Abstract
Oxidative tailoring of C40 carotenoids by double bond-specific cleavage enzymes (carotenoid cleavage dioxygenases, CCDs) gives rise to various apocarotenoids. AtCCD1 generating C13 and C14 apocarotenoids and orthologous enzymes in other plants are the only CCDs acting in the cytosol, while the hitherto presumed C40 substrate is localized in the plastid. A new model for CCD1 action arising from a RNAi-mediated CCD1 gene silencing study in mycorrhizal hairy roots of Medicago truncatula may solve this contradiction. This approach unexpectedly resulted in the accumulation of C27 apocarotenoids but not C40 carotenoids suggesting C27 as the main substrates for CCD1 in planta. It further implies a consecutive two-step cleavage process, in which another CCD performs the primary cleavage of C40 to C27 in the plastid followed by C27 export and further cleavage by CCD1 in the cytosol. We compare the specificities and subcellular locations of the various CCDs and propose the plastidial CCD7 to be the first player in mycorrhizal apocarotenoid biogenesis.
Key words: carotenoid cleavage dioxygenase CCD1, C13 apocarotenoids, norisoprenoids, arbuscular mycorrhiza, aroma compounds, strigolactones, abscisic acid
Carotenoids are isoprenoids (mostly C40) synthesized by bacteria, algae, fungi and plants. Coordinated tailoring by specific enzymes is thought to be the main principle for generation of defined carotenoid cleavage products (apocarotenoids), which have important functions in their own right.1 These enzymes, called carotenoid cleavage dioxygenases (CCDs), exhibit a high degree of regio- and stereospecificity for certain double bond positions as opposed to their frequent promiscuity towards substrates. In Arabidopsis, the CCD family consists of nine members forming the basis for CCD classification in plants. Five of them exhibit specificity for 9-cis-epoxycarotenoids and are designated nine-cis-epoxycarotenoid dioxygenases (NCEDs). They are involved in ABA biosynthesis.1–3 The remaining 4 CCDs (AtCCD1, AtCCD4, AtCCD7, AtCCD8) cleave a variety of transcarotenoid substrates.1,4 The majority of CCDs/NCEDs has been shown to reside in plastids. The only exception is AtCCD1 and orthologous enzymes in other plants, which act in the cytosol to generate C13 and C14 apocarotenoids.1,4 This contribution will compare current knowledge on enzymatic carotenoid cleavage pathways with recently obtained new insights resulting from silencing the expression of a CCD1 gene in plant roots colonized by arbuscular mycorrhizal (AM) fungi.5
Known Pathways of Carotenoid Cleavage Leading to C15 and C18 Apocarotenoids
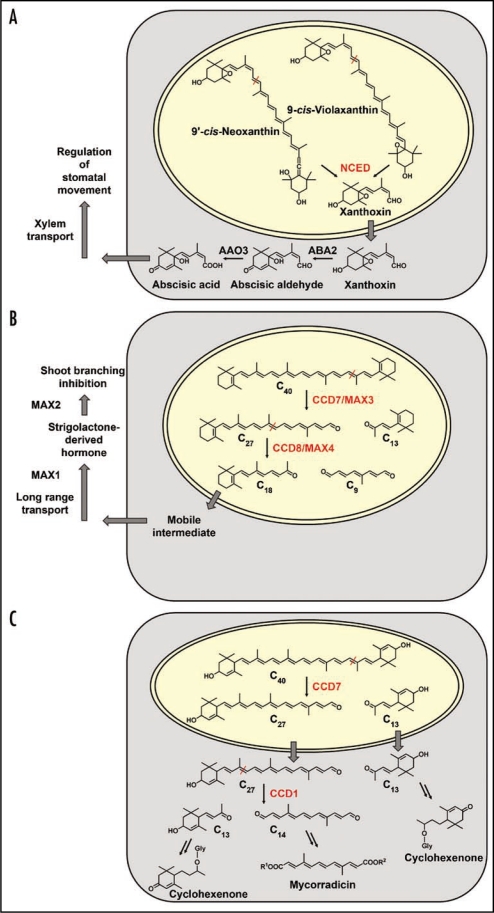
The phytohormone ABA is the best-studied member of plant apocarotenoids. Single-step cleavage of the 11,12 double bond of 9-cis violaxanthin and 9′-cis neoxanthin by NCEDs results in formation of C15 xanthoxin inside plastids (Fig. 1A).2 The next steps of xanthoxin conversion to abscisic aldehyde and abscisic acid are known to take place in the cytosol. Thus, considering the plastidial location of NCEDs, a transport of xanthoxin into the cytosol must be postulated but the mechanisms by which this occurs are still unknown.6
Figure 1.
