Abstract
In C. elegans, four asymmetric divisions, beginning with the zygote (P0), generate transcriptionally repressed germline blastomeres (P1–P4) and somatic sisters that become transcriptionally active. The protein PIE-1 represses transcription in the later germline blastomeres, but not in the earlier germline blastomeres P0 and P1. We show here that OMA-1 and OMA-2, previously shown to regulate oocyte maturation, repress transcription in P0 and P1 by binding to and sequestering in the cytoplasm TAF-4, a component critical for assembly of TFIID and the pol II preinitiation complex. OMA-1/2 binding to TAF-4 is developmentally regulated, requiring phosphorylation by the DYRK kinase MBK-2, which is activated at meiosis II following fertilization. OMA-1/2 are normally degraded after the first mitosis, but ectopic expression of wildtype OMA-1 is sufficient to repress transcription in both somatic and later germline blastomeres. We propose that phosphorylation by MBK-2 serves as a developmental switch, converting OMA-1/2 from oocyte to embryo regulators.
Introduction
Germ cells retain developmental totipotency, whereas somatic cells undergo developmental potential and proliferative restriction, culminating in terminal differentiation. Primordial germ cells (PGCs) are set aside early during development and kept quiescent until they are needed for reproduction (review Extavour and Akam, 2003). In mice, PGCs are specified around E6.5, at which time they become transcriptionally silent, concurrent with the removal of chromatin modifications associated with transcription and differentiation, and the addition of other epigenetic markers associated with silenced chromatin (review Surani et al., 2004). In Drosophila and C. elegans, PGCs are specified much earlier during embryogenesis by maternally-supplied factors. In Drosophila, before activation of zygotic transcription, the inclusion of germplasm during the cellularization of a small number of nuclei at the posterior pole specifies these cells as PGCs. These PGCs remain transcriptionally repressed and lack epigenetic markers associated with active chromatin (Kobayashi et al., 1988; Seydoux and Dunn, 1997; Schaner et al, 2003).
In C. elegans, primordial germ cells are specified through a series of four asymmetric divisions beginning with the zygote, P0, the first germline blastomere. Each division gives rise to a transcriptionally repressed germline blastomere (P1–P4) and a corresponding somatic sister cell which quickly becomes transcriptionally active (Figure. 1) (Seydoux and Dunn, 1997; Seydoux and Fire, 1994). Transcriptional repression in C. elegans germline blastomeres must, therefore, involve a mechanism that is either very transient, or readily reversible. P4, the first germline-restricted blastomere, divides symmetrically to generate Z2 and Z3, which go on to generate the entire germline.
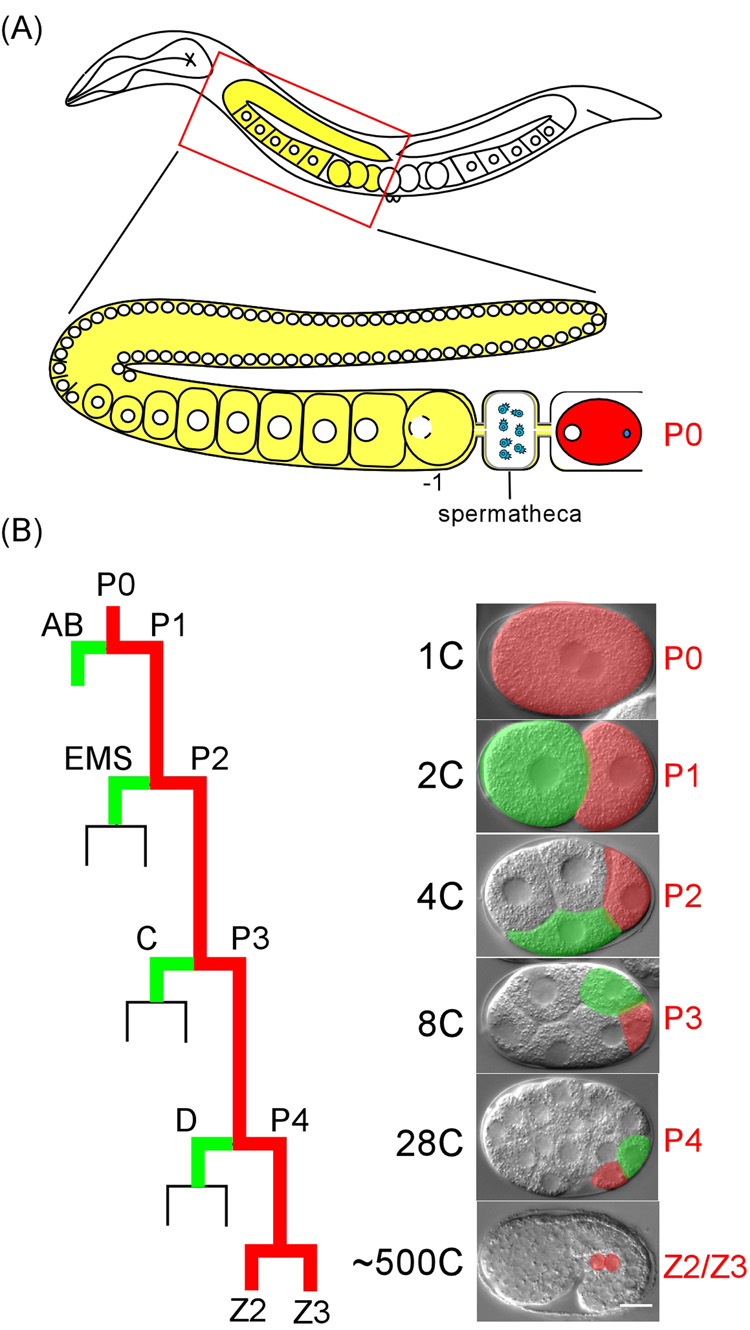
Figure 1. Schematic of C. elegans hermaphrodite gonad and early embryonic divisions.
(A). Oocyte development and maturation schematized in one of the two gonad arms. Germ nuclei (white circles) are in a syncitial cytoplasm of the gonad arm before cellularization to form oocytes. The oocyte adjacent to the spermatheca (−1) undergoes maturation, and is then immediately ovulated and fertilized. (B). Early embryonic divisions, highlighting the germline precursors P0 to P4 (red) and their somatic sisters (green). Left, schematic; Right, DIC images. Embryo images in this and all subsequent figures are oriented with the anterior pole to the left. Bar: 10 µm.
Transcriptional repression in germline blastomeres requires maternally-supplied PIE-1 protein, which localizes exclusively to germline blastomeres (Mello et al., 1996; Seydoux et al., 1996). Studies using C. elegans embryos as well as mammalian tissue culture cells demonstrated that PIE-1 can repress transcriptional elongation (Batchelder et al., 1999; Ghosh and Seydoux, 2008; Zhang et al., 2003). Progression from transcription initiation to elongation requires phosphorylation of serine 2 within heptapeptide repeats of the RNA polymerase II C-terminal domain (CTD) by the p-TEFb kinase complex (Komarnitsky et al., 2000). A region of PIE-1 which resembles an unphosphorylatable form of the CTD heptapeptide sequence serves as a competitive inhibitor of Ser2 phosphorylation (Batchelder et al., 1999; Zhang et al., 2003). A recent report shows that PIE-1 also inhibits transcription initiation in germline blastomeres through an unknown mechanism (Ghosh and Seydoux, 2008).
PIE-1 protein, although present at high levels from P0 to P4, is only essential for transcriptional repression in P2 and P3, and partially required in P4 (Seydoux and Dunn, 1997; Seydoux et al., 1996). In pie-1 null mutant embryos, germline transcription remains repressed in P0 and P1. In addition, phosphorylation at serine 5 of pol II CTD, a modification required for promoter clearance and transcription initiation, was detected at low levels in P2, P3, and P4, but was not detected in P0 or P1 (Seydoux and Dunn, 1997; Walker et al., 2001). These results indicate that 1), transcription repression in P0 and P1 involves a mechanism(s) independent of, or in addition to, PIE- 1, and 2), that unlike P2-P4, repression in P0 and P1 occurs prior to transcription initiation.
PIE-1 contains two CCCH TIS11 zinc fingers (Mello et al., 1996; Varnum et al., 1989), domains which have been implicated in RNA-binding (Lai et al., 1999; Pagano et al., 2007). PIE-1 promotes translation of the NOS-2 protein, a function ascribed to one of the zinc fingers (Tenenhaus et al., 2001). OMA-1 and OMA-2 each also contain two TIS11 zinc fingers, but otherwise share no sequence similarity to PIE-1. OMA proteins are redundantly required for oocyte maturation and the earliest stages of embryonic development (Detwiler et al., 2001; Lin, 2003; Nishi and Lin, 2005; Shimada et al., 2002). OMA-1 and -2 are cytoplasmic proteins detected only in oocytes and the very early embryo. OMA-1/2 levels increase in the proximal oocytes, peak in the maturing (−1) oocyte and the 1-cell embryo, and then rapidly decrease after the first mitotic division (Detwiler et al., 2001; Lin, 2003; Shimada et al., 2002). Timely degradation of OMA proteins requires phosphorylation by a DYRK2 kinase, MBK-2, at T239. Mutations that prevent or reduce phosphorylation at T239 lead to the maintenance of high OMA-1 levels beyond the 1-cell stage and embryonic lethality (Nishi and Lin, 2005; Shirayama et al., 2006). Animals with reduced combined OMA-1 and OMA-2 activity, as opposed to complete depletion, are not fully penetrant for the oocyte maturation defects and do produce some dead embryos (Nishi and Lin, 2005). For simplicity, unless otherwise noted, OMA- 1/2 will refer to OMA-1 and OMA-2.
Here we demonstrate that OMA-1/2 globally repress transcription in early germline blastomeres, P0 and P1, by a novel mechanism. We show that OMA-1/2 bind to and sequester in the cytoplasm TAF-4 (TATA-binding protein associated factor 4), an essential factor required for TFIID formation and function, thereby repressing all RNA polymerase II-dependent transcription. TAF-4 and TAF-12 form specific heterodimers through their histone fold domains (HFD), and this interaction is required for proper nuclear localization of TAF-4. OMA-1 binds to the HFD of TAF-4 via a domain predicted to resemble the structure of the TAF-12 HFD. We also show that the interaction between TAF-4 and OMA-1/2 is essential for embryonic viability and is developmentally regulated, via phosphorylation of OMA-1 at T239 by the kinase MBK-2. This is the same phosphorylation event that promotes OMA-1 timely degradation. Finally, ectopic OMA-1 at the 4- cell stage can repress transcription in somatic cells as well as substitute for PIE-1 in transcriptional repression in the P2 germline blastomere.
