Abstract
A diet high in fat induces cardiac hypertrophy, inflammation, and oxidative stress. Although such actions have largely been ascribed to fat deposition, the accumulation of advanced glycation end products (AGEs) and subsequent activation of the receptor for AGEs (RAGE) may also represent important mediators of cardiac injury following exposure to a Western diet. In this study, male C57BL6J and RAGE knockout mice were placed on either a standard diet (7% fat) or a Western “fast-food” diet (21% fat). Animals receiving a high-fat diet were further randomized to receive the AGE inhibitor alagebrium chloride (1 mg·kg−1·day−1) and followed for 16 wk. A Western diet was associated with cardiac hypertrophy, inflammation, mitochondrial-dependent superoxide production, and cardiac AGE accumulation in wild-type mice. Although RAGE-KO mice fed a Western diet also became obese and accumulated intramyocardial lipid, cardiomyocyte hypertrophy, inflammation, and oxidative stress were attenuated compared with wild-type mice. Similarly, mice of both strains receiving alagebrium chloride had reduced levels of inflammation and oxidative stress, in association with a reduction in cardiac AGEs and RAGE. This study suggests that AGEs represent important mediators of cardiac injury associated with a Western fast-food diet. These data point to the potential utility of AGE-reducing strategies in the prevention and management of cardiac disease.
Keywords: advanced glycation end products, receptor for advanced glycation end products, inflammation, cardiac disease
the classical western diet, which is high in fat and processing, has a number of adverse effects on cardiovascular physiology. Most research has focused on atherogenesis and vascular stiffness. However, a range of direct cardiac effects may also be observed in the absence of large vessel changes and prior to the onset of diabetes, including inflammation, hypertrophy (2), fibrosis (2), and contractile dysfunction (21, 32). The mechanisms underlying these cardiac changes are many and varied and include lipotoxicity (8, 21), oxidative stress, and mitochondrial dysfunction (4). We hypothesized that the accumulation of advanced glycation end products (AGEs) and subsequent activation of the receptor for AGEs (RAGE) may also represent important mediators of cardiac injury associated with a high-fat diet.
A number of pathways may contribute to the accumulation of AGEs following ingestion of a high-fat diet. Some AGEs may be formed endogenously due to the induction of dyslipidemia, dysglycemia, and oxidative (carbonyl) stress (33). However, a significant quantity of AGEs may also be obtained directly from the diet (29). In particular, diets rich in fat often have high levels of AGEs due to chemical interactions between oxidized lipids and protein during high-temperature processing (29). Previous studies have shown that increasing the content of AGEs in the diet can increase circulating levels of AGEs. It is also known that circulating levels of AGEs are correlated with cardiac dysfunction (3). Furthermore, inhibition of cardiac AGE accumulation, via a range of different strategies, prevents diabetes- and age-associated increases in myocardial stiffness (6, 7, 9, 16, 19).
Some of the pathogenic effects of AGEs appear to be mediated by interactions with AGE receptors (33), of which the receptor for AGEs (RAGE) is the best characterized (13). The expression of RAGE is upregulated in diabetes, aging, and other conditions associated with elevated AGE levels, where it is strongly associated with impaired cardiac function (26). Activation of RAGE is known to influence myocardial calcium homeostasis (22) and contribute to interstitial fibrogenesis in the diabetic heart (6). RAGE is also involved in the activation of inflammatory cascades, including the production of cytokines and chemokines (13). The present study demonstrates the pivotal role of the AGE-RAGE axis in cardiac disease associated with a high-fat diet and the cardioprotective actions of the AGE-reducing agent alagebrium chloride.
MATERIALS AND METHODS
Animals.
RAGE knockout (KO) mice were generated on a C57Bl6 background as previously described (15). Briefly, essential elements encoding the extracellular domain of RAGE (exons 2-7) were flanked by two loxP sites in the same orientation. Mice heterozygous for Cre recombination were bred to homozygosity to generate the RAGE−/− mouse line. To confirm the abrogation of RAGE expression, different primers covering the entire RAGE molecule were used and detected no transcripts (data not shown).
Adult (8-wk-old) male C57Bl6J mice (Precinct Animal Centre, Melbourne, Australia) and RAGE KO mice were pair fed with either a standard diet (SD, AIN-93G; Specialty feeds, Perth Australia; n = 10/group) or a high-fat (HF) diet formulated to mimic a “Western fast-food diet”, (SF05-031; Specialty Feeds; n = 10/group). Both diets contained the same vitamin and total calorie content; however, 21% of total energy in the SF05-031 diet was derived from fat (vs. 7% in AIN-93G), based on the addition of clarified butter (ghee, 210 g/kg). The SF05-031 diet also contained higher levels of AGEs, including carboxymethyllysine (65 nmol CML·mol lysine−1·100 mg chow−1) compared with standard chow (21 nmol CML·mol lysine−1·100 mg chow−1). Animals receiving a HF diet were further randomized to receive the AGE inhibitor, alagebrium chloride (Synvista, NJ) at a dose of 1 mg·kg−1·day−1 delivered by oral gavage. All groups were followed for 16 wk. All animal protocols were approved by the Institutional Animal Care and Use Committee and followed US National Institutes of Health guidelines.
