Abstract
The sympathetic nervous system is a critical regulator of cardiac function (heart rate and contractility) in health and disease. Sympathetic nervous system agonists bind to adrenergic receptors that are known to activate protein kinase A, which phosphorylates target proteins and enhances cardiac performance. Recently, it has been proposed that protein kinase A–mediated phosphorylation of the cardiac ryanodine receptor (the Ca2+ release channel of the sarcoplasmic reticulum at a single residue, Ser2808) is a critical component of sympathetic nervous system regulation of cardiac function. This is a highly controversial hypothesis that has not been confirmed by several independent laboratories. The present study used a genetically modified mouse in which Ser2808 was replaced by alanine (S2808A) to prevent phosphorylation at this site. The effects of isoproterenol (a sympathetic agonist) on ventricular performance were compared in wild-type and S2808A hearts, both in vivo and in isolated hearts. Isoproterenol effects on L-type Ca2+ current (ICaL), sarcoplasmic reticulum Ca2+ release, and excitation–contraction coupling gain were also measured. Our results showed that isoproterenol caused significant increases in cardiac function, both in vivo and in isolated hearts, and there were no differences in these contractile effects in wild-type and S2808A hearts. Isoproterenol increased ICaL, the amplitude of the Ca2+ transient and excitation–contraction coupling gain, but, again, there were no significant differences between wild-type and S2808A myocytes. These results show that protein kinase A phosphorylation of ryanodine receptor Ser2808 does not have a major role in sympathetic nervous system regulation of normal cardiac function.
Keywords: ryanodine receptor, PKA phosphorylation, excitation-contraction coupling, β-adrenergic regulation
The sympathetic nervous system (SNS) is the primary regulator of cardiac contractility in the normal cardiovascular system.1,2 Regular daily activities that require activation of the SNS include actions as simple as assuming an upright posture to aerobic exercise.1,3 These reflex control systems are also involved in what has also been termed the “fight or flight” response. In all of these scenarios, activation of the SNS results in an increase in heart rate and cardiac contractile function to maintain blood pressure and/or increase cardiac output.
The bases of sympathetic-agonist induced increases in cardiac myocyte contractility have been thoroughly studied and are thought to be well understood.1 Sympathetic agonists, released from activated sympathetic nerves, bind to β-adrenergic receptors and ultimately lead to activation of protein kinase A, which phosphorylates target proteins.1 Phosphorylation of these molecules leads to an increased rate and magnitude of contraction and acceleration of relaxation.1,4 Protein kinase A (PKA) target proteins known to be involved in SNS-mediated increases in cardiac contractility include the L-type Ca2+ channel,5–8 phospholamban,9–11 and troponin.9,12,13
PKA-mediated phosphorylation of L-type Ca2+ channels increases their open probability, so that a greater influx of Ca2+ takes place during each action potential.5 This increase in Ca2+ influx provides a greater trigger for Ca2+ release from the sarcoplasmic reticulum (SR) by activation of the cardiac ryanodine receptor (RyR) and provides a source of Ca2+ to increase SR Ca2+ loading and to directly activate contractile proteins.1 Phosphorylation of phospholamban increases Ca2+ uptake by the SR, which speeds relaxation and increases SR Ca2+ loading, which, in turn, increases the magnitude of the Ca2+ transient and shortens its duration.14–16 Phosphorylation of troponin speeds relaxation.17 These finely orchestrated responses are essential because the SNS simultaneously increases both contractility and heart rate, and, therefore, the duration of systole must be shortened to maintain optimal diastolic filling of the heart. These 3 (L-type Ca2+ channel, phospholamban, and troponin) PKA-regulated molecules are thought to be primarily responsible for sympathetic regulation of cardiac contractile function.
Recently, Marks and colleagues have suggested that PKA-mediated regulation of cardiac contractility involves phosphorylation of RyR, which is proposed to increase RyR Ca2+-mediated open probability.18–20 This group has proposed that PKA phosphorylation of RyR at a single serine, Ser2808, is a major component of SNS regulation of cardiac contractility and that “hyperphosphorylation” of this serine in heart failure produces hyperactivity of the RyR at diastolic [Ca2+], resulting in diastolic SR Ca2+ leak and depressed contractility.18,21,22 This hypothesis is not universally accepted because a number of published studies from independent laboratories, including the laboratory that initially supported the hypothesis,23 have been unable to confirm many of its critical components.23–29 In addition, the idea that changing the Ca2+-mediated open probability of RyR can cause a steady-state increase in the contractility of the normal heart is not consistent with many published studies.30–32
A recent study by Benkusky et al (from a laboratory that is also involved in the present report) used a RyR-S2808A knock-in mouse, in which PKA cannot phosphorylate RyR at Ser2808, to critically test the RyR-Ser2808 phosphorylation hypothesis.26 The results of this study did not support the idea that Ser2808 is involved in the physiological regulation of cardiac contractility. In an associated editorial, Lehnart and Marks33 criticized the study by Benkusky et al26 because it relied on single cells to examine normal physiological regulation of contraction, rather than more intact systems, and because excitation– contraction coupling (ECC) “gain” (the relationship between Ca2+ influx and the magnitude of SR Ca2+ release) was not measured.
The purpose of the present research was to further address the controversial hypothesis that RyR-Ser2808 PKA phosphorylation is a critical regulator of cardiac function. In our study, inotropic responses to the sympathetic agonist isoproterenol (ISO) were compared in vivo in wild-type (WT) and RyR-S2808A mice. To eliminate potential confounding effects of ISO-induced increases in heart rate, effects of ISO were also compared in isolated, rate-controlled hearts from WT and RyR-S2808A animals. Finally, the effects of ISO on ECC gain were determined in myocytes isolated from these hearts.
