differentiating oligodendrocytes. Oligodendrocytes were isolated by O4 immunopanning and double-immunostained 4 days after plating for the O4 antigen and for LPA1, 2 or 3. Scale Bar: 20 μm.
One of the extracellular factors long suspected to play a critical role during the later stages of oligodendrocyte maturation is the lipid signaling molecule lysophosphatidic acid (LPA). Extracellular LPA exerts its physiological functions by activating specific G protein-coupled receptors (GPCRs; for review see: [9–11]). GPCRs of the endothelial differentiation gene family were the first to be recognized as LPA receptors, and they are designated LPA1 (Edg-2), LPA2 (Edg-4) and LPA3 (Edg-7). In addition to these classical LPA receptors three GPCRs of the purinergic family, GPR23/LPA4, GPR92/LPA5 and GPR87, have been recently identified to also mediate signaling via LPA [12–15]. Out of the above, LPA 1 and LPA3 have been described to be expressed by differentiating oligodendrocytes [16–20]. The in vivo expression pattern of LPA1 was found to coincide with myelination, and it was this observation that prompted a number of investigations into the role of LPA for oligodendrocyte differentiation and function [21–24]. Somewhat disappointingly, no significant effects on morphology and/or gene expression were identified in these studies.
Differentiating oligodendrocytes express not only the receptors for extracellular LPA, but also the major enzyme responsible for the production of extracellular LPA, namely the lysophospholipaseD (lysoPLD) phosphodiesterase-Iα/autotaxin (PD-Iα/ATX) [25–29]. Our previous studies identified PD-Iα/ATX as an extracellular factor that is released by differentiating oligodendrocytes during the initial stages of myelination and that stimulates the establishment of a complex process network independently of its lysoPLD activity via a second functionally active domain, i.e. the modulator of oligodendrocyte remodeling and focal adhesion organization (MORFO) domain [30–33]. In addition, however, it is reasonable to assume that the presence of PD-Iα/ATX in the extracellular environment of differentiating oligodendrocytes results in the extracellular production of LPA and in the stimulation of LPA-mediated effects. Thus, the limited responses observed so far when adding LPA exogenously to differentiating oligodendrocytes may at least be in part due to PD-Iα/ATX precluding or masking the physiological effects of LPA.
In the present study we have, therefore, revisited the role of LPA on differentiating oligodendrocytes, and we have assessed its effects under conditions where the expression of PD-Iα/ATX was down-regulated. Under these conditions, exogenous application of LPA was found to support the formation of membranous structures and to stimulate an increase in mRNA levels coding for MBP but not MOG. Taken together, these findings demonstrate that LPA can stimulate the later maturation steps of differentiating oligodendrocytes.
Experimental Procedures
Materials
Antibodies: Hybridoma clone A2B5 (ATCC, Manassas, VA), hybridoma clone O4 (gift from S.E. Pfeiffer [34, 35]), anti-LPA1 (Edg-2) antibodies (Abcam Inc., Cambridge, MA), anti-LPA2 (Edg-4) and anti-LPA3 (Edg-7) antibodies (Santa Cruz Biotechnology Inc., Santa Cruz, CA), anti-acetylated α-tubulin antibodies (Zymed Laboratories Inc., South San Francisco, CA), anti-MBP antibodies (Covance Berkeley, CA), secondary Alexa 488- and Alexa 594-conjugated antibodies (Invitrogen/Molecular Probes, Carlsbad, CA), secondary horseradish peroxidase (HRP)-conjugated antibodies (Vector Laboratories, Burlingame, CA). The tyramid signal amplification (TSA) Plus Cyanine 3 system was from Perkin Elmer (Waltham, MA). SMARTpool siRNA directed against rat PD-Iα/ATX and control non-targeting SMARTpool siRNA were obtained from Dharmacon Inc. (Lafayette, CO). Tissue culture and transfection reagents were from Invitrogen (Carlsbad, CA) unless stated otherwise. LPA (18:1; 1-oleoyl-2-hydroxy-sn-glycero-3-phosphate) was purchased from Avanti Polar Lipids Inc. (Alabaster, AL) as a 54.5 mM stock solution prepared in chloroform. Oligonucleotide primers for PCR analysis were from MWG-BIOTECH Inc (High Point, NC). RNA purification and RT kits were obtained from Qiagen (Valencia, CA). The SYBR Green PCR mix was from BioRad (Hercules, CA). All other reagents and supplies were from Fisher Scientific (Atlanta, GA) unless noted otherwise.
Animals
Sprague-Dawley female rats with early postnatal litters were obtained from Zivic Miller (Pittsburg, PA) and Harlan (Indianapolis, IN). All animal studies were approved by the Institutional Animal Care and Use Committee at Virginia Commonwealth University.
Cell Culture, siRNA and LPA Treatment
Primary cultures of differentiating oligodendrocytes were prepared from brains of postnatal day 4 to 5 rat pups as described previously [30, 36]. Briefly, cerebral hemispheres were dissected out and single cell suspensions were prepared by incubation in Hank’s Balanced Salt Solution supplemented with 0.25% trypsin/1 μg/ml DNase (Sigma, St Louis, MO) and subsequent trituration. Differentiating oligodendrocytes where then isolated by O4 immunopanning. Isolated cells were plated on fibronectin (10 μg/ml)-coated coverslips and cultured in serum-free defined medium [Dulbeccos’s Modified Eagle’s Medium (DMEM) containing 40 ng/ml tri-iodo-thyronine (T3; Sigma, St Louis, MO) and 1x N2 supplement (DMEM/T3/N2)]. After two days in culture, cells were transfected with siRNA as described previously [33]. siPD-Iαs/ATX- and siControl-treated cells were analyzed after an additional 48 hours in culture. Knock-down of PD-Iα/ATX was assessed by real-time qRT-PCR. When cells were treated with exogenous LPA, it was done for 2 hours at a concentration of 1 μM (diluted in DMEM/N2). Immediately before use, the required aliquot of the LPA stock solution was dried down under sterile conditions, and the lipid film was resuspended by sonication in 0.4% fatty acid-free BSA (Sigma-Aldrich, St. Louis, MO) dissolved in tissue culture medium.
PD-Iα/ATX-lysoPLD Activity Assay
PD-Iα/ATX’s lysoPLD activity was determined using the fluorogenic assay described by Ferguson et al. (2006). Cell culture supernatants were incubated with 2.5 μM FS-3 substrate (Echelon Biosciences Inc., Salt Lake City, UT) in 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 5 mM Tris/HCl (pH 8.0), 1 mg/ml fatty acid-free BSA (Sigma-Aldrich, St. Louis, MO) for 5.0 hours at 37°C. Increase in fluorescence with time was measured at an excitation wavelength of 485 nm and an emission wavelength of 520 nm using a PHERAstar multimode microplate reader (BMG LABTECH Inc. Durham, NC).
