Abstract
A significant number of youths use cigarettes, and more than half of the youths who smoke daily also use illicit drugs. The focus of these studies is on how exposure to nicotine affects subsequent responses to both nicotine and cannabinoids in adolescents compared with adults. We have shown previously that chronic treatment with nicotine produces sensitization to its locomotor-activating effects in female and adult rats but not male adolescent rats. To better understand the effects of nicotine on adolescent and adult rats, rats were injected with nicotine or saline for 7 days and, on day 8, either challenged with delta-9-tetrahydrocannabinol (Δ9-THC) or the cannabinoid agonist CP 55,940 and tested for locomotor activity, or the brains were removed for quantitative autoradiography studies of the cannabinoid1 receptor. A separate group of rats was treated with nicotine plus the cannabinoid antagonist AM 251 and then challenged with CP 55,940. In adolescent male rats, nicotine administration led to sensitization to the locomotor-decreasing effects of both Δ9-THC and CP 55,940, but in adult male rats, the response to either drug was unchanged compared to controls. The effect of nicotine on CP 55,940-mediated locomotor activity was blocked by co-administration of AM 251 with the nicotine. Further, cannabinoid receptor density was increased in the prelimbic prefrontal cortex, ventral tegmental area, and select regions of the hippocampus in adolescent male rats pretreated with nicotine compared to vehicle-treated controls. There were no significant changes in cannabinoid receptor binding, however, in any of the brain regions examined in adult males pretreated with nicotine. The prelimbic prefrontal cortex and the hippocampus have been shown previously to be involved in stimulant reinforcement; thus it is possible that these changes contribute to the unique behavioral effects of chronic nicotine and subsequent drug administration in adolescents compared with adults.
Keywords: autoradiography, cannabinoid, locomotor activity, nicotine, adolescent
Adolescents who smoke are 11 times more likely to try marijuana than those that do not smoke, and although adolescents who already are cigarette smokers make up only 12% of marijuana non-users, they make up almost half of those who initiate marijuana use (reviewed by Hornik, 2003). In addition, nicotine use between ages 14 and 17 yrs predicted progression to cannabis use (Hofler et al., 1999) and high school seniors who used cigarettes before the age of 13 used marijuana 3.3 times more than those who had never smoked (Merrill et al., 1999). Because many adolescents begin with nicotine and progress to other drugs of abuse (Kandel, 1975), it has been theorized that use of cigarettes in adolescents leads to the use of marijuana. However, an earlier study did not establish causality between nicotine and marijuana (Kandel et al., 1992). In a later long term study of smoking patterns of Israeli smokers, an economic model was used to study progression from cigarette smoking to marijuana use (Beenstock and Rahav, 2002), and that study showed that when cigarettes were less expensive, marijuana use was higher. Other researchers have postulated a reverse pattern: that marijuana use leads to smoking (Tullis et al., 2003).
Laboratory studies have shown that adolescent rats have a unique response to nicotine compared with adult rats, although the findings vary. For example, exposure to nicotine changes nicotine receptor density, and these changes vary according to age of the subject during exposure, as well as the type of nicotinic receptor measured. It has been reported that, in general across brain regions, periadolescent male rats show higher densities of α4β2* compared with adult counterparts, but that nicotine pretreatment caused a greater upregulation of that receptor subtype in adults than in adolescents (Doura et al., 2008). In contrast, there was a down-regulation of the α6 receptor subtype, and this down-regulation was greater in adolescents treated with nicotine compared with adults. An earlier study showed that α7 nicotinic receptors were upregulated in the striatum after 17 days of nicotine administration during adolescence (Slotkin et al., 2004). There also are differences between adolescent and adult rats in their behavioral responses to nicotine. One study found that adolescent male rats showed conditioned place preference to nicotine (Vastola et al., 2002), while another showed that conditioned place preference to nicotine only occurred during late adolescence and not during early adolescence (Belluzzi et al., 2004). It also has been reported that adolescent male rats became sensitized to the locomotor-activating effects of nicotine (Faraday et al., 2003; Green et al., 2003). In other studies, however, periadolescent male rats did not become sensitized to the locomotor-stimulant effects of nicotine during a 7-day pretreatment period, in contrast to the sensitization that developed in periadolescent female and adult male and female rats (Collins and Izenwasser, 2004; Collins et al., 2004b; Schochet et al., 2004). In addition, female rats that began self-administering nicotine during adolescence administered more nicotine over a 4-week period that extended into adulthood than rats that began self-administration as adults (Levin et al., 2003). Similar findings later were observed in male rats (Levin et al., 2007). This suggests that nicotine exposure in adolescence has effects on behavior that last into adulthood.
Nicotine also has effects on responses to other drugs at a neurochemical and behavioral level. Our laboratory has demonstrated that treatment with nicotine for 7 days during adolescence led to an increased locomotor response to both cocaine (Collins and Izenwasser, 2004) and amphetamine (Collins et al., 2004a) and that these changes persisted into adulthood. In addition, nicotine administered during adolescence increased dopamine transporter and decreased serotonin transporter densities in the nucleus accumbens and caudate putamen, but had no effect during adulthood (Collins et al., 2004b). In contrast, nicotinic receptors were increased in adults, but not in adolescents. Together, these data suggest that there are changes that occur in the adolescent brain in response to nicotine that may produce unique behavioral responses to subsequent drug exposure within the adolescent period and at later time points past the adolescent period and into adulthood. In addition to these studies on nicotine, it also has been shown that the effects of cannabinoid agonists can be different during adolescence from their effects in adults. For example, studies have shown that administration of a cannabinoid agonist during adolescence leads to differential long-term neuroadaptations in regulating the effects of both opioids (Ambrosio et al., 1999; Crespo et al., 2001) and cocaine (Higuera-Matas et al., 2008; Higuera et al., 2005). Thus, there are indications that both the nicotinic and cannabinoid systems may be differentially regulated in adolescents and in adults.
Although studies suggest that smoking may lead to marijuana use in adolescents, there are very few preclinical studies that demonstrate any effect of nicotine on the cannabinoid system, and even fewer using a model of adolescence. The adolescent rat can be used to model adolescent-onset drug use and evaluate the consequences of early nicotine exposure. In the present studies, periadolescent and adult rats were treated for seven days with nicotine or vehicle. One day later, the effect of Δ9-THC or the cannabinoid agonist CP 55,940, in the presence or absence of the cannabinoid antagonist AM 251 was examined on locomotor activity, or cannabinoid receptor density was measured.
2. Experimental Procedures
The animals used in this study were maintained and the studies were conducted in accordance with the guidelines of the Guide for Care and Use of Laboratory Animals, National Research Council, Department of Health, Education and Welfare, NIH Publication 85-23, revised 1996. Sprague-Dawley male periadolescent (postnatal day 30) and adult (postnatal day 60) rats (Charles River, Wilmington, MA) were used in all studies. Periadolescence is a period of early adolescence between approximately postnatal day (PND) 30-42 (Spear and Brake, 1983). Rats were housed two per cage in a temperature and humidity-controlled environment under a 12 h light/dark cycle. Food and water were available ad libitum.
