Abstract
BACKGROUND
This study evaluated the therapeutic efficacy of interleukin 12 (IL-12) gene therapy in Ewing’s sarcoma and whether murine mesenchymal stem cells (MSCs) could serve as vehicles for IL-12 gene delivery.
METHODS
MSCs were isolated from murine bone marrow cells. Cells were phenotyped using flow cytometry and were then differentiated into osteocytes and adipocytes using the appropriate media. The isolated MSCs were then transfected with adenoviral vectors containing either the β-galactosidase (Ad:β-gal) or IL-12 (Ad:IL-12) gene. Expression of IL-12 was confirmed using RT-PCR. Mice with TC71 Ewing’s sarcoma tumors were then treated intravenously (i.v.) with MSCs transfected with Ad:β-gal or Ad:IL-12. Tumors were measured and analyzed by immunohistochemical analysis for expression of IL-12 protein.
RESULTS
Expression of both p35 and p40 IL-12 subunits was demonstrated in MSCs transfected in vitro with Ad:IL-12. IL-12 expression was seen in tumors from mice treated with MSCs transfected with Ad:IL-12. Tumor growth was also significantly inhibited compared with that in mice treated with MSCs transfected with Ad:β-gal.
CONCLUSIONS
MSCs can be transfected with the IL-12 gene. These transfected cells localize to tumors after i.v. injection and induce local IL-12 protein production and the regression of established tumors.
Keywords: Mesenchymal stem cells, IL-12, Ewing’s sarcoma, gene therapy
Specific and efficient gene delivery to target cells and the subsequent expression of the RNA and protein is crucial to the success of gene-based therapy.1,2 The ability of mesenchymal stem cells (MSCs) to localize to tumors has raised interest in using these cells to deliver genes specifically to tumors as a mode of therapy. Recent advances in isolation techniques, the ability to culture and expand these cells in vitro, the ability to transduce MSCs with viral vectors, and the ability to deliver transduced cells systemically or locally have increased the feasibility of using MSCs for therapy.2–5 In addition, in vivo imaging analysis has demonstrated significant accumulation of MSCs at the site of tumors.6 These findings suggest that transduced MSCs may be effective against metastatic tumors as well as peripheral tumors.6–10
We have previously demonstrated that interleukin 12 (IL-12) gene therapy given by aerosol administration was effective against osteosarcoma (OS) lung metastases.11 We also demonstrated that IL-12 gene therapy administered by intratumor injection suppressed the growth of both local and distant Ewing’s sarcoma.12 Taken together, these data indicate that IL-12 gene therapy may have therapeutic potential for patients with both OS and Ewing’s sarcoma, provided that the gene can be delivered to the tumor area. In addition to our work with IL-12 gene therapy in Ewing’s sarcoma, we have demonstrated that bone marrow stem cells selectively migrate to Ewing’s sarcoma tumors.13–15 The purpose of this study was to determine whether MSCs transduced with the IL-12 gene can migrate into established Ewing’s sarcoma tumors and induce the production of IL-12 protein locally. The effect of these transduced MSCs on tumor growth was also evaluated. Our study demonstrated that MSCs can serve as cellular vehicles for cancer gene therapy.
MATERIALS AND METHODS
Isolation and culture of MSCs
Primary bone MSC cultures were established from mouse bone marrow (BM) cells obtained from the hind femurs of nude mice.16 The bones were aseptically removed, dissected clean of attached muscles, and flushed with phosphate-buffered saline (PBS). Cells were then washed with PBS and suspended in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS). BM cells (1×107/ml) were placed in 75-cm2 tissue culture flasks (BD Biosciences, Bedford, MA) and incubated at 37°C. After 3 days, nonadherent cells were removed, and 5 mL of fresh culture medium was added. MSCs have been shown to express several cell lineage-specific antigens.17–19 Therefore, 4 weeks later, the adherent cells were phenotyped as MSCs by flow cytometry using CD11b, CD29, CD31, CD34, CD44, CD45, CD49e, CD62E, and Sca-1 (Pharmingen, San Diego, CA, USA). Mouse isotype antibodies (Pharmingen, San Diego, CA, USA) served as respective controls. Using these expression criteria, the cultured BM-derived cells were >98% MSCs.
Differentiation Studies
Osteoblastic differentiation
The cultured MSCs (2×103/cm2) were seeded into four-chamber slides. Osteogenic differentiation medium consisting of DMEM containing dexamethasone 1×10−8 M, β- glycerophosphate 10 mM, ascorbate 0.3 mM (all from Sigma), and 10% FBS was added to the chamber slides. Osteocyte formation was evaluated 3 weeks later by calcium accumulation. The accumulation of mineralized calcium phosphate was assessed by von Kossa staining.17, 20
Adipogenic differentiation
Cultured MSCs were seeded into four-chamber slides. Adipogenic induction medium containing 1 µM dexamethasone, 10 µg/mL recombinant insulin, 0.5 mM 3-isobutyl-1-methyl-xanthine, and 10% FSC was added to the culture chambers. The induction of adipocytes was assessed using Oil Red O stain (Sigma-Aldrich Corp. St. Louis, MO) as an indicator of intracellular lipid accumulation.17, 21 The cells were fixed with 10% formalin, washed, and stained with a working solution of 0.18% Oil Red O for 5 minutes.
