Abstract
Recognizing the value of including complex pyridines in small-molecule screening collections, we developed a previously unexplored [2 + 2 + 2]-cycloaddition of silyl-tethered diynes with nitriles. The tether provides high regioselectivity, while the solvent THF allows catalytic CpCo(CO)2 to be used without exogenous irradiation. One of the resulting bicyclic and monocyclic (desilylated) pyridines was identified as an inhibitor of neuregulin-induced neurite outgrowth (EC50 = 0.30 µM) in a screen that probes a pathway likely to be involved in breast cancers and schizophrenia.
We are contributing to the complete synthesis of a small-molecule screening collection whose members have properties that facilitate discovery, optimization, and manufacturing of biologically active small molecules.(1) Recognizing the value of including complex pyridines(2) in this resource, we conceived of a previously unexplored transition-metal-mediated [2 + 2 + 2]-cycloaddition of silyl ether-tethered diynes with nitriles as a route to bicyclic pyridines. The tether was used to control regioselectivity and to enable modification of the products into complex monocyclic pyridines. Here, we report an irradiation-free Co(I)-catalyzed [2 + 2 + 2]-cycloaddition reaction yielding complex, bicyclic pyridines. Assaying the resulting compounds in a cell-based screen that reveals a visual phenotype of a targeted signaling pathway resulted in the discovery of an inhibitor of neuregulin-1/ErbB4-mediated neuronal differentiation.
The discovery of well-defined metal complexes (Co, Rh, Ir, Ru, Ti, and Ni) as catalysts that induce cycloaddition has advanced the synthesis of pyridines considerably.(3) The CpCo(CO)2 catalyzed reaction has been widely used in recent years.(4) These reactions typically require high temperature and irradiation to generate active Co(I) by liberating CO, limiting overall generality. Alternatives (CpCo(cod)(5) and CpCo(ethylene)2(6)) exist that liberate active catalyst under milder conditions, but these precatalysts are difficult to handle.(7) While studies have explored the effect of altering the ligand sphere around cobalt,(8) the effect of exogenous ligands and solvents on catalyst performance has not been studied extensively.(9)
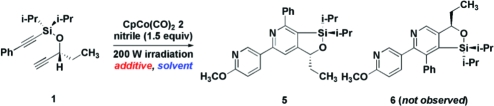
When 1(10) was treated with 2-methoxy-5-pyridinecarbonitrile and stoichiometric CpCo(CO)22 in xylenes (140 °C, 200 W irradiation), a moderate conversion to 5 was achieved (Table 1, entry 1), while the regioisomeric pyridine 6 was not detected. The regiochemistry of the pyridines was determined by NoE; data are provided in the Supporting Information.(11) We were unable to drive the reaction to completion by increasing nitrile stoichiometry (1.5−5.0 equiv). When 25 mol % of 2 was used, only 17% conversion was observed. Due to the hindered nature of these substrates,(12) we postulated that catalyst decomposition may be competitive. Accordingly, we attempted to stabilize CpCo(I). Surprisingly, a dramatic solvent effect was noted, despite the reports of nonpolar, noncoordinating solvents as being optimal in these reactions. 1,2-Dimethoxyethane (entry 3), 1,2-dichloroethane (entry 4), 2-methyltetrahydrofuran (entry 9), and THF (entry 10) all resulted in catalytic reactions using 25 mol % of 2. Attempts to stabilize CpCo(I) further by introducing chelating ligands resulted in lower conversions relative to the control reaction in THF, suggesting a possible trade-off between ligand stabilization and substrate coordination (entries 11−15).(13) Gratifyingly, when the reaction was performed in THF in the absence of irradiation, conversion increased to >95%. Attempts to reduce the temperature abolished catalytic activity, which is expected due to the stability of the CO ligands. NiCl2(dppp)/Zn,(14) CoCl2/Zn,(15) and RhCl(PPh3)3,(16) resulted in recovery of starting materials or complex mixtures of products.
Table 1. Development of a Catalytic [2 + 2 + 2] Cycloaddition.
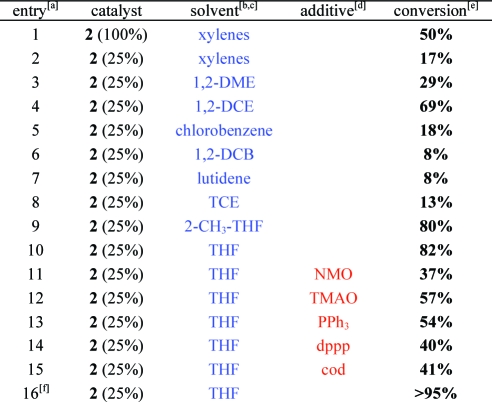 |
Experimental procedures provided in the Supporting Information. Nitrile: 2-methoxy-5-pyridine carbonitrile.