Results
OMA-1/2 interact with TAF-4, a component of the TFIID complex, in yeast
In yeast two-hybrid screens for OMA-1 interacting proteins, using the N-terminal 117 amino acids of OMA-1 (OMA-1-N) as bait, we isolated multiple TAF-4 partial cDNAs. TAFs, along with TATA box binding protein (TBP), comprise the TFIID transcription complex (Green, 2000; Walker et al., 2001). The interaction with TAF-4 appears to be specific, as OMA-1-N did not interact with seven other TAF proteins examined (Suppl. Figure 1). OMA-2-N also interacted with TAF-4 with the same specificity (not shown). The isolation of TAF-4 as an OMA-1/2 interacting protein suggested that OMA-1/2 might function in a process that involves transcriptional activation or repression.
OMA proteins sequester TAF-4 in the cytoplasm
The transition from oocyte to embryo is rapid and dynamic in C. elegans. Immediately after fertilization, the oocyte nucleus completes two rounds of meiosis, generating the oocyte pronucleus. Following oocyte and sperm pronuclei decondensation, the oocyte pronucleus migrates toward the sperm pronucleus, and soon after the two pronuclei meet, the nuclear envelope breaks down and the first mitosis begins. Previous antibody staining showed TAF-4 to be nuclear in oocytes and early blastomeres, although nuclear TAF-4 staining was not obvious in 1- and 2-cell embryos (Walker et al., 2001). We repeated the staining using the same anti-TAF-4 antibody and observed a ubiquitous staining in all 1-cell and early 2-cell embryos, and nuclear-enriched staining at all other embryo stages (Figure 2A). This transient decrease in nuclear TAF-4 in the early embryo coincided closely with the brief period during which OMA proteins were detected (Figure 2A) (Detwiler et al., 2001; Lin, 2003; Nishi and Lin, 2005; Shimada et al., 2002). The level of OMA-1 protein is high in 1-cell embryos soon after meiosis but decreases during the first mitotic cycle. In 2-cell embryos, while the majority of OMA-1 protein has been degraded, a low level (<10%) can still be detected (Suppl. Figure 2).
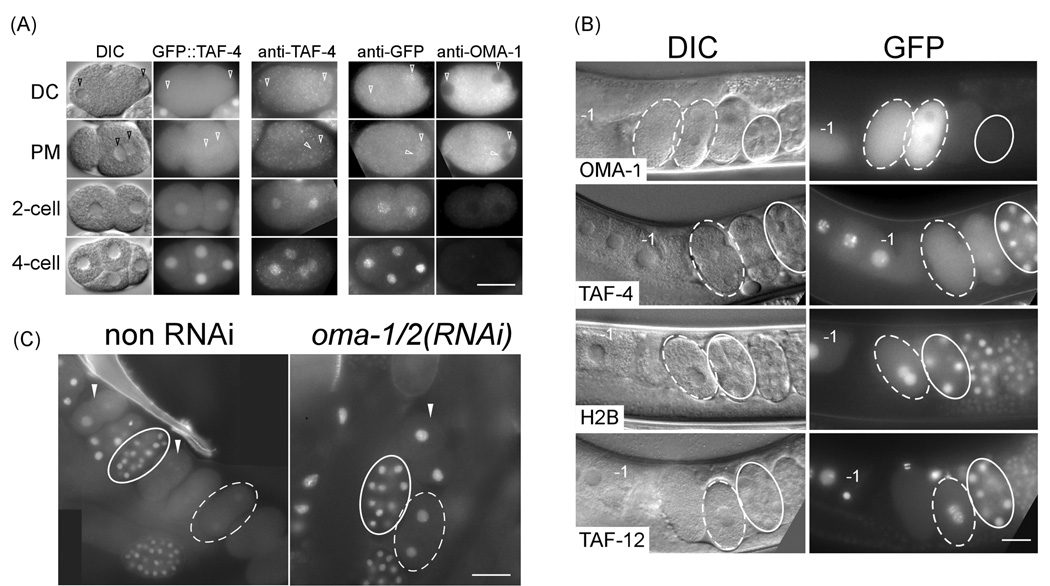
Figure 2. OMA-1 and OMA-2 are required for nuclear enrichment of GFP::TAF-4 in 1-cell embryos.
(A) DIC and GFP fluorescence micrographs of GFP::TAF-4-expressing embryos (first two columns), anti-TAF-4 staining of wildtype embryos (third column), and anti-GFP and anti-OMA-1 double staining of GFP::TAF-4-expressing embryos (last two columns). DC: pronuclei decondensation. PM: pronuclei meeting. Arrowhead points to pronuclei in 1-cell embryos. (B) DIC and GFP fluorescence micrographs of animals expressing various GFP fused transgenic proteins, as indicated below the DIC image. Embryos in the uterus arrange in a developmental progression from youngest (left) to oldest (right). −1, maturing oocyte. (C) GFP fluorescence in fixed embryos from transgenic animals expressing GFP::TAF-4 either without (left) or following (right) oma-1(RNAi);oma-2(RNAi). White arrowheads: 2-cell embryos. The 2-cell and two 1-cell embryos not circled in non-RNAi samples are dividing. 1- and 12-cell embryos are outlined in dashed and solid white lines in (B) and (C). Bar: 25 µm.
We generated a transgenic strain expressing GFP::TAF-4, which recapitulated the anti-TAF- 4 staining pattern: strong nuclear GFP fluorescence in oocytes and all blastomeres in early embryos, with the exception of 1-cell and early 2-cell stage embryos, where GFP fluorescence appeared ubiquitous throughout the blastomeres (Figure 2A, 2B; Suppl. Figure 3). Quantification of GFP::TAF-4 revealed that both the intensity of nuclear GFP and the ratio of nuclear to cytoplasmic GFP were lower in 1-cell embryos compared to the most proximal (−1) oocyte and later stage embryos (Suppl. Figure 3). This inverse correlation with OMA-1 level is specific to GFP::TAF-4 because GFP::histone H2B or another TAF, GFP::TAF-12, expressed from the same expression vector, were predominantly nuclear throughout early embryogenesis (Figure 2B and Suppl. Figure 3D).
The inverse correlation between cytoplasmic OMA and nuclear TAF-4 protein levels suggested that OMA-1/2 might regulate TAF-4 subcellular localization, or vise versa, in vivo. Depletion of taf-4 by RNAi in a strain expressing OMA-1::GFP did not result in any observable defect in OMA-1::GFP localization (data not shown). However, depletion of oma-1/2 did affect TAF-4 localization. We observed an increase in nuclear, relative to cytoplasmic, levels of GFP::TAF-4 in 1-cell and early 2-cell oma-1(RNAi);oma-2(RNAi) embryos (Figure 2C; Suppl. table 1). oma-1/2 depletion did not affect the overall level of GFP::TAF-4 or a control GFP-fusion protein, GFP::PGL-1, expressed from the same vector (Suppl. Table 1 and Suppl. Figure 4). This result is consistent with OMA-1/2 preventing nuclear enrichment of TAF-4 by sequestering it in the cytoplasm in 1-cell and early 2-cell embryos.
OMA-1 and OMA-2 are required for transcriptional repression in germline blastomeres
C. elegans TAF-4 has been shown to be essential for all RNA pol II transcription examined in the embryo. In taf-4(RNAi) embryos, no zygotic transcription was detected (Walker et al., 2001). A low level of nuclear TAF-4 in 1- and 2-cell embryos could account for the lack of transcription in these two stages (Seydoux and Fire, 1994; Walker et al., 2001). We asked whether depletion of oma-1 and oma-2, which results in elevated nuclear GFP::TAF-4 levels, would lead to transcription in 1- and 2-cell embryos.
In wildtype embryos, markers of transcriptional activity or products of transcription are first detected in 4-cell stage embryos, and then only in somatic blastomeres. This is true whether the assay is performed using an antibody to phosphorylated serine 2 of pol II CTD (anti-Ser2P antibody), a marker for transcriptional elongation (Komarnitsky et al., 2000; Seydoux and Dunn, 1997), or by in situ hybridization to known early zygotic transcripts (Seydoux and Fire, 1994). In pie-1 mutant or RNAi embryos, ectopic transcription was detected in germline blastomeres P2 and P3, but never in embryos prior to the 4-cell stage (Seydoux et al., 1996). We have reproduced these results (Figure 3A, 3B).
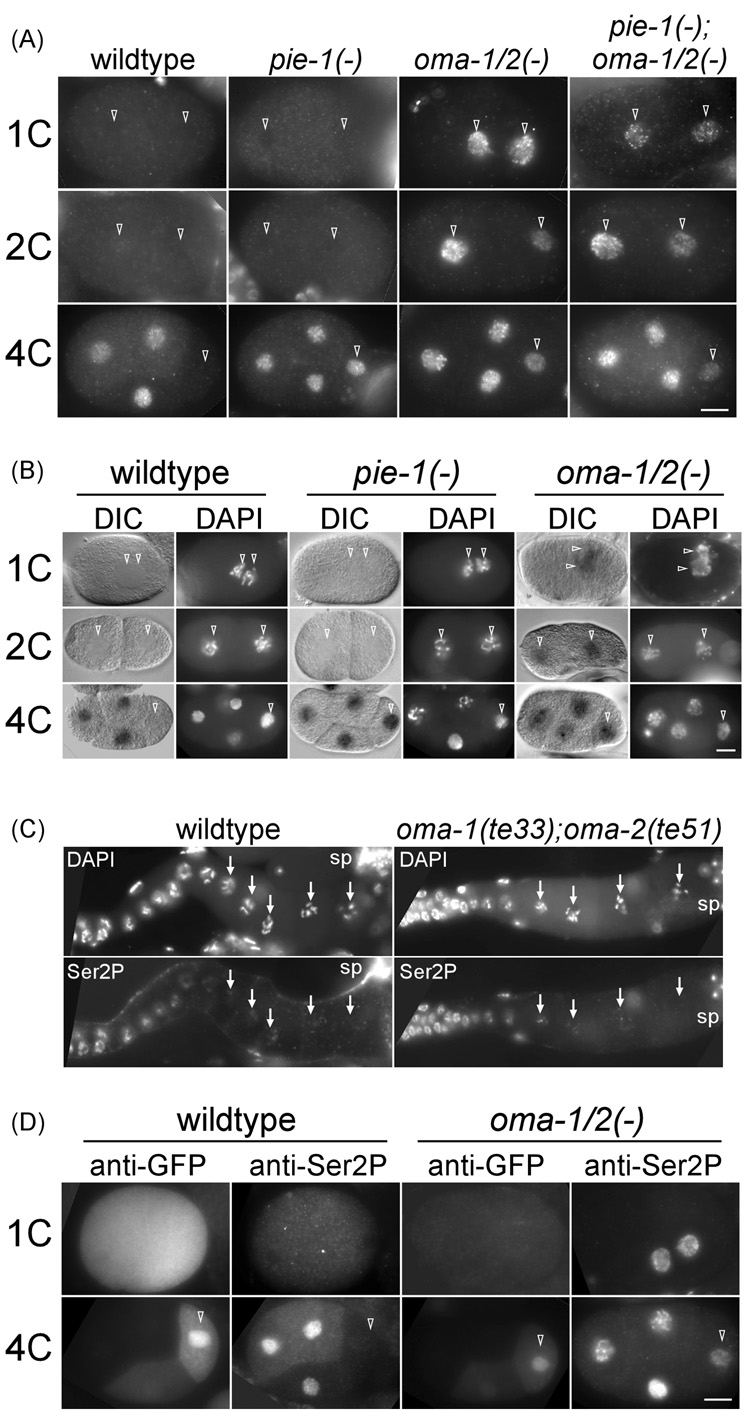
Figure 3. OMA-1 and OMA-2 are required for transcriptional repression in germline blastomeres.
