Abstract
Retinitis pigmentosa (RP) is a group of diseases in which many different mutations cause rod photoreceptor cells to die and then gradually cone photoreceptors die due to progressive oxidative damage. In this study, we have shown that peroxynitrite-induced nitrosative damage also occurs. In the rd1 mouse model of RP, there was increased staining for S-nitrosocysteine and nitrotyrosine protein adducts that are generated by peroxynitrite. Peroxynitrite is generated from nitric oxide (NO) and superoxide radicals. After degeneration of rods, injection of hydroethidine resulted in strong fluorescence in the retina of rd1 mice indicating high levels of superoxide radicals, and this was reduced, as was nitrotyrosine staining, by apocynin suggesting that over action of NADP(H) oxidase is at least partially responsible. Treatment of rd1 mice with a mixture of nitric oxide synthase (NOS) inhibitors markedly reduced S-nitrosocysteine and nitrotyrosine staining and significantly increased cone survival, indicating that NO-derived peroxynitrite contributes to cone cell death. Treatment with 7-nitroindazole, a relatively specific inhibitor of neuronal NOS, also significantly reduced cone cell death, but aminoguanidine, a relatively specific inhibitor of inducible NOS, did not. These data suggest that NO generated by neuronal NOS exacerbates oxidative damage to cones in RP and that combined therapy to reduce NO and oxidative stress should be considered.
Keywords: antioxidants, apoptosis, photoreceptors, reactive nitrogen species, retina, retinal dystrophies
Introduction
Retinitis pigmentosa (RP) is a group of inherited primary rod cell degenerations that share a similar phenotype. RP originates from one of hundreds of mutations that result in a gene product that is damaging to rods or homozygous mutations that cause lack of a protein that is needed for rod survival. The death of rods is inevitably followed by cone cell death predominantly from oxidative damage [1–3]. Rod photoreceptors are the most numerous cell type in the retina and the largest consumers of oxygen and after rods die, the level of oxygen in the outer retina is markedly elevated [4,5] providing the source for oxidative damage. Transgenic pigs that express Pro347Leu mutant rhodopsin undergo degeneration of rod photoreceptors and by 9 months of age there are almost no remaining rods [6,7]. After 9 months of age, there is progressive staining in cones and other retinal cells for the biomarkers of oxidative damage acrolein, 4-hydroxynonenal, and 8-deoxyhydroxyguanosine. There is also progressive staining for 3-nitrotyrosine, which is a specific marker for peroxynitrite-induced nitrosative damage [8].
Peroxynitrite is a strong oxidant that can damage macromolecules by reacting with them directly [9–18] or it can generate other strong oxidants such as hydroxyl or carbonate radicals [19]. Peroxynitrite is generated from nitric oxide (NO) and superoxide radicals [20]. Normally NO is rapidly removed from tissues by diffusion and levels of superoxide are kept low by superoxide dismutases (SODs), but when oxidative stress overwhelms the SODs and levels of superoxide rise, the likelihood of a meeting between NO and superoxide is increased. The reaction rate is so high that almost all meetings between NO and superoxide result in peroxynitrite [21] providing a major amplification step in the oxidative/nitrosative damage cascade, because peroxynitrite is not easily detoxified. The best way to deal with peroxynitrite is to prevent its formation by keeping levels of NO and superoxide as low as possible.
In the nervous system where levels of NO are high compared to other tissues, the formation of peroxynitrite can play a particularly important role in disease pathogenesis. Peroxynitrite has been implicated as an important contributor to neuronal cell death in several neurodegenerative diseases (for reviews see [22,23]). In this study, we sought to determine if generation of peroxynitrite from NO contributes to cone cell death in RP.
Materials and Methods
Injections with nitric oxide synthase (NOS) inhibitors or apocynin
Mice were treated in accordance with the recommendations of the Association for Research in Vision and Ophthalmology. Litters of homozygous rd1 mice were separated into two groups. Mice in one group were given twice daily (9:30am and 6:30pm) intraperitoneal injections of a mixture of NOS inhibitors including NG-nitro-L-arginine (L-NNA, 400mg/kg), N(omega)-nitro-L-arginine methyl ester (L-NAME,400mg/kg), N-monomethyl-L-arginine (L-NMMA, 200mg/kg), and aminoguanidine bicarbonate (400mg/kg). All four NOS inhibitors were obtained from Sigma Aldrich (Saint Louis, MO). L-NAME and L-NMMA were dissolved in phosphate-buffered saline (PBS) and L-NNA and aminoguanidine were injected as a suspension in PBS. Rd1 mice in the control group were given injections of PBS. In separate experiments, rd1 mice in one group were given twice daily intraperitoneal injections of aminoguanidine bicarbonate (1250mg/kg) in PBS or 7-nitroindazole (30mg/kg; Cayman Chemical Co. Ann Arbor, MI) in dimethylsulfoxide (DMSO) and control groups were given injections of PBS or DMSO, respectively. Apocynin (Sigma Aldrich, St. Louis, MO) was dissolved in PBS and 10 mg/kg/day or PBS alone (controls) was given by intraperitoneal injection to rd1 mice between P15 through P30.
Measurement of cone cell density
Cone density was measured as previously described [2]. Briefly, rd1 mice were euthanized at P35 and eyes were rapidly removed. After removal of the cornea, iris, and lens, a small cut was made at 12:00 in the retina for future orientation. Eyecups were fixed in 4% paraformaldehyde for 1–2 hours and then the entire retina was carefully dissected away from the retinal pigmented epithelium (RPE), severed at the optic nerve, and removed from the eye. Retinas were placed in 10% normal goat serum in PBS for 30 minutes at room temperature (RT), incubated for 1 hour at RT in 1:30 rhodamine-conjugated peanut agglutinin (PNA; Vector Laboratories, Burlingame, CA) in PBS containing 1% normal goat serum, and flat mounted with the photoreceptors facing upward. The retinas were examined with a Zeiss LSM 510 META confocal microscope (Carl Zeiss, Oberkochen, Germany) with a Zeiss Plan-Apochromat 20x/0.75NA objective using an excitation wavelength of 543 nm to detect rhodamine fluorescence. Retinas were examined by oil immersion with a 63x/1.4NA Zeiss Plan-Apochromat objective to evaluate cone morphology in detail. Images were acquired in the frame scan mode. The number of cones present within four 230µm × 230µm (512 × 512 pixels) squares located 1 mm superior, temporal, inferior, and nasal to the center of the optic nerve was determined.
Real time reverse transcriptase-polymerase chain reaction (RT-PCR)
In some experiments, one eye was used for measurement of cone density and the fellow eye was used to measure levels of m-cone opsin or s-cone opsin mRNA. One µg of total retinal RNA was incubated with 200 units of reverse transcriptase (SuperScript II, Invitrogen) and the cDNA was used for real time PCR using the QuantiTect SYBR Green PCR Kit (QIAGEN, Valencia, CA) and the Roche Lightcycler 2.0 (Roche Applied Science, Indianapolis, IN). A total volume of 20 µl was loaded into LightCycler capillaries and contained 2 µl of cDNA sample and 0.5 µM of primers specific for m-cone opsin (forward: 5’-TCA TTT CCT GGG AGA GAT GG-3’ and reverse: 5’-AGG CCA TAA GGC CAG TAC CT-3’) or s-cone opsin (forward: 5’-GCC TCA GTA CCA CCT TGC TC-3’ and reverse: 5’-CTG GCG ATG AAG ACT GTG AA-3’). For normalization, cyclophilin was amplified (forward: 5’-CAG ACG CCA CTG TCG CTT T-3’ and reverse: 5’-TGT CTT TGG AAC TTT GTC TGC AA-3’). The PCR reaction quality and specificity were verified by melting-curve dissociation analysis. For quantification, a standard curve was generated from a cDNA template for each gene. Titrations were performed to ensure that PCR reactions were carried out in the linear range of amplification. Relative transcript levels of each gene were calculated using the second derivative maximum values from the linear regression of cycle number versus log concentration of the amplified gene.