ISO increased heart rate and cardiac contractile function in vivo and increased developed pressure in isolated hearts in every animal studied. There were no differences between WT and S2808A hearts. ISO also caused equivalent increases in the L-type Ca2+ current (ICaL), SR Ca2+ release, and ECC gain in WT and S2808A myocytes. These results strongly support the idea that PKA-mediated phosphorylation of RyR-Ser2808 has little or no role in normal SNS regulation of cardiac contractility.
Materials and Methods
Mice
WT and RyR-S2808A mice were used in these studies. The methods used to generate the RyR-S2808A mouse have been described previously.26 All animals received humane care in compliance with University standards and “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the “Guide for the Care and Use of Laboratory Animals” prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH publication 85-23, revised 1996). Animals were coded so that all experimenters were blinded to the type of mouse being studied.
In Vivo Studies
Cardiac contractile function was measured before and after ISO using the VisualSonics Velvo 770. Mice were anesthetized with 1% isoflurane during the echocardiographic measurements to maintain the heart rate between 350 and 400 bpm. Hearts were viewed in the short axis between the 2 papillary muscles and analyzed in M-mode. M-mode images were recorded before and after intraperitoneal injection of ISO (2 μg/g body weight) as described previously (Sigma, St Louis, Mo).34 Continuous measurements were made and images were analyzed offline with the Velvo software. Basal values and peak responses are reported. Parameters measured included: ejection fraction, heart rate, and fractional shortening.
Isolated Heart Studies
Mice were anesthetized with sodium pentobarbital and heparinized intravenously. Hearts were excised, trimmed of excess tissue, weighed, and rapidly immersed in cold (4°C) Ca2+-free Krebs–Henseleit buffer. Hearts were then placed on a Langendorff perfusion apparatus (ML785B2, ADInstruments, Colorado Springs, Colo), paced at 8.0 Hz, and perfused at a constant pressure of 80 mm Hg (STH pump controller ML175, ADInstruments) with a modified Krebs–Henseleit buffer solution containing (in mmol/L); 2.0 Ca2+Cl2, 130 NaCl, 5.4 KCl, 11 dextrose, 2 pyruvate, 0.5 MgCl2, 0.5 NaH2PO4, and 25 NaHCO3 and aerated with 95% oxygen and 5% carbon dioxide, pH 7.35 to 7.4. A balloon was inserted into the left ventricular (LV) cavity, and the balloon volume was adjusted to 10 mm Hg of LV end-diastolic pressure (LVEDP); no further alterations in balloon volume were made. All hearts were immersed in a water-jacketed organ chamber to maintain a temperature of 37°C. Preagonist baseline data were recorded following a 20-minute equilibration period. Subsequently, an ISO (10 nmol/L) infusion was initiated, and peak response was recorded. LV pressure (LVP), LVEDP, the maximum rate of positive and negative change in LV pressure (±LV dP/dt), and coronary flow rate were continuously recorded by means of a data acquisition system (Powerlab/8SP, ADInstruments). LV developed pressure was calculated by subtracting the LVEDP from the LV systolic pressure.
Western Blot Analysis
Calcium regulatory protein abundance and levels of phosphorylation were analyzed using Western blot analysis as reported previously.35 The following antibodies: SR Ca2+ ATPase (SERCa) (Sigma-Aldrich, St Louis, Mo), phospholamban (Upstate, Lake Placid NY), ryanodine receptor (ABR, Golden, Colo), α-sarcomeric actin (Sigma-Aldrich), glyceraldehyde phosphate dehydrogenase (GAPDH; Serotec, Raleigh, NC), Na+/Ca2+ exchanger (Swant, Switzerland), L-type Ca2+ channel α1C subunit (Chemicon, Temecula, Calif), and phosphorylation-specific RyR (serine 2808/2809) (Badrilla, Leeds, UK) were used for the analysis.
Electrophysiological Experiments
Ventricular myocytes were isolated from wild-type and RyR2-S2808A mice as previously described.26 L-type Ca2+ currents were recorded with the patch clamp technique in whole-cell configuration. A voltage ramp from −80 to −45 mV was used to inactivate Na+ channels, and cesium was present in both the internal and external solution to prevent current through K+ channels. Test pulses (200 ms) were applied in 10-mV increments from −40 to +50 mV, followed by a repolarizing pulse to −80 mV. Series resistance was electronically compensated, and the pClamp software (Axon Instruments) was used in data acquisition and analysis. The patch pipette contained 50 μmol/L Fluo-3 pentapotassium salt to simultaneously record the associated [Ca2+]i transient. External and internal solutions were as previously described.36 [Ca2+]i transients were recorded with a Zeiss LSM510 META confocal microscope in line-scan mode (1.5 ms/line) and a ×60 water immersion objective, numeric aperture 1.2. The 488-nm line of the argon laser was used to excite Fluo-3, and fluorescence emission was collected at >505 nm. The scanning line was placed parallel to the longitudinal cell axis to simultaneously measure cell shortening. Images were analyzed with a custom-made program running in IDL (Research Systems Inc). These experiments were performed at 25°C.
Statistical Analysis
Data are presented as means±SEM. Two-sample comparisons were performed by using the paired t test, and P<0.05 was used as a measure of statistical significance. Electrophysiological data were compared with ANOVA for repeated measures followed by 1-way ANOVA and Tukey post hoc at each voltage tested. Significance was set at an α level of P<0.05.
Results
Animal Characteristics
As noted in previous reports,26,37 the RyR-S2808A mice had no obvious basal phenotype. The heart and body weights of the WT and RyR-S2808A mice used in this study were not significantly different (Table 1).
Table 1.
