Abstract
Plant mitochondria are typically portrayed as being small, oval organelles. However, a recent study has demonstrated that the chondriome of shoot apical meristem (SAM) cells of Arabidopsis thaliana is unique in having two types of mitochondria, a large, central, tentaculate mitochondrion and variable numbers of small, oval mitochondria in the cell cortex that fuse with and fission from the tentaculate mitochondrion.1 The tentaculate mitochondrion wraps around the nucleus and persists throughout the cell cycle, undergoing distinct changes in morphology and size in a cell cycle-dependent manner. Here we demonstrate that SAM cell plastids, which also contain DNA, do not reticulate, and address the question as to why SAM cell mitochondria but not plastids form reticulate structures. We postulate that the presence of a large, tentaculate mitochondrion in SAM cells provides an efficient means for homogenizing the mitochondrial DNA and proteins during vegetative life prior to gamete production, and that this mitochondrial architecture prevents speciation. The lack of plastid reticulation in the same cells most likely reflects on the fact that the individual plastids are much larger than the small mitochondria and therefore do not need to fuse to achieve efficient intermixing of their genomes.
Key words: 3D reconstruction, arabidopsis, chloroplasts, DNA-containing organelles, mitochondria, plant, plastids
Introduction
Mitochondria are highly dynamic organelles that can rapidly change their form and their distribution in response to changing cellular energy needs. In plants they have been reported to be oval or sausage-like organelles capable of undergoing rounds of fusion and fission.2 With some exceptions,1,3 the observation of reticular-type morphologies has been restricted to mitochondrial mutants or to cells subjected to experimental perturbations.2,4–10 Although mitochondria contain their own DNA, mtDNA, and can synthesize proteins, they are only semi-autonomous organelles, since most of their proteins are encoded by nuclear genes and have to be imported from the cytoplasm.11
Genetic studies have shown that the mtDNA of flowering plants has a high frequency of recombination and that the mitochondrial genome has evolved to become recombinationally active.12,13 It has been hypothesized that this recombination involves corresponding high rates of transient mitochondrial fusion to enable intermixing of the mitochondrial genomes in each cell. For this reason, Logan6,14 has proposed that the population of mitochondria (the chondriome) of higher plant cells be termed a “discontinuous whole.” However, whether the actual rates of mitochondrial fusion and fission in plant cells could account for the recorded rates of mtDNA intermixing has yet to be tested experimentally.
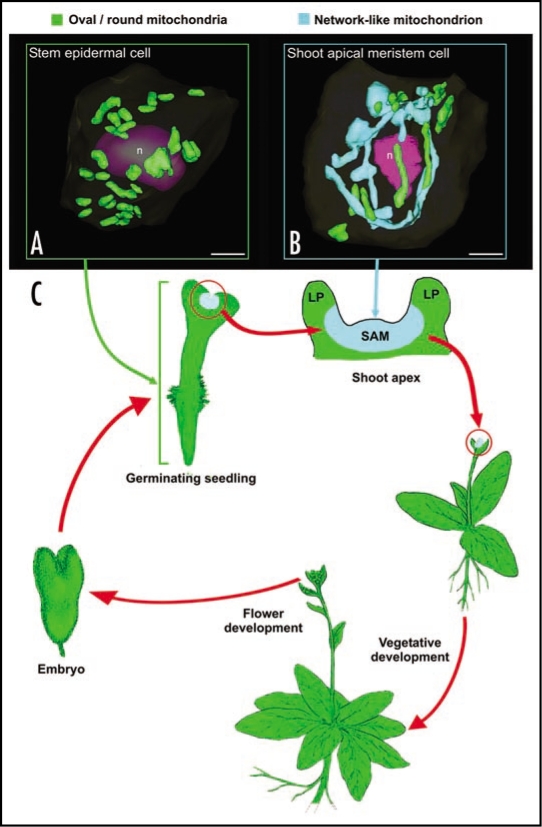
We have recently shown that in wild type Arabidopsis thaliana plants most, but not all cells possess small, oval or sausage-shaped mitochondria. In particular, our microscopical data demonstrated that shoot apical meristem (SAM) and leaf primordial (LP) meristematic cells are unique in that they contain a large, “reticulate-type” of mitochondrial system that allows for the efficient intermixing of mtDNA1 (Fig. 1B). In contrast, all of the other cell types we have examined—non-dividing, differentiated leaf primordial cells, mature leaf and stem epidermal cells, root meristematic, embryo, endosperm and male gametophyte cells—produce typical small mitochondria as reported in the literature1 (Fig. 1A). Similar results have been reported previously in a morphometric study of curcurbita mitochondria by Bendich and Gauriloff,3 who demonstrated that SAM cells contained branched mitochondria, whereas hypocotyl, cotyledon and leaf cells only possessed small, unbranched mitochondria.