Measurement of physiological and biochemical parameters.
The following parameters were serially measured in all groups: body weight; blood glucose, measured using a glucometer (Accutrend; Boehringer Mannheim Biochemica, Mannheim, Germany); and systolic blood pressure, measured by tail cuff plethysmography in conscious, warmed mice.
Assessment of cardiac hypertrophy.
The total cardiac and isolated left ventricular masses were measured after the anuimals ewre killed, and expressed adjusted for body surface area in square meters (m2). Cardiomyocyte hypertrophy was assessed by measuring cross-sectional area of 100 cardiomyocytes in the left ventricle near the endocardial region, assessing only those with nearly circular capillary profiles. Cardiac hypertrophy in diabetes and obesity reactivates the fetal gene program, characterized by a switch to β-myosin heavy chain (β-MHC) expression in the adult murine heart (17). Expression of these markers of pathological hypertrophy were assessed by real-time quantitative RT-PCR. This was performed using the TaqMan system based on real-time detection of accumulated fluorescence (ABI Prism 7500; PerkinElmer, PE Biosystems, Foster City, CA), as previously utilized by our group (6, 28). Briefly, the left ventricle was homogenized using the Ultra-Turrax (Janke & Kunkel IKA, Labortechnik, Germany) in TRIzol (Life Technologies, Gaithersburg, MD), and total RNA was isolated. RNA purity and concentration were determined spectrophotometrically by measuring the absorbance of 1 μl of total RNA at 260 and 280 nm. Total RNA was then treated for DNAse contamination with a DNAse treatment kit (Ambion, TX) according to the manufacturer's recommendations. cDNA was synthesized with reactions consisting of 1.5 μg of RNA, Superscript (First Strand Synthesis System for RT-PCR, Life Technologies), random hexamers, and dNTPs according to the manufacturer's recommendations. Fifty nanograms of the resulting single-stranded cDNA was used in the real-time PCR experiments as described below. To assess genomic DNA contamination, controls without reverse transcriptase were included.
Gene-specific 5′-oligonucleotide primer, 3′-oligonucleotide primer, and MGB/FAM probe corresponding to each gene (see supplementary data online) were designed using the software program Primer Express (PE Applied Biosystems) to identify primer/probe combinations that meet criteria for an annealing temperature of 58–60°C.
The RT-PCR reaction took place with 500 nmol/l forward and reverse primer and 50 nmol/l FAM/MGB probe and VI 18S ribosomal probe, in 1× Taqman universal PCR master mix (PE Biosystems). Each sample was run and analyzed in triplicate. Cycle conditions were an initial denaturation at 95°C for 20 s, followed by 40 cycles of 95°C for 3 s and 60°C for 30 s. The generation of amplicons was defined by the point during cycling when amplification of the PCR product was first detected above the threshold setting. Each sample was run and analyzed in triplicate. Gene expression was normalized to 18S mRNA, and samples from C57Bl6J heart were then used as the calibrator with a given value of 1, and all other groups were compared with this calibrator group (27).
Assessment of myocardial fibrosis.
Previous studies have shown that both diabetes and obesity are associated with an increase in intramyocardial fibrosis. Gene expression of markers of fibrosis, including collagens and fibronectin were assessed by real-time quantitative RT-PCR, as detailed above. Perivascular collagen deposition was assessed by Van Giesen's stain, and interstitial fibrosis was assessed by staining with Picrosirus red staining for organized collagen. Paraffin-fixed cardiac sections were also stained for collagen IV by immunohistochemistry, as previously described (6).
Assessment of myocardial inflammation.
The expression of inflammatory cytokines, tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6), along with macrophage infiltration, is augmented following cardiac stress. In our study, the gene expression of IL-6, TNF-α, the intercellular adhesion molecule ICAM-1, and the chemokine macrophage chemoattactant factor (MCP-1) in left ventricular homogenates were assessed by real-time quantitative RT-PCR. The myocardial expression of IL-6 protein was further quantitated by ELISA (R&D Systems, Minneapolis, MN).
Assessment of superoxide production.
The production and accumulation of superoxide and other reactive oxygen species (ROS) are key mediators of cardiac injury and cellular dysfunction associated with HF feeding (4). Superoxide production was assesses as previously described (11). Briefly, left ventricular tissues was rapidly excised and homogenized in oxygen-saturated Krebs buffer (containing 118 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4·7H2O, 1.2 mM KH2PO4, 11 mM d-glucose, 0.03 mM EDTA, and 2.5 mM CaCl2, pH 7.4). Fresh homogenates were then divided to determine superoxide production in the presence of 125 μM NADH and 30 μM of the NADPH oxidase inhibitor diphenylene iodinium (designated NADH-dependent superoxide production) and superoxide production in the presence of 125 μM NADPH and 30 μM of the respiratory chain inhibitor rotenone (designated NADPH-dependent superoxide production). This latter activity was not inhibited by l-NG-nitroarginine or allopurinol (data not shown). Each of the samples was processed in triplicate following incubation for 60 min at 37°C. The rate of superoxide anion formation was determined by the addition of 3.8 μM lucigenin (bis-N-methylacridinium nitrate, Sigma Chemical). Chemiluminescence was monitored every 6 min for 60 min, and the integral over this period was expressed as relative light units (RLUs) normalized to 10 mg of tissue weight. The expression of the NADPH subunits, NOX2 (gp91phox), p47phox, NOX-4, and RAC-1 in left ventricular homogenates, along with the expression of heme oxygenase (HO) and glutathione peroxidases (GPX-1, GPX-3), key defence mechanisms against oxidative stress, were further assessed by real-time quantitative RT-PCR, as detailed above.