Immunocytochemistry
For immunocytochemical detection of the O4 antigen as a marker for differentiating oligodendrocytes in combination with one of the three classical LPA receptors, cells were fixed in 4% paraformaldehyde dissolved in Phosphate Buffered Saline (PBS). Unspecific binding sites were blocked using DMEM/10% Fetal Calf Serum (FCS) and cells were incubated overnight at 4°C with O4 hybridoma supernatants (1:1 diluted in DMEM/10% FCS). Bound O4 antibodies were detected using Alexa 488-conjugated secondary antibodies (1:250 diluted in PBS). Cells were then fixed again and incubated for 30 min in blocking/permeabilization solution (PBS containing 5% normal goat serum, 2% non fat dry milk and 0.05% Tween-20). Primary antibodies to each of the classical LPA receptors were applied at a concentration of 1:100 (in PBS containing 1% normal goat serum, 0.05% Tween-20) overnight at 4°C. Bound primary antibodies were detected using the TSA Plus Cyanine 3 system. The primary antibodies to the three classical LPA receptors used here have been employed successfully by others (e.g. [37, 38]). When tested for specificity by us using Western blot analysis of whole brain lysates, all three antibodies were found to react with a single band of approximately 40 kDa, which is consistent with the size of the receptor monomers (data not shown). Furthermore, all antibodies detected an additional band of approximately 80 kDa, likely representing previously described LPA receptor homo- and/or heterodimers [39].
For immunocytochemical detection of MBP, cells were fixed in 4% paraformaldehyde dissolved in PBS and permeabilized using 0.5% triton/0.4 M sucrose in PBS. Subsequently, cells were incubated for 30 min in DMEM/10% FCS and then overnight with anti-MBP antibodies (1:250 diluted in DMEM/10% FCS). Bound primary antibodies were detected using Alexa 488-conjugated secondary antibodies (1:250 diluted in PBS) and nuclei were stained using Hoechst (1 μg/ml; Calbiochem, San Diego, CA).
For immunocytochemical detection of the O4 antigen in combination with acetylated α-tubulin the treatment for O4 immunostaining was as described above. Bound O4 antibodies were detected using Alexa 594-conjugated secondary antibodies (1:250 diluted in PBS). Cells were then fixed again and permeabilized using 0.5% triton in PBS. Following permeabilization cells were incubated in DMEM/10% FCS and then overnight with anti-acetylated α-tubulin antibodies. Bound antibodies were detected using Alexa 488-conjugated secondary antibodies (1:250 diluted in PBS).
Cells were analyzed using an Olympus BX51 inverted fluorescent microscope (Olympus America Inc., Center Valley, PA) or a confocal laser scanning microscope (TCS SP2 AOBS, Leica Microsystems, Exton, PA or LSM 510 META, Carl Zeiss MicroImaging, Inc., Thornwood, NY).
Real-time qRT-PCR analysis
Total RNA was isolated from oligodendrocyte cultures using the RNeasy Micro Kit. Purified RNA samples were quantified by fiber optic spectrophotometry using the Nanodrop ND-1000 (Nanodrop Inc., Wilmington, DE) and their quality was assessed using Experion RNA HighSens Chips (BioRad, Hercules, CA). For real-time qRT-PCR, oligo(dT)-primed cDNAs were synthesized using the Sensiscript or Omniscript RT kit. PCR was performed on a Chromo 4 Four-Color Real-Time System (BioRad, Hercules, CA) using the iQ SYBR Green Supermix. The following primer pairs were used at the indicated annealing temperatures: PD-Iα/ATX: forward (5′-GACCCTAAAACCATTATTGCTAA-3′), reverse (5′-GGGAAGGTGCTGTTTCATGT-3′), 60°C; MBP (exon 2 containing isoforms) forward (5′-ACTTGGCCACAGCAAGTACCATGGACC-3′), reverse (5′-TTGTACATGTGGCACAGCCCGGGAC-3′), 60°C; MOG: forward (5′-CTCATTGCCCTTGTGCCTAT-3′), reverse (5′-GCACGGAGTTTTCCTCTCAG-3′), 60°C; LPA1 (Edg-2): forward (5′-GACACCATGATGAGCCTTCTGA-3′), reverse (5′-CCCGGAGTCCAGCAGACA-3′), 65°C; LPA2 (Edg-4): forward (5′-CGCTCAGCCTAGTCAAGACA-3′), reverse (5′-TTGCAGGATTTACAGTCCAGAC-3′), 65°C; LPA3 (Edg-7): forward (5′-CACACGAGTGGCTCCATCAG-3′), reverse (5′-GGTCCAGCACACCACGAA-3′), 65°C. For normalization, amplification of 18S rRNA was performed: forward (5′-TTCGGAACTGAGGCCATGAT-3′), reverse (5′-TTTCGCTCTGGTCCGTCTTG-3′), 60°C or 65°C. PCR conditions were as follows: 95°C for 15 min followed by 34 cycles at 94°C for 15s, annealing temperature for 20s, and 72°C for 20s. For quantification of relative LPA receptor mRNA levels, the method described by Peirson et al. [40] was used. For relative comparison of MBP and MOG mRNA levels in the presence of siPD-Iα/ATX or siControl, the ΔΔCT method was used [41].
Oligodendrocyte Morphology Analysis
For morphology analysis, cells were immunostained using the O4 antibody and an anti-acetylated α-tubulin antibody. Images of approximately 40 cells were taken randomly for each treatment group in each experiment using an Olympus BX51 inverted fluorescent microscope (Olympus America Inc., Center Valley, PA). IP Lab imaging software (BD Biosciences Bioimaging, Rockville, MD) was used to determine process index (total amount of O4-positive process surfaces per cell minus the cell body), network area (total area within the radius of the process network surrounding the cell body minus the cell body) and complexity index (1 minus process index divided by network area) as previously described [33]. In addition, a membrane index was defined as follows: [(O4 network area minus acetyl-tubulin network area) divided by the O4 network area]. A 1:1 correlation of network area to microtubule area yields values approaching 0, while a membranous network area considerably larger than the microtubule network area produces a membrane index approaching 1.
Cell count analysis (MBP-positive cells)
Composite images of MBP-positive cells were collected by tile scan using a Leica TCS SP2 AOBS confocal microscope (Leica Microsystems, Exton, PA). The total number of detectable nuclei and MBP-positive oligodendrocytes was determined using the particle count plugin (Wright Cell Imaging Facility) to the ImageJ software package [42] and the following parameters: nuclei (threshold size: 0–10, circularity: 0–1.0); MBP (threshold size: 0.5–5.0, circularity: 0–1.0).
Results
Differentiating oligodendrocytes express enzymatically active PD-Iα/ATX and all three classical LPA receptors
Prior to analyzing the role of LPA on differentiating oligodendrocytes, studies were undertaken to confirm the enzymatic activity of secreted PD-Iα/ATX and the expression of LPA receptors in the culture system to be used. Differentiating oligodendrocytes were isolated from brains of 4 to 5 day-old rats by O4 immunopanning and allowed to mature for four days in vitro. The antigenic marker O4 has been previously demonstrated to be present on the membrane surface of oligodendrocytes that are considered post-migratory, premyelinating cells [34, 35, 43, 44]. In addition, such O4-positive oligodendrocytes release endogenous PD-Iα/ATX and start to express MBP and MOG (Fig. 1A and [5, 30, 45]). To determine lysoPLD activity of the secreted PD-Iα/ATX, cell culture supernatants were tested using a fluorogenic assay in which enzymatic activity is measured in a concentration and time dependent manner via an increase in fluorescence (see supplemental Fig. 1 and [46]). Using this assay, cell culture supernatants of differentiating oligodendrocytes were found to contain significant amounts of enzymatically active PD-Iα/ATX (see Fig. 1B). This finding is in agreement with our previous data demonstrating an increase in PD-Iα/ATX expression levels during the initial stages of myelination [30]. In contrast, oligodendrocyte progenitor cells were found to be negative for PD-Iα/ATX mRNA, and only low levels of PD-Iα/ATX mRNA were detected in oligodendrocytes present in the adult CNS (unpublished observations and [26]).
