Abstract
We report the rationale, design, methods and details of participation of a community-based, double blind, randomized clinical trial of an HPV 16 and 18 vaccine conducted in two provinces of Costa Rica to investigate the efficacy and population impact of the vaccine in the prevention of cervical cancer precursors. More than 24,000 women between 18 and 25 years of age were invited to participate and pre-screened for eligibility, with recruitment of 7,466 women (30% of those prescreened, 59% of those eligible) who were randomized to receive 3 doses of the HPV vaccine or hepatitis A vaccine as control. A complex protocol of data and specimen collection was applied, including an interview, pelvic exam for sexually active women, blood for serology and cell-mediated immunity, cervical secretions for local immunity and cells for HPV, Chlamydia trachomatis and Gonorrhea testing. Eighty percent of the women received 3 doses, 12.4% two doses and 7.4% one dose. At visits, compliance with data and specimen collection was close to 100%. Baseline characteristics and age-specific prevalence of HPV and cervical neoplasia are reported. Overall prevalence of HPV was high (50%), with 8.3% of women having HPV 16 and 3.2% HPV 18. LSIL was detected in 12.7% of women at baseline and HSIL in 1.9%. Prevalence of Chlamydia was 14.2%. There was very good agreement in HPV detection between clinician-collected and self-collected specimens (89.4% agreement for all types, kappa 0.59). Follow up will continue with yearly or more frequent examinations for at least 4 years for each participant.
Introduction
Cervical cancer is diagnosed in approximately half a million women every year, and at constant rates, this number is expected to increase almost 50% by the year 2020, solely as a consequence of demographic changes (1).
Despite the success of cytology-based screening programs to reduce cervical cancer incidence and mortality in developed countries, efforts to control this problem in developing countries have failed, with a few exceptions (2). New screening approaches appear very promising, but are hampered by high cost, and their necessary validation is still underway. In fact, cervical cancer incidence is increasing in some countries and there is an increase in cervical adenocarcinoma, even in places where squamous cervical cancer is under control (3).
The loss of life and associated costs of cervical cancer are a large burden to both high and low resource societies, and the development of new, effective and ultimately affordable methods to prevent this disease are needed by the millions of families worldwide that could suffer the consequences of cervical cancer in the future.
The relatively recent knowledge that sexually transmitted HPV is a necessary cause of cervical cancer, and its association with cancers of the anus, vulva, vagina, penis, and oropharynx (4), opens the possibility of preventing these cancers by preventing HPV infection. It has been estimated that, in addition to cervical cancer, cancers of those locations associated with HPV represent an additional 70,000 cases per year (1).
The development of virus-like particles (VLPs) and their testing as vaccine antigens in several clinical trials have generated enormous enthusiasm. In results published to date, they seem to protect virtually 100% of uninfected women against subsequent persistent infection and cervical neoplasia associated with the HPV types included in the formulation (5–8). The alum-adjuvanted, yeast-derived vaccine produced by Merck that contains antigens from HPV types 6, 11, 16 and 18 has been licensed in many countries and is being recommended for application in young women (9). HPV 6 and 11 are responsible for most genital warts and HPV 16 and 18 are responsible for up to 70% of cervical cancers in most areas of the world. GlaxoSmithKline (GSK) produces a bivalent VLP-based vaccine, which contains antigens from HPV 16 and 18 and is formulated with an adjuvant different from that used by Merck (ASO4). This vaccine has been licensed recently in Europe and other countries and is expected to be approved by the FDA in the near term (10).
Guanacaste is a rural area in Costa Rica with a traditionally high incidence of cervical cancer where we have been studying the epidemiology of HPV for the last 20 years, including a recently completed 10,000 women cohort study of the natural history of HPV and cervical neoplasia (11,12). In that study, women were screened and followed with pap smears and a variety of other screening methods and extensive HPV testing for up to 10 years. The study has shed light on many aspects of the natural history of cervical disease and screening methods, and has been extensively published (13–20).
Given the historically elevated rates of cervical cancer in the region, the ample knowledge of the epidemiology of HPV in this area, and the existence of a highly trained staff fully dedicated to the study of HPV and cervical cancer, with an accumulated experience of more than 20 years, this was an ideal area to conduct an independent evaluation of the HPV vaccine.
In this paper we report on the rationale and design of a double-blind, randomized controlled efficacy trial of the prophylactic VLP-based vaccine against HPV types 16 and 18 developed by researchers of the US National Cancer Institute and other institutions and manufactured by GSK Biologicals.
Materials and Methods
Design and objectives of the study
The study described in this document is a community-based double-blind randomized controlled phase III trial of a virus-like particle (VLP) vaccine against HPV types 16 and 18 among healthy women 18–25 years old. The control vaccine is inactivated antigen of hepatitis A virus (three dose formulation used in Twinrix® that allowed blinding to be maintained).
The main objective of the trial is to investigate the efficacy of the vaccine to prevent cervical cancer precursors (defined as persistent HPV infection and/or high grade cervical intraepithelial neoplasia, CIN2 or CIN3).
We are particularly interested in evaluating the potential effectiveness of the vaccine at the population level, the impact of the HPV vaccine in relation to previous HPV exposure and age at vaccination, and will explore in detail efficacy against non-vaccine HPV types, duration of protection, characteristics of the immune response to the vaccine and potential impact of less than 3 doses.
We have recently reported on the lack of therapeutic efficacy among women who were HPV positive at enrollment into the study (21). In addition, multiple tertiary objectives are envisioned, to address issues of relevance to HPV vaccination, to understand immunological responses to vaccination and to further investigate the natural history of HPV and HPV-associated outcomes.
The trial included eligible women 18 to 25 years of age residing in Guanacaste Province, Costa Rica or selected areas of Puntarenas Province. Consenting women were randomized to receive the candidate vaccine (40 µg of L1 protein VLPs of HPV 16 and 18 formulated with ASO4 adjuvant, consisting of alum and monophosphoryl lipid A) or the vaccine against hepatitis A consisting of 720 ELISA Units of inactivated viral antigen with Alum, formulated in 0.5 ml doses for this study. Randomization was carried out in a 1:1 ratio (figure 1). The vaccination schedule consisted of 3 doses, one at the enrollment visit, one a month later and one 6 months after recruitment.
Figure 1.
Design of the HPV 16/18 vaccine trial in Guanacaste
After receiving the vaccines, women are being followed once a year for at least 4 years, as long as they have normal cytology. If they have minor HPV-related cytological abnormalities they are transferred to a 6 month schedule and if the lesion persists they are referred to colposcopy. Also, if they have high grade cytology at any time during the study, they are referred to colposcopy for diagnosis and treatment (see below).
Organization of the study
During the vaccination phase of the study, 7 study clinics were established with appropriate facilities to conduct the trial procedures. The clinics were located in Liberia, Cañas, La Cruz, Nicoya, Tilarán, Santa Cruz and Puntarenas. The staff at each clinic included a physician, a microbiologist who functioned as team coordinator, a nurse and a nurse aide, an assistant team coordinator, a supervisor, a driver, an interviewer and a receptionist. The clinics were equipped to provide emergency care and the staff was extensively trained for medical emergencies, particularly anaphylactic reactions.
The headquarters in Liberia coordinated appointments and supervised the field work, using a data management system developed specifically to assist the process, and also housed the repository for vaccines and specimens, document center, resident doctors, data entry, information technology and quality control. All participant records, vaccines and specimens were centralized at the Liberia headquarters and transported daily to and from the clinics in study vehicles. The cytology and histopathology laboratories were also in Liberia, although interpretation of cytology was carried out at the private laboratory of the study cytopathologist (MA). Similarly, interpretation of histology was done at the lab of our pathologist (DG). The processing of blood samples and the CBC at enrollment was contracted to a local laboratory (Trilab Sci, Liberia). HPV DNA, Chlamydia trachomatis (CT) DNA and Neisseria gonorrhea (GC) DNA detection by Hybrid Capture 2 (HC2) on the remaining PreservCyt after cytology slide preparation were performed at the University of Costa Rica (UCR) in San José. Heparinized blood samples were transported every day from study clinics to the UCR for cryopreservation of lymphocytes.
Selection of the population and invitation to participate
The study is being conducted in Guanacaste, Costa Rica, an area in the northwest part of the country with a population of 292,500 inhabitants (2005), where we have been conducting our studies for many years. We also invited women from selected towns in nearby Puntarenas (total population 399,500). These areas are traditionally rural and agricultural, but tourism-related activities are expanding rapidly. Cervical cancer incidence and mortality have been traditionally high (>20 per 100,000 women per year).
A complete census of women ages 12–22 was conducted specifically for this study between February and July 2000 (anticipating a subsequent start a few years later within the age range of 18–25), with an update of urban areas in 2005. During the census, all households in the selected areas were visited by study outreach workers, to obtain name, date of birth, cédula (national unique identification number) or other ID number, exact address, geographic location (cantón, district, censal segment (like a census tract) and house number), details of a contact person, telephone number and information on stability of residence for all resident women ages 12–22. A total of 68,662 households in Guanacaste and 22,294 in Puntarenas were visited. Intensive duplicate checks were carried out as an ongoing process throughout the study. Detailed maps were created for each of the censal segments, including location and number of each house to facilitate subsequent location of the women by the outreach workers.
All women in the census were randomly assigned a study-specific individual personal identification number (PID) in the database, and when the study initiated, attempts were made to visit all women in the census who were at that time 18 to 25 years old, to invite them to participate in the study.
Outreach workers visited potentially eligible women at home to give them a personalized letter with an appointment to the nearest of our clinics and an informational brochure (see web page www.proyectoguanacaste.org for educational materials). Women who appeared eligible and expressed an interest in participating were given a copy of the informed consent document to read and discuss with their families before the appointment. Women were offered transportation in the study vehicles or reimbursement of travel expenses, but they were not paid for participating in the study.
Eligibility criteria and informed consent
The day of the visit, the receptionist at the clinic verified the identity of the potential participant, updated personal information, showed her a video (see web page) explaining the design and procedures of the study and administered an initial eligibility screener. Women who appeared potentially eligible had an extensive discussion of the informed consent document with a trained interviewer, with nurses and doctors always available to answer questions by the participants. The consent form, approved by the Costa Rica and NCI IRBs (see below), included details on HPV, the study, the meaning of participating, risks and benefits associated with participation, use of biological specimens and confidentiality among other topics (available at www.proyectoguanacaste.org). Women who decided not to participate or who were deemed ineligible for any reason were offered a physical exam and a Pap smear with a follow-up colposcopic evaluation and treatment if needed at no cost to them.
