Abstract
The occurrence of total hip impingement, whether or not accompanied by frank dislocation, holds substantial untoward clinical consequences, especially as less-forgiving advanced bearing implant designs come into ever more widespread use. Biomechanical aspects of impingement and dislocation have historically received relatively little scientific attention, although that situation is now rapidly changing. The present article reviews contemporary laboratory and clinical research on the impingement/dislocation phenomena, focusing particularly on how implant design variables, surgical implantation factors and patient activity each act individually and in concert to pose impingement and dislocation challenges. In recent years, several powerful new research methodologies have emerged that have greatly expanded the scope for clinical translation of systematic laboratory study. Transferring the findings from such research into yet better implant designs, and even better surgical procedures, offers encouragement that the clinical impact of this troublesome complication can be further reduced.
Keywords: Impingement, Dislocation, Total Hip, Finite Element Analysis, Capsule
Clinical background
Ater aseptic loosening, recurrent dislocation (usually due to impingement) has historically ranked second1 in terms of frequency as a cause of failure in total hip arthroplasty.§2 One or more dislocations are experienced by typically 2% to 11% of all patients in primary series, and by typically 4 % to 25% of all patients in revision series.3,4 Approximately one-third of all patients with recurrent dislocations require re-operation.5 Stability is achieved in only about half of re-operated cases.6 Besides the worst-case scenario of recurrent dislocation leading to revision, additional problems include sudden acute severe pain, functional impairment, soft tissue damage, and dissociation of modular components. Moreover, dislocation can be devastating to a patient’s confidence in his or her hip replacement and in his or her surgeon.
THA dislocations are costly to the health care system. A recent American study of 58,521 Medicare claims for primary THA procedures done in 1996 noted that 3.9% of patients experienced one or more dislocations in the first six months after implantation.7 In our own centre, non-surgically reducing each instance of dislocation— and for many patients, such episodes are recurrent—involves a fluoroscopic study (≈ $1,000), nursing and pharmaceutical costs (≈$500), a brace (≈$1,000), the surgeon’s fee (≈$1,000), and often a general anesthetic (≈$1,500). This typical figure of about $5,000 per dislocation episode escalates to at least $25,000 per case if surgical revision is required. This experience is corroborated by the recent formal analysis of Mayo Clinic financial records by Sanchez-Sotelo et al.8, who ascertained that each dislocation patient treated by closed reduction incurred an additional 27% of the cost of an uncomplicated primary THA, and that each dislocation patient eventually requiring revision incurred an additional 148% of uncomplicated primary THA cost. Scaled to a (conservative) figure of approximately 220,000 primary THA cases performed per year in the US, the direct costs to society of dislocation extrapolates to approximately $200 million.†
At present, the quantitative mechanisms of THA dislocation are incompletely understood. For this reason, and given the limited and often anecdotal information in the clinical literature regarding influencing factors (much of which is in dispute, often including directly contradictory observations from study to study), progress has been slow in reducing this serious source of morbidity. Many observers have strongly-held opinions, but objective factual evidence has been very limited regarding which parameters influence the propensity to dislocation, how and to what degree. This is an area where the weaknesses of traditional retrospective studies are particularly evident, due to the unpredictable episodic nature of dislocation events being superimposed against the backdrop of a great many confounding factors.
The bulk of the literature on THA dislocation consists of retrospective studies of plausible trends and risk factors. Several of the major registry series have involved patient populations which are unusually large by orthopaedic standards5,9, but statistical power in this area has nevertheless tended to be relatively weak owing to the high degree of variability that characterises dislocation rates. Perhaps the only issue on which agreement is nearly unanimous is that previous hip surgery is the greatest predisposing factor. In the largest single series5, for example, prior hip surgery was associated with increased dislocation rate at a significance level of p<0.001. There also exists preponderant9,10—but not unanimous11,12—support of acetabular component malposition as another major risk factor. The choice of surgical approach is also widely regarded as a major consideration4, but here the evidence is somewhat softer because of the lack of well-controlled comparisons. For example, one of the largest registry series9 (3,935 cases with anterior or anterio-lateral approaches versus 2,527 cases with posterior approaches) provides compelling statistical evidence that surgical approach is not a significant factor. Other supposed risk factors are matters of overt controversy. For example, data exist both to support5 and to refute1 larger femoral head sizes as enhancing stability. In some series, dislocation rates for females have substantially exceeded those for males5,13, whilst in other series exactly the opposite has held true14. Appropriate myofascial tension has often been viewed as a key factor in maintaining stability, but laxity inferred objectively from preoperative versus postoperative greater trochanter height changes has failed to significantly correlate with increased dislocation rate.5
Quite apart from the varying levels of consensus or controversy that exist on issues of causality, retrospective studies of dislocation can unfortunately provide very little guidance on ways to improve the situation. Given the many confounders, it is difficult to know which implant designs actually work best, or which component positions really are intrinsically the safest. This quandary has grown more severe in recent years as available surgical options have multiplied: more liner choices (extended liners, offset liners, lateralising liners, version change liners, constraining liners, etc.), more shell size options (e.g., jumbo cementless shells), more head size options, more neck diameter and neck cross-section options, more bearing surface options (conventional MOP, highly cross-linked MOP, MOM, COC), and more approach/technique options (especially with the introduction of minimally invasive and/or computer-assisted procedures.) Even more so now than historically, new technologies continue to be introduced far more rapidly than their efficacy can be documented by rigorous outcome measures. Further accelerating the pace of unproven change is the fact that many of these new technologies are extremely lucrative financially for their manufacturers, especially with the trend toward more aggressive marketing and direct-to-consumer advertising. Given these realities, systematic study in disinterested laboratory settings, using clinically realistic models, has an important role for identifying which innovations truly constitute steps forward, versus merely steps sideways or even steps backward.
Laboratory Studies
Most laboratory research dealing with THA impingement and dislocation has involved only geometric range of motion. Landmark early work was done by Amstutz et al.15, who developed a test apparatus, which they termed a three-dimensional protractor. They used that instrument to measure range of motion prior to impingement for excised pelvis/femur preparations in which various total hip systems had been implanted, including various head sizes ranging from 22 mm (Charnley) to 41 mm (McKee-Farrar), and including various component orientations. Key findings were that the Mueller design (32 mm) provided 16° more flexion range of motion than the Charnley design, and that increasing acetabular component anteversion decreased the abduction and external rotation ranges of motion, whereas increasing femoral component anteversion increased the flexion motion range. Also, the motion range was found to be increased by larger head:neck ratio, by smaller socket-depth-to-ball-radius ratio, and by what they termed “optimisation” of the neck and socket rim. Chandler et al.16 subsequently performed cadaver bench studies using a similarly conceived test apparatus. They found that both head:neck ratio and neck length affected the pre-impingement range of motion, and that as head size increased, the impingement tendency shifted from component-on-component to bone-on-bone.
An alternative approach to studying pre-impingement range of motion was initiated by Robinson et al.17, who simulated total hip implantation in a three-dimensional geometric computer model developed from CT scans of a normal hip. Their model was implemented on a personal computer, using specialty software written to detect geometric overlap of distinct objects in the model. At 0° of abduction and 0° of internal rotation, the flexion range of motion increased as acetabular abduction, acetabular anteversion, and/or femoral anteversion increased. These same changes of component positioning also improved the range of internal rotation when the hip was in 90° of flexion. The model’s output also included the apparent area of bearing surface contact, which was found to increase as acetabular adduction and/or acetabular anteversion were decreased. This purely geometric model by definition could not report forces or moments. Neither was it feasible to address the role of soft tissues in the impingement process, or to simulate the complex multi-axial rotations characteristic of dislocation-prone patient motions. The latter consideration was subsequently addressed in a more sophisticated geometric computer model developed by Jaramaz et al., which was later extended to document range of motion changes accompanying plausible surgical imprecision of cup placement.18 Another 3D geometric computer model was developed by Herrlin and colleagues19, noteworthy not because of its technical sophistication (simple trigonometric transforms of component silhouettes apparent on plane film x-rays), but rather because it was applied to long-term follow-up of impingement in dislocating versus non-dislocating patients in several large cohorts. This general class of purely geometric models of impingement-free range of motion has continued to further improve, particularly in versatility of kinematic input and in graphic interface user-friendliness.