To assess LPA receptor expression, RNA was isolated from differentiating oligodendrocyte cultures and analyzed using real-time qRT-PCR. Amplification products for all three classical LPA receptors were noted. Quantification of the mRNA expression levels revealed highest expression of LPA1 followed by LPA3 and LPA2 (Fig. 1C). The data related to LPA1 and LPA3 expression are consistent with previous findings [20, 24]. Expression of LPA2, however, may have been overlooked so far due to its very low expression levels. To further confirm the expression of the three classical LPA receptors by differentiating oligodendrocytes, we took advantage of the availability of specific antibodies. As shown in Fig. 1D all three receptors were detected in O4-positive cells of both simple and complex process morphology. In addition, a punctate distribution was noted that may be indicative of receptor localization to intracellular vesicles. Taken together, the above data confirm and extend the finding that differentiating oligodendrocytes secrete enzymatically active PD-Iα/ATX and express receptors known to mediate signaling by extracellular LPA.
Extracellular LPA can support an increase in oligodendrocyte network area via stimulation of the formation of membranous structures
As introduced above, the presence of PD-Iα/ATX in the extracellular environment of differentiating oligodendrocytes may preclude and/or mask some of the physiologic effects of LPA when it is added exogenously and/or in excess. Thus, we analyzed the effect of exogenous LPA on the morphology of differentiating oligodendrocytes under conditions where PD-Iα/ATX expression was down-regulated. Differentiating oligodendrocytes were isolated by O4 immunopanning from postnatal day 4 to 5 rat brains and treated with a siRNA pool specific to PD-Iα/ATX two days after plating. We have previously established that under these conditions PD-Iα/ATX mRNA and protein levels are reduced by at least 50% 48 hours after siRNA treatment [33]. For LPA treatment a concentration of 1 μM was chosen. At this concentration significant effects on Ca2+ mobilization and ERK1/2 activation have been described by others [20, 21, 24]. Furthermore, for the LPA-induced increase in pERK an EC50 of 1 μM has previously been established [20]. LPA was added 48 hours after siRNA treatment and its effects were evaluated two hours after application. We first determined the network area as a measure for morphological changes (Fig. 2A). In agreement with previous studies, no effects were noted under control conditions (Fig. 2B and [24]). At reduced levels of PD-Iα/ATX mRNA and protein, however, the network area was significantly reduced, and addition of LPA led to an increase to almost control levels (Fig. 2B).
Fig. 2.
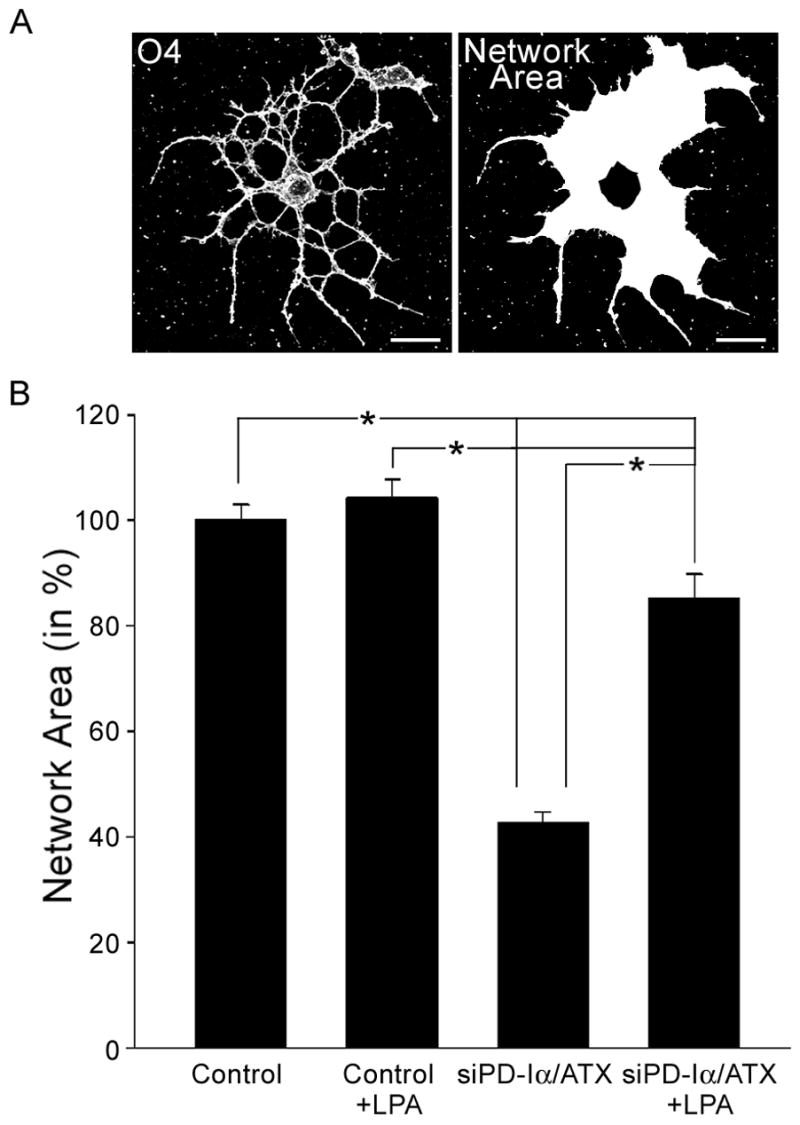
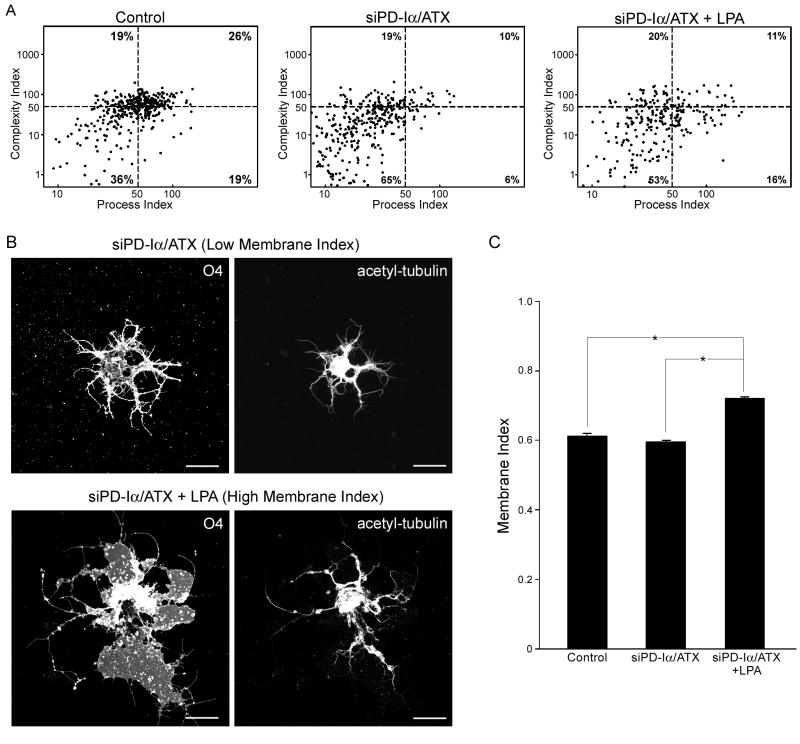
LPA can induce an increase in network area under conditions where PD-Iα/ATX expression is down-regulated. Differentiating O4-positive oligodendrocytes isolated from brains of 4 to 5 day-old rats were treated with a control (Control) or PD-Iα/ATX-specific (siPD-Iα/ATX) siRNA SMARTpool. 48 hours after siRNA transfection, LPA was applied at a concentration of 1 μM for two hours. Cells were then immunostained with the O4 antibody and the area covered by each cell’s process network was determined. A. Representative example of an oligodendrocyte labeled for the O4 antigen (left panel; confocal image representing a 2D maximum projection of stacks of 0.5 μm optical sections) and its network area (right panel). Scale Bar: 20 μm. B. Bar graph depicting the network area in % of control (mean of control = 100%). Means ± SEM of four independent experiments are shown. Stars indicate overall two-tailed significance levels of p<0.05 as determined by Student’s t-test.
