Abstract
Nitric oxide (NO) and carbon monoxide (CO) seem to be neurotransmitters in the brain. The colocalization of their respective biosynthetic enzymes, neuronal NO synthase (nNOS) and heme oxygenase-2 (HO2), in enteric neurons and altered intestinal function in mice with genomic deletion of the enzymes (nNOSΔ/Δ and HO2Δ/Δ) suggest neurotransmitter roles for NO and CO in the enteric nervous system. We now establish that NO and CO are both neurotransmitters that interact as cotransmitters. Small intestinal smooth muscle cells from nNOSΔ/Δ and HO2Δ/Δ mice are depolarized, with apparent additive effects in the double knockouts (HO2Δ/Δ/nNOSΔ/Δ). Muscle relaxation and inhibitory neurotransmission are reduced in the mutant mice. In HO2Δ/Δ preparations, responses to electrical field stimulation are nearly abolished despite persistent nNOS expression, whereas exogenous CO restores normal responses, indicating that the NO system does not function in the absence of CO generation.
Nitric oxide (NO) is a neurotransmitter in the central, peripheral, and enteric nervous systems (ENS; refs. 1–3). In the ENS, NO is produced in enteric neurons by neuronal NO synthase (nNOS) and acts as a intercellular inhibitory neurotransmitter by diffusing from neurons to adjacent smooth muscle cells where it activates soluble guanylate cyclase, resulting in smooth muscle relaxation. Carbon monoxide (CO) has been postulated to be a second gaseous neurotransmitter (4, 5). CO is produced together with ferrous iron and bilirubin by the action of heme oxygenase, in collaboration with cytochrome P450 reductase and biliverdin reductase (6). Like NOS, heme oxygenase occurs in inducible and constitutive isoforms; heme oxygenase-1 (HO1) is highly inducible and is involved in cellular responses to stress (6, 7), whereas heme oxygenase-2 (HO2) is constitutively expressed (6) and discretely localized to neurons in the brain (4), enteric neurons (8–11), and interstitial cells of Cajal (ICC) in the mouse intestine (12). Because nNOS and HO2 are colocalized in some enteric neurons, NO and CO may function as coneurotransmitters (8, 13–15). Several studies show that CO can modulate important physiologic functions. Thus, exogenous CO modulates the release of hypothalamic hormones (16, 17), regulates vascular tone (18), and is a protective factor in hypoxia (19). In the intestine, CO relaxes the opossum internal anus sphincter (20) and hyperpolarizes isolated human and canine jejunal circular smooth muscle cells (10, 21). These previous investigations have relied on nonspecific inhibitors of heme oxygenase (22) or the application of exogenous CO. Thus, these studies do not provide direct evidence for CO as an endogenously produced neurotransmitter. Recently, we found attenuated intestinal smooth muscle relaxation and delayed intestinal transit in mice genetically deficient in HO2, suggesting that CO may be produced endogenously as an enteric neurotransmitter (8).
In the present study, we employ mice with targeted genomic deletions of HO2 (HO2Δ/Δ), nNOS (nNOSΔ/Δ), or both (HO2Δ/Δ/nNOSΔ/Δ) to evaluate the physiologic roles of CO and NO in gastrointestinal neuromuscular transmission. We report that the resting membrane potential (RMP) of gastrointestinal smooth muscle cells is significantly depolarized in HO2Δ/Δ, nNOSΔ/Δ, and HO2Δ/Δ/nNOSΔ/Δ mice and that mechanical and electrical responses to nerve stimulation in these mutant mice are substantially attenuated. Our results show that CO is an inhibitory neurotransmitter and indicate that CO and NO collaborate as cotransmitters to mediate intestinal inhibitory neurotransmission.
Materials and Methods
Solutions and Drugs.
Krebs' solution was freshly prepared daily and had the following ionic composition (in mM): Na+ 127.4, K+ 5.9, Ca2+ 2.5, Mg2+ 1.2, Cl− 134, HCO3− 15.5, H2PO4− 1.2, and glucose 11.5. The solution was continuously bubbled with 97% oxygen, 3% CO2 and maintained at pH 7.4. Atropine (1 mM), propranolol (1 mM), and phentolamine (1 mM) were present in all solutions used in this study to block adrenergic and cholinergic neurotransmission. Unless otherwise indicated, all chemicals were obtained from Sigma. CO was obtained from Scott Specialty Gasses (Troy, MI).