Staining of MSCs
MSCs were incubated with the fluorescent cell tracker dye CM-Dil (Molecular Probes, Eugene, OR) at a concentration of 10 mM for 5 min at 37°C and then for 15 min at 4°C. Cells were washed and resuspended in fresh HBSS immediately before use. Cells were kept on ice and protected from light until they were injected into the tail veins of nude mice, within 1 hour after staining.13
Vectors and Cell Transfection
The bicistronic adenoviral vector carrying both p35 and p40 cDNA for the IL-12 heterodimer (Ad:mIL-12) was used as previously described.11–12 Ad:mIL-12 was propagated in 293 cells and purified twice by cesium chloride gradient centrifugation. The viral titers were determined using a plaque-forming assay. An adenoviral vector containing the β-galactosidase gene (Ad:β-gal) was used as a control.7,8 Murine MSCs were infected with Ad:IL-12 at a multiplicity of infection (MOI) of 50, 100, 200, 300, and 500 or with Ad:β-gal at an MOI of 100. The cells were cultured for 2 days in the presence of Ad:mIL-12 and then assayed by RT-PCR for expression of IL-12. These 100 MOI-transfected cells were used in the in vivo experiments. Murine MSCs also were infected with adenovirus-GFP (Ad:GFP) at an MOI of 100. The cells were cultured for 2–3 days in the presence of Ad:GFP and then examined by fluorescence microscopy to confirm transduction.
Ewing’s Sarcoma Tumor Model
TC71 human Ewing’s sarcoma cells were cultured in Eagle’s modified essential medium with 10% FBS, 2 mM L-glutamine, 1 mM nonessential amino acids, and 2-fold vitamin solution (Life Technologies, Inc., Grand Island, NY). Cells were free of Mycoplasma infection as verified by Gen-Probe screening (Gen-Probe, San Diego, CA). 13 Six-week-old specific pathogen-free male athymic nude mice were purchased from Charles River Breeding Laboratories (Kingston, MA), and TC71 cells were injected subcutaneously (2.5×106 in 100 µl of HBSS) into the flank of each mouse. Tumors were palpable within 6 days. The mice were maintained and fed in a pathogen-free animal facility approved by the American Association of Laboratory Animals in accordance with current regulations and standards of the United States Department of Agriculture, the Department of Health and Human Services, and the National Institutes of Health. All animal protocols were approved by the Institutional Animal Care and Use Committee.
Cell Administration
To evaluate migration of MSCs into the tumors, murine MSCs transfected with Ad:GFP (2×105 cells) were injected twice, 4 days apart, into the lateral tail vein of mice bearing TC71 tumors. After 10 days, the tumors were removed and assayed by immunohistochemistry (IHC). To determine the distribution of MSCs in different organs after i.v. injection, MSCs stained with CM-Dil (105 cells) were injected into the lateral tail vein of nude mice twice, 4 days apart. Mice were euthanized by with carbon dioxide gas10 days later. Lungs, livers, spleens, kidneys, hearts, and brains were removed 1 week later and examined by fluorescence microscopy. For evaluation of therapeutic efficacy, murine MSCs transfected with Ad:IL-12 (2×105 cells) or Ad:β-gal (2×105 cells) were injected into the lateral tail vein twice weekly for 2.5 weeks. Tumors were measured by calipers, and the tumor area was calculated as the geometric mean of the perpendicular diameters. Mice were euthanized using carbon dioxide gas on day 30 and tumors were removed for IHC as described below.
Tissue Processing and IHC
Tumors were embedded in OCT compound (Miles, Inc., Elkhart, IN), snap frozen in liquid nitrogen, and sectioned (6–8 µm). For detection of GFP, tumor sections were incubated with rabbit anti-GFP (Santa Cruz Biotechnology, Santa Cruz, CA) and then with goat anti-rabbit Alexa Fluor 488 (Molecular Probes, Eugene, OR) as the secondary antibody. For the detection of IL-12, tissue was incubated with rat anti-mouse IL-12 (Biosource International, Inc., Camarillo, CA) and then with goat anti-rat horseradish peroxides as the second antibody. The slides were developed with 3,3’-diaminobenzidine (DAB) and counterstained with hematozylin.
RT-PCR
Total RNA was purified using Trizol reagent (Invitrogen, Carlsbad, CA). A nonspecific reverse transcriptase reaction was performed using oligo (dT) primers using the reverse transcriptase system (Promega, Madison, WI). For strand-specific PCR, the sequences of primers were sense, 5’-ATGTGTCAATCACGCTACCTC=3’ and, antisense 5’-TCAGGCGGAGCTCAGATAG=3’ for murine IL-12 p35, and sense 5’-ATGTGTCCTCAGAAGCTAAC=3’, and antisense 5’- TCCTAGGATCGGACCCTG=3’ for murine IL-12 p40. The primers were designed to produce a 648-bp fragment for mIL-12 p35 and a 1010-bp fragment for mIL-12 p40. The PCR products were isolated by electrophoresis on 2% agarose gel stained with ethidium bromide and were visualized under UV light.11
Statistical Analysis
A two-tailed Wilcoxon matched-pairs signed rank test was used to statistically evaluate the tumor size in the various treatment groups. A P value of < .05 was considered statistically significant.