(A) Immunofluorescence micrographs of anti-Ser2P staining in wildtype (non RNAi), pie-1(RNAi), oma-1(RNAi);oma-2(RNAi), and oma-1(te33);oma-2(RNAi);pie-1(RNAi) embryos. The background cytoplasmic signals in 1-cell (1C) and 2-cell (2C) non-RNAi and pie-1(RNAi) embryos were enhanced to reveal the outline of embryos. 4C: 4-cell. (B) In situ hybridization of the vet-5 gene. For each embryo, DIC and DAPI images are shown. Open arrowheads point to pronuclei in 1C, nuclei in 2C, and the germline nucleus in 4C embryos, respectively. (C) anti-Ser2P in the gonad of wildtype or oma-1(te33);oma-2(te51) animals. Oocyte nuclei are indicated by arrows. sp: spermatheca. (D) Immunofluorescence micrographs of anti-GFP and anti-Ser2P double staining in embryos expressing GFP::PIE-1. left two columns: non RNAi. right two columns: oma-1(RNAi);oma-2(RNAi). Bar: 10 µm.
Double loss-of-function mutants for oma-1 and oma-2 are sterile. Embryos diminished for oma-1/2 can be obtained however from animals carrying reduction of function mutations [oma- 1(te21);oma-2(te50)] or by RNAi [oma-1(RNAi);oma-2(RNAi)] (Detwiler et al., 2001; Nishi and Lin, 2005). We analyzed both Ser2P staining, as well as the expression of vet-5 (very early transcript 5) by in situ hybridization, in oma-1(RNAi);oma-2(RNAi) embryos. vet-5 is an early zygotic pol II transcript of unknown function which accumulates in the nucleus (Schauer and Wood, 1990; Tenenhaus et al., 1998). These RNAi embryos have severe defects in cell divisions (Nishi and Lin, 2005), and therefore we only analyzed 1-cell oma-1(RNAi);oma-2(RNAi) embryos with two clear pronuclei, prior to the first mitotic division. We detected a clear anti-Ser2P staining and vet-5- specific signal in 1-cell nuclei of oma-1(RNAi);oma-2(RNAi) embryos (Figure 3A, 3B). The observed ectopic transcription is not a consequence of derepressed transcription in oocytes of oma-1(RNAi);oma-2(RNAi) animals, as we detected no Ser2P staining in proximal oocytes of oma-1(te33);oma-2(te51) animals, similar to the lack of Ser2P staining in wildtype proximal oocytes (Walker et al., 2007) (Figure 3C). These observations indicate that OMA-1 and OMA-2 are required for transcriptional repression in 1-cell embryos.
By performing weaker oma-1;oma-2 RNAi, we could obtain embryos that divided relatively normally beyond the 1-cell stage. In some of these weaker RNAi embryos, we observed Ser2P staining and vet-5 RNA in all blastomeres, including AB, P1, P2, and P3 (Figure 3A, 3B, and not shown). This result demonstrates that oma-1/2 are required for transcriptional repression not only at the 1–2-cell stage, but also in germline blastomeres of later-staged embryos. Because little or no OMA proteins are detected after the 2-cell stage, this requirement for oma-1/2 for transcriptional repression in P2 and P3 is likely indirect. We believe that OMA-1/2 repress transcription in P2 and P3 indirectly by maintaining PIE-1 levels in P blastomeres. Depletion of oma-1/2 results in a loss or dramatic decrease in PIE-1 levels in RNAi embryos (Figure 3D). Double staining of GFP::PIE-1 expressing embryos depleted of oma-1/2 by RNAi revealed an inverse relationship between the level of GFP::PIE-1 and Ser2P staining in P2 (Figure 3D). As depletion of pie-1 does not result in transcription in 1- or 2-cell embryos (Seydoux et al., 1996), a reduced PIE-1 level can not account for the precocious transcription we observed in 1- and 2-cell oma-1(RNAi);oma-2(RNAi) embryos.
Genetic interaction between OMA-1/2 and TAF-4
Our model predicts that a strain with elevated TAF-4 levels would be more sensitive to a reduction in OMA levels. In a strain expressing GFP::TAF-4 in addition to endogenous TAF-4, we observed an increased sensitivity to depletion of oma-1/2 by RNAi compared to control strains expressing either no transgenic protein (N2) or a control protein (GFP::CYCLIN) driven by the same regulatory sequences. Under RNAi conditions that resulted in no sterile animals, and no or few dead embryos in the two control strains, the GFP::TAF-4 expressing strain exhibited approximately 50% sterile animals and 50% embryonic lethality amongst those embryos that were laid (Suppl. Table 2). These three strains showed similar sensitivity to control RNAi using an unrelated gene, wrm-1 (data not shown) (Rocheleau et al., 1997). In the GFP::TAF-4 expressing strain, we observed a strict correlation between the severity of embryonic lethality with enriched nuclear GFP in 1-cell embryos. We interpret the increased sensitivity of the GFP::TAF-4 expressing strain to oma-1/2 RNAi to be the result of elevated TAF-4 levels. The in vivo localization and genetic interaction described here are consistent with the physical interaction between OMA proteins and TAF-4 observed in yeast.
OMA-1/2 and TAF-12 compete for binding to the histone fold domain of TAF-4
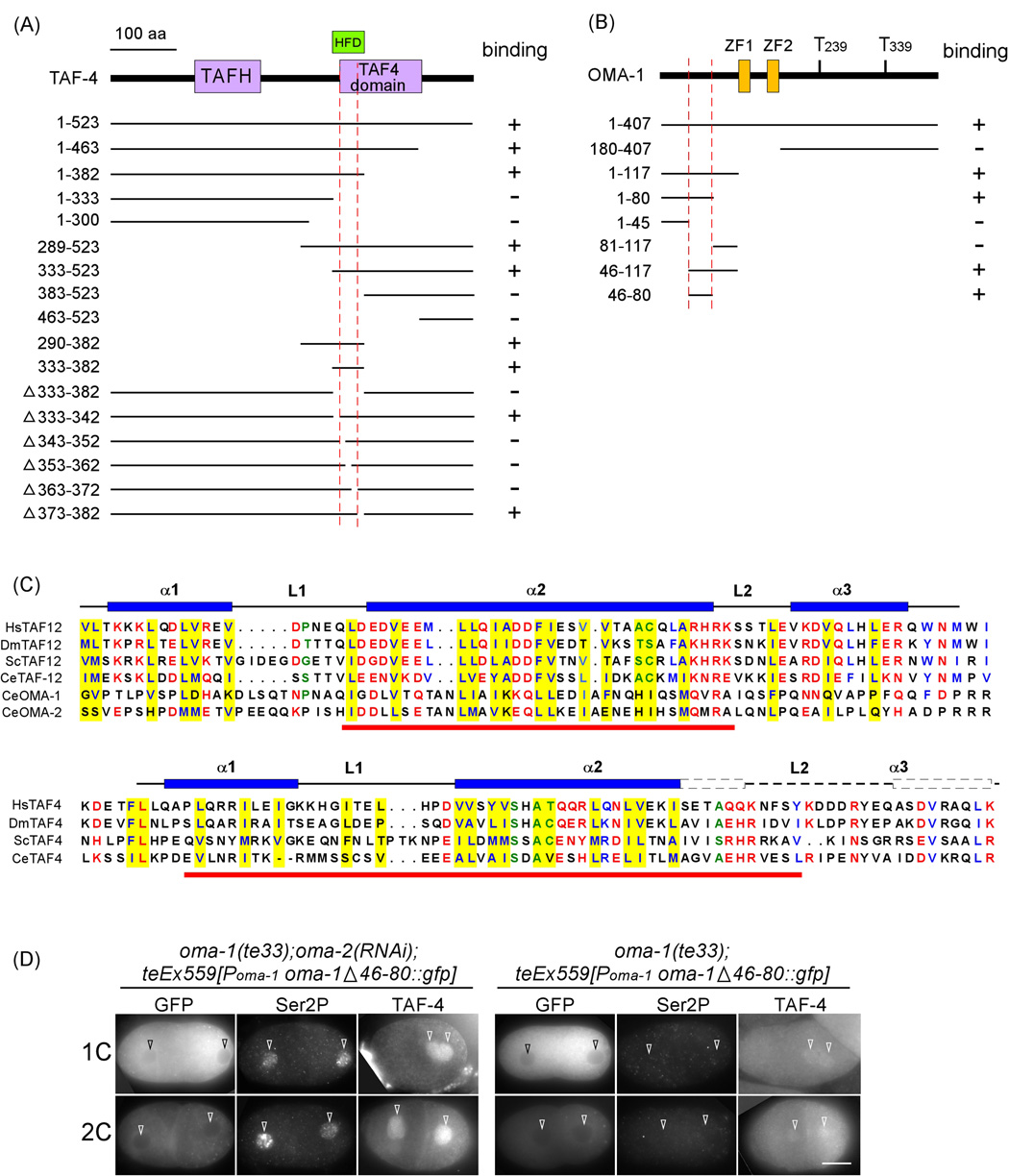
C. elegans TAF-4 contains two domains conserved among TAF4 proteins, the N-terminal TAFH/NHR1 domain (amino acids 135–220) and the C-terminal TAF4 domain (amino acids 346– 471) (Walker et al., 2001). Most TAFs contain an identifiable histone fold domain, helical structures similar to those found in all four core histones, which mediate specific interactions among the TAFs (review by Gangloff et al., 2001). The HFD of TAF-4, which resides within the TAF4 domain, resembles that of histone H2A and interacts specifically with the TAF-12 HFD, which resembles that of H2B (Gangloff et al., 2000; Hoffmann and Roeder, 1996; Werten et al., 2002). We have confirmed that C. elegans TAF-4 and TAF-12 interact in both a yeast 2-hybrid assay as well as an in vitro pull-down assay (Suppl. Figure 1). All taf-4 partial cDNAs isolated in the yeast 2-hybrid screen contained the HFD. We further mapped the region of TAF-4 necessary and sufficient for OMA-1/2 interaction to amino acids 333–382, which overlaps the histone fold domain (HFD) (Figure 4A and Suppl. Figure 1B). We found that the HFD region of TAF-4 was both necessary and sufficient for interaction with OMA-1, OMA-2, or TAF-12 (data not shown).