Until relatively recently, almost no laboratory work had been done to study THA dislocation per se (as distinct from geometric impingement). Prior to 1995, there had been only one such full paper in the English language archival literature, a study by Nicholas et al.20 involving a rocker apparatus housing a potted acetabular component. That work documented stability differences for several means of augmenting Charnley acetabular components. However, the numerical values of resisting moment reported were almost an order of magnitude below those of more recent work21,22, suggesting the possibility of an undetected systematic measurement error. In the last few years, several biomechanics research groups have responded to the increasingly evident need for more and better information about dislocations. Noble’s group developed a sophisticated physical model, using a cadaver preparation that included computer-controlled muscle force simulations and a conductive foil technique to detect the instant of dislocation. Factors studied with that model included head size, cup anteversion, and patient-specific cup positioning23. They documented the existence of three distinct modes of dislocation: following component-on-component impingement, following bone-on-bone impingement, and sliding out in the absence of impingement. Guarding against impingement-free dislocation had seldom been considered in the clinical literature, despite fluoroscopic evidence24 underscoring the ease of impingement-free femoral component head subluxation in low-contact-force activities.
Impingement per se
Besides its role in prelude to frank dislocation, impingement in its own right has recently come to the fore due to increased usage of new bearing couples: metal-on-metal (MOM), ceramic-on-ceramic (COC), and highly cross-linked polyethylenes (HXPEs). First-generation MOM designs such as the McKee-Farrar, which date from the 1950s, were largely abandoned soon after Charnley’s breakthrough work with metal-on-polyethylene articulation. However, interest in MOM rekindled in the 1980s with increasing recognition of the polyethylene wear debris problem. Contemporary MOM designs date from laboratory work begun in 1983 in Switzerland, based on B.G. Weber’s observations from a subset of well-performing first-generation (Müller) MOM prostheses that had been implanted in the late 1960s. Widespread European usage of the flagship second-generation (Metasul®) MOM design began in the late 1980s, and the implanted base now extends to an estimated 275,000 patients worldwide.25 Spearheaded by Dorr in Los Angeles, investigational usage in the U.S. began in the early 1990s, with formal FDA clearance coming in 1999. As regards COC, extremely low-wear alumina (Al2O3)-on-alumina bearing couples were first utilised clinically in 1970 by Boutin, in France. These devices have overcome a series of substantial early technical problems (notably, fracture risk and inconsistent bony fixation), to achieve their present position of widespread usage in Europe and Asia. The US orthopaedic community’s attitude toward COC has historically been guarded, due to litigation concerns regarding fracture risk. However, after continued favorable European/Asian experience and ongoing design and material improvements, several large US investigational trials were finally initiated in the late 1990s, leading to FDA approval in 2003.
The pathway to current widespread US acceptance of highly cross-linked polyethylene has been quite different. Rather than being grounded in longstanding European clinical experience, as with MOM and COC, HXPE’s achieved their dominant position in the US marketplace extremely quickly, and almost entirely on the basis of their performance in pre-clinical laboratory wear testing. The various proprietary HXPE’s currently on the market have been the subject of intense competition between manufacturers, and have sparked strong controversy as to the most appropriate process parameters, especially radiation dosage and thermal treatment. Clinical usage specifically of highly cross-linked polyethylenes dates only from 1998, but of course as a general class these implants the continuity with conventional-polyethylene MOP implants constitutes an extensive precedent.
There is good cause for optimism that these three alternative bearing surfaces indeed represent a cure for the “man-made worldwide disease” of particulate wear osteolysis.26 Collectively, however, these alternative bearing surfaces also represent an unprecedentedly large and abrupt bolus of technologic change and challenge for the arthroplasty community. No small part of this challenge involves dealing with the accompanying complications. In that context, impingement comes very prominently to the fore. Even for traditional THA designs involving conventional-polyethylene MOP bearings, local contact stresses at neck-liner impingement sites are typically increased by 10-fold or more over those at the bearing surface.27 Seemingly, this already adverse situation would be greatly compounded when both impinging members have a high elastic modulus, although actual such data are lacking.
For metal-on-metal, the greatest clinical concern lies with the potentially adverse effects of elevated metal ion concentrations systemically, and with metal particle accumulation in distant organs, especially the liver and spleen. Both such effects have been documented to dramatically increase in MOM patients who experience impingements or other abnormal articulations28, with corroborating implant damage being clearly evident visually upon retrieval.29 Greatly increased MOM wear rates have also been observed for other unintended motions such as micro-separation30 and subluxation.31 Unfortunately, despite intensive ongoing work in research settings, it has not yet proven feasible to routinely obtain (or at least to meaningfully interpret) blood/urine metal ion concentration data in large numbers of MOM patients, despite plausible linkage with immuno-mediated hypersensitivity, tissue bed apoptosis and necrosis, chromosomal damage, or even neoplasms. Improved understanding of the wear-consequential effects of MOM impingement clearly is needed as one element of progress in this area.
The clinical concerns are somewhat different for COC, but also relate heavily to impingement. Smaller grain size alumina, along with improved proof testing and other modern quality control measures, have now reduced the incidence of fracture to very low levels. Bearing surface wear rates are also extremely low, at least under circumstances of normal articulation. Alumina wear particles tend to be slightly smaller in size range than UHMWPE particles, and they elicit generally similar cell and tissue reactions. So, overall osteolytic potency for normally functioning COC implants tends to be much reduced relative to UHMWPE, owing simply to the much smaller number of particles. However, that situation changes appreciably in the presence of third body debris32, which can arise both from the impaction process of liner seating33 and of course from impingement. Avoiding impingement is particularly challenging for COC, because contemporary cup designs need to have metal backing for purposes of bony fixation, which in turn requires compromise of head size. Also, in part because of the conservatism in head/trunion design, motivated by head fracture avoidance, the range of modular variants tends to be quite restricted, leading in turn to limited options for construct fine-tuning intraoperatively. Besides debris generation from impingement at range-of-motion limits, COC implants are also recognised to undergo impingement/impaction at reseating after swing-phase separation, a phenomenon often difficult to avoid owing to the limited modularity options for soft tissue tensioning. Besides 3rd body liberation, COC stress concentrations from edge loading during reseating are also implicated in the stripe phenomenon, which is associated with substantial increase of wear debris burden.34
Radiation-induced cross-linking of UHMWPE, in conjunction with additional thermal processing to reduce free radicals, has resulted in dramatic improvements in wear performance both in laboratory simulators and now clinically. Unfortunately, this improved wear performance comes at the expense of substantial reductions in fatigue strength and fracture toughness.35 This increased fracture/fatigue risk is substantial enough to have largely precluded HXPE usage thus far in total knees, pending possible further material improvements. For well functioning total hips, habitual contact stresses at the bearing surface are low enough that fracture/fatigue is of relatively little concern, even for the somewhat thinner liners needed to accommodate the more dislocation-resistant larger head sizes enabled by cross-linking’s “wear dividend.” An important caveat is that under various abnormal conditions, especially impingement situations, UHMWPE contact stresses can be many-fold higher22, constituting a substantial challenge for material failure. One variant, most catastrophically, is frank fracture of the liner, particularly a concern for sub-optimal component placement. Another variant is so-called impingement wear, liberating vastly increased amounts of debris and triggering a rapid osteolytic cascade.36 Also (but less well appreciated), for lever-out subluxation during impingement, a near-line-contact situation develops at the opposite side of the cup, involving egress-site stresses that can reach levels even higher than those at the impingement site per se21, thus also being of concern for fracture or highly accelerated wear.
The damaging effects of repeated impingements are necessarily cumulative. At present, there are no rigorously grounded impingement simulation protocols, on which basis meaningful implant performance standards can be prescribed. Large numbers of potentially impingement-vulnerable HXPE components are currently being implanted, with very little long-term direct clinical precedent, based instead primarily on favorable performance in laboratory wear simulators whose duty cycles have been impingement-free. Thus, critical need exists to quantitatively characterize HXPE impingement biomechanics, particularly to identify the patient factors and surgical implantation factors that most sensitively influence this potentially adverse situation.