Assessment of cardiac lipid metabolism.
Changes in myocardial energy metabolism, due to altered substrate handling, characterize diabetes and obesity-related cardiac disease (4). In particular, intramyocardial accumulation of triacylglycerol metabolites mediated by activation of peroxisome proliferator-activated receptor-α (PPARα) has been strongly associated with cardiac dysfunction in rodents fed a HF diet (20). To assess the interaction of AGE/RAGE with lipotoxicity pathways in this model, the ventricular expression of PPARα and the fatty acid translocase CD36 were assessed by real-time quantitative RT-PCR. Intramyocardial accumulation of neutral lipids was assessed by staining with Oil red-O in frozen cardiac sections.
Accumulation of RAGE ligands and AGE receptor expression.
Cardiac levels of AGEs were estimated using a indirect in-house ELISA, as previously described (11), with a monoclonal AGE antibody that recognizes the nonfluorescent AGE carboxymethyl-lysine (CML) at its primary epitope. The expression of the non-AGE RAGE ligand S100 A8/A9 (calprotectin, MRP 8/14) (12) was also assessed by commercial ELISA (R&D Systems). The expression of RAGE, together with the AGE receptors AGE-R1 and AGE-R3 were further assessed by real-time quantitative RT-PCR.
Statistical analysis.
Continuous data are expressed as means ± SE except where otherwise specified. Differences in continuous variables were compared using Student's t-tests (2 groups) or one-way ANOVA (3 or more groups). Spearman rank order correlation was used to analyze associations between continuous variables. Differences in categorical variables were compared using the Mann-Whitney rank sum test. A P value of <0.05 was considered statistically significant.
RESULTS
Physiological parameters.
Feeding with the HF diet resulted in a significant increase in body weight, in both C57Bl6J and RAGE KO mice, compared with animals fed the SD diet (Table 1). Treatment with alagebrium chloride, modestly reduced weight gain associated with a Western diet but did not return it to control levels in either C57Bl6J or RAGE KO mice. This effect on weight was not due to differences in food intake, which were unaffected by the addition of alagebrium chloride (data not shown). Blood lipid levels were significantly increased in wild-type mice fed the HF diet (total cholesterol 3.0 ± 0.2 mM) compared with animals fed the SD diet (1.5 ± 0.3 mM, P < 0.01). A similar elevation was observed in RAGE KO mice (SD 1.7 ± 0.2 mM, HF 3.4 ± 0.3 mM, P < 0.01), with no significant difference observed between C57Bl6J and RAGE KO mice. Fifteen weeks of HF feeding did not influence systolic blood pressure levels in C57Bl6J or RAGE KO mice, and the systolic blood pressure was not modified in mice treated with alagebrium chloride (data not shown).
Table 1.
Biophysical parameters in study groups
|
Wild Type |
RAGE KO
|
|||||
|---|---|---|---|---|---|---|
| SD | HF | HF + AL | SD | HF | HF + AL | |
| Weight gain, %baseline | 10±3 | 40±3* | 27±2*# | 35±3* | 64±7*# | 44±7* |
| Total cardiac mass, g/m2 | 14.9±0.7 | 13.8±0.6 | 14.5±0.4 | 14.8±0.5 | 14.3±0.6 | 13.7±0.6 |
| Total LV mass, g/m2 | 9.4±0.5 | 8.8±0.6 | 9.5±0.3 | 10.0±0.6 | 10.0±0.5 | 9.7±0.3 |
| Mean myocyte diameter, μm | 16.8±0.5 | 20.5±0.8* | 19.7±0.8* | 17.0±0.6 | 18.0±0.5 | 18.5±0.6 |
Values are means ± SE. RAGE, receptor for advanced glycation end products; LV, left ventricle; KO, knockout; SD, standard diet; HF, high-fat diet; AL, alagebrium chloride.
Significant difference vs. wild-type SD;
vs. wild-type HF, P < 0.05.
Cardiac hypertrophy in HF-fed mice.
There was no significant change in the surface area adjusted total cardiac or isolated left ventricular mass in C57Bl6J or RAGE KO mice following 15 wk of feeding with a HF diet (Table 1). However, the cross-sectional area of cardiomyocytes in the left ventricular free wall was significantly increased following feeding with a HF diet in C57Bl6J mice (Table 1). In addition, the cardiac expression of β-myosin heavy chain was elevated following 15 wk of HF feeding in C57Bl6J, whereas the expression of α-myosin heavy chain was unaltered (Table 2). HF feeding RAGE KO mice failed to induce cardiomyocyte hypertrophy (Table 1) or increase the expression of β-myosin heavy chain gene (Table 2). Treatment with alagebrium chloride had no effect on hypertrophy or the expression of β-myosin heavy chain gene in HF-fed animals but reduced the expression of α-myosin heavy chain in both wild-type and RAGE KO animals.