Comparison of substrates, enzymes and their compartmentation in three carotenoid cleavage pathways. (A) ABA biosynthesis involves cleavage of cis-carotenoid substrates by NCEDs in the plastid, C15 xanthoxin export to the cytosol followed by further metabolization steps and transport.6 (B) Strigolactone biosynthesis is assumed to start from β-carotene and to proceed via two consecutive cleavage steps (CCD7 and CCD8) inside the plastid as exemplified by the MAX3 and MAX4 proteins of Arabidopsis.4 The C18 cleavage product of CCD8 or a derivative of it is predicted to serve as mobile strigolactone precursor undergoing export to the cytosol, further modification steps, transport and eventually perception as a regulator of shoot branching.7 (C) Proposed organization of local C13 and C14 apocarotenoid biosynthesis in a mycorrhizal root cell. Lactucaxanthin as the tentatively proposed C40 carotenoid precursor containing two α-ionone rings is tailored by two consecutive cleavage steps in the plastid (CCD7) and subsequently, following export of the C27 intermediate, in the cytosol (CCD1). The C27 intermediate has only been detected upon silencing CCD1 expression.5 Additional modification steps in the cytosol lead to the various C13 cyclohexenone and C14 mycorradicin derivatives accumulating in mycorrhizal roots. Abbreviations: MAX, more axillary branching; Gly, glycoside.
In addition to ABA, another carotenoid-derived phytohormone exists, whose long-sought chemical nature was recently identified as strigolactone.7–9 Mutants in its biosynthesis or its perception display a striking increase in shoot branching.7 One CCD involved is CCD7 converting C40 trans-carotenoids to C27 apocarotenoids (Fig. 1B).10,11 A second cleavage activity is contributed by CCD8. Several lines of evidence argue for a consecutive action of these CCDs with CCD8 converting C27 to C18 and C9 (Fig. 1B).7,11 Both CCDs have transit peptides indicative of their action in plastids.4 The C18 reaction product of CCD8 and strigolactone precursor subsequently undergoes still uncharacterized steps of export from the plastid, further metabolization and transport to the shoot (Fig. 1B).
Strigolactones were previously known as germination stimulants for parasitic weeds and as signaling molecules to promote hyphal branching of AM fungi.12,13 Most recent data might point to additional roles of strigolactones in roots. A C18 β-apo-13-carotenone called “D'orenone” blocks the growth of root hairs by interfering with PIN2-mediated auxin transport.14 The synthetic compound “D'orenone” is structurally identical to the proposed C18 apocarotenoid precursor of strigolactone biosynthesis (Fig. 1B). The effects observed might therefore be strigolactone-related.