Solvents were degassed by bubbling argon for 20 min prior to use. 1,2-DME: dimethoxyethane. 1,2-DCE: dichloroethane. 1,2-DCB: dichlorobenzene. TCE: trichloroethylene. DMA: dimethylacetamide.
1,4-Dioxane, pyridine, and DMA gave <2% conversion.
Additives at 50 mol %.
Conversions determined by 1H NMR spectroscopy.
Reaction ran without irradiation.
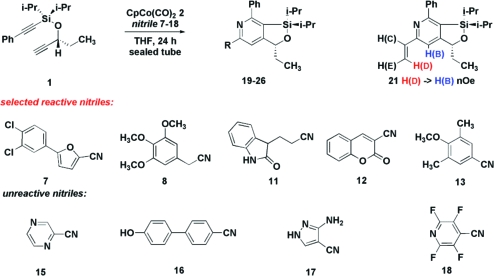
Based on the above data, we adopted a standard procedure using degassed THF at 140 °C in a sealed tube without irradiation. Using these conditions, nitriles 7−18 were screened to determine the generality of the reaction (Table 2). The electronics of the nitrile did not have a predictable effect on either conversion or yield. Aromatic (entry 10), heteroaromatic (entries 1 and 9), benzylic (entry 3), as well as primary (entry 8) and secondary (entry 12) aliphatic nitriles were effective reactants. Pyrazine-containing nitrile 15, nitriles bearing free hydroxyl and amine groups (16 and 17), and strongly deactivated nitriles (e.g., 18) failed to give detectable amounts of pyridines. In selected cases where conversions were low or where products coeluted chromatographically with diyne 1, the desired heterocycles were obtained with >95% conversion by increasing loading of 2 (entries 5 and 6). Attempts to react 2π-systems other than nitriles (e.g., alkynes, isocyanates, and thioisocyanates) failed.(17)
Table 2. Effect of the Nitrile Component.
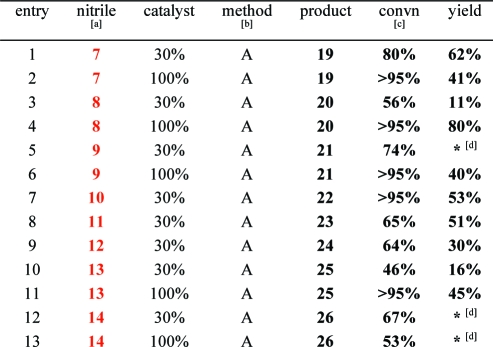 |
Nitriles: 9: acrylonitrile. 10: 5-methylfuran-2-carbonitrile. 14: cyclopropylnitrile.
Method A: crude reaction mixtures were filtered through a 4 g silica plug to remove insoluble cobalt species; combined fractions were assayed for conversion by 1H NMR. Organics were then chromatographed to obtain yields.
Conversions determined by 1H NMR spectroscopy.
∗ = coelution with diyne using standard chromatographic conditions. Regioselectivity was determined on the basis of 1D NOE studies of 21 (Supporting Information).
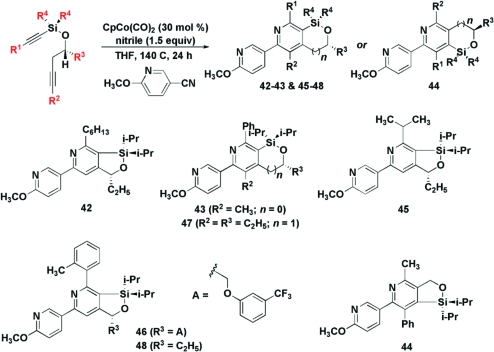
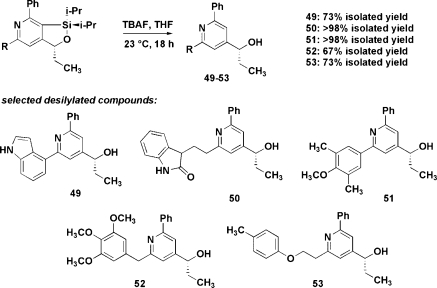
The silylated diynes used in this study possess several sites that can be modified to provide pyridines with different appendages and fused rings of various sizes (Table 3 and Figure 1).(18) Even large substituents on the silylated acetylene were accommodated (R1 = i-Pr, entry 5; R1 = o-tol, entry 7). Likewise, the distal alkyne need not be terminal (entries 3 and 4 (R2 = Me) and 9 (R2 = Et), allowing fully substituted pyridines to be accessed. In the case of 37, a mixture of regioisomers (43−44) was observed in a 2.7:1 ratio; this is the only case in which the alternative mode of addition was observed. Separation of the alkyne moieties by the addition of an extra methylene in the tether provided a fused silabicyclic compound 47 in 50% yield (entry 9). A modified protocol was devised that dispenses with the intermediate filtration step (methods A vs B, Table 3), often resulting in elevated yields. The diisopropylsilyl moiety could be easily extruded (Figure 1, five examples).