Immunofluorescent staining
Nitrotyrosine immunofluorescence staining was done as previously described [1]. Briefly, frozen sections of mouse eyes were dried at room temperature and post-fixed in 4% paraformaldehyde for 15 minutes. Sections were washed with phosphate-buffered saline (PBS), and antigen retrieval was done in with 0.02M citrate buffer, pH 6.0. After blocking with normal donkey serum, slides were stained with a rabbit anti-nitrotyrosine IgG (1:300, Upstate Biotechnologies, Lake Placid, NY), at 4°C overnight. To evaluate the specificity of nitrotyrosine staining, the primary antibody was incubated with more than a 3-fold molar excess of Nitrotyrosine-BSA (Cayman Chemical Company, Ann Arbor, Michigan) overnight at 4°C prior to incubation with sections at a final dilution of 1:300. To control for nonspecific binding of secondary antibody, primary antibody was eliminated in initial incubations prior to incubating with secondary antibody. For S-Nitroso-Cysteine (SNO-Cys) immunofluorescent staining, slides were dried and fixed with 4% paraformaldehyde for 15 minutes. After blocking with 7% normal donkey serum in PBS for 30 minutes at room temperature, the slides were incubated overnight at 4°C with rabbit anti-SNO-Cys antibody (1:200, Sigma-Aldrich, St. Louis, MO). To evaluate the specificity of SNO-Cys staining, the primary antibody was incubated with more than a 3-foldmolar excess of NO-Cysteine-G-BSA (Advanced Targeting Systems, San Diego, CA) overnight at 4°C prior to incubation with sections at a final dilution of 1:200. To control for nonspecific binding of secondary antibody, primary antibody was eliminated in initial incubations prior to incubating with secondary antibody.
For both procedures, secondary antibody incubations were done in the same way. After the primary antibody incubation, sections were washed 3 times with PBS/0.05% Tween-20 and incubated for 45 minutes at room temperature with Cy3-conjugated donkey anti-rabbit antibody (1:800, Jackson ImmunoResearch Lab). Sections were thoroughly washed with PBS/Tween-20 and counterstained for 3 minutes at room temperature with the nuclear dye Hoechst 33258 (1:1,200; Sigma, St. Louis, MO). Slides were mounted with Aquamount solution and viewed with a Nikon Fluorescence Microscope (Nikon Instruments, Inc., NY). Using the same exposure time for each section, images were captured using a Nikon digital camera and SPOT RT 3.4 software. Pictures were obtained by merging an image of a Hoechst-stained slide and the fluorescent image from the same field.
Quantification of fluorescence intensity was done as previously described [24]. With the observer masked with respect to treatment group, image analysis was used to measure optical density of the retina in the entire 200x field (immunofluorescent signal) and the inner plexiform layer in the same field (internal control for background). The immunofluorescent signal was divided by the background signal for 4 different sections for each mouse and the mean was used for one experimental value. Experimental values were obtained from 4 mice in each group and results are reported as the mean (± SD) ratio of immunofluorescent signal:background signal for each treatment group.
Assessment of superoxide radicals with hydroethidine
In situ production of superoxide radicals was evaluated using hydroethidine, which is taken up into cells and in the presence of superoxide radicals is converted to ethidium, which binds DNA and emits red fluorescence [25]. Fluorescence emission occurs at around 600nm. Hydroethidine imaging was performed as previously described [26]. Briefly, mice were given two intraperitoneal injections (27mg/kg) of freshly prepared hydroethidine (Invitrogen, Carlsbad, CA) 30 minutes apart and euthanized after 18 hours. Eyes were rapidly removed and frozen in OCT compound. Ten µm ocular frozen sections were fixed in 4% paraformaldehyde for 15 minutes, rinsed with PBS, and counterstained for 3 minutes at room temperature with the nuclear dye Hoechst 33258 (1:1200; Sigma, St. Louis, MO). After rinsing in PBS, slides were mounted with Aquamount solution and were evaluated for fluorescence (excitation wavelength: 543nm, emission wavelength: >590nm) with a LSM 510 Meta confocal microscope using the same exposure time.
Recording of electroretinograms (ERGs)
An Espion ERG Diagnosys machine (Diagnosys LLL, Littleton, MA) was used to record ERGs as previously described [27]. Mice were adapted for 10 minutes to a background of white light at an intensity of 30 cd/m2. The background intensity was then changed to10 cd/m2, and photopic ERGs were performed under dim red illumination. Mice were anesthetized with an intraperitoneal injection of Avertin (Aldrich, Milwaukee, WI) and topical administration of 0.5% proparacaine hydrochloride (Alcon Labs, Forth Worth, TX). Pupils were dilated with 1% tropicamide (Alcon Labs) and 2.5% phenylephrine hydrochloride (Bausch and Lomb, Tampa, FL). Mice were placed on a pad heated to 39°C, and platinum loop electrodes were placed on each cornea after application of Gonioscopic prism solution (Alcon Labs). A reference electrode was placed subcutaneously in the anterior scalp between the eyes, and a ground electrode was inserted into the tail. The head of the mouse was held in a standardized position in a ganzfeld bowl illuminator that ensured equal illumination of the eyes. Recordings for both eyes were made simultaneously with electrical impedance balanced. Sixty photopic measurements were taken at 1.48 log cd-s/m2 and the average value was recorded.
