Abstract
Background
Increasing evidence, derived mainly from animal models, supports the existence of endogenous cardiac renewal and repair mechanisms in adult mammalian hearts that could contribute to normal homeostasis and the responses to pathological insults.
Methods and Results
Translating these results, we isolated small c-kit+ cells from 36 of 37 human hearts using primary cell isolation techniques and magnetic cell sorting techniques. The abundance of these cardiac progenitor cells was increased nearly 4-fold in patients with heart failure requiring transplantation compared with nonfailing controls. Polychromatic flow cytometry of primary cell isolates (<30 μm) without antecedent c-kit enrichment confirmed the increased abundance of c-kit+ cells in failing hearts and demonstrated frequent coexpression of CD45 in these cells. Immunocytochemical characterization of freshly isolated, c-kit–enriched human cardiac progenitor cells confirmed frequent coexpression of c-kit and CD45. Primary cardiac progenitor cells formed new human cardiac myocytes at a relatively high frequency after coculture with neonatal rat ventricular myocytes. These contracting new cardiac myocytes exhibited an immature phenotype and frequent electric coupling with the rat myocytes that induced their myogenic differentiation.
Conclusions
Despite the increased abundance and cardiac myogenic capacity of cardiac progenitor cells in failing human hearts, the need to replace these organs via transplantation implies that adverse features of the local myocardial environment overwhelm endogenous cardiac repair capacity. Developing strategies to improve the success of endogenous cardiac regenerative processes may permit therapeutic myocardial repair without cell delivery per se.
Keywords: action potentials, heart failure, myocytes, stem cells, cell differentiation, myogenesis
In the midst of an explosion of clinical trails aimed at therapeutic cardiac regeneration using cellular therapeutics, recent studies in rodents support the existence of endogenous cardiac renewal and repair mechanisms that may contribute to normal homeostasis and the responses to pathological insults. Several studies have used bone marrow ablation and reconstitution with green fluorescent protein (GFP)–labeled cells and then reported GFP-labeled cardiac myocytes (CMs), vascular cells, and fibroblasts within and around experimentally infarcted myocardium.1–4 Other studies used direct myocardial injection of enriched and labeled progenitor cells derived from autologous progenitor cell pools5,6 or from allogeneic donors, including humans.7–9 Although some studies suggest a high rate of attrition for these newly engrafted cells,10 most studies suggest that local myocardial injury augments cell engraftment into the recipient myocardium. Indeed, using a “pulse-chase” strategy in which adult murine CMs were permanently labeled by GFP expression, Hseih et al11 demonstrated that endogenous CM replacement was significantly upregulated by myocardial infarction or pressure overload. These studies also demonstrated that cells expressing the stem cell marker c-kit were extremely rare in normal myocardium (0.004% of cells) but were substantially increased within the infarcted mouse myocardium (0.7% of nuclei) in association with increases in myocardial c-kit mRNA abundance.
In humans, early data supporting the existence of endogenous myocardial repair processes came from sex-mismatched transplanted hearts (male recipient) in which Y-chromosome–positive CMs (as well as endothelial cells and smooth muscle cells) were found within cardiac allografts obtained from female donors. Despite quantitative variation,12–16 higher rates of allograft chimerism were observed when the donor hearts suffered acute ischemic events,14 suggesting that host-derived progenitor cell recruitment might be injury responsive. Extending these observations, recent studies have reported the isolation and expansion of cardiac progenitor cells (CPCs) derived from biopsies of human hearts.7,17,18 After many weeks of in vitro processing and expansion, each study demonstrated that a small subset of the cells derived from cultured human heart biopsies expressed the stem cell surface marker c-kit and were multipotent, with the capacity to differentiate into endothelial cells, smooth muscle cells, fibroblasts, and CMs both in vitro and when engrafted into the hearts of immunotolerant rodents.7–9
Accordingly, the purpose of these studies was to define the abundance and characteristics of c-kit–expressing CPCs in the human myocardium. Recognizing that prolonged periods of tissue culture and cell expansion routines may induce distortions of progenitor cell populations and phenotype, we performed cell isolation and characterization immediately after cardiac explantation in failing and nonfailing hearts. Using this approach, we isolated c-kit+ CPCs from nearly every heart studied, observed increases in CPCs in failing hearts, and demonstrated their capacity for in vitro CM differentiation. While validating the clinical relevance of endogenous cardiac repair capacity reported in animal models, these new findings suggest a therapeutic paradigm aimed at enhancing endogenous cardiac repair mechanisms without cell therapy per se.
Methods
Tissue Procurement
After approval by the Institutional Review Board at the University of Pennsylvania, human myocardium was obtained from patients with end-stage heart failure undergoing heart transplantation and from nonfailing donors deemed unsuitable for transplant. Prospective informed consent for research use of heart tissue was obtained from transplant recipients or their next of kin (for brain-dead donors). Relevant clinical data were collected from all subjects providing heart tissue. Myocardial perfusion with cold 4:1 blood cardioplegia and prompt transport to the laboratory on ice were performed as previously described.19
Perfusion-Based Cell Isolation
Immediately after arrival in the laboratory, an epicardial branch artery was cannulated, and a 3-step perfusion digestion was performed as previously described.19 All digestions included a 30-minute perfusion with crystalloid buffer to clear the myocardial vasculature of retained blood cells, a 30-minute collagenase perfusion, and a final rinse phase. After initial passage through a stainless steel mesh (200 μm), the crude tissue digest from the mid myocardium of the left ventricular free wall was collected, diluted in 150 to 250 mL resuspension buffer, and sampled to allow subsequent measurement of DNA and protein content for normalization of the CPC yield. After centrifugation at 50g for 2 minutes, the crude tissue digest was filtered several times through a series of nylon filters (Millipore, Danvers, Mass) starting at 80 μm and finishing at 30 μm. The cells were washed twice with Ca/Mg-free PBS for 5 minutes each (460g at 4°C) and resuspended with buffer (PBS, 2 mmol/L EDTA, 0.5% BSA).