After signature of the informed consent, an interview on risk factors was administered by trained interviewers, with responses entered directly into pre-coded data fields on a computer screen. The questionnaire elicited information on years of education, marital status, income, household facilities, menstrual history, sexual, reproductive and contraceptive history, smoking and family history of cancer. Among lifetime sexually monogamous women, additional questions were asked about their sexual partner, including age, education, circumcision, sexual history and smoking (interview available upon request).
The process continued with a complete medical history and physical exam. A urine sample was collected to conduct a pregnancy test. After this was completed, study doctors assessed final eligibility for the vaccine trial. To be eligible for the study, women had to be non-pregnant and, if they reported having started sexual activity, had to be using some form of contraception at least one month before the application of the vaccine and be willing to use it until 2 months after the last dose. Acceptable methods included abstinence, condoms, hormonal contraceptives, IUDs and female surgical sterilization. Additional eligibility criteria included being in good general health as ascertained by the study clinicians. Exclusion criteria comprised chronic diseases, history of severe allergic reactions to vaccines, history of hepatitis A or previous vaccination against it, history of chronic administration of immunosuppressive drugs or immunosuppressive conditions, hysterectomy, use of other investigational products in the past 30 days, previous administration of the adjuvant in the candidate vaccine (ASO4), previous HPV vaccination, allergy to 2 phenoxyethanol or neomycin (components of the vaccine), or latex hypersensitivity (component of syringe).
Participation was deferred if a woman was sexually active and not using but willing to use contraception as per the protocol, was pregnant or less than 3 months post partum, was lactating, had an acute condition expected to resolve soon or had recent administration of a vaccine or immunoglobulin.
Clinical procedures and specimen collection
As mentioned above, after the interview a medical history and physical exam were carried out by the study doctors. After eligibility determination, a pelvic exam was performed on sexually experienced women. During the pelvic exam, cervical secretions were collected with polyvinyl acetate-based Mero-cell sponges (Medtronic Solan, Jacksonville, FL, USA), by placing one sponge on the cervical os gently for 30 seconds; and then a second one for the same length of time. The sponges were placed into empty 10 ml tubes and frozen in the vapor phase of liquid nitrogen immediately.
Exfoliated cells for cytology, HPV DNA, CT DNA, GC DNA and other testing were collected with a Cervex brush by firmly rotating the brush 5 times 360° around the cervical os. In women whose cervix exhibited extensive ectopy, the cervex brushing was also used on the ectocervix to insure sampling of the squamo-columnar junction. The brush was vigorously rinsed in liquid transport medium (PreservCyt) and stored in coolers at about 20° Celsius. An additional Dacron swab was used to obtain more cells for experimental HPV and other testing, by rotating it 360° in the cervical os, and placing the swab in UCM (universal collection medium) that allows preservation of RNA (Digene Corporation, Gaithersburg, MD (now Qiagen)). The swab, in medium, was placed immediately into a portable liquid nitrogen transport tank to optimize the quality of RNA.
The PreservCyt vial was processed as follows: first, two 0.5 ml aliquots were extracted following PCR-safe procedures for type-specific HPV detection. Then, a cytology slide was prepared with a ThinPrep 2000 processor, to obtain a thin-layer, liquid-based cytology preparation that was stained with a modified Pap stain. The remainder of enrollment specimens was sent to the HPV lab at the University of Costa Rica to test for HPV, CT and GC by hybrid capture 2 (HC2, Digene –Qiagen). The hybrid capture HPV results were used to triage women with ASC-US (see below) and CT and GC testing was done as a service for participants, who received treatment for themselves and their partners as appropriate. An analysis of the epidemiologic determinants of CT infection, found at baseline in 14.2% of participants, has been published (22). During follow-up, HPV testing is restricted to women with ASC-US for triage, and CT and GC testing is done on women who initiate sexual activity or post-treatment to verify treatment efficacy.
Blood was collected at every visit regardless of previous sexual activity. At the enrollment visit, blood was collected to obtain serum and plasma. The specimen with citrate as anticoagulant was aliquotted to obtain buffy coats and plasma, and ascorbic acid and metaphosphoric acid buffers were added to preserve folic acid (whole blood) and vitamin C, respectively. Also, as a benefit for the participants, a specimen for a CBC was collected. At subsequent visits, only blood for serum is collected. Among 10% of all women and women developing HPV related cervical disease, an additional 40 ml of blood were collected in heparinized tubes, and sent to a laboratory at the University of Costa Rica for cryopreservation of lymphocytes using the ficoll-hypaque gradient method for studies of cell-mediated immunity.
Randomization procedure
Randomization (1:1) occurred in a masked fashion at the field site at the time the participant received her first vaccine dose. To allow for this, a range of vaccine ID numbers were randomly assigned to two groups by the Data Management Center. One group of random vaccine ID numbers was used at the manufacturing plant located in Belgium to label the HPV-16/18 bivalent vaccine. The other was used to label the control Hepatitis A vaccine at the same facility. Vaccine syringes for doses one, two and three were labeled in this manner. Doses two and three vaccine IDs were individually linked to dose one vaccine IDs using a vaccine ID numbering system that utilized an alphanumeric root ID number (e.g., VX12345) followed by a three digit sequence number that identified the dose number (i.e., 001, 002, and 003). Following the labeling process, vaccine vials from the two groups were combined in sequential order. This was done separately for each of the three doses. Vaccines were then shipped from the manufacturing plant to Costa Rica. In Costa Rica, first dose vaccine vials were dispensed in sequential order. Once a woman had been linked to a dose one vaccine ID, her vaccine ID for the remaining doses was fixed to the same root vaccine ID number (e.g., if a woman received vaccine VX12345-001 for dose 1, she was linked to VX12345-002 and VX12345-003 for doses two and three). This ensured that the same material type was administered to each woman at all three vaccination visits. To allow for replacement of vaccine vials that were damaged or otherwise unusable, additional doses were always available at the site. In cases where replacement was necessary, a web-based system developed and maintained by the Data Management Center was accessed by authorized personnel with valid logon and password information and a request made for a replacement vaccine. The web-based system provided the site with a replacement vaccine ID number without providing treatment arm information to ensure masking was maintained.
Vaccination (windows)
After the exams and specimen collection, women received their first vaccine most of the times in the non-dominant deltoid muscle, with a 1-inch syringe needle in most instances but a 1.5-inch syringe needle for large women. Extensive verification procedures were in place to make sure the women received the correct vaccine (i.e., the next available for the first dose and the vaccine with the same ID number as the first one for booster doses).
The first vaccination was given at the enrollment visit, and the second and third doses were targeted at one and six months after the initial dose was received. Two types of windows (allowable periods to be eligible for each study visit) were defined for each booster: the desirable window that complied with recommendations of the manufacturer and an allowable range that permitted vaccination outside the desirable window when necessary. The desirable window for the 1 month visit was from 21 days to 90 days after enrollment (first dose), and the allowable window was from 21 days to 120 days after enrollment. Similarly, for the 6 month visit, the desirable window was from 90 days to 210 days after the 1 month visit, and the allowable window was from 121 days to 300 days after enrollment. A woman who missed her allowable window moved to the next window and missed that vaccine dose.
During the 6-month visit, no pelvic exam was done unless the woman had an ASC-US positive for HPV DNA or LSIL interpretation of the enrollment cytology. However, all sexually active women were asked to self-collect a vaginal specimen for HPV testing. Detailed explanation of the procedure and a brochure were used to instruct the women on the collection of this specimen, which consisted of inserting a Dacron swab as high as possible into the vagina, trying to avoid touching the external genitalia, rotating it 5 times and placing it in an empty wide-mouth 50-ml collection cup. The nurse transferred the swab to a 10-ml vial with 3 ml of PreservCyt solution and placed it immediately in liquid nitrogen. Women who required a pelvic exam had both the clinician-collected specimen and the self-collected one (see results section).
A subset of women was invited to participate at a 7-month visit to obtain blood to investigate the maximum peak of immunogenicity. The target was to obtain 600 women for this component of the study
Monitoring of adverse events and pregnancies
All adverse events (any untoward medical condition occurring to a trial participant), independent of their possible relationship with vaccination are fully documented and followed through resolution using different mechanisms. The first assessment of reactogenicity was carried out immediately after each vaccination. Women were asked to stay in an observation room, initially for 30 minutes and, beginning in October 2005, for 60 minutes. After the observation period, women were asked about the occurrence of any reactogenicity symptoms, including fatigue, myalgia, arthralgia, headache, gastrointestinal symptoms, fever, malaise, difficulty breathing or rash. Ten percent of participants (those with a randomly assigned Participant ID [PID] ending in “2”) had a home visit scheduled by a study nurse 3–6 days after each vaccination. In addition, a toll-free number (800-doctora) with direct access to one of our resident doctors was available 24 hours a day, 7 days a week for reporting of adverse events or to answer questions by the participants. During subsequent visits, the participants are asked about hospital admissions or consultation with clinicians which, if reported, prompt the completion of an adverse event form. A rapid reporting system is in place to report serious adverse events within 24 hours to the IRBs and to a regulatory associate (Westat) for reports within regulatory timelines to NCI, the investigational new drug (IND) application holder (GSK) and the US Food and Drug Administration (FDA).
Pregnancies reported to any member of the study team are documented and reported to the NCI-contracted regulatory associates within 5 days of the study team learning about them. The regulatory associate communicates the information to NCI and the IND holder (GSK). All pregnant women are followed until resolution of their pregnancies and the outcome is documented, including characteristics of the delivery and the babies (duration of pregnancy, outcome, type of delivery, sex, weight, length, and Apgar score). Any abnormalities of a baby are reported as serious adverse events. In addition, all adverse events related to the pregnancy are reported. Statistics of non-serious adverse events are reported periodically to the corresponding authorities.