Table 2.
Expression of mediators of cardiac dysfunction associated with HF diet in C57Bl6J and RAGE KO mice, measured by real time RT-PCR
| Gene Expression (AU/18 s) |
Wild Type |
RAGE KO
|
||||
|---|---|---|---|---|---|---|
| SD | HF | HF + AL | SD | HF | HF + AL | |
| RAGE | 1.0±0.2 | 2.5±0.4* | 1.0±0.2# | ND | ND | ND |
| Hypertrophy | ||||||
| α-MHC | 1.0±0.2 | 1.0±0.1 | 0.7±0.2* | 0.8±0.1 | 1.0±0.2 | 0.6±0.2*# |
| β-MHC | 1.0±0.2 | 3.2±0.5* | 2.5±0.3* | 0.9±0.2 | 1.3±0.3# | 1.6±0.2*# |
| Inflammation | ||||||
| Il-6 | 1.0±0.3 | 18.0±5.3* | 0.2±0.1*# | 0.9±0.2 | 0.3±0.1*# | 0.4±0.1*# |
| TNF-α | 1.0±0.2 | 2.0±0.3* | 0.5±0.2*# | 1.4±0.3 | 0.3±0.1*# | 0.4±0.1*# |
| ICAM-1 | 1.0±0.1 | 5.6±0.8* | 0.2±0.1*# | 1.0±0.2 | 0.6±0.1*# | 0.2±0.1*# |
| MCP-1 | 1.0±0.1 | 3.0±0.6* | 0.4±0.1*# | 1.0±0.1 | 0.7±0.2*# | 0.2±0.1*# |
| p65 | 1.0±0.1 | 1.5±0.2* | 0.8±0.2*# | 0.5±0.1* | 0.5±0.1*# | 0.3±0.1*# |
| Fibrosis | ||||||
| Collagen I | 1.0±0.1 | 1.6±0.1* | 1.6±0.1* | 0.4±0.1* | 0.7±0.1*# | 0.8±0.1# |
| Collagen IV | 1.0±0.1 | 1.3±0.1* | 1.3±0.1* | 0.6±0.1* | 0.7±0.1*# | 0.9±0.1# |
| Oxidative stress | ||||||
| gp91phox | 1.0±0.1 | 1.8±0.3* | 0.8±0.2# | 1.0±0.1 | 1.0±0.1 | 0.9±0.1 |
| RAC-1 | 1.0±0.1 | 1.0±0.1 | 1.0±0.2 | 0.7±0.1* | 0.7±0.1* | 0.7±0.1* |
| HO | 1.0±0.2 | 0.2±0.1* | 0.1±0.1* | 1.0±0.2 | 0.2±0.1* | 0.1±0.1* |
| Metabolic | ||||||
| PPARα | 1.0±0.2 | 3.0±0.5* | 1.9±0.4*# | 1.0±0.2 | 3.0±0.6* | 1.2±0.3# |
Values are means ± SE. MHC, myosin heavy chain; ICAM, intercellular adhesion molecule; MCP, macrophage chemoattactant factor; HO, heme oxygenase; ND, not detected.
Significant difference vs. wild-type SD;
vs. wild-type HF, P < 0.05.
Myocardial fibrosis in HF-fed mice.
Previous studies have shown that diabetes is associated with an increase in intramyocardial fibrosis, which can be prevented by inhibition of AGE accumulation (6). In our study, feeding a HF diet also resulted in a significant increase in the gene expression of collagen I and collagen IV in C57Bl6J mice (Table 2). Untreated RAGE KO mice had a lower baseline level of collagen I and collagen IV gene expression than untreated C57 controls. However, HF feeding in these animals failed to significantly increase cardiac collagen expression beyond that seen in animals receiving a normal diet (Table 2). The differences were confirmed with significant interstitial collagen deposition in HF-fed mice (Fig. 1), which was reduced in RAGE KO animals fed the same diet (Fig. 1). However, collagen deposition was unaffected by treatment with alagebrium chloride in HF-fed animals.
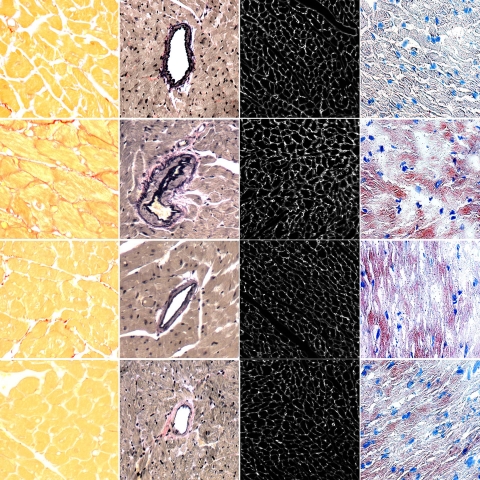
Fig. 1.
