Abstract
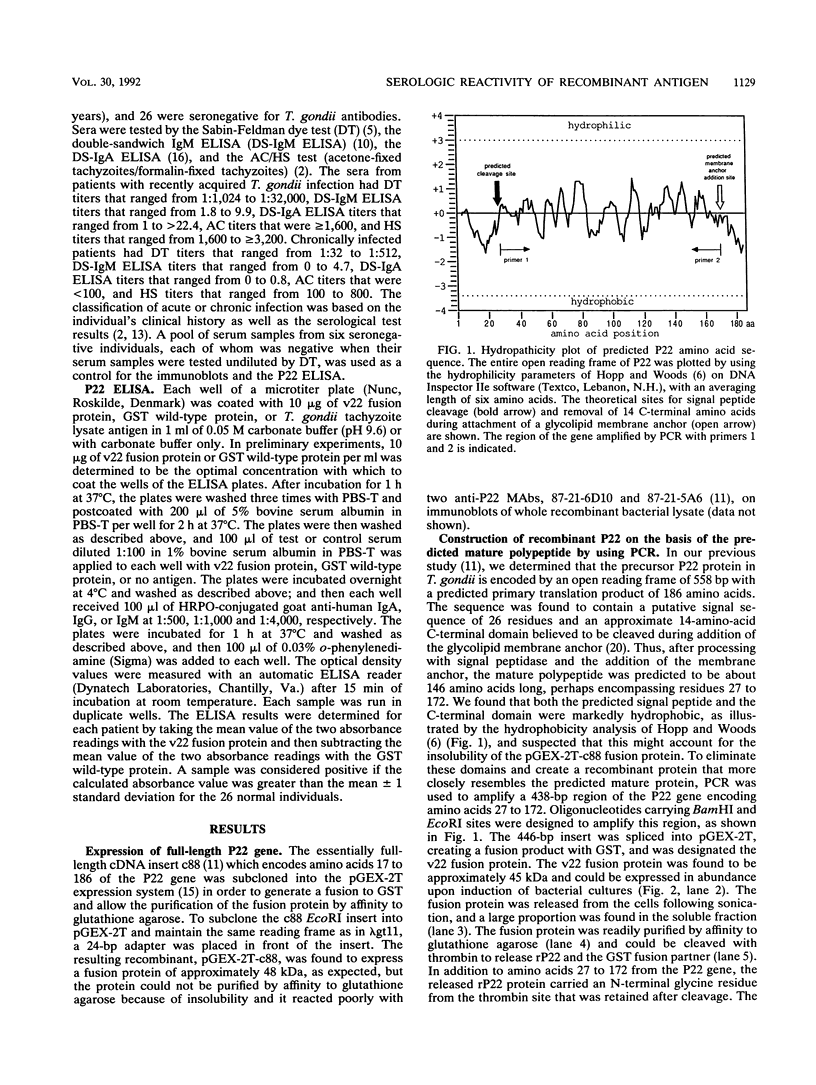
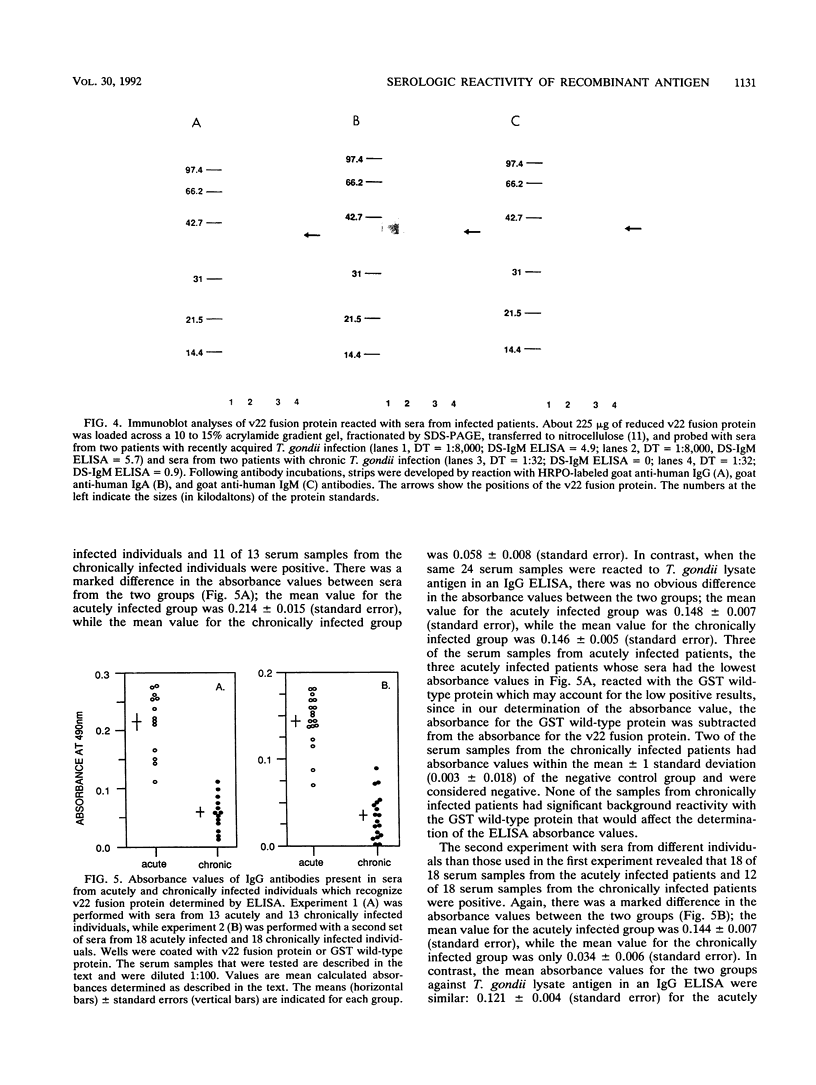
The utility of recombinant Toxoplasma gondii surface antigen P22 for the detection of specific T. gondii antibodies in human sera was evaluated. Polymerase chain reaction was used to produce a 438-bp fragment of the P22 gene; the fragment corresponded to the amino acids predicted to be in the processed, native antigen. The fragment was subcloned into pGEX-2T and was expressed in Escherichia coli as a glutathione-S-transferase (GST) fusion protein. The fusion protein was purified in a soluble form and was found to be recognized by sera from infected individuals in immunoblots and an enzyme-linked immunosorbent assay. Immunoglobulin G antibodies in sera from 31 acutely infected patients in general reacted more strongly to the fusion protein than did those in sera from 31 patients with the chronic infection. None of the sera from a panel of 26 seronegative controls reacted with the fusion protein was separated from the GST partner by cleavage with thrombin, it retained its immunoreactivity and its electrophoretic mobility in polyacrylamide gels was found to be similar to that of native P22. By a modification of the published method for purification of the foreign polypeptide from the GST carrier, the recombinant P22 was readily purified to homogeneity by thrombin cleavage of the fusion protein while it was adsorbed to glutathione agarose.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Couvreur G., Sadak A., Fortier B., Dubremetz J. F. Surface antigens of Toxoplasma gondii. Parasitology. 1988 Aug;97(Pt 1):1–10. doi: 10.1017/s0031182000066695. [DOI] [PubMed] [Google Scholar]
- Dannemann B. R., Vaughan W. C., Thulliez P., Remington J. S. Differential agglutination test for diagnosis of recently acquired infection with Toxoplasma gondii. J Clin Microbiol. 1990 Sep;28(9):1928–1933. doi: 10.1128/jcm.28.9.1928-1933.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Handman E., Goding J. W., Remington J. S. Detection and characterization of membrane antigens of Toxoplasma gondii. J Immunol. 1980 Jun;124(6):2578–2583. [PubMed] [Google Scholar]
