Abstract
Objective:
This study determined the mechanisms and time-course of recovery of vascular relaxation in middle cerebral arteries (MCA) of salt-fed Sprague-Dawley rats returned to low salt (LS) diet (0.4% NaCl) or infused with low-dose angiotensin II (ANG II).
Methods:
Rats were fed high salt (HS) diet (4% NaCl) for 3 days or 4 weeks before return to LS diet for various periods. Other rats fed HS diet (HS + ANG II) received a chronic (3 days) i.v. infusion of a low dose of ANG II (5 ng·kg−1·min−1) to prevent salt-induced ANG II suppression.
Results:
HS diet eliminated the increase in cerebral blood flow in response to acetylcholine (ACh) infusion and the relaxation of MCA in response to ACh, iloprost, cholera toxin, and reduced PO2. Recovery of vascular relaxation was slow, requiring at least 2 weeks of LS diet, regardless of the duration of exposure to HS diet. Hypoxic dilation was mediated by cyclooxygenase metabolites and ACh-induced dilation was mediated via NO in LS rats and in HS rats returned to LS diet or receiving ANG II infusion.
Conclusions:
Return to LS diet for 2 weeks or chronic 3-day ANG II infusion restore the mechanisms that normally mediate cerebral vascular relaxation.
Keywords: angiotensin II, salt, vascular relaxation, vasodilation, endothelium, vascular smooth muscle
INTRODUCTION
In recent years, many studies have demonstrated that elevated dietary salt intake adversely affects vascular structure and function independent of its association with the pathogenesis of hypertension (10; 20; 21; 28; 30; 32; 42; 46). For example, normotensive animals fed a high-salt (HS) diet display microvascular rarefaction (20; 21), medial-intimal dissociation (20), inward remodeling (10; 20), potentiation of certain vasoconstrictor responses (10; 54), reduced endothelial Ca2+ signaling (59), and a dramatic attenuation of vascular relaxation responses (28; 30; 59).
This abrogation of vascular function is mediated by suppression of circulating angiotensin II (ANG II), since normalizing plasma ANG II levels via intravenous infusion of a low (subpressor) dose of ANG II restores vascular relaxation in response to vasodilator stimuli (30; 55; 56) in rats fed a HS diet. Importantly, acute vascular exposure to ANG II in vitro does not restore vasodilation, as this effect of ANG II requires chronic i.v. infusion of the peptide to exert its effects (30; 55; 56). Returning HS animals to a low-salt (LS) diet for 4 weeks also restores impaired vascular relaxation in rats fed HS diet. However, while reducing salt intake is known to restore dilator responses, the exact time-course of this reversal has yet to be determined, and it is unknown whether the duration of exposure to HS diet affects the rate of recovery of vascular relaxation mechanisms.
Another question regarding the restoration of vasodilator responses by return to low salt diet and, especially, low dose ANG II infusion, is whether vasodilator responses are mediated by the same mechanisms that are normally responsible for vascular relaxation, or whether alternate compensatory mechanisms emerge to mediate vascular relaxation under these conditions. This is important because a variety of studies have demonstrated that alternate mechanisms of vascular relaxation can emerge to mediate vasodilator responses in rats (6; 7; 11; 43; 48) and mice (24; 49) when the mechanisms that normally mediate these responses are compromised.
The goals of the present study were: (1) to evaluate the effect of high salt diet on cerebral blood flow responses to ACh and hypercapnia using Laser-Doppler flowmetry; (2) to determine the time-course of the restoration of vascular relaxation following return to a LS diet after exposure to short-term (3 days) and chronic (4 weeks) elevation of dietary salt intake and (3), to elucidate the mechanisms of restored vascular relaxation that emerge when rats fed HS diet are returned to a LS diet or receive a low dose ANG II infusion to prevent salt-induced suppression of circulating ANG II levels.
MATERIALS AND METHODS
Experimental Groups
Age-matched, male Sprague-Dawley rats (Harlan Teklad, Madison, WI) were used for all experiments. The rats were maintained on a LS diet (0.4% NaCl; Dyets; Bethlehem, PA) prior to the experiments. The studies evaluating the effect of high salt on cerebral blood flow utilized Sprague-Dawley rats of approximately 400-500 grams (14-16 weeks of age) in order to increase our success rate by providing a longer and larger segment of internal carotid artery. For these experiments, the animals were maintained on a low salt (0.4% NaCl, Dyets; LS) diet or switched to a high (4.0% NaCl, Dyets; HS) for 4 weeks, as described in previous studies by our laboratory and others (31; 34; 47; 54-56). Although rats in this group were slightly older than the other rats used in this study for practical considerations, Uchida et al. (53) have shown that rats of this age exhibit similar reactivity to ACh as younger animals.
To examine the effect of returning to LS diet after short-term or chronic exposure to a HS diet, the rats were placed on HS diet (4% NaCl) for either 3 days or 4 weeks. Following exposure to HS diet, the rats were returned to a LS diet for 3 days, 1 week, 2 weeks, or 4 weeks prior to the in vitro vessel experiments. A separate control group was maintained on low salt diet for an equivalent period of time (8 weeks). Rats in the short-term HS groups were 8-10 weeks old at the time of the isolated vessel experiment, and rats in the long-term HS groups were 11-14 weeks old at the time of the experiment.
For studies examining the mechanisms of the restored dilation of the MCA, animals were divided into four experimental groups: (1) LS diet; (2) 3 days HS diet; (3) 3 days HS diet followed by a return to LS for 2 weeks; and (4) 3 days HS diet followed by 3 days ANG II infusion (5 ng·kg−1·min−1 on HS diet) to override the salt-induced suppression of ANG II and to return plasma ANG II levels to the values present in animals fed low salt diet (19; 42; 59). Rats used in those experiments were 8-10 weeks old at the time of the isolated vessel experiment.
Laser-Doppler Studies of Cerebral Blood Flow Responses to ACh and Hypercapnia
The cerebral blood perfusion experiments utilized a previously described thinned-skull laser Doppler protocol (1; 15; 16; 58). The rats were anesthetized initially with 50 mg/kg i.p. sodium pentobarbital and anesthesia was maintained with a continuous i.v. infusion of pentobarbital at a rate of approximately 10 mg·kg−1·hour−1 throughout the experiment. After placement in the stereotaxic apparatus, the animal was artificially ventilated utilizing a small animal ventilator and CO2 analyzer (Harvard Apparatus). Inspiratory rate and minute ventilatory volume were monitored and carefully adjusted to maintain end tidal CO2 (ETCO2) at a constant value of approximately 35 mmHg throughout the experiment, and supplemental oxygen (30% O2 - balance N2) was provided in the inhalational gas, as described previously (29). Body temperature was maintained at 37°C with a heating pad supplied by a circulating warm water pump. Arterial blood pressure, ETCO2, and laser Doppler flow (LDF) signal in blood perfusion units (BPU) from an Oxford Optronix Oxy Flo 2000 laser Doppler flow meter (Oxford Instruments, Oxford, UK) were recorded for later analysis by automated data acquisition systems (WINDAQ & BIOPAC systems) connected to a personal computer.
Internal Carotid Artery Drug Infusion
Acetylcholine or saline vehicle was infused randomly for 10 minutes at 0.03 mL/minute into the cerebral circulation via the internal carotid artery. The ACh infusion rate (15 μg/minute) is the same one used by Panza's group to assess ACh-induced dilation by strain gauge plethysmography in the human forearm (4; 38-41) Cerebral blood flow was compared to resting values obtained during a one minute control period immediately prior to drug or PSS vehicle infusion. After ACh infusion, the catheter was flushed with warm saline to remove any residual drug in the line.
To assess the ability of the cerebral microcirculation to increase blood flow in response to elevated plasma PCO2, animals were acutely ventilated with a 5% CO2, 95% O2 gas mixture. Minute ventilation during induction of experimental hypercapnia was maintained at a constant level by increasing the ventilation rate while slowly decreasing inspiratory volume. Changes in brain blood flow were measured over a 10-minute period, during which the ETCO2 increased to approximately 60 mmHg in both LS and HS rats. Laser Doppler flow values during hypercapnia were compared to values obtained from a ten-minute resting period recorded immediately prior to stimulation with hypercapnia.