Follow-up
Follow-up is planned to continue for at least 4 years with yearly visits for most women. During the yearly visits, a pelvic exam is performed with collection of the same specimens as in the first visit, with the exception of citrated blood for plasma and whole blood for CBC. During follow-up, the 40-ml of blood for cryopreservation are collected in the subset of women with PID ending in “5” (immunogenicity subcohort) at visit 12 and 36, from women referred to 6 months follow-up (see below) and from women referred to colposcopy who require a biopsy or a LEEP. Women with LSIL or with ASC-US HPV positive are followed every 6 months until they have 3 consecutive normal cytologies (see below). The 4-year follow-up visit colposcopy referral algorithm (see below) will be modified using all available information (including HPV typing data) to assure the safety of the women before they are released from the study or transferred to a new follow-up schedule for optional long term follow-up (see below) under a separate protocol that has been developed.
Diagnosis and management of cytological abnormalities and colposcopic referral
Liquid-based cytologic preparations are made and stained at the Liberia laboratory following strict standard operating procedures and subject to extensive quality control methods. In particular, extensive measures to control humidity are essential to maintain the quality of the staining in tropical weather regions. Results are classified using the Bethesda system recording squamous and glandular changes.
Interpretation is carried out by a local laboratory with double reading by cytotechnologists and adjudication of all possibly abnormal interpretations by the cytopathologist (MA). The clinical management of the study participants relies on the Costa Rican cytopathology interpretation but, for quality control, all slides read as abnormal in Costa Rica (ASC or worse) and a 10% sample of the slides read as Negative (either totally Normal or Reactive Changes) in Costa Rica are re-screened and re-interpreted in the United States (led by cytotechnologist Claire Eklund and pathologist Martha Hutchinson). The 10% set of negatives selected for evaluation in the United States is randomly selected, without replacement and without regard to HPV test results. This sampling scheme was designed to result in approximately 50% of subjects with a negative cytology having an expert review over the course of the 4-year study (see results section).
Women with LSIL or HPV positive ASC-US are transferred from a yearly to a six-monthly follow-up schedule, until 3 consecutive normal cytologic results refer them back to yearly follow-up. A repeat LSIL or HPV positive ASC-US prompts referral to colposcopy, as does a single ASC-H or an HSIL+ at any time. The uncommon glandular abnormalities also lead to immediate referral to colposcopy. These guidelines are consistent with current American Society for Colposcopy and Cervical Pathology (ASCCP)-sponsored consensus guidelines for this age group (23). Cytologic preparations interpreted as “Unsatisfactory” are treated as LSIL, referring women to semi-annual follow-up if previous interpretations were normal or to colposcopy if there was a previous LSIL or HPV positive ASC-US.
Colposcopic examinations are carried out by our expert colposcopist (JM), following strict diagnostic and treatment algorithms that involve biopsy of women with evident low-grade lesions and active surveillance with repeat colposcopy and cytology for women with apparently normal colposcopic impression. Women with histologic CIN2 or CIN3 lesions are treated with LEEP. Any woman with cancer would be referred for appropriate treatment at the local referral hospitals under the Social Security of Costa Rica. Women referred to colposcopy usually require several visits to complete their diagnostic workup; they do not attend regular study visits until released from colposcopy. After the last visit in the study, a modified algorithm for even more aggressive colposcopic referral, biopsy, and treatment will be established to assure safety of participants who might subsequently have less intensive surveillance than the trial provides.
Specimen handling, data management and quality control
Detailed standard procedures were developed for the proper labeling, transportation, processing and shipment of specimens, with particular emphasis on maintenance and monitoring of the cold chain. The vaccine and biospecimen repository operated in Liberia and the tens of thousands of specimens were tracked using the NCI biospecimen inventory system (BSI). Large vapor phase liquid nitrogen tanks were used for storage, small dry shippers for transportation from the clinics and large dry shippers for transportation to laboratories or repositories outside Costa Rica.
A data-management system was developed in collaboration with Information Management systems (IMS) and our computer experts. All data on case report forms were double-keyed and extensive data cleaning was carried out in real time by checking for inconsistencies. The data management system was designed to track the investigational product, handle appointments, permit data entry, and organize follow-up of adverse events and pregnancies. It also permitted a series of reports to assess the field effort. A strict safety and back-up protocol was established to assure integrity of the data, which were transferred periodically to IMS in the US, where extensive computer edits were conducted and queries produced to resolve all apparent discrepancies.
An extensive quality-control process was established, including continued training of the staff, careful documentation and tracking of deviations from procedures. Monitors from the NCI contracted regulatory associate reviewed 100% of informed consents, eligibility criteria, all serious adverse events and pregnancies, selected items in all charts after data entry and a sample of approximately 20% of all charts. Two of the NCI investigators served as medical monitors (MS and DS). They received safety reports, assisted the investigators with the adjudication of difficult cases and served as liaison between the Investigational New Drug (IND) holder (GSK) and Costa Rica.
HPV testing
HC2™ testing was performed on enrollment (pre-vaccination) specimens using a 2ml aliquot of exfoliated cells stored in Preservcyt. Testing was performed using Probe B (designed to detect 13 oncogenic HPV types including types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68; and with known cross-reactivity to another carcinogenic type, HPV66) as per manufacturer’s instructions in a laboratory located at the University of Costa Rica in San José, Costa Rica.
Broad spectrum PCR-based HPV DNA testing was performed at Delft Diagnostics Laboratory (DDL, Delft, the Netherlands) using a previously described procedure based on amplification using the SPF10 primers followed by typing using the LiPA line detection system (24–26). In addition, to ensure that HPV-16 and HPV-18 infections were not missed, all specimens that screened positive for HPV DNA using SPF10 but that were negative for HPV-16 or HPV-18 by LiPA were tested for the presence of HPV-16 and HPV-18 DNA using type specific primers, as previously described (26,27). One of the two 0.5 ml aliquots of exfoliated cells stored in Preservcyt was used for PCR-based testing. Both enrollment specimens (pre-vaccination) and specimens collected at the semi-annual and annual visits were tested by the PCR method. For all except the 6-month visit, clinician administered cervical specimens collected during the pelvic examination were used. For the 6-month visit, self-collected cervicovaginal specimens were used. In addition, for the subset of women who had a pelvic examination performed at the time of the 6-month visit, a clinician-administered cervical specimen collected during the pelvic examination was used for PCR-based HPV DNA testing.
The SPF10/LIPA system in our study has shown very good agreement both with Hybrid Capture 2 (28) and with Linear Array (29).
Regulatory supervision
The primary IRB reviewing and following the study was the INCIENSA (Instituto Costarricense de Investigación y Enseñanza en Nutrición y Salud) IRB. In addition, the CONIS (Costa Rican National Council for Health Research), the NCI IRB, and the IRB of the University of Costa Rica approved the study. A data and safety monitoring board (DSMB) was in place with members from Costa Rica and the US to carry out periodic evaluations of safety. In addition, an external Working Group was established; the CR and US members had varied expertise to provide scientific advice to the NCI.
Statistical methods
In this article we present a comparison of the demographic characteristics of women included in both study arms. Statisitcal significance of the difference between arms was assessed with the chi square test, which was also used to test the significance of the difference between the Costa Rican census and the population included in our study.
To assess agreement between different laboratory tests or concordance between laboratories in the US and Costa Rica we used linear weighted kappa statistic and McNemar’s tests
Results
Participation rates
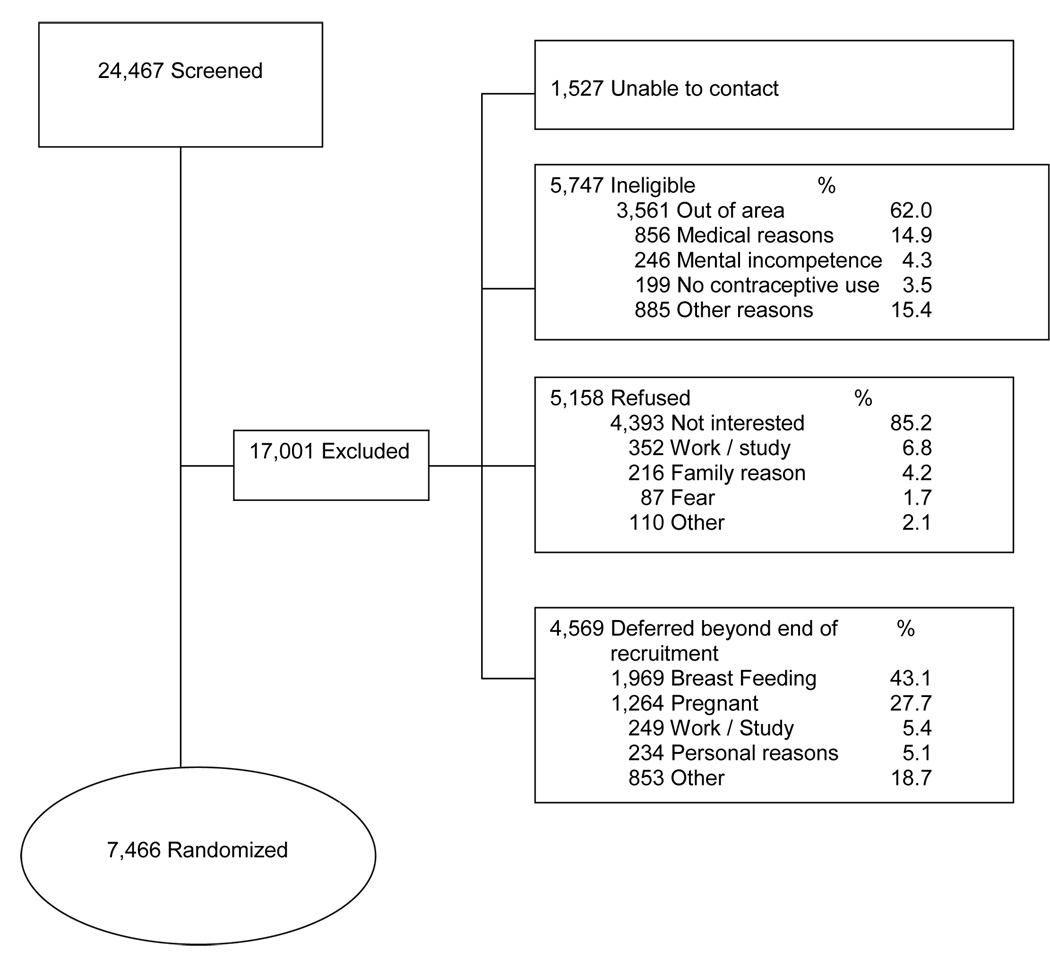
Inclusion of women in the study started on 28 June 2004 and was completed on December 21, 2005. Of a total 24,467 women who were pre-screened, 7,466 were recruited (30.5%) and 17,001 were excluded (Figure 2). Despite repeated visits to their expected homes, we were unable to contact 1,527 women (6.2%). A total of 5,747 women (23.5%) were coded as ineligible. Most of them (3,561, 62.0% of ineligibles) had moved or were out of the area for long periods. An additional 856 (14.9% of ineligibles) were not eligible for physical medical reasons, and 246 (4.3%) were considered mentally incompetent.