Animals and Initial Dissection of Jejunal Smooth Muscle Specimens.
Adult male 129SVEV (wild-type) mice from The Jackson Laboratory and HO2Δ/Δ, nNOSΔ/Δ, and HO2Δ/Δ/nNOSΔ/Δ mice were killed at 6–8 weeks of age with CO2 gas with the prior approval of the Mayo Animal Care and Use Committee. After the abdomen was opened, a segment of jejunum approximately 10 cm distal to the pylorus was removed and placed in preoxygenated Krebs' solution. The jejunal segments were opened along their anti-mesenteric borders and transferred to fresh oxygenated Krebs' solution. The mucosal layer was removed under direct vision by using a binocular microscope, and full thickness muscle strips (0.5 mm × 0.2 mm) were prepared, with the long axis cut parallel to the circular muscle layer for subsequent use in immunostaining, histochemical staining, or physiologic recordings (described below). For immunochemical or histochemical staining and for electrophysiological studies, the mucosa was peeled away from the jejunal smooth muscle layers of all specimens before further preparation as described below.
Immunostaining.
The mucosa-free dissected muscle layers were fixed in 4% (vol/vol) paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) by incubation at 4°C for 12 h. Next, tissues were rinsed with PBS before immunostaining by using antibodies to c-kit, PGP 9.5, or HO2 or histochemical staining for NADPH-diaphorase. The antibodies, amounts or titers, and their sources were as follows: rat monoclonal anti-c-kit (GIBCO; 5 μg/ml), rabbit polyclonal anti-PGP 9.5 (Biogenesis, Bournemouth, U.K.; 1/400), rabbit polyclonal anti-HO2 (StressGen Biotechnologies, Victoria, Canada; 1/1,000), and rabbit polyclonal anti-GST-HO2 (1/1,200). Secondary antibodies (donkey anti-rabbit IgG; Chemicon; 1/100) were conjugated to Cy2, rhodamine, and Cy5. NADPH-diaphorase histochemistry was performed either directly after fixation or after immunohistochemistry for HO2 by incubating tissues for 15–20 min in 0.1 M Tris (pH 7.4, 24°C) containing 0.3% Triton X-100, 2 mg/ml nitroblue tetrazolium, and 1 mg/ml β-NADPH. Staining was analyzed by examining the tissues with an epifluorescent (Zeiss Axiophot) light and laser scanning confocal microscopy (Zeiss LSM510).
Electrophysiological Measurements.
After initial dissection as described above, muscle strips were placed in a recording chamber with the circular muscle facing upward. One end of the muscle strip was pinned down to a sylgard-coated (Dow-Corning) chamber to record intracellular electrical activity, and the other end was attached to an isometric force transducer to record contractile activity of the entire muscle strip. The chamber (3-ml total volume) was perfused with warmed (37°C) and oxygenated Krebs' solution at a constant flow rate of 3 ml/min. After an equilibration period of at least 2 h, the muscle strips were stretched to an initial tension of 50–100 mg above the baseline tension. Recordings of intracellular electrical activity from smooth muscle cells were obtained by using glass capillary microelectrodes filled with 3 M KCl and with resistances ranging from 30 to 80 MΩ. Intracellularly recorded potentials were amplified by using a WPI M-707 amplifier (WPI Instruments, New Haven, CT) and displayed on an oscilloscope (Tektronix 5113). Force was measured isometrically and amplified with a bridge circuit amplifier. Both electrical signals and mechanical activity were recorded on chart paper (Gould 220, Gould, Cleveland) and also on an FM tape recorder (3964A, Hewlett–Packard). For electrical field stimulation (EFS) of nerves in the muscle strips, two platinum wires were placed parallel to the long axis of the preparation and were connected through a square wave stimulator (Grass 588, Grass Instruments, Quincy, MA) and a stimulus isolation unit (Grass SIU 5A). In all cases, EFS was applied as a train of six pulses of 0.35-ms duration, 100 V, and 30 Hz, for a total duration of 200 ms.
Statistical Analysis.
All observed values are expressed as means ± SEM. The number of cells recorded is designated (n) in the results or figure legends. Statistical significance was determined by using paired and nonpaired Student's t tests. A P value of less than 0.05 was considered significant.
Results
Myenteric Neurons and ICC Appear Normal in HO2Δ/Δ, nNOSΔ/Δ, and HO2Δ/Δ/nNOSΔ/Δ Mice.