To further define the nature of the morphological changes observed in response to LPA, process and complexity indices were determined (see experimental procedures and [33]). As shown in Fig. 3A, treatment of differentiating oligodendrocytes with a siRNA pool specific to PD-Iα/ATX resulted in a decrease in both process and complexity indices. Thus, siPD-Iα/ATX-treated cells displayed a simple process morphology (Fig. 3B; upper panel). Upon addition of exogenous LPA to these cells, an increase in process but not complexity index was noted (Fig. 3A). Such an increase could either be due to an increase in long, unbranched processes or in membranous structures. When visually inspecting the cells, the latter appeared to be the case (an example of maximal effect is depicted in Fig. 3B; lower panel). To quantify this effect, cells were double stained for O4 and acetylated α-tubulin, a protein associated with stabilized microtubules [47]. In oligodendrocytes, stabilized microtubules are found in processes of differentiating cells but not within their membranous structures [48–52]. Thus, the differential in distribution of the O4 antigen versus acetylated a-tubulin allowed us to define a membrane index for which a value close to 1 is characteristic for cells extending large membranous areas while a lower value denotes the presence of fine processes (for details see experimental procedures). As shown in Fig. 3C, the membrane index remained unaffected when PD-Iα/ATX expression was down-regulated. This finding is in agreement with our previous studies, and it reflects a decrease in the number of cells extending a complex network of fine processes [33]. Application of LPA to these cells of relatively simple process morphology significantly increased the membrane index (Fig. 3C). Thus, LPA can induce an increase in the oligodendrocyte’s network area by stimulating the formation of membranous structures.
Fig. 3.
LPA-induced increase in network area is due to an increase in the establishment of membranous structures. Differentiating oligodendrocytes were isolated and treated as described in Fig. 2. A. Normalized values of the cells’ process indices were plotted against normalized values of their complexity indices on logarithmic scales (mean control values = 50; see dashed lines). The numbers in each of the quadrants represent % cells. Four independent experiments with at least 30 cells per condition are shown. B. Upper panel: representative example of a siPD-Iα/ATX-treated cell that is characterized by a low membrane index. Lower panel: representative example of a siPD-Iα/ATX plus LPA-treated cell that is characterized by a high membrane index. Images were obtained by confocal microscopy and represent 2D maximum projections of stacks of 0.5 μm optical sections. Scale Bar: 20 μm. C. Bar graph depicting membrane indices. Means ± SEM of two independent experiments are shown. Stars indicate overall two-tailed significance levels of p<0.05 as determined by Student’s t-test.
Extracellular LPA can support an increase in the number of MBP-positive cells via stimulation of an increase in MBP mRNA levels
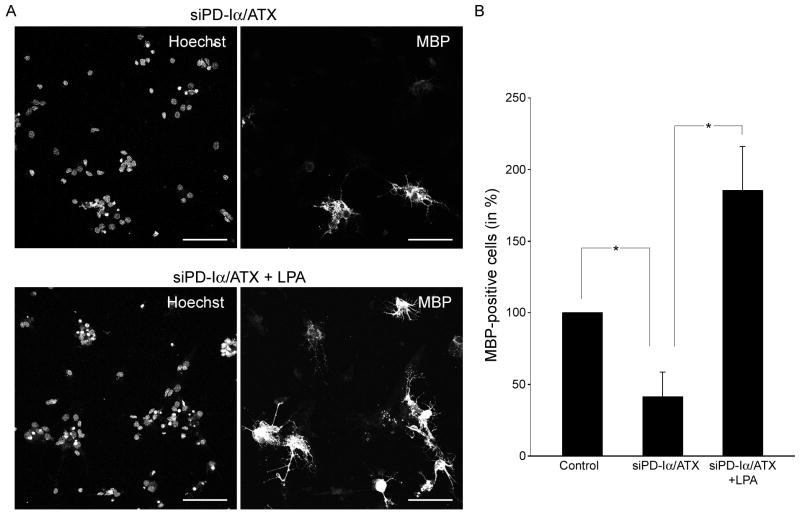
In an attempt to gain insight into potential molecular mechanisms underlying the above observed effect of LPA on the formation of membranous structures, the expression of the classic isoforms of the myelin protein MBP was analyzed (Figs. 4 and 5). These MBP isoforms are highly expressed during the active stages of myelination and are thought to have regulatory effects on the myelination program [53–58]. As shown in Fig. 4, down-regulation of PD-Iα/ATX expression led to a decrease in the number of MBP-positive cells. Upon addition of LPA, the number of MBP-positive cells returned to at least control levels.
Fig. 4.
LPA can induce an increase in the number of MBP-positive cells under conditions where PD-Iα/ATX expression is down-regulated. Differentiating oligodendrocytes were isolated and treated as described in Fig. 2. A. Representative images of cultures of differentiating oligodendrocytes treated with siPD-Iα/ATX (upper panel) or siPD-Iα/ATX plus LPA (lower panel). Cells expressing the classic isoforms of MBP were visualized by immunocytochemistry and nuclei were detected by Hoechst staining. Images were obtained by confocal microscopy and represent 2D maximum projections of stacks of 0.5 μm optical sections. Scale Bar: 75 μm B. Bar graph depicting the number of MBP-positive cells as % of control. Control values were set to 100%. Means ± SEM of three independent experiments are shown. Stars indicate overall two-tailed significance levels of p<0.05 as determined by Student’s t-test.
Fig. 5.
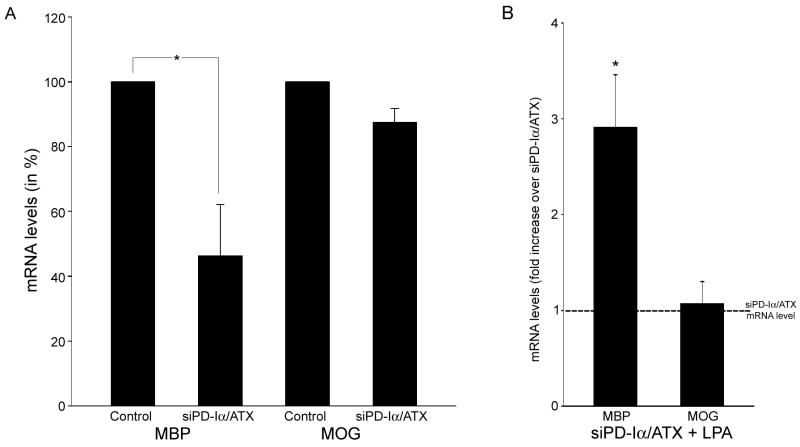
LPA-induced increase in the number of MBP-positive cells is due to an increase in MBP mRNA levels. Differentiating oligodendrocytes were isolated and treated as described in Fig. 2. mRNA levels for MBP and MOG were determined by real-time qRT-PCR [41]. 18S rRNA was used for normalization. A. Bar graph depicting mRNA levels as % of control (control levels were set to 100%). B. Bar graph illustrating the fold increase in mRNA levels upon LPA treatment (mRNA levels under siPD-Iα/ATX conditions were set to 1; see dotted line). For both graphs, means ± SEM of three independent experiments done in duplicates are shown. Stars indicate an overall two-tailed significance level of p<0.05 as determined by Student’s t-test analysis.