Figure 2.
Flow diagram of recruitment results
A total of 5,158 women (21.1% of those pre-screened) were considered refusals. Most women who refused indicated lack of interest in participation (85.2%), while others reported work, study or family reasons.
Another group of 4,569 women (18.7% of those pre-screened) had to be deferred beyond the end of the recruitment period. Most of them were breastfeeding (43.1%) or pregnant (27.7%). Although overall participation was 30.5%, when calculated as a percentage of 12,624 women potentially eligible for recruitment during the study period, participation was 59.1%.
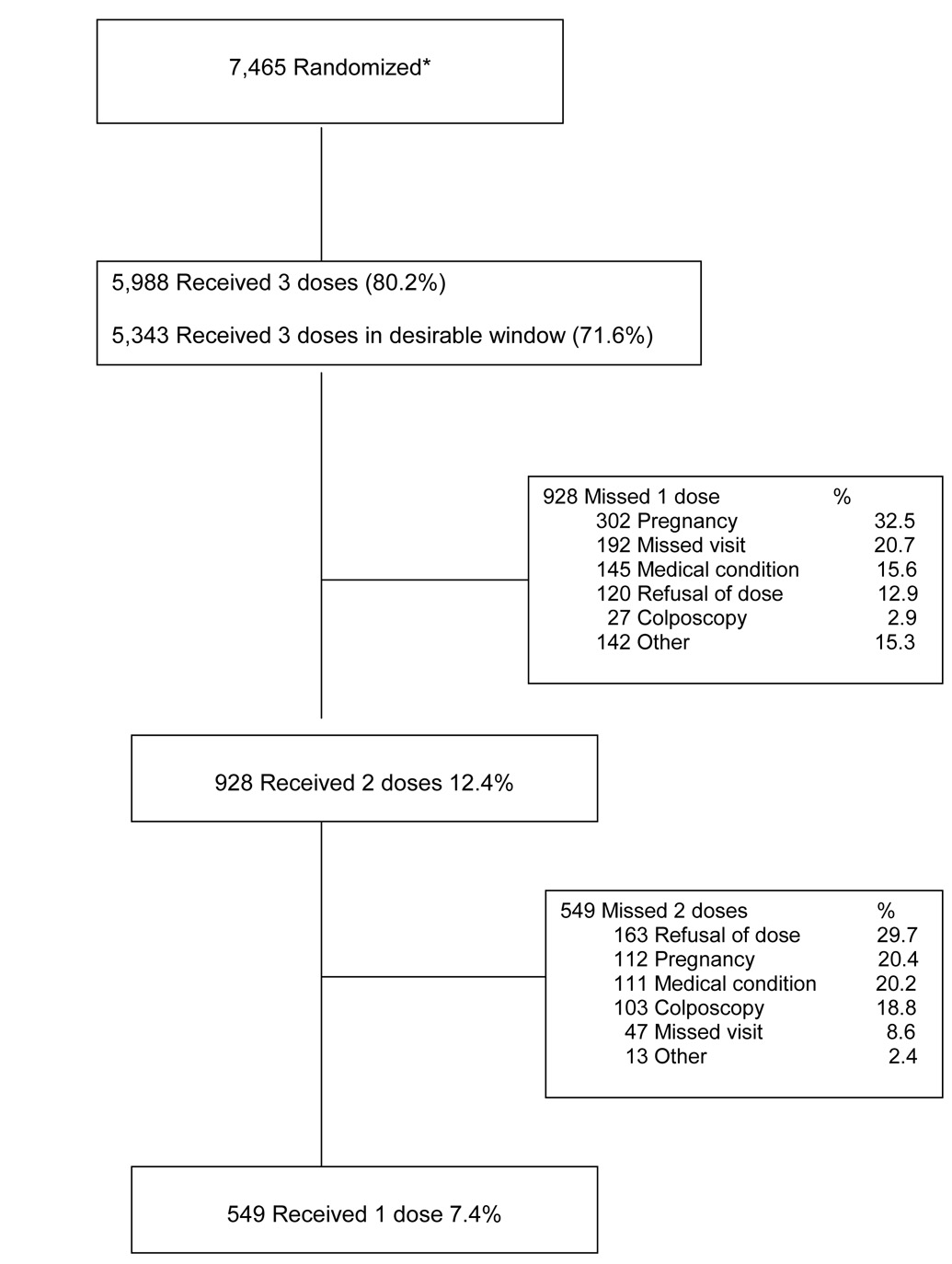
Compliance with vaccination, visit 07 and post vaccination home visits
Of the 7,466 women recruited, one woman was excluded from the vaccine compliance diagram because she received discordant vaccines, for a total of 7,465. Of these, 5,988 women (80.2%) received 3 doses, and 5,343 (71.6%) received 3 doses within the desirable windows. A total of 928 women missed one dose (the second or the third), mainly because of pregnancy (32.5%), missed visits (20.7%), medical conditions (15.6%), or refusal to get that dose (12.9%). An additional 549 women received only one vaccine dose, with refusal to get the vaccine being the main reason (29.7%), followed by pregnancy (20.4%), medical conditions (20.2%), or referral to colposcopy (18.8%).
The first 600 women recruited were initially defined as the immunogenicity sub-cohort, and were invited to a visit at month 7 (defined in relation to the 6 months visit), when the immune response is thought to peak after vaccination. Of the original 600 women, only 353 attended this visit. However, a protocol amendment was made later during the study to recruit more women into this sub-cohort. In all, 557 women were recruited into the immunogenicity sub-cohort (93% of the original planned).
Ten percent of randomly selected women were selected for a home visit between days 3–6 after vaccination to document solicited reactogenicity. The home visits after the first dose were completed for 98% of participants and those after the second and third were completed for 97% of participants.
Compliance with interview, pelvic exam and specimen collection
Table 1 presents results of compliance with interview and specimen collection during the vaccination period. The enrollment visit (V00) was the most complex. Interview and blood were obtained from non-sexually active women and multiple types of cervical specimens were collected from sexually experienced women.
Table 1.
Compliance with interview and specimen collection during vaccination
| V00 | V01 | V06 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Eligible | Collected - available | % | Eligible | Collected | % | Eligible | Collected | % | |
| Interview | 7466 | 7458 | 99.9 | 6484 | 6465 | 99.7 | |||
| Pelvic exam | 5874 | 5871 | 99.9 | 669 | 675 | 100.9 | |||
| Cervical secretions | 5871 | 5871 | 100.0 | 669 | 674 | 100.7 | |||
| PreservCyt | 5871 | 5871 | 100.0 | 669 | 674 | 100.7 | |||
| Aliquots | 5871 | 5871 | 100.0 | 669 | 674 | 100.7 | |||
| Cytology results | 5871 | 5871 | 100.0 | 674 | 673 | 99.9 | |||
| HC2 HPV test results | 5871 | 5686 | 96.8 | ||||||
| Chlamydia test results | 5871 | 5829 | 99.3 | ||||||
| Gonorrhea test results | 5871 | 5831 | 99.3 | ||||||
| PCR HPV test results | 5871 | 5871 | 100.0 | ||||||
| Cells in UCM | 5871 | 5871 | 100.0 | ||||||
| Violet top blood | 7466 | 7452 | 99.8 | ||||||
| Hematocrit | 7466 | 7445 | 99.7 | ||||||
| Red top blood | 7466 | 7428 | 99.5 | 6851 | 6843 | 99.9 | 6484 | 6474 | 99.8 |
| Yellow top blood | 7466 | 7398 | 99.1 | ||||||
| Green top blood | 757 | 739 | 97.6 | ||||||
| Self-collected cells for HPV | 5225 | 5219 | 99.9 | ||||||
For the enrollment visit (V00), most women had a valid interview (99.9%), and of sexually active women, 99.9% had a pelvic exam (3 missing, two presented excessive bleeding before collection and one had an intact hymen). Of those who had a pelvic exam, cervical secretions, exfoliated cells in PreservCyt, and additional cells in UCM were obtained for 100% of women. Laboratory results generated from the PreservCyt vial were available as follows: cytology 100%, HC2 HPV 96.8%, CT-GC 99.3% and PCR (assay done in the Netherlands), 100%. Only 0.5% of enrollment cytologies were considered inadequate, mainly because of a scant squamous epithelial component, while 12.8% were considered satisfactory but limited, mainly because of a lack of a transformation zone component.
Blood specimens were also obtained from more than 97.5% of women at enrollment, and CBC results were available for 99.7% of women who provided blood.
At the one-month visit (V01), only blood for serum was collected from 99.9% of women. A similar pattern of very high compliance was noted for the 6-month visit (V06), and at this visit a self-collected vaginal specimen for HPV testing was also requested from participants, with 99.9% compliance.
Quality indicators of the handling of the specimens demonstrated high adherence to the procedures, particularly in relation to maintenance of the cold chain. For example, the median time until placement of the cervical secretions into liquid nitrogen was 4 minutes (range 0–34). Minor issues following collection of cervical specimens were annotated for 53.3% of women, mainly bleeding during collection (80.3%), infection or discharge (10.4%) or a combination of both (6.4%).