Obesity, and peri-articular soft tissue compromise
As in many other areas of health care, longstanding experience in joint replacement surgery has shown that the prospects for a successful outcome depend heavily upon appropriate patient selection. Procedures or constructs that are well suited for some patients can be sub-optimal or even contraindicated in others. Specifically in the context of THA impingement/dislocation, the implant construct per se should not be viewed in isolation, even from the standpoint of biomechanical function. Certainly, a THA construct’s vulnerability to dislocation, and its likelihood of incurring complication-predisposing damage from impingement, depend on the mechanical demands to which it is subjected. Intuitively, patient body weight and hip capsule integrity are two factors that sensitively influence construct mechanical demand during an impingement/dislocation event. Excess body weight—especially situations of obesity—obviously involves higher demands for the THA Newtonian force/moment equilibrium for any given activity, including impingement/dislocation challenges. Also, motor control strategies for overweight patients are likely to differ because of the higher body segment masses needing to be moved, and the concurrent muscle capacity deficits accompanying a sedentary lifestyle. Furthermore, especially for obesity situations, local tissue girth may interfere with normal kinematic pathways, due to bulk pinching/entrapment effects.
Deterministic studies of lower extremity biomechanical abnormalities due to excessive weight have thus far mainly focused on locomotion activities of non-implanted individuals, owing to the increasingly evident linkage with osteoarthritis. Quite apart from its causal role in degeneration of the natural joint, however, understanding the extra demands that excess patient body weight poses for total joint replacement constructs is increasingly important because of the well-recognised demographic shift toward increased body mass index (BMI) and toward general aging of Western populations.37 Moreover, BMI among individuals in the age ranges most commonly undergoing total hip replacement 38 is increasing even more rapidly than among the US population as a whole. Although formal linkages via Newtonian mechanical analysis are (as of yet) lacking, the statistically significant associations of increased patient body weight both with increased polyethylene wear and with worse overall clinical outcome are certainly plausible on intuitive biomechanical grounds. Moreover, initially overweight patients tend to gain further weight after total hip replacement.39 Given these unfortunate realities, closer attention to patient body weight as a factor influencing THA complications seems amply justified.
The broadly recognised association between THA dislocation and prior hip surgery is usually attributed to reduced mechanical competence of peri-articular soft tissues, especially the capsule. Although formal mechanistic linkages again are largely lacking, indirect supporting evidence exists both from laboratory cadaver models40 and from clinical series 41,42 that repair of capsular releases or of capsule incisions improves construct stability. As a practical matter, of course, a great many patients predisposed to dislocation have generalised or regional capsule deterioration not lending itself well to effective surgical repair. As a point of reference, recent finite element work from our own group 43 indicates that situations of profound generalised capsule atrophy can compromise THA dislocation resistance by almost 70%, and that regional atrophy and/or local capsule defects can markedly alter both impingement site contact stresses and the kinematic pathways of femoral head subluxation and egress during posterior dislocation challenges. Thus, appreciably differing situations of irreparable capsule compromise, that usually first become apparent only intra-operatively, would have very different dislocation vulnerabilities, and they would differ greatly in terms of the optimally dislocation-protective THA construct. Similarly, implants which are vulnerable to impingement damage would seemingly be at heightened risk of causing complications when used in situations where the protective structural contribution of the capsule is compromised.
Development of a finite element model of dislocation
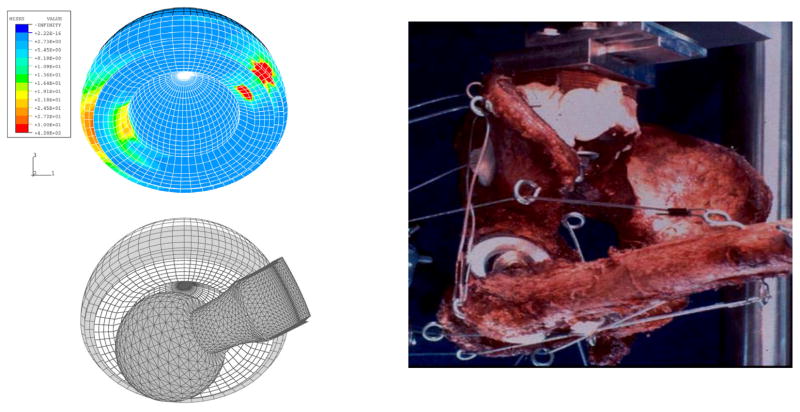
Component-on-component impingement, followed by levering out of the femoral head, is by far the most common mode of dislocation in total hip arthroplasty. There had been many registry-based studies of dislocation incidence, but confounding factors and sources of variability made it difficult to identify specific parameter influences. Development of a three-dimensional nonlinear finite element model (Figure 1) was therefore undertaken in our laboratory for the purpose of detailed mechanical study of the dislocation event itself. This allowed determination of how individual factors of component design and surgical implantation position affect the propensity for dislocation. Also, a first-generation laboratory testing apparatus was developed to provide physical validation for the computational model. The finite element model predicted the experimentally observed range of motion to frank dislocation to within an accuracy of 2.5%. Under even a modest joint load of 200N, the von Mises stresses developed in the polyethylene insert reached 13 MPa, and the contact stresses rose to as high as 30 MPa, values approaching or exceeding the material’s yield strength. Deleterious stress elevations occurred not only at the site of neck impingement, but also at the site of head egress from the liner.
Figure 1.
3-D Finite element model of total hip dislocation, illustrating the initial relative position of the implant components (left), the acetabular component bearing surface stress during stable articulation (center), and the corresponding stresses just prior to a posterior dislocation event (right). From: Scifert CF, Brown TD, Pedersen DR, Heiner AD, Callaghan JJ: Development and Physical Validation of a Finite Element Model of Total Hip Dislocation. Computer Methods in Biomechanics and Biomedical Engineering,1999;2(2):139–147.
Parametric effects of implant design variables
The finite element model was used to study how various total hip component design and surgical placement parameters contribute to resisting the propensity for posterior dislocation, for the case of leg crossing in an erect seated position. The primary outcome measures were the peak intrinsic moment developed to resist dislocation and the ranges of motion before neck-on-liner impingement and before frank dislocation. Modifications of acetabular liner design (chamfer bevel angle, lip breadth, head center inset) involved trading off improved peak resisting moment for compromised range of motion, and vice versa (Figure 2a). Increases of head size led to substantial improvements in peak resisting moment, but had almost no influence on the component range of motion if the head:neck diameter ratio was held constant (Figure 2b). For the leg crossing event studied, increased component anteversion, and even more so increased abduction (tilt), achieved improvements in range of motion and in peak resisting moment.
Figure 2.
Figure 2a. Trade-off between improvement of peak resisting moment versus range of motion, as a function of component design parameter changes (here illustrated for cup rim chamfer.)
Figure 2b. Effects of head size (for constant neck diameter) and of head/neck diameter ratio on impingement/dislocation behavior. From Scifert CF, Brown TD, Pedersen DR, Callaghan JJ: Finite Element Analysis of Factors Influencing Total Hip Dislocation. Clin. Orthop. Rel. Res. 355: 152–162, 1998.
Working collaboratively with implant designers at the Hospital for Special Surgery in New York, we explored a new design concept44 aimed at reducing the propensity for dislocation in total hip patients. The new design involved a convex-curved acetabular lip, extending from the hemispherical articulating surface to the outer edge of the cup. The femoral component had a matching, reverse curve. Our finite element formulation was used to study the dislocation event for this new design, compared to an otherwise nominally corresponding conventional implant design. The results showed that the new design achieved 28% more resisting moment build-up during dislocation, and that it had a higher range of motion from impingement to onset of subluxation. The new curved lip design also developed 50% lower polyethylene von Mises stress in the impingement zone.
Kinetics and kinematics of dislocation-prone manoeuvers
To enhance the clinical realism of biomechanical studies of dislocation, motion data were collected for ten THA-aged (but non-implanted) subjects, each repeatedly performing seven manoeuvers known to be dislocation-prone. The manoeuvers studied were: rising from a low seat (toilet) and from a normal height (chair), leg-crossing while seated erect, tying a shoe from a seated position, bending at the hip from an erect stance to retrieve an object from the floor (stooping), pivoting while standing, and rolling over in bed. The latter two manoeuvers tend to cause the femoral head to dislocate anteriorly, a largely unstudied failure mechanism. An optoelectronic motion tracking system and a recessed force plate (Figure 3) captured the kinematics and ground reaction forces of these maneuvers.