Results
Increased S-nitrosylation of cysteine thiols in rd1 mice after rods die prevented by NOS inhibitors
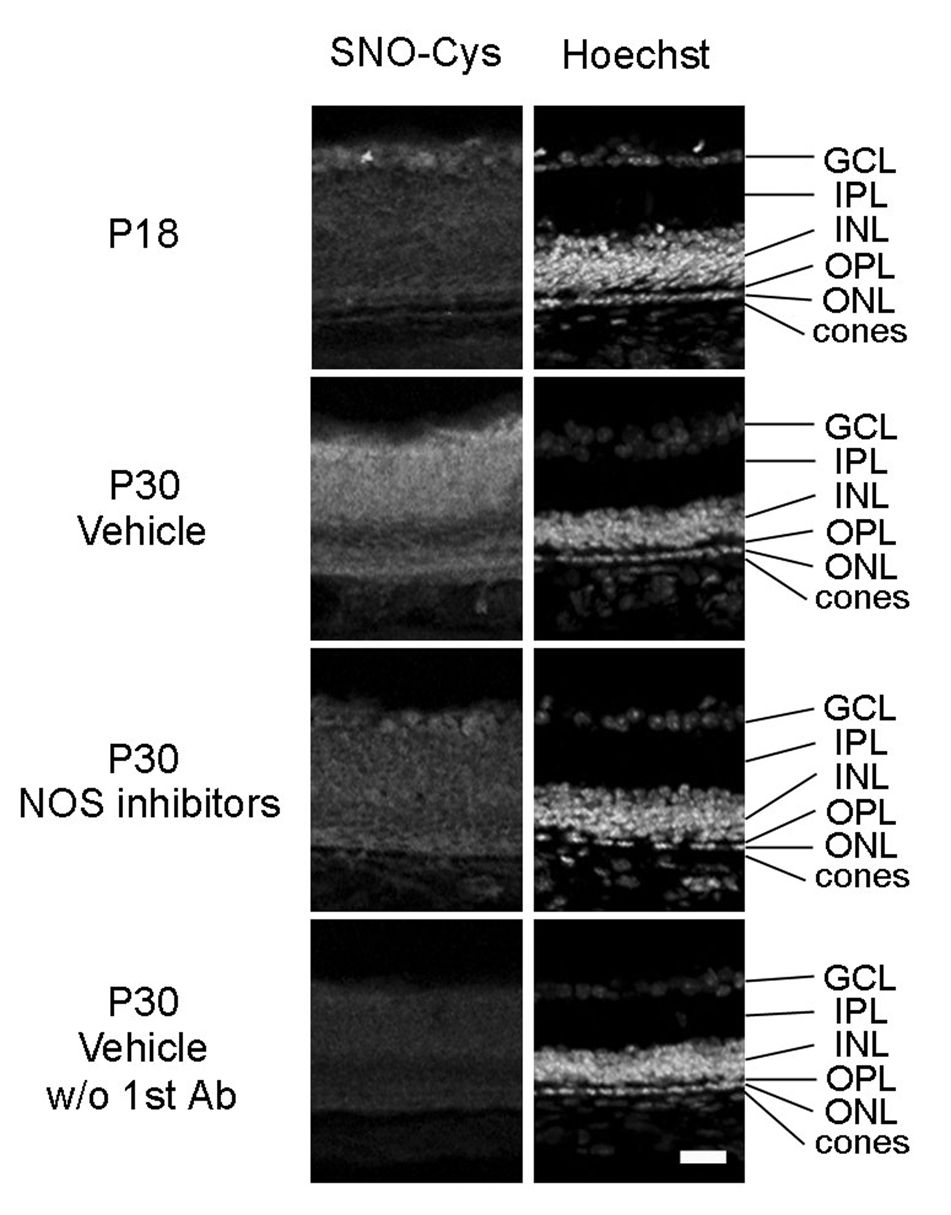
In mice homozygous for the rd1 mutation, rod photoreceptor degeneration begins around postnatal day (P) 10 and is almost completed by P21, while cone cell numbers are normal at P21 [28]. In a previous study, we confirmed that the number of cones in the retinas of rd1/rd1 mice was similar to that in wild type mice at P21, but by P35 it was less than 25% and during this period of cone cell loss there was progressive oxidative damage to cones and other cells in the retina [2]. In the central nervous system where NO levels are high, superoxide radicals react with NO to generate peroxynitrite which causes nitrosylation of tyrosine residues, and in a previous study, we found progressive increased staining for nitrotyrosine in cones after degeneration of rods in a pig model of RP [1]. It should also cause S-nitrosylation of cysteine residues. We tested for this in rd1 mouse retinas by immunohistochemistry with an antibody that specifically recognizes SNO-Cys. There was minimal staining in P18 rd1 mouse retinas (Figure 1, row 1), but at P30, there was very prominent staining for SNO-Cys throughout the entire retina, including cones (Figure 1, row 2). Treatment of rd1 mice with a mixture of NOS inhibitors between P18 and P30 appeared to reduced staining for SNO-Cys (Figure 1, third row).
Figure 1. A mixture of nitric oxide synthase (NOS) inhibitors prevents S-nitrosylation of cysteine thiols in proteins in the retinas of rd1 mice.
Between postnatal day (P) 18 and P30, rd 1 mice were given twice daily intraperitoneal injections of vehicle or vehicle containing a mixture of four NOS inhibitors, L-NNA, L-NAME, L-NMMA, and aminoguanidine. Ocular sections were stained for S-nitrosocysteine (SNO-Cys, column 1) and Hoechst, which stains all cell nuclei (column 2). In rd1 mouse retina, there was minimal staining for SNO-Cys at P18 (column 1) that was substantially increased in P30 vehicle-treated mice throughout the entire retina including the area of remaining photoreceptors (column 2). This increase was blunted by treatment with NOS inhibitors (column 3). In the absence of primary antibody, there was essentially no staining in the retinas of P30 vehicle-treated mice. Results were identical in 2 mice for each time point/condition. Scale bar = 50 µm
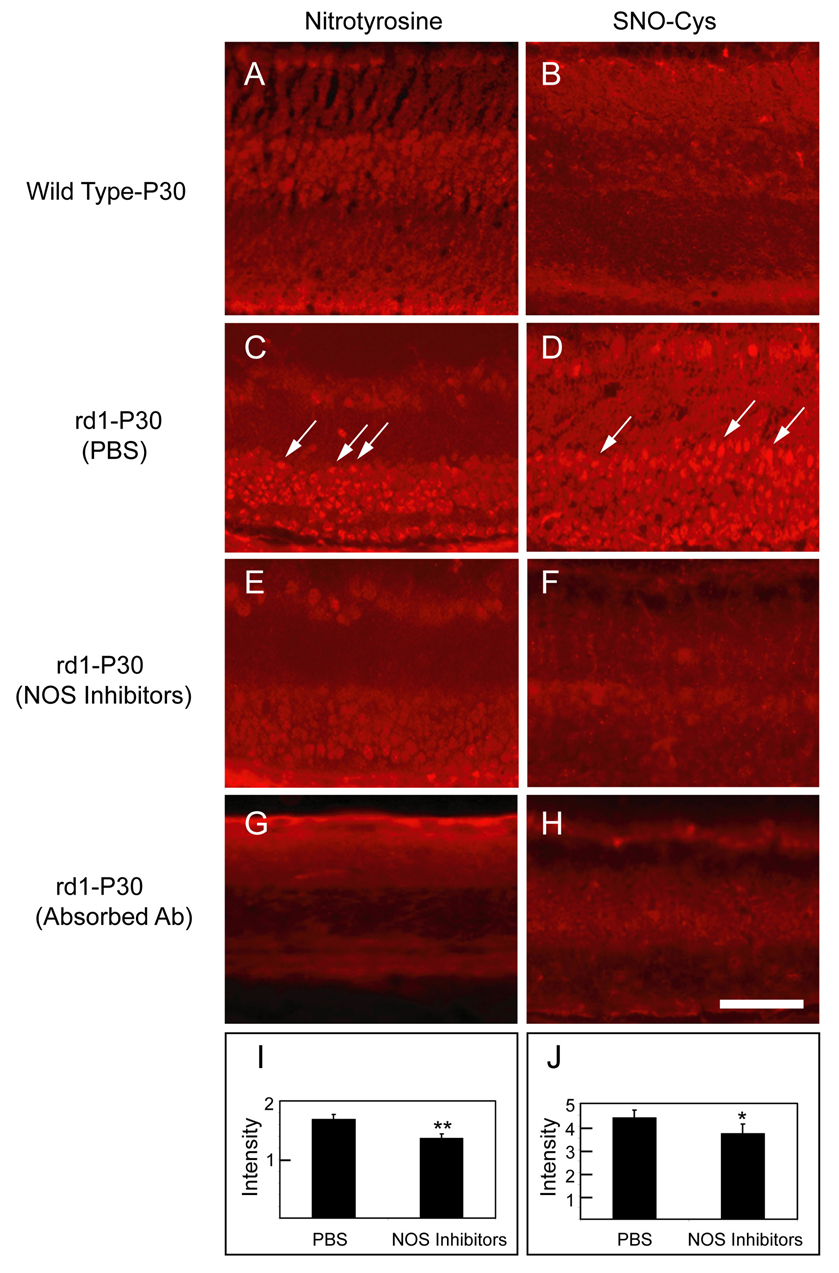
To determine more definitively if NO was contributing to nitrosylation of proteins in the retina, a group of rd1 mice treated with the mixture of NOS inhibitors between P18 and P30 were compared to rd1 mice treated with vehicle. Compared to P30 wild type mice, which showed essentially no staining for nitrotyrosine (Figure 2A) or SNO-Cys (Figure 2B) in the retina, rd1 mice treated with vehicle showed prominent staining for nitrotyrosine (Figure 2C) and SNO-Cys (Figure 2D) that appeared reduced in mice treated with NOS inhibitors (Figures 2E and F). The intense staining of cell bodies in the retina was specific for nitrotyrosine (Figure 2C, arrows) and SNO-Cys (Figure 2D, arrows), because in each case incubation of primary antibody with BSA containing nitrotyrosine or SNO-Cys adducts reduced the staining to background levels. Quantification of staining by measurement of fluorescence intensity with image analysis confirmed that the mixture of NOS inhibitors significantly reduced staining for nitrotyrosine and SNO-Cys in rd1 mouse retinas (Figures 2G and H).