Demographic and sexual characteristics of participating women by study arm
Table 2 presents demographic and selected sexual characteristics of participating women by study arm, indicating that there were no differences in the distribution of these variables by study arm. Overall, the age distribution indicates a trend of decreasing number of participants with each year of increasing age, mainly reflecting the Costa Rican population pyramid and the fact that younger women had more time to be recruited before reaching age 26. In terms of education, about 30% attended only primary schooling, 20% incomplete secondary (9th grade) and 50 % 10th grade or more. About 40% of women were married or cohabiting and 60% were single. Of the 7,466 women recruited, 21.3% were virgins, and 5,874 had already initiated sexual activity. Among the sexually active, the median age of first intercourse was 17 years, with 14% having started at or before age 14, 17% at age 15, 17% at age 16, 19% at age 17 and the rest at age 18 or older. Forty-two percent of sexually active women reported only one sexual partner in their lifetime, and the median number was 2. Less than 10% reported 5 or more partners. For the frequency of sexual intercourse, the mode was 2–4 times a month.
Table 2.
Demographic and sexual characteristic of study participants by study arm
| Study arm | ||||
|---|---|---|---|---|
| HPV-16/18 Vaccine | Control Vaccine | |||
| Age | n | % | n | % |
| 18 years | 634 | 17.0 | 653 | 17.5 |
| 19 years | 558 | 15.0 | 591 | 15.8 |
| 20 years | 516 | 13.8 | 490 | 13.1 |
| 21 years | 431 | 11.6 | 415 | 11.1 |
| 22 years | 417 | 11.2 | 448 | 12.0 |
| 23 years | 400 | 10.7 | 397 | 10.6 |
| 24 years | 395 | 10.6 | 384 | 10.3 |
| 25 years | 375 | 10.1 | 358 | 9.6 |
| Total | 3,726 | 100.0 | 3,736 | 100.0 |
| p = 0.80 | ||||
| Years of education | ||||
| < 7 years | 1047 | 28.1 | 1068 | 28.7 |
| 7 – 9 years | 868 | 23.3 | 828 | 22.2 |
| 10 + technical | 1,178 | 31.6 | 1,240 | 33.3 |
| University | 632 | 17.0 | 591 | 15.9 |
| Total | 3,725 | 100.0 | 3,727 | 100.0 |
| p = 0.25 | ||||
| Marital status | ||||
| Married | 1,532 | 41.1 | 1,543 | 41.4 |
| Divorce – widow | 102 | 2.7 | 98 | 2.6 |
| Single | 2,091 | 56.1 | 2,088 | 56.0 |
| Total | 3,725 | 100.0 | 3,729 | 100.0 |
| p = 0.94 | ||||
| Age at first sexual intercourse (among sexually active) | ||||
| 18 + | 961 | 32.6 | 946 | 32.5 |
| 17 | 553 | 18.7 | 561 | 19.3 |
| 16 | 483 | 16.4 | 503 | 17.3 |
| 15 | 525 | 17.8 | 503 | 17.3 |
| 14 years or less | 430 | 14.6 | 397 | 13.6 |
| Total | 2,952 | 100.0 | 2,910 | 100.0 |
| p = 0.72 | ||||
| Number of lifetime sexual partners (among sexually active) | ||||
| 1 | 1,231 | 41.8 | 1,242 | 42.8 |
| 2 | 775 | 26.3 | 749 | 25.8 |
| 3 | 446 | 15.1 | 441 | 15.2 |
| 4 | 201 | 6.8 | 200 | 6.9 |
| 5 | 140 | 4.7 | 132 | 4.5 |
| 6 + | 155 | 5.3 | 140 | 4.8 |
| Total | 2,948 | 100.0 | 2,904 | 100.0 |
| p = 0.95 | ||||
| Monthly frequency of sexual intercourse | ||||
| 1 or less | 547 | 18.8 | 499 | 17.4 |
| 2 – 4 | 775 | 26.7 | 782 | 27.2 |
| 5 – 9 | 642 | 22.1 | 646 | 22.5 |
| 10 – 13 | 610 | 21.0 | 618 | 21.5 |
| 14 + | 330 | 11.4 | 328 | 11.4 |
| Total | 2,904 | 100.0 | 2,873 | 100.0 |
| p = 0.71 | ||||
Statistics for 4 women excluded for discordant vaccinations: Age - 19, 22, & 2 at 23; Education - <7 years, 2 at 7–9years, & University; Marital status - 2 married & 2 single; Age at first sex - 14 or less, 16, & 18+; Lifetime sex partners - 1, 3, & 4; Monthly frequency of sex - 1 or less & 2 at 5–9.
Unknowns excluded.
Other characteristics of study participants
In table 3, selected reproductive, contraceptive and smoking behavior characteristics are presented. Almost 50% of the women had already been pregnant, most of them with one pregnancy. Almost 80% of women had used oral contraceptives, with current use by 53%, while only 20% reported use of condoms in the last month. Among sexually active women, 68% had had a pap smear. Less than 10% were current smokers.
Table 3.
Reproductive, contraceptive and smoking behavior of study participants.
| Number of pregnancies | ||
|---|---|---|
| n | % | |
| 0 | 3,917 | 52.5 |
| 1 | 2,185 | 29.3 |
| 2 | 950 | 12.7 |
| 3 | 319 | 4.3 |
| 4 | 67 | 0.9 |
| 5 + | 28 | 0.4 |
| Total | 7,466 | 100.0 |
| Ever used contraceptive method (among sexually active) | ||
| Yes | 5,683 | 96. 8 |
| No | 186 | 3.2 |
| Total | 5,869 | 100.0 |
| Use of oral contraceptives (among sexually active) | ||
| No | 1360 | 23.2 |
| Former user | 1374 | 23.4 |
| Current used (last month) | 3126 | 53.3 |
| Total | 5860 | 100.0 |
| Use of condom (among sexually active) | ||
| No | 2324 | 39.6 |
| Former user | 2393 | 40.8 |
| Current user (last month) | 1149 | 19.6 |
| Total | 5866 | 100.0 |
| Has had a Pap (among sexually active) | ||
| Yes | 4002 | 68.1 |
| No | 1870 | 31.8 |
| Total | 5872 | 100.0 |
| Has been told that cytology was abnormal (among those with a cytology) | ||
| Yes | 364 | 10.2 |
| No | 3205 | 89.8 |
| Total | 3569 | 100.0 |
| Smoking | ||
| Never smoked | 6,441 | 86.4 |
| Past | 427 | 5.7 |
| Current intermittent smoker | 425 | 5.7 |
| Current regular smoker | 165 | 2.2 |
| Total | 7,458 | 100.0 |
Unknowns excluded
Table 4 presents selected characteristics of the sexual partners of women reporting lifetime monogamy, as reported by the women. In terms of education, about 60% of the partners had 9 years of education or less, and about 40% had more education. As reported by the monogamous women, only 22% of their partners had only them as their lifetime sexual partner. Circumcision of their partners was reported by about half the women, but despite the use of diagrams during the interview, we question the validity of this information given that the practice of circumcision is known to be rare in this area.
Table 4.
Characteristics of partners of monogamous women
| Currently living with partner | ||
|---|---|---|
| n | % | |
| Yes | 1525 | 61.6 |
| No | 949 | 38.4 |
| Total | 2474 | 100.0 |
| Years of education | ||
| < 6 years | 936 | 39.1 |
| 7 – 9 | 545 | 22.7 |
| 10 + technical | 554 | 23.1 |
| University | 361 | 15.1 |
| Total | 2,396 | 100.0 |
| Numbers of partners | ||
| 1 | 492 | 22.1 |
| 2 | 533 | 23.9 |
| 3 | 371 | 16.7 |
| 4 | 383 | 17.2 |
| 5 | 111 | 5.0 |
| 6 | 153 | 6.9 |
| 7 + | 184 | 8.3 |
| Total | 2227 | 100.0 |
| Circumcision | ||
| Yes | 1,181 | 52.7 |
| No | 1,061 | 47.3 |
| Total | 2,242 | 100.0 |
| Smoking | ||
| Never | 1,775 | 71.8 |
| Before | 70 | 2.8 |
| Currently | 626 | 25.3 |
| Total | 2,471 | 100.0 |
Unknowns excluded
In table 5, we present selected characteristics detected in the physical exam and cytology. About 65% of women had normal or lower than normal weight according to WHO classification using the body mass index, 22% were overweight and 13% obese. Contrary to our expectations, less than 2% of women had anemia, while 30% had some degree of ectopy and 44% moderate or severe inflammation in the cytologic interpretation, which is common in Guanacaste (30) Prevalence of CT infection was high at 14.2% and prevalence of GC infection was 0.8% (22).
Table 5.
Physical exam and selected laboratory characteristics
| Body mass index | ||
|---|---|---|
| n | % | |
| < 20 | 1,395 | 18.7 |
| 20 – 25 | 3,468 | 46.5 |
| 25 – 30 | 1,658 | 22.2 |
| 30 + | 945 | 12.7 |
| Total | 7,466 | 100.0 |
| Hematocrit | ||
| 24 – 31.9 | 50 | 0.7 |
| 32 – 32.9 | 50 | 0.7 |
| 33 + | 7,344 | 98.7 |
| Total | 7,444 | 100.0 |
| Location of the transformation zone (among women with a pelvic exam) | ||
| Not visible | 1911 | 32.5 |
| External os | 2200 | 37.5 |
| Moderate Ectropion | 1312 | 22.3 |
| Extensive Ectropion | 448 | 7.6 |
| Total | 5871 | 100.0 |
| Inflammation in cytology (among women with a pelvic exam) | ||
| Not present or Mild | 3282 | 55.9 |
| Moderate | 2048 | 34.9 |
| Severe | 541 | 9.2 |
| Total | 5871 | 100.0 |
| Chlamydia trachomatis | ||
| Negative | 5002 | 85.8 |
| Positive | 827 | 14.2 |
| Total | 5829 | 100.0 |
| Neisseria gonorrhea | ||
| Negative | 5782 | 99.2 |
| Positive | 49 | 0.8 |
| Total | 5831 | 100.0 |
Unknowns excluded
Table 6 presents HPV and cytology results by age. HPV positivity by HC (13 targeted carcinogenic types, with some known cross-reactivity (31) was 35% overall, with some indication of a decline with increasing age. PCR positivity was 50% with a clearer decline with age. HPV 16 and 18 positivity were 8.3% and 3.2%, respectively, and did not have a clear age trend. Other oncogenic types (30.3%) and non oncogenic types (15.3%) showed clear trends of reduction with age as did LSIL. On the other hand, HSIL increased to the upper end of the limited age range of study participants. Histologic CIN1 was detected rarely and did not show a clear age pattern, because LSIL cytology in this protocol did not prompt referral to colposcopy. Histologic confirmation of CIN 2+ showed a clear pattern of increase with age, with a prevalence among 25 year old women more than 10 times that of 18 years old women.
