Abstract
Background
There is growing evidence that individual differences among depressed patients on electrophysiologic (EEG), neuroimaging, and neurocognitive measures are predictive of therapeutic response to antidepressants. This study replicates prior findings of pretreatment differences between SSRI responders and nonresponders in EEG alpha power or asymmetry, and examines whether these differences normalize or are stable following treatment.
Methods
Resting EEG (eyes open and closed) was recorded from 28 electrodes (nose reference) in 18 depressed patients when off medication and at the end of 12 weeks of fluoxetine treatment. Clinical response was assessed by an independent rater using the Clinical Global Improvement scale. EEG data were also obtained for 18 healthy adults matched to patients in gender and age.
Results
Treatment responders had greater alpha power compared to nonresponders and healthy controls, with largest differences at occipital sites where alpha was largest. There were also differences in alpha asymmetry between responders and nonresponders at occipital sites. Responders showed greater alpha (less activity) over right than left hemisphere, whereas nonresponders tended to show the opposite asymmetry. Neither alpha power nor asymmetry changed following treatment and test-retest correlations were high, particularly for alpha power. Alpha power and asymmetry showed reasonable positive predictive value but less negative predictive value.
Discussion
The findings confirm reports of alpha differences between antidepressant responders and nonresponders, and raise hopes for developing EEG tests for selecting effective treatments for patients. The stability of alpha power and asymmetry differences between SSRI responders and nonresponders following treatment suggests that they represent state-independent characteristics.
Clinical Trials
Prozac treatment of major depression: discontinuation study; registration #NCT00447128; and Dichotic listening as a predictor of placebo and medication response in depression; registration #NCT00296725; http://www.clinicaltrials.gov/.
Keywords: depression, SSRI, EEG, alpha power, hemispheric asymmetry
An abundance of antidepressants with distinct pharmacologic profiles are available for treating depression and yet clinicians have no way of knowing whether a patient will benefit from a specific medication. Studies using neuroimaging (1–4), neurocognitive (5–7), and electrophysiologic (8–12) measures have found that pretreatment differences among depressed patients are related to subsequent clinical response to antidepressants.
The ability of quantitative electroencephalographic (qEEG) measures of “spontaneous” brain electrical activity in a resting state to predict response to a selective serotonin reuptake inhibitor (SSRI) or other antidepressants has been suggested in several studies. Ulrich et al. (13) found increased posterior alpha in patients who eventually responded to amitriptyline, suggesting that there may be two subtypes of depression having different pathophysiology and antidepressant response. Prichep et al. (14) similarly found evidence for two subgroups of patients having an obsessive-compulsive disorder, one with relative excess of alpha responded well to an SSRI and one with increased relative theta showed little treatment response. Knott et al. (15) found that depressed patients who responded to imipramine showed a trend for more alpha, but had significantly less theta compared to nonresponders. Cook et al. (16) did not find pretreatment differences between fluoxetine responders and nonresponders in theta, but did find group differences in “cordance”, a measure based on a form of surface Laplacian (see Tenke and Kayser [17] for a discussion of methodological limitations). We found a difference in alpha asymmetry between fluoxetine responders and nonresponders (8), which was predicted on the basis of dichotic listening findings (18). Fluoxetine nonresponders showed greater alpha power (less activity) over the left hemisphere than the right, whereas responders tended to have the opposite asymmetry. Two studies have used tomographic (LORETA) analyses to infer theta current density in specific brain regions. Pizzagalli et al. (12) localized pretreatment theta increases to rostral anterior cingulate cortex (ACC) in responders to nortriptyline. Similarly, Mulert et al. (19) reported that depressed patients responding to either citalopram or reboxetine had increased pretreatment activity localizable to rostral ACC. These findings suggest that pretreatment alpha or theta measures may be of value as predictors of clinical response to SSRI or other antidepressants.
EEG in healthy adults has high test-retest reliability for power in the alpha and theta bands (20), whereas it is somewhat lower for alpha asymmetry (21). Less is known about stability of qEEG in depressed patients during treatment with antidepressants. Studies have reported changes in qEEG following acute administration of antidepressants and suggested that they may be of value for identifying patients who are most likely to benefit from treatment. Knott et al. (15) found that depressed patients who responded to 2 weeks of imipramine treatment differed from nonresponders in showing acute increases in theta 3 hours after a test dose, as well as an increase in frontal theta 2 weeks after treatment. In contrast, Cook et al. (9) measured qEEG during treatment with fluoxetine or venlafaxine and found no difference between responders and nonresponders in either baseline or acute change in theta power. Patients who responded to 8 weeks of treatment did show an acute decrease in prefrontal “cordance” after 48 hours and one week of treatment. Fewer studies have examined longer-term effects of treatments on qEEG. Knott et al. (22) reported that male depressed patients showed a decrease in alpha power and an increase in relative theta/delta after 6 weeks of treatment with the SSRI paroxetine. Deldin and Chiu (23) did not find changes in alpha power or asymmetry in depressed patients following a cognitive intervention to improve mood.
The present study measured resting EEG in a new sample of depressed patients before and after 12 weeks of treatment with the SSRI fluoxetine. The purpose was twofold: (1) to replicate prior findings of pretreatment differences in alpha power and asymmetry between SSRI responders and nonresponders, as well as to examine differences in their theta power. We hypothesized that responders would differ from nonresponders in showing greater alpha power and the opposite alpha asymmetry; (2) to determine whether pretreatment differences between responders and nonresponders normalize during treatment or are stable, state-independent characteristics.
Methods and Materials
Subjects
Outpatients between the ages of 20 and 56 attending a university-affiliated depression research clinic were included. Patients were excluded for any of the following reasons: serious suicide risk, seizure disorder, mental disorders secondary to a general medical condition, substance use disorders (including alcohol abuse) within the last 6 months, psychotic disorders, history of significant head trauma, or other neurologic disorder. All patients signed informed consent forms before participating in the study. All aspects of their diagnostic assessment and treatment were carried out by research psychiatrists.