RESULTS
MSCs Migrate to Subcutaneous TC71 Tumors
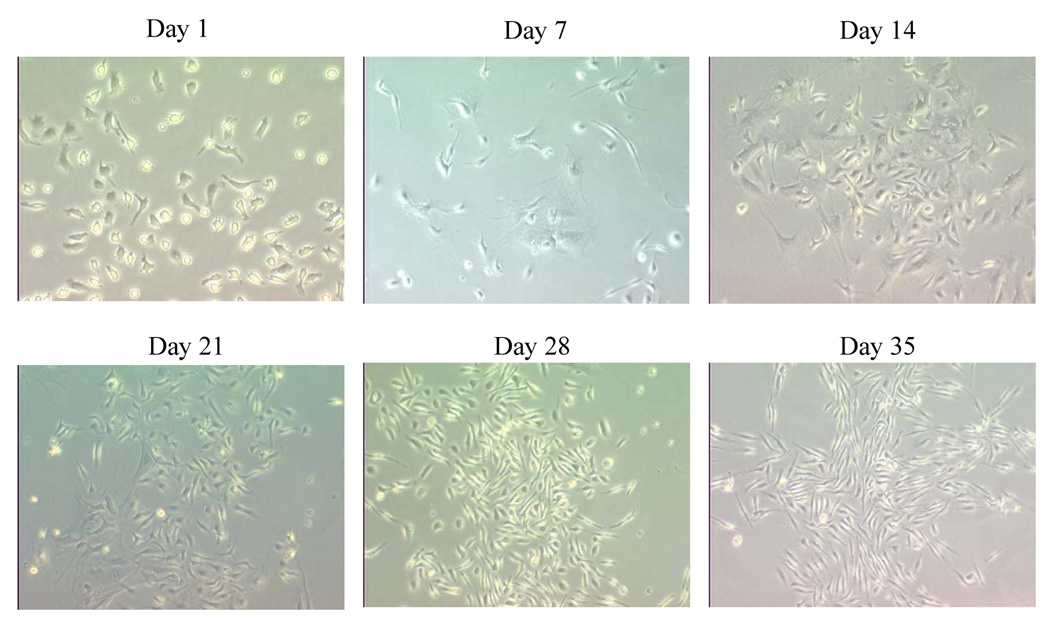
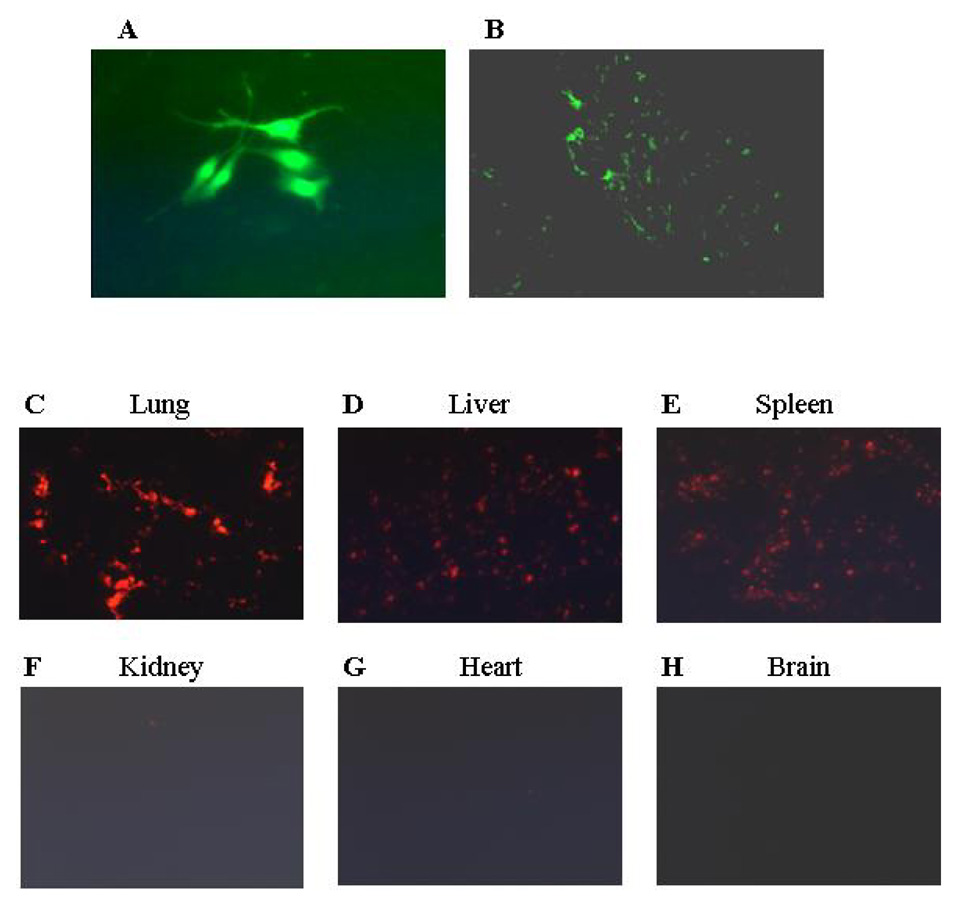
When cultured in vitro, MSCs derived from murine BM showed the defined spindle-shaped fibroblastic morphology over time (Fig. 1). These cells expressed the phenotypic markers shown in Figure 2. Phenotypic characterization of these cells were found to be positive for CD29, CD44, CD49E, and Sca-1 and to be negative for CD31, CD11b, CD34, CD45, and CD62E. The cultured MSCs differentiated into osteocytes and adipocytes depending on the culture medium used (data not shown). To determine whether transfected MSCs can migrate into the tumor area, MSCs were transfected with Ad:GFP. GFP expression was confirmed at 48 hours in the transfected MSCs (Fig. 3A). Mice with TC71 subcutaneous tumors were then treated with 2 doses of GFP-transfected MSCs given i.v. 4 days apart. Mice were sacrificed 10 days later, and tumors were excised and analyzed by IHC. Expression of GFP was observed in the TC71 tumors (Fig. 3B), indicating that the transfected MSCs were localized in the tumor after i.v. administration. MSCs were stained with CM-Dil to track the distribution of these cells into other organs. Lungs, livers, spleens, kidneys, and brains were also removed 10 days after injection, and examined under fluorescence microscopy (Fig.3C-3H). CM-Dil-positive cells were detected in the lung, liver, and spleen only.
Figure 1. MSCs were established from BM marrow cells.
BM adherent cells were isolated from the hind femurs of nude mice. Cells were cultured in tissue-culture flasks for various time periods as described. On day 35, cells were identified as MSCs by immunophenotyping and by osteogenic and adipogenic differentiation. Magnification = 20x.