Table 3. Scope and Limitations with Respect to the Diyne.
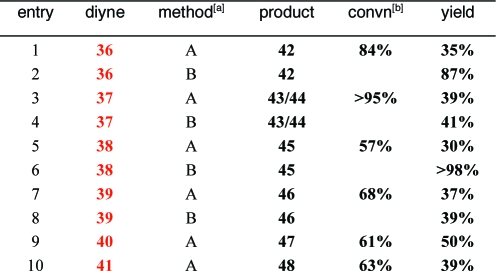 |
Method A is described in Table 2. Method B: Organics were directly chromatographed to obtain yields for 42−46.
Conversions determined by 1H NMR (method A). Since method B involves immediate chromatography, conversion was not determined for these reactions.
Figure 1.
Modification of cycloaddition products.
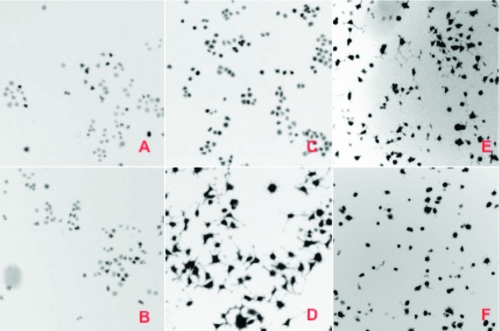
Pyridine-containing small molecules (e.g., Gleevec(19) and 2-methyl-6-(phenylethynyl)pyridine (MPEP)(20)) are known that have a wide range of biological activities. Twenty-five bicyclic and monocyclic pyridines were included in a small-molecule screening collection that was assayed using a live-cell, image-based, high-content screen. PC12 cells were engineered to express the ErbB4 receptor tyrosine kinase in order to monitor ErbB4-dependent cell differentiation upon activation by neuregulin-1 (Nrg1), a secreted activator of ErbB4.(21) ErbB4 (Figure 2) belongs to the family of receptor tyrosine kinases, which are membrane receptors that regulate cell proliferation, invasion, and differentation in response to extracellular protein factors. ErbB4 is the least studied of the epidermal growth factor receptor (EGFR) subfamily, yet both gene expression and genetic analyses suggest that ErbB4 is involved in the progression of breast cancer and in the pathogenesis of brain disorders such as schizophrenia.22,23 Discovering specific small-molecule modulators of ErbB4 receptor signaling could facilitate both an understanding of the functions of ErbB4 and the development of novel therapeutics. In the ErbB4-expressing PC12 cells, but not in the wild-type PC12 cells lacking ErbB4, neuregulin induces neurite outgrowth. Using this assay, pyridine 52 was discovered to be a potent inhibitor of the neuregulin/ErbB4 pathway, with an approximate EC50 of 0.30 µM (Figure 2F), while its bicyclic precursor (Figure 2E) is inactive at 20 µM, the highest concentration tested.
Figure 2.
(a) PC12 cells. (b) PC12 cells treated with neuregulin. (c) PC12 cells transfected with ErbB4. (d) PC12 + ErbB4 cells treated with neuregulin showing neurite outgrowth. (e) PC12 + ErbB4 cells treated with neuregulin in the presence of the silylated precursor to 52. (f) PC12 + ErbB4 cells treated with neuregulin in the presence of 52. Pyridine 52 was found to be a potent inhibitor of this cellular activity (EC50 = 0.30 µM). See the Supporting Information for details.