- Handman E., Remington J. S. Antibody responses to toxoplasma antigens in mice infected with strains of different virulence. Infect Immun. 1980 Jul;29(1):215–220. doi: 10.1128/iai.29.1.215-220.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huskinson J., Stepick-Biek P., Remington J. S. Detection of antigens in urine during acute toxoplasmosis. J Clin Microbiol. 1989 May;27(5):1099–1101. doi: 10.1128/jcm.27.5.1099-1101.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. M., Illana S. Cloning of Toxoplasma gondii gene fragments encoding diagnostic antigens. Gene. 1991 Mar 1;99(1):127–132. doi: 10.1016/0378-1119(91)90044-c. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Naot Y., Remington J. S. An enzyme-linked immunosorbent assay for detection of IgM antibodies to Toxoplasma gondii: use for diagnosis of acute acquired toxoplasmosis. J Infect Dis. 1980 Nov;142(5):757–766. doi: 10.1093/infdis/142.5.757. [DOI] [PubMed] [Google Scholar]
- Prince J. B., Auer K. L., Huskinson J., Parmley S. F., Araujo F. G., Remington J. S. Cloning, expression, and cDNA sequence of surface antigen P22 from Toxoplasma gondii. Mol Biochem Parasitol. 1990 Nov;43(1):97–106. doi: 10.1016/0166-6851(90)90134-8. [DOI] [PubMed] [Google Scholar]
- Prince J. B., Koven-Quinn M. A., Remington J. S., Sharma S. D. Cell free synthesis of Toxoplasma gondii antigens. Mol Biochem Parasitol. 1985 Nov;17(2):163–170. doi: 10.1016/0166-6851(85)90015-5. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Stepick-Biek P., Thulliez P., Araujo F. G., Remington J. S. IgA antibodies for diagnosis of acute congenital and acquired toxoplasmosis. J Infect Dis. 1990 Jul;162(1):270–273. doi: 10.1093/infdis/162.1.270. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Thulliez P., Remington J. S. Use of acute-stage-specific antigens of Toxoplasma gondii for serodiagnosis of acute toxoplasmosis. J Clin Microbiol. 1990 Aug;28(8):1734–1738. doi: 10.1128/jcm.28.8.1734-1738.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenter A. M., Johnson A. M. Human antibody response to the nucleoside triphosphate hydrolase of Toxoplasma gondii. J Immunoassay. 1990;11(4):579–590. doi: 10.1080/01971529008055051. [DOI] [PubMed] [Google Scholar]
- Tenter A. M., Johnson A. M. Recognition of recombinant Toxoplasma gondii antigens by human sera in an ELISA. Parasitol Res. 1991;77(3):197–203. doi: 10.1007/BF00930858. [DOI] [PubMed] [Google Scholar]
- Tomavo S., Schwarz R. T., Dubremetz J. F. Evidence for glycosyl-phosphatidylinositol anchoring of Toxoplasma gondii major surface antigens. Mol Cell Biol. 1989 Oct;9(10):4576–4580. doi: 10.1128/mcb.9.10.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]