Figure 1.
Diagram illustrating the tissues and the stages of the life cycle of Arabidopsis plants in which small, oval and network-type mitochondria are observed. (A) shows a 3D reconstruction of a stem epidermal cell in which the small, round/oval-type of mitochondria are randomly distributed (green structures). (B) illustrates a SAM cell during prometaphase. The chondriome consists of a mixture of a few individual, oval or sausage-like mitochondria (green), and a massive, cage-like mitochondrion (light blue) that wraps around the dismantling nucleus (n, purple). (C) represents a typical Arabidopsis life cycle, where the spatial and temporal confinement of the tentaculate/cage-like mitochondrion is represented by coloring vegetative SAM regions in light blue. Bars in (A and B): 2 µm.
The reticulate mitochondrial system of Arabidopsis also undergo characteristic, cell cycle-dependent changes in morphology during the cell cycle. During the G1 through S phases of the cell cycle, the reticulate mitochondrion in each cell has a tentaculate morphology and wraps around one nuclear pole, with the remaining smaller mitochondria scattered throughout the cortical cytoplasm. In G2, both types of mitochondria increase their volume, and as this occurs, the large tentaculate mitochondrion extends around the nucleus to establish a second cap-like domain on the opposite site of the nucleus. This expansion is achieved, in part, by fusion of ∼60% of the smaller mitochondria with the large mitochondrion, whose volume increases to ∼80% of the total mitochondrial volume. During mitosis, the large mitochondrion is transformed into a cage-like structure that first encompasses the mitotic spindle and later the entire cytokinetic apparatus. This cage-like mitochondrion then divides during cytokinesis into two tentacular mitochondria that wrap around the opposite poles of the daughter nuclei, while simultaneously giving rise to new, small mitochondria by fission. By early G1 phase, the large, tentaculate mitochondrion contributes ∼40% of the mitochondrial volume and the small mitochondria ∼60%.
The finding that the above mitochondrial cycle has only been observed in SAM and LP meristem cells has led us to ask two questions: (1) Why is the mitochondrial cycle limited to the above-mentioned cell types? (2) Do the DNA-containing plastids also exhibit a reticulation pattern in SAM and LP meristematic cells?
Why do Vegetative Shoot Apical Meristem Cells possess Reticulate Mitochondria?
The fact that reticulate mitochondria are found in shoot (SAM) but not in root apical meristem cells suggests that a reticulate mitochondrial system is not essential to accomplish cell division in plants. This leads us to ask what features distinguish SAM from root apical meristem cells and from the non-dividing cell types that contain small, oval or sausage-shaped mitochondria? The function of SAM cells is to produce the aerial parts of plants, including leaves and flowers. Interestingly, with the exception of the young LP meristematic cells, all of the cell types generated by SAM cells such as differentiated leaf and floral organ cells, possess small, conventional mitochondria. Thus, it appears that the capability to form reticulate mitochondria is specific to vegetative SAM, and is progressively lost as the derived meristematic cells become physically displaced from the vegetative SAM region and adopt their primary function. This loss even seems to occur during the vegetative-to-floral meristem transition, given its absence in flower cells.
Considering this temporal and spatial confinement of cell types with reticulated mitochondria,1 we conceive mitochondrial reticulation as a mechanism to allow for an efficient homogenization of the mitochondrial content, including mitochondrial DNA (mtDNA) and mtDNA products, during the vegetative growth of the SAM. It can be argued that other mechanisms, such as the elevated frequencies of transient fusion and fission reported for small, oval mitochondria in other cell types,15 could also account for the elevated rate of recombination that defines the plant mtDNA.12 However, this mechanism does not guarantee an equal distribution of the mtDNA after fission.15 We rather believe that the permanent presence of a significantly larger, common space may expedite the process of nucleoid encounter and thus make recombination more efficient. Furthermore, not only mtDNA recombination can be positively affected by the presence of a permanent, large mitochondrion to fuse with and to divide from. This larger space has the potential to act as a pool of mitochondrial components that small, individual units may use to homogenize their protein content, prevent the accumulation of undesired mutations, and minimize the chances of containing no mtDNA. Additionally, it is also a means to ensure an equal partitioning of the chondriome between daughter cells during cytokinesis.