C13 and C14 Apocarotenoid Biogenesis via CCD1: Single-Step or Stepwise Cleavage and the Importance of Compartmentation
Strigolactones exert their signaling functions in low amounts and can act in early stages of the AM symbiosis. Conversely, two other classes of apocarotenoids with unknown functions accumulate in large amounts in mycorrhizal roots and at later stages of the AM interaction.15,16 These AM-induced colorless C13 cyclohexenone and yellow linear polyene derivatives have been C14 identified independently but probably originate from a common precursor (Fig. 1C).15–17 Both types of compounds accumulate locally in cells harbouring arbuscules, which are the symbiotic organs of the AM symbiosis mediating nutrient exchange between plant and fungus.18 To identify a function for these apocarotenoids in the symbiosis, both a CCD1 gene and an AM-induced MEP pathway isogene were targeted by gene silencing approaches.5,19
CCD1 is, next to the NCEDs, the best-studied CCD due to its involvement in C13 apocarotenoid-based flower scent as well as fruit and wine aroma biosynthesis.20–22 Recombinant CCD1 enzymes from several plants have been shown to preferentially catalyze a single-step symmetrical cleavage at the 9,10 and the 9′,10′ double bonds of various C40 carotenoids.1,4,22,23 Cleavage activity on 5,6 (5′,6′) double bonds in vitro has also been reported.24 However, being able to convert a substrate in vitro does not mean that this activity must be the main in vivo functional role of the enzyme. Indeed, in planta studies with CCD1 mutants or gene silencing transgenics have raised doubts in an exclusive role of CCD1 in C13 apocarotenoid generation.4,20,21 Strong suppression of CCD1 transcript accumulation resulted in near 50% C13 apocarotenoid levels compared to wild type plants implicating additional players in C13 apocarotenoid biogenesis.20,21
Given this background knowledge of presumed single-step symmetrical CCD1 action on C40 carotenoid substrates we performed a CCD1 RNAi knock-down approach in hairy roots of Medicago truncatula. Surprisingly, HPLC analyses of mycorrhizal hairy roots clearly indicated a differential reduction of C13 and C14 apocarotenoids. C14 mycorradicin derivatives were strongly reduced (3–6% residual amounts relative to mycorrhizal empty vector controls) but C13 cycohexenone derivatives exhibited an actual decrease to only 30–47% of empy vector controls.5 This result is incompatible with the assumption of a symmetrical cleavage action of CCD1 in planta. Moreover, a striking color change to yellow-orange was observed in mycorrhizal RNAi roots. Analysis of the corresponding chromophore indicated that its chemical nature is a C27 apocarotenoid. This suggests that C27 but not C40 derivatives as previously thought17 are the main substrates for CCD1 in mycorrhizal roots. Based on these data a new scheme of carotenoid cleavage and CCD1 action in mycorrhizal roots is proposed, in which CCD1 catalyzes only the second of at least two carotenoid cleavage steps from C40 carotenoids to the C13/C14 apocarotenoid end-products (Fig. 1C). As in the case of strigolactone biosynthesis a consecutive action of two CCDs on C40 carotenoids and on the primary cleavage product, respectively, is predicted. Interestingly, the two pathways have a C27 intermediate in common (Fig. 1B and C). In strigolactone biosynthesis the generation of C27 apocarotenoids is due to CCD7 activity and it is tempting to speculate that CCD7 is the first cleavage enzyme in AM-induced C13/C14 apocarotenoid biosynthesis as well. Preliminary evidence for such an involvement has been obtained.5 CCD7 might thus constitute a crosspoint where the two pathways meet but subsequently branch into different directions towards CCD8 or CCD1, respectively (Fig. 1B and C). This is supported by the wide substrate specificity of CCD7,10 as opposed to the narrow specificity reported for CCD8.25
However, compartmentation of the second cleavage step in both pathways is different. The C27 compound tentatively identified in mycorrhizal CCD1-RNAi roots was a 3-hydroxy-α-apo-10′carotenoic acid glycosylated by two hexose moieties at the 3-hydroxyl position.5 Glycosylation is a modification usually carried out by cytosolic enzymes. Therefore, the C27 compound is most likely exported from the plastid to the cytosol, where it is further cleaved by CCD1. In this new view of carotenoid cleavage and its compartmentation (Fig. 1A–C) the cytosolic location of CCD1 finally makes sense.