EEGs were obtained for 18 depressed patients at baseline and again after 12 weeks of open treatment with fluoxetine. All but one patient was treated as part of a clinical trial in which they received 10 mg of fluoxetine during week 1, 20 mg during weeks 2–4, and 40 mg during weeks 5–8, and if still no response, a further increase to 60 mg was permitted during weeks 9–12. Dose increases after week 2 were optional, based on clinical response and tolerability of medication. The end dose of fluoxetine at week 12 was 60mg, except for two patients whose end dose was 40mg (one treatment responder and one nonresponder). The remaining patient was tested before receiving a 6-week single-blind placebo period and, after not responding to placebo, received 12 weeks of fluoxetine treatment beginning at 20 mg and increasing biweekly up to a final dose of 60 mg. A clinician, blind to the patient’s EEG data, rated each patient at the end of 12 weeks of treatment using the Clinical Global Impression Improvement scale (CGI-I; 24). Patients who had a CGI-I rating of “much or very much” improved were considered to be responders and all other patients were considered as nonresponders. A 21-item Hamilton Depression scale (HAM-D21; 25) was obtained before and during treatment. EEGs were also obtained for 18 right-handed healthy adults matched to the depressed patients in gender and age. They were screened to exclude those having current or past Axis I psychopathology, substance abuse, history of significant head trauma, and neurological disorders.
Table 1 gives the characteristics of the responders, nonresponders and healthy controls. These groups did not differ significantly in gender or age, but nonresponders had somewhat less education than responders and healthy controls (p<.05). Education was not, however, significantly associated with alpha power (r= .11, ns) or asymmetry (r= .32, ns). Also, group differences in alpha power and asymmetry reported below remained the same when education was included as a covariate. The groups did not differ significantly in handedness, as indicated by their laterality quotient (LQ) on the Edinburgh Inventory (26). Two responders and three nonresponders were left handed and the remaining patients and controls were right handed (LQ>0). Handedness LQ scores were not significantly associated with either alpha power (r= .07, ns) or asymmetry (r= − .22, ns). There was no difference between responders and nonresponders in pretreatment severity of depression on the HAM-D21. Following treatment, responders had markedly lower HAM-D21 scores than nonresponders (t= 3.57, df=16, p<.01) and were essentially in remission (i.e., all had HAM-D21 score ≤ 7 except for one patient who had score of 8). Among responders, 8 met DSM-IV criteria for major depressive disorder (MDD), 2 for both MDD plus dysthymia, and 1 for dysthymia with past MDD. Among nonresponders, 6 met criteria for MDD and 1 for MDD plus dysthymia. Three responders also met DSM-IV criteria for an anxiety disorder (1 social phobia, 1 panic disorder and 1 obsessive-compulsive disorder). Two nonresponders met criteria for panic disorder.
Table 1.
Characteristics of Treatment Responders, Nonresponders and Healthy Controls
| Responders | Nonresponders | Controls | |
|---|---|---|---|
| Gender | |||
| M/F | 7/4 | 6/1 | 13/5 |
| Age (years) | |||
| M | 38.0 | 33.7 | 31.7 |
| SD | 9.4 | 9.7 | 7.6 |
| Education (years)a | |||
| M | 16.5 | 13.4 | 15.5 |
| SD | 2.6 | 1.6 | 1.8 |
| Handedness (Laterality Quotient) | |||
| M | 61.8 | 19.7 | 72.9 |
| SD | 64.5 | 91.1 | 19.9 |
| Pretreatment HAM-D | |||
| M | 19.0 | 19.7 | |
| SD | 2.1 | 6.6 | |
| Post-Treatment HAM-Db | |||
| M | 5.4 | 15.4 | |
| SD | 2.5 | 8.6 |
N = 10 for responders. Significant difference among groups in education (F = 4.27, df = 2, 32, p < .05). Newman-Keuls post-hoc test, nonresponders < responders = controls.
Responders differ significantly from nonresponders in post-treatment HAM-D (t = 3.57, df = 16, p < .01).
Procedures
Patients were initially tested during a baseline session after being unmedicated for a minimum of 7 days (6 weeks if receiving fluoxetine), but most patients were drug-free for considerably longer. Four responders and 4 nonresponders were not previously treated with an antidepressant, and 3 responders and 2 nonresponders had not received an antidepressant for over 8 months. All patients were retested at end of the 12th week of fluoxetine treatment using the same procedures as the baseline session. Resting EEG was recorded while subjects sat quietly in a sound-attenuated booth. EEGs were recorded during four 2-min periods, half with eyes open (O) and half with eyes closed (C) in a counterbalanced order (OCCO or COOC). Subjects were instructed to remain still and to avoid blinks or eye movements during the recording period. During the O condition, subjects fixated on a central cross.
Electrophysiologic Recording
Scalp EEG was recorded using a 30 channel electrode cap (Electro Cap International, Eaton, OH) with a nose reference. Ag/AgCl electrodes (Grass, West Warwick, RI) at supra- and infraorbital sites surrounding the right eye were used to monitor eye blinks and vertical eye movements (bipolar), and electrodes at right and left outer canthi monitored horizontal eye movements (bipolar). All electrode impedances were below 5 KΩ. EEGs were recorded through a Grass Neurodata (West Warwick, RI) acquisition system at a gain of 10 K (5 K and 2 K for horizontal and vertical eye channels), with a bandpass of 0.1–30 Hz. A PC-based EEG acquisition system (NeuroScan, Sterling, VA) acquired and digitized the data continuously at 200 samples/sec over each recording period.