Figure 3.

Subject reflective marker array and custom bench for sit-to-stand motion tracking.
Using an established inverse dynamics model to estimate hip joint loading, 354 motion trials were evaluated using the three-dimensional nonlinear finite element model of THA dislocation. Worst-case-scenario THA constructs were simulated (22mm femoral head, acetabular cup orientations at the limit of the accepted safe zone) in order to predispose toward impingement and dislocation. The results showed a high incidence of computationally predicted dislocation for all movements studied. However, dislocation risk was very manoeuver-dependent, with patients being six times more likely to dislocate from a low-sit-to-stand manoeuver than from stooping.
Under an IRB-approved protocol, three-dimensional motion data were subsequently collected for ten THA patients during early recovery from surgery, when performing sit-to-stand activity. The subjects rose from a normal-height chair (47 cm), at a self-selected speed and with minimal assistance. The focus was on the instant of peak hip flexion, to characterise the potential for impingement and dislocation. The patients were assessed at 6 weeks (+/− 1 wk) and at 6 months (+/− 3wks) post-op. Eight control subjects also participated. Kinematic data were collected for the operated side (right side for control subjects) using an Optotrak® motion analysis system; concurrent kinetic data were collected by force plate. These movement data were combined with anthropometric measurements to estimate net joint moments, using an inverse dynamics approach. Three-dimensional position data at peak hip flexion were then plotted against the impingement limit plane estimated by Robinson et al.17 for an acetabular component at 30° abduction and 10° anteversion, and a femoral component at 10° anteversion. Three of the 10 THR patients and 6 of the 8 control subjects either penetrated or came within 3.4° (1 std dev) of the impingement plane (Figure 4).
Figure 4.
Propensity for impingement for THA patients for sit-to-stand motion. The estimated impingement envelope is shown shaded.
Complementary cadaver and computational dislocation models
Synergistic development of new THA dislocation models (Figure 5) was undertaken by two independent research groups, ours at the University of Iowa focusing on finite element computations, and the other from Baylor University, focusing on cadaver studies.45 Because both groups’ programs were motivated by similar pragmatic clinical issues, the major questions targeted had substantial overlap: When does dislocation occur from component versus bony impingement, and how do their respective mechanisms differ? What is the effect of head size on construct stability? How does the orientation of the acetabular component influence stability? Can stability be enhanced through changes to the geometry of the acetabular component? The experimental data showed mainly neck-on-liner impingement for small heads, and mainly bone-on-bone impingement for large heads. Computationally, there was a 3.6% resisting moment increase per mm of head size increase. ROM increased by 0.27° per mm of head size for constant neck diameter, but there was no effect if head:neck ratio was held constant. Skirt inclusion for a 22-mm head caused a 10° ROM decrease and a 12.6% resisting moment decrease. Cadaverically, there was a 1.26° ROM increase per degree of anteversion, versus a 0.6° increase computationally. Cadaverically, increased cup adduction led to ROM decreases for 22, 26, and 28-mm components. Computationally, lip chamfer caused a 0.47° decrease in ROM, and a 0.45% increase of resisting moment, per degree of increased bevel. Each millimeter of lip broadening achieved a 1.26° increase of ROM and a 3.9% decrease in resisting moment. Computationally, for flexion/exorotation-dominated motion challenges, standard-offset stem motion simulations were usually terminated by femoral component collar hooking on the cup rim, whereas large-offset stems usually progressed straightforwardly to dislocation.
Figure 5.
Complementary FEA and cadaver model analysis of THA impingement/dislocation. From: Scifert CF, Noble PC, Brown TD, Bartz RL, Kadakia N, Sugano N, Johnston RC, Pedersen DR, Callaghan JJ: Experimental and Computational Simulation of Total Hip Arthroplasty Dislocation. Orthopaedic Clinics of North America. 2001 Oct;32(4):553–567
Effects of surgical positioning of the acetabular component
The finite element dislocation model was further programmed to represent a widely-used total hip implant design and was exercised to measure peak resisting moment and range of motion both prior to impingement and prior to the onset of frank dislocation, for a range of acetabular component surgical alignments. Twenty-five combinations of cup abduction (30,40,50,60,70°) and anteversion (0,10,20,30,40°) were considered, with the resultant resisting moment about the cup centre being tracked in each case. A novel dislocation resistance index was developed for multi-factorial comparison. Increasing tilt and/or anteversion resulted in a monotonically increasing range of motion prior to impingement, as well as increased peak resisting moment. Range of motion was more sensitive to tilt, whereas peak resisting moment was more sensitive to anteversion. A 4-mm increase in head size typically achieved about 25% increase in peak resisting moment.
Clinically, most assessments of cup position suitability for dislocation avoidance rest upon subjective surgeon impressions, based on semi-quantitative impingement ROM assessments performed intra-operatively. To help elucidate optimal cup positioning more objectively, and to clarify the distinction between impingement avoidance and dislocation avoidance, inputs for the seven dislocation-prone activities described above were programmed for finite element models of a contemporary 22-mm modular total hip reconstruction.46 The same twenty-five cup placement positions were studied (5 abduction angles × 5 anteversion angles), straddling the conventional ‘safe zone’ of 30–50° of tilt and 5–25° of anteversion. Neck-on-liner impingement occurred during one or more of the seven dislocation-prone activities at all 25 cup positions. Dislocation preceded by impingement occurred for 51 of the 175 total simulations (25 cup positions × 7 activities), whereas impingement without dislocation occurred in 45 of the simulations, and dislocation without impingement occurred in 10. Neither dislocation nor impingement occurred in the remaining 69 simulations. Kappa analysis showed that impingement and dislocation as distinct outcome measures exhibited little more than chance agreement, indicating that intra-operative assessment of impingement range of motion is only marginally predictive of a given cup position’s vulnerability to frank dislocation.
Mechanical properties of the hip capsule
Finite element analysis in principle allows incorporating the effects of the hip capsule on both THA construct stability and load transmission through the joint. However, the necessary capsule mechanical properties had not been available for this purpose. Both hip joints were excised from each of five fresh-frozen cadavers that were free of hip joint pathology. Following dissection and potting of each hemi-pelvis, distraction of the intact joint was performed to measure the tensile stiffness of the overall joint capsule. Anatomical insertion loci of the hip capsule were then recorded, after which a complete capsulectomy was performed. Once excised, each capsule was sectioned into eight circumferential sectors. Tensile testing of these individual sectors then allowed determination of the capsule’s site-specific mechanical properties (structural tangent stiffness, failure load, ultimate strength, tangent modulus.) Mostly because of reduced thickness, the capsule’s structural stiffness was significantly lower (p<0.01) in the posterior-inferior sector than elsewhere.
Capsule representation in the dislocation finite element model
Our hardware-only finite element analyses had provided extensive insight into how specific design and surgical placement variables influence a THA implant’s intrinsic ability to resist dislocation. To broaden the picture, experimentally based capsule representation and anatomic bony structures were next added to the FE model (Figure 7). This was done in a manner that provided for large-deformation multi-body contact, including capsule wrap-around on the bone and/or the implant, capsule infolding upon itself, and/or capsule pinching/entrapment by the bone or by the implant. Moreover, the numerical formulation incorporated large-displacement interfacial sliding, and large-deformation nonlinear (hyperelastic) capsule mechanical properties. In addition, the model was configured for rapid incorporation of implant design variants (from manufacturers’ CAE data), and for turn-key analyst designation of implant placement (position and orientation) relative to the bony anatomy. Complex physiologic motion inputs were accommodated. Three-dimensional stress distributions throughout the implant and the capsule were output, before and during the impingement and dislocation events. Results from the model showed that a fully intact capsule provided about three-fold higher resistance to dislocation than that attributable to the implant hardware alone.
Figure 7.
Representation of sectoral capsule properties in a finite element model of THA impingement/dislocation. From: Stewart KJ, Pedersen DR, Callaghan JJ, Brown TD: Implementing Capsule Representation in a Total Hip Dislocation Finite Element Model. Iowa Orthopaedic Journal. 2004;24:1–8.