Chronic Animal Preparation for ANG II Infusion
Rats receiving ANG II infusion were anesthetized with an injection of a 70:30 mixture of ketamine:acepromazine (1 mg/kg, i.m). A catheter (micro-renathane PE10; Braintree Scientific; Braintree, MA) was inserted into a femoral vein under aseptic conditions, tunneled subcutaneously to the mid-scapular region, externalized, and attached to a swivel to allow free movement during infusion. Infusion of sterile isotonic saline (0.5 mL/hr) was begun after surgery to maintain catheter patency, while animals were fed a LS diet and allowed a 3-day recovery period. Following this recovery period, animals were placed on a HS diet for 6 days. After 3 days of saline infusion, ANG II infusion (5 ng·kg−1·min−1) was begun and continued for 3 days immediately prior to the acute in vitro experiment, in order to override the salt-induced suppression of ANG II and return plasma ANG II levels to the values present in animals fed low salt diet (19; 42; 59).
Preparation of Isolated Middle Cerebral Arteries
On the day of the experiment, animals were anesthetized with sodium pentobarbital (60 mg/kg, i.p.), mean arterial pressures were determined via a carotid artery cannula, and the brain was removed and immersed in physiological salt solution (PSS) having the following ionic composition: 119.0 mM NaCl, 4.7 mM KCl, 1.6 mM CaCl2, 1.18 mM NaH2PO4, 1.17 mM MgSO4, 24.0 mM NaHCO3, 5.5 mM D-glucose, and 0.03 mM ethylenediaminetetraacetic acid (EDTA). The MCA was carefully excised under a dissecting microscope, cannulated at the proximal and distal ends using glass micropipettes (80-120 μm; FHC, Brunswick, ME), and extended to its approximate in situ length. Side branches were ligated to prevent leaks and to allow the vessel to be pressurized. The vessel was continuously perfused and superfused with PSS (37° C) equilibrated with a 21% O2-5% CO2-74% N2 gas mixture, intraluminal pressure was maintained at 80 mmHg to approximate in vivo conditions, and internal diameter of the artery was measured using a video micrometer (model IV-550, FOR-A Company; Tokyo, Japan). Vessels lacking intrinsic tone during resting conditions were excluded from the study.
Vessel Response to Reduced PO2
After allowing the cannulated vessel a one-hour equilibration period with 21% O2, the response to reduced PO2 was assessed by simultaneous equilibration of the perfusion and superfusion solutions with a 0% O2-5% CO2-95% N2 gas mixture for 10 minutes. In this protocol, the PO2 of PSS equilibrated with 21% O2 is ∼140 mmHg and this value falls to 35-45 mmHg during equilibration with 0% O2. (8)
Responses to Vasodilator Stimuli and Ca2+-Free Solution
Vascular diameter changes in response to the endothelium-dependent muscarinic agonist acetylcholine (ACh; 10−9 M-0−5 M), the NO donor sodium nitroprusside (SNP; 10−9 M-10−5 M), the stable prostacyclin analogue iloprost (10−16 g/mL-10−11 g/mL), the stimulatory G protein (Gs) activator cholera toxin (10−13 g/mL-10−7 g/mL), and the adenylyl cyclase activator forskolin (10−12 M-10−6 M) were assessed in MCA from each group of rats. At the end of the experiment, active tone in the MCA was assessed by superfusing the vessel with Ca2+-free PSS having the following composition: 119.0 mM NaCl, 20.0 mM MgCl2, 4.7 mM KCl, 1.18 mM NaH2PO4, 1.17 mM MgSO4, 24.0 mM NaHCO3, 5.5 mM D-glucose, and 2.0 mM ethylene glycol-bis (μ-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA).
To determine the mechanisms mediating vascular relaxation in response to reduced PO2 and ACh, the arteries were incubated with either the cyclooxygenase (COX) inhibitor indomethacin (10−6 M) or the nitric oxide synthase (NOS) inhibitor Nω-nitro-L-arginine methyl ester (L-NAME; 10−4 M) for 20 minutes. After this incubation period, vessel responses to reduced PO2 and vasodilator agonists were assessed as described above.
Copper/Zinc Superoxide Dismutase (Cu/Zn SOD) Expression in Cerebral Arteries from Rats fed Low Salt Diet and High Salt Diet (± ANG II Infusion)
To verify that HS diet reduced Cu/Zn SOD expression, the middle cerebral arteries and as many other small arterial vessels as possible were isolated from the ventral surface of the brain from rats fed LS diet + saline vehicle infusion, HS diet + saline vehicle infusion, and HS diet + ANG II infusion. The arteries were removed and quickly frozen in liquid nitrogen. The vessels were homogenized on ice using a tissue protein extraction reagent (T-PER; Pierce; Rockford, IL). The protein concentration was determined using the Pierce MicroBCA Protein Assay kit (Pierce, Rockford, IL) with bovine serum albumin as a standard; and 2.5 μg of each sample was loaded on an 8-16% SDS-polyacrylamide gel and separated by electrophoresis. The samples were transferred to a nitrocellulose membrane (0.45 μm) and blocked for one hour with 5% non-fat dried milk. The membrane was incubated overnight with a polyclonal antibody against Cu/Zn SOD (Stressgen; Ann Arbor, MI) in 2.5% non-fat dried milk. The following day, the nitrocellulose membrane was washed and incubated with a horse radish peroxidase-conjugated goat anti-rabbit antibody for 2 hours (Santa Cruz Biotechnology; Santa Cruz, CA). After incubation with the secondary antibody, the membranes were washed and protein bands were visualized using chemiluminescence (Super Signal Pico; Pierce; Rockford IL).
Statistical Methods
Data are presented as mean value ± SEM. For all concentration-response curves, differences between groups were determined at each concentration using a two-way, repeated-measures analysis of variance (ANOVA). Active tone (%) in the resting arteries was calculated as: [(DMax−DC)/DMax) × 100] where DMax is the maximum diameter of the artery in Ca2+ free solution and DC is the resting diameter of the vessel in PSS. Cerebral blood flow was expressed as % of the control blood flow measured during a resting 1-minute period of time immediately preceding experimental maneuvers. Cerebral perfusion, blood pressure, resting diameter, maximum vessel diameter and active resting tone in the vessels were compared using one way ANOVA. A post hoc Student-Newman-Keuls test was used for comparison of multiple (>2) means. A P value of <0.05 was considered to be statistically significant.
RESULTS
High Salt Diet Impairs Cerebral Blood Flow Responses to Acetylcholine Infusion
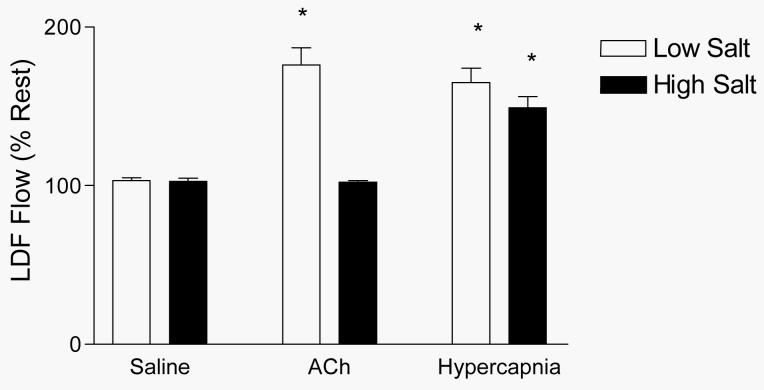
Figure 1 summarizes the responses of the pial circulation to acetylcholine and hypercapnia. In these studies, cerebral blood flow increased in response to ACh infusion in rats fed low salt diet, but not in rats fed high salt diet. However, both groups of rats exhibited equivalent increases in perfusion of the pial circulation during exposure to hypercapnia, demonstrating that the failure of blood flow to increase in response to acetylcholine in salt-fed animals was not due to a nonspecific reduction in the ability of the cerebral circulation to dilate.
Figure 1.
Changes in blood flow in the pial microcirculation (evaluated via laser-Doppler flowmetry) in response to saline vehicle infusion, ACh infusion, and hypercapnia in rats fed LS or HS diet for 4 weeks. Values are expressed as mean percent of resting control prior to infusion or hypercapnia (± SEM) for n=6 rats in each group. * P < 0.05 vs. resting control.