Electrophysiologic Analyses
Data were segmented into consecutive 1.28-sec epochs (50% overlap) yielding a frequency resolution of 0.78 Hz. Epochs contaminated by blinks, eye movements, and movement-related artifacts were excluded from analyses by direct visual inspection of the raw data. The DC offset of each epoch was then removed, and the EEG was tapered over the entire 1.28 sec using a Hanning window to suppress spectral side lobes (27). By overlapping epochs by 50% the attenuated data are restored in the adjacent record, preserving data with minimal redundancy. EEG data were subjected to a power spectrum analysis using a Fast-Fourier Transform. At each electrode, alpha power was averaged for artifact-free epochs spanning each recording period for each subject, and subsequently integrated over 7.8 – 12.5 Hz. Common logarithms of alpha power were computed to normalize the data. Secondary analyses also examined group differences in theta power (4–7 Hz). There was a difference among groups in total number of minutes of artifact-free EEG data (F=3.31, df=2,33, p<.05). Normal controls (Mean=3.4±1.1) had fewer minutes of EEG than responders (Mean=4.8±2.1; p<.05), but there was no significant difference between responders and nonresponders (Mean=4.3±1.0). However, number of minutes of EEG was not significantly related to either alpha power (r= .22, ns) or asymmetry (r= −.14, ns).
Statistical Analyses
Analyses focused on alpha because of its inverse relation to cortical activity (28) and prior findings of differences between antidepressant responders and nonresponders. Previous EEG studies have indicated the importance of regional (e.g., anterior vs. posterior) and hemispheric (left vs. right) differences when comparing alpha in depressed and nondepressed subjects. To examine these regional differences, log alpha power during pretreatment session was computed at medial sites over each hemisphere at frontal (left, F3; right, F4), central (C3; C4), parietal (P3; P4), and occipital (O1;O2) regions. These topographic measures were then used as orthogonal factors in a repeated-measures ANOVA, using three within-subject factors: Hemisphere (left, right), Region (F, C, P, O), and Condition (eyes open, eyes closed), and one between-subjects factor: Group (responder, nonresponder, controls). The sources of significant interactions were further examined by analysis of simple effects. F ratios were evaluated using degrees of freedom computed using the Greenhouse–Geisser ε correction (29) where appropriate to counteract heterogeneity of variance– covariance matrices associated with repeated measures. The same ANOVA model was also used to examine theta power. To determine whether pretreatment differences in alpha between responders and nonresponders remained stable or changed following fluoxetine treatment, a repeated-measures ANOVA was performed on log alpha power at occipital sites, where pretreatment differences were maximum. This ANOVA used three within-subject factors: Hemisphere (left, right), Condition (eyes open, eyes closed), and session (pretreatment, fluoxetine), and one between-subjects factor: Group (responder, nonresponder).
Results
Pretreatment session in responders, nonresponders and controls
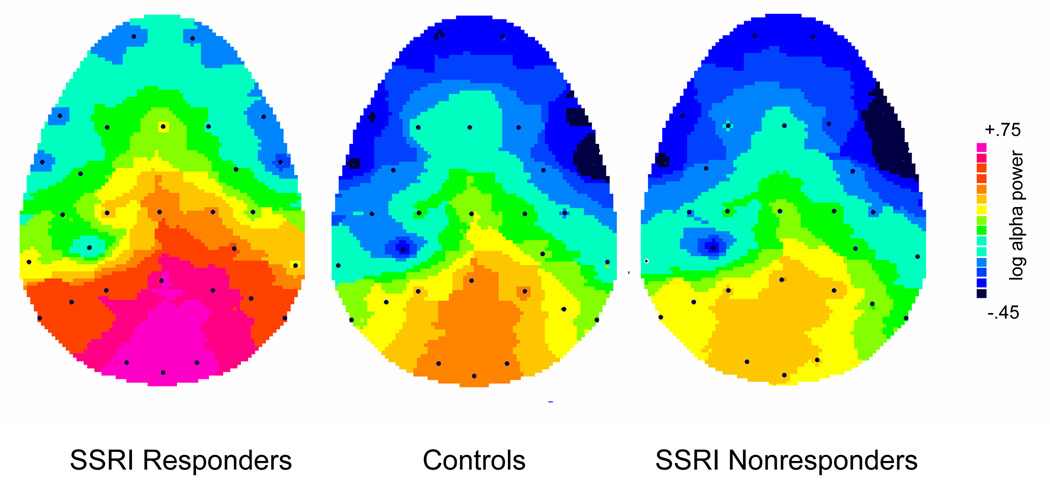
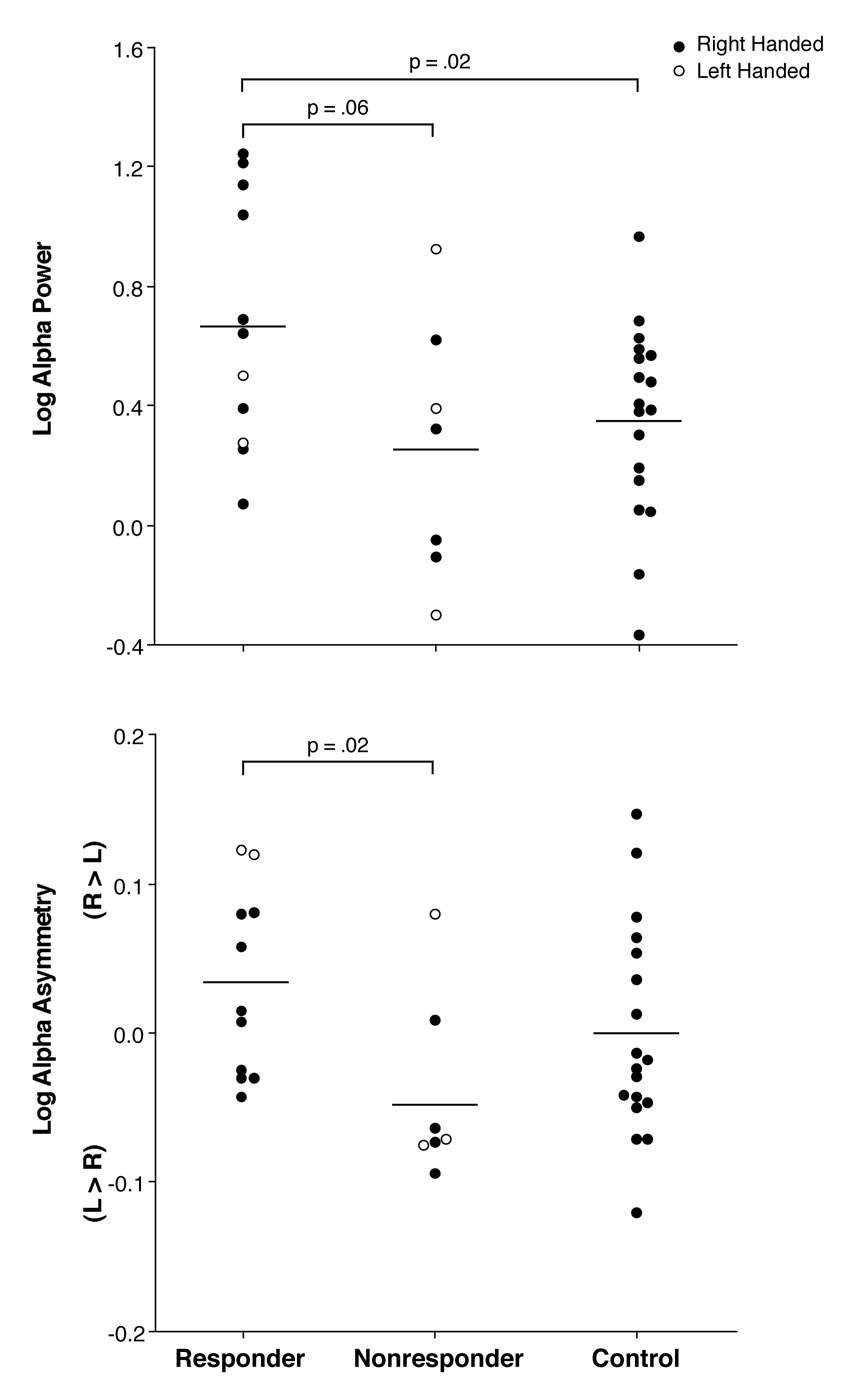