Figure 2. Immunophenotypic profile of MSCs derived from adult murine BM.
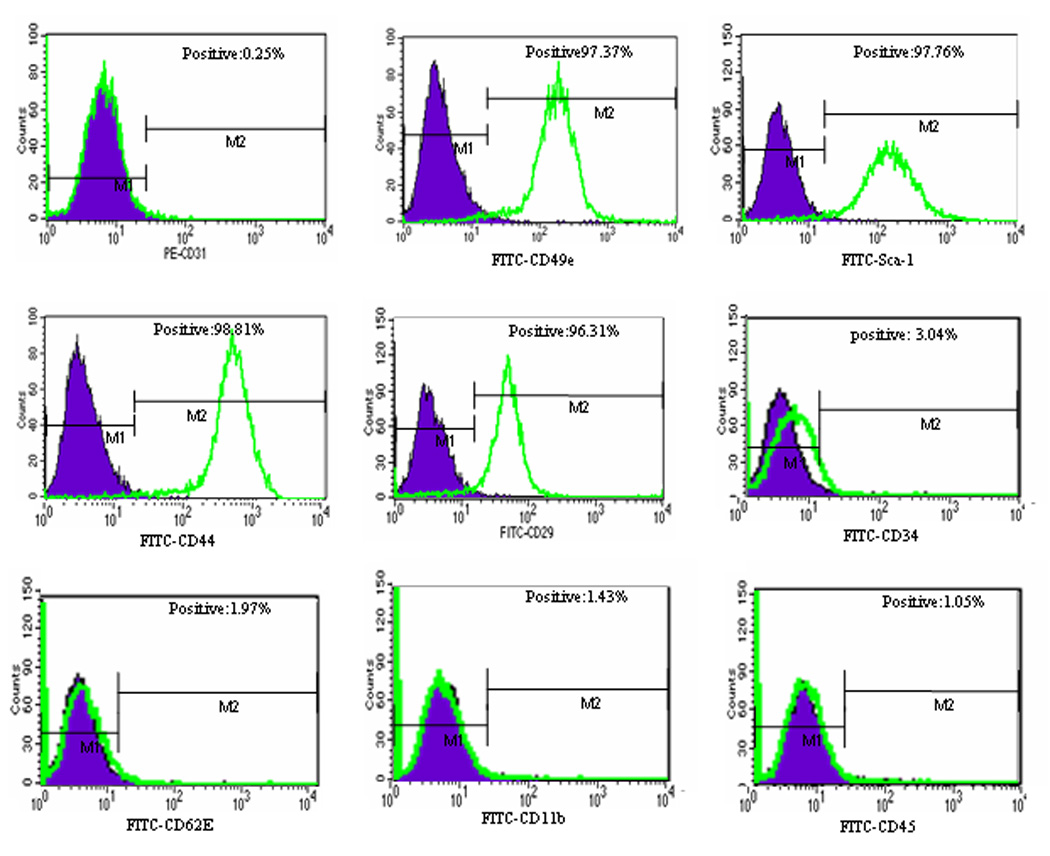
Flow-cytometric histograms show the expression (unshaded) of CD29, CD44, CD49E, and Sca-1. Cells were negative for CD31, CD11b, CD34, CD45, and CD62E. FITC, fluorescein isothiocyanate; PE, phycoerythrin.
Figure 3. Transfected MSCs migrate into the peripheral TC71 tumor.
(A) MSCs were transfected with Ad:GFP for 48 h. (B) Mice with subcutaneous TC71 Ewing’s sarcoma tumors received 2 i.v. injections of Ad:GFP MSCs given 4 days apart. Mice were sacrificed 10 days later, and tumors were excised, frozen, and analyzed by IHC. (C–H) CM-Dil-stained MSCs were injected in the tail veins of nude mice. Organ tissue was examined under fluorescence microscopy 10 days later. CM-Dil-positive cells were detected in lungs, livers, and spleens but not kidney, heart or brain.