Because loss of certain genes, such as GDNF, c-kit, and c-ret, leads to failure of enteric neurons or ICC to migrate and/or develop (23–26), we explored the morphology and function of the ENS and ICC in mutant mice. We monitored PGP 9.5 and c-kit expression by immunostaining as markers for ENS and ICC, respectively. HO2Δ/Δ, nNOSΔ/Δ, and HO2Δ/Δ/nNOSΔ/Δ intestinal preparations stain normally for PGP 9.5 and c-kit, indicating normal development of enteric neurons and intact ICC network (data not shown). NADPH-diaphorase histochemistry, reflecting nNOS expression, appears normal in HO2Δ/Δ mice but is absent in nNOSΔ/Δ and HO2Δ/Δ/nNOSΔ/Δ intestine. HO2 immunoreactivity is present in a subset of enteric neurons, with 25–50% of these also staining for NADPH-diaphorase in wild-type mice. We also observe HO2 but not NADPH-diaphorase staining in ICC of wild-type mice, consistent with our previous findings (12, 27). HO2 staining is absent in the HO2Δ/Δ and the HO2Δ/Δ/nNOSΔ/Δ specimens.
Functional ICC are required for normal electrical slow waves and smooth muscle contraction (28–30). We recorded phasic contractions and electrical slow waves in jejunum and observe no significant abnormalities in HO2Δ/Δ, nNOSΔ/Δ, or HO2Δ/Δ/nNOSΔ/Δ mice, consistent with functionally normal ICC in these mice.
Jejunal Smooth Muscle Cells Are Depolarized in HO2Δ/Δ, nNOSΔ/Δ, and HO2Δ/Δ/nNOSΔ/Δ Mice.
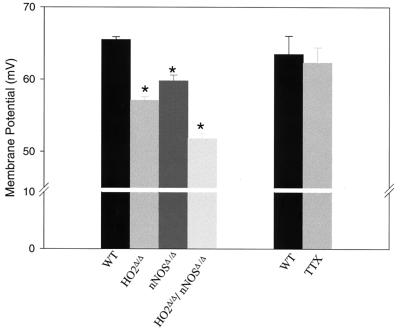
We measured the RMP in smooth muscle cells of the jejunum from wild-type and mutant mice (Fig. 1). In comparison to wild-type cells, the RMP is depolarized about 8 and 5 mV in HO2Δ/Δ and nNOSΔ/Δ smooth muscle cells, respectively. Compared with the RMP of wild-type mice, the RMP of HO2Δ/Δ/nNOSΔ/Δ mice is depolarized by about 13 mV, reflecting an apparent additive effect of the two genomic deletions. Thus, HO2 and nNOS seem to be crucial for maintenance of a normal RMP, and NO and CO formed in ICC or enteric neurons act together to determine the RMP.
Figure 1.
Targeted genomic deletion of HO2 and nNOS results in depolarization of the RMP of jejunal circular smooth muscle cells. The RMP of smooth muscle cells from HO2Δ/Δ, nNOSΔ/Δ, HO2Δ/Δ/nNOSΔ/Δ, and wild-type (WT) mice was determined as described in Materials and Methods. HO2Δ/Δ smooth muscle cells are depolarized compared with wild-type cells with RMPs of −57.1 ± 0.5 mV (n = 115) and −65.4 ± 0.4 mV (n = 118; ∗, P < 0.001), respectively. nNOSΔ/Δ smooth muscle cells are also depolarized with respect to wild-type cells with an RMP of −59.2 ± 0.7 mV (n = 45; ∗, P < 0.05). HO2Δ/Δ/nNOSΔ/Δ cells are even more depolarized with an RMP of −51.8 ± 0.7 mV (n = 55; ∗, P < 0.01). As shown, tetrodotoxin (TTX) had no effect on the RMP with an RMP of −63.5 ± 3 mV (n = 5) in the absence of tetrodotoxin and of −62.3 ± 2 mV (n = 5; P > 0.05) in the presence of tetrodotoxin (500 μM).
We wondered whether the NO and CO that contribute to establishing the RMP of the muscle cells are derived from ICC or enteric neurons. Action potentials of enteric neurons can be abolished by treatment with tetrodotoxin, which should not affect ICC. Tetrodotoxin treatment has no influence on jejunal muscle cell RMP (Fig. 1). This lack of influence suggests that action potentials of the enteric neurons are not required for regulation of RMP; thus, CO or NO regulating RMP comes either from the basal activity of these neurons or from ICC.