Figure 2. A mixture of NOS inhibitors reduces staining for S-nitrosocysteine (SNO-Cys) and nitrotyrosine in the retinas of rd1 mice in the presence of high levels of superoxide radicals.
Ocular sections were stained for SNO-Cys or nitrotyrosine in P30 wild type C57BL/6 mice or P30 rd1 mice in a C57BL/6 background that had been given twice daily intraperitoneal injections of PBS or PBS containing a mixture of four NOS inhibitors, L-NNA, L-NAME, L-NMMA, and aminoguanidine between P18 and P30 (n = 4 for each group) and fluorescence intensity was measured as described in Methods. There was very little staining for nitrotyrosine (A) or SNO-Cys (B) in the retinas of P30 wild type C57BL/6 mice. There was increased staining for nitrotyrosine (C, arrows) and SNO-Cys (D, arrows) in cells of the inner nuclear layer of the retinas of P30 rd1 mice that had received PBS injections. This staining was markedly reduced in P30 rd1 mice that had received injections of NOS inhibitors between P18 and P30 (E–F). Staining of sections from the same mouse shown in (C) with anti-nitrotyrosine antibody that had been preincubated with nitrotyrosine-BSA eliminated the staining (G). Staining of sections from the same mouse shown in (D) with anti-SNO-Cys antibody that had been preincubated with NO-Cysteine-G-BSA eliminated the staining (H). Staining was quantified by measurement of fluorescence intensity with image analysis (n=4 for each group) in the area of interest and normalized to background staining on the same slide. Each bar shows the mean (± SD) normalized intensity (I and J). The staining intensity of outer retina for sections from mice treated with NOS inhibitors was significantly less than that for sections from mice treated with PBS (*p<0.05;**p<0.001 by unpaired Student t-test) confirming that the mixture of NOS inhibitors significantly reduced staining for nitrotyrosine and SNO-Cys in rd1 mouse retinas. Scale bar = 50 µm
Hydroethidine demonstrates high levels of superoxide radicals in retinas of rd1 mice
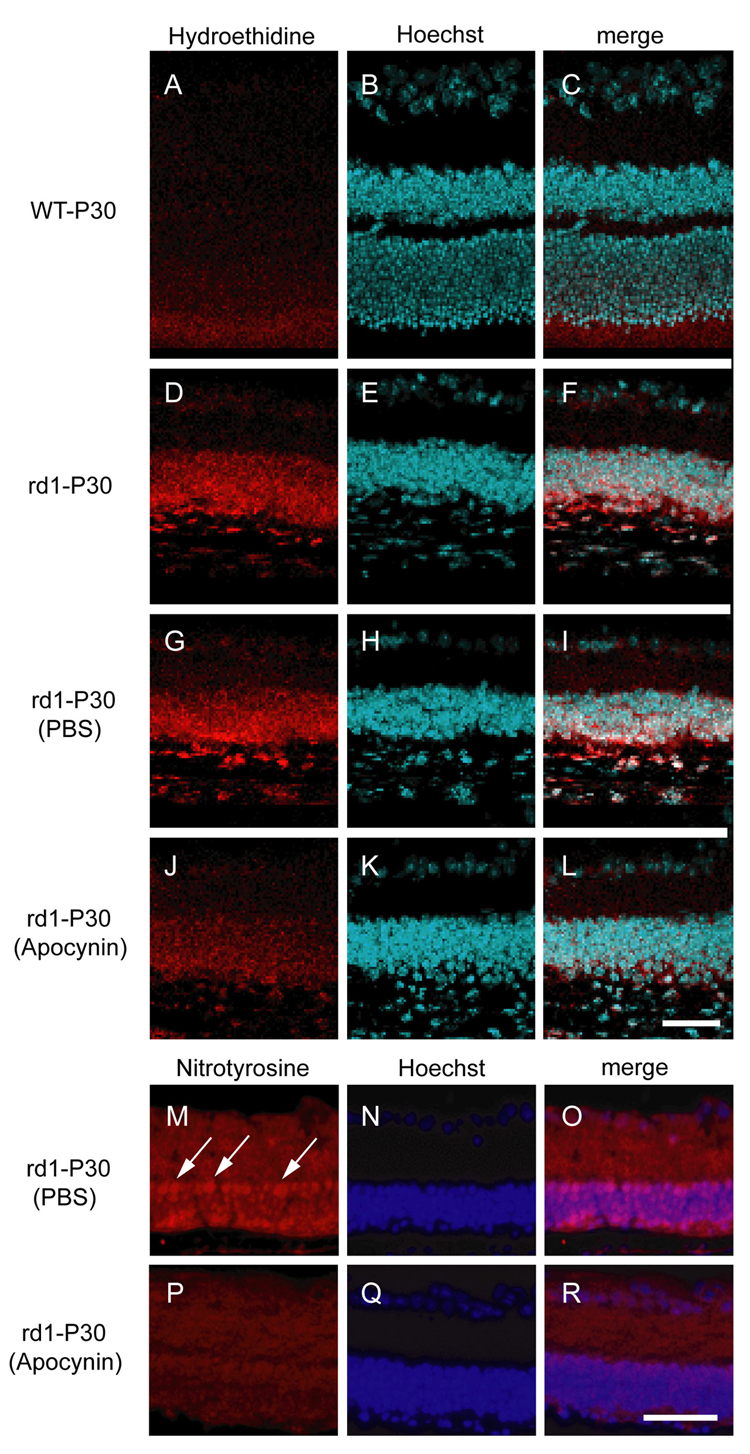
Hydroethidine is taken up into cells and in the presence of superoxide radicals is converted to ethidium, which binds DNA and emits red fluorescence providing a means to visualize production of superoxide radicals in situ [25]. The retinas of P30 wild type mice that had been given a systemic injection of hydroethidine before death, showed minimal fluorescence (Figure 3A–C), but the retinas of P30 rd1 mice that had been injected with hydroethidine showed strong fluorescence in the outer part of the remaining retina (Figure 3D–F). This indicates that after the death of rods, there are high levels of superoxide radicals in the retina which are available to react with NO and generate peroxynitrite.
Figure 3. An inhibitor of NAD(H) oxidase reduces superoxide radicals and protein nitrosylation in the retinas of rd1 mice.