In summary, we report an irradiation-free cycloaddition protocol with broad generality for the synthesis of pyridines from silylated diynes and nitriles using THF to induce catalyst turnover.(24) The silyl ether proved useful for both enabling an expanded set of complex pyridines and illuminating structure/activity relationships, as highlighted by the discovery of compound 52 as a potent inhibitor of the neuregulin/ErbB4-dependent signaling pathway. Studies to expand the use of this reaction in the build/couple/pair strategy of diversity synthesis and to elucidate compound 52ʼs cellular mechanism of pathway modulation are underway.
Acknowledgments
This research was supported by the National Cancer Institute (NCI P01 CA-078048). B.L.G. acknowledges Eli Lilly and the National Science Foundation for predoctoral fellowships. S.L.S. is an investigator at the Howard Hughes Medical Institute at the Broad Institute of Harvard and MIT.
Supporting Information Available
Experimental details, spectral characterization data, data-analysis methods, and dose/response curves. This material is available free of charge via the Internet at http://pubs.acs.org.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Nielsen T. E.; Schreiber S. L. Angew. Chem., Int. Ed. 2008, 47, 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Heller B.; Hapke M. Chem. Soc. Rev. 2007, 36, 1085–1094. [DOI] [PubMed] [Google Scholar]; b Chopade P. R.; Louie J. Adv. Synth. Catal. 2006, 348, 2307–2327. [Google Scholar]; c Nakamura I.; Yamamoto Y. Chem. Rev. 2004, 104, 2127–2198. [DOI] [PubMed] [Google Scholar]; d Varela J. A.; Saá C. Chem. Rev. 2003, 103, 3787–3801. [DOI] [PubMed] [Google Scholar]
- Recent examples; a Doszczak L.; Fey P.; Tacke R. Synlett 2007, 75, 3–756. [Google Scholar]; b Zhou Y.; Porco J. A. Jr.; Synder J. K. Org. Lett. 2007, 9, 393–396. [DOI] [PubMed] [Google Scholar]; c Tanaka K.; Hara H.; Nishida G.; Hirano M. Org. Lett. 2007, 9, 1907–1910. [DOI] [PubMed] [Google Scholar]; d Trost B. M.; Gutierrez A. C. Org. Lett. 2007, 9, 1473–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Kase K.; Goswami A.; Ohtaki K.; Tanabe E.; Saino N.; Okamoto S. Org. Lett. 2007, 9, 931–934. Additional examples are available in supporting information (2004−2006). [DOI] [PubMed] [Google Scholar]
- a Senaiar R. S.; Young D. D.; Deiters A. Chem. Commun. 2006, 1313–1315. [DOI] [PubMed] [Google Scholar]; b Boñaga L. V. R.; Zhang H-C.; Moretto A. F.; Ye H.; Gauthier D. A.; Li J.; Leo G. C.; Maryanoff B. E. J. Am. Chem. Soc. 2005, 127, 3473–3485. [DOI] [PubMed] [Google Scholar]; c Groth U.; Huhn T.; Kesenheimer C.; Kalogerakis A. Synlett 2005, 1758–1760. [Google Scholar]
- a Heller B.; Oehme G. Chem. Commun. 1995, 179–180. [Google Scholar]; b Bönnemann H.; Brinkmann R.; Schenkluhn H. Synthesis 1974, 57, 5–577. [Google Scholar]
- Jonas K.; Deffense E.; Habermann D. Angew. Chem., Int. Ed. 1983, 22, 716–717. [Google Scholar]
- King R. B.; Treichel P. M.; Stone F. G. A. J. Am. Chem. Soc. 1961, 83, 3593–3597. [Google Scholar]
- a Gutnov A.; Heller B.; Fisher C.; Drexler H-J.; Spannenberg A.; Sundermann B.; Sundermann C. Angew. Chem., Int. Ed. 2004, 43, 3795–3797. [DOI] [PubMed] [Google Scholar]; b Fatland A. W.; Eaton B. E. Org. Lett. 2000, 2, 3131–3133. [DOI] [PubMed] [Google Scholar]; c Bönnemann H. Angew. Chem., Int. Ed. Engl. 1985, 24, 248–262. [Google Scholar]
- A reaction of diynes with cyanamides using 2 in 1,4-dioxanes has been reported; Boñaga L. V. R.; Zhang H-C.; Maryanoff B. E. Chem. Commun. 2004, 21, 2394–2395. [DOI] [PubMed] [Google Scholar]
- Petit M.; Chouraqui G.; Aubert C.; Malacria M. Org. Lett. 2003, 5, 2037–2040. [DOI] [PubMed] [Google Scholar]