Muscle Relaxation and Inhibitory Neurotransmission Are Reduced in HO2Δ/Δ, nNOSΔ/Δ, and HO2Δ/Δ/nNOSΔ/Δ Intestine.
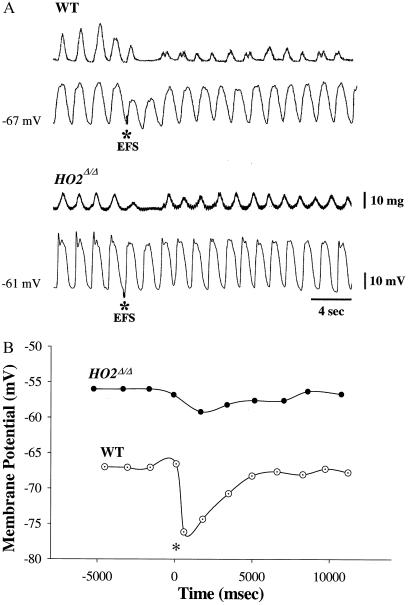
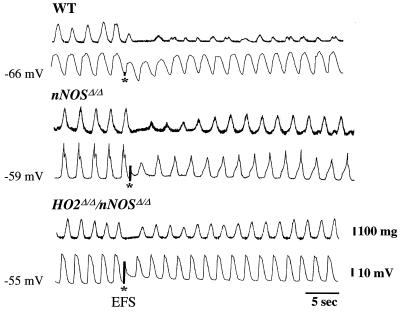
We monitored responses to EFS of jejunal smooth muscle strips by recording mechanical activity with a force transducer, while simultaneously monitoring the electrical activity, by whole cell recording, of individual cells in the muscle strips. The strips were treated with adrenergic and cholinergic inhibitors (see Materials and Methods), permitting us to focus on nonadrenergic noncholinergic (NANC) inhibitory transmission (Figs. 2 and 3). In wild-type preparations, EFS leads to hyperpolarization of the membrane potential, reflected as the IJP, and diminution of muscle contractions. The maximal inhibitory effect occurs with the first slow wave, with lesser effects evident during the second and third slow wave (Table 1). The mechanical and electrical responses to EFS are markedly reduced in HO2Δ/Δ preparations (Fig. 2A). The slow wave frequency in mouse intestine is significantly higher than in other species, tending to obscure the EFS-evoked IJP. Accordingly, we graphed the membrane potential reached at the bottom of each electrical slow wave before, during, and after EFS to observe the influences of EFS more clearly and to compare wild-type and mutant mice (Fig. 2B). In nNOSΔ/Δ preparations, a similar reduction in mechanical and electrical response to EFS is evident (Fig. 3). Deletion of both nNOS and HO2 leads to a profound reduction in effects of EFS (Fig. 3). Indeed, in HO2Δ/Δ/nNOSΔ/Δ preparations, we observe depolarization of the membrane potential presumably reflecting the unmasking of NANC excitatory transmission (Fig. 3).
Figure 2.
HO2 is required for normal inhibitory neurotransmission in intestinal smooth muscle. (A) Targeted genomic deletion of HO2 results in attenuated mechanical and electrical responses to EFS. Mechanical (Upper traces) and intracellular electrical (Lower traces) activities were recorded from jejunal circular smooth muscle cells derived from wild-type (WT) and HO2Δ/Δ mice. EFS (six pulses each of 0.35 ms, 100 V, and 30 Hz) was applied as indicated by the asterisk (*). Under NANC conditions, EFS elicited a hyperpolarization, an inhibitory junctional potential (IJP), with an amplitude of 7.4 ± 0.4 mV (n = 26 measurements; 17 preparations) in wild-type specimens. Inhibition of electrical slow wave activity and contractile activity follows the EFS-evoked IJP in wild-type specimens with recovery of activity in 15–25 s. In HO2Δ/Δ-derived samples, EFS evokes an initial IJP of only 2.6 ± 0.4 mV (n = 110 cells; 22 preparations; P < 0.05) with an attenuation in the reduction of electrical slow waves. Moreover, EFS did not abolish, but only modestly decreased, contractile activity in HO2Δ/Δ-derived muscle strips with a mean decrease in contractile activity of 12 ± 3% (n = 10 preparations). Recovery of contractile activity was significantly faster than in wild-type specimens, with full recovery in less than 10 s. (B) Graph of the most hyperpolarized portion of each electrical slow wave before, during, and after EFS. The voltage corresponding to the most negative potential for each electrical slow wave was plotted before, during, and after EFS. EFS (six pulses each of 0.35 ms, 100 V, and 30 Hz) was given at the time indicated (see asterisk). The RMP is depolarized in the HO2Δ/Δ-derived specimen compared with the wild type.