A Western high-fat diet (HF) was associated with increased accumulation of interstitial collagen, as demonstrated by picrosirus staining (column 1); increased perivascular collagen, as demonstrated on Van-Giesen stained sections (column 2); and expression of collagen IV (column 3). Collagen accumulation associated with HF feeding was reduced in RAGE knockout (KO) mice (HF+KO, bottom row) and in mice receiving alegebrium chloride (HF+AL, 3rd row). Cardiac fat accumulation was increased in fat-fed mice, as demonstrated by staining with Oil red O (right column 4). This increase was not altered in RAGE KO mice and mice receiving alegebrium chloride.
Myocardial inflammation in HF-fed mice.
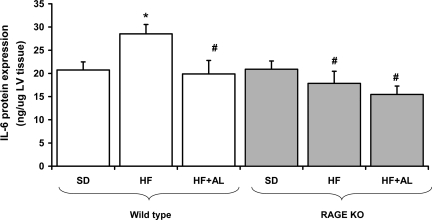
Myocardial inflammation is significantly increased in animals fed a HF diet. In our study, 15 wk of HF feeding resulted in a significant increase in the gene expression of IL-6 and TNF-α in C57Bl6J mice (Table 2). This was associated with a significant increase in myocardial IL-6 expression at a protein level (Fig. 2). Untreated RAGE KO mice had reduced expression of inflammatory cytokines compared with untreated C57Bl6J controls (Table 2 and Fig. 2). In addition, HF feeding in RAGE KO animals failed to induce cardiac inflammation. Indeed, there was a paradoxical decline in the expression of these inflammatory cytokines following HF feeding compared with RAGE KO mice receiving a SD low in AGEs. The increase in these cytokines in wild-type fed a HF diet was also reduced by alagebrium chloride, although this agent had no significant effect in RAGE KO mice.
Fig. 2.
Cardiac expression of IL-6 protein, as measured by ELISA. *Significant difference vs. wild-type standard diet (SD); # vs. wild-type HF, P < 0.05.
Cardiac expressions of MCP-1 and ICAM-1 were also increased in C57Bl6J mice fed a HF diet. Following the same trend observed for inflammatory cytokines, the expressions of both ICAM-1 and MCP-1 were significantly reduced in RAGE KO mice (Table 2). Treatment with the AGE inhibitor alagebrium chloride also reduced the expression of ICAM-1 and MCP-1 in both wild-type and RAGE KO mice.
It is known that activation of RAGE triggers the activation of inflammatory pathways via NF-κB. In this study, the HF feeding significantly increased NF-κB p65 mRNA levels (Table 2). This increase was prevented in animals treated with alagebirum chloride. Expression of p65 and signaling through NF-κB-dependent pathways was downregulated in RAGE KO mice, consistent with previous reports in these animals (15).
Myocardial AGE accumulation and receptor expression in HF-fed mice.
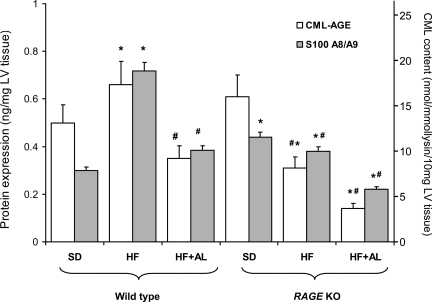
HF feeding was associated with a significant increase in the intake of AGEs (4 μmol CML·lysine−1·day−1) compared with a normal chow diet (1 μmol CML·lysine−1·day−1). This resulted in a small but significant increase in cardiac AGE accumulation, as demonstrated by ELISA for CML-modified protein (Fig. 3). RAGE KO mice fed a SD diet had a similar level of cardiac CML-AGE to wild-type animals. However, HF feeding in RAGE KO mice was associated with a paradoxical reduction in the AGE content of cardiac tissues. AGE accumulation was also markedly reduced following treatment with the AGE inhibitor alagebrium chloride in both wild-type and RAGE KO mice.
Fig. 3.
Quantification of RAGE ligands, CML-modified protein, and S100 A8/A9 (calprotectin, MRP 8/14) by ELISA in cardiac homogenates. *Significant difference vs. wild-type SD; # vs. wild-type HF, P < 0.05.
HF feeding was also associated with a significant accumulation of the non-AGE RAGE ligand S100 A8/A9 (Fig. 3), correlating with changes in other inflammatory cytokines, as detailed above. This increase in S100 A8/A9 following HF feeding, was also attenuated in RAGE KO mice and in animals receiving alagebrium chloride. Interestingly, basal levels of S100 A8/A9 were increased in the RAGE KO mice compared with wild-type animals receiving a normal diet (Fig. 3).
Paralleling changes in RAGE ligands, the gene and protein expression of RAGE was significantly increased in HF-fed C57Bl6J mice compared with mice fed a SD diet (Table 2). This increase in RAGE expression was prevented in C57Bl6J mice treated with alagebrium chloride (Table 2). There was no detectable RAGE expression in RAGE KO mice. Neither a HF diet nor alagebrium influenced the cardiac expression of the AGE receptors AGE-R1 and AGE-R3, and there was no difference in the cardiac expression of AGE-R1 or AGE-R3 between RAGE KO mice and C57Bl6J mice (data not shown).
Superoxide production in HF-fed mice.