2.1. Chemicals
Isotopes and drugs were obtained from the following sources: 1-(2,4-dichlorophenyl)-5-(4-iodophenyl]])-4-methyl-N-(1-piperidyl)pyrazole-3-carboxamide1-(2,4-dichlorophenyl)-5-(4-iodophenyl]])-4-methyl-N-(1-piperidyl)pyrazole-3-carboxamide (AM 251), Δ9-THC, and 2-[(1S,2R,5S)-5-hydroxy-2-(3-hydroxypropyl) cyclohexyl]-5-(2-methyloctan-2-yl)phenol (CP 55, 940) from the National Institute on Drug Abuse (Rockville, MD). (-)-Nicotine hydrogen tartrate salt from Sigma Chemical Co. (St. Louis, MO); [3H]CP 55,940 (approximately 168 Ci/mmol) from PerkinElmer Life and Analytical Sciences, Inc. (Boston, MA).
2.2. Locomotor activity
Animals were maintained on a 12 h light/dark schedule with lights on at 7 a.m. and off at 7 p.m. All behavioral testing was done during the light schedule between 9 a.m. and 4 p.m. with each group tested at the same hour each day and the groups randomized over the course of the day.
We have shown previously that the effects of once daily injections of nicotine (0.4 mg/kg) or twice daily injections of nicotine (2 × 0.4 mg/kg 4 h apart for a total daily dose of 0.8 mg/kg) for 7 days produced sensitization to nicotine in adult male rats and in adult and periadolescent female rats. In contrast, sensitization to nicotine did not develop subsequent to either dosing schedule in periadolescent male rats (Collins and Izenwasser, 2004; Collins et al., 2004a). The difference between the adult and adolescent male rats has since been confirmed by another laboratory (Schochet et al., 2004). Thus, it does not appear that the different behavioral adaptations in response to nicotine in the adult and adolescent rats are due to the dose used in the study. Based upon these previous data, we selected a dose of 0.4 mg/kg nicotine/day administered in a single daily injection for the present studies.
To measure locomotor activity, rats were placed in clear acrylic chambers (40 × 40 cms) inside Digiscan activity monitors (Omnitech Electronics, Columbus, OH) that were equipped with infrared light sensitive detectors mounted 2.5 cm apart along two perpendicular walls. Mounted along the opposing walls were infrared light beams that were directed at the detectors.
Experiment 1
Periadolescent male rats at PND 30 (average weight 99 ± 2 g at the start of the experiment) and adult male rats at PND 60 (average weight 265 ± 1 g at the start of the experiment) were used in the nicotine/cannabinoid study. There were two groups at each age, with 8 rats in each group. One group was injected once daily for 7 days with 0.4 mg/kg nicotine/day (dose was based on weight of the base; i.p.) and the other was injected daily with vehicle (saline). On the first 2 days and the last 2 days of the treatment period, each rat was placed in a locomotor activity chamber for a 15 min habituation period followed by an injection of nicotine or saline and activity was measured 1 h. On day 8, all rats were put in the locomotor chambers for 15 min after which they were injected with vehicle or Δ9-THC (3.0 mg/kg) and locomotor activity was measured for 1 h.
Prior to this experiment, a dose-effect curve for Δ9-THC was run to determine the effects of increasing doses so that an appropriate dose of this drug in adolescent and adult males could be selected for the nicotine study. All rats then were injected with vehicle (saline), followed by 0.3,1.0, 3.0, 5.6 and 10 mg/kg Δ9-THC (i.p.) in a cumulative dosing regimen (actual injections of 0.3, 0.7, 2.0, 3.6, and 4.4 mg/kg Δ9-THC). Fifteen minutes after each injection, locomotor activity was measured for a total of 30 min for vehicle and for each cumulative dose of Δ9-THC. This cumulative dosing procedure allowed us to measure the effects of multiple doses of Δ9-THC in a single day without the use of additional animals. Thus, all doses could be tested in a single animal at the same point in development. It has been shown that this dosing regimen produces data that are not significantly different from that found when each dose is given to individual animals (Terry, 1992).
Experiment 2
An initial test was done to determine the time-course of the locomotor-decreasing effects of the cannabinoid agonist CP 55,940 so that the correct pretreatment time could be used. To do this, rats were injected with the cannabinoid agonist CP 55,940 at a dose of 0.3 mg/kg, or vehicle (6 rats/group), and placed in a locomotor testing chamber for 60 min. Rats were placed in clear acrylic chambers (40.64 × 40.64 cm) inside Digiscan activity monitors (Omnitech Electronics, Columbus, OH) that were equipped with infrared light sensitive detectors mounted 2.5 cm apart along two perpendicular walls. Mounted along the opposing walls were infrared light beams that were directed at the detectors. One count of horizontal activity was registered each time the subject interrupted a beam. Activity was monitored for 60 min and beam breaks were measured during 12 consecutive 5 min time periods.
Periadolescent male rats at PND 30 (average weight 103 ± 2 g at the start of the experiment) and adult male rats at PND 60 (average weight 257 ± 2 g at the start of the experiment) were used. Rats were treated as in experiment 1. On day 8, all rats were put in the locomotor chambers for 15 min prior to the first injection. All rats then were injected with vehicle (saline), followed by 0.1, 0.3, 0.56 and 1.0 mg/kg CP 55,940 (i.p.) in a cumulative dosing regimen (actual injections of 0.1, 0.2, 0.26, 0.44 mg/kg CP 55,940). Following each injection, locomotor activity was measured for a total of 15 min for vehicle and for each cumulative dose of CP 55,940 (based upon the determination that CP 55,940 had produced effects within that time period). Testing began 5 min after each injection. This cumulative dosing procedure allowed us to measure the effects of multiple doses of CP 55,940 in a single day without the use of additional animals.
Experiment 3
Male adult rats at PND 60 (average weight 277 ± 2 g at the start of the experiment) and periadolescent rats at PND 30 (average weight 101 ± 1) were treated once daily for seven days with 3 mg/kg AM 251 followed 15 minutes later by nicotine (0.4 mg/kg i.p.) or vehicle. Each group had 6 rats. Five min later, locomotor activity was measured for 60 min. On day 8 (one day after the last pretreatment), the effects of CP 55,940 on locomotor activity were examined using cumulative dosing as described for experiment 2.
2.3. Quantitative Autoradiography
Periadolescent male rats at PND 30 (average weight 90 ± 4 g at the start of the experiment) and adult male rats at approximately PND 60 (average weight 293 ± 3 g at the start of the experiment were housed two per cage in a temperature and humidity-controlled environment under a 12 h light/dark cycle. Food and water were available ad libitum. Rats (6/group) were injected for 7 days with 0.4 mg/kg nicotine/day (dose was based on weight of the base; i.p.) or vehicle (saline) once daily for seven days and decapitated on day 8, and brains removed for neurochemistry. Their brains were removed quickly and frozen in isopentane at -35°C, then stored at -70°C prior to slicing. Slices (20μm) from the prefrontal cortex, caudate putamen, nucleus accumbens, substantia nigra, ventral tegmental area, and hippocampus were thaw-mounted on gelatin/chromate-coated slides and stored at -70°C prior to assay. These brain regions were chosen because the medial prefrontal cortex (Piazza et al., 1991; Schenk et al., 1991; Steketee, 2003; Weissenborn et al., 1997), hippocampus (Nestler, 2002; Robbins and Everitt, 2002; Volkow et al., 2002), ventral tegmental area (Stratta et al., 1995; Vezina, 2004), and substantia nigra (Stratta et al., 1995; Unterwald et al., 1994) have been shown to be involved in mediating various behaviors related to drug abuse. Further, it has been shown that cannabinoid receptor levels are high in the hippocampus and the prefrontal cortex (Mailleux and Vanderhaeghen, 1992; Tsou et al., 1998).