The observed increase in the number of MBP-positive cells could be mediated by stimulation of gene expression or by regulatory posttranscriptional events such as mRNA transport and/or translational regulation. To evaluate the contribution of an increase in mRNA levels, real-time qRT-PCR was performed. In these studies, mRNAs coding for the exon 2-containing classic isoforms of MBP were assayed since these are expressed more abundantly during the initial stages of myelination. In contrast, exon 2-deficient MBP isoforms have been implicated primarily in the regulation of myelin maintenance and/or compaction [59, 60]. Cells treated with a siRNA pool specific to PD-Iα/ATX displayed a reduction in MBP mRNA levels that was similar to the reduction in the number of MBP-positive cells (compare Fig. 5A with 4B). In contrast to MBP, MOG, which is specifically expressed by myelinating oligodendrocytes, was not affected by the alteration in PD-Iα/ATX expression (Fig. 5A and [45]). These findings demonstrate that a reduction in PD-Iα/ATX expression affects mRNA levels for some but not all myelin genes. Upon treatment with LPA, MBP mRNA levels were increased by a factor of 2.9, while MOG mRNA levels remained unchanged (Fig. 5B). Interestingly, MBP mRNA levels reached above control levels after LPA application. This enhancement in MBP mRNA levels could either reflect a more efficient direct effect of LPA on MBP under low PD-Iα/ATX expression conditions, or it could, in part, be a consequence of an indirect or off-target effect of the siPD-Iα/ATX-treatment on other LPA-responsive genes. Nevertheless, these data demonstrate that under certain conditions LPA can induce an increase in the mRNA levels encoding classic isoforms of MBP.
Discussion
The well coordinated sequence of events leading to the final maturation of differentiating oligodendrocytes is crucial for proper functioning of the CNS. Our data presented here indicate that the lipid signaling molecule LPA may play a crucial role in regulating these events. In particular, we found that LPA can promote an increase in the area occupied by the oligodendrocyte’s process network by stimulating the extension of membranous structures. In addition, LPA can increase the appearance of MBP-positive cells via stimulation of an increase in MBP mRNA levels. mRNA levels for the later stage marker MOG, however, appeared unaffected. Increases in neither the network area nor the MBP mRNA levels were observed under control conditions. Detection of these exogenous LPA-mediated effects required prior down-regulation of PD-Iα/ATX expression. Thus, our data demonstrate that under conditions where PD-Iα/ATX expression is down-regulated LPA can promote the transition from a network of fine processes to the formation of myelin sheets as well as an increase in MBP mRNA levels (Fig. 6).
Fig. 6.
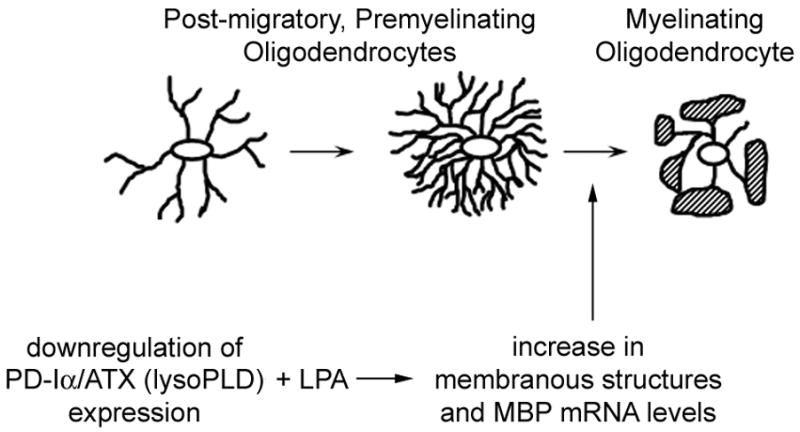
Potential effects of LPA on differentiating oligodendrocytes. Our data presented here demonstrate that extracellular LPA can stimulate the formation of membranous structures and an increase in mRNA levels encoding classic isoforms of MBP at least under conditions where PD-Iα/ATX expression is down-regulated.
In the studies presented in Fig. 3, the formation of membranous structures was induced by LPA in cells of simple process morphology. In vivo and under control in vitro conditions, however, membrane sheath formation is preceded by the extension of a complex process network [4, 6]. Thus, for earlier differentiation stages of oligodendrocytes with simple morphology LPA does not appear to exert a similar effect as the one observed here. These cells may lack components of the signaling pathway leading to membrane sheath formation and/or this pathway may be inhibited by the expression of “silencing” factors. In either case, down-regulation of PD-Iα/ATX does not seem to affect the crucial components of these pathways. In an extended interpretation, these findings suggest that down-regulation of PD-Iα/ATX does not block the entire differentiation program in maturing cells of the oligodendrocyte lineage.
LPA-mediated effects on oligodendrocyte network area and MBP mRNA levels were only revealed after down-regulation of PD-Iα/ATX expression. Interestingly, in the case of the membrane index, a slight increase was observed upon LPA application under control conditions, i.e. without prior down-regulation of PD-Iα/ATX expression (data not shown). This effect was, however, not associated with a concomitant increase in network area (see Fig. 2). Thus, the increase in membrane index under control conditions appears primarily due to a decrease in acetylated α-tubulin that is likely associated with a rearrangement from fine and complex process networks to membranous structures. Myelin sheath formation has been recently suggested to involve destabilization of microtubules and deacetylation of a-tubulin [61]. Thus, the LPA-mediated effect on oligodendrocyte morphology may be associated with cytoskeletal rearrangements involved in the transition from process outgrowth to membrane sheath formation. In support of this idea, it has been previously shown that LPA induces rearrangements of microtubules during remodeling of neuronal processes [62, 63].
Down-regulation of PD-Iα/ATX was found here to negatively affect MBP expression. Subsequent application of LPA reverted this effect. These data point toward a role of LPA on the expression of certain genes known to be important for myelination. Up-regulation of gene expression within hours of LPA application has been previously reported to occur and to be mediated by signaling through LPA receptors [64–67]. Accordingly, the LPA-mediated effects described here are thought to be mediated by signaling through one or more of the cell surface LPA receptors expressed by differentiating oligodendrocytes. Upon agonist stimulation, most GPCRs are rapidly internalized into cells through endocytic pathways. Indeed, LPA receptor activation has been associated with vesicle-mediated endocytosis [68–71]. Our immunostaining for LPA1 through 3 revealed a punctate pattern indicative of LPA receptor localization to intracellular vesicles. Thus, LPA receptor activation is likely operative in cultures of primary oligodendroglial cells as utilized here. We consider it unlikely that the effects we observe are mediated via the recently discovered nuclear LPA receptor (PPAR ) since extracellular LPA has long been known to have access to the intracellular compartment only when applied in a carrier (albumin)-free manner [72, 73].
Knock-out mice for LPA receptors have so far not revealed any specific myelination defects [74–76]. This observation, however, does not minimize the predicted importance of LPA receptor signaling for oligodendrocyte differentiation and myelination. The existence of an increasing number of LPA receptors and the redundancy of lysophospholipid-evoked cellular responses create a scenario of great complexity [15]. Thus, more sophisticated strategies may have to be employed to be able to dissect in vivo the physiological roles for each of the LPA receptors.