- In other intramolecular reactions, the length of the tether affects the regioselectivity; see; Moretto A. F.; Zhang H-C.; Maryanoff B. E. J. Am. Chem. Soc. 2001, 123, 3157–3158. [Google Scholar]
- Examples often append the silyl group externally:; a McCormick M. M.; Duong H. A.; Zuo G.; Louie J. J. Am. Chem. Soc. 2005, 127, 5030–5031. [DOI] [PubMed] [Google Scholar]; b Gutnov A.; Abaev V.; Redkin D.; Fischer C.; Bonrath W.; Heller B. Synlett 2005, 1188–1190. [Google Scholar]; c Varela J. A.; Castedo L.; Saá C. J. Org. Chem. 1997, 62, 4189–4192. [Google Scholar]
- Coordination of pyridines with CpCo is also possible. Examples of coordination to products of [2 + 2 + 2]-cycloaddition exist. See:Gandon V.; Leboeuf, D.; Amslinger S.; Vollhardt K. P. C.; Malacria M.; Aubert C. Angew. Chem., Int. Ed. 2005, 44, 7114–7118. [DOI] [PubMed] [Google Scholar]
- Turek P.; Novák P.; Pohl R.; Hocek M.; Kotora M. J. Org. Chem. 2006, 71, 8978–8981. [DOI] [PubMed] [Google Scholar]
- Saino N.; Kogure D.; Okamoto S. Org. Lett. 2005, 7, 3065–3067. [DOI] [PubMed] [Google Scholar]
- Novák P.; Pohl R.; Kotora M.; Hocek M. Org. Lett. 2006, 8, 2051–2054. [DOI] [PubMed] [Google Scholar]
- For a [2 + 2 + 2] example with CO2, see:; Louie J.; Gibby J. E.; Farnworth M. V.; Tekavec T. N. J. Am. Chem. Soc. 2002, 124, 15188–15189. [DOI] [PubMed] [Google Scholar]; b Diyne cycloaddition with isocyanates:Yamamoto Y.; Takagishi H.; Itoh K. Org. Lett. 2001, 3, 2117–2119. [DOI] [PubMed] [Google Scholar]
- For the synthesis of a collection of diverse silylated diynes, see:Gray B. L.; Schreiber S. L. J. Comb. Chem. 2007, 9, 1028–1035. [DOI] [PubMed] [Google Scholar]
- Schiffer C. A. N. Engl. J. Med. 2007, 357, 258–265. [DOI] [PubMed] [Google Scholar]
- Lea P. M. IV; Faden A. I. CNS Drug Rev. 2006, 12, 149–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The EGF domain of Neuregulin 1β1 (corresponding to amino acid residues 176−246 of heregulin-1β1), was expressed and purified from E. coli (R&D Systems; no. 396-HB) and reconstituted in phosphate-buffered saline (PBS) with 0.1% bovine serum albumin as a nonspecific carrier and frozen in aliquots at −20 °C.
- a Junttila T. T.; Sundvall M.; Lundin M.; Lundin J.; Tanner M.; Härkönen P.; Joensuu H.; Isola J.; Elenius K. Cancer Res. 2005, 65, 1384–1393. [DOI] [PubMed] [Google Scholar]; b Corfas G.; Roy K.; Buxbaum J. D. Nature Neurosci. 2004, 7, 575–580. [DOI] [PubMed] [Google Scholar]
- Kuai, L. et al. Manuscript in preparation.
- Procedure for pyridine synthesis: Diisopropyl(pent-1-yn-3-yloxy)(phenylethynyl)silane 1 (10.0 mg, 0.033 mmol) was placed into an oven-dried sealed tube and dissolved in degassed THF (0.67 mL). Nitriles 7−18 (0.05 mmol, 1.5 equiv) were added to the stirring solution. After complete dissolution, a solution of cyclopentadienylcobalt(I) dicarbonyl (1.8 mg, 0.010 mmol, 30 mol %) in degassed xylenes (50 µL) was introduced by syringe, giving a pale yellow solution that was submerged into an oil bath preheated to 140 °C. After 24 h, the dark brown solution was cooled to ambient temperature and loaded onto a 4 g silica plug. Filtration was performed using an Isco Combiflash system using a gradient solvent commencing with hexanes and ending with 1/1 hexanes/ethyl acetate (total volume of approximately 40 mL), which effectively removed insoluble cobalt byproducts. Pooled fractions were concentrated in vacuo and assayed for conversion by 1H NMR. Purification was performed by silica gel chromatography using an Isco Combiflash 12 g column with 20:1 hexanes/EtOAc as eluant, providing the corresponding pyridines 19−26
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.