For the cannabinoid receptor autoradiography assay, sections were thawed to room temperature and incubated for 2 hr with 3 nM [3H]CP 55,940 in binding buffer (50 mM Tris-HCl, pH 7.4, with 5% BSA), essentially as described previously (Oviedo et al., 1993; Westlake et al., 1994). Sections were then washed 4 × 30 min in ice-cold buffer (50 mM Tris-HCl with 1% BSA), followed by 5 min at 25°C in buffer containing 50 mM Tris-HCl with 0.5% formaldehyde solution. Slides were then dipped quickly in ice-cold deionized water and dried. Nonspecific binding was defined as binding in the presence of 10 μM CP 55,940. Slides and standards (3H-labeled microscales, Amersham Corp., Arlington Heights, IL) were apposed to tritium sensitive film for 3 days for the caudate putamen/nucleus accumbens and prefrontal cortex sections, and 4 days for the hippocampus/SN/VTA sections. Films were developed in Kodak GBX developer and fixative.
2.4. Data Analysis
Nicotine-stimulated locomotor activity was analyzed using a two-way analysis of variance (ANOVA) with treatment (nicotine vs vehicle) and day as the main effects. Post hoc analysis using Fisher's Protected Least Significant Difference (PLSD) was used when warranted. P values less than 0.05 were considered significant for all tests.
For the challenge locomotor activity studies, data from the challenge day (day 8) were analyzed by a two-factor (pretreatment drug × CP 55,940 dose) ANOVA with repeated measures for each of the groups tested (periadolescent males and adult males). Post hoc analysis using Fisher's Protected Least Significant Difference (PLSD) was used when warranted. P values less than 0.05 were considered significant for all tests.
For the autoradiography studies, data were analyzed using a Macintosh-based image analysis system (NIH Image 1.60 software). Brain images were quantified using curves generated from the labeled standards. Data were analyzed by a two-factor ANOVA (pretreatment × brain region) and post hoc analysis using Fisher's Protected Least Significant Difference (PLSD) was used when warranted. P values less than 0.05 were considered significant for all tests.
3. Results
3.1. Locomotor Activity
Experiment 1
Nicotine increased activity over vehicle on all of the days tested in both adult male (ADM) and adolescent male (PAM) rats (Fig. 1). There was a significant effect of treatment (F(1,240)=220.2, P<0.0001), of age (F(1,240)=5.559, P<0.0192) and of day (F(3,240)=22.46, P<0.0001). In addition, there were significant treatment × day and treatment × age interactions. Sensitization to the locomotor-activating effects of nicotine developed in the adult rats such that activity levels were significantly higher on day seven than on day one (P≤0.008). In contrast, there was no significant change in the activity in the adolescent males as a function of the baseline (vehicle) level, with nicotine-stimulated activity being similar on day 1 and day 7 in the adolescent rats.
Figure 1.
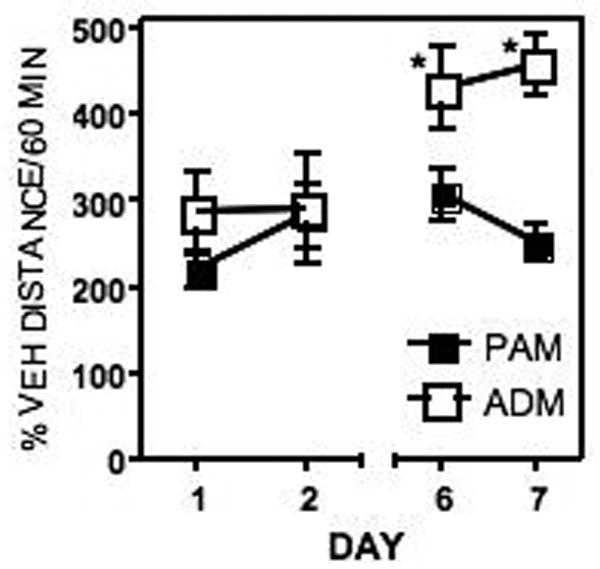
Locomotor activity in response to daily injections of nicotine (0.4 mg/kg/day). Activity was measured on the first 2 days (days 1 and 2) and last 2 days of the treatment period (days 6 and 7). Nicotine increased activity in both adolescent (PAM) and adult (ADM) rats, however, only the adult rats developed a significant sensitization to the locomotor-activating effects of nicotine. Data are expressed as % total distance traveled with vehicle injection (baseline) per 60 min test for adult male (ADM, unfilled squares) or periadolescent male (PAM, filled squares) rats. *Significantly different from day 1.
Figure 2 shows the dose-effect curves for Δ9-THC in adult (ADM) and adolescent (PAM) rats expressed as % baseline. The dose-effect curves for Δ9-THC are similar at both ages, with Δ9-THC decreasing activity in both groups in a dose-dependent manner. A dose of 3.0 mg/kg Δ9-THC was chosen as the challenge dose for subsequent experiments.
Figure 2.
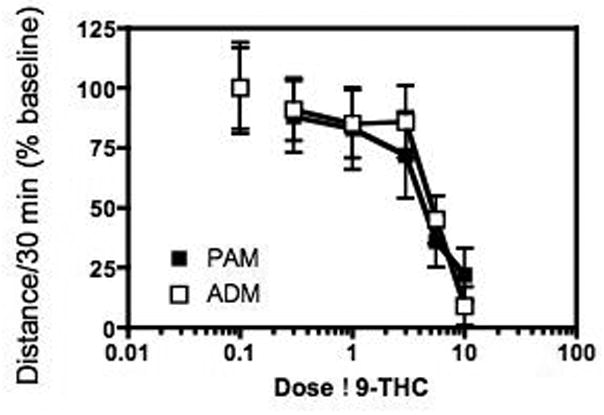
Effect of Δ9-THC on locomotor activity in naïve adult (ADM) and adolescent (PAM) rats. Activity was measured using a cumulative dosing procedure and Δ9-THC decreased activity in both groups of animals. The curves for decreasing locomotor activity were not significantly different in adolescent and adult rats.
In control animals, 3.0 mg/kg Δ9-THC had no effect on locomotor activity in either adolescent (Fig. 3A) or adult (Fig. 3B) rats (compare VEH/THC to VEH/VEH). In the adolescent rats, prior treatment with nicotine significantly decreased locomotor activity compared to vehicle pretreatment (t(14)=2.987, P≤0.0098; Fig. 3A). In contrast, there was no effect of Δ9-THC on locomotor activity in the adult rats after nicotine treatment compared with vehicle (Fig. 3B).
Figure 3.
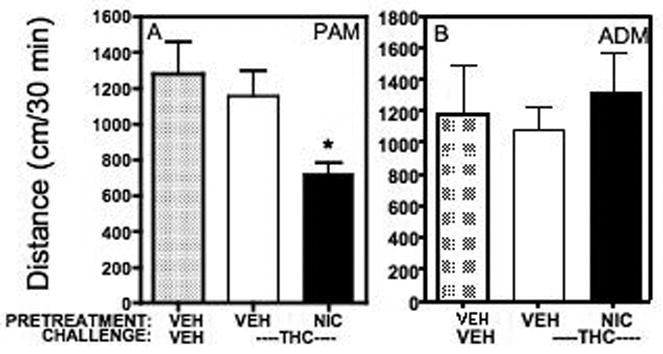
Effect of 3.0 mg/kg Δ9-THC after 7 days of administration of nicotine in (A) adolescent or (B) adult rats. Δ9-THC had no effect on locomotor activity after vehicle treatment (compare VEH/VEH to NIC/VEH). However, Δ9-THC significantly decreased activity in adolescent rats after treatment with nicotine compared to vehicle (compare VEH/THC to NIC/VEH), but still had no effect in adult rats. *significant decrease in activity compared with vehicle.