Clinical experience with constrained liners
Between April 1988 and February 1993, fifty-six tri-polar acetabular components were implanted in fifty-five patients who had sustained recurrent dislocations (average, six dislocations; range, two to twenty). All patients had additional factors contributing to the instability of the implant, including absence or disruption of the abductor mechanism, poor health, mental retardation, confusion, and/or Alzheimers disease. One patient was lost to follow-up. The remaining patients were followed clinically for a minimum of three years (average, sixty-four months; range thirty-seven to ninety-seven months) or until the time of death.47 During this follow-up interval, only two (four per cent) of the fifty-five patients had a subsequent dislocation. We concluded that the use of this type of component should be considered for patients who have recurrent dislocation, if other treatment modalities are unlikely to be effective.
Building on this five-year average follow-up, we subsequently reported on this same group of patients after an average of 10.2 years (range, 7.0 to 13.2 years) or until death.48 At this later time point, four (7%) of the fifty-six hips had sustained a subsequent dislocation or mechanical failure of the device. Three femoral components (5%) and two acetabular components (4%) were revised because of aseptic loosening. One hip was revised because of osteolysis. We concluded that this constrained acetabular component continued to provide durable protection against recurrent dislocations without substantial deleterious effects on component fixation, and we continued our advocacy of this device to treat recurrent dislocation when other modalities are unlikely to be effective.
Experimental testing of constrained liners
Bench testing was undertaken to study dislocation resistance for two conceptually distinct revision THA designs used to treat recurrent dislocation: tri-polar replacements inserted into metal shell polyethylene constructs, and constrained liners. The outcome measures utilized were: peak resisting moment, range of motion to impingement, range of motion to the orientation for peak resisting moment, range of motion to frank dislocation, and peak value of fixation interface spatial mean shear stress. Relative to an otherwise comparable conventional (mono-polar) unconstrained design, the tri-polar replacement achieved increased range of motion to full impingement (by 6°) and to dislocation (by 7°), while achieving a similar peak resisting moment. The constrained liners had ≈20° less range of motion to impingement, especially with 28-millimeter constructs, but showed no significant difference in the range of motion to dislocation. However, the constrained liners achieved order-of-magnitude increases in peak resisting moment, while still dislocating at torque levels unlikely to pose risk of fixation interface failure.
Finite element analysis of constrained liners
Clinical experience in managing recurrent dislocation by means of constrained acetabular liners has varied appreciably from series to series. There is limited understanding of the intrinsic mechanical characteristics of these specialty devices. We augmented our three-dimensional finite element model to include the interference fit effects and multi-surface contact effects specific to constrained liner implants (Figure 8). The model was physically validated, and then was used for parametric testing to explore the effects of individual design features. The model was exercised for both intra-operative push-in assembly and for lever-out dislocation. The data showed that the coefficient of friction between the femoral head and the liner substantially affected both the force required to seat the femoral head into the liner during assembly, and the peak moment resisting dislocation (226% increase in assembly force for friction coefficients of 0.2 vs. 0.0; 49% reduction in dislocation moment for friction coefficients of 0.013 vs. 0.135). As expected, the cup opening radius also had a dominant effect on both maneuvers: decreasing the opening radius from 13.9 to 13.6 mm (for 28 mm head diameter) increased assembly force by 506 N and increased the dislocation moment by over 3.5 N-m, whereas the influence of other design parameters was much more modest.
Figure 8.
Finite element analysis of a constrained-liner THA, showing stress contours at the instant of incipient dislocation (A,B), and the effect of cup opening radius for a 28-mm head diameter. From: Bouchard SM, Stewart KJ, Pedersen DR, Callaghan JJ, Brown TD: Design Factors Influencing Performance of Constrained Acetabular Liners: Finite Element Characterization. Journal of Biomechanics. 2006;39(5):885–893.
FEA simulations of dislocation for capsule deficits
Toward objectively characterising the influences of compromised capsule integrity, the capsule-augmented three-dimensional contact finite element model was used to study posterior dislocation events occurring due to flexion-dominated challenge manoeuvers. Parametric series included graded levels of overall atrophy of the capsule and loss of attachment of differing circumferential fractions of the capsule’s femoral insertion postero-laterally. For the atrophy series, full loss of capsule stiffness resulted in approximately a 66% reduction in peak resisting moment, with nominally proportional reductions for the three intermediate levels of stiffness attenuation. Implant range of motion to dislocation did not appreciably change with increasing degrees of capsule atrophy, although peak polyethylene contact stress, especially at the head egress site, progressively diminished to about 45% of full-capsule-stiffness levels. Circumferential attachment deficits also greatly diminished the resisting moment (Figure 9), but the most striking effect was severe capsule stress concentrations at attachment sites immediately adjacent to the defect.
Figure 9.
Computed stress distributions in the capsule during head subluxation (left) and after frank dislocation (right), for situations of 50% (upper) and 25% (lower) circumferential detachment of the capsule at its insertion on the femur, centered posteriorly.
Servo-hydraulic system for impingement/dislocation simulations
Custom equipment was designed and fabricated (Figure 10), by means of which THA-implanted cadaveric hip preparations could be subjected to a range of realistic impingement and dislocation challenges. This unit interfaces with the load frame of an MTS 858 Bionix testing machine. The custom unit consists of two heavy-duty aluminum yokes, mounted in series on the Bionix’s (spline-coupled) axial/rotary actuator. The inner of these two yokes controls hip flexion-extension, while the outer yoke controls hip abduction-adduction. The Bionix’s rotary channel controls hip endorotation-exorotation, and its axial channel controls resultant contact force magnitude. Control routines were written for normal locomotion activities and for various dislocation challenges as described above. Test specimens are mounted to the inner yoke such that the hip center is coincident with the three independently controlled rotary actuators. The femur is mounted on the load cell of the MTS, in series with a two-degree-of-freedom translation stage, permitting the femoral head to subluxate or dislocate from the acetabulum if the joint position/loading so dictates.
Figure 10.
Four-degree-of-freedom hip loading system. For visual clarity, a loaded THA construct is shown in isolation.
Surrogate validation of capsule impingement/dislocation FEA model
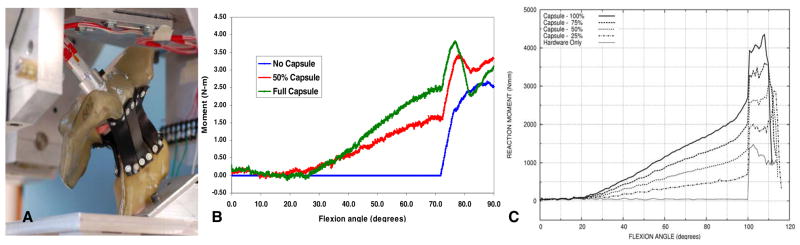
A laboratory surrogate was developed to systematically induce incremental degradations of capsule integrity, and to measure corresponding decrements of THA construct stability. Based upon earlier measurements of hip capsule mechanical properties, we identified 60A durometer polyurethane rubber as being optimally suitable. “Anatomic” strips of this material were cut from 1/16th inch-thick sheets, and were arrayed on a Sawbones pelvis and proximal femur. THA components were implanted, mounted in the servo-hydraulic system, and the preparation was subjected to simulated leg-cross dislocation challenges. Resisting moment was continually recorded for an “intact”capsule, and for progressive reductions in capsule stiffness achieved by successively removing strips. The resisting moment curves (Figure 11) showed progressive joint stiffening from capsule tensioning during normal hip flexion, followed by an abrupt increase at the instant of impingement, behavior very analogous to that obtained computationally. It was encouraging that experimental versus computational behavior was similar not only in terms of qualitative trends, but also in terms of quantitative magnitudes.
Figure 11.
A) Surrogate pelvis mounted in the dislocation simulator. B) Experimentally measured resisting moment versus hip flexion angle, as a function of capsule stiffness attenuation. C) Finite element computations of resisting moment versus flexion angle, as a function of capsule stiffness attenuation.