Animal Characteristics
| Wild Type (n=10) | RYR-S2808A (n=11) | |
|---|---|---|
| BW (g) | 23.5±1.2 | 24.4±1.7 |
| HW (mg) | 165.7±11.1 | 168.5±11.5 |
| HW/BW (mg/g) | 7.20±0.29 | 6.97±0.28 |
Data are presented as means±SEM. BW indicates body weight; HW, heart weight.
Effects of ISO In Vivo
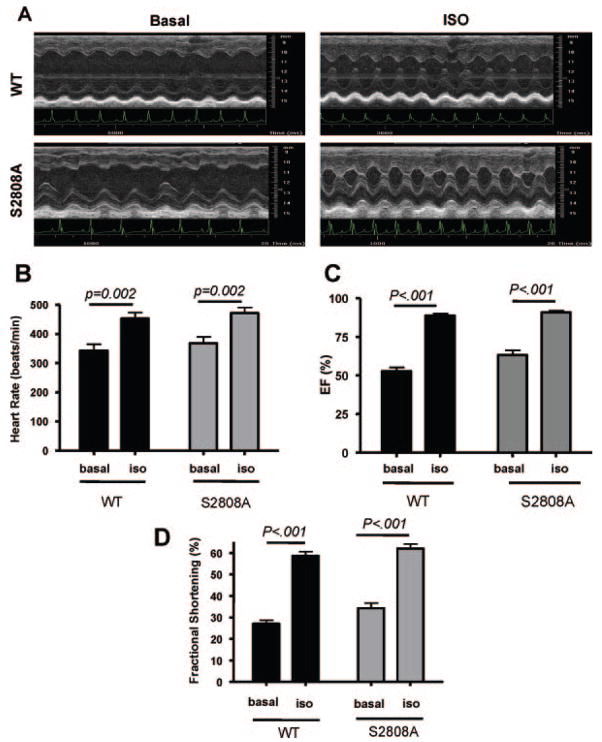
Baseline cardiac function was similar in WT and RyR-S2808A mice (Figure 1). ISO caused the heart rate and cardiac contractility to increase in every animal. Representative effects of ISO on cardiac function in WT and RyR-S2808A mice are shown in Figure 1A, and average data are shown in Figure 1B through 1D. There were no significant differences in the effects of ISO on heart rate, fractional shortening or ejection fraction in WT versus RyR-S2808A mice. These studies show that eliminating RyR phosphorylation at Ser2808 does not alter ISO effects on heart rate and cardiac contractile performance in vivo.
Figure 1.
Effects of ISO on cardiac function in vivo. A, Representative echocardiographic measurements in WT and S2808A animals before and after ISO. B, ISO caused significant increases in heart rate in both WT and S2808A mice. There were no differences in the response between the 2 groups. C, ISO caused significant increases in ejection fraction (EF) in WT and S2808A mice. There were no differences in ISO effects between groups. D, ISO caused significant increases in fractional shortening in WT and S2808A mice, with no differences between the response in the 2 groups.
Isolated Heart Studies
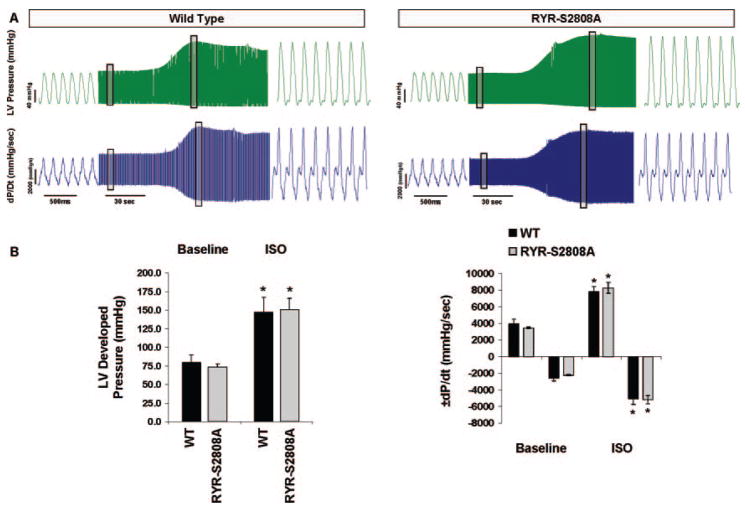
In the in vivo studies, ISO caused an increase in heart rate, and this could have potentially obscured differences in PKA-mediated inotropic responses between WT and RyR-S2808A animals because of heart rate dependent increases in Ca2+-calmodulin kinase II activity.38 Therefore, an additional series of in vitro studies was performed using isolated hearts, in which heart rate was controlled. Under these conditions, baseline isovolumic cardiac performance was identical in WT and RyR-S2808A isolated hearts (Table 2, Figure 2). ISO increased the rate and magnitude of pressure generation and the rate of pressure decay in all animals and there were no differences between WT and RyR-S2808A hearts (Table 2). Representative baseline pressure recordings and ISO responses are shown in Figure 2A, and average data are shown in Figure 2B. These results are consistent with our in vivo findings and do not support a major role for RyR-Ser2808 phosphorylation in the regulation of normal cardiac function.
Table 2.
LV Performance
| Wild Type (n=5) |
RYR-S2808A (n=6) |
Percentage Change |
||||
|---|---|---|---|---|---|---|
| Baseline | ISO | Baseline | ISO | WT | RYR-2808A | |
| LV Dev P (mm Hg) | 79±10 | 147±20* | 74±4 | 151±16* | 87±12 | 102±15 |
| LVEDP (mm Hg) | 9±0.7 | 10.5±0.9 | 10±0.5 | 10±0.5 | ||
| +dP/dt (mm Hg/sec) | 3917±584 | 7799±647* | 3444±87 | 8259±664* | 104±21 | 138±14 |
| −dP/dt (mm Hg/sec) | −2569±385 | −5052±691* | −2225±87 | −5180±499* | 102±19 | 132±20 |
| Perfusion pressure (mm Hg) | 80±0.4 | 80±0.4 | 80±3 | 80±4 | ||
| Heart rate (bpm) | 465±14 | 460±13 | 466±12 | 469±12 | ||
Data are presented as means±SEM. Concentration of ISO was 10 nmol/L. LV Dev P indicates LV developed pressure; LVEDP, LV end-diastolic pressure.