Experiment 2
The time-course study for a single dose of 0.3 mg/kg CP 55,940 showed that activity was reduced in response to the drug for the first 15 min of testing compared with vehicle administration (Fig. 4). After this time, activity levels in the vehicle-injected animals were so low that it was difficult to measure a decrease in activity. Thus, for the cumulative dosing curve (Fig 5), the rats were injected with each dose and activity was measured for 15 min, followed by administration of the next dose.
Figure 4.
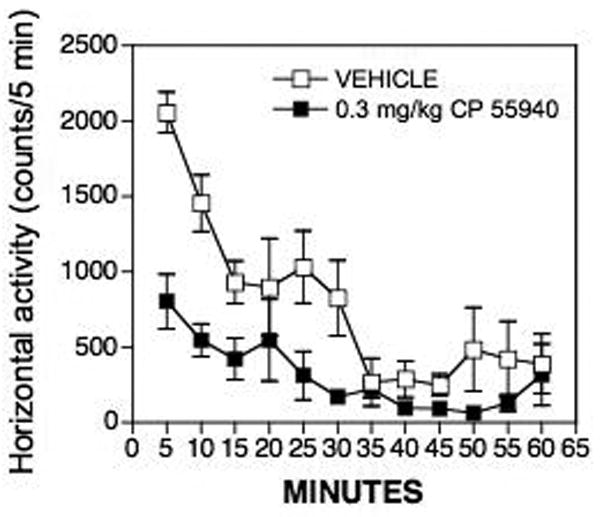
Time course of locomotor activity in response to an acute injection of 0.3 mg/kg CP 55,940 or vehicle in adult male rats. Activity was measured at 5 min intervals. Rats given CP 55,940 exhibited lower levels of activity about 15 min after injection compared with rats given vehicle.
Figure 5.
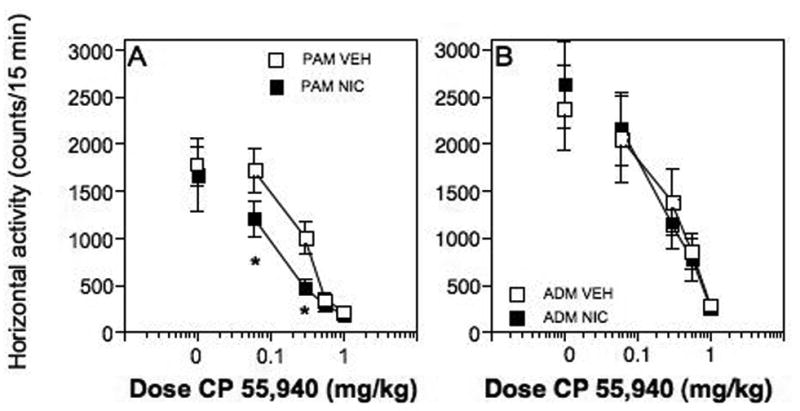
Locomotor activity in response to a CP 55,940 challenge on day 8, one day after a 7-day pretreatment with daily injections of nicotine or vehicle. (A) Periadolescent male rats pretreated with nicotine had significantly decreased activity levels in response to 0.1 and 0.3 mg/kg CP 55,940 compared to periadolescent male rats pretreated with vehicle (p < 0.05). (B) Activity levels were not significantly different in response to CP 55,940 in adult male rats pretreated with nicotine or vehicle. *significant difference compared with vehicle controls (p < 0.05).
There was a significant interaction of pretreatment drug × CP 55,940 dose in periadolescent male rats pretreated with nicotine compared to periadolescent male rats pretreated with vehicle on day 8 (F[3,32] = 3.49, p ≤ 0.03; Fig 5A). Overall, there was a significant decrease in horizontal activity at lower doses of CP 55,940 in the rats pretreated with nicotine compared to the rats pretreated with vehicle. Post-hoc testing showed that 0.1 and 0.3 mg/kg CP 55,940 produced significantly reduced locomotor activity in the nicotine-treated rats compared to the controls (p ≤ 0.05). In contrast to the periadolescent male rats, there were no significant differences in adult male rats (F[3,30] = 0.07, p ≤ 0.98) pretreated with nicotine compared to vehicle controls in response to CP 55,940 on day 8 (Fig 5B). Further, there were no significant differences in response to saline in any of the groups (shown on the graphs as the 0 mg/kg dose of CP 55,940), thus basal levels of activity did not differ across groups.
Experiment 3
The ability of the cannabinoid antagonist AM251 to block the depressant effect of CP 55,940 on activity also was measured. On day 8, there were no differences in the effects of CP 55,940 on locomotor activity in either the adolescent or adult rats after treatment with nicotine + AM 251 compared to vehicle + AM 251 (Fig. 6).
Figure 6.
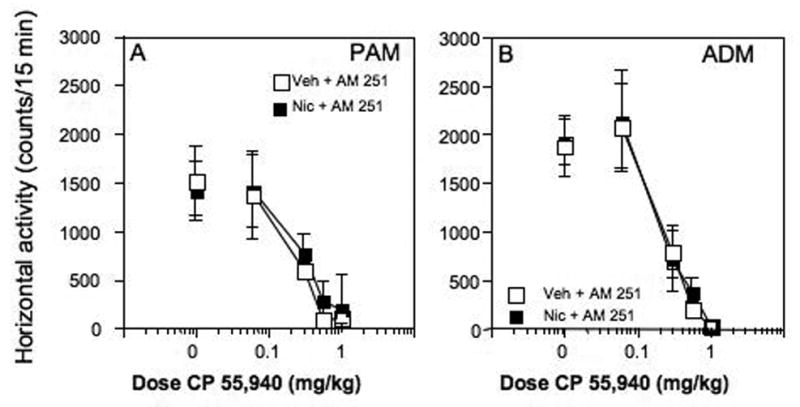
(A)Co-administration of AM 251 with nicotine blocked the development of sensitization to CP 55,940 in periadolescent male rats. (B) As with nicotine alone, there were no effects of nicotine + AM 251 on the locomotor-decreasing effects of CP 55,940 in the adult male rats.
3.2. Quantitative Autoradiography
Cannabinoid receptor binding was significantly increased in the prelimbic areas of the medial prefrontal cortex (CG3, CG1, and Fr2) but not in the lateral (AID) or infralimbic (IL) regions of the frontal cortex in adolescent male rats treated with nicotine for 7 days (Fig. 7A). Cannabinoid receptor density was significantly increased by 24% in CG1 (F[1,7] = 18.85, p ≤ 0.003), 30% in CG3 (F[1,7] = 19.03, p ≤ 0.003), and 23% in FR2 (F[1,7] = 18.85, p ≤ 0.003). In contrast, there were no significant differences in cannabinoid receptor binding in any region of the prefrontal cortex in adult male rats treated with nicotine compared with their vehicle controls (Fig 7B).