Measurement of DNA and Protein Content
Cells from a 0.5-mL aliquot of the crude tissue digest were pelleted and washed once with PBS. Total DNA was isolated with a DNeasy Tissue Kit (Qiagen, Valencia, Calif). DNA content was quantified by ultraviolet absorbance at 260 nm. Cellular protein was extracted with 10% SDS, and protein was measured with the BCA assay (Pierce, Rockford, Ill). DNA and protein concentrations were multiplied by the tissue digest volume to obtain the total DNA and protein content of the tissue digested for CPC isolation.
Magnetic Affinity Cell Enrichment
After resuspension, both human IgG Fc receptor blocking reagent and MicroBeads conjugated to human CD117 (c-kit) antibodies (Miltenyi Biotec, Auburn, Calif) were added to the filtered cell suspension at a ratio of 1:1:3, respectively, and incubated for 45 minutes at 4°C. After incubation, the cells were washed, centrifuged (300g for 10 minutes at 4°C), and resuspended. C-kit+ cells were separated with the OctoMACS Separation Unit (Miltenyi Biotec).
Flow Cytometry
Using small cells (<30 μm) isolated after tissue digestion and sequential filtration without antecedent magnetic cell sorting, we stained cells with a multicolor antibody panel composed of 7-AAD (viability dye, catalog No. A1310, Invitrogen, Molecular Probes, Carlsbad, Calif), CD117 (catalog No. A3C6E2, Miltenyi Biotec), CD45 (catalog No. 558441, BD PharMingen, San Diego, Calif), and CD133 (catalog No. 293C3, Miltenyi). Instrument compensation for all fluorochromes was performed with human peripheral blood mononuclear cells before each experiment. Data were collected on a 14-fluorescent-parameter BD LSRII (BD Biosciences, Sparks, Md), and a minimum of 500 000 live events (based on 7-AAD) were processed for each sample. Fluorescence-minus-one samples were used to define background fluorescence for each stain. Unstained controls confirmed that cell autofluorescence fell far below the thresholds defined by the fluorescence-minus-one analysis. Experiments using cells negative for CD45, CD133, and CD177 demonstrated minimal nonspecific labeling with the antibodies used.
Immunocytochemistry
Cells enriched via magnetic cell sorting were fixed in 4% paraformaldehyde at room temperature for 10 minutes, and their immunophenotype was evaluated by double staining with antibodies against CD117, CD45, CD34, and CD133. In coculture experiments, cells were fixed with 4% paraformaldehyde and permeabilized with 0.5% Triton X-100 immediately before labeling with antibodies directed against the following molecules: α-actinin, cardiac troponin T, Ki67, or Nkx2.5.
Neonatal Rat Ventricular Myocyte Coculture
Primary cultures of neonatal rat ventricular myocytes (NRVMs) were derived from 1- to 2-day-old Sprague-Dawley rat pups as previously described20 with minor modifications. Immediately after euthanasia, hearts were removed, ventricles were minced, and myocytes were dissociated with trypsin (1.5 mg/mL, Difco, Pittsburgh, Pa). Dispersed cells were plated on fibronectin-coated 18-mm coverslips placed in a 12-well culture dish at a density of 160 per 1 mm2 (200 000 per well). The NRVMs were precultured for 1 to 2 days with DMEM (GIBCO, Carlsbad, Calif) supplemented with 5% FBS (Hyclone Laboratories, Logan, Utah), BrdU (0.1 mmol/L; Sigma Chemical, St Louis, Mo), vitamin B12 (1.5 mmol/L; Sigma), and antibiotics (penicillin 500 000 U/L, streptomycin 50 mg/L, gentamicin 50 mg/L; Sigma). Before the coculture with CPCs, the preculture medium was replaced with coculture medium that included DMEM (GIBCO) supplemented with 5% FBS (Hyclone), insulin (10 μg/mL; Sigma), transferrin (10 μg/mL; Sigma), vitamin B12 (1.5 mmol/L; Sigma), and the antibiotics used in the preculture medium.
CPCs enriched for c-kit were infected with adenovirus containing a GFP gene (AdGFP) at a multiplicity of infection of 50 to 100 to allow human-derived cells to be distinguished from rat-derived cells. C-kit–enriched cells were incubated with GFP for 20 to 40 hours and then washed 4 times with the coculture medium before being added to precultured NRVMs. In all cases, human cells were added within 72 hours of plating the NRVMs.
Electrophysiological Recordings from Cocultured Cells
Cocultured human c-kit–enriched GFP+ cells and NRVMs were bathed in Tyrode’s solution containing (in mmol/L) CaCl2 1, glucose 10, HEPES 5, KCl 5.4, MgCl2 1.2, NaCl 150, and sodium pyruvate 2, pH 7.4. GFP+ CPCs were examined after 24 to 36 hours of coculture with NRVMs. Whole-cell patch-clamp recordings were performed at 37°C with the Axoclamp 2A amplifier (Molecular Devices, Sunnyvale, Calif) and pipettes (resistance, 5 to 7 mol/LΩ) filled with a solution containing (mmol/L) KOH 120, aspartic acid 120, KCl 20, Na2ATP 5, MgCl2 1, and HEPES 10, pH 7.2. Action potentials (APs) were recorded in the current-clamp mode and analyzed with pClamp8 software. AP waveshape was recorded from GFP+ cells in close contact with GFP− (rat-derived) cells and in GFP+ cells spatially removed from NRVMs. Spontaneous APs also were recorded in isolated GFP− NRVMs as an additional control. For each cell, the AP duration at 90% repolarization was derived from 3 APs.