In summary, the data presented here reveal a potentially crucial role for LPA during the later stages of oligodendrocyte maturation. Due to the heterogeneity of the oligodendrocyte cultures with respect to their developmental stage, we cannot rule out the possibility that the LPA-mediated effects observed here are limited to a narrow developmental window. Nevertheless, these effects were only revealed after down-regulation of PD-Iα/ATX expression. In light of the well established role of PD-Iα/ATX as the major enzyme generating exogenous LPA, it is, therefore, tempting to speculate that during normal development LPA is generated in the environment surrounding the maturing oligodendrocyte via PD-Iα/ATX’s lysoPLD active site. This generation of exogenous LPA can, at the appropriate developmental time point, promote the transition from a highly branched network of fine processes to myelin sheath formation associated with an increase in MBP expression. Interestingly, the prior establishment of the complex network of fine processes is facilitated by PD-Iα/ATX’s second functionally active site, the MORFO domain [33]. Thus, PD-Iα/ATX seems to promote oligodendrocyte maturation via the concerted action of its functionally active sites. Future studies will, however, be necessary to dissect the exact roles of PD-Iα/ATX for myelination during normal development and during myelin repair under pathological demyelinating conditions.
Supplementary Material
Acknowledgments
The authors thank S. Pfeiffer for the hybridoma cell line O4 and S. Spiegel for helpful discussions and comments. Confocal and fluorescent microscopy was performed at VCU’s Department of Neurobiology and Anatomy Microscopy Facility, which was supported, in part, through NIH-NINDS Center Core grant 5P30NS047463. This work was supported by grants from the National Institute of Health (B.F), the National Multiple Sclerosis Society (B.F.), a postdoctoral fellowship award from the National Multiple Sclerosis Society (J.D.) and a predoctoral fellowship award from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior of the Brazilian Ministry of Education (L.N.).
References
- 1.Miller RH. Regulation of oligodendrocyte development in the vertebrate CNS. Prog Neurobiol. 2002;67:451–67. doi: 10.1016/s0301-0082(02)00058-8. [DOI] [PubMed] [Google Scholar]
- 2.Richardson WD, Kessaris N, Pringle N. Oligodendrocyte wars. Nat Rev Neurosci. 2006;7:11–8. doi: 10.1038/nrn1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kachar B, Behar T, Dubois-Dalcq M. Cell shape and motility of oligodendrocytes cultured without neurons. Cell Tissue Res. 1986;244:27–38. doi: 10.1007/BF00218378. [DOI] [PubMed] [Google Scholar]
- 4.Knapp PE, Bartlett WP, Skoff RP. Cultured oligodendrocytes mimic in vivo phenotypic characteristics: cell shape, expression of myelin-specific antigens, and membrane production. Dev Biol. 1987;120:356–65. doi: 10.1016/0012-1606(87)90238-7. [DOI] [PubMed] [Google Scholar]
- 5.Pfeiffer SE, Warrington AE, Bansal R. The oligodendrocyte and its many cellular processes. Trends Cell Biol. 1993;3:191–97. doi: 10.1016/0962-8924(93)90213-k. [DOI] [PubMed] [Google Scholar]
- 6.Hardy RJ, Friedrich VL., Jr Progressive remodeling of the oligodendrocyte process arbor during myelinogenesis. Dev Neurosci. 1996;18:243–54. doi: 10.1159/000111414. [DOI] [PubMed] [Google Scholar]
- 7.Dugas JC, Tai YC, Speed TP, et al. Functional genomic analysis of oligodendrocyte differentiation. J Neurosci. 2006;26:10967–83. doi: 10.1523/JNEUROSCI.2572-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81:871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- 9.Choi JW, Lee CW, Chun J. Biological roles of lysophospholipid receptors revealed by genetic null mice: An update. Biochim Biophys Acta. 2008 doi: 10.1016/j.bbalip.2008.03.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyer zu Heringdorf D, Jakobs KH. Lysophospholipid receptors: signalling, pharmacology and regulation by lysophospholipid metabolism. Biochim Biophys Acta. 2007;1768:923–40. doi: 10.1016/j.bbamem.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 11.Birgbauer E, Chun J. New developments in the biological functions of lysophospholipids. Cell Mol Life Sci. 2006;63:2695–701. doi: 10.1007/s00018-006-6155-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noguchi K, Ishii S, Shimizu T. Identification of p2y9/GPR23 as a novel G protein-coupled receptor for lysophosphatidic acid, structurally distant from the Edg family. J Biol Chem. 2003;278:25600–6. doi: 10.1074/jbc.M302648200. [DOI] [PubMed] [Google Scholar]
- 13.Lee CW, Rivera R, Gardell S, et al. GPR92 as a new G12/13- and Gq-coupled lysophosphatidic acid receptor that increases cAMP, LPA5. J Biol Chem. 2006;281:23589–97. doi: 10.1074/jbc.M603670200. [DOI] [PubMed] [Google Scholar]
- 14.Tabata K, Baba K, Shiraishi A, et al. The orphan GPCR GPR87 was deorphanized and shown to be a lysophosphatidic acid receptor. Biochem Biophys Res Commun. 2007;363:861–6. doi: 10.1016/j.bbrc.2007.09.063. [DOI] [PubMed] [Google Scholar]
- 15.Valentine WJ, Fujiwara Y, Tsukahara R, et al. Lysophospholipid signaling: Beyond the EDGs. Biochim Biophys Acta. 2007;1780:597–605. doi: 10.1016/j.bbagen.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiner JA, Hecht JH, Chun J. Lysophosphatidic acid receptor gene vzg-1/lpA1/edg-2 is expressed by mature oligodendrocytes during myelination in the postnatal murine brain. J Comp Neurol. 1998;398:587–98. [PubMed] [Google Scholar]
- 17.Allard J, Barron S, Diaz J, et al. A rat G protein-coupled receptor selectively expressed in myelin-forming cells. Eur J Neurosci. 1998;10:1045–53. doi: 10.1046/j.1460-9568.1998.00117.x. [DOI] [PubMed] [Google Scholar]
- 18.Allard J, Barron S, Trottier S, et al. Edg-2 in myelin-forming cells: isoforms, genomic mapping, and exclusion in Charcot-Marie-Tooth disease. Glia. 1999;26:176–85. doi: 10.1002/(sici)1098-1136(199904)26:2<176::aid-glia8>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 19.Handford EJ, Smith D, Hewson L, et al. Edg2 receptor distribution in adult rat brain. Neuroreport. 2001;12:757–60. doi: 10.1097/00001756-200103260-00029. [DOI] [PubMed] [Google Scholar]
- 20.Yu N, Lariosa-Willingham KD, Lin FF, et al. Characterization of lysophosphatidic acid and sphingosine-1-phosphate-mediated signal transduction in rat cortical oligodendrocytes. Glia. 2004;45:17–27. doi: 10.1002/glia.10297. [DOI] [PubMed] [Google Scholar]
- 21.Moller T, Musante DB, Ransom BR. Lysophosphatidic acid-induced calcium signals in cultured rat oligodendrocytes. Neuroreport. 1999;10:2929–32. doi: 10.1097/00001756-199909290-00010. [DOI] [PubMed] [Google Scholar]