P<0.01 vs group baseline.
Figure 2.
Effects of ISO on cardiac function in isovolumic Langendorff perfused hearts. A, Representative LV pressure (mm Hg) and ±dP/dt (mm Hg/sec) tracings acquired during both steady-state baseline (end-diastolic pressure, 10 mm Hg; 8.0Hz) and ISO (10 nmol/L) infusions. Boxed areas are shown at an expanded time scale. B, Group averages. No differences in baseline performance or peak ISO responses were observed between WT (n=5) and RyR-S2808A (n=6) mice. Data are presented as means±SEM. *P<0.01 vs respective group baseline.
Western Blot Analysis
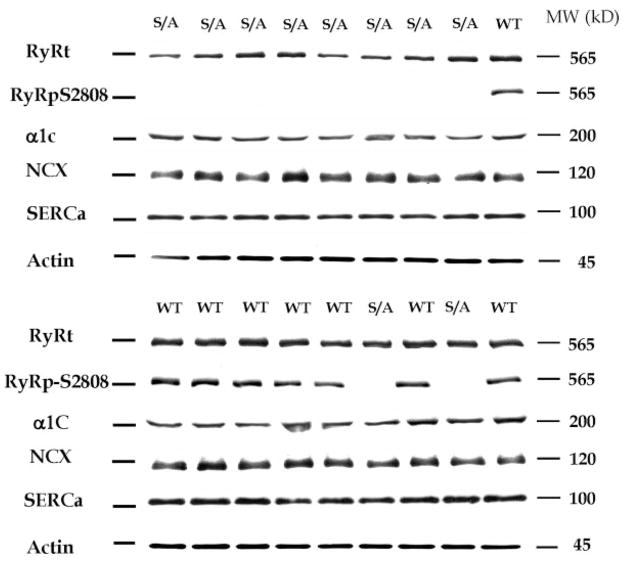
If PKA-mediated RyR-S2808 phosphorylation is of fundamental importance to the physiological regulation of contractility in the normal mouse, then eliminating this critical factor may be expected to cause compensatory changes in other Ca2+ regulatory processes. Otherwise, a significant basal phenotype would be expected. These compensations may underlie our inability to observe any differences in ISO effects in WT versus RyR-S2808A mice. Representative Western blots for Ca2+ regulatory proteins (L-type Ca2+ channel α1C subunit, Na+/Ca2+ exchanger, and SERCa) are shown in Figure 3. No differences in the abundance of any of the Ca2+ regulatory proteins were observed. An antibody against phosphorylated RyR-Ser2808 did not recognize the RyR-S2808A samples but yielded robust signals against WT samples, documenting the RyR-S2808A genotype, the phosphorylation of S2808 in WT mice under our conditions, and the lack of ISO-induced RyR phosphorylation at 2808 in the RyR-S2808A mice. These results do not support the idea that there are adaptive changes in Ca2+ regulatory protein abundance to compensate for the putative critical role of PKA-mediated phosphorylation of RyR-Ser2808 role in the regulation of normal cardiac performance.
Figure 3.
Western blot analysis of Ca2+ regulatory proteins. Two representative Western blots are shown. S2808A animals were identified by the failure of binding of an antibody (RyRpS2808) that detects phosphorylation at S2808. The following were measured: Na+/Ca2+ exchanger (NCX); the α subunit of the L-type Ca2+ channel (α1C); SERCa; and RyR (total) (RyRt). There were no significant differences in Na+/Ca2+ exchanger, α1C, and SERCa abundance (normalized to either GAPDH or cardiac actin) between samples from WT and S2808A (labeled WT and S/A, respectively) hearts.
ICaL, SR Ca2+ Release, and ECC Gain
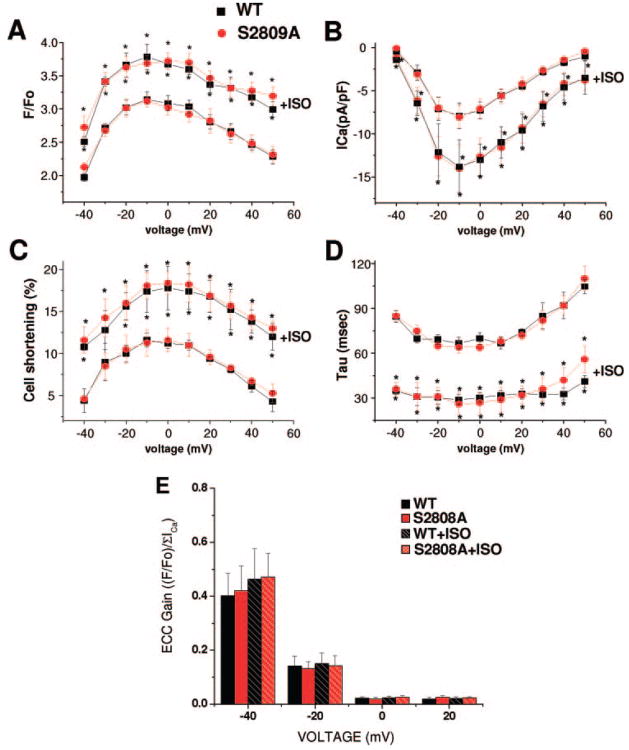
The unaltered density of key Ca2+ regulatory proteins in the RyR-S2808A mice does not rule out the possibility that there may be a compensatory change in the activity of these proteins. We thus measured functional parameters of ECC in WT and RyR-S2808A ventricular myocytes. The density and voltage dependence of activation of the ICaL was not different in WT versus RyR-S2808A myocytes under basal conditions (Figure 4A). ISO increased ICaL to the same extent in WT and RyR-S2808A mice. The amplitude of Ca2+ influx–induced cytosolic Ca2+ transients and contractions, which involve the activation of RyRs, were not different in WT and RyR-S2808A myocytes under basal conditions. ISO increased the amplitude of the Ca2+ transient and contractions at all voltages tested, and there were no differences in these effects in WT versus RyR-S2808A mice (Figure 4C). ECC gain was calculated at each voltage step and was identical in WT and RyR-S2808A mice under basal conditions (Figure 4C). ISO increased ECC gain, especially at negative test potentials, but there changes were identical in WT and RyR-S2808A mice. These results do not support a central role for PKA-mediated phosphorylation of RyR-Ser2808 in adrenergically mediated regulation of ECC.