There was a difference in overall alpha power among groups (F=3.19, df=2,33, p=.05), with responders having significantly greater alpha when compared to controls (p=.02). The enhanced alpha in responders was most evident at posterior sites where alpha is typically largest (see Figure 1). When separately examined at frontal, central, parietal and occipital regions, the difference in alpha among groups was significant only at occipital sites (F= 3.51, df=2,33, p=.04). Responders had significantly greater alpha when compared to controls (p=.02) at occipital sites and also tended to have greater alpha than nonresponders (p=.06) (see top portion of Figure 2). Although only approaching a conventional level of significance, the alpha difference between responders and nonresponders had a relatively large effect size of 0.98. There was also a trend for the predicted difference in alpha asymmetry among groups at occipital sites (Group by Hemisphere interaction, F=2.68, df=2,33, p=.08). Responders differed significantly from nonresponders in alpha asymmetry (p=.02), with responders showing greater alpha (less activity) over right than left occipital sites and nonresponders tending to show the opposite asymmetry (see bottom of Figure 2). Healthy controls had essentially no alpha asymmetry and did not differ significantly from either patient group.
Figure 1.
Topography of pretreatment alpha power for responders, nonresponders, and healthy controls, illustrating that group differences in alpha are most evident over posterior sites (bottom portion of maps).
Figure 2.
Log alpha power and asymmetry for the responders, nonresponders and healthy controls.
There was also a difference among the responder, nonresponder and control groups in theta power at occipital sites (F=3.26, df= 2,33, p=.05). Responders had greater theta power (Mean= .29±.22) when compared to controls (Mean= .13±.18; p=.02), whereas nonresponders (Mean= .25±.20) did not differ significantly from the other groups. The difference in theta between responders and controls was also significant at the midline occipital site (p=.02), but not at other midline sites. Although there was a difference in theta across left and right occipital sites (F= 6.88, df=1,33, p=.01), there was no significant group difference in the theta asymmetry.
Pretreatment versus Fluoxetine Treatment Sessions in responders and nonresponders
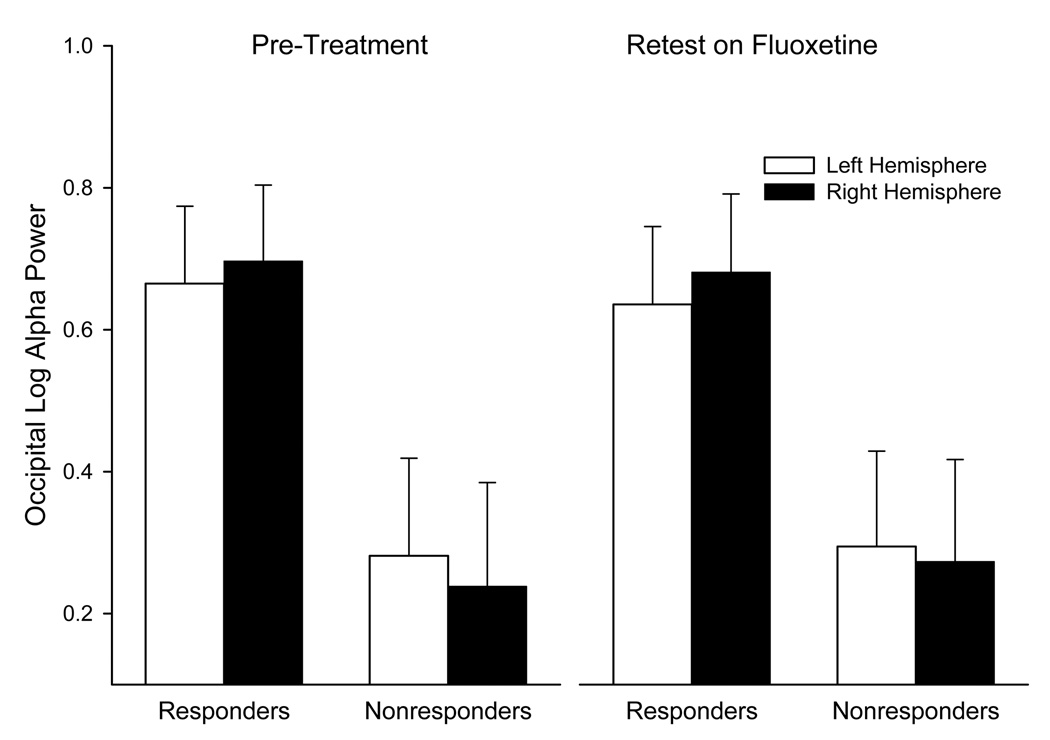
At occipital sites, where group differences in alpha were most evident, responders tended to have greater alpha power than nonresponders across the pre and post treatment sessions (see Figure 3; F= 3.85, df=1,16, p=.07). There was no significant change in alpha power across sessions and the difference in alpha between responders and nonresponders did not change (i.e., no Group by Session interaction). There was a Group by Hemisphere interaction (F= 6.66, df=1,16, p=.02), with responders showing greater alpha (less activity) over right than left occipital site (F= 5.09, df= 1,16, p=.04) and nonresponders showing a nonsignificant asymmetry in opposite direction. This group difference in occipital asymmetry did not change across sessions, which is indicated by the lack of a Group by Hemisphere by Session interaction. There was a significant difference in occipital alpha asymmetry in both pretreatment (F=6.16, df=1,16, p= .02) and fluoxetine treatment (F=5.83, df= 1,16, p=.03) sessions. Test-retest correlations confirmed that alpha power was extremely stable across pretreatment and fluoxetine treatment sessions, ranging from r=.92 at frontal to .97 at occipital sites. Test-retest correlations were lower for alpha asymmetry, being .63 at frontal, .56 at central, .73 at parietal, and .86 at occipital sites.