Effect of IL-12-Transfected MSCs on the Growth of Established TC71 Tumors
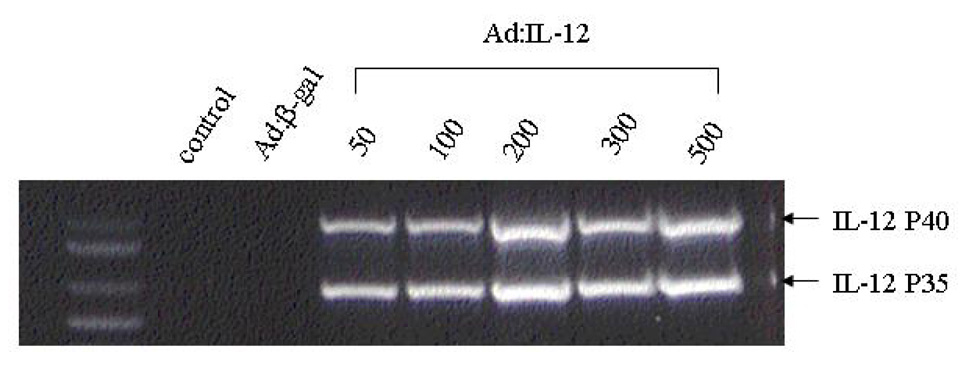
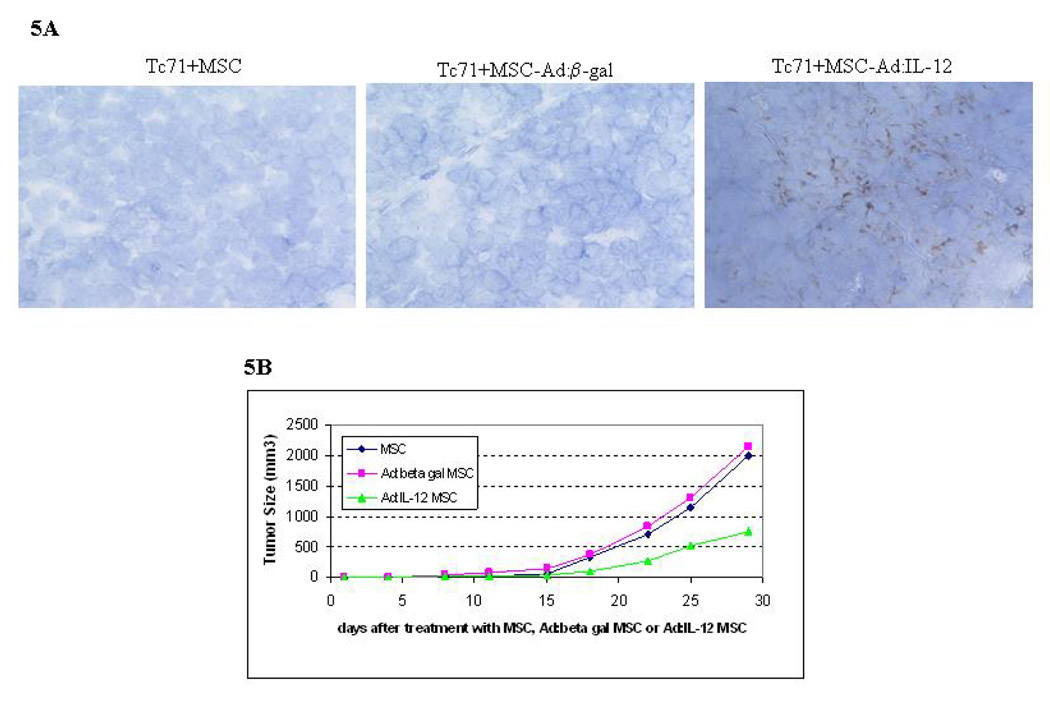
We have previously demonstrated that IL-12 gene therapy administered by aerosol delivery can induce regression of established sarcoma lung metastases.11 Having demonstrated that transfected MSCs can migrate into the peripheral tumor area after i.v. injection, we next wished to assess the ability of MSCs to deliver the IL-12 gene to subcutaneous TC71 tumors after i.v. injection. MSCs were transfected with Ad:IL-12 at various MOIs and then analyzed by RT-PCR for expression of p35 and p40 IL-12 (Fig. 4) to confirm expression the of subunits. Expression of p35 and p40 was seen at all MOIs. We elected to use the 100 MOI dose for the in vivo investigations. To evaluate therapeutic efficacy, TC71 cells were injected into nude mice. Mice were divided into 3 groups 4 days after tumor cell inoculation. Mice were then treated i.v. with Ad:IL-12 MSCs, Ad:β -gal-transfected MSCs (Ad:β gal MSCs), and untransfected MSCs. Tumor size was monitored: tumors were excised on day 30 and analyzed by IHC. IL-12 expression was only seen in the tumors from mice treated with Ad:IL-12 MSCs (Fig. 5A). Tumor growth was inhibited in mice treated with Ad:IL-12 MSCs, compared with all other groups (Fig. 5B, P <.05).
Figure 4. Expression of IL-12 after transfection.
MSCs were incubated with Ad: β-gal at 100 MOI or various MOIs of Ad:IL-12 for 48 h. Total RNA was extracted from 106 MSCs after transfection. IL-12 expression was determined by RT-PCR.
Figure 5. Effect of Ad:IL-12 MSCs on tumor growth.
Mice bearing TC71 tumors were treated i.v. with Ad:IL-12 MSCs, Ad: β-gal MSCs, or untransfected MSCs. (A) Frozen sections of tumors were analyzed by IHC. Expression of IL-12 was only observed in the Ad:IL-12 MSC-treated group. (B) Tumors were measured by calipers and the tumor area calculated as the geometric mean of perpendicular diameters. Day 25: MSC vs. Ad:IL-12 MSC, P = .024; and Ad: β-gal MSC vs. Ad:IL-12 MSC, P = .041. Day 29: MSC vs. Ad:IL-12 MSC, P = .04; and Ad: β-gal MSC vs. Ad:IL-12 MSC, P = .038.
DISCUSSION
MSCs are multipotent stem like cells that have been shown to migrate to sites of injured tissue in response to inflammatory mediators produced after tissue damage. 22–24 Similarly, migration of these cells into irradiated tumors has been demonstrated after i.v. injection.25 This migration into tumors and injured tissues is presumed to be mediated by specific cytokines, such as VEGF. Indeed, we have previously demonstrated that BM stem cells specifically migrate to Ewing’s sarcoma tumors13 and that VEGF165 is the chemotactic stimulus.14, 15 This was shown using a BM transplant model in which human CD34+ cells were injected into lethally irradiated nude mice. Tumor growth was then induced following engraftment. This allowed us to distinguish bone marrow derived (donor) cells (which were human) in the tumor from locally derived mouse host cells.13–14 Identical results were seen in another model in which specific GFP-labeled mouse BM subsets were injected i.v. into animals with established tumors.15 In that study, GFP-positive cells in the tumor identified the cells that had migrated from the BM into the tumor. Since MSCs are multipotent BM stem-like cells, these data suggested that MSCs may be capable of migrating into Ewing’s sarcoma tumors and of serving as a gene delivery system for therapeutic benefit in Ewing’s sarcoma.