There was minimal fluorescence in the retinas of P30 wild type mice after systemic Injection of hydroethidine (A–C), but the retinas of P30 rd1 mice showed prominent fluorescence in the remaining outer retina (D–F). Rd1 mice treated with intraperitoneal injections of PBS between P15 and P30 also showed strong fluorescence in the retina (G–I), but rd1 mice treated with intraperitoneal injections of apocynin between P15 and P30 showed only mild fluorescence (J–L). Rd1 mice treated with subcutaneous injections of PBS between P15 and P30 showed strong staining for nitrotyrosine in cells of the inner and remaining outer nuclear layer (M–O, arrow), but rd1 mice treated with apocynin showed mild staining (P–R). Scale bars = 50 µm
Over action of NADP(H) oxidase contributes to excess superoxide radicals and nitrosative damage in rd1 mouse retinas
Rd1 mice that received intraperitoneal injections of PBS between P15 and P30 showed strong fluorescence in the outer retina after injection of hydroethidine (Figure 3H–G) like that seen in untreated rd1 mice (Figure 3D–F). In contrast, treatment of rd1 mice with apocynin, an inhibitor of NADP(H) oxidase between P15 and P30 reduced fluorescence in the outer retina seen after injection of hydroethidine (Figure 3J–L). Compared to P30 rd1 mice treated with PBS in which there was strong staining for nitrotyrosine (Figure 3M–O, arrows), there was reduction in nitrotyrosine staining in rd1 mice treated with apocynin (Figure 3P–R). This suggests that over action of NADP(H) oxidase contributes to the excess of superoxide radicals in the retinas of rd1 mice, which in turn lead to nitrosative damage to proteins.
NOS inhibitors reduce cone degeneration and loss of cone function in rd1 mice
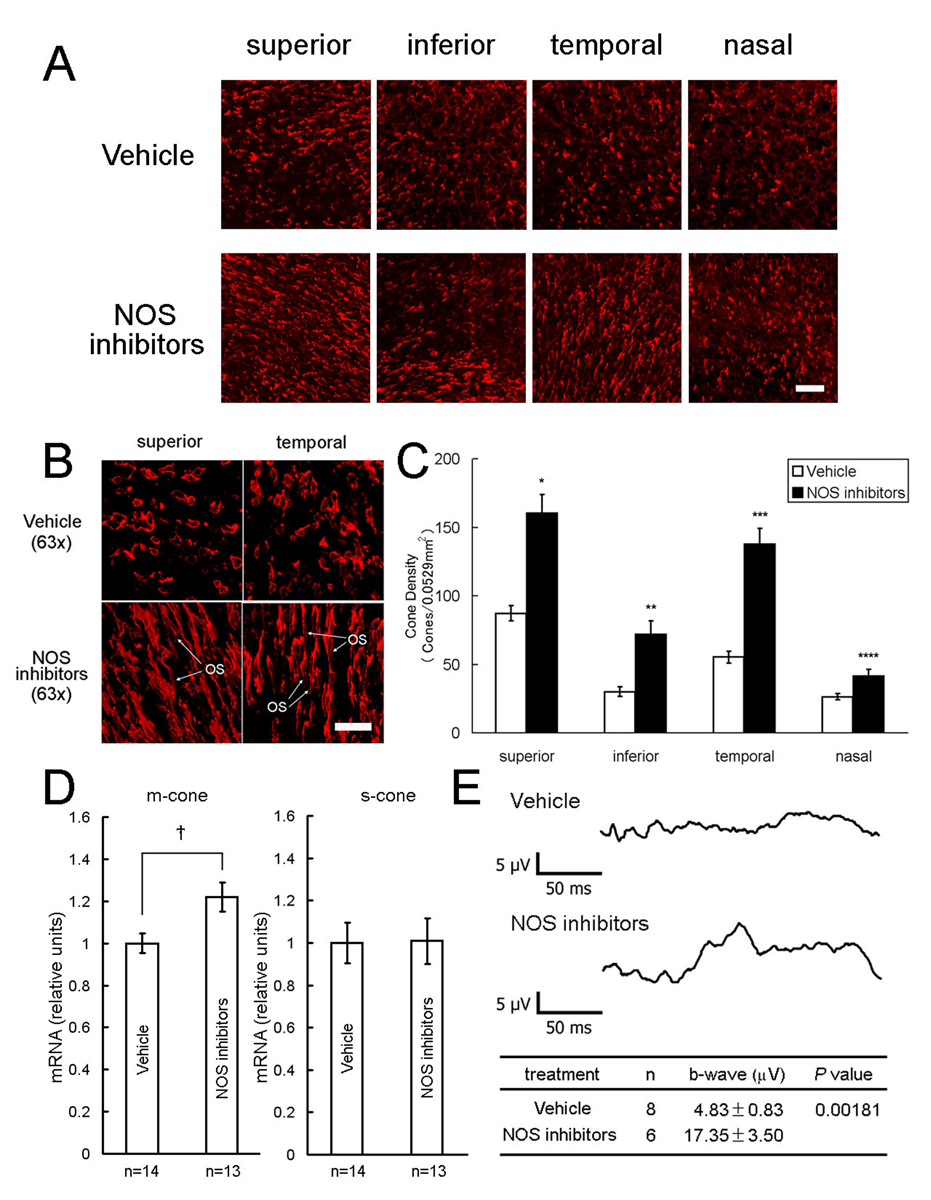
Starting at P18, rd1 mice received twice daily intraperitoneal injections of vehicle or vehicle containing a mixture of four NOS inhibitors and at P35, cone density was measured in 0.0529 mm2 bins 1 mm superior, inferior, temporal, and nasal to the optic nerve. Confocal images appeared to show a higher density of cone inner segments, particularly superior and temporal to the optic nerve (Figure 4A). Three-dimensional reconstruction of confocal images showed preservation of cone outer segments (OS, Figure 4B, bottom row) in 3 of 9 NOS inhibitor-treated mice, whereas 17 of 17 vehicle-treated mice showed small flattened inner segments and no OS (Figure 4B, top row). At P35, cone density was significantly higher in the NOS inhibitor group compared to the vehicle-treated group in all 4 regions of the retina (Figure 4C). Mice treated with NOS inhibitors also had significantly more m-cone opsin mRNA per retina than vehicle controls at P35, but there was no difference in s-cone opsin mRNA (Figure 4D). Photopic electroretinograms (ERGs) showed significantly greater b-wave amplitudes in mice treated with NOS inhibitors compared to those treated with vehicle (Figure 4E).
Figure 4. Nitric oxide synthase (NOS) inhibitors promote cone survival in rd1 mice.