Reduced Salt Intake Restores Vascular Relaxation In Rats Fed a High Salt Diet
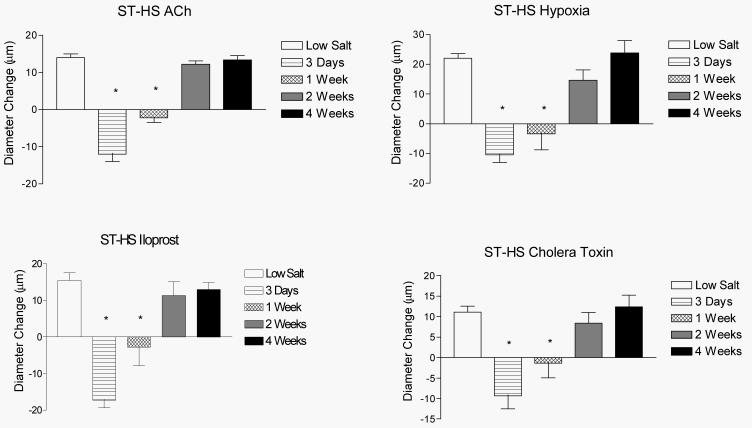
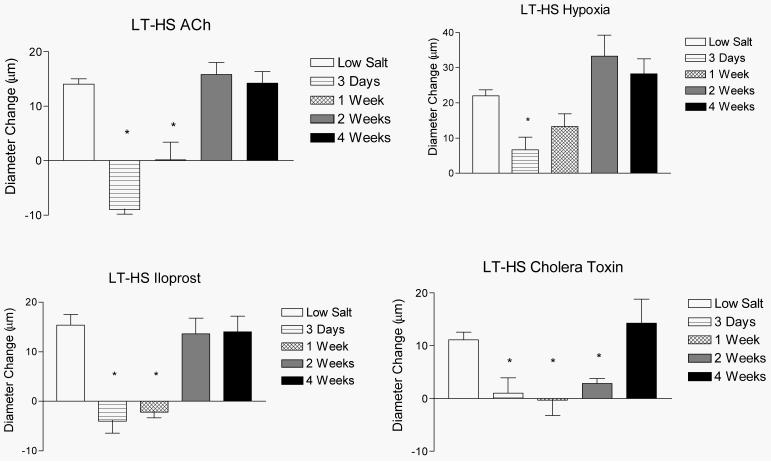
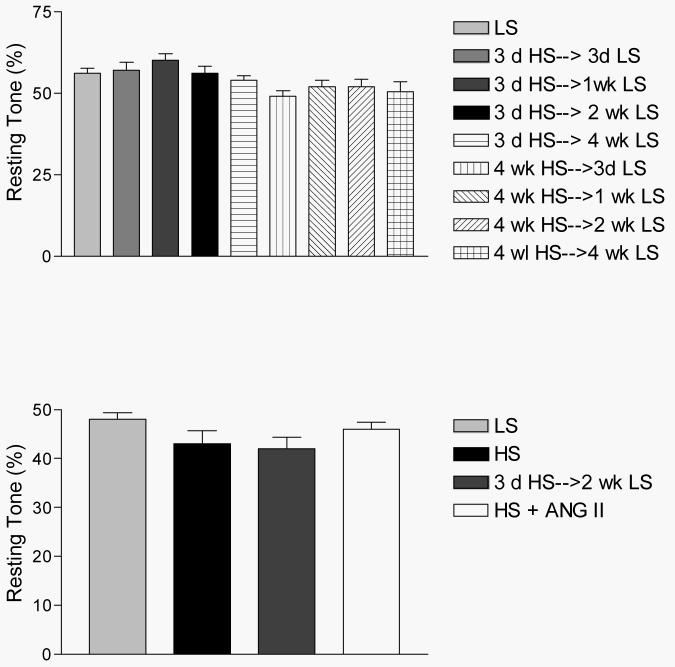
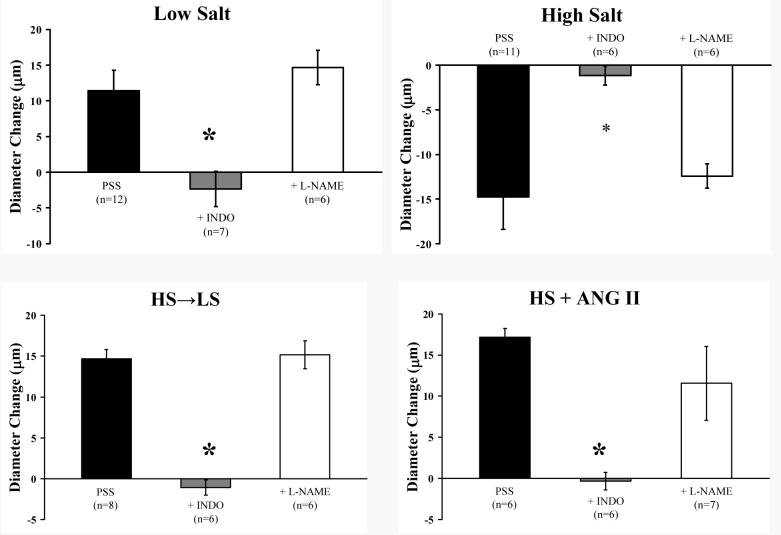
Figures 2 and 3 summarize the time-course of recovery of vasodilator responses in animals that were fed HS diet for either 3 days (short-term; ST-HS) or 4 weeks (long-term; LT-HS) before being returned to a LS diet for either 3 days, 1 week, 2 weeks, or 4 weeks. In both the ST-HS (Figure 2) and LT-HS (Figure 3) groups, the MCA either failed to dilate or exhibited a paradoxical vasoconstriction to ACh, hypoxia, iloprost, and cholera toxin 3 days after return to LS diet. The vessels exhibited no change in diameter or a reduced dilation (LT-HS hypoxia response only) in response to these stimuli 1 week after return to LS diet. Vasodilation returned to control values after 2 weeks of LS diet for all stimuli (except cholera toxin in the LT-HS group), with no further increase after returning to LS for 4 weeks. In the case of cholera toxin (LT-HS group), vasodilator responses returned 4 weeks after restoration of a LS diet. There were no significant differences in resting diameter, arterial pressure, or maximum diameter between the experimental groups (data not shown). Active tone in the vessels under resting conditions was similar in all the experimental groups (Figure 4-upper panel).
Figure 2.
Time-course for the restored vascular relaxation to ACh (upper left), reduced PO2 (upper right), iloprost (lower left), and cholera toxin (lower right) when rats fed HS diet for 3 days (ST-HS) were returned to a LS diet for either 3 days, 1 week, 2 weeks, or 4 weeks. Values are expressed as mean ± SEM. * p < 0.05 vs. 8 weeks Low Salt.
Figure 3.
Time-course for the restored vascular relaxation to ACh (upper left), reduced PO2 (upper right), iloprost (lower left), and cholera toxin (lower right) when rats fed HS diet for 4 weeks (LT-HS) were returned to a LS diet for either 3 days, 1 week, 2 weeks, or 4 weeks. Values are expressed as mean ± SEM. * p < 0.05 vs. 8 weeks Low Salt.
Figure 4.
Active resting tone (%) in isolated middle cerebral arteries of rats fed LS or HS diet in the time course studies (upper panel) and combined studies of mechanisms of vasodilator responses (lower panel). Values are expressed as mean ± SEM.
Effect of ANG II Infusion and Reduced Salt Intake on Endothelium-Dependent Relaxation Mechanisms
Similar to the time course studies, there were no differences in blood pressure, resting diameters, or maximum vessel diameter between the experimental groups in this component of the study. Active resting tone was virtually identical in all the experimental groups used to evaluate endothelium-dependent and endothelium-independent (see below) vasodilator mechanisms (Figure 4-lower panel).