In conclusion, we have presented here a new scheme of C13 and C14 apocarotenoid biogenesis and of the role of CCD1 in this process, which illustrates both the importance of consecutive cleavage steps and the decisive role of compartmentation. The pivotal question is now, whether this scheme is also applicable to the biosynthesis of C13 apocarotenoids (also referred to as “C13 norisoprenoids”22) involved in flower scent, fruit aroma and wine bouquet.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: www.landesbioscience.com/journals/psb/article/7840
References
- 1.Auldridge ME, McCarty DR, Klee HJ. Plant carotenoid cleavage oxygenases and their apocarotenoid products. Curr Opin Plant Biol. 2006;9:315–321. doi: 10.1016/j.pbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz SH, Tan BC, Gage DA, Zeevaart JA, McCarty DR. Specific oxidative cleavage of carotenoids by VP14 of maize. Science. 1997;276:1872–1874. doi: 10.1126/science.276.5320.1872. [DOI] [PubMed] [Google Scholar]
- 3.Tan BC, Joseph LM, Deng WT, Liu L, Li QB, Cline K, et al. Molecular characterization of the Arabidopsis 9-cis epoxycarotenoid dioxygenase gene family. Plant J. 2003;35:44–56. doi: 10.1046/j.1365-313x.2003.01786.x. [DOI] [PubMed] [Google Scholar]
- 4.Auldridge ME, Block A, Vogel JT, Dabney-Smith C, Mila I, Bouzayen M, et al. Characterization of three members of the Arabidopsis carotenoid cleavage dioxygenase family demonstrates the divergent roles of this multifunctional enzyme family. Plant J. 2006;45:982–993. doi: 10.1111/j.1365-313X.2006.02666.x. [DOI] [PubMed] [Google Scholar]
- 5.Floss DS, Schliemann W, Schmidt J, Strack D, Walter MH. RNA interference-mediated repression of MtCCD1 in mycorrhizal roots of Medicago truncatula causes accumulation of C27 apocarotenoids, shedding light on the functional role of CCD1. Plant Physiol. 2008;148:1267–1282. doi: 10.1104/pp.108.125062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nambara E, Marion-Poll A. Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol. 2005;56:165–185. doi: 10.1146/annurev.arplant.56.032604.144046. [DOI] [PubMed] [Google Scholar]
- 7.Mouchel CF, Leyser O. Novel phytohormones involved in long-range signaling. Curr Opin Plant Biol. 2007;10:473–476. doi: 10.1016/j.pbi.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pagès V, Dun EA, Pillot JP, et al. Strigolactone inhibition of shoot branching. Nature. 2008;455:189–194. doi: 10.1038/nature07271. [DOI] [PubMed] [Google Scholar]
- 9.Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N, et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature. 2008;455:195–200. doi: 10.1038/nature07272. [DOI] [PubMed] [Google Scholar]
- 10.Booker J, Auldridge ME, Wills S, McCarty D, Klee HJ, Leyser O. MAX3/CCD7 is a carotenoid cleavage dioxygenase required for the synthesis of a novel plant signaling molecule. Curr Biol. 2004;14:1232–1238. doi: 10.1016/j.cub.2004.06.061. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz SH, Qin X, Loewen MC. The biochemical characterization of two carotenoid cleavage enzymes from Arabidopsis indicates that a carotenoid-derived compound inhibits lateral branching. J Biol Chem. 2004;279:46940–46945. doi: 10.1074/jbc.M409004200. [DOI] [PubMed] [Google Scholar]
- 12.Matusova R, Rani K, Verstappen FWA, Franssen MCR, Beale MH, Bouwmeester H. The strigolactone germination stimulants of the plant parasitic Striga and Orobanche spp. are derived from the carotenoid pathway. Plant Physiol. 2005;139:920–934. doi: 10.1104/pp.105.061382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akiyama K, Matsuzaki K-I, Hayashi H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature. 2005;435:824–827. doi: 10.1038/nature03608. [DOI] [PubMed] [Google Scholar]