- 22.Dawson J, Hotchin N, Lax S, et al. Lysophosphatidic acid induces process retraction in CG-4 line oligodendrocytes and oligodendrocyte precursor cells but not in differentiated oligodendrocytes. J Neurochem. 2003;87:947–57. doi: 10.1046/j.1471-4159.2003.02056.x. [DOI] [PubMed] [Google Scholar]
- 23.Cervera P, Tirard M, Barron S, et al. Immunohistological localization of the myelinating cell-specific receptor LP(A1) Glia. 2002;38:126–36. doi: 10.1002/glia.10054. [DOI] [PubMed] [Google Scholar]
- 24.Stankoff B, Barron S, Allard J, et al. Oligodendroglial expression of Edg-2 receptor: developmental analysis and pharmacological responses to lysophosphatidic acid. Mol Cell Neurosci. 2002;20:415–28. doi: 10.1006/mcne.2002.1129. [DOI] [PubMed] [Google Scholar]
- 25.Narita M, Goji J, Nakamura H, et al. Molecular cloning, expression, and localization of a brain-specific phosphodiesterase I/nucleotide pyrophosphatase (PD-I alpha) from rat brain. J Biol Chem. 1994;269:28235–42. [PubMed] [Google Scholar]
- 26.Fuss B, Baba H, Phan T, et al. Phosphodiesterase I, a novel adhesion molecule and/or cytokine involved in oligodendrocyte function. J Neurosci. 1997;17:9095–103. doi: 10.1523/JNEUROSCI.17-23-09095.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanaka M, Okudaira S, Kishi Y, et al. Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J Biol Chem. 2006;281:25822–30. doi: 10.1074/jbc.M605142200. [DOI] [PubMed] [Google Scholar]
- 28.van Meeteren LA, Ruurs P, Stortelers C, et al. Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol Cell Biol. 2006;26:5015–22. doi: 10.1128/MCB.02419-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuelling LM, Fuss B. Autotaxin (ATX): A multi-functional and multi-modular protein possessing enzymatic lysoPLD activity and matricellular properties. Biochim Biophys Acta. 2008 doi: 10.1016/j.bbalip.2008.04.009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fox MA, Colello RJ, Macklin WB, et al. Phosphodiesterase-Ialpha/autotaxin: a counteradhesive protein expressed by oligodendrocytes during onset of myelination. Mol Cell Neurosci. 2003;23:507–19. doi: 10.1016/s1044-7431(03)00073-3. [DOI] [PubMed] [Google Scholar]
- 31.Fox MA, Alexander JK, Afshari FS, et al. Phosphodiesterase-I alpha/autotaxin controls cytoskeletal organization and FAK phosphorylation during myelination. Mol Cell Neurosci. 2004;27:140–50. doi: 10.1016/j.mcn.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Dennis J, Nogaroli L, Fuss B. Phosphodiesterase-Ialpha/autotaxin (PD-Ialpha/ATX): a multifunctional protein involved in central nervous system development and disease. J Neurosci Res. 2005;82:737–42. doi: 10.1002/jnr.20686. [DOI] [PubMed] [Google Scholar]
- 33.Dennis D, White MA, Forrest AD, et al. Phosphodiesterase-I/Autotaxin’s MORFO domain regulates oligodendroglial process network formation and focal adhesion organization. Mol Cell Neurosci. 2008;37:412–24. doi: 10.1016/j.mcn.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sommer I, Schachner M. Cell that are O4 antigen-positive and O1 antigen-negative differentiate into O1 antigen-positive oligodendrocytes. Neurosci Lett. 1982;29:183–8. doi: 10.1016/0304-3940(82)90351-2. [DOI] [PubMed] [Google Scholar]
- 35.Bansal R, Warrington AE, Gard AL, et al. Multiple and novel specificities of monoclonal antibodies O1, O4, and R-mAb used in the analysis of oligodendrocyte development. J Neurosci Res. 1989;24:548–57. doi: 10.1002/jnr.490240413. [DOI] [PubMed] [Google Scholar]
- 36.Barres BA, Hart IK, Coles HS, et al. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992;70:31–46. doi: 10.1016/0092-8674(92)90531-g. [DOI] [PubMed] [Google Scholar]
- 37.Horak CE, Lee JH, Elkahloun AG, et al. Nm23-H1 suppresses tumor cell motility by down-regulating the lysophosphatidic acid receptor EDG2. Cancer Res. 2007;67:7238–46. doi: 10.1158/0008-5472.CAN-07-0962. [DOI] [PubMed] [Google Scholar]
- 38.Park SY, Schinkmann KA, Avraham S. RAFTK/Pyk2 mediates LPA-induced PC12 cell migration. Cell Signal. 2006;18:1063–71. doi: 10.1016/j.cellsig.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 39.Zaslavsky A, Singh LS, Tan H, et al. Homo- and hetero-dimerization of LPA/S1P receptors, OGR1 and GPR4. Biochim Biophys Acta. 2006;1761:1200–12. doi: 10.1016/j.bbalip.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 40.Peirson SN, Butler JN, Foster RG. Experimental validation of novel and conventional approaches to quantitative real-time PCR data analysis. Nucleic Acids Res. 2003;31:e73. doi: 10.1093/nar/gng073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 42.Abramoff MD, Magelhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- 43.Sommer I, Schachner M. Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfaces: an immunocytological study in the central nervous system. Dev Biol. 1981;83:311–27. doi: 10.1016/0012-1606(81)90477-2. [DOI] [PubMed] [Google Scholar]
- 44.Warrington AE, Barbarese E, Pfeiffer SE. Stage specific, (O4+GalC-) isolated oligodendrocyte progenitors produce MBP+ myelin in vivo. Dev Neurosci. 1992;14:93–7. doi: 10.1159/000111652. [DOI] [PubMed] [Google Scholar]
- 45.Solly SK, Thomas JL, Monge M, et al. Myelin/oligodendrocyte glycoprotein (MOG) expression is associated with myelin deposition. Glia. 1996;18:39–48. doi: 10.1002/(SICI)1098-1136(199609)18:1<39::AID-GLIA4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 46.Ferguson CG, Bigman CS, Richardson RD, et al. Fluorogenic phospholipid substrate to detect lysophospholipase D/autotaxin activity. Org Lett. 2006;8:2023–6. doi: 10.1021/ol060414i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Piperno G, LeDizet M, Chang XJ. Microtubules containing acetylated alpha-tubulin in mammalian cells in culture. J Cell Biol. 1987;104:289–302. doi: 10.1083/jcb.104.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lunn KF, Baas PW, Duncan ID. Microtubule organization and stability in the oligodendrocyte. J Neurosci. 1997;17:4921–32. doi: 10.1523/JNEUROSCI.17-13-04921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Richter-Landsberg C. Organization and functional roles of the cytoskeleton in oligodendrocytes. Microsc Res Tech. 2001;52:628–36. doi: 10.1002/jemt.1047. [DOI] [PubMed] [Google Scholar]
- 50.Liu A, Muggironi M, Marin-Husstege M, et al. Oligodendrocyte process outgrowth in vitro is modulated by epigenetic regulation of cytoskeletal severing proteins. Glia. 2003;44:264–74. doi: 10.1002/glia.10290. [DOI] [PubMed] [Google Scholar]