Figure 3.
Targeted genomic deletion of nNOS, HO2, or both genes attenuates electrical and mechanical responses to EFS. Mechanical (Upper) and electrical (Lower) response to EFS (*) recorded from jejunal circular smooth muscle cells from wild-type (WT), nNOSΔ/Δ, and HO2Δ/Δ/nNOSΔ/Δ mice. EFS evokes an IJP of only −0.75 ± 0.8 mV in nNOSΔ/Δ-derived specimens compared with −7.6 ± 0.6 mV in wild-type muscles (n = 4; P < 0.05). However, the decrease in slow wave amplitude was not different between nNOSΔ/Δ and wild-type specimens, with a decreased amplitude of 45 ± 5% and 47 ± 3%, respectively (n = 12; P > 0.05). Both electrical and mechanical inhibitory responses to EFS were nearly abolished in the HO2Δ/Δ/nNOSΔ/Δ-derived samples, and an excitatory electrical response was unmasked.
Table 1.
Targeted genomic deletion of HO2 attenuates the electrical response to EFS
| Wild type (n = 12) | HO2Δ/Δ (n = 8) | |
|---|---|---|
| Hyperpolarization, mV | 7.6 ± 0.6 | 2.6 ± 1.02∗ |
| Reduction in amplitude of first slow wave, % | 47 ± 3.3 | 15 ± 3.8∗ |
| Reduction in amplitude of second slow wave, % | 22 ± 4.6 | 1.6 ± 1.6∗ |
| Reduction in amplitude of third slow wave, % | 9.7 ± 2 | 0 ± 0∗ |
Targeted genomic deletion of HO2 results in attenuation of electrical response to EFS in jejunal smooth muscle preparations. The initial hyperpolarization or IJP in response to EFS is only 2.6 mV in HO2Δ/Δ-derived specimens compared to 7.6 mV in wild-type samples. In addition, the reduction in the amplitude of the first three electrical slow waves after EFS is significantly attenuated in the HO2Δ/Δ-derived specimens compared to wild-type samples. The number of independent measurements is indicated by (n), and statistical significance is indicated (∗, P < 0.05).
Exogenous CO Restores NANC Inhibitory Transmission in HO2Δ/Δ Jejunal Muscle.
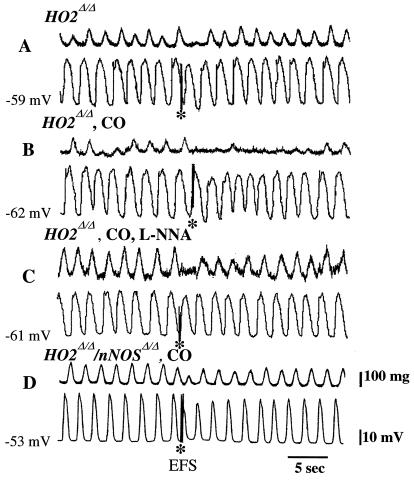
HO2 activity gives rise to three products: iron, biliverdin, which is rapidly reduced to bilirubin, and CO (6). Our previous study established that CO is most likely the HO2 product that is responsible for enteric neurotransmission (8). To examine this question directly, we monitored smooth muscle activity neurophysiologically after administration of CO gas to intestinal preparations of mutant mice (Fig. 4). We confirm that EFS-evoked inhibitory neurotransmission and muscle relaxation are both greatly reduced in HO2Δ/Δ preparations (Fig. 4A). After perfusing muscle strips with 10% CO, EFS-evoked electrical response is partially restored, whereas muscle relaxation is nearly normal, mimicking wild-type specimens (Fig. 4B).
Figure 4.