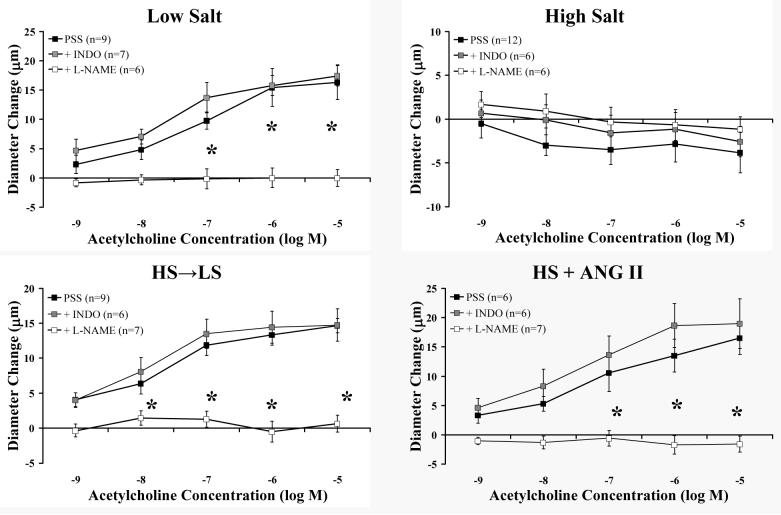
The results of studies to identify the mechanisms of restored vascular relaxation following return to LS diet for 2 weeks or during low dose ANG II infusion are summarized in Figures 5 (ACh) and 6 (reduced PO2). In control animals fed a LS diet, the concentration-dependent dilation to ACh was eliminated by inhibiting NOS with L-NAME and unaffected by inhibiting cyclooxygenase (COX) with indomethacin. Vessels from rats fed a HS diet did not dilate in response to ACh and were unaffected by treatment with either indomethacin or L-NAME. Vessels from animals fed HS diet for 3 days before being returned to LS for 2 weeks (HS→LS) and animals receiving an intravenous infusion of ANG II for 3 days while remaining on a HS diet (HS + ANG II) exhibited a concentration-dependent dilation to ACh that was eliminated with L-NAME and unaffected by indomethacin.
Figure 5.
Effect of NOS inhibition (L-NAME) and cyclooxygenase inhibition with indomethacin (INDO) on responses to ACh in middle cerebral arteries from LS-fed control rats (upper left), HS-fed rats (upper right), rats fed HS diet for 3 days before returning to a LS diet for 2 weeks (HS→LS) (lower left), and HS-fed rats receiving ANG II infusion (HS + ANG II) (lower right). Values are expressed as mean ± SEM. * p < 0.05 vs. PSS control in same group.
Figure 6.
Effect of NOS inhibition (L-NAME) and cyclooxygenase inhibition with indomethacin (INDO) on responses to reduced PO2 in MCA middle cerebral arteries from the LS (upper left), HS (upper right), HS→LS (lower left), and HS + ANG II (lower right) groups. Values are expressed as mean ± SEM. * p < 0.05 vs. PSS control in the same group.
Dilation of the MCA in response to reduced PO2 (Figure 6) was eliminated with indomethacin and unaffected by L-NAME in control animals fed a LS diet. As previously reported (30), MCA from rats on HS diet displayed a paradoxical vasoconstriction in response to hypoxia. The restored dilations to reduced PO2 in the HS→LS and HS + ANG II groups were eliminated by indomethacin and unaffected by L-NAME. The paradoxical vasoconstrictor response to hypoxia in rats fed HS diet was eliminated by COX inhibition with indomethacin, and unaffected by NOS inhibition with L-NAME. However, indomethacin failed to restore vascular relaxation to reduced PO2 in rats fed HS diet.
Effect of ANG II Infusion and Reduced Salt Intake on Endothelium-Independent Vascular Relaxation Mechanisms
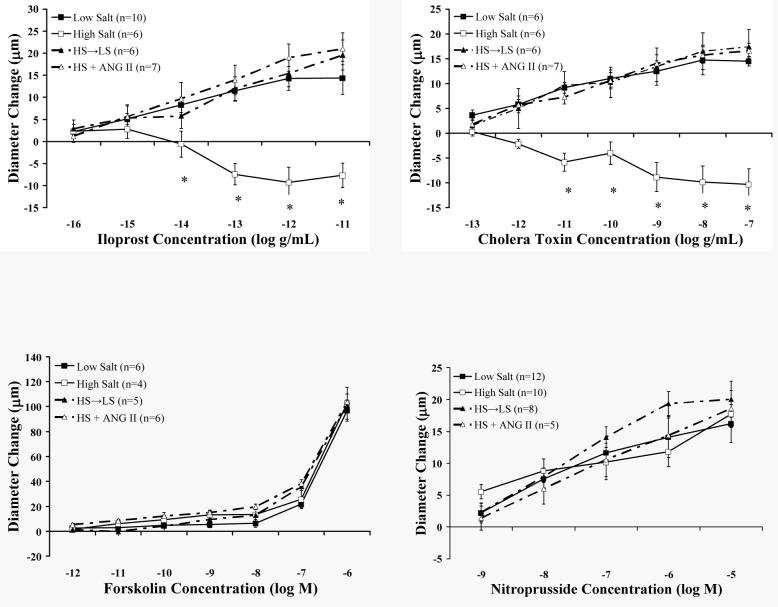
Figure 7 summarizes the response of MCA in the various groups to the different endothelium-independent vasodilator stimuli tested in these experiments. Previous studies have shown that HS diet disrupts the prostacyclin (IP) receptor signaling pathway in MCA by functionally uncoupling the Gs protein from membrane-bound adenylyl cyclase (30). In these experiments, we sought to determine if this signal transduction pathway is restored in the HS→LS and HS + ANG II groups, and also evaluated vessel responses to the NO donor sodium nitroprusside in the various groups. MCA isolated from control animals fed a LS diet exhibited a concentration-dependent vasodilation to iloprost, while vessels taken from rats fed a HS diet constricted in response to iloprost. Vasodilator responses to iloprost were restored to control values in the HS→LS and HS + ANG II groups. Stimulation with the Gs protein activator cholera toxin elicited a dilation in the LS, HS→LS, and HS + ANG II groups that was absent in HS animals. Vasodilatations in response to the adenylyl cyclase activator forskolin and sodium nitroprusside were similar in all the experimental groups.
Figure 7.
Responses to the stable prostacyclin analogue iloprost (upper left); the Gs protein activator cholera toxin (upper right), the adenylyl cyclase activator forskolin (lower left) and the NO donor sodium nitroprusside (lower right) in middle cerebral arteries from LS, HS, HS→LS, and HS + ANG II groups. Values are expressed as mean ± SEM. *- P<0.05 vs. low salt.
Effect of Dietary Salt Intake and ANG II Infusion on Cu/Zn SOD Expression in Cerebral Arteries
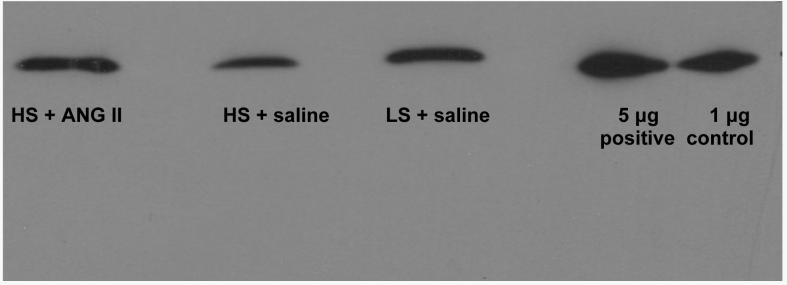
Figure 8 shows a Western blot comparing Cu/Zn SOD expression in cerebral arteries from rats fed LS diet + saline vehicle infusion, HS diet + saline vehicle infusion, and HS diet with low dose ANG II infusion. Consistent with a role for increased oxidative stress in contributing salt-induced impairment of vascular relaxation (26), HS diet led to a reduced expression of Cu/Zn SOD and ANG II infusion restored Cu/Zn SOD expression to a level similar to that in rats fed LS diet.
Figure 8.
Western blot comparing Cu/Zn SOD expression in cerebral arteries of rats fed LS diet (+ saline infusion) and HS diet (+ saline infusion or low dose ANG II infusion). See text for details.