- 51.Lee J, Gravel M, Zhang R, et al. Process outgrowth in oligodendrocytes is mediated by CNP, a novel microtubule assembly myelin protein. J Cell Biol. 2005;170:661–73. doi: 10.1083/jcb.200411047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li W, Zhang B, Tang J, et al. Sirtuin 2, a mammalian homolog of yeast silent information regulator-2 longevity regulator, is an oligodendroglial protein that decelerates cell differentiation through deacetylating alpha-tubulin. J Neurosci. 2007;27:2606–16. doi: 10.1523/JNEUROSCI.4181-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barbarese E, Carson JH, Braun PE. Accumulation of the four myelin basic proteins in mouse brain during development. J Neurochem. 1978;31:779–82. doi: 10.1111/j.1471-4159.1978.tb00110.x. [DOI] [PubMed] [Google Scholar]
- 54.Campagnoni CW, Carey GD, Campagnoni AT. Synthesis of myelin basic proteins in the developing mouse brain. Arch Biochem Biophys. 1978;190:118–25. doi: 10.1016/0003-9861(78)90258-8. [DOI] [PubMed] [Google Scholar]
- 55.Staugaitis SM, Colman DR, Pedraza L. Membrane adhesion and other functions for the myelin basic proteins. Bioessays. 1996;18:13–8. doi: 10.1002/bies.950180106. [DOI] [PubMed] [Google Scholar]
- 56.Kimura M, Sato M, Akatsuka A, et al. Overexpression of a minor component of myelin basic protein isoform (17.2 kDa) can restore myelinogenesis in transgenic shiverer mice. Brain Res. 1998;785:245–52. doi: 10.1016/s0006-8993(97)01383-8. [DOI] [PubMed] [Google Scholar]
- 57.Campagnoni AT, Campagnoni C. Myelin basic protein gene. In: Lazzarini RA, editor. Myelin biology and disorders. Elsevier Academic Press; San Diego: 2004. pp. 387–400. [Google Scholar]
- 58.Boggs JM. Myelin basic protein: a multifunctional protein. Cell Mol Life Sci. 2006;63:1945–61. doi: 10.1007/s00018-006-6094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Campagnoni AT. Molecular biology of myelin proteins from the central nervous system. J Neurochem. 1988;51:1–14. doi: 10.1111/j.1471-4159.1988.tb04827.x. [DOI] [PubMed] [Google Scholar]
- 60.Allinquant B, Staugaitis SM, D’Urso D, et al. The ectopic expression of myelin basic protein isoforms in Shiverer oligodendrocytes: implications for myelinogenesis. J Cell Biol. 1991;113:393–403. doi: 10.1083/jcb.113.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Southwood CM, Peppi M, Dryden S, et al. Microtubule deacetylases, SirT2 and HDAC6, in the nervous system. Neurochem Res. 2007;32:187–95. doi: 10.1007/s11064-006-9127-6. [DOI] [PubMed] [Google Scholar]
- 62.Sayas CL, Avila J, Wandosell F. Regulation of neuronal cytoskeleton by lysophosphatidic acid: role of GSK-3. Biochim Biophys Acta. 2002;1582:144–53. doi: 10.1016/s1388-1981(02)00149-x. [DOI] [PubMed] [Google Scholar]
- 63.Fukushima N, Morita Y. Actomyosin-dependent microtubule rearrangement in lysophosphatidic acid-induced neurite remodeling of young cortical neurons. Brain Res. 2006;1094:65–75. doi: 10.1016/j.brainres.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 64.Klemm S, Zimmermann S, Peschel C, et al. Bcl10 and Malt1 control lysophosphatidic acid-induced NF-kappaB activation and cytokine production. Proc Natl Acad Sci U S A. 2007;104:134–8. doi: 10.1073/pnas.0608388103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee J, Park SY, Lee EK, et al. Activation of hypoxia-inducible factor-1alpha is necessary for lysophosphatidic acid-induced vascular endothelial growth factor expression. Clin Cancer Res. 2006;12:6351–8. doi: 10.1158/1078-0432.CCR-06-1252. [DOI] [PubMed] [Google Scholar]
- 66.Cui MZ, Laag E, Sun L, et al. Lysophosphatidic acid induces early growth response gene 1 expression in vascular smooth muscle cells: CRE and SRE mediate the transcription. Arterioscler Thromb Vasc Biol. 2006;26:1029–35. doi: 10.1161/01.ATV.0000214980.90567.b5. [DOI] [PubMed] [Google Scholar]
- 67.Lee H, Lin CI, Liao JJ, et al. Lysophospholipids increase ICAM-1 expression in HUVEC through a Gi- and NF-kappaB-dependent mechanism. Am J Physiol Cell Physiol. 2004;287:C1657–66. doi: 10.1152/ajpcell.00172.2004. [DOI] [PubMed] [Google Scholar]
- 68.Luttrell LM, Daaka Y, Della Rocca GJ, et al. G protein-coupled receptors mediate two functionally distinct pathways of tyrosine phosphorylation in rat 1a fibroblasts. Shc phosphorylation and receptor endocytosis correlate with activation of Erk kinases. J Biol Chem. 1997;272:31648–56. doi: 10.1074/jbc.272.50.31648. [DOI] [PubMed] [Google Scholar]
- 69.Kranenburg O, Verlaan I, Moolenaar WH. Gi-mediated tyrosine phosphorylation of Grb2 (growth-factor-receptor-bound protein 2)-bound dynamin-II by lysophosphatidic acid. Biochem J. 1999;339(Pt 1):11–4. [PMC free article] [PubMed] [Google Scholar]
- 70.Murph MM, Scaccia LA, Volpicelli LA, et al. Agonist-induced endocytosis of lysophosphatidic acid-coupled LPA1/EDG-2 receptors via a dynamin2- and Rab5-dependent pathway. J Cell Sci. 2003;116:1969–80. doi: 10.1242/jcs.00397. [DOI] [PubMed] [Google Scholar]
- 71.Urs NM, Jones KT, Salo PD, et al. A requirement for membrane cholesterol in the beta-arrestin- and clathrin-dependent endocytosis of LPA1 lysophosphatidic acid receptors. J Cell Sci. 2005;118:5291–304. doi: 10.1242/jcs.02634. [DOI] [PubMed] [Google Scholar]
- 72.Tokumura A, Tsutsumi T, Tsukatani H. Transbilayer movement and metabolic fate of ether-linked phosphatidic acid (1-O-Octadecyl-2-acetyl-sn-glycerol 3-phosphate) in guinea pig peritoneal polymorphonuclear leukocytes. J Biol Chem. 1992;267:7275–83. [PubMed] [Google Scholar]
- 73.McIntyre TM, Pontsler AV, Silva AR, et al. Identification of an intracellular receptor for lysophosphatidic acid (LPA): LPA is a transcellular PPARgamma agonist. Proc Natl Acad Sci U S A. 2003;100:131–6. doi: 10.1073/pnas.0135855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Contos JJ, Ishii I, Fukushima N, et al. Characterization of lpa(2) (Edg4) and lpa(1)/lpa(2) (Edg2/Edg4) lysophosphatidic acid receptor knockout mice: signaling deficits without obvious phenotypic abnormality attributable to lpa(2) Mol Cell Biol. 2002;22:6921–9. doi: 10.1128/MCB.22.19.6921-6929.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang AH, Ishii I, Chun J. In vivo roles of lysophospholipid receptors revealed by gene targeting studies in mice. Biochim Biophys Acta. 2002;1582:197–203. doi: 10.1016/s1388-1981(02)00172-5. [DOI] [PubMed] [Google Scholar]
- 76.Ye X, Hama K, Contos JJ, et al. LPA3-mediated lysophosphatidic acid signalling in embryo implantation and spacing. Nature. 2005;435:104–8. doi: 10.1038/nature03505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.