Statistical Analysis
All data are expressed as mean ± SEM. Intergroup comparisons were made by unpaired Student t test. Differences with a value of P<0.05 were considered statistically significant.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Clinical Characteristics
As summarized in Table 1, the demographic and clinical features of the individuals whose hearts were examined were diverse, including significant representation of both failing and nonfailing hearts, a wide variation in subject age (range, 14 to 72 years), and the duration of heart failure (1 to 244 months). Of the 25 failing hearts, 17 required continuous intravenous milrinone support.
Table 1.
Clinical Characteristics of Patients Donating Hearts for This Research
| All Subjects | Nonfailing Controls | Transplant Recipients | |
|---|---|---|---|
| N | 37 | 12 | 25 |
| Age, y | 51±3 | 55±6 | 49±3 |
| Sex, M/F | 30/7 | 8/4 | 22/3 |
| Heart weight, g | 524±31 | 448±78 | 548±140 |
| HF origin | … | NA | 10 ICM, 18 DCM |
| HF duration, mo | NA | … | 87±75 |
HF indicates heart failure; ICM, ischemic cardiomyopathy; and DCM, idiopathic dilated cardiomyopathy. Data are mean±SEM.
Abundance of Putative Progenitor Cells in Human Heart
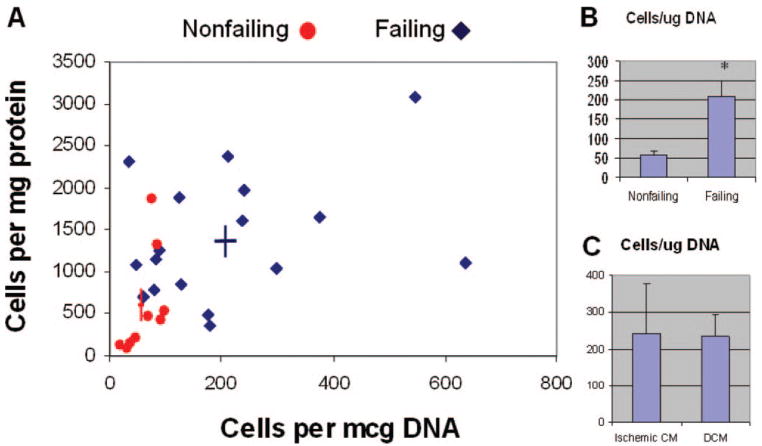
Using the isolation, filtration, and magnetic cell sorting techniques described above, we successfully isolated and enriched small c-kit+ cells from 30 of 31 consecutive hearts made available to our laboratory during a 7-month period. Figure 1A shows the normalized yields using either DNA content (x axis) or protein content (y axis) as the basis for normalization. The abundance of c-kit+ cells was nearly 4-fold greater in failing hearts compared with nonfailing controls (209±42 versus 59±10 c-kit+ cells per 1 μg DNA; P<0.005) using DNA content for normalization (Figure 1B) and 2.4-fold increased (1380±190 versus 575±205 c-kit+ cells per 1 mg protein; P<0.01) using protein content for normalization. However, among failing hearts, the cause of end-stage cardiomyopathy did not affect the abundance of c-kit+ cells obtained (Figure 1C). These results show that c-kit+ CPCs are more abundant in the failing human heart.
Figure 1.
Abundance of c-kit+ cells derived from human myocardium. A, Normalized yields of c-kit–enriched cells after perfusion-based isolation, sequential filtration, and magnetic-assisted cell sorting. Cell yields were normalized on the basis of the DNA content (x axis) and protein content (y axis) of the crude tissue digest from which the cells were derived. Color-coded cross-hairs indicate mean±SEM for each group. B, Intergroup comparison of DNA-normalized c-kit cell abundance in nonfailing vs failing hearts. C, Comparison of normalized c-kit abundance in hearts from patients with ischemic cardiomyopathy (Ischemic CM; n=6) vs idiopathic dilated cardiomyopathy (DCM; n=15). Data are mean±SEM. *P<0.005.
Characterization of CPCs From Human Hearts
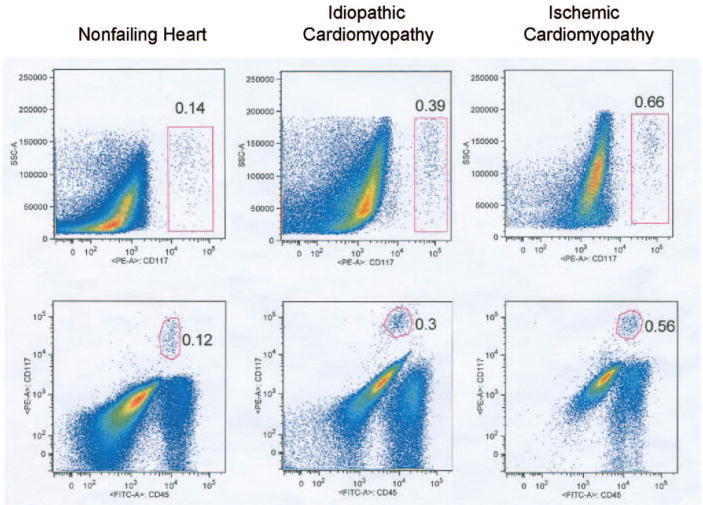
To characterize c-kit+ CPCs, we used flow cytometry in 10 freshly isolated live cell suspensions not subjected to magnetic-assisted c-kit enrichment after sequential filtration. As illustrated in Figure 2, flow cytometry confirmed an identifiable population of c-kit+ cells in each human heart consistent with undifferentiated CPCs. In failing hearts, 0.41±0.09% of small cells were c-kit+ compared with 0.11±0.02% in nonfailing hearts. This analysis also demonstrated that a high proportion of c-kit+ CPCs are CD45+ (88.3±2.0% in nonfailing hearts, 86.9±7.1% in failing hearts). Moreover, most of these c-kit+/CD45+ CPCs were CD45dim/moderate as opposed to the bright CD45 fluorescence intensity characteristic of mature leukocytes and many of the c-kit−/CD45+ cells found in the human heart. On the other hand, after gating based on the fluorescence-minus-one analysis, every human heart studied contained some c-kit+/CD45− cells, and none of the c-kit+ cells were CD133+.
