Abstract
Leguminous plants form root nodules, in which symbiotic rhizobia fix atmospheric nitrogen and supply the fixation products to their host plants as a nitrogen source. On the process of establishing the symbiosis, rhizobia induce genes involved in the defense system of their host plants. However, the host defense responses will be cancelled by unknown mechanism. We focused on nitric oxide (NO) as a key molecule of plant defense system and class 1 plant hemoglobin (Hb) as a scavenger of NO. The inoculation of a symbiotic rhizobium, Mesorhizobium loti MAFF303099, induced transiently NO production and expression of a class 1 Hb gene LjHb1 in the roots of a model legume Lotus japonicus. In this addendum, we show that the lipopolysaccharide of M. loti induces NO production and expression of LjHb1 in L. japonicus, and we propose the role of NO and Hb at the early stage of symbiosis.
Key words: nitric oxide, hemoglobin, rhizobium, symbiosis, defense, Lotus japonicus, Mesorhizobium loti, lipopolysaccharide
Symbiotic interactions between leguminous plants and rhizobia have strict host specificity. The nodulation process of legume-rhizobium symbiosis consists of a series of events initiated by an exchange of specific signaling compounds between both partners.1,2 The early stages of the interaction between plants and symbiotic and pathogenic bacteria have much in common;3 the reactions against symbiotic rhizobia resemble plant defense response to pathogens. In many plants, infection by pathogens induces the expression of pathogenesis-related genes, including the phenylalanine ammonialyase (PAL) and chalcone synthase (CHS) genes.4 When Glycine max was inoculated with the symbiotic rhizobia Bradyrhizobium japonicum, PAL and CHS were induced during the early stages of the interaction.5 However, it is unclear how the induction of the plant defense response is cancelled or avoided to establish the symbiosis. We focused on two molecules, nitric oxide (NO) and class 1 plant hemoglobin (Hb), because NO is one of the key molecules in the plant defense system6 and class 1 Hb controls various NO-regulated physiological processes of plants by modulating NO levels7,8 with its extremely high affinity for NO.9,10
In our experiments, NO production of the inoculated roots was observed by fluorescent microscope using 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM DA) as a specific probe for NO. Planchet and Kaiser (2006)11 reported that DAF fluorescence was not a good indicator of NO, because DAF might react with unidentified DAF-reactive compound which was initially formed inside cells. Vitecek et al. (2008)12 showed that the reaction between DAF-FM and NO were both time- and pH-dependent observations. To examine whether the fluorescence of DAF-FM DA indicates NO production in roots of L. japonicus, we used NO scavenger carboxy-2-phenyl-4,4,5,5-tetramethylimidazoline-3-oxide-1-oxyl (c-PTIO). The fluorescence intensity of roots treated with c-PTIO was very low compared with that of roots without c-PTIO. The very deep color of c-PTIO solution sometimes masks the green fluorescence of DAF-FM DA. However, c-PTIO did not mask the fluorescence of roots expressing the green fluorescent protein gene under experimental conditions we employed.13 Furthermore, expression of LjHb1, which was induced by a NO donor (SNAP, S-nitroso-N-acetyl-D, L-penicillamine) and by inoculation with M. loti MAFF303099, was strongly inhibited by c-PTIO.13 We concluded that the fluorescence of DAF-FM DA indicated NO production in our experimental conditions.
The inoculation of M. loti induced transiently NO production and the expression of LjHb1. If LjHb1 converts NO to NO3−, LjHb1 may play a role as a NO scavenger and repress the induction of plant defense system. It is unclear whether the host legumes produce NO against all strains of their microsymbionts. However, once NO is produced by the host plant, it will be essential to reduce the NO level not to induce defense system (Fig. 1A).
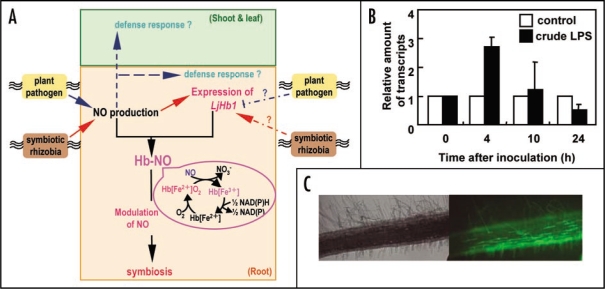
Figure 1.
(A) A schematic representation of a model for the role of NO and LjHb1 at the recognition between plant and its microsymbiont and plant pathogens. (B) LjHb1 expression and (C) NO production by inoculation with crude LPS of M. loti MAFF303099. Control plants were treated with sterilized water. Total RNAs were isolated from whole plants at indicated time after inoculation. Relative amounts of transcripts normalized by control plants are shown. Data represent the average of three independent experiments with standard error. Images of the roots in (C) were taken at 4 h after inoculation by fluorescence microscopy using DAF-FM DA as a NO detector.