Exogenous CO restores NANC inhibitory transmission in HO2Δ/Δ jejunal smooth muscles. Mechanical and electrical responses to EFS were monitored in HO2Δ/Δ and HO2Δ/Δ/nNOSΔ/Δ-derived jejunal smooth muscles as indicated. In some cases (B–D), the perfusing buffer was treated with CO (10% vol/vol) for 10 min before the experiment. In one case (C), the buffer contained l-nitroarginine (L-NNA; 100 μM) for 20 min before EFS. The mean hyperpolarization evoked by CO was −3.6 ± 0.7 mV (n = 5) and −3.0 ± 0.6 mV (n = 8) in wild-type and HO2Δ/Δ-derived preparations, respectively, indicating HO2Δ/Δ-derived smooth muscle retains the ability to respond to exogenous CO. (A) EFS evokes only a small IJP and a modest reduction in mechanical activity from HO2Δ/Δ-derived muscles (see also Fig. 2 A and B). (B) After perfusion with CO, the inhibition of mechanical activity in response to EFS is restored, although the amplitude of the EFS-evoked IJP remains small. (C and D) Interestingly, the increase in EFS-evoked inhibition of contractile-mechanical activity after CO application is abolished in the presence of 100 μM l-nitroarginine (C) and is absent in HO2Δ/Δ/nNOSΔ/Δ-derived specimens (D). Thus, the effect of exogenous CO is not apparent when nNOS is absent (HO2Δ/Δ/nNOSΔ/Δ muscles) or when NO production is blocked (by treatment with l-nitroarginine), indicating that CO may require NO for its physiologic effects.
In the HO2Δ/Δ preparations, NO production from nNOS in response to EFS continues, but we detect negligible effects of EFS. The observed pattern is as if nNOS or NO requires HO2-derived CO for normal function. This notion is supported by the partial restoration of NANC inhibitory transmission by CO gas (Fig. 4B), which may allow endogenously produced NO to mediate inhibitory transmission comparable to wild-type preparations. To examine the interaction between NO and CO further, we treated the HO2Δ/Δ strips with l-nitroarginine for 20 min, followed by exposure to 10% CO for 10 min. The l-nitroarginine treatment blocks the effects of CO such that less inhibitory transmission is evident than in the HO2Δ/Δ preparations treated with CO alone (Fig. 4C). We also examined intestinal preparations of HO2Δ/Δ/nNOSΔ/Δ mice in the presence of exogenous CO. The behavior of these muscle strips is essentially the same as that of the HO2Δ/Δ preparations treated with l-nitroarginine in addition to CO (Fig. 4D).
Discussion
One of the most striking findings of the present study is that the RMP of jejunal circular smooth muscle cells is determined by HO2 and nNOS. Smooth muscle cells are depolarized in HO2Δ/Δ and nNOSΔ/Δ, and the HO2Δ/Δ/nNOSΔ/Δ manifest additional depolarization, reflecting additive effects of the two enzymes. Do the NO and CO responsible for the RMP derive from enteric neurons or ICC? Mice with genomic deletion of c-ret lack an ENS (26, 31), but the RMP of their small intestinal smooth muscle cells is the same as that of wild-type mice (S. Ward, personal communication). On the other hand, mice with a mutation in c-kit (W/Wv) lack ICC, and their intestinal smooth muscle cells are depolarized by about 8 mV (23). This level of depolarization is essentially identical to what we observe in HO2Δ/Δ specimens, suggesting that HO2-derived CO from the ICC plays a major role in establishing the RMP. How does NO affect the RMP? Immunohistochemical studies suggest that, although nNOS is abundant in enteric neurons, it may not be present in ICC (12, 27). Conceivably, ICC express low levels of nNOS that were not detected in these studies. Despite the observations that the loss of the entire ENS does not affect the smooth muscle RMP, nNOS in enteric neurons may influence RMP; because loss of the whole ENS results in the loss of many excitatory and inhibitory enteric neurotransmitters that may contribute directly or indirectly to the RMP, the effect of the singular loss of NO may be obscured.
Previously, we found that intestinal relaxation is partially reduced in HO2Δ/Δ and in nNOSΔ/Δ mouse ileum (8). We also found that cGMP levels of ileal muscle strips were reduced in HO2Δ/Δ and nNOSΔ/Δ specimens, and depolarization-induced augmentation of cGMP was also reduced in HO2Δ/Δ and nNOSΔ/Δ mouse ileum (8). The relevance of these findings to intestinal physiology in the intact organism was established by our observation that gastrointestinal transit time was prolonged in HO2Δ/Δ and nNOSΔ/Δ animals (8). These findings suggest that both CO and NO are neurotransmitters in the ENS regulating intestinal motility in mice. Because our earlier study did not monitor the electrical responses of intestinal smooth muscle cells to neural input and did not evaluate the smooth muscle RMP, it was not possible to provide definitive evidence of a neurotransmitter role for CO. In the present study, we have directly demonstrated that, after EFS, IJPs are reduced in HO2Δ/Δ and nNOSΔ/Δ muscles, with evidence for an additive effect in the HO2Δ/Δ/nNOSΔ/Δ specimens. Taken together with our earlier findings, these new observations indicate that both CO and NO are inhibitory neurotransmitters in the ENS.