DISCUSSION
Elevated salt intake is a critical risk factor not only for hypertension (18; 22; 23; 36), but also for cerebrovascular complications such as stroke (5; 23; 37; 51). Recent reviews (2; 3; 35) have noted that “defensive authoritarianism” (35) in support of “entrenched positions” (3) in the debate about dietary salt and blood pressure has detracted from the wider and more important question of whether HS diet has adverse consequences on vascular function independent of its effects on blood pressure. The existence of such pressure-independent effects of HS diet is supported by long term clinical studies in humans showing that salt-sensitive normotensive individuals not only have a greater risk of developing hypertension than salt-resistant subjects, but also have a mortality rate that is similar to hypertensive individuals, while mortality rates are lower in salt-resistant subjects (57).
Although previous studies have demonstrated that dilation of cerebral resistance arteries (30; 50) and pial arterioles (29) is compromised by high salt diet, it is essential to demonstrate that elevated dietary salt intake also compromises the ability of the cerebral circulation to regulate its blood flow. In the present study, we have shown that a chronic elevation in dietary salt intake eliminates the increase in cerebral blood flow that occurs in response to directed infusion of the endothelium-dependent vasodilator acetylcholine, which has been extensively used to evaluate vascular dysfunction in humans (39; 41). In contrast, the ability of the cerebral circulation to dilate in response to hypercapnia is unaffected by HS diet. Taken together, these findings indicate that HS diet compromises endothelium-dependent regulation of blood flow and NO-dependent vasodilation in the cerebral microcirculation, but this does not occur because of a generalized inability of the microcirculation to increase its blood flow. This loss of ACh-induced vasodilation in the cerebral circulation points to endothelial dysfunction and reduced bioavailability of NO, which has been proposed to result from increased vascular oxidative stress (26). Our studies provide support for that hypothesis, but also suggest that endothelium-independent vasodilator mechanisms lying upstream in the signal transduction pathways are compromised as well. The latter finding is consistent with evidence indicating that oxidative stress can impair the coupling between receptors and downstream signal transduction steps (52; 59).
Previous reports indicate that the suppression of plasma ANG II levels that occurs with elevated dietary salt intake dramatically attenuates vascular relaxation to many vasodilator stimuli, since these responses can be rescued by a chronic i.v. infusion of a low dose of ANG II (30; 55; 56) or returning animals to a reduced salt diet (10). ANG II infusion in this specific dose range (3-5 ng/mg/min) has been shown by several laboratories (19; 42; 59) to restore plasma ANG II levels to normal with this specific level of elevated dietary salt intake. Plasma ANG II levels also recover rapidly following a reduction in dietary salt intake, as demonstrated by rat studies (33) showing that plasma renin activity (PRA) increases sharply and reaches a plateau within 8 hours of Na restriction; and human studies demonstrating significant increases in plasma ANG II 24 hours after Na restriction (45).
While earlier studies (10) have shown that relaxation of skeletal muscle arterioles of rats fed HS diet for 4 weeks can be restored by returning the animals to a LS diet for 4 weeks, little is known regarding the precise time-course of this restoration of vascular relaxation following return to a LS diet. It is also unknown whether the duration of exposure to HS diet affects the time required for vascular relaxation to recover. Another important question regarding the reversibility of salt-induced loss of vascular relaxation is whether the mechanisms that mediate vascular relaxation during ANG II infusion or following return to LS diet are the same ones that normally mediate vascular relaxation in response to various vasodilator stimuli, or whether alternate compensatory mechanisms of vascular relaxation emerge. The latter question is important because a number of studies have shown that alternate mechanisms can emerge to mediate vasodilator responses under conditions where the normal mechanisms of vascular relaxation are compromised (7; 11; 24; 43; 48; 49).
In the context of these questions, the present study reports two new findings. First, both short-term and long-term exposure to HS diet lead to a profound reduction in vasodilator responses and restoration of vascular relaxation following return to LS diet requires a prolonged period of recovery that is similar, regardless of the duration of exposure to HS diet. A related and highly important observation is that high salt diet, low-dose ANG II infusion, and restoration of LS diet after salt feeding had no significant effect on resting diameter, maximum diameter, or active resting tone in the vessels. Therefore the loss of vascular relaxation with HS diet and the ability of ANG II infusion (or return to low salt diet) to restore vascular relaxation are not due to any effect on the reserve capacity of the vessel to dilate, because active resting tone is virtually identical in all the experimental groups (Figure 4). The other new finding in this study is that both ANG II infusion and reduced salt intake restore the mechanisms that normally mediate vascular relaxation in response to a variety of vasodilator stimuli, rather than evoking alternate compensatory mechanisms of vasodilation.
Time-Course of Recovery of Vasodilator Responses
A novel and important finding of the present study is that restoration of vasodilator responses (including vascular relaxation in response to Gs protein activation with cholera toxin) requires a prolonged period of recovery following the return to LS diet. It is especially important that neither the time course of recovery of vascular relaxation, active resting tone, or maximum arterial diameter differed in the LT-HS and ST-HS groups. These findings indicate that the length of the initial HS insult is not a critical factor in determining the rate of recovery of vascular relaxation following the restoration of LS diet, and that neither changes in active resting tone nor structural remodeling of the vessel are responsible for the reduced response to the vasodilator stimuli that are impaired by HS diet.
Taken together, these findings demonstrate that even short-term exposure to HS diet leads to profound decreases in the ability of vessels to relax and to striking alterations in signal transduction mechanisms that are not easily reversible by salt restriction. These fundamental observations raise a number of interesting questions. For example, why does vascular relaxation recover more quickly following ANG II infusion vs. return to low salt diet? One potential explanation is that, even though PRA and ANG II recover very rapidly (within hours) following salt restriction (33; 45), direct infusion of ANG II would still be expected to restore plasma ANG II levels faster than return to a LS diet. Previous studies of the time course of salt-induced changes in gene expression in the kidney of Dahl salt-sensitive rats and salt-resistant SS.13BN consomic controls (27) have shown that many genes are differentially expressed only at early time points (16 hours or 3 days) while other genes exhibit differences in expression only after prolonged (2 weeks) salt exposure. In addition, a recent study by Reed and coworkers (44) showed that the level of reactive oxygen species (ROS) is a crucial determinant of coronary collateral growth following myocardial ischemia in normotensive WKY rats, that coronary collateral growth is augmented by subpressor doses of ANG II and abrogated by hypertensive doses of ANG II, and that the temporal patterns of p38 and Akt activation are crucial determinants of whether or not coronary collateral growth occurs following myocardial ischemia. This raises the possibility that different levels of ROS or temporal differences in the expression of genes during ANG II infusion vs. salt restriction could exert differential effects on the regulatory mechanisms that determine the rate of recovery of vascular relaxation under the two different experimental conditions (27).
Another interesting question is whether low dose ANG II infusion will restore normal vascular function during chronic ingestion of a high salt diet in the same way that it restores vascular relaxation following short term increases in dietary salt intake. The similarity in resting tone and the lack of a difference in resting and maximum diameters of the arteries in animals fed short term vs. long term high salt diet suggest that ANG II infusion could restore vascular relaxation after prolonged exposure to HS diet. However, it is also possible that prolonged exposure to increased oxidative stress and/or differences in gene expression in key regulatory pathways could interfere with the ability of ANG II infusion to restore vascular relaxation in the face of long term increases in dietary salt intake. Another complicating factor would be the possible occurrence of hypertension in response to ANG II infusion in animals fed HS diet for a prolonged period of time, which did not occur in ANG II infused rats fed short term HS diet. A final consideration that could affect the degree of vascular relaxation in ANG II-infused rats maintained on chronic HS diet would be any changes in vessel distensibility or structural remodeling of the vessel that would interfere with vascular relaxation. While structural remodeling of the vessel was not present in the MCA used in the present studies, structural narrowing and reduced vessel distensibility does occur to some extent in skeletal muscle resistance arteries (13; 55) ([although the latter changes are not sufficient to eliminate vasodilator responses to forskolin, sodium nitroprusside, or Ca2+-free solution (55)].