Starting at P18, rd1 mice received twice daily intraperitoneal injections of vehicle or vehicle containing a mixture of four NOS inhibitors, L-NNA, L-NAME, L-NMMA, and aminoguanidine. At P35, cone density was measured in 0.0529 mm2 bins 1 mm superior, inferior, temporal, and nasal to the optic nerve, or the retina was used for real time RT-PCR. Confocal images appeared to show a higher density of cone inner segments, particularly superior and temporal to the optic nerve (A). Three-dimensional reconstruction of confocal images showed preservation of cone outer segments (OS) in some NOS inhibitor-treated mice (B, bottom row), whereas all vehicle-treated mice showed small flattened inner segments and no OS (B, top row). Cone density was significantly higher in the NOS inhibitor group (n=9) in all 4 regions of the retina (C, *p<5.0×10−6; **p<5.0×10−5; ***p<5.0×10−8; ****p<0.01 by unpaired Student t-test for difference from corresponding vehicle control (n=17). The mean (±SEM) amount of m-cone or s-cone opsin mRNA per retina was normalized to the P35 vehicle-treated group, which was set to 1.00. The amount of m-cone opsin mRNA, but not s-cone opsin mRNA, was significantly greater in NOS inhibitor-treated mice compared to vehicle-treated mice (D, *p<0.02 by unpaired Student t-test). Photopic electroretinograms done on rd1 mice treated with NOS inhibitors at P25 showed greater b-wave amplitudes than those seen in vehicle-treated mice (E). Scale bars = 50 µm for (A) and 20 µm for (B)
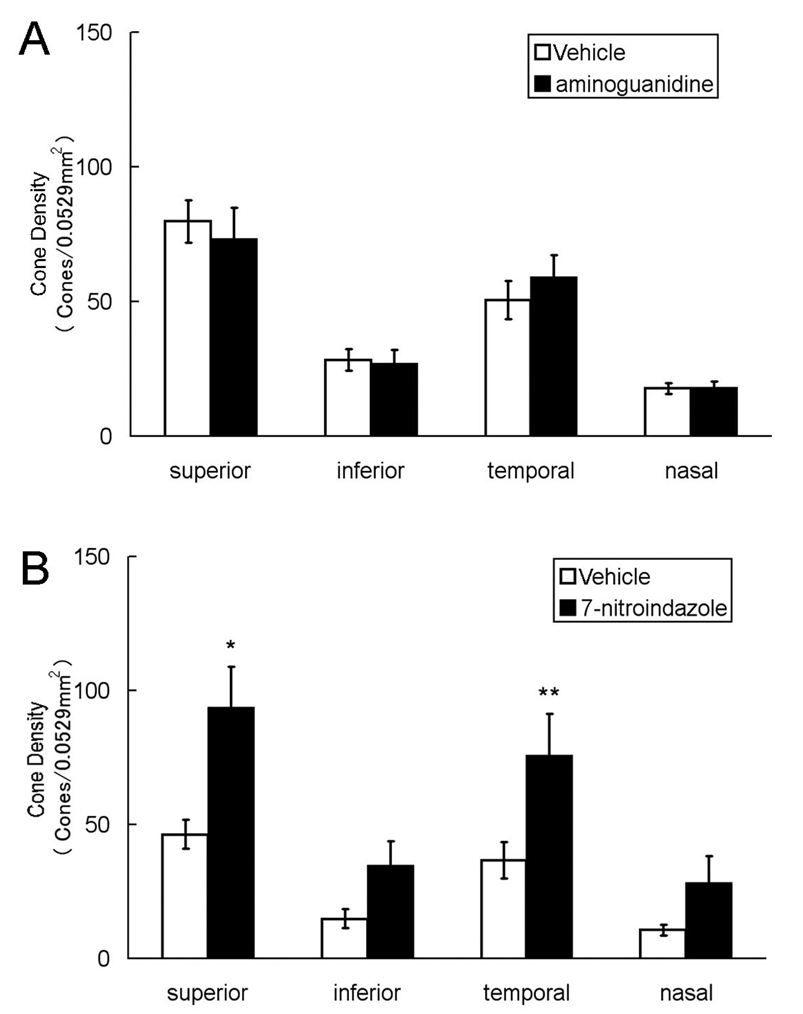
Blockade of nNOS, but not iNOS promotes cone survival in rd1 mice
Aminoguanidine is a relatively specific inhibitor of iNOS [29] and 7-nitroindazole is a relatively specific inhibitor of nNOS [30,31]. Twice a day injections of a relatively high dose of aminoguanidine between P18 and P35 had no significant effect on cone survival in rd1 mice (Figure 5A), but mice treated with 7-nitroindazole showed significantly more cones in the superior and temporal regions of the retina than vehicle treated mice (Figure 5B). The vehicle used for 7-nitroindazole, DMSO, may have been deleterious, because it was noted that mice treated with DMSO alone consistently had lower cone densities than mice treated with PBS.
Figure 5. 7-Nitroindazole, but not aminoguanidine, reduces cone cell death in rd1 mice.
At P18, rd1 mice were given intraperitoneal injections twice a day of high-dose aminoguanidine (1250 mg/kg/injection in PBS, n=10) or PBS (n=10). Another group of P18 rd1 mice were given intraperitoneal injections twice a day of 7-nitroindazole (30 mg/kg/injection in DMSO, n=10) or DMSO (n=10). At P35, cone density was measured in 0.0529 mm2 bins 1 mm superior, inferior, temporal, or nasal to the center of the optic nerve. Compared to PBS-treated mice, there was no significant difference in cone density in any region of the retina in mice treated with aminoguanidine (A), but cone density was significantly higher in 2 of 4 regions of the retina (*p<0.02, **p<0.05 by unpaired Student t-test) in mice treated with 7-nitroindazole compared to those treated with DMSO.
Discussion
NO is an important signaling molecule involved in regulation of vascular tone and neurotransmission. Because of its role in neurotransmission, levels of NO in the nervous system are high compared to most other tissues. These high levels are a potential hazard in the setting of oxidative stress, because build up of superoxide radicals in the vicinity of NO results in rapid generation of peroxynitrite resulting in amplification of oxidative/nitrosative damage. In this study, we have demonstrated that like neurodegenerative diseases in the brain and spinal cord in which oxidative stress plays a major role [32–36], NO acts as an exacerbating factor in the retina by contributing to cone cell death in the rd1 mouse model of RP. After rods die in rd1 mice, there is accumulation of superoxide radicals in the outer retina, due at least in part to over action of NADP(H) oxidase, because apocynin reduces the superoxide radical accumulation. Apocyanin also reduced excess staining for nitrotyrosine in rd1 mouse retinas suggesting that the excess in superoxide radicals lead to nitrosative damage to proteins. Treatment of rd1 mice with a combination of NOS inhibitors reduced nitrosative damage as shown by reduced staining for nitrotyrosine. It also reduced S-nitrosylation reactions which can either be harmful or productive [10,12,17,37–39]. In this situation the harmful effects of nitrosylation reactions seem to outweigh any potential benefits, because the mixture of NOS inhibitors which decreased nitrosylation also significantly reduced cone cell death. This suggests that NO acting through peroxynitrite plays an important pathogenic role in RP. The major source of the NO that contributes to the cone cell damage is nNOS rather than iNOS, because 7-nitroindazole, a relatively specific inhibitor of nNOS [30,31], provided significant protection for cones, but aminoguanidine, a selective inhibitor of iNOS, had no effect.
Peroxynitrite alters protein structure and function by reacting with transition metal centers or various amino acids in the peptide chain. It oxidizes the ferrous heme group in hemoglobin, myoglobin, or cytochrome c to the ferric form [11,16,18]. It inactivates mitochondrial aconitase by interacting with its iron-sulfur cluster [9]. The most common peroxynitrite-induced change in peptide chains is thiol oxidation of cysteines resulting in disulfide bonds or thiyl radicals that can react with oxygen to generate more reactive oxygen species or with NO to form nitrosothiols [15]. Cysteine modifications by peroxynitrite cause inactivation of several enzymes involved in generation of ATP including glyceraldehyde-3-phosphate dehydrogenase, NADH dehydrogenase, succinate dehydrogenase, cytochrome c reductase, and ATP synthase [10,12,17]. However, as noted above, S-nitrosylation of some proteins can impart new signaling functions and thereby have a beneficial effect [37–39]. Nitration of tyrosine residues by peroxynitrite is quite selective for tyrosines in which the aromatic ring is near the surface, there is nearby negative charge, and there are no proximal cysteines [13]. Nitration of Tyr-34 leads to complete inactivation of SOD2 and promotes additional peroxynitrite formation [14], and has been seen in patients with neurodegenerative diseases [40]. Therefore, in some situations peroxynitrite activates a positive feedback loop that increases oxidative stress.