The presence of this improved mechanism for homogenization of mitochondrial distribution and contents appears essential in SAM meristematic cells of Arabidopsis and most likely other plants, because these cells give rise to the floral meristem (Fig. 1C), the germ lines, and ultimately—and most importantly—the female gametes. In Arabidopsis and many other species, the female gametes are responsible for the exclusive transmission of all of their chondriome to the newly formed zygote and thus to the new generations.16,17 In this context, the presence of such a homogenizing mechanism in SAM cells is critically important for preserving the stability of the mitochondrial traits that have to be passed on to future gametes and embryos, i.e., to prevent speciation.
Thus, our hypothesis (Fig. 1C) postulates that mitochondrial reticulation starts as soon as the embryo germinates and produces a SAM in the seedling, and that this feature of SAM cells is maintained through the multiple rounds of SAM cell divisions. However, once a cell lineage develops into an aerial organ, this mitochondrial feature is no longer needed and disappears progressively. This process also applies to floral organs. During the vegetative to floral meristem transition, reticulation might be retained, but at the latest would disappear when the floral organs are formed, since comparatively few rounds of division are needed to generate the gametophytes and subsequently the gametes. Finally, after meiosis and microspore compartmentalization, the developmental need for a mechanism for generating a homogeneous mtDNA pool to be transmitted to gametes would no longer be needed. Thus, during gamete, embryo and seed development, this characteristic mitochondrial feature would be silenced and would only be reactivated during germination of the new seedling when a new SAM is formed and vegetative development starts.
At the molecular level, the different mitochondrial morphologies in vegetative, reproductive and embryo tissues might be governed by different sets of tissue-specific machineries for mitochondrial fusion and fission. In plants, there are many examples of alternative tissue or organ-specific protein expression patterns. For example, in Arabidopsis there are eight actin isovariants, five reproductive and eight vegetative,18 that perform specific functions in each type of tissue. Since actin is also involved in mitochondrial motility and shape,19,20 it is tempting to speculate that one set of actin and actinrelated protein isoforms might help maintain the reticulated type of mitochondria in SAM cells during vegetative development. On the other hand, a different set would serve to allow for the maintenance of a homogeneous population of small, oval mitochondria in the rest of vegetative tissues as well as in the reproductive and embryo tissues.
In SAM Cells of Arabidopsis, the Plastids, Unlike the Mitochondria, do not Undergo Reticulation during the Cell Cycle
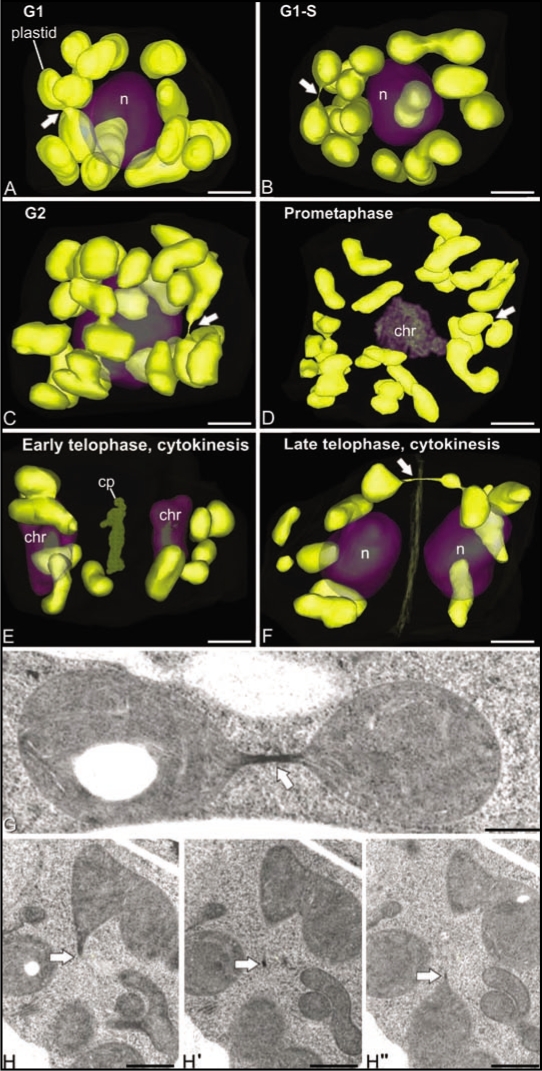
To shed light on the question whether the DNA-containing plastids (chloroplasts) also exhibit a reticulation pattern in SAM cells, we have modeled and analyzed the 3D structure of plastids in fully reconstructed Arabidopsis SAM cells at different stages of the cell cycle. The set of cells employed for this analysis was the same as used previously for the analysis of the 3D structure, number and distribution of other organelles and cellular compartments, including mitochondria, nuclei, Golgi stacks, vacuoles, and multivesicular bodies,1,21 thereby providing a reliable system for correlating the cell cycle-dependent changes of the plastids with those of other cellular organelles. In Figure 2A–F, the plastids are seen to maintain the same oval/lenticular morphology and the same random distribution as leaf cell chloroplasts22 throughout the cell cycle. At all cell cycle stages, plastids interconnected through thin, tubular structures of variable length were also detected. As documented in Figure 2G and H these thin tubular connections contained stroma material but no thylakoids. Thus, we postulate that they correspond to transient, stromule-type links.23 What did change during the cell cycle was the number of plastids per cell, with the late telophase, G1 and S stage cells containing from 9–12 plastids, and the G2 and prometaphase cells we reconstructed possessing 23–25 plastids. This indicates that in SAM cells the doubling of plastid numbers occurs during G2, i.e., at the same time as the doubling of the number of Golgi stacks21 and the doubling of mitochondrial volume1 is observed. However, at no stage of the cell cycle did we detect any large-scale fusion of plastids into reticulate structures. During cell division (Fig. 2D and F) the plastids appeared to be partitioned in similar numbers between the daughter cells.