Another intriguing question regarding the depression of vascular relaxation with HS diet is how does HS diet lead to the profound alterations in vascular signaling (12; 30; 59) that eventually culminate in the reduced vascular relaxation that occurs with elevated dietary salt intake? In our estimation, a likely mechanism for these alterations in vascular function is a chronic increase in vascular superoxide levels, which can adversely affect several different membrane signaling mechanisms (30; 59; 60). Relevant to the latter hypothesis, Lenda et al. (25; 26) proposed that reduced activity of antioxidant enzymes such as Cu/Zn SOD contributes to increased oxidative stress in skeletal muscle arterioles, leading to an impaired ability of the vessels to dilate in response to acetylcholine (and presumably other NO dependent vasodilators).
While increased oxidative stress in the face of reduced ANG II levels appears to be paradoxical at first, there is growing evidence that physiological levels of ANG II are essential to maintain normal expression and activity of the crucial antioxidant enzyme superoxide dismutase (SOD). This potential link between circulating ANG II and SOD expression/activity is supported by several findings. These include the observation by Fukai et al. (14) that ANG II infusion up-regulates extracellular SOD (ecSOD) expression in mouse aorta and human aortic smooth muscle cells; the report of Gongora et al. (17), who showed that ANG II infusion increases Cu/Zn SOD activity and improves endothelium-dependent vascular relaxation in aortas of ecSOD-deficient mice; and our finding that ANG II infusion prevents the reduced expression of Cu/Zn SOD in salt-fed rats (Figure 8). The importance of physiological levels of ANG II in maintaining normal vascular function is further supported by the findings of Reed and coworkers (44), who reported that subpressor levels of ANG II augment coronary collateral growth following myocardial ischemia while hypertensive doses of ANG II abrogate ischemia-induced collateral growth.
Mechanisms of Restored Relaxation to ACh and Reduced PO2 with ANG II Infusion or Return to Low-Salt Diet
Our data clearly indicate that the normal mechanism of ACh-induced MCA dilation in LS control animals is NO release from the endothelium (Figure 5). ACh-induced dilation via NO release is completely lost during both ST-HS and LT-HS diet, and this mechanism returns in the HS + ANG II and HS→LS groups (Figure 5).
In contrast to ACh-induced dilation, the normal mechanism of vasodilation is response to reduced PO2 in MCA from rats fed LS diet is endothelial release of the cyclooxygenase (COX)-dependent metabolite prostacyclin, which stimulates receptor-mediated signaling within the VSM cells (9; 30) that can be mimicked with stable prostacyclin analogues such as iloprost. Consistent with previous reports (9; 30), COX inhibition with indomethacin eliminated the hypoxic dilation in control rats fed LS diet, and reduced PO2 led to a paradoxical constriction of MCA from rats fed HS diet (Figure 6). COX-sensitive dilation was restored in both the HS + ANG II and the HS→LS groups. Interestingly, the vasoconstriction of MCA in response to reduced PO2 in rats fed HS diet was eliminated by indomethacin, indicating that a vasoconstrictor metabolite of COX [most likely thromboxane A2, with a possible contribution of prostaglandin H2 (30)] contributes to the paradoxical constriction in response to reduced PO2 in rats fed HS diet. NOS inhibition with L-NAME had no effect on vessel responses to reduced PO2 in any of the groups.
It is also worth noting that, while indomethacin eliminated hypoxic vasoconstriction in MCA from rats fed HS diet, it did not unmask a vasodilation. These findings contrast to the report of Drenjancevic-Peric et al. (7), who reported that MCA from Dahl salt-sensitive (SS) rats exhibited a paradoxical constriction in response to reduced PO2 that is converted to a vasodilation mediated by epoxyeicosatrienoic acids (EETs) when the vessel is treated with indomethacin. The latter study emphasizes that the emergence of various compensatory mechanisms of vascular relaxation is not uniform among different strains of rats, in different species (24; 49), and under different experimental conditions.
Effect of ANG II Infusion and Restoration of Low-Salt Diet on Endothelium-Independent Dilator Responses
In the present experiments, vasodilation in response to both the prostacyclin (IP) receptor agonist iloprost and the Gs protein activator cholera toxin were eliminated in MCA from rats fed HS diet, while dilation in response to the adenylyl cyclase activator forskolin was unaffected (Figure 7). These results suggest that HS diet impairs the endothelium-independent dilation in response to prostacyclin by uncoupling the Gs protein from downstream signaling components or via direct effects on the Gs protein itself while downstream mediators of vascular relaxation such as adenylyl cyclase are unaffected by HS diet.
In a final series of experiments, we determined the responses of MCA from the various groups to the NO donor sodium nitroprusside (SNP), in order to determine whether vessel sensitivity to NO was affected by any of the treatments. Vessel responses to SNP were similar in all groups (Figure 7), demonstrating that HS diet, ANG II infusion, and return to LS diet do not affect vessel sensitivity to NO in the MCA.
Summary and Conclusions
The present study demonstrates that a HS diet not only leads to an impaired ability of the cerebral circulation to increase its blood flow in response to the endothelium-dependent vasodilator acetylcholine, but also impairs endothelium-dependent and –independent vascular relaxation mechanisms in a manner that can be completely reversed by chronic low dose ANG II infusion or by returning the animals to a LS diet (although the recovery of vascular relaxation following return to LS diet is surprisingly prolonged, regardless of the duration of exposure to HS diet). These observations provide further support for the hypotheses that HS diet can compromise normal blood flow regulation independent of changes in arterial blood pressure, and that the mechanisms that are adversely affected by HS diet lie upstream in the signal transduction pathways in endothelial and vascular smooth muscle cells. Taken together, the results of this study indicate that normal circulating levels of ANG II play an important role in maintaining normal vascular relaxation mechanisms in cerebral resistance arteries, and that salt-induced ANG II suppression can lead to a profound impairment of vascular control mechanisms. These factors could be important contributors to the increased likelihood of normotensive salt-sensitive humans to eventually develop hypertension and to the higher mortality of normotensive salt-sensitive individuals compared to salt-resistant subjects (57).
Acknowledgements
The authors thank Tianjian Huang and Lynn Dondlinger for their outstanding assistance in this study. Supported by NIH grants #HL-65289 and #HL-72920.
Supported by NIH #HL-65289; and #HL72920.