Table 6.
HPV positivity and cervical neoplasia by age among sexually experienced women*
| HC2 | PCR | HPV16 | HPV18 | Other oncogenic** | Non oncogenic | Cytology LSIL | Cytology HSIL | Histology CIN 1 | Histology CIN 2+ | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AGE | N* | % Positive | % Positive | % | % | % | % | ||||
| 18 | 717 | 36.8 | 53.7 | 7.9 | 2.4 | 34.6 | 20.2 | 16.6 | 1.1 | 1.0 | 0.1 |
| 19 | 796 | 38.7 | 53.1 | 9.8 | 3.6 | 31.9 | 16.3 | 16.0 | 0.8 | 0.3 | 0.4 |
| 20 | 769 | 37.7 | 51.5 | 7.4 | 4.3 | 29.5 | 15.9 | 12.5 | 1.2 | 1.0 | 0.3 |
| 21 | 689 | 37.9 | 52.8 | 11.8 | 3.6 | 33.7 | 18.7 | 15.7 | 1.6 | 0.7 | 0.6 |
| 22 | 765 | 33.2 | 51.1 | 7.7 | 2.1 | 29.7 | 14.4 | 10.5 | 2.2 | 0.5 | 0.8 |
| 23 | 738 | 31.5 | 46.7 | 6.8 | 4.5 | 28.3 | 13.4 | 10.7 | 2.2 | 0.4 | 1.1 |
| 24 | 714 | 33.5 | 48.9 | 8.3 | 2.0 | 29.6 | 12.2 | 11.5 | 3.0 | 1.3 | 1.1 |
| 25 | 683 | 25.9 | 41.7 | 6.9 | 3.1 | 25.3 | 11.6 | 8.0 | 3.3 | 1.0 | 1.2 |
| Total | 5871 | 34.5 | 50.0 | 8.3 | 3.2 | 30.3 | 15.3 | 12.7 | 1.9 | 0.8 | 0.7 |
The numbers of results for each test are slightly different
Includes HPV 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68
In order to determine if the characteristics of the population included in the study were similar to those of women from the general population, we compared our data on age, education and marital status with those from the Costa Rican National Census of 2000, for women of the same ages and living in the regions of the study (Table 7). Women in the trial tended to be slightly younger, more educated and more likely to be single than women in the national census. All these differences were statistically significant.
Table 7.
Comparison of cohort recruited in the HPV vaccine trial with women 18–25 years old in the 2000 national census of Costa Rica
| Age | Census | HPV vaccine trial | |||
|---|---|---|---|---|---|
| n | % | N | % | ||
| 18 | 3780 | 14.2 | 1287 | 17.2 | |
| 19 | 3609 | 13.5 | 1150 | 15.4 | |
| 20 | 3662 | 13.7 | 1006 | 13.5 | |
| 21 | 3106 | 11.6 | 846 | 11.3 | |
| 22 | 3364 | 12.6 | 866 | 11.6 | |
| 23 | 3140 | 11.8 | 799 | 10.7 | |
| 24 | 3011 | 11.3 | 779 | 10.4 | |
| 25 | 3023 | 11.3 | 733 | 9.8 | |
| Total | 26695 | 100.0 | 7466 | 100.0 | p=0.000 |
| Mean age | 21.3 years | 21.1 years | |||
| Median age | 21.1 years | 21.0 years | |||
| Education | |||||
| n | % | N | % | ||
| < 7 | 11994 | 44.9 | 2116 | 28.4 | |
| 7 – 9 | 5310 | 19.9 | 1698 | 22.8 | |
| 10 – 11 | 5377 | 20.1 | 2418 | 32.4 | |
| > 11 | 4014 | 15.0 | 1224 | 16.4 | |
| Total | 26695 | 100.0 | 7456 | 100.0 | p=0.000 |
| Mean years education | 8.5 years | 9.6 years | |||
| Median years education | 8.0 years | 9.0 years | |||
| Marital status | |||||
| n | % | n | % | ||
| Married | 12959 | 48.5 | 3077 | 41.3 | |
| Divorced/ separated/ widowed | 746 | 2.8 | 200 | 2.7 | |
| Single | 12990 | 48.7 | 4181 | 56.1 | |
| Total | 26695 | 100.0 | 7458 | 100.0 | p=0.000 |
Agreement between self-collected and clinician collected HPV testing
For women who had both self-administered and clinician-administered exfoliated cell collection at the 6-month visit, a high degree of concordance was observed in HPV results from these two specimen types. Percent agreement and kappa values for HPV detection between the self administered and clinician administered specimens was 96.0% and 0.86 (McNemar’s p = 0.56) for HPV-16 and 97.6% and 0.81 (McNemar’s p = 1.00) for HPV-18, respectively. Overall agreement (all types) was 89.4% with a kappa value of 0.59 (McNemar’s p = 0.19). Given this high level of agreement, HPV testing results using the self administered specimens, available for all sexually active women, will be used to define HPV status at the 6-month visit.
Agreement between local cytology and US quality control readings
As described above, all abnormal cytologies (ASC +) and a randomly selected 10% sample of negatives (normal) are read in the US for quality control.
Of the 5871 women who had cytology performed at enrollment, 28 had rare glandular changes. All were reviewed by our protocol, showing the expected poor agreement for these difficult readings. Many yielded concurrently abnormal squamous interpretations that were often high-grade (n=12). Of the remaining slides, 38 were judged to be inadequate for review in the US. Reactive Changes (grouped as normal) comprised the most common cytologic interpretation (3742/5805 = 64.5%). Reactive changes, e.g., inflammation of the cervix is extremely common in Costa Rica as noted in our previous natural history studies there, although the cause(s) is unknown.
Agreement between the Costa Rican and U.S. cytopathologists over six levels of interpretation was very good, with a linear weighted kappa of 0.70 (95% CI = 0.68 – 0.73). The Costa Rican pathologist tended not to use the ASC-US or ASC-H interpretations as much as the U.S. reviewers. In dichotomized results, the U.S. raters called many more slides HSIL+ (McNemar’s test of p < 0.001). However, given the study protocol, few if any cases would be missed by our follow-up and referral algorithm. We found zero cases reported in the US as HSIL and no histologic CIN2+ when the Costa Rican cytology was Normal (0/117) or when Costa Rican cytology was Reactive Changes with a negative HC2 result (0/280). Histologic CIN2 was found in only 1/81 cases of HC2-negative ASC-US.
Discussion
As part of a longstanding collaboration between NCI and Costa Rican researchers to investigate the natural history of HPV and cervical neoplasia and to evaluate new preventive strategies, we have now successfully completed the recruitment and vaccination phase of a double blind randomized clinical trial of a vaccine against HPV 16 and 18, the two viral types associated with up to 70% of cervical cancers.
The trial was conducted in Guanacaste and Puntarenas, two provinces of Costa Rica with traditionally high incidence of cervical cancer. The study is community-based and all women 18–25 residing in the selected areas were invited to participate. The community-based design will allow a natural history study within the trial and better extrapolation of the vaccine efficacy and effectiveness data. A population census was conducted and more than 24,000 women were invited at home to participate. However, the clinical trial required the application of a strict set of eligibility criteria, including being in good health, willing to use contraception, and not being pregnant or breastfeeding. After application of the eligibility criteria and excluding those we could not locate and those who refused to participate, approximately 30% of women were recruited. When considering only eligible women, the fraction recruited was close to 60%. The number of women recruited (7,466) provides adequate statistical power for the analyses anticipated according to the protocol objectives. One of the reasons why many women could not be found when they were visited at home is that the original census was done several years before initiating the study, due to serious delays in initiation unrelated to the field effort.
A comparison of the demographic and sexual characteristics of women assigned to each study arm revealed a very similar distribution, indicating that the randomization process was successful. We compared some characteristics of the population of women included in the study with data for women of the same areas and ages from the national census, and the group selected was slightly younger and considerably more educated than women from the same areas in the census.
An extensive specimen collection protocol was in place, including specimens for cytology, HPV testing, local immunity and blood at every visit. The specimens were transferred to laboratories in San José, USA or The Netherlands. Virtually 100% of women donated all specimens and results were obtained for all laboratory tests, reflecting the sophisticated logistics and organization that the Guanacaste Project has developed over more than 20 years of collaborative research.
The population included in the study is comprised of young women with more than basic education and high access to medical services under the universal coverage of the Costa Rican Social Security, as exemplified by the extended use of hormonal contraceptives and pap smears. The women are characterized by a relatively conservative sexual behavior, with a majority of women reporting one or two sexual partners in their lifetime, and fewer than 10% reporting more than 5 partners. On the other hand, only 20% of the sexual partners of monogamous women were considered by their female partners to have had only one partner. The prevalence of HPV (50.0 %), inflammation on cytology (44.1 %) and Chlamydia infection (14.2%) are very high. A history of pregnancy is common (60% of sexually active women), but smoking is very rare (13.6% ever). Anemia is uncommon (2%) and obesity is 13%.
An overall HPV positivity of 50% is on the upper end of the expected range for this age group, and is higher than all areas reported in a worldwide prevalence survey in which a less sensitive GP5+/6+ PCR method was used (32). The good concordance between the two HPV test methods (HC2 and PCR) confirms the reality of the infections (data not shown). The decline with age of LSIL and both carcinogenic and non-carcinogenic HPV types has been described as well as the upswing in the HSIL rate found after several years of sexual activity. In our previous work in the same region, we found that rates of HSIL fall again by the early thirties (17).
The areas included in the study are predominantly rural and the realization of the study required a very large operation with complex logistics that included outreach workers to maintain communication with the women at all times. Women were transported to many of their clinic visits or offered transportation, contributing to the high compliance with the visits.