Figure 3.
Mean log alpha power over right and left hemisphere for responders and nonresponders before treatment and after 12 weeks on fluoxetine (bars= standard errors).
Prediction of Treatment Response
We examined the value of alpha power and asymmetry at occipital sites for predicting treatment response. As in our prior studies, we used the mean asymmetry for healthy controls (Figure 2) as a cutoff to divide patients into those having alpha asymmetry greater than normal (predicted to be responders) or less than normal (predicted to be nonresponders). Similarly, we used mean alpha power for healthy controls as a cutoff for dividing patients into those with greater or less than normal alpha. Indices evaluating predictions of treatment response are given in Table 2. Both alpha power and asymmetry showed reasonable positive predictive value (i.e., response rate of 72.7 % and 77.8 % for patients predicted to be responders), but less negative predictive value (i.e., nonresponse rate of 57.1 % and 55.6 % for patients who were predicted to be nonresponders). Although alpha power and asymmetry were positively correlated (r=.34, p=.04), the small magnitude of this correlation suggests that each provides somewhat independent information for predicting treatment response. We therefore evaluated whether combined use of both would improve the predictive value. In two-thirds of the patients, there was agreement as to the prediction of treatment response across the alpha power and asymmetry measures (i.e., both above vs. below normal). In these cases, using agreement across measures improved the sensitivity and negative predictive value to 83.3 % and 80.0 %.
Table 2.
Indices Evaluating Predictions of Response Using Log Alpha Power and Log Alpha Asymmetry at Occipital Sites
| Alpha Power | Alpha Asymmetry | Combined | |
|---|---|---|---|
| Sensitivity a | 72.7 | 63.6 | 83.3 |
| Specificity b | 57.5 | 71.4 | 67.7 |
| Positive predictive value c | 72.7 | 77.8 | 71.4 |
| Negative predictive value d | 57.1 | 55.6 | 80.0 |
Sensitivity = percentage of responders who were predicted to be responders.
Specificity = percentage of nonresponders who were predicted to be nonresponders.
Positive predictive value = response rate for patients who were predicted to be responders.
Negative predictive value = nonresponse rate for patients who were predicted to be nonresponders.
Right-Handed Responders, Nonresponders and Controls
Although there were too few left-handed patients to allow meaningful conclusions as to the impact of handedness (Figure 2), a comparison was made of the data for only right-handed responders, nonresponders and controls. Overall, all findings reported for the full samples were as strong or stronger for right handers only. Thus, there was a significant difference among groups in alpha power (F=5.50, df= 2,28, p<.01), with responders having greater alpha when compared to both nonresponders (p=.04) and controls (p<.01). Group differences in alpha were present at only occipital sites (Figure 2 top; F= 4.56, df= 2,28, p=.02), where responders had greater alpha than nonresponders (p=.05) and controls (p=.02). At these sites, responders also differed from nonresponders in alpha asymmetry (Figure 2 bottom; F= 5.65, df= 1,11, p=.04). At occipital sites, the same group difference in theta power evident for the full samples was seen for right handers (F=3.54, df= 2,28, p=.04). Responders had greater theta than controls (p=.01), but did not differ significantly from nonresponders.
Discussion
Patients who responded to fluoxentine had greater EEG alpha when compared to healthy controls, whereas nonresponders did not differ from controls. An excess of alpha has previously been reported for patients having an obsessive-compulsive disorder who responded to an SSRI (14) and in affectively-disordered patients who responded to treatment with an antidepressant or secondary treatment with an anticonvulsant or lithium (13,30). Early studies of resting EEG reported finding greater alpha with eyes closed in depressed patients when compared to controls (31,32). Given the inverse relation of alpha power and cortical activity, increased alpha was viewed as evidence of reduced activity in depressed patients. Our findings indicate that this reduced cortical activity is evident in a subgroup of depressed patients who respond to an SSRI and is a predictor of those who most benefit from this treatment. This may not, however, be specific to SSRI antidepressants, in that depressed patients who responded to tricyclic antidepressants also showed increased alpha (13,15). In contrast, decreased alpha has been found to be predictive of improvement in mood following cognitive restructuring (23). The increase in occipital power in fluoxetine responders was also found in the theta band, which suggests that this qEEG difference is not specific to alpha, but includes somewhat lower frequencies as well. It is noteworthy that a distinct spectral component with a peak that spans both the alpha and theta bands is identifiable in the resting EEG using a reference-free approach (17).