HO2 and nNOS have overlapping expression in 25–50% of myenteric neurons, implying that CO and NO can be produced by the same neurons (8, 13–15). Our present study provides insight into the role of CO and NO as coneurotransmitters. Thus, inhibitory transmission seems to be almost totally abolished in HO2Δ/Δ intestine despite the persistence of nNOS expression and, presumably, NO production. Perfusion with CO gas partially restores NANC inhibitory transmission to the wild-type phenotype, strongly suggesting that the nNOS-NO system is not functional in the absence of HO2 and that CO itself suffices to restore physiologic effects of NO. CO might sensitize intestinal smooth muscle cells to the effects of NO. Alternatively, CO may enhance nNOS catalytic activity or facilitate NO release from enteric neurons.
ICC, enteric neurons, and intestinal smooth muscle cells interact to mediate physiologic inhibitory transmission and smooth muscle relaxation. Our studies of CO and NO interactions may clarify such relationships. nNOS has not been reliably demonstrable in ICC, suggesting that the major role of ICC in regulating muscle RMP involves influences of CO on neuronal NO as well as potential sensitization of muscle cells to NO. It is also possible that CO and NO from enteric neurons affect ICC, perhaps regulating CO production in ICC.
Acknowledgments
The authors thank Gary Stoltz for technical assistance and Kristy Zodrow for secretarial assistance. This work was supported by U.S. Public Health Service Grants DK17238, DK52766, MH-18501, and DA-00266 and Research Scientist Award DA-00074 (to S.H.S.). C.D.F. has a Howard Hughes Fellowship for Physicians.
Abbreviations
- ENS
enteric nervous system
- nNOS
neuronal nitric oxide synthase
- HO1 and HO2
heme oxygenase-1 and -2
- ICC
interstitial cells of Cajal
- RMP
resting membrane potential
- EFS
electrical field stimulation
- NANC
nonadrenergic noncholinergic
- IJP
inhibitory junctional potential
References
- 1.Goyal R K, Hirano I. N Engl J Med. 1996;334:1106–1115. doi: 10.1056/NEJM199604253341707. [DOI] [PubMed] [Google Scholar]
- 2.Stark M E, Szurszewski J H. Gastroenterology. 1992;103:1928–1949. doi: 10.1016/0016-5085(92)91454-c. [DOI] [PubMed] [Google Scholar]
- 3.Jaffrey S R, Snyder S H. Annu Rev Cell Dev Biol. 1995;11:417–440. doi: 10.1146/annurev.cb.11.110195.002221. [DOI] [PubMed] [Google Scholar]
- 4.Verma A, Hirsch D J, Glatt C E, Ronnett G V, Snyder S H. Science. 1993;259:381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- 5.Dawson T M, Snyder S H. J Neurosci. 1994;14:5147–5159. doi: 10.1523/JNEUROSCI.14-09-05147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maines M D. Annu Rev Pharmacol Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 7.Ferris C D, Jaffrey S R, Sawa A, Takahashi M, Brady S D, Barrow R K, Tysoe S A, Wolosker H, Baranano D E, Dore S, Poss K D, Snyder S H. Nat Cell Biol. 1999;1:152–157. doi: 10.1038/11072. [DOI] [PubMed] [Google Scholar]
- 8.Zakhary R, Poss K D, Jaffrey S R, Ferris C D, Tonegawa S, Snyder S H. Proc Natl Acad Sci USA. 1997;94:14848–14853. doi: 10.1073/pnas.94.26.14848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ny L, Alm P, Ekstrom P, Larsson B, Grundemar L, Andersson K E. Br J Pharmacol. 1996;118:392–399. doi: 10.1111/j.1476-5381.1996.tb15415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farrugia G, Miller S M, Rich A, Liu X, Maines M D, Rae J L, Szurszewski J H. Am J Physiol. 1998;274:G350–G358. doi: 10.1152/ajpgi.1998.274.2.G350. [DOI] [PubMed] [Google Scholar]