IL-12, a Th1 cytokine, is a heterodimer composed of 2 subunits (p35 and p40) linked by a disulfide bond. Expression of both subunits is necessary for biologic activity. IL-12 augments NK activity, facilitates the induction of cytotoxic T cells, stimulates IFN-γ production26–28 and inhibits angiogenesis.29–30 IL-12 has been shown in several tumor models to have significant antitumor activity.31–33 Unfortunately, severe toxic effects have been seen with systemic administration of IL-12 protein.34, 35, 36 These toxicities were linked to high levels of IFN-γ. To circumvent systemic toxicities, we investigated the use of organ-specific delivery of the IL-12 gene as an alternative approach. Aerosol IL-12 gene therapy resulted in the expression of both IL-12 p35 and p40, the production of localized IL-12 protein only in the lung (the liver, heart, kidney were negative for IL-12), and eradication of established OS lung metastases.11 We also demonstrated that intratumor IL-12 gene therapy suppressed the growth and metastasis of subcutaneous Ewing’s sarcoma and prolonged survival.12 Although serum levels of IL-12 in the mice treated with intratumor IL-12 gene therapy were elevated, serum IFN-γ levels were identical to those seen in control treated mice. These data indicate that IL-12 gene therapy may be less toxic and, therefore, a potential therapy for Ewing’s sarcoma.
Because we demonstrated that BM stem cells selectively migrate to Ewing’s tumors and that IL-12 gene therapy can suppress the growth and metastasis of this tumor, we investigated whether MSCs could be used as a gene delivery system. Using a GFP label, we first demonstrated that transfected MSCs migrate into subcutaneous tumors after i.v. injection. The expression of GFP was visualized 10 days after 2 injections of the GFP-transfected MSCs (Fig. 3A). We demonstrated that MSCs can be transduced with the IL-12 gene, with subsequent expression of both the p35 and p40 subunits of IL-12. This is critical, given that the biologic activity is dependent on the expression of both subunits.37–39 When these IL-12-transduced MSCs were injected i.v., migration to subcutaneous tumors was demonstrated, with subsequent production of IL-12 protein in the tumor tissue and tumor growth inhibition (Fig. 5). In addition to localizing to the subcutaneous tumor, MSCs injected i.v. were also found to localize in the lungs, liver, and spleen but not the brain, kidney, or heart. This is not surprising, given that MSCs have been shown to reside in the former.40 We found no tissue damage in the liver and spleen of mice treated with IL-12-transfected MSCs. We have already demonstrated that production of IL-12 in the lung resulted in no long-term pathologic sequelae.11 These data indicate that MSCs can be used as a gene delivery system and that IL-12 gene therapy is effective against Ewing’s sarcoma.
A limitation of the model used in these investigations is that it is not an orthotopic model. Subcutaneous tumors may behave differently. However, in terms of BM cell migration into the Ewing’s tumor, we have demonstrated similar results in mice with soft tissue and subcutaneous tumors.15 Since Ewing’s sarcoma is a tumor that often presents in the soft tissue, our previous data indicate that MSC gene delivery will be effective regardless of tumor location. The tumor microenvironment may also contribute to the ability of MSCs and stem cells to chemotax into the tumor. Indeed, using our TC-71 Ewing’s sarcoma model we demonstrated that tumor production of VEGF165 is the critical factor.14,41 Downregulation of VEGF165 resulted in a significant reduction in BM cell infiltration which impacted tumor growth and tumor vessel density and could not be overcome by the overexpression of VEGF189 which has no chemotactic activity for BM cells.14,41–43 Furthermore, VEGF165 played a key role in the differentiation pathway once these cells reached the tumor.42 Therefore, the therapeutic approach of using MSCs to target a specific gene to the tumor may depend on the biology of the tumor and thus not appropriate for all solid tumors. Our present study did not investigate the chemotactic stimulus responsible for MSC migration. We hypothesize that since MSCs are stem cells the migration of injected MSCs into the tumor is mediated by a factor(s) produced by the tumor cells such as VEGF165. Another short coming of our model is that it necessitates using immunodeficient mice as TC-71 are human cells. At the present time there is no murine Ewing’s sarcoma model. Tissue distribution and the side effects of MSC-IL-12 gene therapy may be different in immunocompetent mice. We have however demonstrated that aerosol PEI-IL-12 gene therapy given twice a week for 8 weeks to immunocompetent mice resulted in no long-term damage to the lung.
The current treatment for patients with Ewing’s sarcoma consists of multiagent chemotherapy with surgery and/or radiotherapy. Despite this aggressive approach, the 2-year disease-free survival rate has remained at 60–70% for patients with localized disease and at only 10–30% for those with metastatic disease.44 The cure rate for those patients who present with metastases is less than <10%. The dearth of therapeutic options for patients with metastatic or relapsed Ewing’s sarcoma supports the need for new therapeutic approaches. In addition, the long-term sequelae of dose-intensive chemotherapy and radiation and the risk of secondary neoplasms have created an interest in identifying effective, less-toxic therapeutic approaches. New treatment regimens for this disease will likely come from results of preclinical investigations such as those presented here. Our data suggest that IL-12 gene therapy may be a novel therapeutic approach for patients with Ewing’s sarcoma. The ability of MSCs to infiltrate into the tumor parenchyma and our data showing that gene transcription and protein production can be achieved suggest that MSCs are an ideal gene delivery system for antitumor gene therapy. Further studies are warranted to determine the clinical utility of such an approach.
Acknowledgments
Supported by NIH grant CA108922 (to E.S.K.) and the institutional core grant, CA16672
Abbreviations
- MSC
mesenchymal stem cell
- mIL-12
murine interleukin-12
- β-gal
β-galactosidase
- GFP
green fluorescent protein
- BM
bone marrow
- OCT compound
Optimal Cutting Temperature compound
Footnotes
The authors have no potential conflicts of interest.