Figure 2.
Cell cycle-dependent changes in distribution and morphology of SAM cell plastids in Arabidopsis. (A–F) show 3D reconstructions of all of the plastids in six different SAM cells at representative stages of the cell cycle. Note that the plastids maintain their sausage-like morphology throughout the cell cycle and that they do not form reticulate structures. Arrows point to stromule-like connections between plastids. As seen in (G and H), these narrow bridging structures have highly variable lengths, contain stroma materials, but are devoid of thylakoids. (H and H″) are three consecutive serial sections extracted from a whole-cell 3D reconstruction of a SAM cell. Bars in (A–F): 2 µm, (G and H): 500 nm.
Why do Mitochondria but not Plastids of Arabidopsis SAM Cells Reticulate?
Two of the principal architectural differences between SAM cell mitochondria and chloroplasts are their size and their numbers. In interphase cells, the mitochondria contribute ∼10% of the cell volume,1 whereas the volume contribution of plastids is closer to 20%. Since the number of plastids is also smaller than the total number of mitochondria, the average plastid is much larger in volume and contains a greater number of copies of plastid DNA. Thus, one explanation for the absence of plastid reticulation is that the individual plastids are large enough to permit efficient intermixing of chloroplast DNA and therefore no reticulation of the plastids is needed. To what extent the plastids fuse and fission in SAM cells is unknown. However, since the chloroplast DNA always seems to be anchored to thylakoids, and stromules do not contain any thylakoids, it is unlikely that significant amounts of DNA intermixing occurs via stromule-type bridges.24 In conclusion, we postulate that organelle size might explain why mitochondria but not plastids form reticulate structures in SAM cells.
Acknowledgements
The authors want to thank Dr. Arnold Bendich for his helpful comments. This work was supported by NIH grant GM 61306 to L.A.S and by Spanish MEC grant AGL2006-06678 to J.M.S.S.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/7755
References
- 1.Seguí-Simarro JM, Coronado MJ, Staehelin LA. The mitochondrial cycle of Arabidopsis shoot apical meristem and leaf primordium meristematic cells is defined by a perinuclear tentaculate/cage-like mitochondrion. Plant Physiol. 2008;148:1380–1393. doi: 10.1104/pp.108.126953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bereiter-Hahn J. Behaviour of mitochondria in the living cell. Int Rev Cytol. 1990;122:1–63. doi: 10.1016/s0074-7696(08)61205-x. [DOI] [PubMed] [Google Scholar]
- 3.Bendich AJ, Gauriloff LP. Morphometric analysis of cucurbit mitochondria—the relationship between chondriome volume and DNA content. Protoplasma. 1984;119:1–7. [Google Scholar]
- 4.Arimura S, Tsutsumi N. A dynamin-like protein (ADL2b), rather than FtsZ, is involved in Arabidopsis mitochondrial division. Proc Natl Acad Sci USA. 2002;99:5727–5731. doi: 10.1073/pnas.082663299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Logan DC. Mitochondrial dynamics. New Phytol. 2003;160:463–478. doi: 10.1046/j.1469-8137.2003.00918.x. [DOI] [PubMed] [Google Scholar]
- 6.Logan DC. The mitochondrial compartment. J Exp Bot. 2006;57:1275–1280. doi: 10.1093/jxb/erj151. [DOI] [PubMed] [Google Scholar]
- 7.Van Gestel K, Verbelen JP. Giant mitochondria are a response to low oxygen pressure in cells of tobacco (Nicotiana tabacum L.) J Exp Bot. 2002;53:1215–1218. doi: 10.1093/jexbot/53.371.1215. [DOI] [PubMed] [Google Scholar]