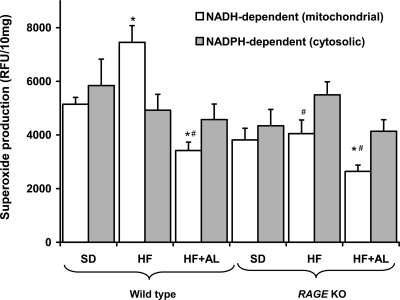
HF feeding was associated with a significant increase in NADH-dependent (mitochondrial) superoxide production in cardiac homogenates from wild-type mice (Fig. 4). However, HF feeding in RAGE KO mice failed to increase mitochondrial superoxide production in cardiac homogenates. Treatment with the AGE inhibitor alagebrium chloride also significantly reduced mitochondrial superoxide production in both wild-type and RAGE KO mice.
Fig. 4.
NADH-dependent (mitochondrial) superoxide production and NADPH-dependent (cytosolic) superoxide production in cardiac homogenates. *Significant difference vs. wild-type SD; # vs. wild-type HF, P < 0.05.
With our detection methods, NADPH-dependent superoxide production was not altered by a HF diet or in RAGE KO mice (Fig. 4). However, cardiac expression of the NADPH oxidase subunit gp91phox (Nox-2) was increased in wild-type mice fed a HF diet. This increase was not observed in RAGE KO animals or in animals treated with alagebrium chloride. Expression of other NADPH subunits, such as p47phox, Nox-4, and rac-1, were not altered by HF feeding (data not shown). However, cardiac expression of the rac-1 gene was lower in RAGE KO mice (Table 2).
A HF diet also resulted in a decline in the expression of heme oxygenase (HO-1), a key protector against superoxide-dependent injury in the heart (1). Interestingly, this decline was observed in both C57Bl6J and RAGE KO mice. HO-1 expression was not influenced by treatment with alagebrium chloride (Table 2). The expression of the antioxidant enzymes GPX-1 and GPX-3 were not affected by feeding with a HF diet (data not shown).
Cardiac lipid metabolism in HF-fed mice.
Diet-associated dyslipidemia following HF feeding was also associated with the intramyocardial accumulation of neutral lipid particles, as demonstrated by Oil-red O staining (Fig. 1). Lipid accumulation was similar in wild-type and RAGE KO mice and unaffected by treatment with alagebrium chloride. Similarly, the fat-dependent induction of PPARα was similar in wild-type and RAGE KO mice fed a HF diet (Table 2). However, treatment with the AGE inhibitor alagebrium chloride reduced the expression of PPARα in both wild-type and RAGE KO animals without modifying fat accumulation. Gene expression of the fatty acid translocase CD36 was not altered by HF feeding and was similar in wild-type and RAGE KO mice and following treatment with alagebrium chloride (data not shown).
DISCUSSION
The Western diet has a range of adverse effects on the heart, including enhanced inflammation, hypertrophy (2), fibrosis (2), and contractile dysfunction (21, 32). Such actions have been ascribed largely to the fat content of such diets. However, processed diets that are high in fat are also high in AGEs (29). Moreover, we have shown by two different strategies, inhibition of AGE accumulation following treatment with alagebrium chloride and prevention of RAGE activation in RAGE KO animals, are able to prevent the induction of inflammation, oxidative stress, and mitochondrial dysfunction in the heart associated with HF feeding. These data suggest an important role for the AGE/RAGE axis in cardiac disease associated with a Western diet.
Recent research on AGEs has focused largely on their pathogenic role in the development and progression of diabetic complications, as chronic hyperglycaemia leads to augmented AGE accumulation in vivo (5). However, AGEs are able to induce end-organ damage in the absence of hyperglycemia (24, 27), and indeed, inhibitors of AGE accumulation are able to prevent organ injury in diabetes without normalizing glucose levels (6). Consequently, other states in which AGEs are elevated may also see the development of “diabetes-like” AGE-induced injury, as well as being able to benefit from inhibition of the AGE/RAGE axis. For example, inhibition of cardiac AGE accumulation in the aging heart reduces myocardial damage (6, 7, 9, 16, 19). Similarly, reducing the amount of AGEs in the diet lowers markers of systemic inflammation in humans (30). Consistent with these findings, in mice pair fed a HF diet, another state associated with high exposure to AGEs, we have now shown that inhibition of AGE accumulation and/or signaling via the RAGE receptor also reduces myocardial inflammation, hypertrophy, and oxidative stress.
It is known that activation of RAGE by AGEs and other non-RAGE ligands triggers the activation of secondary messenger pathways, such as NF-κB and oxidative stress, both mechanisms that have been strongly implicated in the development and progression of cardiac disease (4). In particular, in this study we show that a diet high in fat (and AGEs) produced sustained NF-κB activation and upregulation of NF-κB p65 and NF-κB-dependent signaling pathways such as the expression of MCP-1. ROS produced by the mitochondrial respiratory chain have been described as a major mediator of fat, AGE, and hyperglycemia-dependent NF-κB activation (4, 18). Indeed, in our fat-fed animals, mitochondrial superoxide production was significantly increased in the heart. This increase was prevented in animals treated with the AGE inhibitor alagebrium chloride or in RAGE KO animals. Although no detectable change in NADPH-dependent oxidase activity in any of the groups was detected, studies in endothelial (31) and smooth muscle cells (25) and in the kidney (10) have demonstrated that activation of RAGE by AGEs stimulates NADPH oxidase activity. However, cardiac expression of the NADPH oxidase subunit gp91phox (NOX-2) was increased in wild-type mice fed a HF diet, and normalized following treatment with alagebrium chloride and in RAGE KO animals.