Figure 4. OMA-1 interacts with TAF-4 histone fold domain.
(A, B) Deletion analysis to map the TAF-4 (A) and OMA-1 (B) interaction domains. HFD: histone fold domain. TAFH: TAF homology domain. ZF: TIS11 zinc finger. Numbers to the left of each construct indicate amino acid positions. Red dashed lines demarcate those regions that are necessary and sufficient for the interaction. (C) Above: Sequence alignment of the HFD from various TAF-12 proteins compared to OMA-1 and OMA-2 sequences including the TAF-4 interacting domain. Below: Sequence alignment of various TAF-4 proteins. Underlined in red are the regions necessary and sufficient for interaction. Hs: human, Dm: D. melanogaster, Sc: S. cerevisiae, Ce: worm. Yellow highlight: residues located in the hydrophobic core of the TAF-4/TAF-12 heterodimer (Werten et al., 2002). α: alpha helix. L: loop. Amino acids conserved among TAF proteins are highlighted. Blue = hydrophobic; red = charged; green = small. (D) Immunofluorescence micrographs of anti-GFP and anti-Ser2P double staining or anti-TAF-4 staining in oma-1(te33) embryos expressing OMA-1 Δ46– 80::GFP that are treated with either no RNAi or oma-2(RNAi). Bar10 µm.
The TAF-4/TAF-12 interaction appears to play a key role in TAF-4 nuclear enrichment. taf-12(RNAi) resulted in GFP::TAF-4 not being enriched to any particular subcellular compartment in the gonad or early blastomeres (Figure 5A). Similar ubiquitous localization was observed for TAF-4 deleted of amino acids 333–382 (GFP::TAF-4ΔHFD) (Figure 5A). These results demonstrate that the HFD of C. elegans TAF-4 mediates interaction with TAF-12, and that this interaction is important for nuclear enrichment of TAF-4.
Figure 5. OMA-1 competes with TAF-12 for TAF-4 binding.
(A) GFP fluorescence in the gonad (dashed line) and embryos of transgenic worms expressing GFP::TAF-4, GFP::TAF-4ΔHFD, or GFP::TAF-4 with taf-12(RNAi). (B) HEK293 cells expressing TAF-4 (green) alone, TAF-4+TAF-12 (magenta), TAF-4+MYR::OMA-1-N (red), or TAF-4+TAF-12+MYR::OMA-1-N. (C). TAF-4ΔHFD expressed alone, with TAF-12, or with Myr::OMA-1-N. Bar: 25 µm.
The observation that OMA-1 and TAF-12 bind to the same domain of TAF-4 suggested that OMA-1/2 would compete with TAF-12 for binding to TAF-4. We examined this possibility in mammalian HEK293 tissue culture cells (Figure 5B). When expressed in HEK293 cells, GFP::TAF-4 was exclusively cytoplasmic (100% of GFP-positive cells, n>1,000), whereas HA::TAF-12 was primarily nuclear (~70% of HA-staining cells, n>1,000). Coexpression of GFP::TAF-4 and HA::TAF-12 resulted in nuclear localization of GFP::TAF-4 in almost all cells that exhibited nuclear HA::TAF-12 (~100%, n>300). The successful recapitulation of TAF-4 and TAF-12 subcellular localization in HEK293 cells suggests that, while TAF-4 and TAF-12 are evolutionarily conserved, their interaction is species specific. A similar species-specific interaction of evolutionarily conserved proteins was previously reported (Francis et al., 2002).
We next asked whether coexpressing OMA-1-N in HEK293 cells could prevent GFP::TAF-4 from undergoing the TAF-12-dependent nuclear enrichment. FLAG::OMA-1-N is both cytoplasmic and nuclear when expressed in HEK239 cells. Therefore, we tagged FLAG::OMA-1-N at the N-terminus with a myristylation sequence (Myr::FLAG::OMA-1-N), which tethered OMA-1-N to the underside of the cytoplasmic membrane (Figure 5B). In ~80% of cells expressing both Myr::FLAG::OMA-1-N and GFP::TAF-4, we observed GFP fluorescence localized predominantly to the cytoplasmic membrane (n>200). The recruitment of GFP::TAF-4 to the cytoplasmic membrane occurred even when HA::TAF-12 was also co-expressed in the same cell (Figure 5B). We observed membrane GFP::TAF-4 and low or no nuclear GFP::TAF-4 in ~40% (n>200) of cells expressing all three proteins.
The cytoplasmic sequestration of GFP::TAF-4, its TAF-12-dependent nuclear localization, and its Myr::FLAG::OMA-1-N-dependent membrane localization all required the TAF-4ΔHFD. GFP::TAF-4ΔHFD was ubiquitous throughout the cell (100%, n>1,000), and remained so regardless of whether TAF-12 or Myr-OMA-1-N was co-expressed (Figure 5C). Our results in worms and HEK293 cells both demonstrate that OMA-1/2 and TAF-12 compete for binding to the same domain of TAF-4.
The TAF-4-binding domain of OMA-1 is essential for transcriptional repression but not for oocyte maturation
The region of OMA-1 necessary and sufficient for TAF-4 binding was mapped to a 35 amino acid region (residues 46–80) near the N-terminus (Figure 4B). This region is predicted to exhibit an alpha-helical structure by various protein secondary structure prediction programs. Interestingly, this predicted alpha-helix, while sharing no similarity in primary sequence, is similar in secondary structure to the second alpha-helix (alpha-2) of the TAF-12 HFD (Gangloff et al., 2001; Werten et al., 2002). Structural studies identified 13 residues, 11 with hydrophobic and two with a small side chain, within this second alpha helix that are located in the hydrophobic core of the TAF-4/TAF-12 heterodimer (Werten et al., 2002). Alignment reveals that amino acids 46–80 of OMA-1 also exhibit hydrophobic residues at 10 of these 11 positions. This similarity is consistent with our above data suggesting that OMA-1 competes with TAF-12 for TAF-4 binding.
To test whether the observed ectopic transcription in oma-1/2-depleted embryos was indeed due to a defect in TAF-4 sequestration, we assayed the function of OMA-1 lacking the 35 amino acid TAF-4-interacting domain in vivo. We generated transgenic animals expressing either wildtype OMA-1::GFP or OMA-1::GFP deleted of amino acids 46–80 (OMA-1 Δ46–80::GFP) in the oma-1(te33) background. Both transgenic strains are healthy and exhibit no abnormal phenotype. We then depleted oma-2 by RNAi in animals carrying either transgene, or no transgene, and assayed for (1) oocyte maturation defect (Oma), (2) percentage of dead embryos, (3) anti-Ser2P staining, and (4) anti-TAF-4 staining.
We observed that both wildtype OMA-1::GFP and OMA-1 Δ46–80::GFP rescued the oocyte maturation defect, as 100% of transgenic animals (n>500) remained fertile upon oma-2(RNAi), compared to 0% of non-transgenic animals (n>500). However, only wildtype OMA-1::GFP, and not OMA-1 Δ46–80::GFP, rescued the embryonic lethality of oma-1(te33);oma-2(RNAi) embryos (50%, n=551 and 0%, n>2,000, respectively). Embryonic lethality correlated with nuclear enrichment of TAF-4 and ectopic transcription in 1- and 2-cell embryos. The majority of 1-cell (83%, 24 of 29) and 2-cell (96%, 27 of 28) embryos expressing OMA-1 Δ46–80::GFP had detectable Ser2P staining (Figure 4D). This is in contrast to the 0% Ser2P staining observed in non oma-2(RNAi) controls. Embryos expressing wildtype OMA-1::GFP have detectable Ser2P staining in 26% (5 of 19) and 50% (9 of 18) of 1-cell and 2-cell embryos, respectively. These results lead to three major conclusions. First, the function of OMA-1 in oocytes and embryos can be uncoupled, further supporting our earlier conclusion that the observed transcription in oma-1(RNAi);oma-2(RNAi) embryos was not due to compromised oocyte maturation. Second, TAF-4-binding is essential for OMA-1 function in embryos but not in oocytes. Third, OMA-1 represses transcription by binding to TAF-4. This result does not rule out an additional function(s) for OMA-1/2 in 1-cell embryos. In fact, embryos depleted of oma-1 and oma-2 by RNAi exhibit a more severe defect in embryonic divisions than OMA-1 Δ46-80::GFP expressing embryos, suggesting that OMA proteins might have an additional embryonic function(s).