Figure 4.
Effects of ISO on Ca2+ current, contraction, Ca2+ transients, and ECC gain. A, Peak systolic [Ca2+]i (F/F0) plotted against test voltage in WT (n=9) and knock-in (S2808A) (n=11) myocytes. The amplitude of the Ca2+ transient before and after ISO treatment are shown. B, ICaL density plotted against voltage in WT (n=9) and S2808A (n=11) myocytes before and after ISO treatment. C, Voltage dependence of cell shortening in WT (n=7) and S2808A (n=6) and the ISO effect on cell shortening. D, Time constant of [Ca2+]i decay obtained by fitting of the decay fluorescence trace to a single exponential function plotted vs test voltage before and after ISO treatment. E, ECC gain calculated for each voltage dividing the F/F0 by the integral of ICaL (pC/pF). Black bars represent WT cells; red bars, RyR-S2808A cells before and after ISO treatment. Similar results were obtained when peak ICaL was used in the ECC gain calculation. *P≤0.05 before ISO addition within group.
Discussion
The present study examined the idea that PKA-mediated phosphorylation of RyR at serine 2808 is an important component of SNS regulation of cardiac contractility, as proposed previously.37 Marks and colleagues have published a series of reports18,20,37 that provide very strong support for the hypothesis that PKA phosphorylates RyR at (and only at) Ser2808, that this causes the RyR-associated protein FKBP12.6 (also called Calstabin) to dissociate from RyR, and that these events increase Ca2+-activated opening of RyR. However, this hypothesis is not universally accepted because several independent groups have not been able to confirm all of its key elements.24–29 There is significant controversy as to whether or not Ser2808 is the only PKA phosphorylation site on RyR,26,29 whether or not FKBP12.6 dissociates from RyR after PKA-mediated phosphorylation,18,20,23,28 and whether PKA-mediated RyR phosphorylation at Ser2808 has any significant effect on RyR single channel properties26,27 or spontaneous SR Ca2+ release (Ca2+ sparks).24,26,39,40
The present study was designed to address critical aspects of this controversy. Specifically, we focused on certain issues raised by Marks and colleagues in a recent editorial33 that pointed out what they felt were the shortcomings of previous work.26 We performed experiments that we felt were essential for resolving whether or not RyR-Ser2808 phosphorylation is centrally involved in SNS regulation of cardiac contractile performance in the normal heart. Our studies were performed with the RyR-S2808A knock-in mouse developed by Valdivia and colleagues26 (collaborators in the current project), which cannot be PKA-phosphorylated at Ser2808 and, therefore, is a critical reagent to validate the central tenets of the RyR-Ser2808 phosphorylation hypothesis.
Our initial experiments were performed within the context of the intact cardiovascular system, and we were unable to detect any appreciable differences in ISO responsiveness between WT and RyR-S2808A mice. The potential shortcoming of these experiments is that the ISO-induced increase in heart rate may activate Ca2+-regulated signaling pathways (particularly those involving Ca2+/calmodulin kinase II)38 that could have increased cardiac contractility and obscured our ability to see reduced PKA-mediated inotropic effects in the RyR-S2808A mice. Therefore, we went on to define the effects of ISO in isolated, perfused, rate-controlled isovolumic hearts. Again, we were unable to see any differences in ISO responsiveness between WT and RyR-S2808A hearts under our conditions. Finally, we isolated ventricular myocytes from these hearts and measured ICaL and Ca2+-induced SR Ca2+ release. ISO increased the ICaL, increased SR Ca2+ release, and changed the gain of ECC to identical extents in WT and RyR-S2808A myocytes. Collectively, these results do not support the hypothesis that PKA-mediated phosphorylation of RyR-Ser2808 plays a central role in the regulation of cardiac contractility by the SNS. Our results strongly support the already well-established idea that PKA mediated increases in the ICaL and increases in SR Ca2+ uptake, storage and release are the primary mechanisms by which the SNS increases the rate and force of cardiac contraction.1
It is not clear why there is so much controversy in this area and why we were unable to see any alteration in adrenergic regulation of cardiac function in the RyR-S2808A mice. There are at least 2 possible explanations. The simplest explanation is that PKA-mediated phosphorylation of RyR-Ser2808 makes no significant contribution to the changes in Ca2+ handling and cardiac contractility that are hallmark features of adrenergic control of cardiac function. The fact that the RyR-S2808A mouse has no basal phenotype is certainly consistent with the lack of an important role for phosphorylation of this site in the normal regulation of cardiac function. It seems implausible that this mouse would exhibit no phenotype if it were unable to mount a normal SNS response. The fact that we did not observe any changes in other Ca2+ regulatory proteins in the RyR-S2808A mice does not support the idea that these mice somehow adapted to the loss of a major cardiac regulatory mechanism. Instead, these data, like all other components of our results, are consistent with the idea that PKA-mediated phosphorylation of RyR-Ser2808 is immaterial to adrenergic regulation of the heart.