- 11.Werkstrom V, Ny L, Persson K, Andersson K E. Br J Pharmacol. 1997;120:312–318. doi: 10.1038/sj.bjp.0700893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller S M, Farrugia G, Schmalz P F, Ermilov L G, Maines M D, Szurszewski J H. Gastroenterology. 1998;114:239–244. doi: 10.1016/s0016-5085(98)70473-1. [DOI] [PubMed] [Google Scholar]
- 13.Ny L, Alm P, Larsson B, Andersson K E. J Auton Nerv Syst. 1997;65:49–56. doi: 10.1016/s0165-1838(97)00034-9. [DOI] [PubMed] [Google Scholar]
- 14.Snyder S H, Jaffrey S R, Zakhary R. Brain Res Brain Res Rev. 1998;26:167–175. doi: 10.1016/s0165-0173(97)00032-5. [DOI] [PubMed] [Google Scholar]
- 15.Reed D, Miller S M, Farrugia G, Sarr M G, Szurszewski J H. Gastroenterology. 1996;110:A1110. (abstr.). [Google Scholar]
- 16.Lamar C A, Mahesh V B, Brann D W. Endocrinology. 1996;137:790–793. doi: 10.1210/endo.137.2.8593832. [DOI] [PubMed] [Google Scholar]
- 17.Mancuso C, Kostoglou-Athanassiou I, Forsling M L, Grossman A B, Preziosi P, Navarra P, Minotti G. Brain Res Mol Brain Res. 1997;50:267–276. doi: 10.1016/s0169-328x(97)00197-6. [DOI] [PubMed] [Google Scholar]
- 18.Wang R, Wang Z, Wu L. Br J Pharmacol. 1997;121:927–934. doi: 10.1038/sj.bjp.0701222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morita T, Mitsialis S A, Koike H, Liu Y, Kourembanas S. J Biol Chem. 1997;272:32804–32809. doi: 10.1074/jbc.272.52.32804. [DOI] [PubMed] [Google Scholar]
- 20.Rattan S, Chakder S. Am J Physiol. 1993;265:G799–G804. doi: 10.1152/ajpgi.1993.265.4.G799. [DOI] [PubMed] [Google Scholar]
- 21.Farrugia G, Irons W A, Rae J L, Sarr M G, Szurszewski J H. Am J Physiol. 1993;264:G1184–G1189. doi: 10.1152/ajpgi.1993.264.6.G1184. [DOI] [PubMed] [Google Scholar]
- 22.Grundemar L, Ny L. Trends Pharmacol Sci. 1997;18:193–195. doi: 10.1016/s0165-6147(97)01065-1. [DOI] [PubMed] [Google Scholar]
- 23.Ward S M, Burns A J, Torihashi S, Sanders K M. J Physiol (London) 1994;480:91–97. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huizinga J D, Thuneberg L, Kluppel M, Malysz J, Mikkelsen H B, Bernstein A. Nature (London) 1995;373:347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez M P, Silos-Santiago I, Frisen J, He B, Lira S A, Barbacid M. Nature (London) 1996;382:70–73. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- 26.Schuchardt A, D'Agati V, Larsson-Blomberg L, Costantini F, Pachnis V. Nature (London) 1994;367:380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- 27.He C L, Miller S M, Sarr M G, Pemberton J H, Farrugia G, Szurszewski J H. Gastoenterology. 1998;114:A763. (abstr.). [Google Scholar]
- 28.Horowitz B, Ward S M, Sanders K M. Annu Rev Physiol. 1999;61:19–43. doi: 10.1146/annurev.physiol.61.1.19. [DOI] [PubMed] [Google Scholar]
- 29.Sanders K M, Ordog T, Koh S D, Torihashi S, Ward S M. Neurogastroenterol Motil. 1999;11:311–338. doi: 10.1046/j.1365-2982.1999.00164.x. [DOI] [PubMed] [Google Scholar]
- 30.Huizinga J D, Thuneberg L, Vanderwinden J M, Rumessen J J. Trends Pharmacol Sci. 1997;18:393–403. doi: 10.1016/s0165-6147(97)01108-5. [DOI] [PubMed] [Google Scholar]
- 31.Pasini B, Ceccherini I, Romeo G. Trends Genet. 1996;12:138–144. doi: 10.1016/0168-9525(96)10012-3. [DOI] [PubMed] [Google Scholar]