The compliance with vaccination was very good, and the main reasons for noncompliance were those mandated by the protocol, like colposcopy, medical reasons or pregnancy.
This study is unique in that it is financed with US federal funds under a clinical trial agreement with the manufacturer who supplied vaccine for the trial. US and Costa Rican investigators retain autonomy in study decisions and data analysis, which will be fully disseminated in the scientific literature. This is, to our knowledge, the only clinical trial of an HPV vaccine in the public domain. The study also received enthusiastic support from the Costa Rican Ministry of Health, the University of Costa Rica and other government institutions, which also provided close supervision of the study through their Institutional Review Boards.
The investigation is monitored by multiple layers of review (education, standard operating procedures, internal quality assurance, data checks, monitoring, periodic audits, NCI and several Costa Rican IRBs, the Costa Rican Ministry of Health, a DSMB and a working group). It also involves extensive coordination between multiple collaborating institutions in several countries. The staff of the study in Costa Rica included more than 125 members at the most intensive recruitment period. The members of the staff required intensive training on Good Clinical Practices (GCP) and all the study procedures. Additional difficulties encountered to conduct the study included the logistic complexity of inviting large numbers of women at home and their transportation to the study clinics for multiple study visits, the limited countrywide experience in the realization of large clinical trials and the need for adequate communication with the media and health authorities.
Despite the community-based nature of this study, possible limitations of our study include the large number of exclusions and relatively low participation rates that may affect generalizability of the results. In addition, the fact that a sizable fraction of women received less than 3 doses may reduce our statistical power for some analyses. However, we may be able to preliminarily investigate the immunogenicity and efficacy of a reduced-dose schedule.
After completing the 4-years of follow-up, women are expected to be offered the vaccine they did not receive during the vaccination phase, plus hepatitis B vaccine. Since we believe that this large community–based trial provides a unique opportunity to evaluate the impact of the vaccine and to undertake a series of natural history evaluations, we are also planning long term follow-up of trial participants. This is logistically feasible given the low migration rates and the high retention observed to date (approximately 5% study discontinuation).
This study is unique in that, in addition to the data already available from multicentric studies (reviewed in 33) it will provide data on vaccine efficacy and effectiveness in a defined large population in a developing country. In addition, multiple tertiary objectives are envisioned, taking advantage of the fact that this is a large community based cohort. Furthermore, many scientific objectives, including a series of innovative immunological markers of humoral and cell-mediated immunity are envisioned
Finally, the success of our project demonstrates the feasibility of conducting large, complex and rigorous clinical and epidemiologic studies in Latin America.
Figure 3.
Compliance with vaccination
*One woman received discordant vaccines and was excluded
Table 8.
Cytology review agreement between original Costa Rican interpretation and U.S. review*
| CR Interpretation | Inadequate | Negative | ASC-US | ASC-H | LSIL | HSIL | Total |
|---|---|---|---|---|---|---|---|
| Inadequate | 3 | 0 | 0 | 0 | 0 | 0 | 3 |
| Negative* | 33 | 442 | 42 | 15 | 12 | 8 | 519 |
| 85.2% | 8.1% | 2.9% | 2.3% | 0.5% | 100.0% | ||
| ASC-US | 2 | 27 | 79 | 32 | 131 | 19 | 288 |
| 9.4% | 27.4% | 11.1% | 45.5% | 6.6% | 100.0% | ||
| ASC-H | 0 | 0 | 1 | 3 | 2 | 10 | 16 |
| 0.0% | 6.2% | 18.8% | 12.5% | 62.5% | 100.0% | ||
| LSIL | 0 | 2 | 25 | 27 | 407 | 71 | 532 |
| 0.4% | 4.7% | 5.1% | 76.5% | 13.3% | 100.0% | ||
| HSIL | 0 | 0 | 0 | 0 | 12 | 60 | 72 |
| 0.0% | 0.0% | 0.0% | 16.7% | 83.3% | 100.0% | ||
Restricted to squamous intepretations for slides with no glandular abnormalites
10% sample of total negative cytology, which includes reactive changes
Acknowledgements
We would like to extend a special thanks to the women of Guanacaste and Puntarenas, Costa Rica, who gave of themselves in participating in this effort. We also wanted to acknowledge the tremendous effort and dedication of the staff in Costa Rica involved in this project, including Bernardo Blanco and his team (census), Ricardo Cerdas and Ana Hernández (blood processing), Osman López, Johnny Matamoros, Cristian Montero, Rafael Thompson, and Jorge Umaña (field activity coordinators), Su Yen Araya, Hazel Barquero, Hayleen Campos, Muriel Grijalba, Ana Cristina Monge, Ana Peraza, Diana Robles, María Fernanda Sáenz, Dorita Vargas, and Jessica Vindas (clinic coordinators), Paola Alvarez, Dinia Angulo, Ana Live Arias, Betzaida Barrantes, Marianela Bonilla, Jessenia Chinchilla, Marianela Herrera, Andrea Interiano, Viviana Loría, Rebeca Ocampo, Angie Ramírez, Libia Rivas, Jessenia Ruiz, Malena Salas, and Yesenia Vázquez (clinicians), Marta Alvarado, Ana Cristina Arroyo, Gloriana Barrientos, Diana Díaz, Marlen Jara, Maureen Matarrita, María Ester Molina, Elida Ordóñez, Gina Sánchez, and Zihara Villegas (nurses), Arianne Castrillo and Vivian López (education and outreach effort coordinators), Karla Coronado (appointment coordinator), Ricardo Alfaro (quality control coordinator), Charles Sánchez and Livia Romero (document center coordinators), and Eric Alpízar and Carlos Avila (IT coordinators). Special recognition is also extended to Sofía Elizondo, Executive Director of Fundación INCIENSA and her staff for their administrative support. In the United States we would like to extend our appreciation to the team from Information Management Services (IMS) responsible for the development and maintenance of the data system used in the trial and who serve as the data management center for this effort. We would like to specifically acknowledge the invaluable contributions made by Julie Buckland and Laurie Rich. We acknowledge the contributions made by individuals at Westat, Inc., who provided project development and/or monitoring support, including Maribel Gomez, Kirk Midkiff, and Susan Truitt. We acknowledge the assistance provided by Carla Chorley, Troy Moore, Kathi Shea, and Heather Siefers in the establishment of a specimen and vaccine repository for our trial and in their continued assistance with the handling and shipment of specimens. From GSK Biologicals, we would like to acknowledge the contributions of Gary Dubin, Anne Schuind, Kelechi Lawrence, Darrick Fu, and Bruce Innis for their contribution to discussions regarding trial conduct and Francis Dessy and Brigitte Colau for HPV-16/18 antibody testing. We would like to thank members of the Data and Safety Monitoring Board charged with protecting the safety and interest of participants in our trial (Steve Self, Chair, Luis Diego Calzada, Ruth Karron, Ritu Nayar, and Nancy Roach) and members of the external Scientific HPV Working Group who have contributed to the success of our efforts over the years (Joanna Cain, Chair, Diane Davey, David DeMets, Francisco Fuster, Ann Gershon, Elizabeth Holly, Silvia Lara, Raphael Viscidi, Henriette Raventós, Luis Rosero-Bixby, and Kristen Suthers).
Funding/Support and Financial Disclosures
The Costa Rican Vaccine Trial is a longstanding collaboration between investigators in Costa Rica and NCI. The trial is sponsored and funded by NCI (N01-CP-11005) with support from the NIH Office for Research on Women’s Health and conducted in agreement with the Ministry of Health of Costa Rica. The NCI and Costa Rica investigators are responsible for the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation of the manuscript. Vaccine was provided for our trial by GSK Biologicals, under a Clinical Trials Agreement with NCI. GSK also provided support for aspects of the trial associated with regulatory submission needs of the company under FDA BB-IND 7920. Douglas Lowy and John Schiller are named inventors on U.S. government owned HPV vaccine patents that are licensed to GSK and Merck, and so are entitled to limited royalties as specified by federal law. None of the other co-authors have any potential conflicts of interest to report.
Footnotes
Names and Affiliations of investigators in the Costa Rica Vaccine Trial (CVT) group are as follows:
Proyecto Epidemiológico Guanacaste, Fundación INCIENSA, San José, Costa Rica
Mario Alfaro (Cytopathologist)
Manuel Barrantes (Field Supervisor)
M. Concepción Bratti (co-Investigator)
Fernando Cárdenas (General Field Supervisor)
Bernal Cortés (Specimen and Repository Manager)
Albert Espinoza (Head, Coding and Data Entry)
Yenory Estrada (Pharmacist)
Paula González (co-Investigator)
Diego Guillén (Pathologist)
Rolando Herrero (co-Principal Investigator)
Silvia E. Jiménez (Trial Coordinator)
Jorge Morales (Colposcopist)
Lidia Ana Morera (Head Study Nurse)
Elmer Pérez (Field Supervisor)
Carolina Porras (co-Investigator)
Ana Cecilia Rodríguez (co-Investigator)
Maricela Villegas (Clinic M.D.)