SSRI responders also differed from nonresponders in their alpha asymmetry, which is in accord with our prior findings (8). At occipital sites, where alpha is largest, responders showed an asymmetry indicative of greater activity over left than right hemisphere, whereas nonresponders had the opposite direction of asymmetry. The favoring of left over right hemisphere activity in responders is the asymmetry seen for patients having a “pure” MDD in EEG studies (33,34) and SSRI responders in dichotic listening studies (5). Although the specificity of the posterior alpha asymmetry to SSRI responders is unknown, alpha asymmetry at frontal but not posterior sites predicted mood improvement following cognitive restructuring (23). In the present study, frontal alpha asymmetry did not differ between fluoxetine responders and nonresponders.
Neither alpha power nor asymmetry changed following 12 weeks of treatment with fluoxetine. The extremely high test-retest correlations for alpha power in depressed patients (r≥ .90) are comparable to those previously reported for healthy adults for retest periods over one year, and twin studies indicate that alpha power is a stable, heritable trait (20). Elevated alpha power has been found in recovered depressed patients in a euthymic state, which led Pollock and Schneider (35) to hypothesize that it reflects a trait difference in a subgroup of depressed patients. Our findings suggest that this trait is present in patients who respond favorably to an SSRI.
Although there was no significant change in alpha asymmetry after 12 weeks of treatment, test-retest correlations were lower than seen for alpha amplitude, but were still moderately high (r= .56 to .86), particularly at occipital sites. Test-retest correlations in healthy adults are also lower for alpha asymmetry than alpha power, with about 60% of the variance of alpha asymmetry attributed to a temporally stable trait (21). The alpha asymmetry in SSRI responders at occipital sites has been found in depressed adolescents and adults (33,34,36,37), in remitted depressed patients (38), and in offspring of parents concordant for MDD who have increased risk for developing a depressive disorder (39). This alpha asymmetry may therefore be a trait marker of vulnerability to a familial form of depression that responds to an SSRI.
It is known that serotonergic activity is closely related to arousal. In an awake state, serotoneric cells in raphé nuclei display a constant pattern of discharge that decreases in firing rate as arousal decreases to a sleep state (40). We hypothesize that increased alpha in depressed patients who respond to an SSRI reflects low arousal associated with low serotonergic activity. Evidence for the role of right temporoparietal and subcortical regions in mediating arousal (41,42) suggests a possible mechanism that could account not only for increased alpha in SSRI responders, but also their alpha asymmetry. Heller et al. (42) presented a model suggesting that depression is related to dysfunction of right temporoparietal mechanisms mediating emotional arousal. Thus, low serotonergic activity, presumably related to reduced activity of mesencephalic raphé nuclei and cortical afferents, could play a role in both the increased alpha and alpha asymmetry in SSRI responders.
A question remains as to why clinical improvement in SSRI responders did not normalize their alpha. Although a common serotonergic mechanism may underlie both depression and EEG abnormalities in responders, they need not have the same pharmacological properties. A preclinical study (44) found that spontaneous firing of serotonin neurons in dorsal raphé of rats was not altered after two weeks of escitalopram administration, whereas combined treatment with this SSRI plus bupropion resulted in a marked increase in firing rates. Moreover, persistence of alpha abnormalities in treatment responders is compatible with their being an endophenotyic marker of vulnerability to MDD (39).
This study has limitations. First, the sample of SSRI nonresponders was small (n=7). It was, however, larger in our prior study (8) and the difference in alpha asymmetry between fluoxetine responders (n=34) and nonresponders (n=19) was the same as the present study. Also, heightened alpha in responders (n=11) was seen not only when compared to nonresponders, but also compared to healthy controls (n=18). The stability of differences between responders and nonresponders across pre and post treatment sessions also increases confidence in the findings. A second limitation is that patients in this study were predominately men, which may raise a question as to whether findings generalize to women. The number of women was larger in our prior studies (5,8) and the difference in hemispheric asymmetry between responders and nonresponders was even stronger among women than men. Third, the EEG findings were obtained during open-label treatment and placebo effects are unknown. In a study in which a placebo control group was included (43), we found no difference between placebo responders and nonresponders in hemispheric asymmetry for dichotic listening. Lastly, although differences between responders and nonresponders in alpha power and asymmetry appear to represent stable, state-independent traits, their biological basis is unknown. An ongoing study is using a high-density electrode array and current source density measures (17) to provide spatial resolution to more adequately address the neuroanatomical origin of these differences. Studies are also needed to determine their relation to genetic and neurochemical mechanisms that may underlie responsiveness to antidepressants. The findings do raise hopes for developing qEEG tests for selecting effective treatments for depressed patients.
Acknowledgments
This research was supported by National Institute of Mental Health grants MH36295(GEB) and MH56058 (PJM). We gratefully acknowledge Jürgen Kayser for providing us with processing and analysis software.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures. Dr. Bruder, Dr. Tenke and Mr. Sedoruk do not have any financial disclosures. Dr. Stewart has been on the speakers bureau of, been a consultant to, or received research support from Eli Lilly, Forest, GlaxoSmithKline, Organon, Shire, Pfizer, BioVail, and Somerset Pharmaceuticals. Dr. McGrath has provided scientific consultation or served on advisory boards for GlaxoSmithKline and Somerset Pharmaceuticals. He has received research grant support from Eli Lilly, GlaxoSmithKline, Lipha, and Organon Pharmaceuticals. Dr. Quitkin provided scientific consultation or served on advisory boards for Eli Lilly, Organon, and Pfizer and held shares of Cyberonics stock. Dr. Quitkin died in October 2005.