REFERENCES
- 1.Douglas JT. Cancer gene therapy. Technol Cancer Res Treat. 2003;2:51–64. doi: 10.1177/153303460300200107. [DOI] [PubMed] [Google Scholar]
- 2.Pereboeva L, Curiel DT. Cellular vehicles for cancer gene therapy: current status and future potential. Biodrugs. 2004;18:361–385. doi: 10.2165/00063030-200418060-00003. [DOI] [PubMed] [Google Scholar]
- 3.Minguell JJ, Erices A, Conget P. Mesenchymal stem cells. Exp Biol Med. 2001;226:507–520. doi: 10.1177/153537020122600603. [DOI] [PubMed] [Google Scholar]
- 4.Devine SM, Bartholomew AM, Mahmud N, et al. Mesenchymal stem cells are capable of homing to the bone marrow of non-human primates following systemic infusion. Exp Hematol. 2001;29:244–255. doi: 10.1016/s0301-472x(00)00635-4. [DOI] [PubMed] [Google Scholar]
- 5.Liechty KW, MacKenzie TC, Shaaban AF, et al. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000;6:1282–1286. doi: 10.1038/81395. [DOI] [PubMed] [Google Scholar]
- 6.Ozawa K, Sato K, Oh I, et al. Cell and gene therapy using mesenchymal stem cells. J Autoimmun. 2008;30:121–127. doi: 10.1016/j.jaut.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Studenyk M, Marinik FC, Dembinski JL, et al. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004;96:1593–1603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- 8.Pereboeva L, Komarove S, Mikheeve G, Krasnykh V, Curiel DT. Approaches to utilize mesenchymal progenitor cells as cellular vehicles. Stem Cells. 2003;21:389–403. doi: 10.1634/stemcells.21-4-389. [DOI] [PubMed] [Google Scholar]
- 9.Hamada H, Kobune M, Nakamura K, et al. Mesenchymal stem cells as therapeutic cytoreagents for gene therapy. Cancer Sci. 2005;96:149–156. doi: 10.1111/j.1349-7006.2005.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahmood A, Lu D, Chopp M. Intravenous administration of marrow stromal cells (MSCs) increases the expression of growth factors in rat brain after traumatic brain injury. J Neurotrauma. 2004;21:33–39. doi: 10.1089/089771504772695922. [DOI] [PubMed] [Google Scholar]
- 11.Jia S-F, Worth LL, Densmore CL, Xu B, Duan X, Kleinerman ES. Aerosol gene therapy with PEI:IL-12 eradicates osteosarcoma lung metastases. Clin Cancer Res. 2003;9:3462–3468. [PubMed] [Google Scholar]
- 12.Jia S-F, Duan X, Worth LL, Guan H, Kleinerman ES. Intratumor murine interleukin-12 gene therapy suppressed the growth of local and distant Ewing’s sarcoma. Cancer Gene Ther. 2006;13:948–957. doi: 10.1038/sj.cgt.7700968. [DOI] [PubMed] [Google Scholar]
- 13.Bolontrade MF, Zhou R-R, Kleinerman ES. Vasculogenesis Plays a role in the growth of Ewing’s sarcoma in vivo. Clin Cancer Res. 2002;8:3622–3627. [PubMed] [Google Scholar]
- 14.Lee TH, Bolontrade MF, Worth LL, Guan H, Ellis LM, Kleinerman ES. Production of VEGF165 by Ewing’s sarcoma cells induces vasculogenesis and the incorporation of CD34+ stem cells into the expanding tumor vasculature. Int. J. Cancer. 2006;119:839–846. doi: 10.1002/ijc.21916. [DOI] [PubMed] [Google Scholar]
- 15.Reddy K, Zhou Z, Jia S-F, Kleinerman ES. Specific bone marrow subsets differentiate into endothelial cells and pericytes and contribute to tumor vessel formation in Ewing’s sarcoma. Molecular Cancer Res. 2008;6:929–936. doi: 10.1158/1541-7786.MCR-07-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tropel P, Noel D, Platet N, Legrand P, Benabid A-L, Berger F. Isolation and characterization of mesenchymal stem cells from adult mouse bone marrow. Exp Cell Res. 2004;295:395–406. doi: 10.1016/j.yexcr.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 17.Bieback K, Kern S, Kluter H, Eichler H. Critical Parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells. 2004;22:625–634. doi: 10.1634/stemcells.22-4-625. [DOI] [PubMed] [Google Scholar]
- 18.da Silva Meirelles L, Beyer Nardi N. Murine marrow-derived mesenchymal stem cell: isolation in vitro expansion, and characterization. Br J Haematol. 2003;123:702–711. doi: 10.1046/j.1365-2141.2003.04669.x. [DOI] [PubMed] [Google Scholar]
- 19.Lee RH, Kim BC, Choi IS, et al. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem. 2004;14:311–324. doi: 10.1159/000080341. [DOI] [PubMed] [Google Scholar]
- 20.Cheng SL, Yang JW, Rifas L, et al. Differentiation of human bone marrow osteogenic stromal cells in vitro: induction of the osteoblast phenotype by dexamethasone. Endocrinology. 1994;134:277–286. doi: 10.1210/endo.134.1.8275945. [DOI] [PubMed] [Google Scholar]
- 21.Pittenger MF, Mackay AM, Beck CB, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 22.Li H, Fu X, Ouyang Y, Cai C, Wang J, Sun T. Adult bone-marrow-derived mesenchymal stem cells contribute to wound healing of skin appendages. Cell Tissue Res. 2006;326:725–736. doi: 10.1007/s00441-006-0270-9. [DOI] [PubMed] [Google Scholar]
- 23.Morigi M, Imberti B, Zoja C, et al. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol. 2004;15:1794–1804. doi: 10.1097/01.asn.0000128974.07460.34. [DOI] [PubMed] [Google Scholar]