Figure 7.
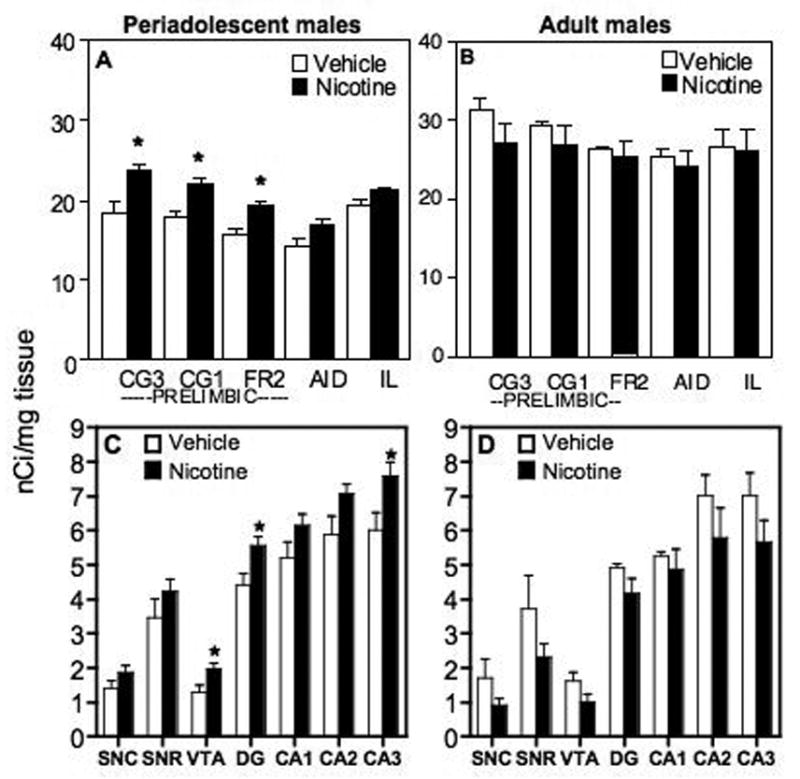
Cannabinoid receptor densities in the prefrontal cortex, substantia nigra, ventral tegmental area, and hippocampus on day 8, one day after a 7-day pretreatment with daily injections of nicotine or vehicle. (A) Periadolescent male rats pretreated with nicotine had significantly higher cannabinoid receptor densities in area 1 and area 3 of the cingulate cortex and area 2 of the frontal cortex compared to periadolescent male rats pretreated with vehicle (p < 0.05). (B) There were no significant changes in cannabinoid receptor densities in the prefrontal cortex in adult male rats pretreated with nicotine compared to adult rats pretreated with vehicle. (Cannabinoid receptor densities were measured in four areas of the prefrontal cortex (CG3: area 3 of the cingulate cortex, CG1: area 1 of the cingulate cortex, FR2: area 2 of the frontal cortex, AID: agranular insular cortex, IL: infralimbic area). (C) Periadolescent male rats pretreated with nicotine had significantly higher cannabinoid receptor densities in the ventral tegmental area, dentate gyrus, and the CA3 region of the hippocampus compared to periadolescent male rats pretreated with vehicle (p < 0.05). (D) There were no significant changes in cannabinoid receptor densities in the substantia nigra, ventral tegmental area, and hippocampus in adult male rats pretreated with nicotine compared to adult rats pretreated with vehicle. Cannabinoid receptor densities were measured in two areas of the substantia nigra (SNC: substantia nigra pars compacta, SNR: substantia nigra pars reticulata), the ventral tegmental area, and five areas of the hippocampus (DG: dentate gyrus, CA1, CA2, CA3). *indicates a significant difference from rats pretreated with vehicle, p ≤ 0.05.
Cannabinoid receptor densities were increased in the ventral tegmental area (VTA), the dentate gyrus (DG) and the CA3 layer of the hippocampus in periadolescent rats treated with nicotine compared to vehicle (Fig 7C). Binding was increased by 52% in the VTA (F[1,11] = 7.00, p ≤ 0.02), 26% in the DG (F[1,11] = 8.05, p ≤ 0.01), and 26% in the CA3 (F[1,11] = 6.58, p ≤ 0.03) region of the hippocampus in periadolescent rats treated with nicotine compared to their controls. There were no significant differences in cannabinoid receptor binding in the substantia nigra pars compacta or reticulata or the CA1 or CA2 regions of the hippocampus in periadolescent male rats treated with nicotine compared to their controls. Further, there were no significant differences in the substantia nigra, ventral tegmental area, or hippocampus in adult male rats pretreated with nicotine compared to their controls (Fig 7D). Cannabinoid receptor densities were not altered significantly in the caudate putamen or nucleus accumbens in any of the groups treated with nicotine compared to their respective controls (data not shown).
4. Discussion
In the current study, nicotine pretreatment produced differential cannabinoid-mediated activity levels and radioligand binding to cannabinoid receptors in periadolescent compared with adult male rats. The activity-decreasing effects of Δ9-THC and the cannabinoid agonist CP 55,940 were potentiated subsequent to repeated nicotine treatment in periadolescent male rats. In contrast, nicotine did not produce any differences in cannabinoid-regulated activity in adult males. These findings support the hypotheses that 1) the cholinergic and cannabinoid systems interact, and 2) the age during which the animal is exposed to nicotine is critical to nicotine's effects on the cannabinoid system.
Other studies have examined the interaction between the nicotinic cholinergic and cannabinoid systems; however most studies were done in adult mice or rats (for review see Viveros et al., 2007), and many studies have utilized acute injections of nicotine. For example, acute injections of nicotine between 0.5 and 6 mg/kg decreased locomotor activity in both adult wild-type and cannabinoid receptor knock-out mice (Castane et al., 2002), suggesting that the cannabinoid receptor is not required for mediation of the locomotor effects of nicotine. In another study, adult male mice given an acute injection of Δ9-THC plus nicotine showed significantly decreased locomotor activity compared with vehicle-treated or Δ9-THC-treated mice (Valjent et al., 2002). Thus, the simultaneous acute administration of a cannabinoid agonist and nicotine may produce different locomotor effects compared with nicotine's effects on the cannabinoid system, or subsequent cannabinoid agonist administration. In contrast to the lack of effect of knocking out the CB1 receptor on nicotine-stimulated locomotor activity, a significant place preference was produced by acute nicotine (0.5-6.0 mg/kg) in wild-type mice, but was not evident in CB1 knockout mice, suggesting that the endogenous cannabinoid system may play a role in mediating the rewarding effects of nicotine (Castane et al., 2002). This appears to be task-specific however, since both adult male CB1 receptor knock-out and wild-type mice had a similar number of nose pokes, where each nose poke resulted in an IV injection of nicotine, in a single 30 min period (Cossu et al, 2001). Thus, it is possible that nicotine reward and reinforcement are regulated differentially by the cannabinoid system.