The inoculation of plant pathogens (Ralstonia solanacearum MAFF730135, Pseudomonas syringae pv. pisi MAFF730032 and pv. glycinea MAFF302690) induced continuous NO production in the roots of L. japonicus. It is reported that NO itself functions as an inducer of LjHb1.14 However, no expression of LjHb1 was detectable in plants inoculated with plant pathogens. It is suggested that inoculation of plant pathogens induces rapid and high amount of NO production and inhibits the expression of LjHb1 in the roots (Fig. 1A). Although NO triggers plant defense system, excess amount of NO will be harmful for plant cells and will create favorable condition for the invasion of plant pathogens. We could not fully exclude another possibility; the host plants inoculated with these plant pathogens may release stable DAF-reactive compounds not related to NO as reported by Planchet and Kaiser (2006).11
To understand the mechanism of NO production in host plants, it is crucial to identify the bacterial factor(s) that induce NO. Plants recognize microbe-derived molecules as elicitors and induce defense responses including production of reactive oxygen species and antimicrobial compounds.15 Cell surface macromolecules or fragments, which are typically derived from microorganisms such as cell wall polysaccharides and flagella protein, serve as potent elicitors of plant defense responses.16,17 The defense systems mediated by the perception of these elicitors in plants have considerable similarities to the mammalian innate immunity networks in both the recognition of microbe associated molecular patterns (MAMPs) as well as the molecules involved in the perception and transduction of these signaling molecules.18 Lipopolysaccharides (LPS) that cover the cell surface of gram-negative bacteria have been shown to activate innate immune responses in both plants and animals.19,20 For instance, LPS from various bacterial strains induce NO production in cultured Arabidopsis thaliana cells.21 We focused on LPS of M. loti as a candidate for an inducer of NO production and LjHb1 expression in L. japonicus. The plants of L. japonicus (14 days after germination) were treated with crude LPS prepared from M. loti by hot-phenol method. RNAs were extracted from L. japonicus at various time intervals after crude LPS addition. Relative amounts of LjHb1 transcripts were estimated by real-time reverse transcriptase (RT)-PCR. Using DAF-FM DA, we also examined the production of NO by rhizobial crude LPS. When L. japonicus was treated with crude LPS of M. loti, transient expression of LjHb1 and the distinct DAF-fluorescence were observed in the roots of L. japonicus as same profile to inoculation of symbiotic rhizobial cells (Fig. 1B and C). These results suggest that LPS of symbiotic rhizobia is one of the candidates for an inducer of NO production in host plants.
In our model, class 1 plant Hb facilitates establishing the symbiosis successfully by modulation of NO level when host plants produce NO as a response against symbiotic rhizobia. The use of plant mutant lines, i.e., overexpression, RNAi and structural mutant of class 1 Hb, will help the future confirmation of our hypothesis. NO is detectable inside the nodules even after the symbiosis is established.22,23 It will be another interest whether NO in nodules contribute to induction of the systemic resistance of host plants.
Acknowledgements
We thank H. Kaku and H. Ochiai (National Institute of Agribiological Sciences, Japan) for providing us information about R. solanacearum and T. Nakagawa and K. Takeuchi (National Institute of Agribiological Sciences, Japan) for providing us information about P. syringae strains.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/7796
References
- 1.Mulligan JT, Long SR. Induction of Rhizobium meliloti nodC expression by plant exudate requires nodD. Proc Natl Acad Sci USA. 1985;82:6609–6613. doi: 10.1073/pnas.82.19.6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lerouge P, Roche P, Faucher C, Maillet F, Truchet G, Promé JC, et al. Symbiotic host-specificity of Rhizobium meliloti is determined by a sulphated and acylated glucosamine oligosaccharide signal. Nature. 1990;344:781–784. doi: 10.1038/344781a0. [DOI] [PubMed] [Google Scholar]
- 3.Baron C, Zambryski PC. The plant response in pathogenesis, symbiosis and wounding: Variations on a common theme? Annu Rev Genetics. 1995;29:107–129. doi: 10.1146/annurev.ge.29.120195.000543. [DOI] [PubMed] [Google Scholar]
- 4.Mishina TE, Zeier J. Pathogen-associated molecular pattern recognition rather than development of tissue necrosis contributes to bacterial induction of systemic acquired resistance in Arabidopsis. Plant J. 2007;50:500–513. doi: 10.1111/j.1365-313X.2007.03067.x. [DOI] [PubMed] [Google Scholar]