Cadaver validation of capsule-dislocation FEA impingement/subluxation computation
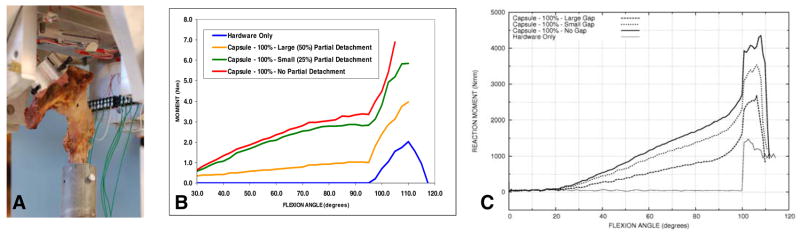
To further reinforce confidence in FEA model performance, pilot trials were next run in a fresh-frozen cadaver hip in which THA components had been implanted using the trans-pelvic procedure. Resisting moment build-up was measured during impingement and subluxation, for progressive reduction of capsule stabilisation, achieved by progressive partial release of the capsule’s femoral attachment. Except for the fully detached case (labeled Hardware Only in Figure 12b), the challenge was deliberately terminated when the femoral head began to sublux, to avoid possible specimen damage. The partial capsule releases were implemented by sharp dissection, visually estimating arc length around the circumference. Hence, these releases were only approximate, as was the specimen’s nominal anatomic alignment relative to the loading yoke axes. Given those approximations, the cadaver specimen’s measured response agreed well with the FEA simulations, both in qualitative trends and nominal quantitative magnitudes. These pilot results provide encouraging evidence (a) that the finite element model performs realistically, and (b) that the model system could reliably detect clinically consequential differences of capsule integrity, and also of implant positioning and patient body weight.
Figure 12.
Dislocation trials for a cadaver hip specimen implanted by a purpose-developed trans-pelvic procedure. (A) Specimen mounted in the loading apparatus, (B) Measured decrement of moment resisting dislocation, as a function of progressive release (centered posteriorly) of the capsule’s femoral attachment, (C) Corresponding FEA simulations.
Summary
Component impingement has long been a problematic aspect of total hip replacement, both in and of itself, and as a precursor to frank dislocation. Usage of conventional clinical registry data to understand the factors and mechanisms involved has historically proven difficult, owing to the many intrinsic variables involved, and also to the uncertainties often surrounding the event itself. Besides the longstanding concern regarding dislocation, impingement per se has come further to the fore in terms of modern advanced bearing systems, whose materials and designs tend to be less forgiving in that regard than has been the case with metal-on-conventional-polyethylene bearings. In recent years, various experimental and computational routes have been pursued in biomechanical laboratory settings, to better understand individual factors bearing upon impingement/dislocation propensity. This growing body of laboratory research has addressed implant design parameters, surgical implantation parameters, and various patient motion challenges. Going forward, the development of increasingly robust measurement and simulation capabilities offers promise that longstanding controversies will continue to be resolved, and that new implant designs and improved surgical techniques will continue to emerge, to further mitigate adverse clinical effects.
Figure 6.
Effects of acetabular component inclination and anteversion on peak resisting moment and on range of motion to dislocation. From: Nadzadi ME, Pedersen DR, Callaghan JJ, Brown TD: Effects of acetabular component orientation on dislocation propensity for small head size total hip arthroplasty. Clinical Biomechanics. 2002;17(1):32–40.
Table 1.
Finite element estimates of the propensity for THA dislocation, for various challenge maneuvers, based on experimentally measured hip joint kinetics and kinematics. From: Nadzadi ME, Pedersen DR, Yack HJ, Callaghan JJ, Brown TD: Kinematics, Kinetics, and Finite Element Analysis of Commonplace Maneuvers at Risk for Total Hip Dislocation. Journal of Biomechanics. 2003;36(4):577–591.
| Maneuver | # of Trials | # of Dislocations | % of Trials Dislocating | % CPA Accuracy |
|---|---|---|---|---|
| Low Sit-to-Stand | 47 | 41 | 87 | 85 |
| Normal Sit-to-Stand | 55 | 33 | 64 | 87 |
| Tie | 69 | 31 | 45 | 62 |
| Leg Cross | 64 | 22 | 34 | 81 |
| Stoop | 42 | 6 | 14 | 62 |
| Post. Disloc. Maneuvers | 277 | 133 | 48 | 75 |
| Pivot | 58 | 23 | 40 | 21 |
| Roll | 19 | 12 | 63 | 13 |
| Ant. Disloc. Maneuvers | 77 | 35 | 45 | 19 |
| Overall Series | 353 | 168 | 47 | 63 |
Table 2.
Regional mechanical properties of the hip capsule. (Sector locations are as defined in the image at left.) From Stewart KJ, Edmonds-Wilson R, Brand RA, Brown TD: Spatial Distribution of Hip Capsule Structural and Material Properties. Journal of Biomechanics. 2002;35 (11):1491–1498.
| Material Property | Units | Hip Capsule Sector Number | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |||

|
Stiffness |
(N/mm) |
45.76 (19.89) |
35.82 (21.82) |
12.71 (11.11) |
31.27 (41.28) |
12.86 (16.64) |
35.92 (42.34) |
60.20 (26.27) |
53.93 (50.75) |
| Modulus |
(Mpa) |
11.16 (8.55) |
8.08 (6.93) |
4.38 (3.48) |
6.95 (4.54) |
8.13 (4.12) |
21.65 (11.6) |
17.52 (10.21) |
21.10 (15.27) |
|
| Ultimate Strength |
(Mpa) |
3.39 (1.65) |
2.99 (.99) |
1.98 (.84) |
3.46 (2.87) |
2.58 (2.16) |
4.03 (1.97) |
5.72 (2.17) |
6.16 (3.64) |
|
| Failure Load |
(N) |
296.46 (111.44) |
232.80 (134.71) |
93.84 (73.33) |
149.86 (141.68) |
78.36 (99.) |
172.17 (209.88) |
313.43 (172.84) |
298.87 (168.17) |
|
Table 3.
| Table 3a. Component specifications of the six different constructs tested. Nadzadi M.E., Heiner A.D., Callaghan J.J., Brown T.D.: Dislocation Mechanics of Constrained Liner and BiPolar THA Designs, Proceedings of the 4th Combined Meeting of the Orthopaedic Research Societies of The U.S.A., Canada, Europe, and Japan, p.280, 2001. | |||||
|---|---|---|---|---|---|
| Construct | Stem | Head | Liner | Shell | # Tested |
| Constrained E-28 | Endurance +3
Standard Offset |
Articul/eze
28mm +5 |
Constrained Poly-Dial 28 mm/0° | ZTT I 54 mm
L Deep Profile |
3 |
| Constrained E-32 | Endurance +3
Standard Offset |
Articul/eze
32 mm +5 |
Constrained Poly-Dial 32 mm/0° | ZTT I 54 mm
L Deep Profile |
3 |
| Constrained UR-28 | UNI-ROM 18
B LRG 13 mm |
S-ROM
28 mm +0 |
Constrained Poly-Dial 28 mm/0°L | ZTT I 52 mm
L Deep Profile |
8 |
| Constrained UR-32 | UNI-ROM 18
B LRG 13 mm |
S-ROM
32 mm + 0 |
Constrained Poly-Dial 32 mm/0°L | ZTT I 52 mm
L Deep Profile |
8 |
| Bipolar | Endurance +3
Standard Offset |
Self-Centering
39/28/1 |
N/A | Duraloc 100
54 mm |
12 |
| Conventional Unconstrained | Endurance +3
Standard Offset |
Articul/eze
28mm +5 |
Duraloc Neutral | Duraloc 52
mm |
|
| Table 3b. Kinematic and kinetic performance of the individual constructs. Tmax is the peak resisting moment. ROM-I, ROM-Tmax, and ROM-D are ranges of motion (starting from a position of the neck axis symmetrically positioned perpendicular to the cup equatorial plane) to full impingement, to the configuration at which Tmax is developed, and to dislocation, respectively. τmax is the spatial mean shear stress on the cup backing corresponding to Tmax. | |||||
|---|---|---|---|---|---|
| Construct | Tmax (Nm) | ROM-I (°) | ROM-Tmax (°) | ROM-D (°) | τmax (MPa) |
| E-28 | 31.7±0.6 | 45.6±0.3 | 65.4±0.4 | 70.8±0.8 | 0.33±0.006 |
| E-32 | 41.9±0.5 | 47.2±0.3 | 66.6±0.6 | 67.1±0.6 | 0.47±0.006 |
| UR-28 | 35.9±0.8 | 43.4±0.5 | 66.0±0.9 | 71.7±0.8 | 0.41±0.009 |
| UR-32 | 40.8±0.7 | 42.7±0.3 | 62.2±0.5 | 62.7±0.5 | 0.43±0.007 |
| Bipolar | 1.9±0.1 | 65.7±0.4 | 70.2±0.6 | 76.6±0.7 | 0.02±0.001 |
| Conventional | 1.7 | 60.0 | 62.0 | 70.0 | 0.02 |
| Table 3c. Individual construct comparisons (A, B, C) which failed to demonstrate statistically significant difference (α=0.05) by one-way ANOVA and Duncan’s multiple range test. | |||
|---|---|---|---|
| Construct | ROM-Tmax | ROM-D | |
| E-28 | A | C | |
| E-32 | B | ||
| UR-28 | A | B | C |
Acknowledgments
The authors gratefully acknowledge the contributions of the many colleagues and students who worked either as formal collaborators, or who provided valuable advice and/or technical assistance behind the scenes. These especially include Dr. Christopher Scifert, Mr. Kristofer Stewart, Mr. Mark Nadzadi, Dr. Douglas Pedersen, Dr. Anneliese Heiner, Dr. John Yack, Dr. Phillip Noble, Mr. Joseph Lipman, Dr. Richard Brand, Dr. Rohan Edmonds-Wilson, Dr. Devon Goetz, Dr. William Capello, Dr. Richard Johnston, Ms. Suzanne Bouchard, Dr. M. James Rudert, Dr. Yuki Tochigi, Mr. Thomas Baer, and Ms. Julie Mock. Financial assistance was provided by research grants from the U.S. National Institutes of Health (AR046601, AR053553) and U.S. Department of Veterans Affairs, and from DePuy Inc.