Both nicotinic and cannabinoid systems have been implicated in nociceptive responses, and interactions between the systems have been identified. A dose-dependent increase in antinociception was reported in response to an acute nicotine injection (0.5 mg/kg) combined with Δ9-THC, and nociception was significantly greater in cannabinoid receptor knock-out mice. Additionally, mice given an acute injection of Δ9-THC plus nicotine exhibited significantly increased jump and tail-flick latencies compared with controls (Valjent et al., 2002). The nicotinic and cannabinoid systems also may converge in mediating anxiety. Mice given an acute injection of Δ9-THC plus nicotine exhibited significantly decreased anxiety compared with controls (Valjent et al., 2002). Together, these data suggest that the cannabinoid system may not mediate the reinforcing effects produced by short-term nicotine self-administration or activity produced by acute or chronic nicotine injections when given alone. It is possible, however, that the cannabinoid system may play a role in the effects of nicotine on antinociception, anxiety, and/or conditioned place preference in adult males.
It has been reported that there is an increase in cannabinoid receptor density in the cerebral cortex and a decrease in cannabinoid receptor mRNA in the septum nucleus, but no changes in the caudate putamen, basolateral amygdala, hippocampus, or cerebellum after once daily injections of 1 mg/kg nicotine (base) for seven days in adult male rats (Gonzalez et al., 2002). Endocannabinoid levels, however, were increased in the limbic forebrain and brainstem and decreased in hippocampus, striatum, and cerebral cortex after this same treatment (Gonzalez et al., 2002). The present data showing that CB1 receptor densities are not changed in the hippocampus, striatum, or prefrontal cortex in adult male rats after seven days of 0.4 mg/kg nicotine administration are consistent with the lack of changes in mRNA in these regions. What is interesting is that in the adolescent rats, however, there are significant changes in receptor binding in numerous brain regions thought to be involved in mediating the effects of drugs of abuse. It is interesting to note that a number of recent publications have suggested that the prefrontal cortex is involved in mediating the regulation of locomotor activity by drugs of abuse. For example, it has been shown that cocaine-stimulated locomotor activity is accompanied by changes in Fos expression in the PFC in young rats (McDougall et al., 2008). Others have shown that injection of a glutamate agonist into the PFC alters cocaine-induced motor activity (Xie and Steketee, 2008) and that prefrontal dopaminergic transmission plays a role in methamphetamine-induced hyperactivity in mice (Ago et al., 2009). Thus, although there were no changes in CB1 binding in the caudate putamen in the present studies, it may be that changes in the prefrontal cortex mediate, at least in part, the changes in the locomotor-decreasing effects of cannabinoid agonists observed after nicotine treatment.
The focus of the current study specifically was on the comparison of behavioral and neurochemical adaptations in response to nicotine in adolescents compared with adults. While nicotine is known to produce unique effects on other neurochemical systems such as nicotinic (Abreu-Villaca et al., 2003; Collins et al., 2004b), dopaminergic (Collins et al., 2004b; Trauth et al., 2001), and serotonergic (Collins et al., 2004b; Jang et al., 2002; Xu et al., 2001; Xu et al., 2002) systems in adolescents compared to adults, the interaction between nicotinic and cannabinoid systems in adolescence has been largely unexplored. However, a recent study showed that subchronic nicotine administration to adolescent male rats elevated the increase in corticosterone seen after an acute injection of CP 55,940, and that the two drugs together produced an anxiogenic effect on the plus maze (Marco et al., 2006). Marco and colleagues hypothesized that the functional interactions they observed between the nicotinic and cannabinoid systems could be subserved by overlapping distributions of receptors for the systems in hippocampus, and such an overlap is consistent with our data, as we see an increase in hippocampal cannabinoid receptor density in the CA3 and dentate gyrus regions.
It has been shown that cannabinoid receptor densities are just reaching adult levels in adolescent males (Belue et al., 1995; Rodriguez de Fonseca et al., 1993), which may lead to the differential neurochemical and behavioral responses observed after nicotine treatment when compared with older age groups. The present study showed that nicotine treatment for seven days produced increases in cannabinoid receptor densities in several areas of the prefrontal cortex, the hippocampus, and the ventral tegmental area in adolescent male rats, but not in adult male rats. Interestingly, in the current study, no changes were observed in cannabinoid receptor densities in the caudate putamen or nucleus accumbens, areas thought to be involved in mediating motor activity. However, the effects of nicotine on cannabinoid-mediated activity were blocked by concomitant administration of the cannabinoid antagonist AM 251. Thus, it may be that cannabinoid receptors in other brain regions either are directly or indirectly involved in mediating the locomotor activity induced by other drugs given subsequently to nicotine. The prefrontal cortex, hippocampus, and ventral tegmental area, brain regions in which significant changes in cannabinoid receptor density changes were observed in periadolescent male rats treated with nicotine, have been implicated in the mediation of other behaviors such as memory tasks (Thierry et al., 2000) and in the reinforcing effects of drugs (Bardo, 1998; Kornetsky and Porrino, 1992). Future studies will determine the role of cannabinoid receptors in mediating other drug-related behaviors after chronic nicotine treatment in adolescent and adult rats.
In summary, these data show that nicotine during adolescence, but not in adults, produces changes in cannabinoid receptors in specific brain regions and that these changes are accompanied by alterations in cannabinoid-mediated behaviors. These data strongly support the idea that there are interactions between nicotinic and cannabinoid systems, and suggest the possibility that the cannabinoid system plays a role in the adaptations that occur to chronic nicotine treatment, especially in adolescents. These findings may help to explain the increased use of marijuana in adolescents who smoke, in comparison to those who do not smoke. A better understanding of the interactions between the nicotinic and cannabinoid system may help in the development of age-specific treatments for dependence on nicotine and/or marijuana.
Acknowledgments
This work was supported by NIDA grant DA 15119. Thanks to Erin Wall for her technical assistance with this project.