References
- 1.Buchsbaum MS, Wu J, Siegel BV, Hackett E, Trenary M, Abel L, et al. Effect of sertraline on regional metabolic rate in patients with affective disorder. Biol Psychiatry. 1997;41:15–22. doi: 10.1016/s0006-3223(96)00097-2. [DOI] [PubMed] [Google Scholar]
- 2.Little JT, Ketter TA, Kimbrell TA, Dunn RT, Benson BE, Willis MW, Luckenaugh DA, Post RM. Bupropion and venlafaxine responders differ in pretreatment regional cerebral metabolism in unipolar depression. Biol Psychiatry. 2005;57:220–228. doi: 10.1016/j.biopsych.2004.10.033. [DOI] [PubMed] [Google Scholar]
- 3.Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, Silva JA, McGinnis S, Glass TG, Martin CC, Fox PT. Cingulate function in depression: a potential predictor of treatment response. NeuroReport. 1997;8:1057–1061. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- 4.Saxena S, Brody AL, Ho ML, Zohrabi N, Maidment KM, Baxter LR. Differential brain metabolic predictors of response to paroxetine in obsessive-compulsive disorder versus major depression. Am J Psychiatry. 2003;160:522–532. doi: 10.1176/appi.ajp.160.3.522. [DOI] [PubMed] [Google Scholar]
- 5.Bruder GE, Stewart JW, McGrath PJ, Deliyannides D, Quitkin FM. Dichotic listening tests of functional brain asymmetry predict response to fluoxetine in depressed women and men. Neurophsychopharmacology. 2004;29:1752–1761. doi: 10.1038/sj.npp.1300519. [DOI] [PubMed] [Google Scholar]
- 6.Dunkin JJ, Leuchter AF, Cook IA, Kasi-Godey JE, Abrams M, Rosenberg-Thompson S. Executive dysfunction predicts nonresponse to fluoxetine in major depression. J Affect Disord. 2000;60:13–23. doi: 10.1016/s0165-0327(99)00157-3. [DOI] [PubMed] [Google Scholar]
- 7.Taylor BP, Bruder GE, Stewart JW, McGrath PJ, Halperin J, Ehrlichman H, Quitkin FM. Psychomotor slowing as a predictor of fluoxetine nonresponse in depressed outpatients. Am J Pyschiatry. 2006;163:73–78. doi: 10.1176/appi.ajp.163.1.73. [DOI] [PubMed] [Google Scholar]
- 8.Bruder GE, Stewart JW, Tenke CE, McGrath PJ, Leite P, Bhattacharya N, Quitkin FM. Electroencephalographic and perceptual asymmetry differences between responders and nonresponders to an SSRI antidepressant. Biol Psychiatry. 2001;48:416–425. doi: 10.1016/s0006-3223(00)01016-7. [DOI] [PubMed] [Google Scholar]
- 9.Cook IA, Leuchter AF, Morgan M, Witte E, Stubbeman WF, Abrams M, Rosenberg S, Uijtdehaage SH. Early changes in prefrontal activity characterize clinical responders to antidepressants. Neuropsychopharmacology. 2002;27(1):120–131. doi: 10.1016/S0893-133X(02)00294-4. [DOI] [PubMed] [Google Scholar]
- 10.Gallinat J, Bottlender R, Juckel G, Munke-Puchner A, Stotz G, Kuss HJ, Mavrogiorgou P, Hegerl U. The loudness dependence of the auditory evoked N1/P2-component as a predictor of the acute SSRI response in depression. Psychopharmacology. 2000;148:404–411. doi: 10.1007/s002130050070. [DOI] [PubMed] [Google Scholar]
- 11.Kalayam B, Alexopoulos GS. A preliminary study of left frontal region error negativity and symptom improvement in geriatric depression. Am J Psychiatry. 2003;60:2054–2056. doi: 10.1176/appi.ajp.160.11.2054. [DOI] [PubMed] [Google Scholar]
- 12.Pizzagalli D, Pascual-Marqui RD, Nitschke JB, Oakes TR, Larson CL, Abercrombie HC, et al. Anterior cingulate activity as a predictor of degree of treatment response in major depression: evidence from brain electrical tomography analysis. Am J Psychiatry. 2001;158:405–415. doi: 10.1176/appi.ajp.158.3.405. [DOI] [PubMed] [Google Scholar]
- 13.Ulrich G, Renfordt E, Frick K. The topographical distribution of alpha-activity in the resting EEG of endogenous-depressive inpatients with and without clinical-response to pharmacotherapy. Pharmacopsychiatry. 1986;19:272–273. [Google Scholar]
- 14.Prichep LS, Mas F, Hollander E, Liebowitz M, John ER, Almas M, DeCaria CM, Levine RH. Quantitative electroencephalographic subtyping of obsessive-compulsive disorder. Psychiatry Res Neuroimaging. 1993;50:25–32. doi: 10.1016/0925-4927(93)90021-9. [DOI] [PubMed] [Google Scholar]
- 15.Knott VJ, Telner JI, Lapierre YD, Browne M, Horn ER. Quantitative EEG in the prediction of antidepressant response to imipramine. J Affect Disord. 1996;39:175–184. doi: 10.1016/0165-0327(96)00003-1. [DOI] [PubMed] [Google Scholar]
- 16.Cook IA, Leuchter AF, Witte E, Abrams M, Uijtdehaage SHJ, Stubbeman W, Rosenberg-Thompson S, Anderson-Hanley C. Neurophysiologic predictors of treatment response to fluoxetine in major depression. Psychiatry Res. 1999;85:263–273. doi: 10.1016/s0165-1781(99)00010-4. [DOI] [PubMed] [Google Scholar]
- 17.Tenke CE, Kayser J. Reference-free quantification of EEG spectra: combining current source density (CSD) and frequency principal components analysis (fPCA) Clin Neurophysiol. 2005;116(12):2826–2846. doi: 10.1016/j.clinph.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Bruder GE, Otto MW, McGrath PJ, Stewart JW, Fava M, Rosenbaum JF, Quitkin FM. Dichotic listening before and after fluoxetine treatment for major depression: relations of laterality to therapeutic response. Neuropsychopharmcology. 1996;15:171–179. doi: 10.1016/0893-133X(95)00180-L. [DOI] [PubMed] [Google Scholar]