Figure 2.
Polychromatic flow cytometry of isolated cells obtained after digestion and filtration to exclude cells >30 μm. All data reflect live cells based on 7-AAD exclusion. For each of the 3 primary cell isolations, the top plot depicts side scatter vs CD117 (c-kit) staining. As observed with the magnetic cell sorting method (Figure 1), a greater abundance of c-kit+ cells was found in failing vs nonfailing hearts. Bottom plots show CD117 staining vs CD45 staining. Each of these plots indicates that the majority of c-kit+ cells also express CD45 within a dim to moderate range of CD45 fluorescent intensity. Fluorescence-minus-one control experiments indicate that CD117 staining and CD45 staining are above the background fluorescent intensity.
To complement these findings, we used immunofluorescence microscopy of the c-kit–enriched cell suspensions obtained immediately after sequential filtration and magnetic sorting. C-kit+ cell enrichment resulted in 38±2% c-kit immunopositivity for the cells retained on the column compared with a rate of 0.02% to 0.64% for the nonenriched small-cell population. Double-labeling immunocytochemistry demonstrated that 87±5% of cells positive for c-kit also expressed the leukocyte lineage marker CD45, whereas 40±12% of CD117+ cells also expressed the hematopoietic/endothelial progenitor marker CD34, and 20±3% of c-kit+ cells expressed the hematopoietic stem/progenitor surface marker CD133. In separate experiments, we examined the abundance of CD45 mRNA by quantitative reverse-transcriptase polymerase chain reaction in sorted CD117+ cells and appropriate positive and negative controls. As shown in Figure I of the online-only Data Supplement, we found that CD45 mRNA is much greater (≈400-fold) in CD117+ cells than in CD117−/CD45−cells but still <25% of that observed in CD45+ leukocytes found within the heart or peripheral circulation. These data are concordant with the flow cytometry findings indicating that most of the c-kit+/CD45+ CPCs were CD45dim/moderate as opposed to the bright CD45 fluorescence intensity of mature leukocytes.
Cardiomyocyte Differentiation in Coculture Experiments
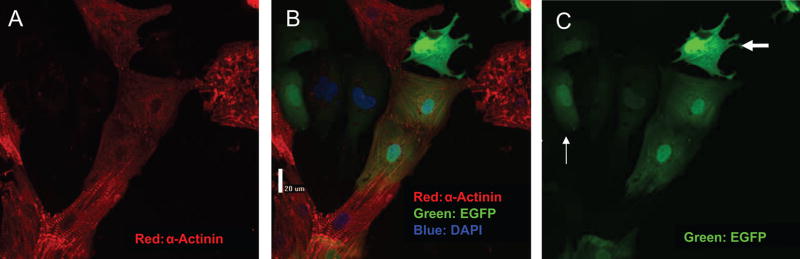
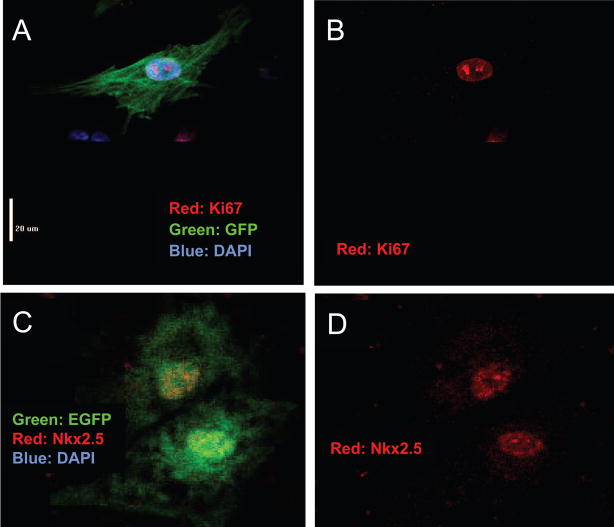
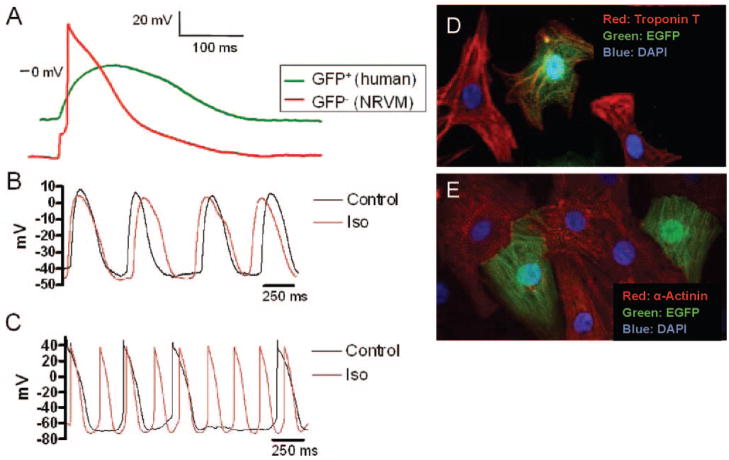
To address the in vitro regenerative capacity of the c-kit+ CPCs within human hearts, we cocultured GFP-expressing c-kit–enriched cell suspensions with established NRVM cultures. On the basis of double-labeling with GFP and myocyte-specific markers (α-actinin, cardiac troponin T, or cardiac myosin heavy chain), we consistently observed that a subset of GFP+ human cells had CM-specific protein expression, suggesting differentiation during these coculture experiments of 1 to 3 days’ duration. On several occasions, ≈50% of GFP+ cells expressed CM-specific proteins (online-only Data Supplement Figure II); lower rates were observed in other cases. The morphology of GFP+ cells expressing CM-specific proteins typically included a single nucleus, clear sarcomeric structure, and a distinct cell-cell interface, with the GFP+ rat cells present in the coculture (Figure 3). A significant fraction of the GFP+ human cells (36±11%, n=4) were Ki67+, indicating cell cycle activity (Figure 4A and 4B). Positive staining for Nkx2.5 in the GFP+ human cells provided further evidence of commitment to a CM fate, even when these cells appeared relatively undifferentiated (Figure 4C and 4D). GFP+ cells that did not express myocyte-specific markers exhibited variable morphology, including round or oval appearance, a stellate morphology, or evidence of cytoplasmic granules, as illustrated in Figure 3. Finally, the absence of rod-shaped, GFP-labeled CMs suggested that significant contamination of the CPC pool with already differentiated CMs was unlikely. Together, these data strongly support the cardiomyogenic potential of c-kit–enriched CPCs derived from the failing human heart.