University of Costa Rica, San José, Costa Rica
Enrique Freer (Director, HPV Diagnostics Laboratory)
José Bonilla (Head, HPV Immunology Laboratory)
Sandra Silva (Head Microbiologist, HPV Diagnostics Laboratory)
Ivannia Atmella (Microbiologist, Immunology Laboratory)
Margarita Ramírez (Microbiologist, Immunology Laboratory)
United States National Cancer Institute, Bethesda, MD, USA
Pam Gahr (Trial Coordinator)
Allan Hildesheim (co-Principal Investigator & NCI co-Project Officer)
Douglas R. Lowy (HPV Virologist)
Nora Macklin (Trial Coordinator)
Mark Schiffman (Medical Monitor & NCI co-Project Officer)
John T. Schiller (HPV Virologist)
Mark Sherman (QC Pathologist)
Diane Solomon (Medical Monitor & QC Pathologist)
Sholom Wacholder (Statistician)
SAIC, NCI-Frederick, Frederick, MD, USA
Ligia Pinto (Head, HPV Immunology Laboratory)
Alfonso García-Piñeres (Scientist, HPV Immunology Laboratory)
Womens and Infants’ Hospital, Providence, RI, USA
Claire Eklund (QC Cytology)
Martha Hutchinson (QC Cytology)
DDL Diagnostic Laboratory, The Netherlands
Wim Quint (HPV DNA Testing)
Leen-Jan van Doorn (HPV DNA Testing)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parkin DM, Bray F. Chapter 2: The burden of HPV – related cancers. Vaccine. 2006;24S3:11–25. doi: 10.1016/j.vaccine.2006.05.111. [DOI] [PubMed] [Google Scholar]
- 2.Denny L, Quinn M, Sankaranarayanan R. Chapter 8: Screening for cervical cancer in developing countries. Vaccine. 2006;(24):71–77. doi: 10.1016/j.vaccine.2006.05.121. [DOI] [PubMed] [Google Scholar]
- 3.Vizcaino AP, Moreno V, Bosch FX, Munoz N, Barros-Dios XM, Borras J, et al. International trends in incidence of cervical cancer: II. Squamous-cell carcinoma. Int J Cancer. 2000;86(3):429–435. doi: 10.1002/(sici)1097-0215(20000501)86:3<429::aid-ijc20>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 4.Cogliano V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F WHO International Agency for Research on Cancer. Carcinogenicity of human papillomaviruses. Lancet Oncol. 2005;6(4):204. doi: 10.1016/s1470-2045(05)70086-3. [DOI] [PubMed] [Google Scholar]
- 5.Koutsky LA, Ault KA, Wheeler CM, Brown DR, Barr E, Alvarez FB, et al. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med. 2002 Nov;347(21):1645–1651. doi: 10.1056/NEJMoa020586. [DOI] [PubMed] [Google Scholar]
- 6.Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli-Martins CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006;367(9518):1247–1255. doi: 10.1016/S0140-6736(06)68439-0. [DOI] [PubMed] [Google Scholar]
- 7.Villa LL, Ault KA, Giuliano AR, Costa RL, Petta CA, Andrade RP, et al. Immunologic responses following administration of a vaccine targeting human papillomavirus Types 6, 11, 16, and 18. Vaccine. 2006;24(27–28):5571–5583. doi: 10.1016/j.vaccine.2006.04.068. [DOI] [PubMed] [Google Scholar]
- 8.Paavonen J, Jenkins D, Bosch FX, Naud P, Salmeron J, Wheeler CM, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369(9580):2161–2170. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 9.Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Quadrivalent Human Papillomavirus Vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2007;56(RR2):1–24. [PubMed] [Google Scholar]
- 10.Paavonen J, Lehtinen M. Introducing human papillomavirus vaccines – questions remain. Ann Med. 2008;40(3):62–66. doi: 10.1080/07853890701802404. [DOI] [PubMed] [Google Scholar]
- 11.Herrero R, Schiffman MH, Bratti C, Hildesheim A, Balmaceda I, Sherman M, et al. Design and methods of a population-based cohort study of cervical neoplasia in a rural province of Costa Rica: The Guanacaste Project. Rev Panam Salud Publica. 1997;1(5):362–375. doi: 10.1590/s1020-49891997000500005. [DOI] [PubMed] [Google Scholar]
- 12.Bratti C, Rodríguez AC, Schiffman M, Hildesheim A, Morales J, Alfaro M, et al. Description of a seven-year prospective study of HPV infection and cervical neoplasia among 10 000 women in Guanacaste, Costa Rica. Rev Panam Salud Publica. 2004;15(2):75–89. doi: 10.1590/s1020-49892004000200002. [DOI] [PubMed] [Google Scholar]
- 13.Schiffman M, Herrero R, Hildesheim A, Sherman ME, Bratti M, Wacholder S, et al. HPV DNA testing in cervical cancer screening: results from women in a high-risk province of Costa Rica. JAMA. 2000;283(1):87–93. doi: 10.1001/jama.283.1.87. [DOI] [PubMed] [Google Scholar]
- 14.Schiffman M, Castle PE, Jeronimo J, Rodríguez AC, Wacholder S. Human papilomavirus and cervical cancer. Lancet. 2007;370(9590):890–907. doi: 10.1016/S0140-6736(07)61416-0. Review. [DOI] [PubMed] [Google Scholar]
- 15.Hildesheim A, Herrero R, Wacholder S, Bratti C, Sherman M, Lorincz AT, et al. HPV cofactors related to the development of cervical cancer: Results from a population-based study in Costa Rica. British J Cancer. 2001;84:1219–1226. doi: 10.1054/bjoc.2001.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferreccio C, Bratti C, Sherman ME, Herrero R, Wacholder S, Hildesheim A, et al. A comparison of single and combined visual, cytologic, and virologic tests as screening strategies in a region at high risk of cervical cancer. Cancer Epidemiol Biomarkers Prev. 2003;12(9):815–823. [PubMed] [Google Scholar]
- 17.Herrero R, Hildesheim A, Bratti C, Sherman ME, Hutchinson M, Morales J, et al. Population-based study of Human Papillomavirus infection and cervical neoplasia in rural Costa Rica. J Natl Cancer Inst. 2000;92(6):464–474. doi: 10.1093/jnci/92.6.464. [DOI] [PubMed] [Google Scholar]
- 18.Herrero R, Castle PE, Schiffman M, Bratti MC, Hildesheim A, Morales J, et al. Epidemiologic profile of type-specific human papillomavirus infection and cervical neoplasia in Guanacaste, Costa Rica. J Infect Dis. 2005;191(11):1796–1807. doi: 10.1086/428850. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Pineres AJ, Hildesheim A, Herrero R, Trivett M, Williams M, Atmetlla I, et al. Persistent human papillomavirus infection is associated with a generalized decrease in immune responsiveness in older women. Cancer Res. 2006;66(22):11070–11076. doi: 10.1158/0008-5472.CAN-06-2034. [DOI] [PubMed] [Google Scholar]
- 20.Rodríguez AC, Schiffman M, Herrero R, Wacholder S, Hildesheim A, Castle P, et al. Rapid clearance of HPV should lead to clinical focus on persistent infections. JNCI. doi: 10.1093/jnci/djn044. (Submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hildesheim A, Herrero R, Wacholder S, Rodríguez AC, Solomon D, Bratti MC, et al. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infeccion. JAMA. 2007;Vol. 298(7):743–753. doi: 10.1001/jama.298.7.743. [DOI] [PubMed] [Google Scholar]
- 22.Porras C, Safaeian M, Gonzalez P, Hildesheim A, Silva S, Schiffman M, et al. Costa Rican HPV Vaccine Trial Group Epidemiology of genital Chlamydia trachomatis infection among young women in Costa Rica. J Infect Dis. 2007 doi: 10.1097/OLQ.0b013e3181644b4c. Submitted to. [DOI] [PubMed] [Google Scholar]
- 23.Wright TC, Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D the 2006 American Society for Colposcopy and Cervical Pathology-sponsored Consensus Conference. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007 Oct;197(4):346–355. doi: 10.1016/j.ajog.2007.07.047. Review. [DOI] [PubMed] [Google Scholar]
- 24.Kleter B, van Doorn LJ, ter Schegget J, Schrauwen L, van Krimpen K, Burger M, et al. Novel short-fragment PCR assay for highly sensitive broad-spectrum detection of anogenital human papillomaviruses. Am J Pathol. 1998;153(6):1731–1739. doi: 10.1016/S0002-9440(10)65688-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kleter B, van Doorn LJ, Schrauwen L, Molijn A, Sastrowijoto S, ter Schegget J, et al. Development and clinical evaluation of a highly sensitive PCR-reverse hybridization line probe assay for detection and identification of anogenital human papillomavirus. J Clin Microbiol. 1999;37(8):2508–2517. doi: 10.1128/jcm.37.8.2508-2517.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Doorn LJ, Molijn A, Kleter B, Quint W, Colau B. Highly effective detection of human papillomavirus 16 and 18 DNA by a testing algorithm combining broad-spectrum and type-specific PCR. J Clin Microbiol. 2006;44(9):3292–3298. doi: 10.1128/JCM.00539-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baay MF, Quint WG, Koudstaal J, Hollema H, Duk JM, Burger MP, et al. Comprehensive study of several general and type-specific primer pairs for detection of human papillomavirus DNA by PCR in paraffin-embedded cervical carcinomas. J Clin Microbiol. 1996 Mar;34(3):745–747. doi: 10.1128/jcm.34.3.745-747.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Safaeian M, Herrero R, Hildesheim A, Quint W, Freer E, Van Doorn LJ, et al. Comparison of the SPF10/LiPA System to the HC2 Assay for Detection of Carcinogenic HPV Genotypes among 5683 Young Women in Guanacaste, Costa Rica. J Clin Microbiol. 2007;45(5):1447–1454. doi: 10.1128/JCM.02580-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castle PE, Porras C, Quint W, Rodriguez AC, Schiffman M, Gravitt P, et al. the CVT Group. Comparing SPF10/LiPA25 and Linear Array Assays for Detection of HPV Genotypes. (Submitted) [Google Scholar]
- 30.Castle PE, Hillier SL, Rabe LK, Hildesheim A, Herrero R, Bratti MC, et al. An association of cervical inflammation with high-grade cervical neoplasia in women infected with oncogenic human papillomavirus (HPV) Cancer Epidemiol Biomarker Prev. 2001;10(10):1021–1027. [PubMed] [Google Scholar]
- 31.Schiffman M, Weeler CM, Dasgupta A, Solomon D, Castle PE the ALTS Group. A comparison of a prototype PCR assay and hybrid capture 2 for detection of carcinogenic human papillomavirus DNA in women with equivocal or mildly abnormal papanicolaou smears. Am J Clin Pathol. 2005;124(5):722–732. doi: 10.1309/E067-X0L1-U3CY-37NW. [DOI] [PubMed] [Google Scholar]
- 32.Franceschi S, Herrero R, Clifford GM, Snijders PJF, Arslan A, Hoang PT, et al. Variations in the age-specific curves of human papillomavirus prevalence in women worldwide. Int.J.Cancer. 2006;119:2677–2684. doi: 10.1002/ijc.22241. [DOI] [PubMed] [Google Scholar]
- 33.Schiller JT, Villa L, Hildesheim A, Castellsague X. An update of HPV VLP vaccine clinical trial results. Vaccine. doi: 10.1016/j.vaccine.2008.06.002. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]