- 24.Wu GD, Nolta JA, Jin YS, et al. Migration of mesenchymal stem cells to heart allografts during chronic rejection. Transplantation. 2003;15:679–685. doi: 10.1097/01.TP.0000048488.35010.95. [DOI] [PubMed] [Google Scholar]
- 25.Klopp AH, Spaeth EL, Dembinski JL, et al. Tumor irradiation increases the recruitment of circulating mesenchymal stem cells into the tumor microenvironment. Cancer Res. 2007;67:11687–11695. doi: 10.1158/0008-5472.CAN-07-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stern AS, Podlaski FJ, Hulmes JD, et al. Purification to homogeneity and partial characterization of cytotoxic lymphocyte maturation factor from human B-lymphoblastoid cells. Proc. Natl Acad Sci. 1990;87:6808–6812. doi: 10.1073/pnas.87.17.6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobayashi M, Fitz L, Ryan M, et al. Identification and purification of atural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170:827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rabinowich H, Herberman RB, Whiteside TL. Differential effects of IL12 and IL2 on expression and function of cellular adhesion molecules on purified human natural killer cells. Cell Immunol. 1993;152:481–498. doi: 10.1006/cimm.1993.1306. [DOI] [PubMed] [Google Scholar]
- 29.Coughlin CM, Salhany KE, Wysocka M, et al. Interleukin-12 and interleukin-18 synergistically induce murine tumor regression which involves inhibition of angiogenesis. J Clin Invest. 1998;101:1441–1452. doi: 10.1172/JCI1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voest EE, Kenyon BM, O'Reilly MS, Truitt G, D'Amato RJ, Folkman J. Inhibition of angiogenesis in vivo by interleukin 12. J Natl Cancer Inst. 1995;87:581–586. doi: 10.1093/jnci/87.8.581. [DOI] [PubMed] [Google Scholar]
- 31.Chen L, Chen D, Block E, O'Donnell M, Kufe DW, Clinton SK. Eradication of murine bladder carcinoma by intratumor injection of a bicistronic adenoviral vector carrying cDNAs for the IL-12 heterodimer and its inhibition by the IL-12 p40 subunit homodimer. J Immunol. 1997;159:351–359. [PubMed] [Google Scholar]
- 32.Brunda MJ, Luistro L, Warrier RR, et al. Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J Exp Med. 1993;178:1223–1230. doi: 10.1084/jem.178.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siders WM, Wright PW, Hixon JA, et al. T cell- and NK cell-independent inhibition of hepatic metastases by systemic administration of an il-12-expressing recombinant adenovirus. J Immunol. 1998;160:5465–5474. [PubMed] [Google Scholar]
- 34.Gately MK, Gubler U, Brunda MJ. Interleukin-12: acytokine with therapeutic potential in oncology and infection disease. Ther Immunol. 1994;1:187–194. [PubMed] [Google Scholar]
- 35.Cohen J. IL-12 death: explanation and a puzzle. Science. 1995;270:908. doi: 10.1126/science.270.5238.908a. [DOI] [PubMed] [Google Scholar]
- 36.Sarmiento UM, Riley JH, Knaack PA, et al. Biologic effects of recombinant human interleukin-12 in squirrel monkeys (Sciureus saimiri) Lab Invest. 1994;71:862–873. [PubMed] [Google Scholar]
- 37.Liu J, Cao S, Herman LM, Ma X. Differential regulation of interleukin (IL)-12 p35 and p40 gene expression and interferon (IFN)-γ-primed IL-12 production by IFN regulatory factor 1. J Exp Med. 2003;198:1265–1276. doi: 10.1084/jem.20030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hayes MP, Wang J, Norcross MA. Regulation of interleukin-12 expression in human monocytes: selective priming by interferon-gamma of lipopolysaccharide-inducible p35 and p40 genes. Blood. 1995;86:646–650. [PubMed] [Google Scholar]
- 39.Plevy SE, Gemberling JH, Hsu S, Dorner AJ, Smale ST. Multiple control elements mediate activation of the murine and human interleukin 12 p40 promoters: Evidence of functional synergy between C/EBP and Rel proteins. Mol Cell Biol. 1997;17:4572–4588. doi: 10.1128/mcb.17.8.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meirelles LS, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Science. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 41.Zhou Z, Reddy K, Guan H, Kleinerman ES. VEGF165 but not VEGF189 stimulates vasculogenesis and bone marrow cell migration into Ewing’s sarcoma tumors in vivo. Mol Cancer Res. 2007;5:1125–1132. doi: 10.1158/1541-7786.MCR-07-0174. [DOI] [PubMed] [Google Scholar]
- 42.Reddy K, Cao Y, Zhou Z, Yu L, Jia S-F, Kleinerman ES. VEGF165 expression in the tumor microenvironment influences the differentiation of bone marrow-derived pericytes that contribute to the Ewing’s sarcoma vasculature. Angiogenesis. doi: 10.1007/s10456-008-9109-1. Epub ahead of print 03/2008. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reddy K, Zhou Z, Jia S-F, Lee TH, Morales-Arias J, Cao Y, Kleinerman ES. SDF-1 stimulates neovasculature development and rescues Ewing’s sarcoma tumor growth in the absence of VEGF. Int J Cancer. 2008;123:831–837. doi: 10.1002/ijc.23582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baker LM, Pendergrass TW, Sanders JE, Hawkins DS. Survival after recurrence of Ewing’s sarcoma family of tumors. J Clin Oncol. 2005;23:4354–4362. doi: 10.1200/JCO.2005.05.105. [DOI] [PubMed] [Google Scholar]