Figure 3.
Heterogeneous responses of c-kit–enriched human cells in coculture. After isolation and incubation with AdGFP for 24 hours, c-kit–enriched human CPCs were cocultured with NRVMs for 36 hours and fixed. A shows staining for the myocyte-specific protein α-actinin. C shows the GFP-stained human cells. B is an overlay of A and C. GFP-positive, α-actinin–positive cells in the center exhibit clear sarcomeric structure and are newly differentiated CMs. These cells appear to be coupled to the GFP-negative rat myocytes. Also visible is a well-attached, intensely GFP-positive cell without sarcomeric structure or α-actinin staining (thick arrow) and less intensely GFP-positive cells (thin arrows) that may represent undifferentiated CPCs. EGFP indicates enhanced GFP.
Figure 4.
Cell cycle activity in differentiating human CPCs. A and B, Ki67 staining demonstrates cell cycle activity in a mononucleated GFP+/Ki67+ cell with clear sarcomeric structure identifying CM differentiation. C and D, Nkx2.5 in GFP+ human CPCs. Nkx2.5 staining indicates cardiac lineage restriction in less differentiated cells. EGFP indicates enhanced GFP.
Functional Phenotype of Newly Differentiated Myocytes
After 1 to 2 days of coculturing, GFP+ cells lacking substantial regions of contact with neonatal myocytes (see Figure 5D for an example) had less polarized resting membrane potentials and longer AP durations than isolated GFP− NRVMs, as illustrated in Figure 5A and summarized in Table 2. These cells usually made contact with neonatal myocytes through cytoplasmic extensions that have been called nanotubules.21 When these cells were exposed to 10−8 mol/L isoproterenol, no significant change was found in their frequency of spontaneous depolarization or AP waveshape as illustrated in Figure 5B. In contrast, GFP+ myocytes in close contact with NRVMs (see Figure 5E for examples) beat synchronously with the GFP− cells, displayed a more polarized resting membrane potential, and exhibited a higher depolarization frequency and shorter AP duration in response to 10−8 mol/L isoproterenol, as shown in Figure 5C. Together, these data indicate that GFP+ cells derived from human CPCs can differentiate into functionally immature CMs that are clearly distinct from isolated NRVMs and that GFP+ new human myocytes are capable of electrically coupling with NRVMs in coculture.
Figure 5.
AP characteristics in cocultured cells. A, Comparison of a representative spontaneous AP from a GFP+ cell derived from human c-kit–enriched CPCs and a stimulated AP from a GFP− NRVM after 48 hours of coculture. Data were obtained via patch pipette in current-clamp mode. B, Spontaneous APs from a different spatially isolated GFP+ human CM before and after application of 10−8 mol/L isoproterenol (Iso). C, Spontaneous APs from a spatially coupled GFP+ human CM. The resting potential and AP waveshape from the spatially isolated GFP+ myocyte are distinct from those observed in NRVMs or adult human myocytes with minimal response to isoproterenol (10−8 mol/L). The spatially coupled GFP+ cell has a resting potential and AP waveshape characteristic of a rat myocyte with increased chronotropy and AP shortening in response to isoproterenol. D and E, Examples of spatially isolated (D) and spatially coupled (E) human CMs. EGFP indicates enhanced GFP.
Table 2.
Electrophysiological Characteristics of Isolated Newly Differentiated Human Myocytes and NVRMs
| Group | n | RMP, mV | APD90, ms | AP Frequency, Hz |
|---|---|---|---|---|
| Human CPCs | 5 | −47.9±9.7* | 370±65* | 1.4±0.6 |
| Neonatal RVMs | 5 | −62.7±4.9 | 197±31 | 1.0 |
RMP indicates resting membrane potential; APD90, AP duration at 90% repolarization. Spontaneous APs were recorded in GFP+ cells derived from human CPCs; triggered APs were recorded in NRVMs after 48 hours in coculture. Data were obtained via patch pipette in current-clamp mode (see Methods for details). Recordings were taken from cells that appeared spatially isolated without obvious cell-cell contact. Data are mean±SEM.
P<0.05 vs NRVMs.
Discussion
The present studies represent the first report characterizing primary isolates of c-kit+ human CPCs obtained within a few hours of cardiac explantation. We show that c-kit+ CPCs can be isolated from nearly every human heart and that their myocardial abundance is increased in patients with advanced heart failure requiring transplantation. A high proportion of freshly isolated CPC c-kit+ cells also express CD45, suggesting a possible bone marrow origin for many of the progenitor cells isolated. Nevertheless, our coculture studies demonstrate the capacity of CPCs to form new human CMs that are functionally immature yet distinct from the NRVMs that induce their myogenic differentiation. At the same time, the need to transplant the failing hearts that yielded these progenitors implies that defects in the progenitor cells themselves and/or adverse features of the local myocardial environment overwhelm endogenous cardiac repair capacity. Together, these findings support a therapeutic paradigm that focuses on augmenting the efficacy of endogenous CPCs, rather than exogenous cell delivery, as a means of promoting cardiac regeneration.