- 14.Schlicht M, Samajova O, Schachtschabel D, Mancuso S, Menzel D, Boland W, et al. D'orenone blocks polarized tip growth of root hairs by interfering with the PIN2-mediated auxin transport network in the root apex. Plant J. 2008;55:709–717. doi: 10.1111/j.1365-313X.2008.03543.x. [DOI] [PubMed] [Google Scholar]
- 15.Maier W, Peipp H, Schmidt J, Wray V, Strack D. Levels of a terpenoid glycoside (blumenin) and cell wall-bound phenolics in some cereal mycorrhizas. Plant Physiol. 1995;109:465–470. doi: 10.1104/pp.109.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klingner A, Bothe H, Wray V, Marner FJ. Identification of a yellow pigment formed in maize roots upon mycorrhizal colonization. Phytochemistry. 1995;38:53–55. [Google Scholar]
- 17.Walter MH, Fester T, Strack D. Arbuscular mycorrhizal fungi induce the non-mevalonate methylerythritol phosphate pathway of isoprenoid biosynthesis correlated with the accumulation of the ‘yellow pigment’ and other apocarotenoids. Plant J. 2000;21:571–578. doi: 10.1046/j.1365-313x.2000.00708.x. [DOI] [PubMed] [Google Scholar]
- 18.Fester T, Hause B, Schmidt D, Halfmann K, Schmidt J, Wray V, et al. Occurrence and localization of apocarotenoids in arbuscular mycorrhizal plant roots. Plant Cell Physiol. 2002;43:256–265. doi: 10.1093/pcp/pcf029. [DOI] [PubMed] [Google Scholar]
- 19.Floss DS, Hause B, Lange PR, Küster H, Strack D, Walter MH. Knock-down of the MEP pathway isogene 1-deoxy-D-xylulose 5-phosphate synthase 2 inhibits formation of arbuscularmycorrhiza-induced apocarotenoids and abolishes normal expression of mycorrhiza-specific plant marker genes. Plant J. 2008;56:86–100. doi: 10.1111/j.1365-313X.2008.03575.x. [DOI] [PubMed] [Google Scholar]
- 20.Simkin AJ, Underwood BA, Auldridge ME, Loucas HM, Shibuya K, Schmelz E, et al. Circadian regulation of the PhCCD1 carotenoid cleavage dioxygenase controls emission of β-ionone, a fragrance volatile of petunia flowers. Plant Physiol. 2004;136:3504–3514. doi: 10.1104/pp.104.049718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simkin AJ, Schwartz SH, Auldridge ME, Taylor MG, Klee HJ. The tomato carotenoid cleavage dioxygenase 1 genes contribute to the formation of the flavor volatiles β-ionone, pseudoionone and geranylacetone. Plant J. 2004;40:882–892. doi: 10.1111/j.1365-313X.2004.02263.x. [DOI] [PubMed] [Google Scholar]
- 22.Mathieu S, Terrier N, Procureur J, Bigey F, Günata Z. A carotenoid cleavage dioxygenase from Vitis vinifera L.: functional characterization and expression during grape berry development in relation to C13-norisoprenoid accumulation. J Exp Bot. 2005;56:2721–2731. doi: 10.1093/jxb/eri265. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz SH, Qin X, Zeevaart JA. Characterization of a novel carotenoid cleavage dioxygenase from plants. J Biol Chem. 2001;276:25208–25211. doi: 10.1074/jbc.M102146200. [DOI] [PubMed] [Google Scholar]
- 24.Vogel JT, Tan BC, McCarty DR, Klee HJ. The carotenoid cleavage dioxygenase 1 enzyme has broad substrate specificity, cleaving multiple carotenoids at two different bond positions. J Biol Chem. 2008;283:11364–11373. doi: 10.1074/jbc.M710106200. [DOI] [PubMed] [Google Scholar]
- 25.Alder A, Holdermann I, Beyer P, Al-Babili S. Carotenoid oxygenases involved in plant branching catalyze a highly specific, conserved apocarotenoid cleavage reaction. Biochem J. 2008;416:289–296. doi: 10.1042/BJ20080568. [DOI] [PubMed] [Google Scholar]