Footnotes
A very recent report by Bozic et al.2, based on 2005 data from the National Inpatient Sample (covering about 20% of all inpatient hospitalizations in the US) now ranks instability (at 24.1%) ahead of aseptic loosening (17.6%), as the most common cause for revision THA.
This assumes a dislocation rate of 3–5% in primaries, costing $5,000 per non-surgical reduction (subtotal=$44 million), plus with 1–2% of these primary cases requiring early revision surgery costing $25,000 per case (subtotal=$82.5 million), plus approximately a 12% average dislocation rate for the 27,500 late revision THA cases performed per year (subtotal $16.5 million for non-surgical reductions, plus $41.3 million for re-revision surgeries).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- 1.Turner RS. Postoperative Total Hip Prosthetic Femoral Head Dislocations - Incidence, Etiologic Factors, and Management. Clin Orthop & Rel Res. 1994;301:196–204. [PubMed] [Google Scholar]
- 2.Bozic KJ, Kurtz SM, Lau E, Ong KL, Vail TP, Berry DJ. Revision Total Joint Arthroplasty: Early Insights into Cause of Failure, Type of Revision from Newly Implemented Diagnosis and Procedure Codes. Trans. 54th ORS: 235; San Francisco CA. March 2–5, 2008. [Google Scholar]
- 3.Grigoris P, Grecula MJ, Amstutz HC. Tripolar Hip Replacement for Recurrent Prosthetic Dislocation. Clin Orthop & Rel Res. 1994;304:148–155. [PubMed] [Google Scholar]
- 4.Morrey BF. Instability After Total Hip Arthroplasty. Orthop Clin N Am. 1992;23:237–247. [PubMed] [Google Scholar]
- 5.Woo RG, Morrey BF. Dislocations after Total Hip Arthroplasty. J Bone & Joint Surg. 1982;64A:1295–1306. [PubMed] [Google Scholar]
- 6.Daly PJ, Morrey BF. Operative Correction of an Unstable Total Hip Arthroplasty. J Bone & Joint Surg. 1992;74A:1334–1343. [PubMed] [Google Scholar]
- 7.Phillips CB, Barrett JA, Losina E, Mahomed NN, Lingard EA, Guadagnoli E, Baron JA, Harris WH, Poss R, Katz JN. Incidence Rates of Dislocation, Pulmonary Embolism, and Deep Infection During the First Six Months After Elective Total Hip Replacement. J Bone & Joint Surg. 2003;85A:20–26. doi: 10.2106/00004623-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Sanchez-Solelo J, Haidukewych GJ, Boberg CJ. Hospital Cost of Dislocation after Primary Total Hip Arthroplasty. Journal of Bone & Joint Surgery. 2006;88A:290–294. doi: 10.2106/JBJS.D.02799. [DOI] [PubMed] [Google Scholar]
- 9.Ali Khan MA, Brackenbury PH, Reynolds ISR. Dislocation Following Total Hip Replacement. J Bone & Joint Surg. 1981;63B:214–218. doi: 10.1302/0301-620X.63B2.7217144. [DOI] [PubMed] [Google Scholar]
- 10.McCollum DE, Gray WJ. Dislocation after Total Hip Arthroplasty - Causes and Prevention. Clin Orthop & Rel Res. 1990;231:159–169. [PubMed] [Google Scholar]
- 11.Paterno SA, Lachiewicz PF, Kelley SS. The Influence of Patient-Related Factors and the Position of the Acetabular Coment on the Rate of Dislocation after Total Hip Replacement. J Bone & Joint Surg. 1997;79A:1202–1210. doi: 10.2106/00004623-199708000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Delaunay CP. Metal-on-Metal Bearings in Cementless Primary Total Hip Arthroplasty. J Arthroplasty. 2004;19 (Suppl 3):35–40. doi: 10.1016/j.arth.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Coventry MB. Late Dislocations in Patients with Charnley Total Hip Arthroplasty. J Bone & Joint Surg. 1985;67A:832–841. [PubMed] [Google Scholar]
- 14.Garcia-Cimbrelo E, Munuera L. Dislocation in Low-friction Arthroplasty. J Arthroplasty. 1992;7:149–155. doi: 10.1016/0883-5403(92)90008-e. [DOI] [PubMed] [Google Scholar]
- 15.Amstutz HC, Lodwig RM, Schurman DJ, Hodgson AG. Range of Motion Studies for Total Hip Replacements. Clin Orthop & Rel Res. 1975;111:124–130. doi: 10.1097/00003086-197509000-00016. [DOI] [PubMed] [Google Scholar]
- 16.Chandler DR, Glousman R, Hull D, McGuire PJ, Kim IS, Clarke IC, Sarmiento A. Prosthetic Hip Range of Motion and Impingement: The Effects of Head and Neck Geometry. Clin Orthop & Rel Res. 1982;166:284–291. [PubMed] [Google Scholar]
- 17.Robinson RP, Simonian PT, Gradisar IM, Ching RP. Joint Motion and Surface Contact Area Related to Component Position in Total Hip Arthroplasty. J Bone & Joint Surg. 1997;79B:140–146. doi: 10.1302/0301-620x.79b1.6842. [DOI] [PubMed] [Google Scholar]
- 18.DiGioia AM, Jaramaz B, Blackwell M, Simon DA, Morgan F, Kischell E, Nikou C, Colgan BD, Aston CA, Moody JE, Labarca RS, Kanade T. An Image Guided Surgical Navigation System for the Accurate Measurement and Alignment of Acetabular Implants. Clin Orthop & Rel Res. 1998;355:8–22. doi: 10.1097/00003086-199810000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Herrlin K, Selvik G, Pettersson H, Kesek P, Onnerfalt R, Ohlin A. Position, Orientation and Component Interaction in Dislocation of the Total Hip Prosthesis. Acta Radiologica. 1988;29:441–444. [PubMed] [Google Scholar]
- 20.Nicholas RM, Orr JF, Mollan RAB, Calderwood JW, Nixon JR, Watson P. Dislocation of Total Hip Replacements - A Comparative Study of Standard, Long Posterior Wall, and Augmented Acetabular Components. J Bone & Joint Surg. 1990;72B:418–422. doi: 10.1302/0301-620X.72B3.2341440. [DOI] [PubMed] [Google Scholar]
- 21.Nadzadi ME, Pedersen DR, Yack HJ, Callaghan JJ, Brown TD. Kinematics, Kinetics, and Finite Element Analysis of Commonplace Maneuvers at Risk for Total Hip Dislocation. J Biomech. 2003;36(4):577–91. doi: 10.1016/s0021-9290(02)00232-4. [DOI] [PubMed] [Google Scholar]
- 22.Scifert CF, Brown TD, Pedersen DR, Heiner AD, Callaghan JJ. Development and Physical Validation of a Finite Element Model of Total Hip Dislocation. Computational Methods in Biomechanics and Biomedical Engineering. 1999;2:139–147. doi: 10.1080/10255849908907983. [DOI] [PubMed] [Google Scholar]
- 23.Sahni IK, Noble PC, Bartz R, Alexander JW, Paravic V, Kadakia NR. Biomechanical Mechanisms of Posterior Hip Dislocation in THR. Trans 44th Orthop Res Soc. 1998;195 [Google Scholar]