By applying established cardiac protection routines, a standardized methodology, and a rational normalization scheme, we are the first to adapt perfusion-based cell isolation techniques for stem/progenitor cell isolation from human hearts. The key advantage of this approach is that it consistently yields a sufficient number of cells for early quantification, characterization, and manipulation of resident CPCs without the requirement for antecedent in vitro propagation. As such, our methodology allows a less distorted view of the in vivo state of endogenous cellular mediators. Using this approach, we observe significantly increased CPC abundance in the left ventricular myocardium of severely failing human hearts, regardless of etiology, compared with nonfailing controls. Increased c-kit+ CPC abundance in failing hearts is demonstrated with 2 independent techniques: cell counting after c-kit enrichment and flow cytometry in the absence of antecedent enrichment. Moreover, this finding of increased CPCs in the failing heart is consistent with a concept of injury/disease responsiveness of endogenous cardiac repair processes that has emerged on the basis of rates of chimerism in sex-mismatched human allografts,14 immunohistological assessment of human hearts,22 bone marrow labeling,23 or a fate-mapping approach in mice.11 The concordance of our findings of increased CPC abundance in severely failing hearts with these previous studies serves to validate our normalization technique while extending previous reports.22
In contrast to recent studies characterizing c-kit+ cardiac cells derived from biopsy specimens and expanded in vitro, our immunophenotypic characterization of freshly isolated CPCs indicates that a minority of the c-kit+ CPCs are lineage negative. Rather, we observe that a high proportion of c-kit+ CPCs also are CD45+, whereas a smaller proportion may be positive for CD34. Despite the low level of double staining for CD133 and CD117 indicated by immunofluorescence cytochemistry, flow cytometry did not detect c-kit+/CD133+ cells. Possible contributors to this discrepancy include the use of paraformaldehyde fixation before staining in cytochemistry versus live cell staining in flow cytometry. Although most CPCs have less intense CD45 expression than c-kit−/CD45+ mature leukocytes, coexpression of these biomarkers suggests a bone marrow origin for many of the c-kit+ CPCs observed in human hearts. This conclusion is consistent with the studies by Mouquet et al,23 who reported injury-induced increases in CPCs originating from the bone marrow (based on GFP labeling) and high rates of CD45 positivity early after infarction with time-dependent decreases during the first week after myocardial infarction in rat. An extracardiac origin for some CPCs also is suggested by the studies in which Y-chromosome–positive cells are observed in allografts from female donors placed in male recipients.12–16 Although our methods cannot conclusively define the origin of increased CPCs in the human heart, our results support the view that normal and failing human hearts contain a variety of c-kit+ subtypes. From our limited characterization of primary isolates, we cannot determine whether resident lineage-negative, c-kit+ cells are altered in failing hearts, yet our results indicate increased CD45+/c-kit+ cells in failing hearts.
Our coculture studies extend recent studies demonstrating the cardiomyogenic potential of CPCs derived from human hearts. In 3 studies from separate laboratories, c-kit+ cells cultured and expanded from heart biopsies have demonstrated the capacity for CM formation after either coculture with NRVMs or engraftment into immunotolerant rodents.7,17,18 Likewise, our primary isolates of noncultivated c-kit–enriched CPCs demonstrate in vitro cardiomyogenic potential. These CPC-derived cells display unequivocal signs of CM differentiation, including cardiac-specific transcription factors and contractile proteins, sarcomeric structure, and spontaneous contractions. Their electrophysiological characteristics and β-adrenergic responses are variable and are related to functional integration with the neonatal myocyte feeder layer. As in many other studies using coculture,7,17,24,25 we have not defined in this study how NRVMs induce CM differentiation in CPCs; however, the presence of spontaneous depolarizations in the absence of electric coupling suggests that electric coupling may not be required for cardiomyogenic commitment.
In human hearts, it remains unknown whether and to what degree CPCs contribute to the native response to myocardial injury and failure. The fact that our failing human hearts required transplantation despite increased abundance of c-kit+ CPCs capable of in vitro cardiomyogenesis seems to indicate an inadequate native regenerative response. Consistent with this interpretation, Fazel et al26 demonstrated that c-kit–dependent homing of bone marrow–derived progenitors to the infarcted myocardium contributes to angiogenesis but few new CMs. On the other hand, Hsieh et al11 recently demonstrated that early increases in myocardial c-kit expression, followed by sustained evidence of new CM formation, albeit at low levels, are observed after experimental myocardial infarction or pressure overload. In fact, the rates of durable myocyte replacement observed by Hsieh et al were much lower than the increases in new cardiac-committed precursors (BrdU+/NKx2.5+), indicating a high in vivo attrition rate for immature CMs that is consistent with our findings in human hearts. Together, recent studies in transgenic models and the present translational studies in humans indicate that myocardial injury and failure induce a robust endogenous repair response, yet the efficiency of durable in vivo cardiomyogenesis is too low to achieve adequate clinical benefit.