Interaction between OMA-1 and TAF-4 is regulated by MBK-2
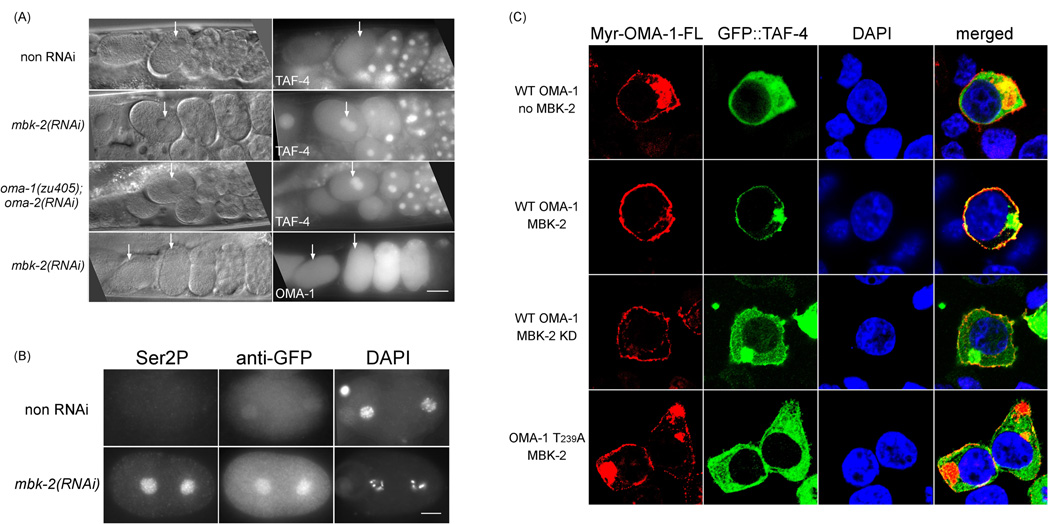
Two observations suggested that the interaction between OMA-1/2 and TAF-4 is regulated. First, while OMA-1/2 proteins are present at a high level in both oocytes and the 1-cell embryo, cytoplasmic sequestration of TAF-4 is only observed in the embryo. Second, we observed that whereas Myr::FLAG::OMA-1-N effectively recruited GFP::TAF-4 to the cell membrane, Myr::FLAG::OMA-1-FL (full length) did so very poorly (8%, n=50, Figure 5B, Figure 6C). One candidate regulator of the OMA-1/TAF-4 interaction is MBK-2, which is activated at meiosis II and has been shown to phosphorylate OMA-1 and OMA-2 (Nishi and Lin, 2005; Pellettieri et al., 2003; Shirayama et al., 2006). The following three results demonstrate that the interaction between OMA-1/2 and TAF-4 is a developmentally-regulated event and that MBK-2 phosphorylation of OMA-1 at T239 facilitates this interaction. First, we performed mbk-2(RNAi) in GFP::TAF-4 expressing animals and observed an increase in nuclear GFP::TAF-4 in 1-cell embryos (Figure 6A). When these embryos were costained with anti-Ser2P and anti-GFP antibodies, we observed a strong correlation between high levels of nuclear GFP::TAF-4 and Ser2P staining in 1-cell embryos (100%, n=30, Figure 6B). Second, we have shown previously that the oma-1(zu405) mutation (P240L) interferes with MBK-2 phosphorylation of OMA-1 at T239, thereby preventing OMA-1 from being degraded after the 1-cell stage. oma-2(RNAi) in the oma-1(zu405) background resulted in a similar increase in GFP::TAF-4 in 1-cell nuclei (Figure 6A). Third, coexpressing MBK-2 with Myr::FLAG::OMA-1- FL and GFP::TAF-4 in HEK293 cells significantly increased cell membrane localization of GFP::TAF-4 (48%, n=50), compared to cells expressing either no MBK-2 or coexpressing a kinase dead version of MBK-2 (16%, n=50) (Figure 6C). This enhancement by MBK-2 was dependent on T239 as no enhancement was observed when Myr::FLAG::OMA-1-FL T239A was expressed (12%, n=50) (Figure 6C).
Figure 6. Interaction between OMA-1 and TAF-4 is regulated by MBK-2.
(A) Top three rows: GFP fluorescence in utero of embryos expressing GFP::TAF-4 in genetic backgrounds listed to the left. Bottom row: persistence of GFP::OMA-1 fluorescence following mbk-2(RNAi). Arrows mark 1-cell embryos. (B) Immunofluorescence micrographs of anti-Ser2P and anti-GFP double staining in 1-cell embryos expressing GFP::TAF-4 either with or without mbk-2(RNAi). (C) Confocal immunofluorescence micrographs of Myr::OMA-1-FL, GFP::TAF-4, DAPI, and all three (merged) in HEK239 cells. Proteins expressed from transfected constructs are indicated to the left. KD: kinase dead. The localization of Myr-OMA-1-FL (cytoplasmic membrane and cytoplasmic aggregates which were observed when high levels of Myr tagged proteins were expressed) is not affected by MBK-2 activity. Bar: 25 µm in (A) 10 µm in (B).
Ectopic OMA-1 expression can repress transcription in later somatic and germline blastomeres
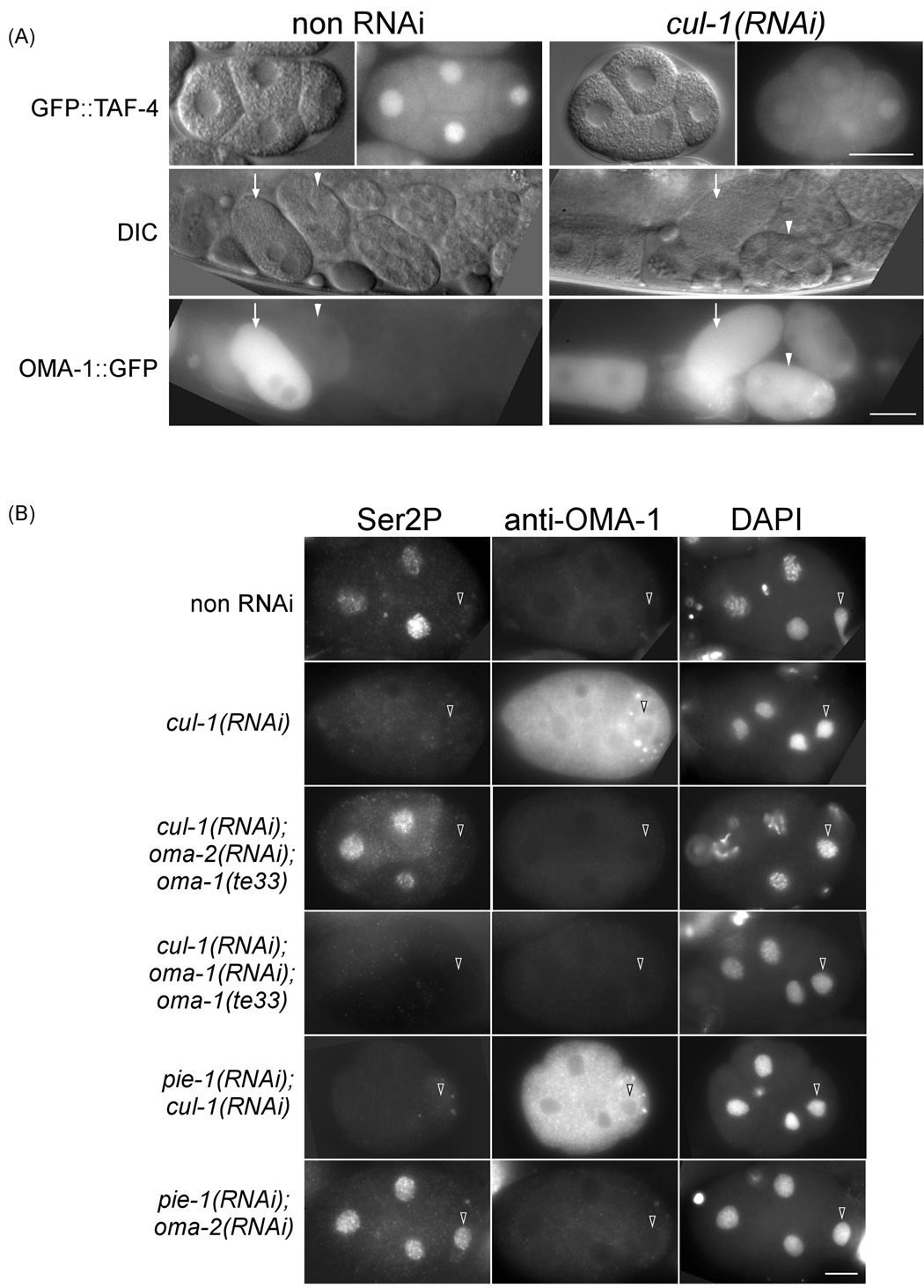
We asked whether OMA-1, under conditions permitting its persistence beyond the 1-cell stage (mbk-2(RNAi), oma-1(zu405);oma-2(RNAi), and cul-1(RNAi)), could sequester TAF-4 or repress transcription in later stage embryos. In both mbk-2(RNAi) and oma-1(zu405);oma-2(RNAi) embryos, in which OMA proteins are compromised in MBK-2 phosphorylation (Nishi and Lin, 2005), we did not observe a reduced nuclear GFP level at 4–12 cell stages (Suppl. Figure 5). CUL-1 has been shown to be required for the timely degradation of OMA-1, but not required for any MBK-2-dependent event (Kipreos et al., 1996; Shirayama et al., 2006). We observed that 100% (n=14) of cul-1(RNAi) animals exhibited OMA-1::GFP in embryos as late as 12-cell (Figure 7A). Unlike mbk-2(RNAi) and oma-1(zu405); oma-2(RNAi), cul-1(RNAi) resulted in an observable reduction in nuclear GFP::TAF-4 in 4-cell embryos (64%, n=14). In addition, very low or no Ser2P staining was detected in all blastomeres in 77% (n=18) of 4-cell cul-1(RNAi) embryos (Figure 7B). When cul-1(RNAi) was performed together with oma-2(RNAi) in the oma-1(te33) background, we detected only 18% (n=17) of 4-cell embryos with low or no Ser2P staining, while the other 82% embryos had Ser2P in somatic nuclei. This result argues that the effect of cul-1(RNAi) is dependent on both oma-1 and oma-2. In control experiments where cul-1(RNAi);oma-1(RNAi) was performed in oma-1(te33) animals, we observed 66% of 4-cell embryos with low or no Ser2P staining (n=8).
Figure 7. Ectopic OMA-1 in cul-1(RNAi) embryos can repress transcription.
(A) GFP fluorescence and corresponding DIC images of 4-cell embryos expressing GFP::TAF-4 and embryos in utero expressing GFP::OMA-1 with or without cul-1(RNAi). Arrow: 1-cell embryo; arrowhead: 4-cell embryo. (B) Immunofluorescence micrographs of anti-Ser2P and anti-OMA-1 double staining in 4-cell embryos of the following backgrounds: no RNAi, cul-1(RNAi), cul-1(RNAi);oma-1(te33);oma-2(RNAi), cul-1(RNAi);oma-1(te33);oma-1(RNAi), pie-1(RNAi);cul-1(RNAi); or pie-1(RNAi);oma-2(RNAi). Bar: 20 µm in (A) 10 µm in (B).
We then asked whether persisting OMA-1 in cul-1(RNAi) embryos could substitute for PIE-1 in germline transcriptional repression. In pie-1(RNAi) embryos, transcription is derepressed in the P2 blastomere, whereas in cul-1(RNAi);pie-1(RNAi) embryos, transcription remains repressed in the P2 blastomere, as indicated by a lack of Ser2P staining (67%, n=12; Figure 7B). This result was not due to inefficient pie-1 RNAi in double RNAi embryos, as we detected Ser2P staining in 100% (n=11, Figure 7B) of P2 blastomeres in pie-1(RNAi);oma-2(RNAi) embryos. All together, these results indicate that persisting OMA-1, if properly phosphorylated by MBK-2, can repress transcription in both somatic and germline blastomeres in later stage embryos.