- 8.Rohr R. Existence of a mitochondrial reticulum in haploid tissue-culture of a vascular plant. Biol Cell. 1978;33:89. [Google Scholar]
- 9.Sheahan MB, McCurdy DW, Rose RJ. Mitochondria as a connected population: ensuring continuity of the mitochondrial genome during plant cell dedifferentiation through massive mitochondrial fusion. Plant J. 2005;44:744–755. doi: 10.1111/j.1365-313X.2005.02561.x. [DOI] [PubMed] [Google Scholar]
- 10.Sheahan MB, Rose RJ, McCurdy DW. Organelle inheritance in plant cell division: the actin cytoskeleton is required for unbiased inheritance of chloroplasts, mitochondria and endoplasmic reticulum in dividing protoplasts. Plant J. 2004;37:379–390. doi: 10.1046/j.1365-313x.2003.01967.x. [DOI] [PubMed] [Google Scholar]
- 11.Alberts B, et al. Molecular biology of the cell. 4th. New York: Garland Science; 2002. [Google Scholar]
- 12.Gillham NW. Organelle genes and genomes. New York: Oxford University Press; 1994. [Google Scholar]
- 13.Gray MW, Burger G, Lang BF. Mitochondrial evolution. Science. 1999;283:1476. doi: 10.1126/science.283.5407.1476. [DOI] [PubMed] [Google Scholar]
- 14.Logan DC. Plant mitochondrial dynamics. Biochim Biophys Acta. 2006;1763:430–441. doi: 10.1016/j.bbamcr.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Arimura S, Yamamoto J, Aida GP, Nakazono M, Tsutsumi N. Frequent fusion and fission of plant mitochondria with unequal nucleoid distribution. Proc Natl Acad Sci USA. 2004;101:7805–7808. doi: 10.1073/pnas.0401077101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsushima R, Hamamura Y, Higashiyama T, Arimura S, Sodmergen, Tsutsumi N, et al. Mitochondrial dynamics in plant male gametophyte visualized by fluorescent live imaging. Plant Cell Physiol. 2008;49:1074–1083. doi: 10.1093/pcp/pcn084. [DOI] [PubMed] [Google Scholar]
- 17.Nagata N, Saito C, Sakai A, Kuroiwa H, Kuroiwa T. The selective increase or decrease of organellar DNA in generative cells just after pollen mitosis one controls cytoplasmic inheritance. Planta. 1999;209:53–65. doi: 10.1007/s004250050606. [DOI] [PubMed] [Google Scholar]
- 18.Meagher RB, Kandasarny MK, McKinney EC. Multicellular development and protein-protein interactions. Plant Signal Behav. 2008;3:333–336. doi: 10.4161/psb.3.5.5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boldogh IR, Pon LA. Interactions of mitochondria with the actin cytoskeleton. Biochim Biophys Acta. 2006;1763:450–462. doi: 10.1016/j.bbamcr.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 20.Foissner I. Microfilaments and microtubules control the shape, motility and subcellular distribution of cortical mitochondria in characean internodal cells. Protoplasma. 2004;224:145–157. doi: 10.1007/s00709-004-0075-1. [DOI] [PubMed] [Google Scholar]
- 21.Seguí-Simarro JM, Staehelin LA. Cell cycle-dependent changes in Golgi stacks, vacuoles, clathrin-coated vesicles and multivesicular bodies in meristematic cells of Arabidopsis thaliana: a quantitative and spatial analysis. Planta. 2006;223:223–236. doi: 10.1007/s00425-005-0082-2. [DOI] [PubMed] [Google Scholar]
- 22.Staehelin LA. Chloroplast structure: from chlorophyll granules to supra-molecular architecture of thylakoid membranes. Photosynth Res. 2003;76:185–196. doi: 10.1023/A:1024994525586. [DOI] [PubMed] [Google Scholar]
- 23.Natesan SKA, Sullivan JA, Gray JC. Stromules: a characteristic cell-specific feature of plastid morphology. J Exp Bot. 2005;56:787–797. doi: 10.1093/jxb/eri088. [DOI] [PubMed] [Google Scholar]
- 24.Kohler RH, Hanson MR. Plastid tubules of higher plants are tissue-specific and developmentally regulated. J Cell Sci. 2000;113:81–89. doi: 10.1242/jcs.113.1.81. [DOI] [PubMed] [Google Scholar]