Study Limitations
Because other recent reports have identified several stem/progenitor populations in the human heart that appear to have cardiomyogenic potential,7,17,25,27 our exclusive focus on c-kit+ cells may tend to underestimate the endogenous repair capacity within the human hearts. Despite the advantages of using freshly isolated, nonexpanded CPCs for these studies, our approach does not permit conclusions about the spatial distribution of CPCs within the heart, including whether the cells we isolated were clustered in discrete niches within the myocardium. Although sufficient to identify immunophenotypic heterogeneity, our methods cannot determine whether this heterogeneity results from CPCs originating from different CPCs pools, time-dependent shifts in lineage commitment, or location-dependent differences in the instructive signals received within the myocardium. Moreover, use of a heterogeneous population of c-kit–enriched cells in coculture experiments does not permit conclusions about the relative cardiomyogenic capacity of particular immunophenotypic subpopulations. Finally, in any coculture experiment, there are concerns that cell fusion or cytoplasmic exchange might have influenced the findings. Nevertheless, factors arguing against cell fusion include the fact that nearly all GFP+ CMs were mononucleated and the high rates of myocyte differentiation we observed in contrast to the low frequency of in vitro cell fusion or cytoplasmic exchange in published reports.21,28 The distinct electrophysiology of electrically isolated GFP+ myocytes also is inconsistent with cell fusion or cytoplasmic exchange.
Conclusions
Although some patients may have insufficient CPC reserves, our data suggest that most severely failing human hearts have and/or attract significant quantities of endogenous CPCs capable of cardiomyogenesis. This observation suggests that local myocardial factors such as ischemia, oxidative stress, and/or inflammation may be thwarting or limiting the success of endogenous cardiac repair. Alternatively, insufficient instructive signaling to foster CPC differentiation may contribute to inadequate in vivo differentiation. These same adverse and/or inadequate local conditions may likewise account for the rather limited success achieved with myocardial cell therapy, including very low levels of myocyte engraftment, to date. Ultimately, better understanding of endogenous repair processes, with particular attention to the role of adverse local myocardial factors, is essential for the development of effective therapeutic strategies for cardiac regeneration with or without exogenous cell therapy.
CLINICAL PERSPECTIVE.
Amid a growing heart failure epidemic, the clinical research community is aggressively pursuing interventional trials involving mobilization and/or administration of putative stem/progenitor cells to promote cardiac regeneration. To date, the therapeutic success and impact of these interventional trials have been limited. In this context, the present studies demonstrate that severely failing human hearts already have and/or attract increased quantities of endogenous stem/progenitor cells that appear capable of new cardiac myocyte formation under favorable in vitro conditions. Our additional finding that many of the stem/progenitor cells in the human heart have biomarkers indicating a bone marrow origin implies that the failing heart may elicit homing signals that promote engraftment of circulating cells. Regardless of their origin, the discovery of increased myogenic progenitors in end-stage failing human hearts highlights a potential therapeutic opportunity to promote myocardial regeneration. However, the fact that unaided endogenous repair is insufficient in these hearts also suggests that the central clinical challenge may not be how to deliver stem/progenitor cells to the heart but rather how to promote their in vivo differentiation, maturation, integration, and survival. With or without supplemental cell delivery to the heart, these data suggest that strategies to identify and ameliorate adverse local myocardial factors are necessary to promote clinically meaningful myocardial regeneration.
Supplementary Material
The online-only Data Supplement can be found with this article at http://circ.ahajournals.org/cgi/content/full/CIRCULATIONAHA.107.761031/DC1.
Acknowledgments
We thank the cardiothoracic surgeons, the heart failure research nurses, and the Gift-of-Life donor program (Philadelphia, Pa) for their assistance with heart tissue procurement. We also appreciate the assistance of Daniel Freitag with reverse-transcriptase polymerase chain reaction experiments.
These studies were supported by funding from the National Institutes of Health, Bethesda, Md (HL033921 to Dr Houser and AG17022 to Dr Margulies).
Footnotes
Disclosures
None.
References
- 1.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 2.Kajstura J, Rota M, Whang B, Cascapera S, Hosoda T, Bearzi C, Nurzynska D, Kasahara H, Zias E, Bonafe M, Nadal-Ginard B, Torella D, Nascimbene A, Quaini F, Urbanek K, Leri A, Anversa P. Bone marrow cells differentiate in cardiac cell lineages after infarction independently of cell fusion. Circ Res. 2005;96:127–137. doi: 10.1161/01.RES.0000151843.79801.60. [DOI] [PubMed] [Google Scholar]
- 3.Goodell MA, Jackson KA, Majka SM, Mi T, Wang H, Pocius J, Hartley CJ, Majesky MW, Entman ML, Michael LH, Hirschi KK. Stem cell plasticity in muscle and bone marrow. Ann N Y Acad Sci. 2001;938:208–218. doi: 10.1111/j.1749-6632.2001.tb03591.x. [DOI] [PubMed] [Google Scholar]