- 24.Northcut E, Komistek RD, Dennis DA. In Vivo Determination of Hip Joint Separation that May Lead to Impulse Loading Conditions. Trans 44th Orthop Res Soc. 1998;197 [Google Scholar]
- 25.Moore D. The First International Hip Resurfacing Forum; Malaga, Spain. 2003. Electronic proceedings at http://www.opnews.com/articles/jul2003/articles.php. [Google Scholar]
- 26.Harris W. Conquest of a Worldwide Human Disease – Particle-Induced Periprosthetic Osteolysis. Presidential Guest Address, 2004 Open Scientific Meeting of the Hip Society Clin Orthop & Rel Res. 2004;429:39–42. doi: 10.1097/01.blo.0000149821.72760.39. [DOI] [PubMed] [Google Scholar]
- 27.Scifert CF. PhD Thesis. Department of Biomedical Engineering, University of Iowa; 1999. A Finite Element Investigation into the Biomechanics of Total Artificial Hip Dislocation. (Supervisor: T.D. Brown) [Google Scholar]
- 28.Urban RM, Tomlinson MJ, Hall DJ, Jacobs JJ. Accumulation in Liver and Spleen of Metal Particles Generated at Nonbearing Surfaces in Hip Arthroplasty. J Arthroplasty. 2004;19 (Suppl 3):94–101. doi: 10.1016/j.arth.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 29.Ritter MA. Dislocation and Subluxation of the Total Hip Replacement. Clin Orthop & Rel Res. 1976;121:92–94. [PubMed] [Google Scholar]
- 30.Williams S, Isaac G, Hatto P, Stone MH, Ingham E, Fisher J. Comparative Wear Under Different Conditions of Surface-Engineered Metal-on-Metal Bearings for Total Hip Arthroplasty. J Arthroplasty. 2004;19 (Suppl 3):112–117. doi: 10.1016/j.arth.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Beaule PE, Le Duff M, Campbell P, Dorey FJ, Park SH, Amstutz HC. Metal-on-Metal Surface Arthroplasty with a Cemented Femoral Component – A 7–10 Year Follow-Up Study. J Arthroplasty. 2004;19 (Suppl 3):17–22. doi: 10.1016/j.arth.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Barrack RL, Burak C, Skinner HB. Concerns About Ceramics in THA. Clin Orthop & Rel Res. 2004;429:73–79. doi: 10.1097/01.blo.0000150132.11142.d2. [DOI] [PubMed] [Google Scholar]
- 33.Skinner HB. Ceramic Bearing Surfaces. Clin Orthop & Rel Res. 1999;369:83–91. doi: 10.1097/00003086-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Stewart TD, Tipper JL, Insley G, Streicher RM, Ingham E, Fisher J. Long-Term Wear of Ceramic Matrix Composite Materials for Hip Prostheses Under Severe Swing Phase Microseparation. J Biomed Mater Res, Appl Biomat. 2003;66(2):567–73. doi: 10.1002/jbm.b.10035. [DOI] [PubMed] [Google Scholar]
- 35.Baker DA, Bellare A, Pruitt L. The Effects of Degree of Crosslinking on the Fatigue Crack Initiation and Propagation Resistance of Orthopedic-Grade Polyethylene. J Biomed Mat Res. 2003;66(1):146–54. doi: 10.1002/jbm.a.10606. [DOI] [PubMed] [Google Scholar]
- 36.Bradford L, Baker D, Ries MD, Pruitt LA. Fatigue Crack Propagation Resistance of Highly Crosslinked Polyethylene. Clin Orthop & Rel Res. 2004;429:68–72. doi: 10.1097/01.blo.0000150124.34906.34. [DOI] [PubMed] [Google Scholar]
- 37.Crowninshield RD, Rosenberg AG, Sporer SM. Changing Demographics of Patients with Total Joint Replacement. Clinical Orthopaedics and Related Research. 2006;443:266–272. doi: 10.1097/01.blo.0000188066.01833.4f. [DOI] [PubMed] [Google Scholar]
- 38.US Bureau of the Census. Statistical Abstract of the United States 2004–2005. Washington DC: US Bureau of the Census Section 3: Health and Nutrition; pp. 89–132. [Google Scholar]
- 39.Heisel C, Silva M, dela Rosa MA, Schmalrried TP. The Effects of Lower-Extremity Total Joint Replacement for Arthritis on Obesity. Orthopaedics. 2005;28:157–159. doi: 10.3928/0147-7447-20050201-18. [DOI] [PubMed] [Google Scholar]
- 40.Mihalko WM, Whiteside LA. Hip Mechanics after Posterior Structure Repain in Total Hip Arthroplasty. Clinical Orthopaedics & Related Research. 2004;420:194–198. doi: 10.1097/00003086-200403000-00027. [DOI] [PubMed] [Google Scholar]
- 41.Dixon MC, Scott RD, Schai PA, Stamos V. A Simple Capsulorraphy in a Posterior Approach for Total Hip Arthroplasty. Journal of Arthroplasty. 2004;19:373–376. doi: 10.1016/j.arth.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 42.Pellicci PM, Bostrom M, Poss R. Posterior Approach to Total Hip Replacement using Enhanced Posterior Soft Tissue Repair. Clinical Orthopaedics & Related Research. 1998;355:224–228. doi: 10.1097/00003086-199810000-00023. [DOI] [PubMed] [Google Scholar]
- 43.Stewart KJ, Pedersen DR, Callaghan JJ, Brown TD. Capsular Atrophy and Detachment Influences on THA Disarticulation Propensity – A Finite Element Exploration. Trans 52d Ann Mtg Orthop Res Soc; Chicago IL. March 19–22, 2006.p. 452. [Google Scholar]
- 44.Scifert CF, Brown TD, Lipman JD. Finite Element Analysis of a Novel Design Approach to Resisting Total Hip Dislocation. Clin Biomech. 1999;14:697–703. doi: 10.1016/s0268-0033(99)00054-6. [DOI] [PubMed] [Google Scholar]
- 45.Scifert CF, Noble PC, Brown TD, Bartz RL, Kadakia N, Sugano N, Johnston RC, Pedersen DR, Callaghan JJ. Experimental and Computational Simulation of Total Hip Arthroplasty Dislocation. Orthopaedic Clinics of North America. 2001;32:553–367. doi: 10.1016/s0030-5898(05)70226-1. [DOI] [PubMed] [Google Scholar]
- 46.Pedersen DR, Callaghan JJ, Brown TD. Activity-dependence of the “safe zone” for impingement versus dislocation avoidance. Medical Engineering and Physics. 2005;27(4):323–328. doi: 10.1016/j.medengphy.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 47.Goetz DD, Capello WN, Callaghan JJ, Brown TD, Johnston RC. Salvage of a Recurrently Dislocating Total Hip Prosthesis with Use of a Constrained Acetabular Component. Journal of Bone and Joint Surgery. 1998;80A:502–509. doi: 10.2106/00004623-199804000-00006. [DOI] [PubMed] [Google Scholar]
- 48.Goetz DD, Barron RB, Bremner DO, Callaghan JJ, Capello WN, Johnston RC. Salvage of a Recurrently Dislocating Total Hip Prosthesis with Use of a Constrained Acetabular Component. A Concise Follow-Up of a Previous Report. Journal of Bone and Joint Surgery. 2004;86-A(11):2419–2423. doi: 10.2106/00004623-200411000-00009. [DOI] [PubMed] [Google Scholar]