Discussion
The paradigm for global transcriptional repression of germline precursors in C. elegans is PIE-1. PIE-1 represses transcription in P2, P3, and, to a certain extent, in P4 (Seydoux et al., 1996) by repressing both transcriptional initiation and elongation (Seydoux and Dunn, 1997; Ghosh and Seydoux, 2008). We propose here that OMA-1 and -2 have redundant functions in globally repressing transcription in P0 and P1 by a distinct mechanism. OMA-1/2 bind to TAF-4, sequestering TAF-4 in the cytoplasm. We further propose that this interaction occurs only in embryos where it is facilitated by phosphorylation of OMA-1 and OMA-2 by MBK-2, a modification also marking both OMA proteins for eventual degradation (Nishi and Lin, 2005). Upon OMA degradation, TAF-4 is released and bound by TAF-12, the TAF-4/12 heterodimer translocates to the nucleus, and transcriptional repression is relieved.
Recognition and binding of the TATA box by TFIID is a major rate-limiting step in transcription initiation by RNA polymerase II (Chatterjee and Struhl, 1995; Klages and Strubin, 1995). There is an approximate 10-minute delay between the time when most OMA protein is degraded (the end of the first mitosis) and when zygotic transcription is first detected (the 4-cell embryo). We propose that this delay reflects the time it takes: first, to degrade the residual amount of OMA proteins in 2-cell embryos (Suppl. Figure 2); second, for the TAF-4/TAF-12 dimer to form in the cytoplasm and translocate to the nucleus; third, for a fully functional TFIID complex to assemble at the TATA box; and fourth, for transcription to reach experimentally detectable levels. Finally, we also propose that OMA-1 and OMA-2 are indirectly required for transcriptional repression in later germline blastomeres by regulating PIE-1 levels.
Developmental regulation of OMA-1 and OMA-2 activity
Regulation of the OMA/TAF-4 interaction via MBK-2-dependent phosphorylation prevents cytoplasmic sequestration of TAF-4 until after meiosis II. This temporal regulation is apparently not to prevent TAF-4 from being sequestered in oocytes, as the oocytes are already transcriptionally repressed through a yet to be determined mechanism (Walker et al, 2007). It is possible that TAF-4 binding is incompatible with OMA-1/2 function(s) in oocyte maturation. For example, binding to TAF-4 might interfere with OMA-1 binding to other proteins in oocytes. Phosphorylation of OMAs by MBK-2 might serve to switch OMA-1 and OMA-2 from being regulators of oocyte maturation to transcriptional repressors, albeit relatively short-lived. We propose that MBK-2 phosphorylation results in either a change in conformation of OMA-1/2 that renders their N-terminal domains more accessible, or changes the affinity between OMA-1/2 and different binding partners. Because OMA-1 P240L persisted past the 1-cell stage and could be suppressed by oma-1(RNAi)) (Lin, 2003; Nishi and Lin, 2005), zu405 was originally considered a gain-of-function mutation. However, our results here demonstrate that zu405 is also a loss-of-function or reduction-of-function allele with respect to TAF-4 sequestration and transcriptional repression.
The interaction between OMA-1/2 and TAF-4 is probably also regulated by breakdown of the oocyte nuclear envelope during oocyte maturation, which occurs slightly before MBK-2 activation (Nishi and Lin, 2005; Pellettieri et al., 2003; Stitzel et al., 2006). Nuclear envelope breakdown would result in OMA-1, OMA-2, and TAF-4 proteins occupying, for the first time, the same subcellular compartment. OMA-1/2 interaction with TAF-4, following OMA phosphorylation by MBK-2, would sequester TAF-4 in the cytoplasm when the nuclear membrane reforms.
Redundancy of transcriptional repression in primordial germ cells
The similarities between PIE-1 and the OMA proteins are intriguing. They are all multifunctional proteins, contain two TIS11 CCCH zinc fingers which may function in RNA-binding, and repress transcription through domains distinct from their zinc fingers (Detwiler et al., 2001; Jadhav et al., 2008; Mello et al., 1996; Tenenhaus et al., 2001; Zhang et al., 2003). We are currently investigating whether OMA-1 and OMA-2 regulate PIE-1 levels through a CCCH finger-dependent, RNA-binding mechanism. We propose that PIE-1 and OMA proteins have partially redundant functions, albeit through different mechanisms, in transcriptional repression in germline blastomeres. Their functions are temporally and spatially restricted by their respective expression patterns. There is increasing evidence that transcriptional repression in PGCs often involves redundant or partially redundant mechanisms. In Drosophila, transcriptional repression in germ cells involves epigenetic chromatin marks as well as several partially redundant factors (Deshpande et al., 2005; Deshpande et al., 2004; Leatherman et al., 2002; Martinho et al., 2004; Schaner et al., 2003). It was recently shown that Drosophila polar granule component (Pgc) also inhibits the p-TEFb complex, thereby repressing transcription in germ cells (Hanyu-Nakamura et al., 2008). However, PIE-1 and Pgc inhibit different subunits of the p-TEFb complex.
TAF-4 as a target for transcriptional repression
TAF-4 is the most crucial TAFs required for the assembly of TFIID complex in vitro (Wright et al., 2006). In C. elegans, taf-4(RNAi) results in the most severe taf depletion phenotype, similar to that from depletion of RNA polymerase II (Walker and Blackwell, 2003; Walker et al., 2001; Walker et al., 2004). Therefore, efficient inactivation of TAF-4 would be an effective way to repress global transcription by RNA pol II. TAF-4 has been proposed as a target of several mutant proteins resulting from polyglutamine expansion (Shimohata et al., 2000), the causative mutation in at least eight neurodegenerative disorders. The polyQ expansion of mutant Huntington protein and atrophin-1 bind to and sequesters TAF-4 within intranuclear inclusions, preventing transcription in certain neurons or tissue culture cells (Dunah et al., 2002; Shimohata et al., 2000). It is important to note that OMA proteins sequester TAF-4 through a domain of TAF-4 distinct from that targeted by polyQ expanded proteins, and under physiological, rather than pathological, conditions.
Transcription repression in C. elegans germline blastomeres
Dynamic and readily reversible mechanisms of transcriptional repression are crucial in C. elegans germline blastomeres. Consistent with this notion, chromatin in germline blastomeres exhibit epigenetic marks characteristic of transcriptionally competent chromatin (Schaner et al., 2003). PIE-1 and OMA-1/2 provide an effective means for repressing transcriptionally competent chromatin in a way that is both dynamic and easily reversed. Asymmetric segregation of PIE-1 to the germline blastomere facilitates rapid resumption of transcription in the somatic sister cell, presumably from preexisting pol II preinitiation complexes (Reese et al., 2000; Seydoux and Dunn, 1997; Seydoux et al., 1996; Zhang et al., 2003). Rapid degradation of OMA-1/2 allows the TAF-4/TAF-12 interaction and transcription to occur within the next cell cycle or two.
C. elegans germline blastomeres resemble stem cells in certain ways. Stem cells are generally defined as cells that can both self-renew and generate progeny that have restricted developmental or differentiation potentials. One common feature of stem cells is their ability to repress the expression of genes associated with lineage specific differentiation. Accumulating data suggest a model whereby important tissue-specific regulator genes are “primed” for expression in pluripotent cells (Giadrossi et al., 2007; Spivakov and Fisher, 2007), but their expression is “held back” by various mechanisms. Mechanisms of “holding back” include opposing histone modifications (Azuara et al., 2006; Bernstein et al., 2006; Stock et al., 2007) and active degradation of specific transcription factors by proteasomes (Szutorisz et al., 2006). Our study on OMA-1/2 and others on PIE-1 could shed light on additional mechanism(s) by which transcription can be repressed in vertebrate stem cells.
Experimental Procedures
Strains
N2 was used as the wildtype strain. Genetic markers are: LGIII, unc-119(ed3); LGIV, oma-1(zu405), oma-1(te33), oma-1(te21); LGV, oma-2(te50); oma-2(te51). Transgenic strains were generated by microparticle bombardment (Praitis et al., 2001) of the respective plasmids: Ppie-1gfp::taf-4 in TX903(teIs90), Ppie-1gfp::taf-4Δ333-382 in TX909(teIs96), Ppie-1gfp::taf-12in TX1014(teIs102), Poma-1oma-1::gfp in TX864 [teIs76; te33], and Poma-1 oma-1Δ46–80::gfp in TX1155 [teEx559; te33] and TX1162[teIs108; te33]. TX189, ET113, and AZ212 contain Poma-1oma-1::gfp, Ppie-1gfp::CYB-1 and Ppie-1gfp::H2B transgenes, respectively, as described (Lin, 2003; Liu et al., 2004; Praitis et al., 2001).
Plasmid construction
Partial cDNA for taf-1 and full-length cDNA for taf-4, taf-5, taf-6.1, and taf-10 were amplified from yk14c2, yk326f12, yk1669h05, yk850e10 and yk1035g02 clones, respectively. Full-length cDNAs for taf-8, taf-9 and taf-12 were amplified from embryos by RT-PCR. Most plasmids were constructed using the Gateway cloning technology. Germline expression constructs were derived from pID3.01B (Reese et al., 2000) or from pRL475 (Lin, 2003).
RNA interference
Feeding RNAi was performed as described (Timmons and Fire, 1998). In all cases, L1 larvae were placed on plates containing feeding RNAi bacteria, incubated either at 20° or 25°C, and scored 3 days later. Serial dilutions with the host strain HT115 were performed to generate weaker RNAi. RNAi of taf-1, taf-5, taf-6.1, taf-7.1, taf-8, taf-9, taf-10, and taf-12 was performed by injection of dsRNA into the TX903 strain, and GFP::TAF-4 expression analyzed 24 and 48 hours later. A dramatic reduction of nuclear and overall GFP was observed 24hrs after injection of taf-12 dsRNA, and 48hrs after injection of the other taf dsRNAs, suggesting that depletion of any of the taf genes eventually affects localization of TAF-4.
Transfection assay
HEK293T cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10% FBS on 1.5mm coverslips and transfected 16 hours later using Fugene 6 (Roche) kit with 1 µg total DNA. Transfected cells were fixed for staining 24h–48h later. TAF-4, TAF-12, and OMA-1 are all driven by the CMV promoter and tagged N-terminally with GFP, 3x HA, and Myr-FLAG, respectively. The Myr sequence used is MGSNKSKPKDASQ. MBK-2 and kinase dead MBK-2 (Y237A) are not tagged (Nishi and Lin, 2005; Shirayama et al., 2006).