A second general possibility is that RyR-Ser2808 phosphorylation increases RyR [Ca2+]-dependent opening, but this effect has no consequence on ISO-induced increases in cardiac function and myocyte Ca2+ regulation. There is support for this idea in the literature.31,32 Eisner and colleagues have clearly shown that in rodents, increasing RyR Ca2+ sensitivity has no significant effect on steady state Ca2+ transients.32 This group has shown that increasing RyR Ca2+ sensitivity only causes a transient increase in SR Ca2+ release because the initial effect to increase the amplitude of the Ca2+ transient causes increased Ca2+ efflux via the Na+/Ca2+ exchanger and reduces Ca2+ influx via the ICaL. Over time, these 2 effects will reduce the amplitude of the Ca2+ transient to normal levels, to reestablish Ca2+ flux balance. Modeling (systems biology approaches) studies from the laboratory of Bers30 also do not support a major role for changes in RyR Ca2+ sensitivity in the regulation of cardiac contractility. In our studies we observed very little effect of ISO on ECC gain, and the small effects we observed were similar in WT and RyR-S2808A myocytes. Collectively these studies support the idea that RyR-Ser2808 phosphorylation has no significant effect on cardiac contractility. The small ISO-induced increases in ECC gain that we observed at negative test potentials are well explained by the fact that ISO increases the open probability of ICaL.5
The present results are consistent with a number of studies from independent laboratories that have found that PKA-mediated phosphorylation of RyR has no significant effect on RyR gating (bilayer studies),25,27 or on spontaneous Ca2+ sparks.24,26,39,40 Collectively these results are most consistent with the idea that PKA-mediated phosphorylation of RyR-Ser2808 has no major effect on RyR biophysical properties.27
The normal Ca2+ transient is the summation of local, regenerative Ca2+ release from the junctional SR.41 For RyR-Ser2808 phosphorylation to induce an increase in SR Ca2+ release, it should produce an increase in the number of SR junctional sites that release their Ca2+. For such a system to be operative, there would need to be portions of the junctional SR that do not release their Ca2+ during the normal heartbeat. This general idea is not supported by previous studies.41,42 Bridge and colleagues have shown that almost every portion of the junctional SR releases its stored Ca2+ with each heart beat.42 These results are at the core of our current understanding of ECC in the heart and show that there is a significant safety factor (ensuring that release takes place) at each junctional couplon (region where the L-type Ca2+ channel is in close proximity to RyR within the junctional SR). The junctional couplon is organized such that there is sufficient Ca2+ influx via L-type Ca2+ channels during every action potential to induce locally regenerative opening of neighboring RyR Ca2+ release channels.41 In our view, this large safety factor keeps ECC from being on the brink of failure under normal physiological conditions. The studies by Bridge and colleagues show that although experimental grading of the amplitude of the ICaL (in voltage clamp studies; also see Figure 4) can change the size of the Ca2+ transient (Ca2+-induced Ca2+ release) by recruiting additional junctional release sites; this is not how the amplitude of the Ca2+ transient is regulated under normal physiological conditions because every site releases its stored Ca2+ with each action potential. Within the context of this established scenario, there is little role for an increase in RyR Ca2+ sensitivity as a regulator of the amplitude of the systolic [Ca2+] transient.
Our experiments were all performed in normal animals without induction of cardiac dysfunction. Therefore, we cannot contribute to the debate concerning the putative role of hyperphosphorylation of RyR-Ser2808 to the diastolic leak of SR Ca2+ that appears to reduce SR Ca2+ loading in congestive heart failure.18 We are preparing to address this question by comparing the response of WT and RyR-S2808A mice to myocardial infarction37 rather than pressure overload, as we have done previously.26
In summary, our data strongly support the idea that PKA-mediated phosphorylation of RyR-Ser2808 has no important role in SNS regulation of cardiac Ca2+ regulation and contractility. Why our data, and those of many other groups,24–27,29 including the group that initially was supportive of this hypothesis,23,28 is so fundamentally different from the data supporting this hypothesis remains a mystery. We will make the RyR-S2808A mice available to other groups, so that any remaining issues can be resolved.
Acknowledgments
Sources of Funding
This study was supported by NIH grants HL089312, HL33921, and HL61495 (to S.R.H.); NIH grants HL55438 and HL76826 (to H.V.); and American Heart Association Postdoctoral Fellowship Grant 0625509U (Pennsylvania/Delaware Affiliate) (to S.M.M.).
Footnotes
Disclosures
None.