HF feeding in wild-type animals resulted in a significant increase in the various markers of cardiac inflammation, including IL-6, TNF-α, and ICAM-1 (Table 2). This response to HF-feeding was prevented in RAGE KO animals, which, indeed, showed a paradoxical reduction in inflammation with HF feeding. One possible explanation for this phenomenon may be unopposed activation of anti-inflammatory pathways by AGEs in RAGE KO mice. For example, it is known that AGEs are able to bind and activate a number of different receptors other than RAGE, including AGE-R1 (p60), AGE-R3 (galectin-3) (14), and lysozyme, all of which have anti-inflammatory properties. Although the cardiac expression of these AGE receptors was not altered in RAGE KO animals, their preferential activation in the setting of RAGE deficiency may have contributed to observed anti-inflammatory actions of a HF diet. Cardiac inflammation may also have been attenuated by the reduction in cardiac AGE levels observed in HF-fed RAGE KO mice (Fig. 3). The underlying mechanism of this paradoxical change remains to be established; however, it may be, in part, an epiphenomenon, as inflammation leads to the generation of AGEs, and anti-inflammatory strategies have been shown to reduce cardiac AGE accumulation (23).
In summary, AGEs represent important mediators of cardiac injury associated with a Western fast-food diet. Inhibition of AGE accumulation following treatment with alagebrium chloride and prevention of RAGE activation in knockout animals are both able to prevent the induction of inflammation, oxidative stress, and mitochondrial dysfunction in the heart associated with HF feeding. The identification of the key role of the AGE/RAGE axis in the response to HF feeding, provides a strong rationale for both dietary and pharmacological strategies directed toward this axis in the prevention and management of cardiac disease.
DISCLOSURES
The alagebrium chloride used in this study was provided by Synvista Inc. M. C. Thomas, M. E. Cooper, and J. M. Forbes have received additional research support of less than $100,000 from Synvista Inc.
Supplementary Material
Acknowledgments
This work was supported in part by National Institutes of Health Grant 5R01-HL-083452-03 and Kidney Health Australia (Bootle bequest).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abraham NG, Kappas A. Heme oxygenase and the cardiovascular-renal system. Free Radic Biol Med 39: 1–25, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Aguila MB, Alberto Mandarim-de-Lacerda C. Blood pressure, ventricular volume and number of cardiomyocyte nuclei in rats fed for 12 months on diets differing in fat composition. Mech Ageing Dev 122: 77–88, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Berg TJ, Snorgaard O, Faber J, Torjesen PA, Hildebrandt P, Mehlsen J, Hanssen KF. Serum levels of advanced glycation end products are associated with left ventricular diastolic function in patients with type 1 diabetes. Diabetes Care 22: 1186–1190, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation 115: 3213–3223, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Brownlee M Biochemistry and molecular cell biology of diabetic complications. Nature 414: 813–820, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Candido R, Forbes JM, Thomas MC, Thallas V, Dean RG, Burns WC, Tikellis C, Ritchie RH, Twigg SM, Cooper ME, Burrell LM. A breaker of advanced glycation end products attenuates diabetes-induced myocardial structural changes. Circ Res 92: 785–792, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Ceylan-Isik AF, Wu S, Li Q, Li SY, Ren J. High-dose benfotiamine rescues cardiomyocyte contractile dysfunction in streptozotocin-induced diabetes mellitus. J Appl Physiol 100: 150–156, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Christoffersen C, Bollano E, Lindegaard ML, Bartels ED, Goetze JP, Andersen CB, Nielsen LB. Cardiac lipid accumulation associated with diastolic dysfunction in obese mice. Endocrinology 144: 3483–3490, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Corman B, Duriez M, Poitevin P, Heudes D, Bruneval P, Tedgui A, Levy BI. Aminoguanidine prevents age-related arterial stiffening and cardiac hypertrophy. Proc Natl Acad Sci USA 95: 1301–1306, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coughlan MT, Cooper ME, Forbes JM. Renal microvascular injury in diabetes: RAGE and redox signaling. Antioxid Redox Signal 9: 331–342, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Coughlan MT, Thallas-Bonke V, Pete J, Long DM, Gasser A, Tong DC, Arnstein M, Thorpe SR, Cooper ME, Forbes JM. Combination therapy with the advanced glycation end product cross-link breaker, alagebrium, and angiotensin converting enzyme inhibitors in diabetes: synergy or redundancy? Endocrinology 148: 886–895, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Ehlermann P, Eggers K, Bierhaus A, Most P, Weichenhan D, Greten J, Nawroth PP, Katus HA, Remppis A. Increased proinflammatory endothelial response to S100A8/A9 after preactivation through advanced glycation end products. Cardiovasc Diabetol 5: 6, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation 114: 597–605, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Iacobini