Immunofluorescence
Immunofluorescence protocols for C. elegans embryos are: anti-Ser2P [1/300, Covance, MMS-129R, (Seydoux and Dunn, 1997)], -PIE-1 [1/50, (Mello et al., 1996)], -OMA-1 [(anti-OMA-1a, 1/100, (Shimada et al., 2002)], -TAF-4 [1/100, (Walker et al., 2001)], and -GFP [1/250, Invitrogen (both rabbit and mouse), (Lin et al., 1998)]. Immunofluorescence staining of HEK293 cells is as described (Hao et al., 2006). Antibodies used are rabbit anti-GFP, mouse anti-FLAG (1/250, Sigma, F3165), rat anti-HA (1/50, Roche, 3F10), Goat anti-rabbit Alexa488, Goat anti-mouse Alexa568, and Goat anti-rat Alexa647 (Invitrogen).
In situ hybridization
In situ hybridization for vet-5 is as described (Seydoux and Fire, 1995) with the exception that one-cell images were obtained by dissecting mothers in PBS on polylysine-treated teflon slides rather than by hypochlorite treatment. oma-1(RNAi);oma-2(RNAi) embryos are not compatible with hypochlorite treatment. The vet-5 probe was synthesized by PCR from pC101 using the DIG probe synthesis kit (Roche). Anti-DIG-AP antibody (Roche, 1/2000) was used, followed by NBT/BCIP (Roche) color reaction.
Analysis of embryos, imaging, and quantification
Imaging of immunofluorescence, in situ, and live embryos was performed using an Axioplan microscope equipped with a MicroMax-512EBFT CCD camera controlled by the Metamorph acquisition software (Molecular Devices, Inc.). Imaging of HEK293 cells was performed using a LSM 510 Meta confocal microscope (Zeiss). The percentage of embryos at meiosis I, meiosis II, 1- cell, 2-cell, and 4-cell stages were determined by DAPI staining and shown to be comparable between oma-1(te33);oma-2(RNAi);teIs108 and oma-1(te33); teIs76 (Suppl. Table3).
Yeast two-hybrid screen and assay
Yeast two-hybrid screens were performed using the GAL4-based transcription system, with OMA-1-N expressed from pGBKT7 as bait. A C. elegans mixed-stage library (unpublished reagent, Z. Zhou and R. Horvitz) was screened on 50mM 3AT (3-amino-1,2,4-triazole) Trp− Leu− His− plates. Four positive clones encoding the C-terminal 235, 287, 317, and 321 amino acids of the TAF-4 protein were isolated from approximately 3.5 million transformants. For all assays described here, pGBKT7 and a pASII-derived Gateway plasmid were used for bait vectors and a pACTII-derived Gateway plasmid was used for prey vector.
Supplementary Material
Acknowledgements
The authors would like to thank Lin lab members for discussions, Angela Collins for technical assistance in the initial isolation of taf-4 clones, Jim Priess for antibodies to MEX-5 and PIE-1, Judith Austin for AZ212, Ed Kipreos for ET113, Geraldine Seydoux for the vet-5 clone and pID3.01B, Andy Fire for vet-5 sequence information, Yuji Kohara for all yk cDNA clones, Zheng Zhou and Bob Horvitz for sharing the yeast 2-hybrid library, Keith Blackwell and Amy Walker for anti-TAF-4 antibody, and Hiroyuki Kawahara for anti-OMA-1a antibody. All other strains used were provided by C. elegans Genome Center (CGC). This work was supported by an NIH grant (HD37933) to R. L.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors maybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Azuara V, Perry P, Sauer S, Spivakov M, Jorgensen HF, John RM, Gouti M, Casanova M, Warnes G, Merkenschlager M, et al. Chromatin signatures of pluripotent cell lines. Nat Cell Biol. 2006;8:532–538. doi: 10.1038/ncb1403. [DOI] [PubMed] [Google Scholar]
- Batchelder C, Dunn MA, Choy B, Suh Y, Cassie C, Shim EY, Shin TH, Mello C, Seydoux G, Blackwell TK. Transcriptional repression by the Caenorhabditis elegans germ-line protein PIE-1. Genes Dev. 1999;13:202–212. doi: 10.1101/gad.13.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Struhl K. Connecting a promoter-bound protein to TBP bypasses the need for a transcriptional activation domain. Nature. 1995;374:820–822. doi: 10.1038/374820a0. [DOI] [PubMed] [Google Scholar]
- Deshpande G, Calhoun G, Jinks TM, Polydorides AD, Schedl P. Nanos downregulates transcription and modulates CTD phosphorylation in the soma of early Drosophila embryos. Mech Dev. 2005;122:645–657. doi: 10.1016/j.mod.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Deshpande G, Calhoun G, Schedl P. Overlapping mechanisms function to establish transcriptional quiescence in the embryonic Drosophila germline. Development. 2004;131:1247–1257. doi: 10.1242/dev.01004. [DOI] [PubMed] [Google Scholar]
- Detwiler MR, Reuben M, Li X, Rogers E, Lin R. Two zinc finger proteins, OMA- 1 and OMA-2, are redundantly required for oocyte maturation in C elegans. Dev Cell. 2001;1:187–199. doi: 10.1016/s1534-5807(01)00026-0. [DOI] [PubMed] [Google Scholar]
- Dunah AW, Jeong H, Griffin A, Kim YM, Standaert DG, Hersch SM, Mouradian MM, Young AB, Tanese N, Krainc D. Sp1 and TAFII130 transcriptional activity disrupted in early Huntington's disease. Science. 2002;296:2238–2243. doi: 10.1126/science.1072613. [DOI] [PubMed] [Google Scholar]
- Extavour CG, Akam M. Mechanisms of germ cell specification across the metazoans: epigenesis and preformation. Development. 2003;130:5869–5884. doi: 10.1242/dev.00804. [DOI] [PubMed] [Google Scholar]
- Francis R, McGrath G, Zhang J, Ruddy DA, Sym M, Apfeld J, Nicoll M, Maxwell M, Hai B, Ellis MC, et al. aph-1 and pen-2 are required for Notch pathway signaling, gamma-secretase cleavage of betaAPP, and presenilin protein accumulation. Dev Cell. 2002;3:85–97. doi: 10.1016/s1534-5807(02)00189-2. [DOI] [PubMed] [Google Scholar]
- Gangloff YG, Romier C, Thuault S, Werten S, Davidson I. The histone fold is a key structural motif of transcription factor TFIID. Trends Biochem Sci. 2001;26:250–257. doi: 10.1016/s0968-0004(00)01741-2. [DOI] [PubMed] [Google Scholar]
- Gangloff YG, Werten S, Romier C, Carre L, Poch O, Moras D, Davidson I. The human TFIID components TAF(II)135 and TAF(II)20 and the yeast SAGA components ADA1 and TAF(II)68 heterodimerize to form histone-like pairs. Mol Cell Biol. 2000;20:340–351. doi: 10.1128/mcb.20.1.340-351.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh D, Seydoux G. Inhibition of transcription by the Caenorhabditis elegans germline protein PIE-1: genetic evidence for distinct mechanisms targeting initiation and elongation. Genetics. 2008;178:235–243. doi: 10.1534/genetics.107.083212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giadrossi S, Dvorkina M, Fisher AG. Chromatin organization and differentiation in embryonic stem cell models. Curr Opin Genet Dev. 2007;17:132–138. doi: 10.1016/j.gde.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Green MR. TBP-associated factors (TAFIIs): multiple, selective transcriptional mediators in common complexes. Trends Biochem Sci. 2000;25:59–63. doi: 10.1016/s0968-0004(99)01527-3. [DOI] [PubMed] [Google Scholar]
- Hanyu-Nakamura K, Sonobe-Nojima H, Tanigawa A, Lasko P, Nakamura A. Drosophila Pgc protein inhibits P-TEFb recruitment to chromatin in primordial germ cells. Nature. 2008;451:730–733. doi: 10.1038/nature06498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, Boyd L, Seydoux G. Stabilization of cell polarity by the C. elegans RING protein PAR-2. Dev Cell. 2006;10:199–208. doi: 10.1016/j.devcel.2005.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann A, Roeder RG. Cloning and characterization of human TAF20/15. Multiple interactions suggest a central role in TFIID complex formation. J Biol Chem. 1996;271:18194–18202. doi: 10.1074/jbc.271.30.18194. [DOI] [PubMed] [Google Scholar]
- Jadhav S, Rana M, Subramaniam K. Multiple maternal proteins coordinate to restrict the translation of C. elegans nanos-2 to primordial germ cells. Development. 2008;135:1803–1812. doi: 10.1242/dev.013656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipreos ET, Lander LE, Wing JP, He WW, Hedgecock EM. cul-1 is required for cell cycle exit in C. elegans and identifies a novel gene family. Cell. 1996;85:829–839. doi: 10.1016/s0092-8674(00)81267-2. [DOI] [PubMed] [Google Scholar]
- Klages N, Strubin M. Stimulation of RNA polymerase II transcription initiation by recruitment of TBP in vivo. Nature. 1995;374:822–823. doi: 10.1038/374822a0. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Mizuno H, Okada M. Accumulation and spatial distribution of poly(A)+RNA in oocytes and early embryos of Drosophila melanogaster. Develop Growth and Differ. 1988;30:251–260. doi: 10.1111/j.1440-169X.1988.00251.x. [DOI] [PubMed] [Google Scholar]
- Komarnitsky P, Cho EJ, Buratowski S. Different phosphorylated forms of RNA polymerase II and associated mRNA processing factors during transcription. Genes Dev. 2000;14:2452–2460. doi: 10.1101/gad.824700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai WS, Carballo E, Strum JR, Kennington EA, Phillips RS, Blackshear PJ. Evidence that tristetraprolin binds to AU-rich elements and promotes the deadenylation and destabilization of tumor necrosis factor alpha mRNA. Mol Cell Biol. 1999;19:4311–4323. doi: 10.1128/mcb.19.6.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leatherman JL, Levin L, Boero J, Jongens TA. germ cell-less acts to repress transcription during the establishment of the Drosophila germ cell lineage. Curr Biol. 2002;12:1681–1685. doi: 10.1016/s0960-9822(02)01182-x. [DOI] [PubMed] [Google Scholar]