References
- 1.Alkayed NJ, Birks EK, Hudetz AG, Roman RJ, Henerson L, Harder DR. Inhibition of brain P-450 arachidonic acid epoxygenase decreases baseline cerebral blood flow. Am J Physiol. 1996;271:H1541–H1546. doi: 10.1152/ajpheart.1996.271.4.H1541. [DOI] [PubMed] [Google Scholar]
- 2.Aviv A. Salt and hypertension: the debate that begs the bigger question. Arch Intern Med. 2001;161:507–510. doi: 10.1001/archinte.161.4.507. [DOI] [PubMed] [Google Scholar]
- 3.Aviv A. Salt consumption, reactive oxygen species and cardiovascular ageing: a hypothetical link. J Hypertens. 2002;20:555–559. doi: 10.1097/00004872-200204000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Barac A, Campia U, Panza JA. Methods for evaluating endothelial function in humans. Hypertension. 2007;49:748–760. doi: 10.1161/01.HYP.0000259601.38807.a6. [DOI] [PubMed] [Google Scholar]
- 5.Coyle P. High NaCl predisposes Dahl rats to cerebral infarction after middle cerebral artery occlusion. Hypertension. 1988;12:96–101. doi: 10.1161/01.hyp.12.2.96. [DOI] [PubMed] [Google Scholar]
- 6.Desai KM, Gopalakrishnan V, Hiebert LM, McNeill JR, Wilson TW. EDHF-mediated rapid restoration of hypotensive response to acetylcholine after chronic, but not acute, nitric oxide synthase inhibition in rats. Eur J Pharmacol. 2006;546:120–126. doi: 10.1016/j.ejphar.2006.06.072. [DOI] [PubMed] [Google Scholar]
- 7.Drenjancevic-Peric I, Phillips SA, Falck JR, Lombard JH. Restoration of normal vascular relaxation mechanisms in cerebral arteries by chromosomal substitution in consomic SS.13BN rats. Am J Physiol Heart Circ Physiol. 2005;289:H188–H195. doi: 10.1152/ajpheart.00504.2004. [DOI] [PubMed] [Google Scholar]
- 8.Fredricks KT, Liu Y, Lombard JH. Response of extraparenchymal resistance arteries of rat skeletal muscle to reduced PO2. Am J Physiol. 1994;267:H706–H715. doi: 10.1152/ajpheart.1994.267.2.H706. [DOI] [PubMed] [Google Scholar]
- 9.Fredricks KT, Liu Y, Rusch NJ, Lombard JH. Role of endothelium and arterial K+ channels in mediating hypoxic dilation of middle cerebral arteries. Am J Physiol. 1994;267:H580–H586. doi: 10.1152/ajpheart.1994.267.2.H580. [DOI] [PubMed] [Google Scholar]
- 10.Frisbee JC, Lombard JH. Development and reversibility of altered skeletal muscle arteriolar structure and reactivity with high salt diet and reduced renal mass hypertension. Microcirculation. 1999;6:215–225. [PubMed] [Google Scholar]
- 11.Frisbee JC, Roman RJ, Krishna UM, Falck JR, Lombard JH. Altered mechanisms underlying hypoxic dilation of skeletal muscle resistance arteries of hypertensive versus normotensive Dahl rats. Microcirculation. 2001;8:115–127. [PubMed] [Google Scholar]
- 12.Frisbee JC, Sylvester FA, Lombard JH. High-salt diet impairs hypoxia-induced cAMP production and hyperpolarization in rat skeletal muscle arteries. Am J Physiol Heart Circ Physiol. 2001;281:H1808–H1815. doi: 10.1152/ajpheart.2001.281.4.H1808. [DOI] [PubMed] [Google Scholar]
- 13.Frisbee JC, Weber DS, Liu Y, DeBruin JA, Lombard JH. Altered structure and mechanics of skeletal muscle arteries with high- salt diet and reduced renal mass hypertension. Microvasc Res. 2000;59:323–328. doi: 10.1006/mvre.1999.2222. [DOI] [PubMed] [Google Scholar]
- 14.Fukai T, Siegfried MR, Ushio-Fukai M, Griendling KK, Harrison DG. Modulation of extracellular superoxide dismutase expression by angiotensin II and hypertension. Circ Res. 1999;85:23–28. doi: 10.1161/01.res.85.1.23. [DOI] [PubMed] [Google Scholar]
- 15.Gebremedhin D, Lange AR, Lowry TF, Taheri MR, Birks EK, Hudetz AG, Narayanan J, Falck JR, Okamoto H, Roman RJ, Nithipatikom K, Campbell WB, Harder DR. Production of 20-HETE and its role in autoregulation of cerebral blood flow. Circ Res. 2000;87:60–65. doi: 10.1161/01.res.87.1.60. [DOI] [PubMed] [Google Scholar]
- 16.Gerrits RJ, Stein EA, Greene AS. Laser-Doppler flowmetry utilizing a thinned skull cranial window preparation and automated stimulation. Brain Research Protocols. 1998;3:14–21. doi: 10.1016/s1385-299x(98)00016-6. [DOI] [PubMed] [Google Scholar]
- 17.Gongora MC, Qin Z, Laude K, Kim HW, McCann L, Folz JR, Dikalov S, Fukai T, Harrison DG. Role of extracellular superoxide dismutase in hypertension. Hypertension. 2006;48:473–481. doi: 10.1161/01.HYP.0000235682.47673.ab. [DOI] [PubMed] [Google Scholar]
- 18.Graudal NA, Galloe AM, Garred P. Effects of sodium restriction on blood pressure, renin, aldosterone, catecholamines, cholesterols, and triglyceride: a meta-analysis. JAMA. 1998;279:1383–1391. doi: 10.1001/jama.279.17.1383. [DOI] [PubMed] [Google Scholar]
- 19.Gross V, Kurth TM, Skelton MM, Mattson DL, Cowley AW., Jr. Effects of daily sodium intake and ANG II on cortical and medullary renal blood flow in conscious rats. Am J Physiol. 1998;274:R1317–R1323. doi: 10.1152/ajpregu.1998.274.5.R1317. [DOI] [PubMed] [Google Scholar]
- 20.Hansen-Smith FM, Morris LW, Greene AS, Lombard JH. Rapid microvessel rarefaction with elevated salt intake and reduced renal mass hypertension in rats. Circ Res. 1996;79:324–330. doi: 10.1161/01.res.79.2.324. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez I, Cowley AW, Jr., Lombard JH, Greene AS. Salt intake and angiotensin II alter microvessel density in the cremaster muscle of normal rats. Am J Physiol. 1992;263:H664–H667. doi: 10.1152/ajpheart.1992.263.3.H664. [DOI] [PubMed] [Google Scholar]
- 22.Hooper L, Bartlett C, Davey SG, Ebrahim S. Systematic review of long term effects of advice to reduce dietary salt in adults. BMJ. 2002;325:628. doi: 10.1136/bmj.325.7365.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karppanen H, Mervaala E. Sodium intake and hypertension. Prog Cardiovasc Dis. 2006;49:59–75. doi: 10.1016/j.pcad.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Lamping KG, Nuno DW, Shesely EG, Maeda N, Faraci FM. Vasodilator mechanisms in the coronary circulation of endothelial nitric oxide synthase-deficient mice. Am J Physiol Heart Circ Physiol. 2000;279:H1906–H1912. doi: 10.1152/ajpheart.2000.279.4.H1906. [DOI] [PubMed] [Google Scholar]
- 25.Lenda DM, Boegehold MA. Effect of a high salt diet on microvascular antioxidant enzymes. J Vasc Res. 2002;39:41–50. doi: 10.1159/000048992. [DOI] [PubMed] [Google Scholar]
- 26.Lenda DM, Sauls BA, Boegehold MA. Reactive oxygen species may contribute to reduced endothelium-dependent dilation in rats fed high salt. Am J Physiol Heart Circ Physiol. 2000;279:H7–H14. doi: 10.1152/ajpheart.2000.279.1.H7. [DOI] [PubMed] [Google Scholar]
- 27.Liang M, Yuan B, Rute E, Greene AS, Olivier M, Cowley AW., Jr. Insights into Dahl salt-sensitive hypertension revealed by temporal patterns of renal medullary gene expression. Physiol Genomics. 2003;12:229–237. doi: 10.1152/physiolgenomics.00089.2002. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Fredricks KT, Roman RJ, Lombard JH. Response of resistance arteries to reduced PO2 and vasodilators during hypertension and elevated salt intake. Am J Physiol. 1997;273:H869–H877. doi: 10.1152/ajpheart.1997.273.2.H869. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Rusch NJ, Lombard JH. Loss of endothelium and receptor-mediated dilation in pial arterioles of rats fed a short-term high salt diet. Hypertension. 1999;33:686–688. doi: 10.1161/01.hyp.33.2.686. [DOI] [PubMed] [Google Scholar]
- 30.Lombard JH, Sylvester FA, Phillips SA, Frisbee JC. High-salt diet impairs vascular relaxation mechanisms in rat middle cerebral arteries. Am J Physiol Heart Circ Physiol. 2003;284:H1124–H1133. doi: 10.1152/ajpheart.00835.2002. [DOI] [PubMed] [Google Scholar]