- 5.Estabrook EM, Sengupta-Gopalan C. Differential expression of phenylalanine ammonia-lyase and chalcone synthase during soybean nodule development. Plant Cell. 1991;3:299–308. doi: 10.1105/tpc.3.3.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klessig DF, Durner J, Noad R, Navarre DA, Wendehenne D, Kumar D, et al. Nitric oxide and salicylic acid signaling in plant defense. Proc Natl Acad Sci USA. 2000;97:8849–8855. doi: 10.1073/pnas.97.16.8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seregélyes C, Barna B, Hennig J, Konopka D, Pasternak TP, Luács N, et al. Phytoglobins can interfere with nitric oxide functions during plant growth and pathogenic responses: A transgenic approach. Plant Sci. 2003;165:541–550. [Google Scholar]
- 8.Seregélyes C, Igamberdiev AU, Maassen A, Hennig J, Dudits D, Hill RD. NO-degradation by alfalfa class 1 hemoglobin (Mhb1): A possible link to PR-1a gene expression in Mhb1-overproducing tobacco plants. FEBS Lett. 2004;571:61–66. doi: 10.1016/j.febslet.2004.06.055. [DOI] [PubMed] [Google Scholar]
- 9.Dordas C, Hasinoff BB, Rivoal J, Hill RD. Class 1 hemoglobins, nitrate and NO levels in anoxic maize cell suspension cultures. Planta. 2004;219:66–72. doi: 10.1007/s00425-004-1212-y. [DOI] [PubMed] [Google Scholar]
- 10.Perazzolli M, Dominici P, Romero-Puertas MC, Zago E, Zeier J, Sonoda M, et al. Arabidopsis nonsymbiotic hemoglobin AHb1 modulates nitric oxide bioactivity. Plant Cell. 2004;16:2785–2794. doi: 10.1105/tpc.104.025379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Planchet E, Kaiser WM. Nitric oxide (NO) detection by DAF fluorescence and chemiluminecence: a comparison using abiotic and biotic NO sources. J Exp Bot. 2006;57:3043–3055. doi: 10.1093/jxb/erl070. [DOI] [PubMed] [Google Scholar]
- 12.Vitecek J, Reinohl V, Jones RL. Measuring NO production by plant tissues and suspension cultured cells. Mol Plant. 2008;1:270–284. doi: 10.1093/mp/ssm020. [DOI] [PubMed] [Google Scholar]
- 13.Nagata M, Murakami E, Shimoda Y, Shimoda-Sasakura F, Kucho K, Suzuki A, et al. Expression of a class 1 hemoglobin gene and production of nitric oxide in response to symbiotic and pathogenic bacteria in Lotus japonicus. Mol Plant-Microbe Interact. 2008;21:1175–1183. doi: 10.1094/MPMI-21-9-1175. [DOI] [PubMed] [Google Scholar]
- 14.Shimoda Y, Nagata M, Suzuki A, Abe M, Sato S, Kato T, et al. Symbiotic rhizobium and nitric oxide induce gene expression of non-symbiotic hemoglobin in Lotus japonicus. Plant Cell Physiol. 2005;46:99–107. doi: 10.1093/pci/pci001. [DOI] [PubMed] [Google Scholar]
- 15.Desaki Y, Miya A, Venkatesh B, Tsuyumu S, Yamane H, Kaku H, et al. Bacterial lipopolysaccharides induce defense responses associated with programmed cell death in rice cells. Plant Cell Physiol. 2006;47:1530–1540. doi: 10.1093/pcp/pcl019. [DOI] [PubMed] [Google Scholar]
- 16.Sharp JK, Valent B, Albersheim P. Purification and partial characterization of a b-glucan fragment that elicites phytoalexin accumulation in soybean. J Biol Chem. 1984;259:11312–11320. [PubMed] [Google Scholar]
- 17.Felix G, Duran JD, Volko S, Boller T. Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 1999;18:265–276. doi: 10.1046/j.1365-313x.1999.00265.x. [DOI] [PubMed] [Google Scholar]
- 18.Nürnberger T, Brunner F, Kemmerling B, Piater L. Innate immunity in plants and animals: striking similarities and obvious differences. Immunol Rev. 2004;198:249–266. doi: 10.1111/j.0105-2896.2004.0119.x. [DOI] [PubMed] [Google Scholar]
- 19.Erridge C, Bennett-Guerrero E, Poxton IR. Structure and function of lipopolysaccharides. Microb Infect. 2002;4:837–851. doi: 10.1016/s1286-4579(02)01604-0. [DOI] [PubMed] [Google Scholar]
- 20.D'Haeze W, Leoff C, Freshour G, Noel KD, Carlson RW. Rhizobium etli CE3 bacteroid lipopolysaccharides are structurally similar but not identical to those produced by cultured CE3 bacteria. J Biol Chem. 2007;282:17101–17113. doi: 10.1074/jbc.M611669200. [DOI] [PubMed] [Google Scholar]
- 21.Zeidler D, Zähringer U, Gerber I, Dubery I, Hartung T, Bors W, et al. Innate immunity in Arabidopsis thaliana: Lipopolysaccharides activate nitric oxide synthase (NOS) and induce defense genes. Proc Natl Acad Sci USA. 2004;101:15811–15816. doi: 10.1073/pnas.0404536101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baudouin E, Pieuchot L, Engler G, Pauly N, Puppo A. Nitric oxide is formed in Medicago truncatula-Sinorhizobium meliloti functional nodules. Mol Plant-Microbe Interact. 2006;19:970–975. doi: 10.1094/MPMI-19-0970. [DOI] [PubMed] [Google Scholar]
- 23.Shimoda Y, Shimoda-Sasakura F, Kucho K, Kanamori N, Nagata M, Suzuki A, et al. Overexpression of class 1 plant hemoglobin genes enhances symbiotic nitrogen fixation activity between Mesorhizobium loti and Lotus japonicus. Plant J. 2009;57:254–263. doi: 10.1111/j.1365-313X.2008.03689.x. [DOI] [PubMed] [Google Scholar]