Peroxynitrite has been implicated in several other animal models of retinal diseases including experimental autoimmune uveitis in which staining for nitrotyrosine, lipid peroxidation, and apoptosis occurs in photoreceptor cells [41,42], diabetic retinopathy [43], and ischemia-reperfusion [44]. The relatively high levels of NO in the retina may be a liability in any disease in which oxidative stress plays a role.
The genetic heterogeneity of RP is a major impediment to development of treatments that prevent rod cell death from all of the many pathogenic mutations. However, the demonstration that oxidative stress is a major contributor to cone cell death provides an important therapeutic target that may apply to patients with RP regardless of the underlying mutation that starts the process [2,3]. Identifying ways to reduce damage initiated by oxidative stress in the retina is an important goal. In this study, we have demonstrated that NO amplifies and exacerbates damage from oxidative stress that occurs from loss of rods and that by blocking nNOS cone cell death is reduced even without reducing the underlying oxidative stress. This suggests that reduction of NO levels in the retina combined with antioxidant therapy may be a good approach that deserves further investigation.
Acknowledgments
Supported by EY05851 from the NEI, a senior scientist award from Research to Prevent Blindness, New York, NY, a grant from Foundation Fighting Blindness, and gifts from Dr. and Mrs. William Lake and Mr. and Mrs. Richard Heffner. PAC is the George S. and Dolores Dore Eccles Professor of Ophthalmology and Neuroscience.
List of Abbreviations
- RP
retinitis pigmentosa
- NO
nitric oxide
- NOS
nitric oxide syntase
- SODs
superoxide dismutases
- L-NNA
NG-nitro-L-arginine
- L-NAME
N(omega)-nitro-L-arginine methyl ester
- L-NMMA
N-monomethyl-L-arginine
- PBS
phosphate-buffered saline
- DMSO
dimethylsulfoxide
- RPE
retinal pigmented epithelium
- RT
room temperature
- PNA
peanut agglutinin
- RT-PCR
real time reverse transcriptase-polymerase chain reaction
- SNO-Cys
S-nitroso-cysteine
- ERGs
electroretinograms
- OS
outer segments
References
- 1.Shen J, Yan X, Dong A, Petters RM, Peng Y-W, Wong F, Campochiaro PA. Oxidative damage is a potential cause of cone cell death in retinitis pigmentosa. J. Cell. Physiol. 2005;203:457–464. doi: 10.1002/jcp.20346. [DOI] [PubMed] [Google Scholar]
- 2.Komeima K, Rogers BS, Lu L, Campochiaro PA. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc. Natil. Acad. Sci. USA. 2006;103:11300–11305. doi: 10.1073/pnas.0604056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Komeima K, Rogers BS, Campochiaro PA. Antioxidants slow photoreceptor cell death in mouse models of retinitis pigmentosa. J. Cell. Physiol. 2007;213:809–815. doi: 10.1002/jcp.21152. [DOI] [PubMed] [Google Scholar]
- 4.Yu DY, Cringle SJ, Su EN, Yu PK. Intraretinal oxygen levels before and after photoreceptor loss in the RCS rat. Invest. Ophthalmol. Vis. Sci. 2000;41:3999–4006. [PubMed] [Google Scholar]
- 5.Yu DY, Cringle SJ, Valter K, Walsh N, Lee D, Stone J. Photoreceptor death, trophic factor expression, retinal oxygen status, and photoreceptor function in the P23H rat. Invest. Ophthalmol. Vis. Sci. 2004;45:2013–2019. doi: 10.1167/iovs.03-0845. [DOI] [PubMed] [Google Scholar]
- 6.Petters RM, Alexander CA, Wells KD, Collins EB, Sommer JR, Blanton MR, Rojas G, Hao Y, Flowers WL, Banin E, Cideciyan AV, Jacobson SG, Wong F. Genetically engineered large animal model for studying cone photoreceptor survival and degeneration in retinitis pigmentosa. Nat. Biotech. 1997;15:965–970. doi: 10.1038/nbt1097-965. [DOI] [PubMed] [Google Scholar]
- 7.Li Z-Y, Wong F, Chang JH, Possin DE, Hao Y, Petters RM, Milam AH. Rhodopsin transgenic pigs as a model for human retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci. 1998;39:808–819. [PubMed] [Google Scholar]
- 8.Beal MF. Oxidatively modified proteins in aging and disease. Free Radic. Biol. Med. 2002;32:797–803. doi: 10.1016/s0891-5849(02)00780-3. [DOI] [PubMed] [Google Scholar]
- 9.Castro L, Rodriguez M, Radi R. Aconitase is readily inactivated by peroxynitrite, but not by its precursor, nitric oxide. J. Biol. Chem. 1994;269:29409–29417. [PubMed] [Google Scholar]
- 10.Radi R, Rodriguez M, Castro L, Telleri R. Inhibiion of mitochondrial electron transport by peroxynitrite. Arch. Biochem. Biophys. 1994;308:89–95. doi: 10.1006/abbi.1994.1013. [DOI] [PubMed] [Google Scholar]
- 11.Thomson L, Trujillo M, Telleri R, Radi R. Kinetics of cytochrome c oxidation by peroxynitrite: implications for superoxide measurements in nitric oxide-producing biological systems. Arch. Biochem. Biophys. 1995;319:491–497. doi: 10.1006/abbi.1995.1321. [DOI] [PubMed] [Google Scholar]
- 12.Souza JM, Radi R. Glyceraldehyde-3-phosphate dehydrogenase inactivation by peroxynitrite. Arch. Biochem. Biophys. 1998;360:187–194. doi: 10.1006/abbi.1998.0932. [DOI] [PubMed] [Google Scholar]
- 13.Souza JM, Daikhin E, Yudkoff M, Raman CS, Ischiropoulos H. Factors determining the selectivity of protein tyrosine nitration. Arch. Biochem. Biophys. 1999;371:169–178. doi: 10.1006/abbi.1999.1480. [DOI] [PubMed] [Google Scholar]
- 14.MacMillan-Crow LA, Thompson JA. Tyrosine modifications and inactivation of active site manganese superoxide dismutase mutant (Y34F) by peroxynitrite. Arch. Biochem. Biophys. 1999;366:82–88. doi: 10.1006/abbi.1999.1202. [DOI] [PubMed] [Google Scholar]
- 15.Ducrocq C, Blanchard B, Pignatelli B, Ohshima H. Peroxynitrite: an ndogenous oxidizing and nitrating agent. Cell Mol. Life Sci. 1999;55:1068–1077. doi: 10.1007/s000180050357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herold S, Exner M, Boccini F. The mechanism of the peroxynitrite-mediated oxidation of myoglobin in the absence and presence of carbon dioxide. Chem. Res. Toxicol. 2003;16:390–402. doi: 10.1021/tx025595l. [DOI] [PubMed] [Google Scholar]
- 17.Buchczyk DP, Grune T, Sies H, Klotz LO. Modifications of glyceraldehyde-3-phosphate dehydrogenase induced by increasing concentrations of peroxynitrite: early recognition by 20S proteasome. J. Biol. Chem. 2003;384:237–241. doi: 10.1515/BC.2003.026. [DOI] [PubMed] [Google Scholar]