References
- 1.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 2.Castellano M, Bohm M. The cardiac beta-adrenoceptor-mediated signaling pathway and its alterations in hypertensive heart disease. Hypertension. 1997;29:715–722. doi: 10.1161/01.hyp.29.3.715. [DOI] [PubMed] [Google Scholar]
- 3.Young MA, Hintze TH, Vatner SF. Correlation between cardiac performance and plasma catecholamine levels in conscious dogs. Am J Physiol. 1985;248:H82–H88. doi: 10.1152/ajpheart.1985.248.1.H82. [DOI] [PubMed] [Google Scholar]
- 4.Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2001. [Google Scholar]
- 5.Tsien RW, Bean BP, Hess P, Lansman JB, Nilius B, Nowycky MC. Mechanisms of calcium channel modulation by beta-adrenergic agents and dihydropyridine calcium agonists. J Mol Cell Cardiol. 1986;18:691–710. doi: 10.1016/s0022-2828(86)80941-5. [DOI] [PubMed] [Google Scholar]
- 6.Hartzell HC. Regulation of cardiac ion channels by catecholamines, acetylcholine and second messenger systems. Prog Biophys Mol Biol. 1988;52:165–247. doi: 10.1016/0079-6107(88)90014-4. [DOI] [PubMed] [Google Scholar]
- 7.McDonald TF, Pelzer S, Trautwein W, Pelzer DJ. Regulation and modulation of calcium channels in cardiac, skeletal, and smooth muscle cells. Physiol Rev. 1994;74:365–507. doi: 10.1152/physrev.1994.74.2.365. [DOI] [PubMed] [Google Scholar]
- 8.Gomez AM, Valdivia HH, Cheng H, Lederer MR, Santana LF, Cannell MB, McCune SA, Altschuld RA, Lederer WJ. Defective excitation-contraction coupling in experimental cardiac hypertrophy and heart failure. Science. 1997;276:800–806. doi: 10.1126/science.276.5313.800. [DOI] [PubMed] [Google Scholar]
- 9.Kranias EG, Solaro RJ. Phosphorylation of troponin I and phospholamban during catecholamine stimulation of rabbit heart. Nature. 1982;298:182–184. doi: 10.1038/298182a0. [DOI] [PubMed] [Google Scholar]
- 10.Li L, Desantiago J, Chu G, Kranias EG, Bers DM. Phosphorylation of phospholamban and troponin I in beta-adrenergic-induced acceleration of cardiac relaxation. Am J Physiol Heart Circ Physiol. 2000;278:H769–H779. doi: 10.1152/ajpheart.2000.278.3.H769. [DOI] [PubMed] [Google Scholar]
- 11.Kiss E, Edes I, Sato Y, Luo W, Liggett SB, Kranias EG. beta-Adrenergic regulation of cAMP and protein phosphorylation in phospholamban-knockout mouse hearts. Am J Physiol. 1997;272:H785–H790. doi: 10.1152/ajpheart.1997.272.2.H785. [DOI] [PubMed] [Google Scholar]
- 12.Zhang R, Zhao J, Mandveno A, Potter JD. Cardiac troponin I phosphorylation increases the rate of cardiac muscle relaxation. Circ Res. 1995;76:1028–1035. doi: 10.1161/01.res.76.6.1028. [DOI] [PubMed] [Google Scholar]
- 13.Zhang R, Zhao J, Potter JD. Phosphorylation of both serine residues in cardiac troponin I is required to decrease the Ca2+ affinity of cardiac troponin C. J Biol Chem. 1995;270:30773–30780. doi: 10.1074/jbc.270.51.30773. [DOI] [PubMed] [Google Scholar]
- 14.MacLennan DH, Kranias EG. Phospholamban: a crucial regulator of cardiac contractility. Nat Rev. 2003;4:566–577. doi: 10.1038/nrm1151. [DOI] [PubMed] [Google Scholar]
- 15.Lorenz JN, Kranias EG. Regulatory effects of phospholamban on cardiac function in intact mice. Am J Physiol. 1997;273:H2826–2831. doi: 10.1152/ajpheart.1997.273.6.H2826. [DOI] [PubMed] [Google Scholar]
- 16.Frank K, Kranias EG. Phospholamban and cardiac contractility. Ann Med. 2000;32:572–578. doi: 10.3109/07853890008998837. [DOI] [PubMed] [Google Scholar]
- 17.Layland J, Solaro RJ, Shah AM. Regulation of cardiac contractile function by troponin I phosphorylation. Cardiovasc Res. 2005;66:12–21. doi: 10.1016/j.cardiores.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 18.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 19.Wehrens XH, Lehnart SE, Huang F, Vest JA, Reiken SR, Mohler PJ, Sun J, Guatimosim S, Song LS, Rosemblit N, D’Armiento JM, Napolitano C, Memmi M, Priori SG, Lederer WJ, Marks AR. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell. 2003;113:829–840. doi: 10.1016/s0092-8674(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 20.Wehrens XH, Marks AR. Altered function and regulation of cardiac ryanodine receptors in cardiac disease. Trends Biochem Sci. 2003;28:671–678. doi: 10.1016/j.tibs.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Yano M, Kobayashi S, Kohno M, Doi M, Tokuhisa T, Okuda S, Suetsugu M, Hisaoka T, Obayashi M, Ohkusa T, Matsuzaki M. FKBP12.6-mediated stabilization of calcium-release channel (ryanodine receptor) as a novel therapeutic strategy against heart failure. Circulation. 2003;107:477–484. doi: 10.1161/01.cir.0000044917.74408.be. [DOI] [PubMed] [Google Scholar]
- 22.Yano M, Ono K, Ohkusa T, Suetsugu M, Kohno M, Hisaoka T, Kobayashi S, Hisamatsu Y, Yamamoto T, Kohno M, Noguchi N, Takasawa S, Okamoto H, Matsuzaki M. Altered stoichiometry of FKBP12.6 versus ryanodine receptor as a cause of abnormal Ca(2+) leak through ryanodine receptor in heart failure. Circulation. 2000;102:2131–2136. doi: 10.1161/01.cir.102.17.2131. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto T, Yano M, Xu X, Uchinoumi H, Tateishi H, Mochizuki M, Oda T, Kobayashi S, Ikemoto N, Matsuzaki M. Identification of target domains of the cardiac ryanodine receptor to correct channel disorder in failing hearts. Circulation. 2008;117:762–772. doi: 10.1161/CIRCULATIONAHA.107.718957. [DOI] [PubMed] [Google Scholar]