Abbreviations
- CB
cannabinoid
- Δ9-THC
delta-9-tetrahydrocannabinol
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abreu-Villaca Y, Seidler FJ, Qiao D, Tate CA, Cousins MM, Thillai I, Slotkin TA. Short-term adolescent nicotine exposure has immediate and persistent effects on cholinergic systems: critical periods, patterns of exposure, dose thresholds. Neuropsychopharmacology. 2003;28:1935–1949. doi: 10.1038/sj.npp.1300221. [DOI] [PubMed] [Google Scholar]
- Ago Y, Arikawa S, Yata M, Yano K, Abe M, Takuma K, Matsuda T. Role of prefrontal dopaminergic neurotransmission in glucocorticoid receptor-mediated modulation of methamphetamine-induced hyperactivity. Synapse. 2009;63:7–14. doi: 10.1002/syn.20575. [DOI] [PubMed] [Google Scholar]
- Ambrosio E, Martin S, Garcia-Lecumberri C, Crespo JA. The neurobiology of cannabinoid dependence: sex differences and potential interactions between cannabinoid and opioid systems. Life Sciences. 1999;65:687–694. doi: 10.1016/s0024-3205(99)00291-x. [DOI] [PubMed] [Google Scholar]
- Bardo MT. Neuropharmacological mechanisms of drug reward: beyond dopamine in the nucleus accumbens. Critical Reviews in Neurobiolgy. 1998;12:37–67. doi: 10.1615/critrevneurobiol.v12.i1-2.30. [DOI] [PubMed] [Google Scholar]
- Beenstock M, Rahav G. Testing Gateway Theory: do cigarette prices affect illicit drug use? Journal of Health Economics. 2002;21:679–698. doi: 10.1016/s0167-6296(02)00009-7. [DOI] [PubMed] [Google Scholar]
- Belluzzi JD, Lee AG, Oliff HS, Leslie FM. Age-dependent effects of nicotine on locomotor activity and conditioned place preference in rats. Psychopharmacology. 2004;174:389–395. doi: 10.1007/s00213-003-1758-6. [DOI] [PubMed] [Google Scholar]
- Belue RC, Howlett AC, Westlake TM, Hutchings DE. The ontogeny of cannabinoid receptors in the brain of postnatal and aging rats. Neurotoxicology and Teratology. 1995;17:25–30. doi: 10.1016/0892-0362(94)00053-g. [DOI] [PubMed] [Google Scholar]
- Castane A, Valjent E, Ledent C, Parmentier M, Maldonado R, Valverde O. Lack of CB1 cannabinoid receptors modifies nicotine behavioural responses, but not nicotine abstinence. Neuropharmacology. 2002;43:857–867. doi: 10.1016/s0028-3908(02)00118-1. [DOI] [PubMed] [Google Scholar]
- Collins SL, Izenwasser S. Chronic nicotine differentially alters cocaine-induced locomotor activity in adolescent vs. adult male and female rats. Neuropharmacology. 2004;46:349–362. doi: 10.1016/j.neuropharm.2003.09.024. [DOI] [PubMed] [Google Scholar]
- Collins SL, Montano R, Izenwasser S. Nicotine treatment produces persistent increases in amphetamine-stimulated locomotor activity in periadolescent male but not female or adult male rats. Developmental Brain Research. 2004a;153:175–187. doi: 10.1016/j.devbrainres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Collins SL, Wade D, Ledon J, Izenwasser S. Neurochemical alterations produced by daily nicotine exposure in periadolescent vs. adult male rats. European Journal of Pharmacology. 2004b;502:75–85. doi: 10.1016/j.ejphar.2004.08.039. [DOI] [PubMed] [Google Scholar]
- Crespo JA, Manzanares J, Oliva JM, Corchero J, Palomo T, Ambrosio E. Extinction of cocaine self-administration produces a differential time-related regulation of proenkephalin gene expression in rat brain. Neuropsychopharmacology. 2001;25:185–194. doi: 10.1016/S0893-133X(01)00221-4. [DOI] [PubMed] [Google Scholar]
- Doura MB, Gold AB, Keller AB, Perry DC. Adult and periadolescent rats differ in expression of nicotinic cholinergic receptor subtypes and in the response of these subtypes to chronic nicotine exposure. Brain Research. 2008;1215:40–52. doi: 10.1016/j.brainres.2008.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraday MM, O'Donoghue VA, Grunberg NE. Effects of nicotine and stress on locomotion in Sprague-Dawley and Long-Evans male and female rats. Pharmacology Biochemistry and Behavior. 2003;74:325–333. doi: 10.1016/s0091-3057(02)00999-1. [DOI] [PubMed] [Google Scholar]
- Gonzalez S, Cascio MG, Fernandez-Ruiz J, Fezza F, Marzo VD, Ramos JA. Changes in endocannabinoid contents in the brain of rats chronically exposed to nicotine, ethanol or cocaine. Brain Research. 2002;954:73–81. doi: 10.1016/s0006-8993(02)03344-9. [DOI] [PubMed] [Google Scholar]
- Green TA, Cain ME, Thompson M, Bardo MT. Environmental enrichment decreases nicotine-induced hyperactivity in rats. Psychopharmacology. 2003;170:235–241. doi: 10.1007/s00213-003-1538-3. [DOI] [PubMed] [Google Scholar]
- Higuera-Matas A, Soto-Montenegro ML, del Olmo N, Miguens M, Torres I, Vaquero JJ, Sanchez J, Garcia-Lecumberri C, Desco M, Ambrosio E. Augmented acquisition of cocaine self-administration and altered brain glucose metabolism in adult female but not male rats exposed to a cannabinoid agonist during adolescence. Neuropsychopharmacology. 2008;33:806–813. doi: 10.1038/sj.npp.1301467. [DOI] [PubMed] [Google Scholar]
- Higuera A, Biscaia M, Fernández B, Miguéns M, Olmo Nd, Torres I, García-Lecumberri C, Viveros MP, Ambrosio E. Pre-exposure to cannabinoid agonist CP 55,940 during rat early adolescence facilitates acquisition of cocaine self-administration behavior in the adulthood. CPDD abstract 2005 [Google Scholar]
- Hofler M, Lieb R, Perkonigg A, Schuster P, Sonntag H, Wittchen HU. Covariates of cannabis use progression in a representative population sample of adolescents: a prospective examination of vulnerability and risk factors. Addiction. 1999;94:1679–1694. doi: 10.1046/j.1360-0443.1999.941116796.x. [DOI] [PubMed] [Google Scholar]
- Hornik R. Alcohol, tobacco, and marijuana use among youth: same-time and lagged and simultaneous-change associations in a nationally representative sample of 9- to 18-year-olds. In: Romer D, editor. Reducing adolescent risk: toward an integrated approach. Sage Publications; Thousand Oaks, CA: 2003. pp. 335–343. [Google Scholar]
- Jang MH, Shin MC, Lee TH, Kim YP, Jung SB, Shin DH, Kim H, Kim SS, Kim EH, Kim CJ. Alcohol and nicotine administration inhibits serotonin synthesis and tryptophan hydroxylase expression in dorsal and median raphe of young rats. Neurosci Lett. 2002;329:141–144. doi: 10.1016/s0304-3940(02)00622-5. [DOI] [PubMed] [Google Scholar]
- Kandel D. Stages in adolescent involvement in drug use. Science. 1975;190:912–914. doi: 10.1126/science.1188374. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Yamaguchi K, Chen K. Stages of progression in drug involvement from adolescence to adulthood: further evidence for the gateway theory. Journal of Studies on Alcohol. 1992;53:447–457. doi: 10.15288/jsa.1992.53.447. [DOI] [PubMed] [Google Scholar]
- Kornetsky C, Porrino LJ. Brain Mechanism of Drug-Induced Reinforcement. In: O'Brien CP, Jaffe JH, editors. Addictive States. Vol. 4. Raven Press Ltd; New York: 1992. pp. 59–77. [PubMed] [Google Scholar]
- Levin ED, Lawrence SS, Petro A, Horton K, Rezvani AH, Seidler FJ, Slotkin TA. Adolescent vs. adult-onset nicotine self-administration in male rats: duration of effect and differential nicotinic receptor correlates. Neurotoxicol Teratol. 2007;29:458–465. doi: 10.1016/j.ntt.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH, Montoya D, Rose JE, Swartzwelder HS. Adolescent-onset nicotine self-administration modeled in female rats. Psychopharmacology. 2003;169:141–149. doi: 10.1007/s00213-003-1486-y. [DOI] [PubMed] [Google Scholar]