C, Amadio L, Oddi G, Ricci C, Barsotti P, Missori S, Sorcini M, Di Mario U, Pricci F, Pugliese G. Role of galectin-3 in diabetic nephropathy. J Am Soc Nephrol 14: S264–S270, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Liliensiek B, Weigand MA, Bierhaus A, Nicklas W, Kasper M, Hofer S, Plachky J, Grone HJ, Kurschus FC, Schmidt AM, Yan SD, Martin E, Schleicher E, Stern DM, Hammerling GG, Nawroth PP, Arnold B. Receptor for advanced glycation end products (RAGE) regulates sepsis but not the adaptive immune response. J Clin Invest 113: 1641–1650, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J, Masurekar MR, Vatner DE, Jyothirmayi GN, Regan TJ, Vatner SF, Meggs LG, Malhotra A. Glycation end-product cross-link breaker reduces collagen and improves cardiac function in aging diabetic heart. Am J Physiol Heart Circ Physiol 285: H2587–H2591, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Ng WA, Grupp IL, Subramaniam A, Robbins J. Cardiac myosin heavy chain mRNA expression and myocardial function in the mouse heart. Circ Res 68: 1742–1750, 1991. [DOI] [PubMed] [Google Scholar]
- 18.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404: 787–790, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Norton GR, Candy G, Woodiwiss AJ. Aminoguanidine prevents the decreased myocardial compliance produced by streptozotocin-induced diabetes mellitus in rats. Circulation 93: 1905–1912, 1996. [DOI] [PubMed] [Google Scholar]
- 20.Ouwens DM, Boer C, Fodor M, de Galan P, Heine RJ, Maassen JA, Diamant M. Cardiac dysfunction induced by high-fat diet is associated with altered myocardial insulin signalling in rats. Diabetologia 48: 1229–1237, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Ouwens DM, Diamant M, Fodor M, Habets DD, Pelsers MM, El Hasnaoui M, Dang ZC, van den Brom CE, Vlasblom R, Rietdijk A, Boer C, Coort SL, Glatz JF, Luiken JJ. Cardiac contractile dysfunction in insulin-resistant rats fed a high-fat diet is associated with elevated CD36-mediated fatty acid uptake and esterification. Diabetologia 50: 1938–1948, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petrova R, Yamamoto Y, Muraki K, Yonekura H, Sakurai S, Watanabe T, Li H, Takeuchi M, Makita Z, Kato I, Takasawa S, Okamoto H, Imaizumi Y, Yamamoto H. Advanced glycation endproduct-induced calcium handling impairment in mouse cardiac myocytes. J Mol Cell Cardiol 34: 1425–1431, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Ren J, Li Q, Wu S, Li SY, Babcock SA. Cardiac overexpression of antioxidant catalase attenuates aging-induced cardiomyocyte relaxation dysfunction. Mech Ageing Dev 128: 276–285, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robinet A, Alouane L, Hoizey G, Millart H. Advanced-glycation end products (AGEs) derived from glycated albumin suppress early beta1-adrenergic preconditioning. Fundam Clin Pharmacol 21: 35–43, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Shaw SS, Schmidt AM, Banes AK, Wang X, Stern DM, Marrero MB. S100B-RAGE-mediated augmentation of angiotensin II-induced activation of JAK2 in vascular smooth muscle cells is dependent on PLD2. Diabetes 52: 2381–2388, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Simm A, Casselmann C, Schubert A, Hofmann S, Reimann A, Silber RE. Age associated changes of AGE-receptor expression: RAGE upregulation is associated with human heart dysfunction. Exp Gerontol 39: 407–413, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Thomas MC, Tikellis C, Burns WM, Bialkowski K, Cao Z, Coughlan MT, Jandeleit-Dahm K, Cooper ME, Forbes JM. Interactions between renin angiotensin system and advanced glycation in the kidney. J Am Soc Nephrol 16: 2976–2984, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Tikellis C, Johnston CI, Forbes JM, Burns WC, Burrell LM, Risvanis J, Cooper ME. Characterization of renal Angiotensin-converting enzyme 2 in diabetic nephropathy. Hypertension 41: 392–397, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Uribarri J, Cai W, Sandu O, Peppa M, Goldberg T, Vlassara H. Diet-derived advanced glycation end products are major contributors to the body's AGE pool and induce inflammation in healthy subjects. Ann NY Acad Sci 1043: 461–466, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Vlassara H, Cai W, Crandall J, Goldberg T, Oberstein R, Dardaine V, Peppa M, Rayfield EJ. Inflammatory mediators are induced by dietary glycotoxins, a major risk factor for diabetic angiopathy. Proc Natl Acad Sci USA 99: 15596–15601, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wautier MP, Chappey O, Corda S, Stern DM, Schmidt AM, Wautier JL. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab 280: E685–E694, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Wilson CR, Tran MK, Salazar KL, Young ME, Taegtmeyer H. Western diet, but not high fat diet, causes derangements of fatty acid metabolism and contractile dysfunction in the heart of Wistar rats. Biochem J 406: 457–467, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan SF, Ramasamy R, Naka Y, Schmidt AM. Glycation, inflammation, and RAGE: a scaffold for the macrovascular complications of diabetes and beyond. Circ Res 93: 1159–1169, 2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.