- 24.Jiang MT, Lokuta AJ, Farrell EF, Wolff MR, Haworth RA, Valdivia HH. Abnormal Ca2+ release, but normal ryanodine receptors, in canine and human heart failure. Circ Res. 2002;91:1015–1022. doi: 10.1161/01.res.0000043663.08689.05. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Kranias EG, Mignery GA, Bers DM. Protein kinase A phosphorylation of the ryanodine receptor does not affect calcium sparks in mouse ventricular myocytes. Circ Res. 2002;90:309–316. doi: 10.1161/hh0302.105660. [DOI] [PubMed] [Google Scholar]
- 26.Benkusky NA, Weber CS, Scherman JA, Farrell EF, Hacker TA, John MC, Powers PA, Valdivia HH. Intact beta-adrenergic response and unmodified progression toward heart failure in mice with genetic ablation of a major protein kinase A phosphorylation site in the cardiac ryanodine receptor. Circ Res. 2007;101:819–829. doi: 10.1161/CIRCRESAHA.107.153007. [DOI] [PubMed] [Google Scholar]
- 27.Stange M, Xu L, Balshaw D, Yamaguchi N, Meissner G. Characterization of recombinant skeletal muscle (Ser-2843) and cardiac muscle (Ser-2809) ryanodine receptor phosphorylation mutants. J Biol Chem. 2003;278:51693–51702. doi: 10.1074/jbc.M310406200. [DOI] [PubMed] [Google Scholar]
- 28.Hunt DJ, Jones PP, Wang R, Chen W, Bolstad J, Chen K, Shimoni Y, Chen SR. K201 (JTV519) suppresses spontaneous Ca2+ release and [3H]ryanodine binding to RyR2 irrespective of FKBP12.6 association. Biochem J. 2007;404:431–438. doi: 10.1042/BJ20070135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao B, Jiang MT, Zhao M, Yang D, Sutherland C, Lai FA, Walsh MP, Warltier DC, Cheng H, Chen SR. Characterization of a novel PKA phosphorylation site, serine-2030, reveals no PKA hyperphosphorylation of the cardiac ryanodine receptor in canine heart failure. Circ Res. 2005;96:847–855. doi: 10.1161/01.RES.0000163276.26083.e8. [DOI] [PubMed] [Google Scholar]
- 30.Puglisi JL, Wang F, Bers DM. Modeling the isolated cardiac myocyte. Prog Biophys Mol Biol. 2004;85:163–178. doi: 10.1016/j.pbiomolbio.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Eisner DA, Trafford AW. No role for the ryanodine receptor in regulating cardiac contraction? News Physiol Sci. 2000;15:275–279. doi: 10.1152/physiologyonline.2000.15.5.275. [DOI] [PubMed] [Google Scholar]
- 32.Venetucci LA, Trafford AW, Eisner DA. Increasing ryanodine receptor open probability alone does not produce arrhythmogenic calcium waves: threshold sarcoplasmic reticulum calcium content is required. Circ Res. 2007;100:105–111. doi: 10.1161/01.RES.0000252828.17939.00. [DOI] [PubMed] [Google Scholar]
- 33.Lehnart S, Marks AR. Regulation of ryanodine receptors in the heart. Circ Res. 2007;101:746–749. doi: 10.1161/CIRCRESAHA.107.162479. [DOI] [PubMed] [Google Scholar]
- 34.Hoit BD, Khoury SF, Kranias EG, Ball N, Walsh RA. In vivo echocardiographic detection of enhanced left ventricular function in gene-targeted mice with phospholamban deficiency. Circ Res. 1995;77:632–637. doi: 10.1161/01.res.77.3.632. [DOI] [PubMed] [Google Scholar]
- 35.Kubo H, Margulies KB, Piacentino V, III, Gaughan JP, Houser SR. Patients with end-stage congestive heart failure treated with beta-adrenergic receptor antagonists have improved ventricular myocyte calcium regulatory protein abundance. Circulation. 2001;104:1012–1018. doi: 10.1161/hc3401.095073. [DOI] [PubMed] [Google Scholar]
- 36.Farrell EF, Antaramian A, Rueda A, Gomez AM, Valdivia HH. Sorcin inhibits calcium release and modulates excitation-contraction coupling in the heart. J Biol Chem. 2003;278:34660–34666. doi: 10.1074/jbc.M305931200. [DOI] [PubMed] [Google Scholar]
- 37.Wehrens XH, Lehnart SE, Reiken S, Vest JA, Wronska A, Marks AR. Ryanodine receptor/calcium release channel PKA phosphorylation: a critical mediator of heart failure progression. Proc Natl Acad Sci U S A. 2006;103:511–518. doi: 10.1073/pnas.0510113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grueter CE, Colbran RJ, Anderson ME. CaMKII, an emerging molecular driver for calcium homeostasis, arrhythmias, and cardiac dysfunction. J Mol Med. 2007;85:5–14. doi: 10.1007/s00109-006-0125-6. [DOI] [PubMed] [Google Scholar]
- 39.Ginsburg KS, Bers DM. Modulation of excitation-contraction coupling by isoproterenol in cardiomyocytes with controlled SR Ca2+ load and Ca2+ current trigger. J Physiol. 2004;556:463–480. doi: 10.1113/jphysiol.2003.055384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valdivia HH, Kaplan JH, Ellis-Davies GC, Lederer WJ. Rapid adaptation of cardiac ryanodine receptors: modulation by Mg2+ and phosphorylation. Science. 1995;267:1997–2000. doi: 10.1126/science.7701323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stern MD, Cheng H. Putting out the fire: what terminates calcium-induced calcium release in cardiac muscle? Cell Calcium. 2004;35:591–601. doi: 10.1016/j.ceca.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 42.Inoue M, Bridge JH. Ca2+ sparks in rabbit ventricular myocytes evoked by action potentials: involvement of clusters of L-type Ca2+ channels. Circ Res. 2003;92:532–538. doi: 10.1161/01.RES.0000064175.70693.EC. [DOI] [PubMed] [Google Scholar]