- Lin R. A gain-of-function mutation in oma-1, a C. elegans gene required for oocyte maturation, results in delayed degradation of maternal proteins and embryonic lethality. Dev Biol. 2003;258:226–239. doi: 10.1016/s0012-1606(03)00119-2. [DOI] [PubMed] [Google Scholar]
- Lin R, Hill RJ, Priess JR. POP-1 and anterior-posterior fate decisions in C. elegans embryos. Cell. 1998;92:229–239. doi: 10.1016/s0092-8674(00)80917-4. [DOI] [PubMed] [Google Scholar]
- Liu J, Vasudevan S, Kipreos ET. CUL-2 and ZYG-11 promote meiotic anaphase II and the proper placement of the anterior-posterior axis in C. elegans. Development. 2004;131:3513–3525. doi: 10.1242/dev.01245. [DOI] [PubMed] [Google Scholar]
- Martinho RG, Kunwar PS, Casanova J, Lehmann R. A noncoding RNA is required for the repression of RNApolII-dependent transcription in primordial germ cells. Curr Biol. 2004;14:159–165. doi: 10.1016/j.cub.2003.12.036. [DOI] [PubMed] [Google Scholar]
- Mello CC, Schubert C, Draper B, Zhang W, Lobel R, Priess JR. The PIE-1 protein and germline specification in C. elegans embryos. Nature. 1996;382:710–712. doi: 10.1038/382710a0. [DOI] [PubMed] [Google Scholar]
- Nishi Y, Lin R. DYRK2 and GSK-3 phosphorylate and promote the timely degradation of OMA-1, a key regulator of the oocyte-to-embryo transition in C. elegans. Dev Biol. 2005;288:139–149. doi: 10.1016/j.ydbio.2005.09.053. [DOI] [PubMed] [Google Scholar]
- Pagano JM, Farley BM, McCoig LM, Ryder SP. Molecular basis of RNA recognition by the embryonic polarity determinant MEX-5. J Biol Chem. 2007;282:8883–8894. doi: 10.1074/jbc.M700079200. [DOI] [PubMed] [Google Scholar]
- Pellettieri J, Reinke V, Kim SK, Seydoux G. Coordinate activation of maternal protein degradation during the egg-to-embryo transition in C. elegans. Dev Cell. 2003;5:451–462. doi: 10.1016/s1534-5807(03)00231-4. [DOI] [PubMed] [Google Scholar]
- Praitis V, Casey E, Collar D, Austin J. Creation of low-copy integrated transgenic lines in Caenorhabditis elegans. Genetics. 2001;157:1217–1226. doi: 10.1093/genetics/157.3.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese KJ, Dunn MA, Waddle JA, Seydoux G. Asymmetric segregation of PIE-1 in C. elegans is mediated by two complementary mechanisms that act through separate PIE-1 protein domains. Mol Cell. 2000;6:445–455. doi: 10.1016/s1097-2765(00)00043-5. [DOI] [PubMed] [Google Scholar]
- Rocheleau CE, Downs WD, Lin R, Wittmann C, Bei Y, Cha YH, Ali M, Priess JR, Mello CC. Wnt signaling and an APC-related gene specify endoderm in early C. elegans embryos. Cell. 1997;90:707–716. doi: 10.1016/s0092-8674(00)80531-0. [DOI] [PubMed] [Google Scholar]
- Schaner CE, Deshpande G, Schedl PD, Kelly WG. A conserved chromatin architecture marks and maintains the restricted germ cell lineage in worms and flies. Dev Cell. 2003;5:747–757. doi: 10.1016/s1534-5807(03)00327-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer IE, Wood WB. Early C. elegans embryos are transcriptionally active. Development. 1990;110:1303–1317. doi: 10.1242/dev.110.4.1303. [DOI] [PubMed] [Google Scholar]
- Seydoux G, Dunn MA. Transcriptionally repressed germ cells lack a subpopulation of phosphorylated RNA polymerase II in early embryos of Caenorhabditis elegans and Drosophila melanogaster. Development. 1997;124:2191–2201. doi: 10.1242/dev.124.11.2191. [DOI] [PubMed] [Google Scholar]
- Seydoux G, Fire A. Soma-germline asymmetry in the distributions of embryonic RNAs in Caenorhabditis elegans. Development. 1994;120:2823–2834. doi: 10.1242/dev.120.10.2823. [DOI] [PubMed] [Google Scholar]
- Seydoux G, Fire A. Whole-mount in situ hybridization for the detection of RNA in Caenorhabditis elegans embryos. Methods Cell Biol. 1995;48:323–337. doi: 10.1016/s0091-679x(08)61394-1. [DOI] [PubMed] [Google Scholar]
- Seydoux G, Mello CC, Pettitt J, Wood WB, Priess JR, Fire A. Repression of gene expression in the embryonic germ lineage of C. elegans. Nature. 1996;382:713–716. doi: 10.1038/382713a0. [DOI] [PubMed] [Google Scholar]
- Shimada M, Kawahara H, Doi H. Novel family of CCCH-type zinc-finger proteins, MOE-1, -2 and -3, participates in C. elegans oocyte maturation. Genes Cells. 2002;7:933–947. doi: 10.1046/j.1365-2443.2002.00570.x. [DOI] [PubMed] [Google Scholar]
- Shimohata T, Nakajima T, Yamada M, Uchida C, Onodera O, Naruse S, Kimura T, Koide R, Nozaki K, Sano Y, et al. Expanded polyglutamine stretches interact with TAFII130, interfering with CREB-dependent transcription. Nat Genet. 2000;26:29–36. doi: 10.1038/79139. [DOI] [PubMed] [Google Scholar]
- Shirayama M, Soto MC, Ishidate T, Kim S, Nakamura K, Bei Y, van den Heuvel S, Mello CC. The Conserved Kinases CDK-1, GSK-3, KIN-19, and MBK-2 Promote OMA-1 Destruction to Regulate the Oocyte-to-Embryo Transition in C. elegans. Curr Biol. 2006;16:47–55. doi: 10.1016/j.cub.2005.11.070. [DOI] [PubMed] [Google Scholar]
- Spivakov M, Fisher AG. Epigenetic signatures of stem-cell identity. Nat Rev Genet. 2007;8:263–271. doi: 10.1038/nrg2046. [DOI] [PubMed] [Google Scholar]
- Stitzel ML, Pellettieri J, Seydoux G. The C. elegans DYRK Kinase MBK-2 Marks Oocyte Proteins for Degradation in Response to Meiotic Maturation. Curr Biol. 2006;16:56–62. doi: 10.1016/j.cub.2005.11.063. [DOI] [PubMed] [Google Scholar]
- Stock JK, Giadrossi S, Casanova M, Brookes E, Vidal M, Koseki H, Brockdorff N, Fisher AG, Pombo A. Ring1-mediated ubiquitination of H2A restrains poised RNA polymerase II at bivalent genes in mouse ES cells. Nat Cell Biol. 2007;9:1428–1435. doi: 10.1038/ncb1663. [DOI] [PubMed] [Google Scholar]
- Surani MA, Ancelin K, Hajkova P, Lange UC, Payer B, Western P, Saitou M. Mechanism of mouse germ cell specification: a genetic program regulating epigenetic reprogramming. Cold Spring Harb Symp Quant Biol. 2004;69:1–9. doi: 10.1101/sqb.2004.69.1. [DOI] [PubMed] [Google Scholar]
- Szutorisz H, Georgiou A, Tora L, Dillon N. The proteasome restricts permissive transcription at tissue-specific gene loci in embryonic stem cells. Cell. 2006;127:1375–1388. doi: 10.1016/j.cell.2006.10.045. [DOI] [PubMed] [Google Scholar]
- Tenenhaus C, Schubert C, Seydoux G. Genetic requirements for PIE-1 localization and inhibition of gene expression in the embryonic germ lineage of Caenorhabditis elegans. Dev Biol. 1998;200:212–224. doi: 10.1006/dbio.1998.8940. [DOI] [PubMed] [Google Scholar]
- Tenenhaus C, Subramaniam K, Dunn MA, Seydoux G. PIE-1 is a bifunctional protein that regulates maternal and zygotic gene expression in the embryonic germ line of Caenorhabditis elegans. Genes Dev. 2001;15:1031–1040. doi: 10.1101/gad.876201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons L, Fire A. Specific interference by ingested dsRNA. Nature. 1998;395:854. doi: 10.1038/27579. [DOI] [PubMed] [Google Scholar]
- Varnum BC, Lim RW, Sukhatme VP, Herschman HR. Nucleotide sequence of a cDNA encoding TIS11, a message induced in Swiss 3T3 cells by the tumor promoter tetradecanoyl phorbol acetate. Oncogene. 1989;4:119–120. [PubMed] [Google Scholar]
- Walker AK, Blackwell TK. A broad but restricted requirement for TAF-5 (human TAFII100) for embryonic transcription in Caenorhabditis elegans. J Biol Chem. 2003;278:6181–6186. doi: 10.1074/jbc.M211056200. [DOI] [PubMed] [Google Scholar]
- Walker AK, Boag PR, Blackwell TK. Transcription reactivation steps stimulated by oocyte maturation in C. elegans. Dev Biol. 2007;304:382–393. doi: 10.1016/j.ydbio.2006.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AK, Rothman JH, Shi Y, Blackwell TK. Distinct requirements for C.elegans TAF(II)s in early embryonic transcription. Embo J. 2001;20:5269–5279. doi: 10.1093/emboj/20.18.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AK, Shi Y, Blackwell TK. An extensive requirement for transcription factor IID-specific TAF-1 in Caenorhabditis elegans embryonic transcription. J Biol Chem. 2004;279:15339–15347. doi: 10.1074/jbc.M310731200. [DOI] [PubMed] [Google Scholar]
- Werten S, Mitschler A, Romier C, Gangloff YG, Thuault S, Davidson I, Moras D. Crystal structure of a subcomplex of human transcription factor TFIID formed by TATA binding protein-associated factors hTAF4 (hTAF(II)135) and hTAF12 (hTAF(II)20) J Biol Chem. 2002;277:45502–45509. doi: 10.1074/jbc.M206587200. [DOI] [PubMed] [Google Scholar]
- Wright KJ, Marr MT, 2nd, Tjian R. TAF4 nucleates a core subcomplex of TFIID and mediates activated transcription from a TATA-less promoter. Proc Natl Acad Sci U S A. 2006;103:12347–12352. doi: 10.1073/pnas.0605499103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Barboric M, Blackwell TK, Peterlin BM. A model of repression: CTD analogs and PIE-1 inhibit transcriptional elongation by P-TEFb. Genes Dev. 2003;17:748–758. doi: 10.1101/gad.1068203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.