- 18.Boccini F, Herold S. Mechanistic studies of the oxidation of oxyhemoglobin by peroxynitrite. Biochemistry. 2004;43:16393–16404. doi: 10.1021/bi0482250. [DOI] [PubMed] [Google Scholar]
- 19.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. USA. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pryor WA, Squadrito GL. The chemistry of peroxynitrite: a product from the reaction of nitric oxide with superoxide. Am. J. Physiol. 1995;268:L699–L722. doi: 10.1152/ajplung.1995.268.5.L699. [DOI] [PubMed] [Google Scholar]
- 21.Huie RE, Padmaja S. The reaction rate of nitric oxide with superoxide. Free Radic. Res. Commun. 1993;18:195–199. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- 22.Szabo C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug Discov. 2007;662–680 doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 23.Moncada S, Bolanos JP. Nitric oxide, cell bioenergetics and neurodegeneration. J. Neurochem. 2006;97:1676–1689. doi: 10.1111/j.1471-4159.2006.03988.x. [DOI] [PubMed] [Google Scholar]
- 24.Dong A, Shen J, Krause M, Hackett SF, Campochiaro PA. Increased expression of glial cell line-derived neurotrophic factor protects against oxidative damage-induced retinal degeneration. J. Neurochem. 2007;103:1041–1052. doi: 10.1111/j.1471-4159.2007.04839.x. [DOI] [PubMed] [Google Scholar]
- 25.Pietch A, Dessy C, Havaux X, Feron O, Balligand JL. Differential regulation of nitric oxide synthases and their allosteric regulators in heart and vessels of hypertensive rats. Cardiovasc. Res. 2003;57:456–467. doi: 10.1016/s0008-6363(02)00676-4. [DOI] [PubMed] [Google Scholar]
- 26.Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL, Dugan LL. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADP(H)-oxidase. Science. 2008;318:1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- 27.Okoye G, Zimmer J, Sung JPG, Deering T, Nambu N, Hackett SF, Melia M, Esumi N, Zack DJ, Campochiaro PA. Increased expression of BDNF preserves retinal function and slows cell death from rhodopsin mutation or oxidative damage. J. Neuosci. 2003;23:4164–4172. doi: 10.1523/JNEUROSCI.23-10-04164.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carter-Dawson LD, LaVail MM, Sidman RL. Differential effects of the rd mutation on rods and cones in the mouse retina. Invest. Ophthalmol. Vis. Sci. 1978;17:489–498. [PubMed] [Google Scholar]
- 29.Wolff DJ, Lubeskie A. Aminoguanidine is an isoform-selective, mechanism-based inactivator of nitric oxide synthase. Arch. Biochem. Biophys. 1995;316:290–301. doi: 10.1006/abbi.1995.1040. [DOI] [PubMed] [Google Scholar]
- 30.Moore PK, Babbedge RC, Wallace P, Gaffen ZA, Hart SL. 7-Nitro indazole, an inhibitor of nitric oxide synthase, exhibits anti-nociceptive activity in the mouse without increasing blood pressure. Br. J. Pharmacol. 1993;108:296–297. doi: 10.1111/j.1476-5381.1993.tb12798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Southan GJ, Szabo C. Selective pharmacological inhibition of distinct nitric oxide synthase isoforms. Biochem. Pharmacol. 1996;51:383–394. doi: 10.1016/0006-2952(95)02099-3. [DOI] [PubMed] [Google Scholar]
- 32.Przedborski S, Jackson-Lewis V, Yokoyama R, Shibatani T, Dawson VL, Dawson TM. Role of neuronal nitric oxide in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced dopaminergic neurotoxicity. Proc. Natl. Acad. Sci. USA. 1996;93:4565–4571. doi: 10.1073/pnas.93.10.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith MA, Harris PLR, Sayre LM, Beckman JS, Perry G. Widespread peroxynitrite-mediated damage in Alzheimer's disease. J. Neurosci. 1997;17:2653–2657. doi: 10.1523/JNEUROSCI.17-08-02653.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beal MF, Ferrante RJ, Browne SE, Matthews RT, Kowall NW, Brown RHJ. Increased 3-nitrotyrosine in both sporadic and familial amyotrophic lateral sclerosis. Ann. Neurol. 1997;42:644–654. doi: 10.1002/ana.410420416. [DOI] [PubMed] [Google Scholar]
- 35.Good PF, Hsu A, Werner P, Perl DP, Olanow CW. Protein nitration in Parkinson's disease. J. Neuropathol. Exp. Neurol. 1998;57:338–342. doi: 10.1097/00005072-199804000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Beal MF. Excitotoxicity and nitric oxide in Parkinson's disease. Ann. Neurol. 1998;44:S110–S114. doi: 10.1002/ana.410440716. [DOI] [PubMed] [Google Scholar]
- 37.Davisson RL, Travis MD, Bates JN, Lewis SJ. Hemodynamic effects of L- and D-S-nitrosocysteine in the rat. Stereoselective S-nitrosothiol recognition sites. Circ. Res. 1996;79:266–262. doi: 10.1161/01.res.79.2.256. [DOI] [PubMed] [Google Scholar]
- 38.Stamler JS, Toone EJ, Lipton SA, Sucher NJ. (S)NO signals: Translocation, regulation, and a consensus motif. Neuron. 1997;18:691–696. doi: 10.1016/s0896-6273(00)80310-4. [DOI] [PubMed] [Google Scholar]
- 39.Lipton AJ, Johnson MA, Macdonald T, Lieberman MW, Gozal D, Gaston B. S-Nitrosothiols signal the ventilatory response to hypoxia. Nature. 2001;413:171–174. doi: 10.1038/35093117. [DOI] [PubMed] [Google Scholar]
- 40.Aoyama K, Matsubara K, Fujikawa Y, Nagahiro Y, Shimizu K, Umegae N, Hayase N, Shiono H, Kobayashi S. Nitration of manganese superoxide dismutase in cerebrospinal fluids is a marker for peroxynitrite-mediated oxidative stress in neurodegenerative diseases. Ann. Neurol. 2000;47:524–527. [PubMed] [Google Scholar]
- 41.Wu G-S, Zhang J, Rao NA. Peroxynitrite and oxidative damage in experimental autoimmune uveitis. Invest. Ophthalmol. Vis. Sci. 1997;38:1333–1339. [PubMed] [Google Scholar]
- 42.Liversidge J, Dick A, Gordon S. Nitric oxide mediates apoptosis through formation of peroxynitrite and Fas/Fas-ligand interactions in experimental autoimmune uveitis. Am. J. Pathol. 2002;160:905–916. doi: 10.1016/S0002-9440(10)64913-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.El-Remmessy AB, Behzadian MA, Abou-Mohamed G, Franklin T, Caldwell RW, Caldwell RB. Experimental diabetes causes breakdown of the blood-retina barrier b y a mechanism involving tyrosine nitration and increases in expression of vascular endothelial growth factor and urokinase plaminogen activator. Am. J. Pathol. 2003;162:1995–2004. doi: 10.1016/S0002-9440(10)64332-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shibuki H, Katai N, Yodoi J, Uchida K, Yoshimura N. Lipid peroxidation and peroxynitrite in retinal ischemia-reperfusion injury. Invest. Ophthalmol. Vis. Sci. 2000;41:3607–3614. [PubMed] [Google Scholar]