- 19.Mulert C, Juckel G, Brunnmeier M, Karch S, Leicht G, Mergl R, Moller HJ, Hegerl U, Pogarell O. Prediction of treatment response in major depression: integration of concepts. J Affect Disord. 2007;98(3):215–225. doi: 10.1016/j.jad.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 20.Smit DJA, Posthuma D, Boomsma DI, De Geus EJC. Heritability of background EEG across the power spectrum. Psychophysiology. 2005;42:691–697. doi: 10.1111/j.1469-8986.2005.00352.x. [DOI] [PubMed] [Google Scholar]
- 21.Hagemann D, Hewig J, Seifert J, Naumann E, Bartussek D. The latent state-trait structure of resting EEG asymmetry: Replication and extension. Psychophysiology. 2005;42:740–752. doi: 10.1111/j.1469-8986.2005.00367.x. [DOI] [PubMed] [Google Scholar]
- 22.Knott V, Mahoney C, Kennedy S, Evans K. EEG correlates of acute and chronic paroxetine treatment in depression. Affect Disord. 2002;69(1–3):241–249. doi: 10.1016/s0165-0327(01)00308-1. [DOI] [PubMed] [Google Scholar]
- 23.Deldin PJ, Chiu P. Cognitive restructuring and EEG in major depression. Biol Psychol. 2005;70:141–151. doi: 10.1016/j.biopsycho.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Guy W. ECDEU Assesment Manual for Psychopharmocology: Publication ADM 76-338. Washington, D.C.: U.S. Department of Health, Education, and Welfare; 1976. pp. 534–537. [Google Scholar]
- 25.Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 27.Bendat JS, Piersol AG. Random data: Analyses and measurement procedures. New York: Wiley; 1971. [Google Scholar]
- 28.Shagass C. Electrical activity of the brain. In: Greenfield NS, Sternbach RA, editors. Handbook of Psychophysiology. New York: Rinehart & Winston; 1972. pp. 263–328. [Google Scholar]
- 29.Jennings JR, Wood CC. The epsilon-adjustment procedure for repeated-measures analyses of variance. Psychophysiology. 1976;13:277–278. doi: 10.1111/j.1469-8986.1976.tb00116.x. [DOI] [PubMed] [Google Scholar]
- 30.Suffin SC, Emory WH. Neurometric subgroups in attentional and affective disorders and their association with pharmacotherapeutic outcome. Clin Electroencephalogr. 1995;26:76–83. doi: 10.1177/155005949502600204. [DOI] [PubMed] [Google Scholar]
- 31.Pollock VE, Schneider LS. Topographic quantitative EEG in elderly subjects with major depression. Psychophysiology. 1990;27:438–444. doi: 10.1111/j.1469-8986.1990.tb02340.x. [DOI] [PubMed] [Google Scholar]
- 32.Shagass C, Roemer RA, Josiassen RC. Some quantitative EEG findings in unmedicated and medicated major depressives. Neuropsychobiology. 1988;19:169–175. doi: 10.1159/000118455. [DOI] [PubMed] [Google Scholar]
- 33.Bruder GE, Fong R, Tenke CE, Leite P, Towey JP, Stewart JE, McGrath PJ, Quitkin FM. Regional brain asymmetries in major depression with or without an anxiety disorder: A quantitative electroencephalographic study. Biol Psychiatry. 1997;41:939–948. doi: 10.1016/S0006-3223(96)00260-0. [DOI] [PubMed] [Google Scholar]
- 34.Kentgen LM, Tenke CE, Pine DS, Fong R, Klein RG, Bruder GE. Electroencephalographic asymmetries in adolescents with major depression: Influence of comorbidity with anxiety disorders. J Abnorm Psychol. 2000;109:797–802. doi: 10.1037//0021-843x.109.4.797. [DOI] [PubMed] [Google Scholar]
- 35.Pollock VE, Schneider LS. Topographic electroencephalographic alpha in recovered depressed elderly. J Abnorm Psychol. 1989;98:268–273. doi: 10.1037//0021-843x.98.3.268. [DOI] [PubMed] [Google Scholar]
- 36.Davidson RJ, Chapman JP, Chapman LJ. Task-dependent EEG asymmetry discriminates between depressed and non-depressed subjects. Psychophysiology. 1987;24:585. [Google Scholar]
- 37.Reid SA, Duke LM, Allen JJB. Resting frontal electroencephalographic asymmetry in depression: Inconsistencies suggest the need to identify mediating factors. Psychophysiology. 1998;35:389–404. [PubMed] [Google Scholar]
- 38.Henriques JB, Davidson RJ. Regional brain electrical asymmetries discriminate between previously depressed and healthy control subjects. J Abnorm Psychol. 1990;99:22–31. doi: 10.1037//0021-843x.99.1.22. [DOI] [PubMed] [Google Scholar]
- 39.Bruder GE, Tenke CE, Warner V, Nomura Y, Grillon C, Hille J, Leite P, Weissman MM. Electroencephalogranphic measures of regional hemispheric activity in offspring at risk for depressive disorders. Biol Psychiatry. 2005;57:328–335. doi: 10.1016/j.biopsych.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 40.Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiological Rev. 1992;72(1):165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- 41.Heilman KM, Watson RT, Valenstein E. Neglect and related disorders. In: Heilman KM, Valenstein E, editors. Clinl Neuropsychol. 4th ed. New York: Oxford University Press; 2003. pp. 296–346. [Google Scholar]
- 42.Heller W, Etienne MA, Miller GA. Patterns of perceptual asymmetry in depression and anxiety: implications for neuropsychological models of emotion and psychopathology. J Abnorm Psychol. 1995;104(2):327–333. doi: 10.1037//0021-843x.104.2.327. [DOI] [PubMed] [Google Scholar]
- 43.Bruder GE, Stewart JW, Mercier MA, Agosti V, Leite P, Donovan S, Quitkin FM. Outcome of cognitive-behavioral therapy for depression: relation to hemispheric dominance for verbal processing. J Abnorm Psychol. 1997;106:138–144. doi: 10.1037//0021-843x.106.1.138. [DOI] [PubMed] [Google Scholar]
- 44.Blier P. Exploiting interactions between monoaminergic neurons to improve the antidepressant response. Biol Psychiatry. 2007;61:196S. [Google Scholar]