- 31.Marvar PJ, Falck JR, Boegehold MA. High dietary salt reduces the contribution of 20-HETE to arteriolar oxygen responsiveness in skeletal muscle. Am J Physiol Heart Circ Physiol. 2007;292:H1507–H1515. doi: 10.1152/ajpheart.00754.2006. [DOI] [PubMed] [Google Scholar]
- 32.Marvar PJ, Nurkiewicz TR, Boegehold MA. Reduced arteriolar responses to skeletal muscle contraction after ingestion of a high salt diet. J Vasc Res. 2005;42:226–236. doi: 10.1159/000085461. [DOI] [PubMed] [Google Scholar]
- 33.Menachery A, Braley LM, Kifor I, Gleason R, Williams GH. Dissociation in plasma renin and adrenal ANG II and aldosterone responses to sodium restriction in rats. Am J Physiol. 1991;261:E487–E494. doi: 10.1152/ajpendo.1991.261.4.E487. [DOI] [PubMed] [Google Scholar]
- 34.Meng S, Cason GW, Gannon AW, Racusen LC, Manning RD., Jr. Oxidative stress in Dahl salt-sensitive hypertension. Hypertension. 2003;41:1346–1352. doi: 10.1161/01.HYP.0000070028.99408.E8. [DOI] [PubMed] [Google Scholar]
- 35.Messerli FH, Schmieder RE. Salt and hypertension: going to the heart of the matter. Arch Intern Med. 2001;161:505–506. doi: 10.1001/archinte.161.4.505. [DOI] [PubMed] [Google Scholar]
- 36.Midgley JP, Matthew AG, Greenwood CM, Logan AG. Effect of reduced dietary sodium on blood pressure: a meta-analysis of randomized controlled trials. JAMA. 1996;275:1590–1597. doi: 10.1001/jama.1996.03530440070039. [DOI] [PubMed] [Google Scholar]
- 37.Nagata C, Takatsuka N, Shimizu N, Shimizu H. Sodium intake and risk of death from stroke in Japanese men and women. Stroke. 2004;35:1543–1547. doi: 10.1161/01.STR.0000130425.50441.b0. [DOI] [PubMed] [Google Scholar]
- 38.Panza JA, Casino PR, Badar DM, Quyyumi AA. Effect of increased availability of endothelium-derived nitric oxide precursor on endothelium-dependent vascular relaxation in normal subjects and in patients with essential hypertension. Circulation. 1993;87:1475–1481. doi: 10.1161/01.cir.87.5.1475. [DOI] [PubMed] [Google Scholar]
- 39.Panza JA, Casino PR, Kilcoyne CM, Quyyumi AA. Impaired endothelium-dependent vasodilation in patients with essential hypertension: Evidence that the abnormality is not at the muscarinic receptor level. J Am Coll Cardiol. 1994;23:1610–1616. doi: 10.1016/0735-1097(94)90664-5. [DOI] [PubMed] [Google Scholar]
- 40.Panza JA, Garcia CE, Kilcoyne CM, Quyyumi AA, Cannon RO., III Impaired endothelium-dependent vasodilation in patients with essential hypertension. Evidence that nitric oxide abnormality is not localized to a single signal transduction pathway. Circulation. 1995;91:1732–1738. doi: 10.1161/01.cir.91.6.1732. [DOI] [PubMed] [Google Scholar]
- 41.Panza JA, Quyyumi AA, Brush JE, Jr., Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 1990;323:22–27. doi: 10.1056/NEJM199007053230105. [DOI] [PubMed] [Google Scholar]
- 42.Petersen MC, Munzenmaier DH, Greene AS. Angiotensin II infusion restores stimulated angiogenesis in the skeletal muscle of rats on a high-salt diet. Am J Physiol Heart Circ Physiol. 2006;291:H114–H120. doi: 10.1152/ajpheart.01116.2005. [DOI] [PubMed] [Google Scholar]
- 43.Phillips SA, Lombard JH. Chronic AT1 receptor blockade alters the mechanisms mediating hypoxic dilation in middle cerebral arteries. J Cardiovasc Pharmacol. 2005;46:706–712. doi: 10.1097/01.fjc.0000184118.76188.8c. [DOI] [PubMed] [Google Scholar]
- 44.Reed R, Kolz C, Potter B, Rocic P. The mechanistic basis for the disparate effects of angiotensin II on coronary collateral growth. Arterioscler Thromb Vasc Biol. 2008;28:61–67. doi: 10.1161/ATVBAHA.107.154294. [DOI] [PubMed] [Google Scholar]
- 45.Rogacz S, Williams GH, Hollenberg NK. Time course of enhanced adrenal responsiveness to angiotensin on a low salt diet. Hypertension. 1990;15:376–380. doi: 10.1161/01.hyp.15.4.376. [DOI] [PubMed] [Google Scholar]
- 46.Simon G. Experimental evidence for blood pressure-independent vascular effects of high sodium diet. Am J Hypertens. 2003;16:1074–1078. doi: 10.1016/j.amjhyper.2003.07.019. [DOI] [PubMed] [Google Scholar]
- 47.Smeda JS, Payne GW. Alterations in autoregulatory and myogenic function in the cerebrovasculature of Dahl salt-sensitive rats. Stroke. 2003;34:1484–1490. doi: 10.1161/01.STR.0000073842.18224.AA. [DOI] [PubMed] [Google Scholar]
- 48.Sofola OA, Knill A, Hainsworth R, Drinkhill M. Change in endothelial function in mesenteric arteries of Sprague-Dawley rats fed a high salt diet. J Physiol. 2002;543:255–260. doi: 10.1113/jphysiol.2002.022277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun D, Huang A, Smith CJ, Stackpole CJ, Connetta JA, Shesely EG, Koller A, Kaley G. Enhanced release of prostaglandins contributes to flow-induced arteriolar dilation in eNOS knockout mice. Circ Res. 1999;85:288–293. doi: 10.1161/01.res.85.3.288. [DOI] [PubMed] [Google Scholar]
- 50.Sylvester FA, Stepp DW, Frisbee JC, Lombard JH. High-salt diet depresses acetylcholine reactivity proximal to NOS activation in cerebral arteries. Am J Physiol Heart Circ Physiol. 2002;283:H353–H363. doi: 10.1152/ajpheart.00127.2002. [DOI] [PubMed] [Google Scholar]
- 51.Tobian L, Hanlon S. High sodium chloride diets injure arteries and raise mortality without changing blood pressure. Hypertension. 1990;15:900–903. doi: 10.1161/01.hyp.15.6.900. [DOI] [PubMed] [Google Scholar]
- 52.Turko IV, Murad F. Protein nitration in cardiovascular diseases. Pharmacol Rev. 2002;54:619–634. doi: 10.1124/pr.54.4.619. [DOI] [PubMed] [Google Scholar]
- 53.Uchida S, Suzuki A, Kagitani F, Hotta H. Effects of age on cholinergic vasodilation of cortical cerebral blood vessels in rats. Neurosci Lett. 2000;294:109–112. doi: 10.1016/s0304-3940(00)01556-1. [DOI] [PubMed] [Google Scholar]
- 54.Weber DS, Frisbee JC, Lombard JH. Selective potentiation of angiotensin II-induced constriction of skeletal muscle resistance arteries by chronic elevations in dietary salt intake. Microvasc Res. 1999;57:310–319. doi: 10.1006/mvre.1999.2147. [DOI] [PubMed] [Google Scholar]
- 55.Weber DS, Lombard JH. Elevated salt intake impairs dilation of skeletal muscle resistance arteries via angiotensin II suppression. Am J Physiol. 2000;278:H500–H506. doi: 10.1152/ajpheart.2000.278.2.H500. [DOI] [PubMed] [Google Scholar]
- 56.Weber DS, Lombard JH. Angiotensin II AT1 receptors preserve vasodilator reactivity in skeletal muscle resistance arteries. Am J Physiol Heart Circ Physiol. 2001;280:H2196–H2202. doi: 10.1152/ajpheart.2001.280.5.H2196. [DOI] [PubMed] [Google Scholar]
- 57.Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension. 2001;37:429–432. doi: 10.1161/01.hyp.37.2.429. [DOI] [PubMed] [Google Scholar]
- 58.Zagorac D, Yamaura K, Zhang C, Roman RJ, Harder DR. The effect of superoxide anion on autoregulation of cerebral blood flow. Stroke. 2005;36:2589–2594. doi: 10.1161/01.STR.0000189997.84161.95. [DOI] [PubMed] [Google Scholar]
- 59.Zhu J, Drenjancevic-Peric I, McEwen S, Friesema J, Schulta D, Yu M, Roman RJ, Lombard JH. Role of superoxide and angiotensin II suppression in salt-induced changes in endothelial Ca2+ signaling and NO production in rat aorta. Am J Physiol Heart Circ Physiol. 2006;291:H929–H938. doi: 10.1152/ajpheart.00692.2005. [DOI] [PubMed] [Google Scholar]
- 60.Zhu J, Huang T, Lombard JH. Effect of high-salt diet on vascular relaxation and oxidative stress in mesenteric resistance arteries. J Vasc Res. 2007;44:382–390. doi: 10.1159/000102955. [DOI] [PubMed] [Google Scholar]