- 4.Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, Entman ML, Michael LH, Hirschi KK, Goodell MA. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001;107:1395–1402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malouf NN, Coleman WB, Grisham JW, Lininger RA, Madden VJ, Sproul M, Anderson PA. Adult-derived stem cells from the liver become myocytes in the heart in vivo. Am J Pathol. 2001;158:1929–1935. doi: 10.1016/S0002-9440(10)64661-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 7.Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, Salio M, Battaglia M, Latronico MV, Coletta M, Vivarelli E, Frati L, Cossu G, Giacomello A. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–921. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 8.Yeh ET, Zhang S, Wu HD, Korbling M, Willerson JT, Estrov Z. Trans-differentiation of human peripheral blood CD34+-enriched cell population into cardiomyocytes, endothelial cells, and smooth muscle cells in vivo. Circulation. 2003;108:2070–2073. doi: 10.1161/01.CIR.0000099501.52718.70. [DOI] [PubMed] [Google Scholar]
- 9.Zhang S, Wang D, Estrov Z, Raj S, Willerson JT, Yeh ET. Both cell fusion and transdifferentiation account for the transformation of human peripheral blood CD34-positive cells into cardiomyocytes in vivo. Circulation. 2004;110:3803–3807. doi: 10.1161/01.CIR.0000150796.18473.8E. [DOI] [PubMed] [Google Scholar]
- 10.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 11.Hsieh PC, Segers VF, Davis ME, Macgillivray C, Gannon J, Molkentin JD, Robbins J, Lee RT. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nature Med. 2007;13:970–974. doi: 10.1038/nm1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quaini F, Urbanek K, Beltrami AP, Finato N, Beltrami CA, Nadal-Ginard B, Kajstura J, Leri A, Anversa P. Chimerism of the transplanted heart. N Engl J Med. 2002;346:5–15. doi: 10.1056/NEJMoa012081. [DOI] [PubMed] [Google Scholar]
- 13.Glaser R, Lu MM, Narula N, Epstein JA. Smooth muscle cells, but not myocytes, of host origin in transplanted human hearts. Circulation. 2002;106:17–19. doi: 10.1161/01.cir.0000021923.58307.8f. [DOI] [PubMed] [Google Scholar]
- 14.Hocht-Zeisberg E, Kahnert H, Guan K, Wulf G, Hemmerlein B, Schlott T, Tenderich G, Korfer R, Raute-Kreinsen U, Hasenfuss G. Cellular repopulation of myocardial infarction in patients with sex-mismatched heart transplantation. Eur Heart J. 2004;25:749–758. doi: 10.1016/j.ehj.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 15.Hruban RH, Long PP, Perlman EJ, Hutchins GM, Baumgartner WA, Baughman KL, Griffin CA. Fluorescence in situ hybridization for the Y-chromosome can be used to detect cells of recipient origin in allografted hearts following cardiac transplantation. Am J Pathol. 1993;142:975–980. [PMC free article] [PubMed] [Google Scholar]
- 16.Laflamme MA, Myerson D, Saffitz JE, Murry CE. Evidence for cardiomyocyte repopulation by extracardiac progenitors in transplanted human hearts. Circ Res. 2002;90:634–640. doi: 10.1161/01.res.0000014822.62629.eb. [DOI] [PubMed] [Google Scholar]
- 17.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marban E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 18.Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N, Cascapera S, Beltrami AP, D’Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, Anversa P. Human cardiac stem cells. Proc Natl Acad Sci USA. 2007;104:14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dipla K, Mattiello JA, Jeevanandam V, Houser SR, Margulies KB. Myocyte recovery after mechanical circulatory support in humans with end-stage heart failure. Circulation. 1998;97:2316–2322. doi: 10.1161/01.cir.97.23.2316. [DOI] [PubMed] [Google Scholar]
- 20.Simpson P, Savion S. Differentiation of rat myocytes in single cell cultures with and without proliferating nonmyocardial cells: cross-striations, ultrastructure, and chronotropic response to isoproterenol. Circ Res. 1982;50:101–116. doi: 10.1161/01.res.50.1.101. [DOI] [PubMed] [Google Scholar]
- 21.Koyanagi M, Brandes RP, Haendeler J, Zeiher AM, Dimmeler S. Cell-to-cell connection of endothelial progenitor cells with cardiac myocytes by nanotubes: a novel mechanism for cell fate changes? Circ Res. 2005;96:1039–1041. doi: 10.1161/01.RES.0000168650.23479.0c. [DOI] [PubMed] [Google Scholar]
- 22.Urbanek K, Torella D, Sheikh F, De Angelis A, Nurzynska D, Silvestri F, Beltrami CA, Bussani R, Beltrami AP, Quaini F, Bolli R, Leri A, Kajstura J, Anversa P. Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure. Proc Natl Acad Sci USA. 2005;102:8692–8697. doi: 10.1073/pnas.0500169102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mouquet F, Pfister O, Jain M, Oikonomopoulos A, Ngoy S, Summer R, Fine A, Liao R. Restoration of cardiac progenitor cells after myocardial infarction by self-proliferation and selective homing of bone marrow-derived stem cells. Circ Res. 2005;97:1090–1092. doi: 10.1161/01.RES.0000194330.66545.f5. [DOI] [PubMed] [Google Scholar]
- 24.Yoon J, Shim WJ, Ro YM, Lim DS. Transdifferentiation of mesenchymal stem cells into cardiomyocytes by direct cell-to-cell contact with neonatal cardiomyocyte but not adult cardiomyocytes. Ann Hematol. 2005;84:715–721. doi: 10.1007/s00277-005-1068-7. [DOI] [PubMed] [Google Scholar]
- 25.Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, Lin LZ, Cai CL, Lu MM, Reth M, Platoshyn O, Yuan JX, Evans S, Chien KR. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fazel S, Cimini M, Chen L, Li S, Angoulvant D, Fedak P, Verma S, Weisel RD, Keating A, Li RK. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J Clin Invest. 2006;116:1865–1877. doi: 10.1172/JCI27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meissner K, Heydrich B, Jedlitschky G, Meyer Zu Schwabedissen H, Mosyagin I, Dazert P, Eckel L, Vogelgesang S, Warzok RW, Bohm M, Lehmann C, Wendt M, Cascorbi I, Kroemer HK. The ATP-binding cassette transporter ABCG2 (BCRP), a marker for side population stem cells, is expressed in human heart. J Histochem Cytochem. 2006;54:215–221. doi: 10.1369/jhc.5A6750.2005. [DOI] [PubMed] [Google Scholar]
- 28.Yoon YS, Wecker A, Heyd L, Park JS, Tkebuchava T, Kusano K, Hanley A, Scadova H, Qin G, Cha DH, Johnson KL, Aikawa R, Asahara T, Losordo DW. Clonally expanded novel multipotent stem cells from human bone marrow regenerate myocardium after myocardial infarction. J Clin Invest. 2005;115:326–338. doi: 10.1172/JCI22326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The online-only Data Supplement can be found with this article at http://circ.ahajournals.org/cgi/content/full/CIRCULATIONAHA.107.761031/DC1.