- Mailleux P, Vanderhaeghen JJ. Distribution of neuronal cannabinoid receptor in the adult rat brain: a comparative receptor binding radioautography and in situ hybridization histochemistry. Neuroscience. 1992;48:655–668. doi: 10.1016/0306-4522(92)90409-u. [DOI] [PubMed] [Google Scholar]
- Marco EM, Llorente R, Moreno E, Biscaia JM, Guaza C, Viveros MP. Adolescent exposure to nicotine modifies acute functional responses to cannabinoid agonists in rats. Behav Brain Res. 2006;172:46–53. doi: 10.1016/j.bbr.2006.04.012. [DOI] [PubMed] [Google Scholar]
- McDougall SA, Charntikov S, Cortez AM, Amodeo DA, Martinez CE, Crawford CA. Persistence of one-trial cocaine-induced behavioral sensitization in young rats: regional differences in Fos immunoreactivity. Psychopharmacology (Berl) 2008 doi: 10.1007/s00213-008-1407-1. [DOI] [PubMed] [Google Scholar]
- Merrill JC, Kleber HD, Shwartz M, Liu H, Lewis SR. Cigarettes, alcohol, marijuana, other risk behaviors, and American youth. Drug and Alcohol Dependence. 1999;56:205–212. doi: 10.1016/s0376-8716(99)00034-4. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Common molecular and cellular substrates of addiction and memory. Neurobiology of Learning and Memory. 2002;78:637–647. doi: 10.1006/nlme.2002.4084. [DOI] [PubMed] [Google Scholar]
- Oviedo A, Glowa JR, Herkenham M. Chronic cannabinoid administration alters cannabinoid receptor binding in rat brain: a quantitative autoradiographic study. Brain Research. 1993;616:293–302. doi: 10.1016/0006-8993(93)90220-h. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Rouge-Pont F, Deminiere JM, Kharoubi M, Moal ML, Simon H. Dopaminergic activity is reduced in the prefrontal cortex and increased in the nucleus accumbens of rats predisposed to develop amphetamine self-administration. Brain Research. 1991;567:169–174. doi: 10.1016/0006-8993(91)91452-7. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Limbic-Striatal memory systems and drug addiciton. Neurobiology of Learning and Memory. 2002;78:625–636. doi: 10.1006/nlme.2002.4103. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Ramos JA, Bonnin A, Fernandez-Ruiz JJ. Presence of cannabinoid binding sites in the brain from early postnatal ages. NeuroReport. 1993;4:135–138. doi: 10.1097/00001756-199302000-00005. [DOI] [PubMed] [Google Scholar]
- Schenk S, Horger BA, Peltier R, Shelton K. Supersensitivity to the reinforcing effects of cocaine following 6-hydroxydopamine lesions to the medial prefrontal cortex in rats. Brain Research. 1991;543:227–235. doi: 10.1016/0006-8993(91)90032-q. [DOI] [PubMed] [Google Scholar]
- Schochet TL, Kelley AE, Landry CF. Differential behavioral effects of nicotine exposure in adolescent and adult rats. Psychopharmacology. 2004;175:265–273. doi: 10.1007/s00213-004-1831-9. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Cousins MM, Seidler FJ. Administration of nicotine to adolescent rats evokes regionally selective upregulation of CNS a7 nicotinic acetylcholine receptors. Brain Research. 2004;1030:159–163. doi: 10.1016/j.brainres.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Spear LP, Brake SC. Periadolescence: age-development behavior and psychopharmacological responsivity in rats. Developmental Psychobiology. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Steketee JD. Neurotransmitter systems of the medial prefrontal cortex: potential role in sensitization to psychostimulants. Brain Research Reviews. 2003;41:203–228. doi: 10.1016/s0165-0173(02)00233-3. [DOI] [PubMed] [Google Scholar]
- Stratta F, Bonci A, Calabresi A, Stefani A, Pisani A, Bernardi G, Mercuri NB. Brain research in substantia nigra and ventral tegmental area: clinical implications. Journal of Neural Transmission. 1995;45:47–55. [PubMed] [Google Scholar]
- Terry P. Differential effects of injection regimen on behavioral responses to cocaine. Pharmacology Biochemistry and Behavior. 1992;41:365–369. doi: 10.1016/0091-3057(92)90112-s. [DOI] [PubMed] [Google Scholar]
- Thierry AM, Gioanni Y, Degenetais E, Glowinski J. Hippocampo-prefrontal cortex pathway: anatomical and electrophysiological characteristics. Hippocampus. 2000;10:411–419. doi: 10.1002/1098-1063(2000)10:4<411::AID-HIPO7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, Ali SF, Slotkin TA. Adolescent nicotine exposure produces immediate and long-term changes in CNS noradrenergic and dopaminergic function. Brain Research. 2001;892:269–280. doi: 10.1016/s0006-8993(00)03227-3. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker J. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Tullis LM, Dupont R, Frost-Pineda K, Gold MS. Marijuana and tobacco: a major connection? J Addict Dis. 2003;22:51–62. doi: 10.1300/J069v22n03_05. [DOI] [PubMed] [Google Scholar]
- Unterwald EM, Ho A, Rubenfeld JM, Kreek MJ. Time course of the development of behavioral sensitization and dopamine receptor up-regulation during binge cocaine administration. Journal of Pharmacology and Experimental Therapeutics. 1994;270:1387–1396. [PubMed] [Google Scholar]
- Valjent E, Mitchell JM, Besson MJ, Caboche J, Maldonado R. Behavioural and biochemical evidence for interactions between D9-tetrahydrocannabinol and nicotine. British Journal of Pharmacology. 2002;135:564–578. doi: 10.1038/sj.bjp.0704479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP. Nicotine-induced conditioned place preference in adolescent and adult rats. Physiology & Behavior. 2002;77 doi: 10.1016/s0031-9384(02)00818-1. [DOI] [PubMed] [Google Scholar]
- Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neuroscience and Biobehavioral Reviews. 2004;27:827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Viveros MP, Marco EM, Llorente R, Lamota L. The role of the hippocampus in mediating emotional responses to nicotine and cannabinoids: a possible neural substrate for functional interactions. Behav Pharmacol. 2007;18:375–389. doi: 10.1097/FBP.0b013e3282d28fb4. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Goldstein RZ. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiology of Learning and Memory. 2002;78:610–624. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- Weissenborn R, Robbins TW, Everitt BJ. Effects of medial prefrontal or anterior cingulate cortex lesions on responding for cocaine under fixed-ratio and second order schedules of reindorcement in rats. Psychopharmacology. 1997;134:242–257. doi: 10.1007/s002130050447. [DOI] [PubMed] [Google Scholar]
- Westlake TM, Howlett AC, Bonner TI, Matsuda LA, Herkenham M. Cannabinoid receptor binding and messenger RNA expression in human brain: an in vitro receptor autoradiography and in situ hybridization histochemistry study of normal aged and alzheimer's brains. Neuroscience. 1994;63:637–652. doi: 10.1016/0306-4522(94)90511-8. [DOI] [PubMed] [Google Scholar]
- Xie X, Steketee JD. Effects of repeated exposure to cocaine on group II metabotropic glutamate receptor function in the rat medial prefrontal cortex: behavioral and neurochemical studies. Psychopharmacology (Berl) 2008 doi: 10.1007/s00213-008-1392-4. [DOI] [PubMed] [Google Scholar]
- Xu Z, Seidler FJ, Ali SF, Slikker WJ, Slotkin TA. Fetal and adolescent nicotine administration: effects on CNS serotonergic systems. Brain Research. 2001;914:166–178. doi: 10.1016/s0006-8993(01)02797-4. [DOI] [PubMed] [Google Scholar]
- Xu Z, Seidler FJ, Cousins MM, Slikker WJ, Slotkin TA. Adolescent nicotine administration alters serotonin receptors and cell signaling mediated through adenylyl cyclase. Brain Research. 2002;951:280–292. doi: 10.1016/s0006-8993(02)03174-8. [DOI] [PubMed] [Google Scholar]


